Abstract
Purpose:
In the phase III CheckMate 238 study, adjuvant nivolumab significantly improved recurrence-free survival (RFS) and distant metastasis-free survival versus ipilimumab in patients with resected stage IIIB–C or stage IV melanoma, with benefit sustained at 4 years. We report updated 5-year efficacy and biomarker findings.
Patients and Methods:
Patients with resected stage IIIB–C/IV melanoma were stratified by stage and baseline programmed death cell ligand 1 (PD-L1) expression and received nivolumab 3 mg/kg every 2 weeks or ipilimumab 10 mg/kg every 3 weeks for four doses and then every 12 weeks, both intravenously for 1 year until disease recurrence, unacceptable toxicity, or withdrawal of consent. The primary endpoint was RFS.
Results:
At a minimum follow-up of 62 months, RFS with nivolumab remained superior to ipilimumab (HR = 0.72; 95% confidence interval, 0.60–0.86; 5-year rates of 50% vs. 39%). Five-year distant metastasis-free survival (DMFS) rates were 58% with nivolumab versus 51% with ipilimumab. Five-year overall survival (OS) rates were 76% with nivolumab and 72% with ipilimumab (75% data maturity: 228 of 302 planned events). Higher levels of tumor mutational burden (TMB), tumor PD-L1, intratumoral CD8+ T cells and IFNγ-associated gene expression signature, and lower levels of peripheral serum C-reactive protein were associated with improved RFS and OS with both nivolumab and ipilimumab, albeit with limited clinically meaningful predictive value.
Conclusions:
Nivolumab is a proven adjuvant treatment for resected melanoma at high risk of recurrence, with sustained, long-term improvement in RFS and DMFS compared with ipilimumab and high OS rates. Identification of additional biomarkers is needed to better predict treatment outcome.
Translational Relevance.
Although adjuvant treatment has improved treatment outcomes for patients with high-risk, resectable stage III/IV melanoma, long-term survival, and safety outcome data are needed to better guide treatment choices. Ipilimumab is the only adjuvant therapy that has been shown to provide a statistically significant increase in overall survival (OS) compared with placebo using prespecified criteria; however, the CheckMate 238 study established the superiority of nivolumab versus ipilimumab for recurrence-free survival (RFS; and an indirect treatment analysis indicated that nivolumab can provide clinically meaningful improvements in OS vs. placebo). Clinically useful biomarkers predictive of disease recurrence with adjuvant therapy have yet to be identified. After 5 years of follow-up in CheckMate 238, RFS remained superior to ipilimumab, and the OS data remained immature. Higher levels of certain biomarkers were associated with improved RFS and OS in both arms. Additional research in identifying clinically useful biomarkers and clinical factors to predict outcome for adjuvant therapy is needed.
Introduction
Recurrence-free survival (RFS) in patients with high-risk resectable stage III/IV melanoma has been improved by adjuvant treatment (1‒4). Long-term survival and safety outcomes are important to guide treatment choice for a population where, historically, approximately two-thirds of placebo-treated patients with stage III melanoma experience cancer recurrence at 5 years (5, 6), and slightly more than one-half of those with stage III disease receiving placebo are alive at 5 years (5). Although modern survival data are still forthcoming in this population, an indirect treatment comparison study that adjusted for subsequent therapy usage after recurrence showed a 4-year overall survival (OS) rate of 63% for patients on placebo (7).
Only ipilimumab adjuvant therapy has been shown to prolong OS compared with placebo in patients with high-risk resected stage III melanoma (5), although dabrafenib + trametinib provided a benefit in the 3-year OS rate of 86% versus 77% for placebo in patients with resected stage III melanoma with BRAF mutations at a first interim analysis, this was not statistically significant by precertified criteria (2). However, the phase III CheckMate 238 study established the superiority of nivolumab versus ipilimumab for RFS (3, 8), and an indirect treatment analysis indicated that nivolumab provides clinically meaningful improvements in OS versus placebo (7). In patients with stage IIIB-C/IV resected melanoma per American Joint Committee on Cancer criteria, seventh edition (AJCC-7) in CheckMate 238, nivolumab showed superiority to ipilimumab with respect to RFS at 4 years (rates of 51.7% for nivolumab and 41.2% for ipilimumab) with similar benefit in distant metastasis-free survival (DMFS; ref. 8). No significant difference was observed between treatment groups for OS, with fewer events than anticipated at 211 of the 302 anticipated events, resulting in an analysis with 73% power instead of the planned 88% needed to confirm a statistically significant difference (8). The safety profile for nivolumab was also more favorable than that for ipilimumab (3).
Thus far, no validated, clinically useful biomarkers predictive of disease recurrence with adjuvant therapy have been identified, although ongoing research suggests that biomarkers indicating a strong adaptive immune response [in particular, tumor mutational burden (TMB) alone or in combination with IFNγ signature] were associated with clinical benefit (9, 10). Biomarkers that would identify patients most at risk from recurrence and those most likely to benefit from checkpoint inhibitor treatment would enhance clinical management of patients with high-risk resected stage III/IV melanoma. Here, we provide 5-year survival outcomes of the CheckMate 238 study and present the results of analyses investigating the association between biomarkers and treatment outcomes.
Patients and Methods
Patients
Eligible patients were aged 15 years or older with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 or 1 and had stage IIIB–C/IV melanoma per AJCC-7, that was histologically confirmed, with metastases to regional lymph nodes, surgically resected distant metastases, or in-transit metastases with or without nodal involvement. Key exclusion criteria were ocular or uveal melanoma, a history of autoimmune disease, previous nonmelanoma cancer without complete remission for more than 3 years, systemic use of glucocorticoids, and previous systemic therapy for melanoma, apart from adjuvant IFN, if completed at least 6 months before randomization. A complete list of inclusion and exclusion criteria, along with more in-depth methods details has been published (3, 8). Additional details, including endpoint definitions and the biomarker analyses, are included in the Supplementary Appendix.
Study design
CheckMate 238 was a randomized, double-blind, phase III trial that enrolled patients at 130 centers in 25 countries. Randomization was stratified by disease stage (stage IIIB or IIIC vs. stage IV M1a or M1b vs. stage IV M1c, according to AJCC-7) and baseline tumor cell programmed cell death ligand 1 (PD-L1) expression (negative or indeterminate vs. positive, on the basis of a 5% cutoff level). Patients were randomized 1:1 to receive i.v. nivolumab 3 mg/kg every 2 weeks or i.v. ipilimumab 10 mg/kg every 3 weeks for four doses and then every 12 weeks, each with corresponding matched placebo, for up to 1 year or until disease recurrence, unacceptable toxicity, or withdrawal of consent.
The primary endpoint was RFS, as assessed by the investigator, and defined as the time from randomization to the date of first recurrence (local, regional, or distant metastasis), new primary melanoma, or death from any cause. Secondary endpoints comprised OS, safety and tolerability, RFS by PD-L1 expression, and health-related quality of life. OS was defined as the time between the date of randomization and the date of death. Exploratory endpoints included DMFS, the association between biomarkers from the tumor microenvironment or periphery and clinical efficacy (i.e., RFS, DMFS, and OS), and the incidence of adverse events (AE). DMFS was defined as the time between the date of randomization and the date of the first distant metastasis (including in those patients with an initial regional recurrence) or of death from any cause.
The trial was approved by the Institutional Review Board or ethics committee at each center and complied with Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. Before enrollment, all patients provided written, informed consent to participating in the trial. This study was registered on ClinicalTrials.gov (identifier NCT02388906).
Clinical assessments
Disease recurrence was assessed by the investigator every 12 weeks for the first 2 years and then every 6 months until 5 years. Disease assessment comprised a physical examination, a CT scan of the chest, abdomen, and pelvis and, as appropriate, a magnetic resonance imaging or CT scan of the brain. All patients were to be followed until death or study conclusion; beyond 5 years, disease was assessed by local standard of care. AEs were reported and graded according to the Common Terminology Criteria for Adverse Events (version 4.0) throughout treatment and at each follow-up visit, to 100 days after the last study treatment administration; investigators were encouraged to report AEs occurring subsequently (late-emergent AEs). All patients were off study treatment for more than 100 days at the primary 18-month follow-up analysis (3), and the study late-emergent AE profile remained unchanged from the 4-year follow-up (8); therefore, safety was not presented here.
All patients were required to provide tumor tissue from the resected disease site for biomarker analyses. Peripheral blood samples taken before study treatment were also used to obtain blood, serum, and plasma biomarkers. Baseline biomarkers included tumor IFNγ signature, intratumoral CD8+ T cells, TMB, peripheral monocytic myeloid–derived suppressor cells (M-MDSC), serum cytokines [C-reactive protein (CRP), IL8], monokine induced by IFNγ; [mitogen inducible gene (MIG, also named C-X-C motif chemokine ligand 9; CXCL9)], and tumor cell PD-L1. Biomarker-evaluated patients provided written informed consent for inclusion in the analysis. Specific methodology for the biomarker studies is provided in the Supplementary Appendix.
Statistical analysis
Sample size determination has been described previously (3). We used a two-sided log-rank test stratified by PD-L1 status and disease stage at screening to compare RFS, DMFS, and OS between the two treatment groups. Statistical tests for efficacy analyses are described in the Supplementary material and have also been published previously (3). Statistical tests for biomarker analyses used R version 4.0.3 and were not adjusted for multiplicity. HRs and their 95% confidence intervals (CI) for RFS or OS between high versus low expression of individual markers were obtained by univariate Cox proportional hazards model. Predictive performance of the biomarkers was evaluated by Harrell concordance index (C-index), with missing biomarkers imputed by the R: mice package with version 3.9.0.
Data availability
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html, including instructions on how to submit a data request. Requests can also be made on the Vivli platform at https://vivli.org/ourmember/bristol-myers-squibb/.
Results
Patients
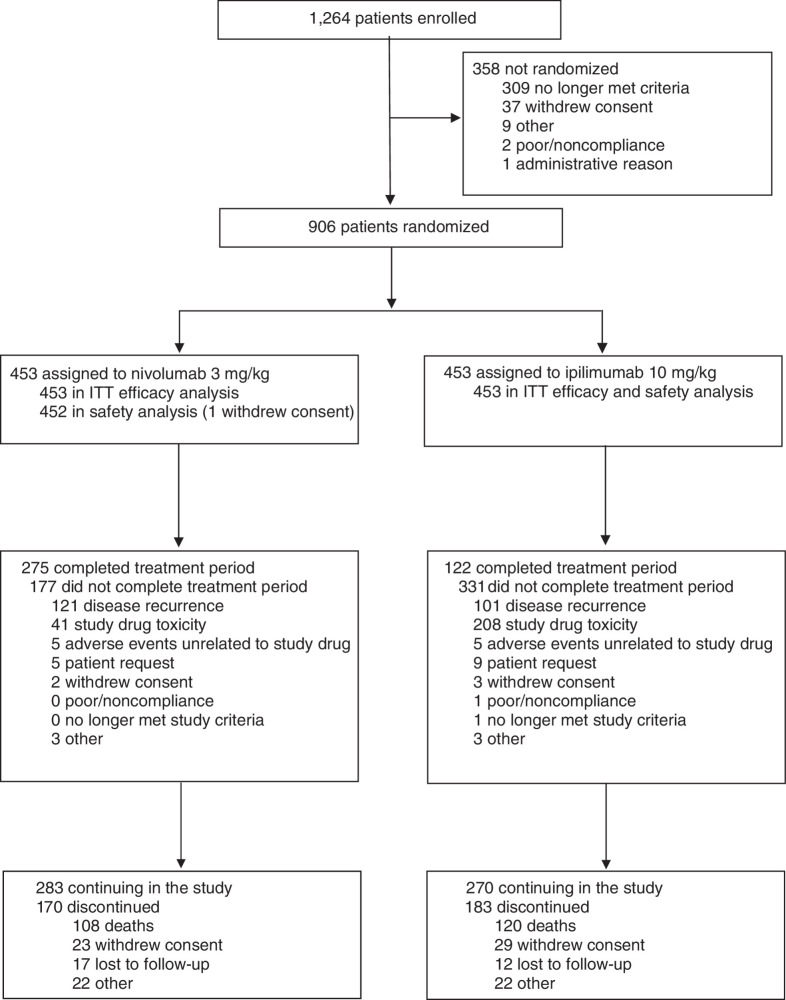
Between March 30 and November 30, 2015, 906 patients were randomized to receive either nivolumab (n = 453) or ipilimumab (n = 453); 452 and 453 patients, respectively, received treatment (Fig. 1). Representativeness of study participants can be found in Supplementary Table S1; baseline characteristics have been reported previously (Supplementary Table S2; ref. 3). At the time of the primary analysis after a minimum of 18 months’ follow-up, all patients had completed or discontinued study treatment; AE data collected up to 100 days after treatment for all patients was reported then and is not updated here (3). At the current database lock of March 16, 2021, 283 and 270 patients in the nivolumab and ipilimumab groups, respectively, remained in follow-up (Fig. 1). In patients who experienced recurrence, 69% and 72% of nivolumab- and ipilimumab-treated patients received subsequent systemic therapy. Subsequent systemic therapy included immunotherapy in 49% and 58% of patients treated with nivolumab and ipilimumab, respectively (Supplementary Table S3).
Figure 1.
CONSORT diagram.
Intention-to-treat efficacy
Minimum follow-up (i.e., from the first dose of the last patient randomized until the database cutoff) was 62.0 months, and median follow-up (i.e., median time between the first dose and date of death or last known alive date for each patient) was 61.5 months for nivolumab and 61.2 months for ipilimumab. The median RFS (nivolumab vs. ipilimumab) was 61.0 months (95% CI, 42.5‒not reached) versus 24.1 months (95% CI, 16.6‒35.1), with a stratified HR favoring nivolumab of 0.72 (95% CI, 0.60‒0.86); 5-year RFS rates were 50% versus 39% (Fig. 2A). Recurrence patterns were similar for each treatment (Supplementary Table S4). Median DMFS was not reached in either group, with 5-year DMFS rates of 58% for nivolumab and 51% for ipilimumab (HR= 0.79; 95% CI, 0.63‒0.99; Fig. 2B). The 228 total deaths remained below the 302 events anticipated at the 4-year primary OS analysis (8). OS did not significantly differ between treatments (HR= 0.86; 95% CI, 0.66‒1.12) with the median not reached in either arm; 5-year OS rates were 76% with nivolumab and 72% with ipilimumab (Fig. 2C).
Figure 2.
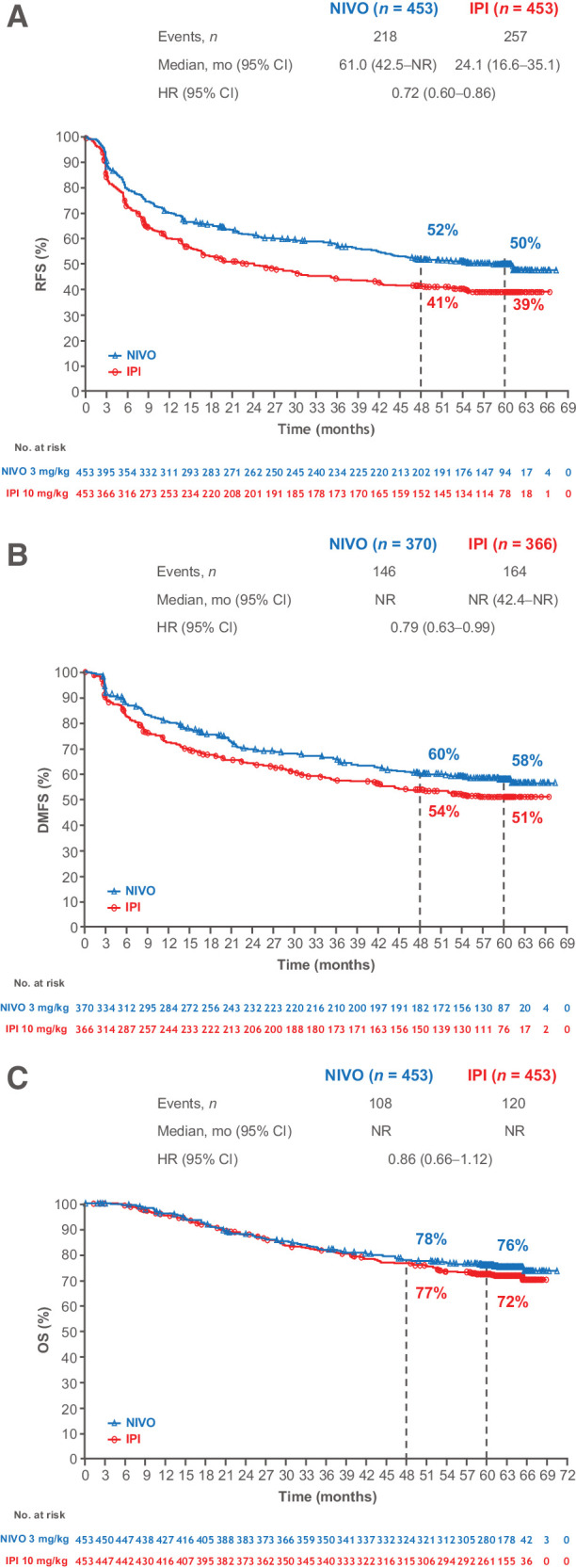
RFS (A), DMFS (B), and OS (C) in patients randomly assigned to nivolumab or ipilimumab. Patients were followed for a minimum of 60 months.
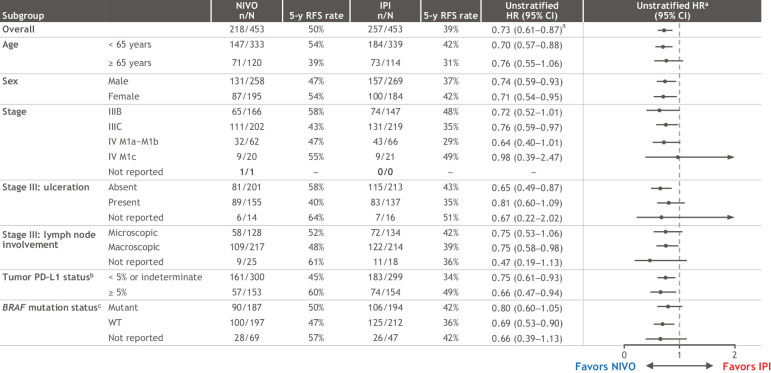
Subgroup efficacy
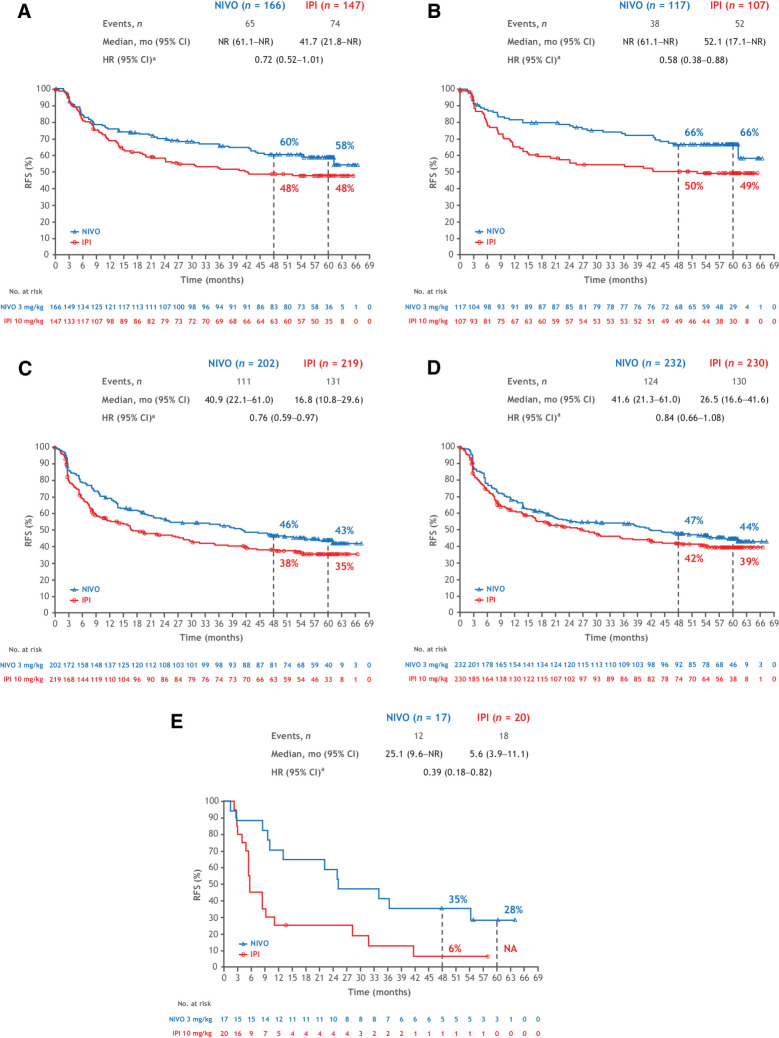
As previously reported (8), the RFS benefit with nivolumab was generally maintained across all subgroups analyzed (Fig. 3), including BRAF status with 5-year rates of 50% with nivolumab and 42% with ipilimumab in patients with BRAFV600-mutant tumors and 47% versus 36% in those with BRAFV600–wild-type (WT) tumors (Supplementary Fig. S1). Five-year RFS benefit reported here per AJCC-7 with nivolumab was also maintained when the data were analyzed per AJCC-8 subgroups criteria (Fig. 4). Recurrence rates for stage IIIB with nivolumab were 58% via AJCC-7 and 66% via AJCC-8 and, for ipilimumab, they were 48% and 49%, respectively; recurrence rates for stage IIIC were 43% and 44% with nivolumab and 35% and 39% with ipilimumab. Only 17 and 20 patients with AJCC-8 stage IIID disease were treated with nivolumab and ipilimumab, 5-year rates were 28% and 0%, respectively; patients with AJCC-8 stage IIIA disease were too few to analyze (3 and 5, respectively).
Figure 3.
RFS in patient subgroups. Results are expressed as unstratified HRs for the risk of recurrence or death in the nivolumab group compared with the ipilimumab group with 95% CIs. aStratified HR = 0.72 (95% CI, 0.60–0.86). bPD–L1 IHC 28–8 pharmDx assay; status determined as percentage of tumor cells. cV600E/K.
Figure 4.
RFS in patients with AJCC-7 stage IIIB disease (A), AJCC-8 stage IIIB disease (B), AJCC-7 stage IIIC disease (C), AJCC-8 stage IIIC disease (D), and AJCC-8 stage IIID disease (E). aUnstratified.
As with RFS, DMFS benefit with nivolumab was generally maintained across all subgroups (Supplementary Fig. S2A). However, as with the intention-to-treat (ITT) population, no treatment difference in OS was observed across subgroups (Supplementary Fig. S2B). In OS multivariate analyses, ECOG PS 1 versus 0, stage IIIC versus IIIB, and tumor PD-L1 expression < 5% versus ≥ 5% were associated with decreased survival (Supplementary Table S5). As a follow-up of that analysis, we investigated three of the subgroups (i.e., ECOG PS 1 vs. 0, aged < 65 years vs. ≥ 65 years, and tumor PD-L1 expression < 5% vs. ≥ 5%) via Kaplan–Meier survival analyses for each treatment: there was relatively equal separation in the curves for each subgroup per treatment except for patients treated with nivolumab who were aged < 65 years versus ≥ 65 years, for which no difference was noted (Supplementary Fig. S3).
Biomarker analysis
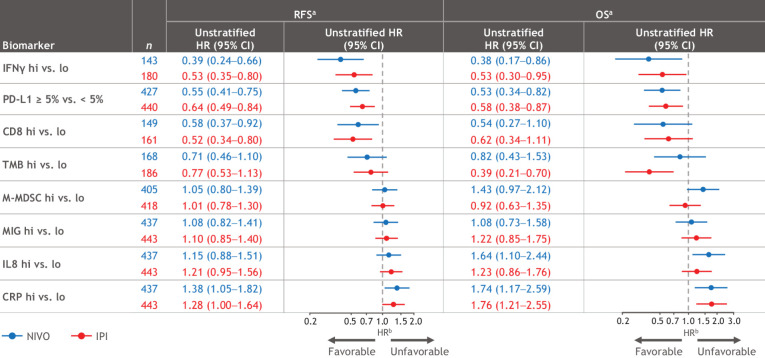
Baseline biomarker analyses included both tissue and blood specimens with evaluability rates ranging from 32% to 98% (Supplementary Table S6). No significant differences in RFS or OS between biomarker-evaluable and the ITT populations were noted in Kaplan–Meier analyses (Supplementary Fig. S4). In a univariate analysis, correlations with favorable RFS and OS for both treatment groups were strongest with higher IFNγ signature, tumor PD-L1 expression (≥ 5%), CD8+ T cells, and TMB; correlations with unfavorable RFS and OS were strongest with higher levels of CRP and IL8 detected in blood (Fig. 5). No substantial differences in biomarker associations were noted between the treatment arms. Supplementary Table S6 shows median values used for each biomarker.
Figure 5.
Univariate biomarker analyses for RFS and OS. aThe evaluated population cohort size for each biomarker is different on the basis of availability of data for the markers. bHR measures the RFS or death (OS) between ≥ median versus < median, except for tumor. PD-L1 between ≥ 5% versus <5%. Supplementary Table S6 shows median values used for each biomarker.
Spearman correlation coefficient pairwise analysis indicated that PD-L1, IFNγ signature, and CD8 were potentially correlated (Supplementary Fig. S5). The prognostic effects of individual biomarkers or biomarker pairs were evaluated by Harrell C-index, a nonparametric statistic used here to assess the concordance between biomarker expression and survival. Individual or pairs of uncorrelated biomarkers showed low predictive accuracy for both RFS and OS, with a C-index ranging from 0.51 to 0.65, considering a value of 0.5 as random and 1.0 as perfect (Supplementary Fig. S6).
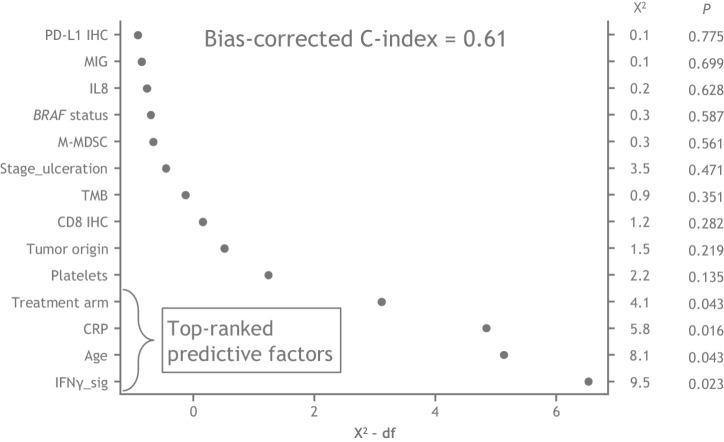
Next, we developed a multivariate model that included both molecular biomarkers and clinical factors to improve accuracy. From a predefined candidate set of clinical factors, we selected those that showed best association with RFS on the basis of the HR and CI measured from univariate Cox proportional hazards model (Supplementary Fig. S7). To avoid potential overfitting of the multivariate model, the population of patients missing all tumor tissue markers (a 490-sample subset) were used to select clinical factors. The remaining patient set with complete clinical factors and partial tumor biomarkers were used to develop a multivariate model. Although melanoma subtype was included in this analysis, > 80% of the patients in CheckMate 238 presented with cutaneous melanoma and thus melanoma subtype was not included in further analyses. The results of the multivariate analysis showed that treatment arm, IFNγ signature, CRP, and age emerged as the strongest factors predicting recurrences (all P < 0.05; Supplementary Figs. S7 and S8; Fig. 6). IFNγ signature score and age showed a nonlinear association with RFS, where patients below median IFNγ signature levels showed higher risks of recurrence, and both younger and older patients show higher risks of recurrence (Supplementary Fig. S8). However, the overall bias-corrected C-index was 0.61 (Fig. 6).
Figure 6.
Multiparameter composite analysis. An RFS prediction model was based on clinical factors and tumor/peripheral biomarkers in a common 415-patient subset with multiple imputations for missing data. The model included targeted biomarkers and potential prognostic clinical factors, which were selected on the basis of univariate analysis in an independent cohort of 490 patients from CheckMate 238.
Discussion
After a minimum of 5 years’ follow-up in patients with resected stage IIIB–C/IV melanoma, nivolumab was superior to ipilimumab for RFS (HR = 0.72; 95% CI, 0.60‒0.86) and DMFS (HR = 0.79; 95% CI, 0.63‒0.99), across all clinical subgroups analyzed. OS data did not differ significantly between treatments at 5 years (75% data maturity), similar to the 4-year data (73% data maturity; ref. 8). Biomarker analyses demonstrated that correlations with favorable RFS and OS were strongest with higher levels of IFNγ signature, tumor PD-L1, CD8, and TMB, and lower levels of CRP, alone or in combination for both treatments, but with limited predictive value on the basis of C-index.
Rates of RFS and DMFS in the ITT population and by stage and BRAF mutation status were all similar to but slightly lower at 5 years than at 4 years (8). The Kaplan–Meier curves continue to fall at a slower rate at years 4 and 5, a trend expected to continue with longer follow-up. The CheckMate 238 recurrence pattern reported at 4 years was maintained through 5 years, demonstrating the durable, long-term benefit of nivolumab over ipilimumab in preventing both locoregional and distant metastases and an associated reduced requirement for subsequent therapy; however, approximately 50% of patients will still have disease recurrence. Thus, additional treatment strategies are needed in this patient population. The recently reported CheckMate 915 trial (11) failed to demonstrate improved RFS with nivolumab 240 mg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks, but efficacy and safety outcomes with nivolumab dosed at 480 mg every 4 weeks were similar to that seen in CheckMate 238. Other phase III studies are ongoing, including a combination of nivolumab with relatlimab, an anti–lymphocyte activation gene-3 (LAG-3) agent (NCT05002569), and pembrolizumab combined with immune modulatory vaccines IO102-IO103 (NCT05155254). Furthermore, the neoadjuvant treatment of melanoma with anti–programmed cell death (PD)-1–based therapy is an emerging area of interest, as noted by several phase II/III trials in this space. For example, the phase II SWOG S1801 recently showed preliminary evidence that the addition of neoadjuvant treatment to adjuvant treatment with single-agent pembrolizumab improves event-free survival compared with adjuvant treatment in patients with high-risk resectable melanoma (12) and the NADINA trial (NCT04949113) is currently recruiting patients to investigate neoadjuvant nivolumab plus ipilimumab versus adjuvant nivolumab in macroscopic stage III melanoma in a phase III setting (13).
Given the challenge of extrapolating efficacy outcomes for patients with AJCC-8 stage III subgroupings from adjuvant trials enrolled under AJCC-7, we show here that similar to the previous analysis (14), RFS outcomes per AJCC-8 staging criteria are consistent with those obtained with AJCC-7, now extended to 5 years. These results are aligned with those for pembrolizumab in KEYNOTE-054, which show that after a median follow-up of 15 months, pembrolizumab treatment demonstrated similar RFS improvement versus placebo, regardless of staging criteria, with the results sustained up to 36 months (15, 16). Overall, RFS rates by AJCC-7 and AJCC-8 staging for nivolumab are similar to those for pembrolizumab, given the caveat that the KEYNOTE-054 population may have had a slightly lower risk for recurrence than that in CheckMate 238 in that no patient had mucosal melanoma (vs. 4% in CheckMate 238), and, according to AJCC-7 staging, approximately 15% of patients had stage IIIA disease (vs. 0%), 47% (vs. 36%) had stage IIIB disease, and 38% (vs. 45%) had stage IIIC, with the remaining patients for CheckMate 238 (18%) having stage IV disease (3, 17). Notably, although stage IIIA patients were not included in the CheckMate 238 study, a real-world study of 183 patients (71 treated with nivolumab) indicated that nivolumab may provide benefit over observation in these patients, with an RFS HR (95% CI) of 0.55 (0.21‒1.49) for nivolumab versus observation with a median follow-up time of 18.8 months versus 25.6 months, respectively (18).
Rates of long-term OS in both treatment groups of CheckMate 238 were higher than anticipated during trial design. The availability of effective subsequent treatment after recurrence may have confounded the between-group comparison (e.g., 58% vs. 49% of patients treated with ipilimumab vs. nivolumab, respectively, received subsequent immunotherapy) and may partially explain why significant OS benefit for nivolumab versus ipilimumab has not been observed. Investigating progression-/recurrence-free survival (PRFS2) through next-line therapy is another endpoint that is increasingly being explored and may represent a good surrogate for OS, because it takes into account potential lack of efficacy of a next-line therapy induced by the treatment under investigation. Recent results from the EORTC 1325/KEYNOTE-054 study of adjuvant pembrolizumab versus placebo in resected stage III melanoma demonstrated a PRFS2 HR of 0.65 (95% CI, 0.53–0.80), providing an encouraging signal that anti–PD-1 may provide an OS advantage over placebo in this patient population (19). It is also possible that the use of 10 mg/kg of ipilimumab instead of the 3 mg/kg used in metastatic studies may have had an impact on the outcome of the study. However, the results of the E1609 study in which both 3 mg/kg and 10 mg/kg ipilimumab were compared with high-dose IFNα showed no difference in 5-year OS rates for the 3 mg/kg dose (72%) and the 10 mg/kg dose (70%), nor were there differences in the RFS between the two doses (20). Although ipilimumab 3 mg/kg has a much better safety profile than ipilimumab 10 mg/kg (5, 21), an anti–PD-1 therapy would still be expected to be better tolerated than ipilimumab 3 mg/kg (22, 23).
In the present study, both tumor and peripheral baseline biomarkers were explored for associations with treatment efficacy. PD-L1 expression has been shown to have predictive and/or prognostic relevance for anti–PD-1 treatment across several tumor types (24, 25). TMB has also been shown to be associated with favorable treatment outcomes (9, 26). Other biomarkers analyzed in this study have not yet reached regulatory approval but are supported by a range of evidence (9, 10, 27, 28). Our analyses suggested that higher levels of IFNγ signature, tumor PD-L1, intratumoral CD8, and TMB, and lower levels of serum CRP, as well as combinations of these biomarkers, correlated with improved survival outcomes with both nivolumab and ipilimumab, although their predictive value of disease course for each treatment was limited. Multivariate analyses showed that IFNγ signature, CRP, and age were the top-ranked factors associated with RFS with both treatments, although below a threshold needed for a clinical decision. Age was the only significant clinical variable in the multivariate model, and the nonlinear relation seen in Supplementary Fig. S7 could possibly lead to the hypothesis that age is a poorer prognostic factor for young patients due to more aggressive disease and for older patients because of comorbidities or immune differences. Despite the multivariate analyses herein suggesting an association of high tumor PD-L1 expression (≥ 5%) with improved OS, PD-L1 has been shown to be an inconsistent prognostic and predictive marker in melanoma. Two recent meta-analyses have suggested no prognostic role for PD-L1 in OS across multiple stages of melanoma, although positive PD-L1 expression was significantly related to prolonged OS when restricted to metastatic melanoma (29, 30).
The ability to distinguish predictive versus prognostic biomarkers in CheckMate 238 is limited because there were two active treatment groups and no placebo control group; however, the information adds to the knowledge necessary to better identify which patients may potentially benefit from adjuvant immunotherapy. IFNγ-related gene expression and TMB showed association with clinical benefit from both nivolumab and ipilimumab, similar to results in the metastatic disease setting in CheckMate 066 and 067 (26), suggesting that pretreatment tumor biomarkers may yield similar results in both the adjuvant and metastatic settings. As the association of biomarkers with outcomes in CheckMate 238 were similar for both nivolumab and ipilimumab, additional biomarkers may be needed to reasonably select for patients who might benefit from one treatment over another. The need for prognostic and treatment predictive biomarkers now extends to adjuvant therapy of earlier stages of melanoma, as in the CheckMate 76K (NCT04099251) and KEYNOTE-716 (NCT03553836) studies in resected stage IIB/C melanoma, in order to select which patients are most likely to benefit.
Despite the addition of biomarkers employed in composite analyses assessing diverse factors associated with tumor cells (e.g., PD-L1 and TMB), immune cells (e.g., IFNγ signature and CD8+ T cells), and serum factors (e.g., CRP), additional investigations to better understand the factors that predict response to therapies or a combination of therapies are required. Although findings to date are difficult to integrate across studies, accumulated data will help to refine predictive biomarker models to maximize the benefit of adjuvant therapy for patients (31). The availability of larger datasets where nivolumab was also used in the stage III–IV adjuvant setting, as in CheckMate 915 (11), provides an opportunity to pool data and develop more robust predictive models.
In conclusion, nivolumab is a proven adjuvant treatment for patients with resected stage III/IV melanoma at high risk of recurrence with a more favorable safety profile than ipilimumab (3). We show here sustained, long-term improvement in 5-year RFS and DMFS for nivolumab compared with ipilimumab. The OS data remain immature, with high OS rates in both groups. Although higher levels of IFNγ signature, tumor PD-L1, CD8, and TMB, and lower levels of CRP were associated with favorable RFS and OS in both groups, their predictive value has limited clinical significance in the assessment of patients’ disease risk or suitability for nivolumab or ipilimumab adjuvant treatment. Further analyses are therefore needed to identify new biomarkers and multiparameter models to predict the efficacy of adjuvant treatment with nivolumab or ipilimumab with adequate clinical significance, thereby enabling a more meaningful benefit/risk dialogue between healthcare providers and patients.
Supplementary Material
DATA SUPPLEMENT Adjuvant Nivolumab Versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results From CheckMate 238
Acknowledgments
Supported by Bristol Myers Squibb Company (Princeton, NJ) and Ono Pharmaceutical Co., Ltd. (Osaka, Japan) We thank the patients and investigators who participated in the CheckMate 238 trial. We also acknowledge Ono Pharmaceutical Company, Ltd., for contributions to nivolumab development and Dako, an Agilent Technologies, Inc. company, for the collaborative development of the PD-L1 immunohistochemistry 28–8 pharmDx assay. We acknowledge the contribution of Abraham Apfel, PhD (BMS), who provided the multivariate analyses of clinical and biomarker factors. Professional medical writing and editorial assistance for the development of this manuscript, under the guidance of the authors, were provided by Melissa Kirk, PhD, Jessica Augello, PhD, and Michele Salernitano of Ashfield MedComms, an Inizio company, and funded by Bristol Myers Squibb.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Selected Articles from This Issue, p. 3251
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
J. Larkin reports grants from Achilles, BMS, MSD, Nektar, Novartis, Pfizer, Roche, Immunocore, Aveo, and Pharmacyclics; personal fees from Eisai, Novartis, Incyte, Merck, touchIME, touchEXPERTS, Pfizer, Royal College of Physicians, Cambridge Healthcare Research, Royal College of General Practitioners, VJOncology, Agence Unik, BMS, iOnctura, Apple Tree, BMS, Calithera, Ultimovacs, Seagen, eCancer, Inselgruppe, Goldman Sachs, and MSD; and other support from BMS, MSD, Novartis, Pfizer, Achilles Therapeutics, Roche, Nektar Therapeutics, Covance, Immunocore, Pharmacyclics, and Aveo during the conduct of the study. M. Mandalá reports personal fees from BMS, MSD, Pierre Fabre, and Sanofi and grants and personal fees from Novartis outside the submitted work. H. Gogas reports grants and personal fees from Bristol Myers Squibb; grants, personal fees, and nonfinancial support from MSD Oncology; personal fees from Pierre Fabre and Sanofi/Regeneron; grants from Roche, Iovance Biotherapeutics, and Novartis; and grants and nonfinancial support from Amgen and Pfizer outside the submitted work. A.M. Arance Fernandez reports other support from Pierre Fabre, Novartis, MSD, Roche, BMS, Merck, and Sanofi outside the submitted work. In addition, A.M. Arance Fernandez reports consultant, advisory, and speaker participation; travel accommodations; expenses; and research funding fees from Pierre Fabre, Novartis, Roche, BMS, MSD, Merck and Sanofi. S. Dalle reports grants from MSD and BMS during the conduct of the study as well as other support from Sanofi and grants from Pierre Fabre outside the submitted work. M. Schenker reports other support from Bristol Myers Squibb during the conduct of the study as well as other support from Bioven, BeiGene, Bayer, Clovis, Gilead, Mylan, Pfizer, PharmaMar, and Samsung Pharmaceuticals outside the submitted work. J.-J. Grob reports personal fees and nonfinancial support from BMS and Novartis and personal fees from Pierre Fabre, Sanofi, MSD, and Philogen outside the submitted work. V. Chiarion-Sileni reports other support from Pierre Fabre outside the submitted work. I. Marquez-Rodas reports grants from BMS during the conduct of the study as well as personal fees from BMS, MSD, Novartis, Pierre Fabre, Sanofi, Sun Pharma, Highlight Therapeutics, AstraZeneca, Merck Serono, Immunocore, and BioLineRx outside the submitted work. M.O. Butler reports personal fees from Bristol Myers Squibb during the conduct of the study as well as personal fees from Novartis, Immunocore, Adaptimmune, GlaxoSmithKline, Sanofi, LaRoche Possey, Iovance, Pfizer, Medison, Ideaya Bio, Regeneron, Merck, Sun Pharma, and Instil Bio; grants from Takara Bio; and grants and personal fees from Novartis outside the submitted work. A.M. Di Giacomo reports personal fees from Bristol Myers Squibb, Pierre Fabre, MSD, and Sanofi during the conduct of the study as well as nonfinancial support from Sun Parma and Immunocore outside the submitted work. M.R. Middleton reports other support from BMS during the conduct of the study as well as grants and other support from Immunocore and other support from Alkermes and other Replimune outside the submitted work. J. Lutzky reports grants from University of Miami during the conduct of the study as well as grants from BMS, Takeda, TriSalus, Dragonfly, Vyriad, Replimune, Agenus, and DayOne and personal fees from Iovance and Replimune outside the submitted work. L. de la Cruz-Merino reports grants from Bristol Myers Squibb during the conduct of the study as well as grants from BMS, Novartis, MSD, and Roche outside the submitted work. P. Arenberger reports nonfinancial support from BMS during the conduct of the study as well as grants from Pfizer; nonfinancial support from MSD, AbbVie, and Sanofi; and other support from Novartis outside the submitted work. V. Atkinson reports personal fees from MSD, BMS, Novartis, Nektar, Immunocore, QBiotics, Provectus, and Limbic and personal fees and nonfinancial support from Pierre Fabre outside the submitted work. L.A. Fecher reports grants from Bristol Myers Squibb during the conduct of the study as well as grants from Kartos, Pfizer, Array, and EMD Serono and personal fees from Elsevier and HCRN outside the submitted work. M. Millward reports personal fees from Bristol Myers Squibb during the conduct of the study as well as personal fees from Roche, AstraZeneca, Novartis, Pfizer, Merck Serono, BeiGene, IQVIA, Eli Lilly, Guardant Health, and Limbic outside the submitted work. P.D. Nathan reports personal fees from BMS and MSD, grants and personal fees from Immunocore, and personal fees and other support from Novartis outside the submitted work. N.I. Khushalani reports grants and personal fees from Bristol Myers Squibb during the conduct of the study as well as grants and personal fees from Regeneron, Merck, Novartis, Replimune, Nektar, and Iovance Biotherapeutics; personal fees from Jounce Therapeutics, Castle Biosciences, Instil Bio, and Genzyme; grants from GlaxoSmithKline, HUYA Bioscience International, Celgene, Modulation Therapeutics, and Amgen; and other support from NCCN (Pfizer), Incyte, AstraZeneca, Bellicum Pharmaceuticals, Asensus Surgical, and Amarin Corporation outside the submitted work. P. Queirolo reports an advisory role with MSD, BMS, Pierre Fabre, Sun Pharma, Sanofi Regeneron, and Novartis. C. Ritchings reports personal fees from Bristol Myers Squibb during the conduct of the study. M. Lobo reports other support from Bristol Myers Squibb outside the submitted work; in addition, M. Lobo is an employee of Bristol Myers Squibb. M. Askelson reports full-time employment by Bristol Myers Squibb (compensation - salary). H. Tang reports personal fees from Bristol Myers Squibb during the conduct of the study. S. Dolfi reports being an employee of Bristol Myers Squibb. P.A. Ascierto reports grants and personal fees from Roche-Genentech and BMS and personal fees from MSD, Novartis, Merck-Serono, Pierre Fabre, AstraZeneca, Sun Pharma, Sanofi, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer/Array, Oncosec, Nouscom, Lunaphore, Seagen, iTeos, Medicenna, Bio-AI Health, ValoTx, Replimmune, Bayer, and Erasca outside the submitted work. J. Weber reports grants and personal fees from BMS during the conduct of the study as well as personal fees from Merck, Pfizer, Regeneron, Genentech, and Astra Zeneca outside the submitted work; in addition, J. Weber has a patent filed by Biodesix for a PD-1 biomarker licensed to Biodesix. No disclosures were reported by the other authors.
Authors' Contributions
J. Larkin: Conceptualization. M. Del Vecchio: Data curation and data interpretation. M. Mandalá: Conceptualization. H. Gogas: Data curation and data interpretation. A.M. Arance Fernandez: Data curation and data interpretation. S. Dalle: Data curation and data interpretation. C.L. Cowey: Data curation and data interpretation. M. Schenker: Data curation and data interpretation. J.-J. Grob: Data curation and data interpretation. V. Chiarion-Sileni: Data curation and data interpretation. I. Marquez-Rodas: Data curation and data interpretation. M.O. Butler: Data curation and data interpretation. A. Di Giacomo: Data curation and data interpretation. M.R. Middleton: Data curation and data interpretation. J. Lutzky: Data curation and data interpretation. L. de la Cruz-Merino: Data curation and data interpretation. P. Arenberger: Data curation and data interpretation. V. Atkinson: Data curation and data interpretation. A.G. Hill: Data curation and data interpretation. L.A. Fecher: Formal analysis. M. Millward: Data curation and data interpretation. P.D. Nathan: Data curation and data interpretation. N.I. Khushalani: Data curation and data interpretation. P. Queirolo: Data curation and data interpretation. C. Ritchings: Formal analysis and data interpretation. M. Lobo: Formal analysis and data interpretation. M. Askelson: Formal analysis and data interpretation. H. Tang: Formal analysis and data interpretation. S. Dolfi: Formal analysis and data interpretation. P.A. Ascierto: Conceptualization, data curation, and data interpretation. J. Weber: Conceptualization, data curation, and data interpretation.
References
- 1. Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522‒30. [DOI] [PubMed] [Google Scholar]
- 2. Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 2017;377:1813‒23. [DOI] [PubMed] [Google Scholar]
- 3. Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824‒35. [DOI] [PubMed] [Google Scholar]
- 4. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789‒1801. [DOI] [PubMed] [Google Scholar]
- 5. Eggermont AM, Chiarion-Sileni V, Grob JJ, Reinhard D, Wolchok J, Schmidt H, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845‒55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dummer R, Hauschild A, Santinami M, Atkinson V, Mandalà M, Kirkwood JM, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med 2020;383:1139‒48. [DOI] [PubMed] [Google Scholar]
- 7. Weber J, Ascierto PA, Middleton M, Hennicken D, Zoffoli R, Pieters A, et al. Indirect treatment comparison of nivolumab versus placebo as adjuvant treatment for resected melanoma. Eur J Cancer 2021;158:225‒33. [DOI] [PubMed] [Google Scholar]
- 8. Ascierto PA, Del Vecchio M, Mandalá M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, Phase 3 trial. Lancet Oncol 2020;21:1465‒77. [DOI] [PubMed] [Google Scholar]
- 9. Dummer R, Brase JC, Garrett J, Campbell CD, Gasal E, Squires M, et al. Adjuvant dabrafenib plus trametinib versus placebo in patients with resected, BRAFV600-mutant, stage III melanoma (COMBI-AD): exploratory biomarker analyses from a randomised, phase 3 trial. Lancet Oncol 2020;21:358‒72. [DOI] [PubMed] [Google Scholar]
- 10. Indini A, Roila F, Grossi F, Massi D, Mandalà M. Impact of circulating and tissue biomarkers in adjuvant and neoadjuvant therapy for high-risk melanoma: ready for prime time? Am J Clin Dermatol 2021;22:511‒22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weber JS, Schadendorf D, Del Vecchio M, Larkin J, Atkinson V, Schenker M, et al. Adjuvant therapy of nivolumab combined with ipilimumab versus nivolumab alone in patients with resected stage IIIB-D or stage IV melanoma (CheckMate 915). J Clin Oncol 2023;41:517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel S, Othus M, Prieto V, Lowe M, Buchbinder E, Chen Y, et al. Neoadjuvant versus adjuvant pembrolizumab for resected stage III-IV melanoma (SWOG S1801). Ann Oncol 2022;33(suppl_7):S808–69. [Google Scholar]
- 13. Lucas MW, Lijnsvelt J, Pulleman S, Scolyer RA, Menzies AM, Van Akkooi ACJ, et al. The NADINA trial: a multicenter, randomized, phase 3 trial comparing the efficacy of neoadjuvant ipilimumab plus nivolumab with standard adjuvant nivolumab in macroscopic resectable stage III melanoma. J Clin Oncol 2022;40(16_suppl):TPS9605. [Google Scholar]
- 14. Larkin J, Weber J, Del Vecchio M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C/IV melanoma: reassessment of 4-year CheckMate 238 efficacy outcomes per AJCC-8 staging criteria. Eur J Cancer 2022;173;285‒96. [DOI] [PubMed] [Google Scholar]
- 15. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from EORTC 1325-MG/KEYNOTE-054 trial. J Clin Oncol 2020;38:3925‒36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eggermont AMM, Blank CU, Mandalà M, Long GV, Atkinson VG, Dalle S, et al. EORTC Melanoma Group. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:643‒54. [DOI] [PubMed] [Google Scholar]
- 17. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, et al. Prognostic and predictive value of AJCC-8 staging in the phase III EORTC1325/KEYNOTE-054 trial of pembrolizumab vs placebo in resected high-risk stage III melanoma. Eur J Cancer 2019;116:148‒57. [DOI] [PubMed] [Google Scholar]
- 18. Moser JC, Pavlick AC, Poretta T, Chan P, Samlowski W, Robert N, et al. Real-world outcomes of patients with resected stage IIIA melanoma treated with adjuvant nivolumab or monitored with observation. Poster presented at the Society for Melanoma Research (SMR), 18th International Congress, New Orleans, LA (Hybrid Congress), October 28–31, 2021. [Google Scholar]
- 19. Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Five-year analysis of adjuvant pembrolizumab or placebo in stage III melanoma. N Engl J Med Evid 2022;1. [DOI] [PubMed] [Google Scholar]
- 20. Tarhini AA, Lee SJ, Hodi FS, Rao UNM, Cohen GI, Hamid O, et al. Phase III study if adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American Intergroup E1609. J Clin Oncol 2020;38:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2017;18:611–22. [DOI] [PubMed] [Google Scholar]
- 22. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;37:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- 24. Healey Bird B, Nally K, Ronan K, Ronan K, Clarke G, Amu S, et al. Cancer immunotherapy with immune checkpoint inhibitors-biomarkers of response and toxicity; current limitations and future promise. Diagnostics (Basel) 2022;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. OPDIVO (nivolumab) injection, for intravenous use. Highlights of Prescribing Information. Bristol Myers Squibb Company, Princeton, NJ, 2022. [Google Scholar]
- 26. Hodi FS, Wolchok JD, Schadendorf D, Larkin J, Long GV, Qian X, et al. TMB and inflammatory gene expression associated with clinical outcomes following immunotherapy in advanced melanoma. Cancer Immunol Res 2021;9:1202‒13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. KEYTRUDA (pembrolizumab) injection, for intravenous use. Highlights of Prescribing Information. Merck & Co., Inc., Whitehouse Station, NJ, 2022. [Google Scholar]
- 28. Yang F, Wang JF, Wang Y, Liu B, Molina JR. Comparative analysis of predictive biomarkers for PD-1/PD-L1 inhibitors in cancers: developments and challenges. Cancers (Basel) 2021;14:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu J, Wang F, Yan Y, Zhang Y, Du Y, Sun G. Prognostic and clinicopathological value of PD-L1 in melanoma: a meta-analysis. Am J Med Sci 2020;359:339‒46. [DOI] [PubMed] [Google Scholar]
- 30. Yang J, Dong M, Shui Y, Zhang Y, Zhang Z, Yin M, et al. A pooled analysis of the prognostic value of PD-L1 in melanoma: evidence from 1062 patients. Cancer Cell Int 2020;20:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res 2020;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DATA SUPPLEMENT Adjuvant Nivolumab Versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results From CheckMate 238
Data Availability Statement
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html, including instructions on how to submit a data request. Requests can also be made on the Vivli platform at https://vivli.org/ourmember/bristol-myers-squibb/.