Abstract
We present a genome assembly from an individual Ailanthus altissima (tree of heaven; Streptophyta; Magnoliopsida; Sapindales; Simaroubaceae). The genome sequence is 939 megabases in span. Most of the assembly is scaffolded into 31 chromosomal pseudomolecules. The mitochondrial and plastid genome assemblies are 661.1 kilobases and 161.1 kilobases long, respectively.
Keywords: Ailanthus altissima, tree of heaven, genome sequence, chromosomal, Simaroubaceae
Species taxonomy
Eukaryota; Viridiplantae; Streptophyta; Embryophyta; Tracheophyta; Spermatophyta; Magnoliopsida; eudicotyledons; Gunneridae; Pentapetalae; rosids; malvids; Sapindales; Simaroubaceae; Ailanthus; Ailanthus altissimus (Mill.) Swingle, 1916 (NCBItxid:23810).
Background
The tree of heaven ( Ailanthus altissima, Simaroubaceae) is a deciduous tree native to China and Taiwan, known for its fast growth and attractive foliage. For these reasons, it has been widely planted as an ornamental tree ( Figure 1). Ailanthus altissima was first introduced to the UK in 1751 as a garden plant ( Hu, 1979), during a period when European fascination with Chinese culture (‘ Chinoiserie’) was at its height. Ailanthus altissima has long been used in Chinese traditional medicine as well as in silk production, as a foodplant for the Ailanthus silk moth ( Samia cynthia) ( Swingle, 1916). Ailanthus altissima has since become an invasive species across North America, southern South America, South Africa, New Zealand and Europe due to its rapid growth, resistance to pollution, fecundity, vegetative reproduction and use of allelopathic chemicals to stifle competition ( Demeter et al., 2021).
Figure 1. Ailanthus altissima (not the sampled specimen).
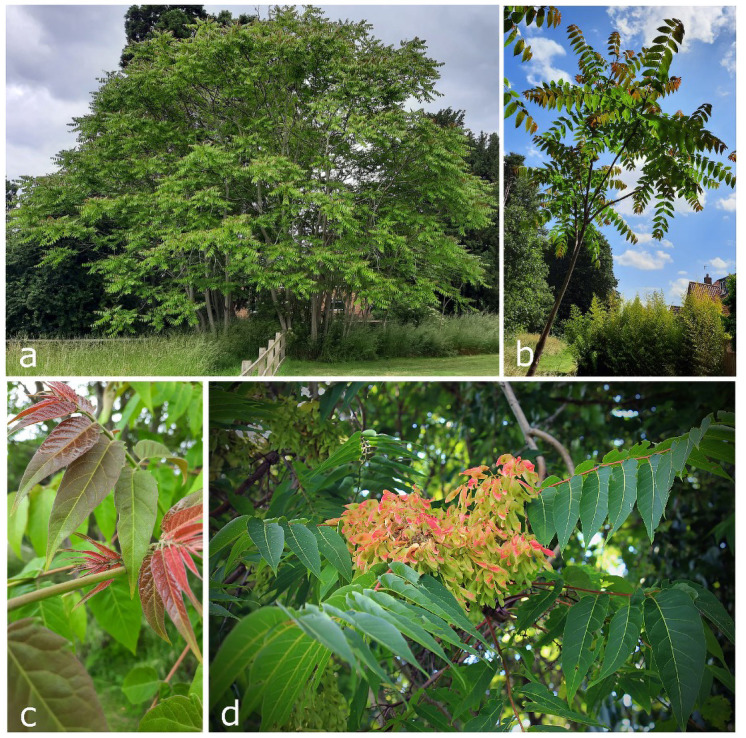
a) mature tree; b) young sapling, and c) new leaves in Nottinghamshire (photographs © Carlton Roberts); d) A. altissima fruits (photograph © Nicholas A. Tonelli).
The genus name Ailanthus is derived from the Ambonese word ‘ailanto’, meaning ‘heaven-tree’, and the specific epithet altissima is Latin for ‘tallest’, referring to the size of the mature tree. Three varieties of A. altissima have been described – A. altissima var. altissima, native to mainland China, A. altissima var. sutchuenensis (Dode) Rehder and E. H. Wilson, restricted to southern China and A. altissima var. tanakae (Hayata) Kaneh. and Sasaki, endemic to northern Taiwan. Multiple chromosome counts have been reported for A. altissima, ranging from 2 n = 80 for arboretum specimens examined at the Royal Botanic Gardens, Kew ( Desai, 1960), down to 2 n = 64 for samples collected from naturalised populations in mainland Europe ( Ivanova et al., 2006), suggesting polyploidy with different cytotypes between individuals.
Here we present the first high-quality genome of A. altissima var. altissima, which we believe will be a useful resource both for those studying the tree in its native range and those aiming to understand what underlies its success as an invasive species. This genome complements existing studies which identified microsatellites for use in population genetic analysis ( Dallas et al., 2005; Saina et al., 2021), and those which sequenced the plastome of the species ( Saina et al., 2018). Fruitful directions for future work may include investigating the population genetics of invasion, building on previous work showing population bottlenecks in invasive populations of A. altissima ( Neophytou et al., 2020).
Genome sequence report
The genome was sequenced from the leaves of an A. altissima specimen (drAilAlti1) collected from the Royal Botanic Gardens, Kew (latitude 51.482, longitude –0.2879). Using flow cytometry, the genome size (1C-value) was estimated as 1.20 pg, equivalent to 1,170 Mb. A total of 45-fold coverage in Pacific Biosciences single-molecule HiFi long reads and 35-fold coverage in 10X Genomics read clouds were generated. Primary assembly contigs were scaffolded with chromosome conformation Hi-C data. Manual assembly curation corrected 17 missing joins or misjoins and removed one haplotypic duplication, reducing the assembly length by 0.11% and the scaffold number by 33.93%, and decreasing the scaffold N50 by 3.45%.
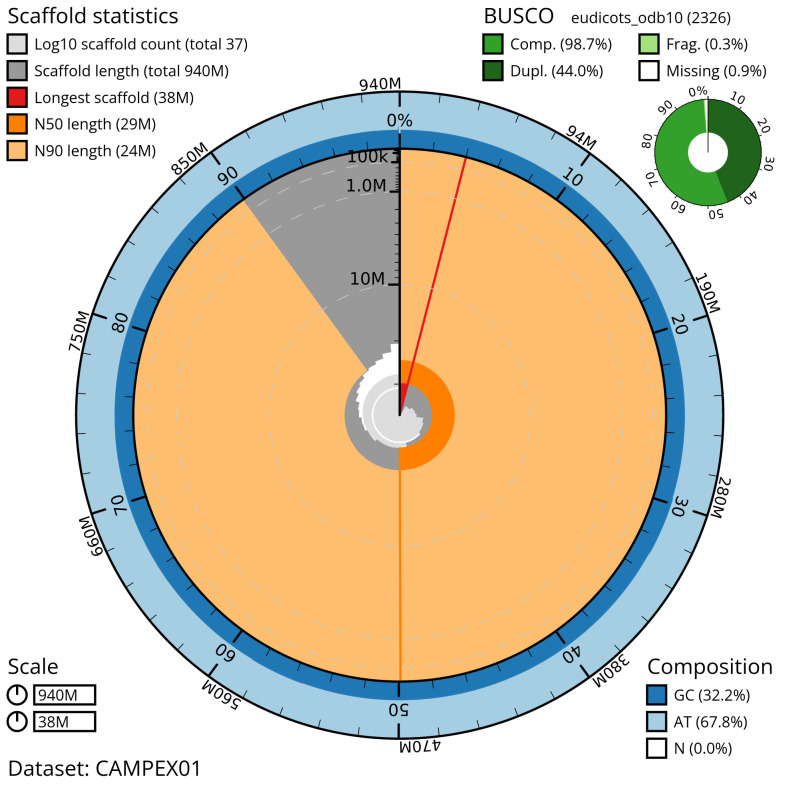
The final assembly has a total length of 939 Mb in 37 sequence scaffolds with a scaffold N50 of 29.4 Mb ( Table 1). Most of the assembly sequence (97.72%) was assigned to 31 chromosomal-level scaffolds ( Figure 2– Figure 5; Table 2). Several small repetitive scaffolds localise equally well to both chromosome 28 and chromosome 9. They have been placed with chromosome 9, although their placement is uncertain. While not fully phased, the assembly deposited is of one haplotype. Contigs corresponding to the second haplotype have also been deposited.
Figure 2. Genome assembly of Ailanthus altissima, drAilAlti1.1: metrics.
The BlobToolKit Snailplot shows N50 metrics and BUSCO gene completeness. The main plot is divided into 1,000 size-ordered bins around the circumference with each bin representing 0.1% of the 939,218,608 bp assembly. The distribution of scaffold lengths is shown in dark grey with the plot radius scaled to the longest scaffold present in the assembly (38,427,504 bp, shown in red). Orange and pale-orange arcs show the N50 and N90 scaffold lengths (29,421,908 and 24,184,688 bp, respectively). The pale grey spiral shows the cumulative scaffold count on a log scale with white scale lines showing successive orders of magnitude. The blue and pale-blue area around the outside of the plot shows the distribution of GC, AT and N percentages in the same bins as the inner plot. A summary of complete, fragmented, duplicated and missing BUSCO genes in the eudicots_odb10 set is shown in the top right. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/CAMPEX01/dataset/CAMPEX01/snail.
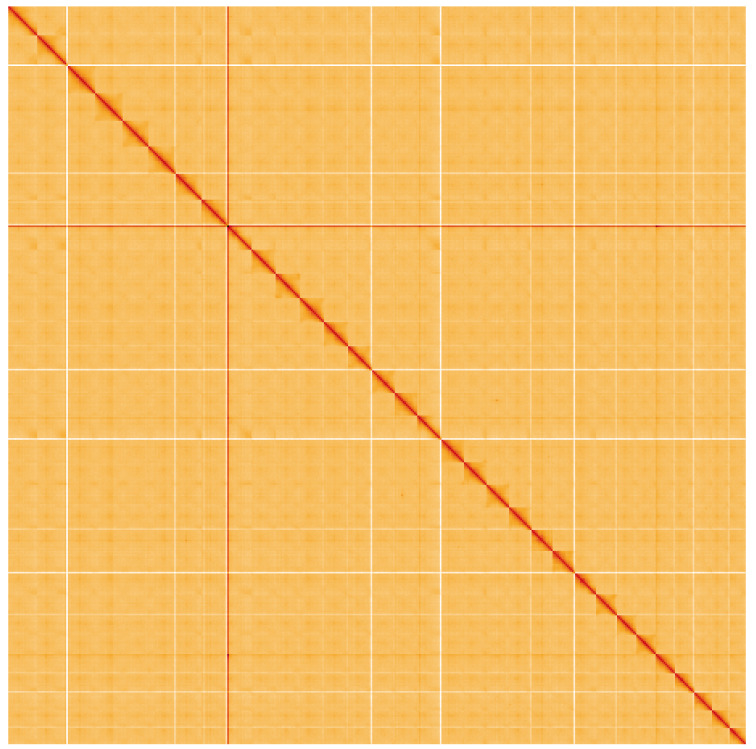
Figure 5. Genome assembly of Ailanthus altissima, drAilAlti1.1: Hi-C contact map.
Hi-C contact map of the drAilAlti1.1 assembly, visualised using HiGlass. Chromosomes are given in order of size from left to right and top to bottom. An interactive version of this figure is available at https://genome-note-higlass.tol.sanger.ac.uk/l/?d=XyRKDNDOTHy19SzkZkKRWw.
Table 1. Genome data for Ailanthus altissima, drAilAlti1.1.
| Project accession data | ||
|---|---|---|
| Assembly identifier | drAilAlti1.1 | |
| Species | Ailanthus altissima | |
| Specimen | drAilAlti1 | |
| NCBI taxonomy ID | 23810 | |
| BioProject | PRJEB47393 | |
| BioSample ID | SAMEA7522284 | |
| Isolate information | Monoecious; leaves | |
| Assembly metrics * | Benchmark | |
| Consensus quality (QV) | 61.1 | ≥ 50 |
| k-mer completeness | 100% | ≥ 95% |
| BUSCO ** | C:98.7%[S:54.7%,D:44.0%],
F:0.3%,M:0.9%,n:2,326 |
C ≥ 95% |
| Percentage of assembly
mapped to chromosomes |
97.72% | ≥ 95% |
| Sex chromosomes | Not applicable | localised homologous pairs |
| Organelles | MT single scaffold of 661,054 bp
Plastid single scaffold of 161,079 bp |
complete single alleles |
| Raw data accessions | ||
| PacificBiosciences SEQUEL II | ERR6808067, ERR6939282 | |
| 10X Genomics Illumina | ERR6745740–ERR6745743 | |
| Hi-C Illumina | ERR6745744 | |
| PolyA RNA-Seq Illumina | ERR9435026, ERR9435027 | |
| Genome assembly | ||
| Assembly accession | GCA_946807835.1 | |
| Accession of alternate haplotype | GCA_946808065.1 | |
| Span (Mb) | 939 | |
| Number of contigs | 49 | |
| Contig N50 length (Mb) | 29.4 Mb | |
| Number of scaffolds | 37 | |
| Scaffold N50 length (Mb) | 29.3 Mb | |
| Longest scaffold (Mb) | 38.4 | |
* Assembly metric benchmarks are adapted from column VGP-2020 of “Table 1: Proposed standards and metrics for defining genome assembly quality” from ( Rhie et al., 2021).
** BUSCO scores based on the eudicots_odb10 BUSCO set using v5.3.2. C = complete [S = single copy, D = duplicated], F = fragmented, M = missing, n = number of orthologues in comparison. A full set of BUSCO scores is available at https://blobtoolkit.genomehubs.org/view/CAMPEX01/dataset/CAMPEX01/busco.
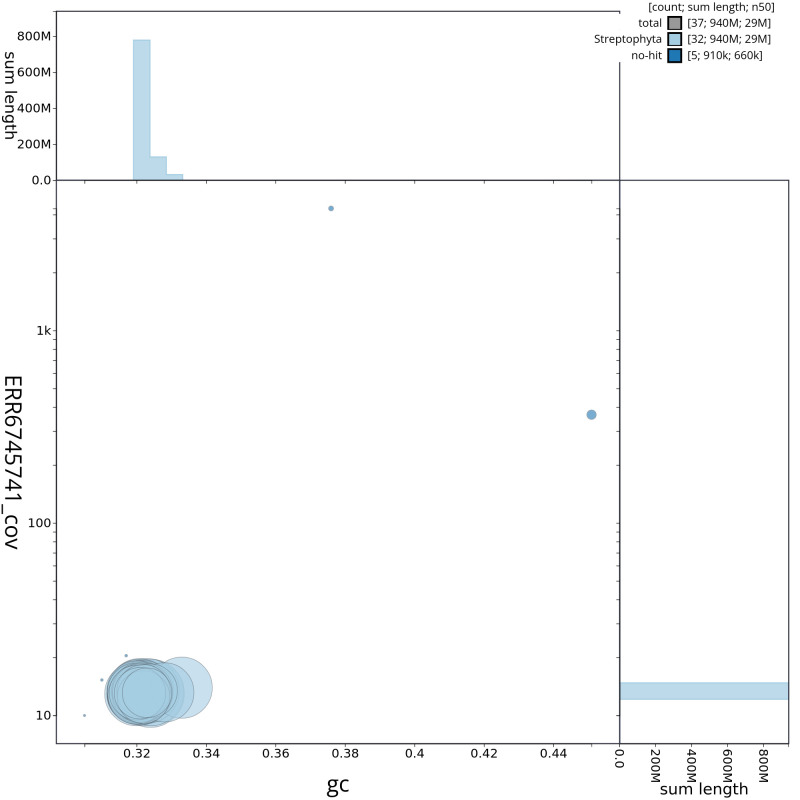
Figure 3. Genome assembly of Ailanthus altissima, drAilAlti1.1: GC coverage.
BlobToolKit GC-coverage plot. Chromosomes are coloured by phylum. Circles are sized in proportion to chromosome length. Histograms show the distribution of chromosome length sum along each axis. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/CAMPEX01/dataset/CAMPEX01/blob.
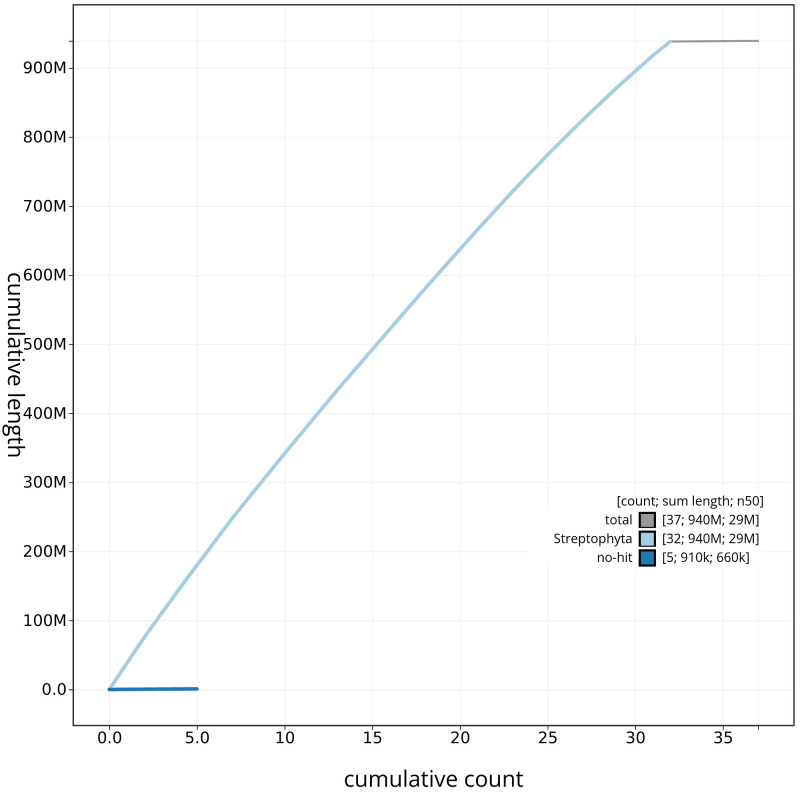
Figure 4. Genome assembly of Ailanthus altissima, drAilAlti1.1: cumulative sequence.
BlobToolKit cumulative sequence plot. The grey line shows cumulative length for all chromosomes. Coloured lines show cumulative lengths of chromosomes assigned to each phylum using the buscogenes taxrule. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/CAMPEX01/dataset/CAMPEX01/cumulative.
Table 2. Chromosomal pseudomolecules in the genome assembly of Ailanthus altissima, drAilAlti1.
| INSDC accession | Chromosome | Size (Mb) | GC% |
|---|---|---|---|
| OX327683.1 | 1 | 38.43 | 32.4 |
| OX327684.1 | 2 | 37.36 | 32.4 |
| OX327685.1 | 3 | 35.13 | 32.1 |
| OX327686.1 | 4 | 35.07 | 32.3 |
| OX327687.1 | 5 | 34.17 | 32 |
| OX327688.1 | 6 | 33.92 | 32.1 |
| OX327689.1 | 7 | 33.54 | 32.1 |
| OX327690.1 | 8 | 31.7 | 32.1 |
| OX327691.1 | 9 | 31.31 | 33.3 |
| OX327692.1 | 10 | 31.18 | 32.1 |
| OX327693.1 | 11 | 30.58 | 32.1 |
| OX327694.1 | 12 | 30.54 | 32.2 |
| OX327695.1 | 13 | 30.47 | 32.1 |
| OX327696.1 | 14 | 29.5 | 32 |
| OX327697.1 | 15 | 29.42 | 32 |
| OX327698.1 | 16 | 29.33 | 32 |
| OX327699.1 | 17 | 29.31 | 32.8 |
| OX327700.1 | 18 | 29.28 | 32 |
| OX327701.1 | 19 | 28.68 | 32 |
| OX327702.1 | 20 | 28.67 | 32.1 |
| OX327703.1 | 21 | 28.14 | 32.2 |
| OX327704.1 | 22 | 27.98 | 32.2 |
| OX327705.1 | 23 | 27.39 | 32 |
| OX327706.1 | 24 | 27.08 | 32.1 |
| OX327707.1 | 25 | 26.09 | 32.1 |
| OX327708.1 | 26 | 25.42 | 32.1 |
| OX327709.1 | 27 | 24.94 | 32 |
| OX327710.1 | 28 | 24.18 | 32.4 |
| OX327711.1 | 29 | 23.78 | 32.2 |
| OX327712.1 | 30 | 23.17 | 32.2 |
| OX327713.1 | 31 | 22.13 | 32.1 |
| OX327714.1 | MT | 0.66 | 45.1 |
| OX327715.1 | Pltd | 0.16 |
The estimated Quality Value (QV) of the final assembly is 61.1 with k-mer completeness of 100%, and the assembly has a BUSCO v5.3.2 completeness of 98.7% (single 54.7%, duplicated 44.0%), using the eudicots_odb10 reference set ( n = 2,326).
Metadata for specimens, spectral estimates, sequencing runs, contaminants and pre-curation assembly statistics can be found at https://links.tol.sanger.ac.uk/species/23810.
Methods
Sample acquisition, genome size estimation and nucleic acid extraction
Ailanthus altissima grows as a weed along pavements and in neglected plots in the UK. Leaves from an A. altissima individual (drAilAlti1) were harvested from a plant growing in the pavement near the Jodrell Gate, Royal Botanic Gardens Kew, Surrey, UK (latitude 51.482, longitude –0.2879). The sample was collected and identified by Maarten Christenhusz (Royal Botanic Gardens, Kew). The leaves were preserved by freezing at –80°C.
The genome size was estimated by flow cytometry using the fluorochrome propidium iodide and following the ‘one-step’ method as outlined in ( Pellicer et al., 2021). Specifically for this species, the General Purpose Buffer (GPB) supplemented with 3% PVP and 0.08% (v/v) beta-mercaptoethanol was used for isolation of nuclei ( Loureiro et al., 2007), and the internal calibration standard was Petroselinum crispum ‘Champion Moss Curled’ with an assumed 1C-value of 2,200 Mb ( Obermayer et al., 2002).
DNA was extracted at the Tree of Life laboratory, Wellcome Sanger Institute (WSI). The drAilAlti1 sample was weighed and dissected on dry ice with tissue set aside for Hi-C sequencing. Leaf tissue was cryogenically disrupted to a fine powder using Qiagen Plant Magattract, receiving multiple impacts. High molecular weight (HMW) DNA was extracted using the Qiagen MagAttract HMW DNA extraction kit. Low molecular weight DNA was removed from a 20 ng aliquot of extracted DNA using 0.8X AMpure XP purification kit prior to 10X Chromium sequencing; a minimum of 50 ng DNA was submitted for 10X sequencing. HMW DNA was sheared into an average fragment size of 12–20 kb in a Megaruptor 3 system with speed setting 30. Sheared DNA was purified by solid-phase reversible immobilisation using AMPure PB beads with a 1.8× ratio of beads to sample to remove the shorter fragments and concentrate the DNA sample. The concentration of the sheared and purified DNA was assessed using a Nanodrop spectrophotometer and Qubit Fluorometer and Qubit dsDNA High Sensitivity Assay kit. Fragment size distribution was evaluated by running the sample on the FemtoPulse system.
RNA was extracted from leaf tissue of drAilAlti1 in the Tree of Life Laboratory at the WSI using TRIzol, according to the manufacturer’s instructions. RNA was then eluted in 50 μL RNAse-free water and its concentration was assessed using a Nanodrop spectrophotometer and Qubit Fluorometer using the Qubit RNA Broad-Range (BR) Assay kit. Analysis of the integrity of the RNA was done using Agilent RNA 6000 Pico Kit and Eukaryotic Total RNA assay.
Sequencing
Pacific Biosciences HiFi circular consensus and 10X Genomics read cloud DNA sequencing libraries were constructed according to the manufacturers’ instructions. Poly(A) RNA-Seq libraries were constructed using the NEB Ultra II RNA Library Prep kit. DNA and RNA sequencing were performed by the Scientific Operations core at the WSI on Pacific Biosciences SEQUEL II (HiFi), Illumina HiSeq 4000 (RNA-Seq) and Illumina NovaSeq 6000 (10X) instruments. Hi-C data were also generated from material from drAilAlti1 using the Arima v2 kit and sequenced on the Illumina NovaSeq 6000 instrument.
Genome assembly, curation and evaluation
Assembly was carried out with Hifiasm ( Cheng et al., 2021) and haplotypic duplication was identified and removed with purge_dups ( Guan et al., 2020). One round of polishing was performed by aligning 10X Genomics read data to the assembly with Long Ranger ALIGN, calling variants with FreeBayes ( Garrison & Marth, 2012). The assembly was then scaffolded with Hi-C data ( Rao et al., 2014) using SALSA2 ( Ghurye et al., 2019). The assembly was checked for contamination and corrected using the gEVAL system ( Chow et al., 2016) as described previously ( Howe et al., 2021). Manual curation was performed using gEVAL, HiGlass ( Kerpedjiev et al., 2018) and Pretext ( Harry, 2022). The mitochondrial and plastid genomes were assembled using MBG ( Rautiainen & Marschall, 2021) from PacBio HiFi reads mapping to related genomes. A representative circular sequence was selected for each from the graph based on read coverage.
A Hi-C map for the final assembly was produced using bwa-mem2 ( Vasimuddin et al., 2019) in the Cooler file format ( Abdennur & Mirny, 2020). To assess the assembly metrics, the k-mer completeness and QV consensus quality values were calculated in Merqury ( Rhie et al., 2020). This work was done using Nextflow ( Di Tommaso et al., 2017) DSL2 pipelines “sanger-tol/readmapping” ( Surana et al., 2023a) and “sanger-tol/genomenote” ( Surana et al., 2023b). The genome was analysed within the BlobToolKit environment ( Challis et al., 2020) and BUSCO scores ( Manni et al., 2021; Simão et al., 2015) were calculated.
Table 3 contains a list of relevant software tool versions and sources.
Table 3. Software tools: versions and sources.
| Software tool | Version | Source |
|---|---|---|
| BlobToolKit | 3.3.8 | https://github.com/blobtoolkit/blobtoolkit |
| BUSCO | 5.3.2 | https://gitlab.com/ezlab/busco |
| FreeBayes | 1.3.1-17-gaa2ace8 | https://github.com/freebayes/freebayes |
| gEVAL | N/A | https://geval.org.uk/ |
| Hifiasm | 0.15.3 | https://github.com/chhylp123/hifiasm |
| HiGlass | 1.11.6 | https://github.com/higlass/higlass |
| Long Ranger ALIGN | 2.2.2 |
https://support.10xgenomics.com/genome-exome/
software/pipelines/latest/advanced/other-pipelines |
| Merqury | MerquryFK | https://github.com/thegenemyers/MERQURY.FK |
| MBG | - | https://github.com/maickrau/MBG |
| PretextView | 0.2 | https://github.com/wtsi-hpag/PretextView |
| purge_dups | 1.2.3 | https://github.com/dfguan/purge_dups |
| SALSA | 2.2 | https://github.com/salsa-rs/salsa |
Wellcome Sanger Institute – Legal and Governance
The materials that have contributed to this genome note have been supplied by a Darwin Tree of Life Partner. The submission of materials by a Darwin Tree of Life Partner is subject to the ‘Darwin Tree of Life Project Sampling Code of Practice’, which can be found in full on the Darwin Tree of Life website here. By agreeing with and signing up to the Sampling Code of Practice, the Darwin Tree of Life Partner agrees they will meet the legal and ethical requirements and standards set out within this document in respect of all samples acquired for, and supplied to, the Darwin Tree of Life Project.
Further, the Wellcome Sanger Institute employs a process whereby due diligence is carried out proportionate to the nature of the materials themselves, and the circumstances under which they have been/are to be collected and provided for use. The purpose of this is to address and mitigate any potential legal and/or ethical implications of receipt and use of the materials as part of the research project, and to ensure that in doing so we align with best practice wherever possible. The overarching areas of consideration are:
• Ethical review of provenance and sourcing of the material
• Legality of collection, transfer and use (national and international)
Each transfer of samples is further undertaken according to a Research Collaboration Agreement or Material Transfer Agreement entered into by the Darwin Tree of Life Partner, Genome Research Limited (operating as the Wellcome Sanger Institute), and in some circumstances other Darwin Tree of Life collaborators.
Funding Statement
This work was supported by Wellcome through core funding to the Wellcome Sanger Institute (206194, <a href=https://doi.org/10.35802/206194>https://doi.org/10.35802/206194</a>) and the Darwin Tree of Life Discretionary Award (218328, <a href=https://doi.org/10.35802/218328>https://doi.org/10.35802/218328</a>).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
Data availability
European Nucleotide Archive: Ailanthus altissima (tree of heaven). Accession number PRJEB47393; https://identifiers.org/ena.embl/PRJEB47393 ( Wellcome Sanger Institute, 2022)
The genome sequence is released openly for reuse. The A. altissima genome sequencing initiative is part of the Darwin Tree of Life (DToL) project. All raw sequence data and the assembly have been deposited in INSDC databases. The genome will be annotated using available RNA-Seq data and presented through the Ensembl pipeline at the European Bioinformatics Institute. Raw data and assembly accession identifiers are reported in Table 1.
Author information
Members of the Royal Botanic Gardens Kew Genome Acquisition Lab are listed here: https://doi.org/10.5281/zenodo.4786680.
Members of the Plant Genome Sizing collective are listed here: https://doi.org/10.5281/zenodo.7994306.
Members of the Darwin Tree of Life Barcoding collective are listed here: https://doi.org/10.5281/zenodo.4893703.
Members of the Wellcome Sanger Institute Tree of Life programme are listed here: https://doi.org/10.5281/zenodo.4783585.
Members of Wellcome Sanger Institute Scientific Operations: DNA Pipelines collective are listed here: https://doi.org/10.5281/zenodo.4790455.
Members of the Tree of Life Core Informatics collective are listed here: https://doi.org/10.5281/zenodo.5013541.
References
- Abdennur N, Mirny LA: Cooler: Scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics. 2020;36(1):311–316. 10.1093/bioinformatics/btz540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis R, Richards E, Rajan J, et al. : BlobToolKit – interactive quality assessment of genome assemblies. G3 (Bethesda). 2020;10(4):1361–1374. 10.1534/g3.119.400908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Concepcion GT, Feng X, et al. : Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18(2):170–175. 10.1038/s41592-020-01056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow W, Brugger K, Caccamo M, et al. : gEVAL – a web-based browser for evaluating genome assemblies. Bioinformatics. 2016;32(16):2508–2510. 10.1093/bioinformatics/btw159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas JF, Leitch M, Hulme PE: Microsatellites for tree of heaven ( Ailanthus altissima). Mol Ecol Notes. 2005;5(2):340–342. 10.1111/j.1471-8286.2005.00920.x [DOI] [Google Scholar]
- Demeter A, Saláta D, Tormáné Kovács E, et al. : Effects of the invasive tree species Ailanthus altissima on the floral diversity and soil properties in the Pannonian region. Land. 2021;10(11):1155. 10.3390/land10111155 [DOI] [Google Scholar]
- Desai S: Cytology of Rutaceae and Simarubaceae. Cytologia. 1960;25(1):28–35. 10.1508/cytologia.25.28 [DOI] [Google Scholar]
- Di Tommaso P, Chatzou M, Floden EW, et al. : Nextflow enables reproducible computational workflows. Nat Biotechnol. 2017;35(4):316–319. 10.1038/nbt.3820 [DOI] [PubMed] [Google Scholar]
- Garrison E, Marth G: Haplotype-based variant detection from short-read sequencing. 2012. 10.48550/arXiv.1207.3907 [DOI] [Google Scholar]
- Ghurye J, Rhie A, Walenz BP, et al. : Integrating Hi-C links with assembly graphs for chromosome-scale assembly. PLoS Comput Biol. 2019;15(8): e1007273. 10.1371/journal.pcbi.1007273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, McCarthy SA, Wood J, et al. : Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 2020;36(9):2896–2898. 10.1093/bioinformatics/btaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry E: PretextView (Paired REad TEXTure Viewer): A desktop application for viewing pretext contact maps. 2022; [Accessed 19 October 2022]. Reference Source
- Howe K, Chow W, Collins J, et al. : Significantly improving the quality of genome assemblies through curation. GigaScience. Oxford University Press,2021;10(1): giaa153. 10.1093/gigascience/giaa153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SY: Ailanthus. Arnoldia. 1979;39(2):29–50. Reference Source [Google Scholar]
- Ivanova D, Dimitrova D, Vladimirov V: Chromosome numbers of selected woody species from the Bulgarian flora. Phytologia Balcanica. 2006;12(1):79–84. Reference Source [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, et al. : HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018;19(1): 125. 10.1186/s13059-018-1486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J, Rodriguez E, Dolezel J, et al. : Two new nuclear isolation buffers for plant DNA flow cytometry: A test with 37 species. Ann Bot. 2007;100(4):875–888. 10.1093/aob/mcm152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, et al. : BUSCO update: Novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol. 2021;38(10):4647–4654. 10.1093/molbev/msab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neophytou C, Pötzelsberger E, Curto M, et al. : Population bottlenecks have shaped the genetic variation of Ailanthus altissima (Mill.) Swingle in an area of early introduction. Forestry: An International Journal of Forest Research. 2020;93(4):495–504. 10.1093/forestry/cpz019 [DOI] [Google Scholar]
- Obermayer R, Leitch IJ, Hanson L, et al. : Nuclear DNA C-values in 30 species double the familial representation in pteridophytes. Ann Bot. 2002;90(2):209–217. 10.1093/aob/mcf167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer J, Powell RF, Leitch IJ: The application of flow cytometry for estimating genome Size, Ploidy Level Endopolyploidy, and Reproductive Modes in Plants.In: Besse, P. (ed.) Methods Mol Biol.New York, NY: Humana,2021;2222:325–361. 10.1007/978-1-0716-0997-2_17 [DOI] [PubMed] [Google Scholar]
- Rao SSP, Huntley MH, Durand NC, et al. : A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–1680. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautiainen M, Marschall T: Minimizer-based sparse de Bruijn Graph construction. Bioinformatics. 2021;37(16):2476–2478. 10.1093/bioinformatics/btab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, McCarthy SA, Fedrigo O, et al. : Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021;592(7856):737–746. 10.1038/s41586-021-03451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, et al. : Merqury: Reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21(1): 245. 10.1186/s13059-020-02134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saina JK, Li ZZ, Gichira AW, et al. : The complete chloroplast genome sequence of Tree of Heaven ( Ailanthus altissima (Mill.) (Sapindales: Simaroubaceae), an important pantropical tree. Int J Mol Sci. 2018;19(4):929. 10.3390/ijms19040929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saina JK, Li ZZ, Mekbib Y, et al. : Transcriptome sequencing and microsatellite marker discovery in Ailanthus altissima (Mill.) Swingle (Simaroubaceae). Mol Biol Rep. 2021;48(3):2007–2023. 10.1007/s11033-020-05402-w [DOI] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, et al. : BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- Surana P, Muffato M, Qi G: sanger-tol/readmapping: sanger-tol/readmapping v1.1.0 - Hebridean Black (1.1.0). Zenodo. 2023a. 10.5281/zenodo.7755665 [DOI] [Google Scholar]
- Surana P, Muffato M, Sadasivan Baby C: sanger-tol/genomenote v1.0.dev (v1.0.dev). Zenodo. 2023b; [Accessed 17 April 2023]. 10.5281/zenodo.6785935 [DOI] [Google Scholar]
- Swingle WT: The early European history and the botanical name of the Tree of Heaven, Ailanthus altissima. J Wash Acad Sci. 1916;6:490–498. Reference Source [Google Scholar]
- Vasimuddin Md, Misra S, Li H, et al. : Efficient Architecture-Aware Acceleration of BWA-MEM for Multicore Systems.In: 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS).IEEE,2019;314–324. 10.1109/IPDPS.2019.00041 [DOI] [Google Scholar]
- Wellcome Sanger Institute: The genome sequence of the tree of heaven Ailanthus altissima (Mill.) Swingle, 1916. European Nucleotide Archive, [dataset], accession number PRJEB47666,2022.