Abstract
Purpose:
We aim to evaluate the prognostic significance of tumor-infiltrating lymphocyte on residual disease (RD-TIL) in HER2+ patients with breast cancer who failed to achieve pathologic complete response (pCR) after anti-HER2+ chemotherapy (CT)-based neoadjuvant treatment (NAT). We assessed the feasibility of combining the prognostic information provided by residual cancer burden (RCB) and RD-TILs into a composite score (RCB+TIL).
Experimental Design:
HER2+ patients with breast cancer treated with CT+anti-HER2-based NAT at three institutions were retrospectively included. RCB and TIL levels were evaluated on hematoxylin and eosin–stained slides from surgical samples according to available recommendations. Overall survival (OS) was used as an outcome measure.
Results:
A total of 295 patients were included, of whom 195 had RD. RCB was significantly associated with OS. Higher RD-TILs were significantly associated with poorer OS as compared with lower RD-TILs (15% cutoff). In multivariate analysis, both RCB and RD-TIL maintained their independent prognostic value. A combined score, RCB+TIL, was calculated from the estimated coefficient of RD-TILs and the RCB index in a bivariate logistic model for OS. The RCB+TIL score was significantly associated with OS. The C-index for OS of the RCB+TIL score was numerically higher than that of RCB and significantly higher than that of RD-TILs.
Conclusions:
We have reported an independent prognostic impact of RD-TILs after anti-HER2+CT NAT, which might underlie an imbalance of the RD microenvironment towards immunosuppressive features. We provided a new composite prognostic score based on RCB+TIL, which was significantly associated with OS and proved to be more informative than the isolated evaluation of RCB and RD-TILs.
Translational Relevance.
Neoadjuvant treatment (NAT) is increasingly being administered to HER2+ breast cancer, as it offers undeniable clinical benefits from a patient perspective. Subsequent adjuvant drugs are tailored on the basis of the presence or absence of residual disease (RD) cells after NAT. In this retrospective study, residual cancer burden (RCB) and tumor-infiltrating lymphocytes (TIL) were evaluated on RD in patients with HER2+ breast cancer treated with NAT. Bot higher RD-TILs and RCB were independently associated with worse overall survival (OS) and their prognostic information was integrated into a composite prognostic score for OS, RCB+TIL, which provided additional prognostic information beyond the isolated evaluation of the two parameters. If validated in larger prospective cohorts, the RCB+TIL prognostic model could help refine the assessment of RD to more reliably predict the outcome of this heterogeneous population of patients with HER2+ breast cancer.
Introduction
Neoadjuvant treatment (NAT) is increasingly administered in patients with HER2+ breast cancer, as it combines the opportunity of expanding locoregional treatment options, assessing the in vivo sensitivity to systemic treatments, prognostically stratifying patients based on pathologic response, and, notably, opening the possibility of personalizing the post-neoadjuvant systemic approach (1). More specifically, pathologic complete response (pCR) is one of the strongest positive prognostic factors in HER2+ breast cancer (2–4), and the presence of residual disease (RD) at surgery currently represents the main criterion for selecting high-risk patients for escalated strategies in the post-neoadjuvant setting (5). Nevertheless, this dichotomic stratification is still suboptimal for estimating, at the patient level, the risk of relapse, as some patients with RD will remain disease-free, and conversely, a fraction of patients achieving pCR will still experience a recurrence event (2–4). Therefore, identification of additional biomarkers to increase prognostication is critical.
Residual cancer burden (RCB), which combines the extent of residual cancer at surgery with more comprehensive histopathological data (6), offers a standardized and reproducible evaluation of RD (7), which can provide more granular prognostic information for all breast cancer subtypes, including HER2+ disease (8). In addition, the immune compartment has a profound impact on HER2+ breast cancer prognosis and response to systemic treatment (9). In patients with HER2+ breast cancer treated with NAT, higher pretreatment tumor-infiltrating lymphocytes (TIL) have been associated with increased pCR rates and survival benefits (10). Nevertheless, TIL evaluation on RD (RD-TIL) in HER2+ breast cancer has provided contradictory results, as high post-NAT TILs have been associated with prognosis in both directions (11–13), and several other reports have failed to establish a prognostic role for RD-TILs (14–17).
This study aimed to evaluate the prognostic significance of RD-TIL levels in a multicentric cohort of retrospectively selected patients with HER2+ breast cancer with RD after anti-HER2 plus chemotherapy (CT) NAT. Furthermore, we assessed the feasibility of combining the prognostic information provided by RCB and RD-TILs into a composite score (RCB+TIL).
Materials and Methods
Population
Patients (female or male ≥18 years) diagnosed with HER2+ breast cancer and treated with CT plus anti-HER2-based NAT at three Italian Institutions (Istituto Oncologico Veneto – IRCCS–Padova, Azienda USL–IRCCS–Reggio Emilia, Humanitas Research Hospital – IRCSS – Milano) between 2001 and 2021 were identified from prospectively maintained databases. Patients with HER2+ breast cancer not receiving chemotherapy or anti-HER2 treatment as part of NAT were excluded. Clinicopathologic, treatment, and follow-up data were also collected.
Pathology
HER2 status was evaluated according to ASCO/CAP recommendations in place at the time of diagnosis, and cases were considered HER2+ in case of IHC score 3+ and/or HER2 gene amplification by ISH.
RCB and TIL levels were evaluated on hematoxylin and eosin (H&E)-stained slides from the surgical samples. The RCB score was calculated according to the standard methodology (6) using online software (http://www.mdanderson.org/breastcancer_RCB), which integrates data regarding primary tumor bed area, overall cancer cellularity, percentage of in situ disease, number of positive lymph nodes, and diameter of the largest metastasis. RCB was considered as a continuous and categorical variable (classes I/II/III) by adopting predefined validated cut-offs (6).
TIL levels were assessed, blinded to patient data, according to the International Immuno-oncology Biomarker Working Group recommendations for TIL evaluation of RD (18, 19).
Statistical analysis
Statistical analyses were performed using IBM SPSS [version 24; (SPSS, RRID:SCR_002865) and R (version 4.2.2; RRID:SCR_001905)].
Descriptive statistics were used to analyze patient demographics and clinical characteristics. For continuous variables, mean, median, range, and quartiles were computed. The distribution of continuous variables across subgroups was assessed using Mann–Whitney and Kolmogorov–Smirnov nonparametric tests. Comparisons of categorical variables across groups were performed using the chi-square test (χ2).
The primary survival endpoint of the present analysis was overall survival (OS), defined as the time from surgery to death from any cause or last follow-up; patients without an OS event were censored at the time of the last follow-up. For descriptive purposes, we also assessed disease-free survival (DFS), defined as the time from surgery to recurrence (local or distant), death from any cause, or last follow-up; patients without a DFS event were censored at the time of the last follow-up. The Kaplan–Meier method was used to estimate survival curves, the log-rank test was used to perform survival analyses, and the Cox regression model to calculate Hazard ratios (HR) and 95% confidence intervals (CI). Harrell's concordance index (C-index; ref. 20) was used to determine the optimal prognostic cut-off for RD-TILs. C-indices were evaluated to compare the performance of the prognostic models (21).
Ethical considerations
The study was approved by the ethics committee of the participating centers, and all relevant ethical regulations were complied with. Tumor samples were collected after approval from the respective Institutional Review Board and in accordance with the Declaration of Helsinki.
Informed written consent was obtained from each subject who was alive at the time of study entry.
Data availability
The datasets that support the findings of this study are not publicly available to protect patient privacy. The data will be available on reasonable request from the corresponding author.
Results
Patients’ characteristics
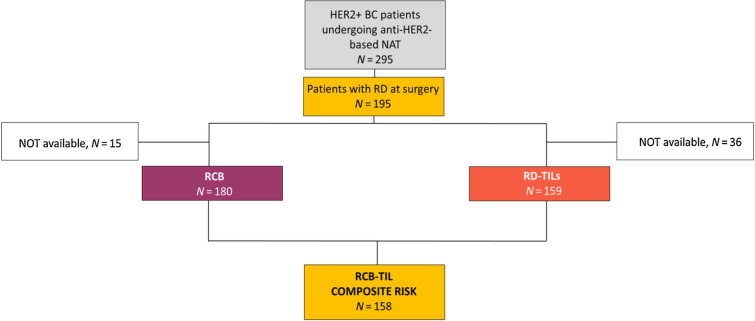
A total of 295 women with HER2+ breast cancer undergoing anti-HER2-based NAT plus CT were selected. A total of 66.1% of patients (n = 195) exhibited RD (Fig. 1). Clinicopathologic characteristics of the overall population are shown in Supplementary Table S1. Clinicopathologic features of the RD cohort are presented in Table 1. The representativeness of study participants is shown in Supplementary Table S2.
Figure 1.
Flow diagram of the study. The flow diagram of the study shows the original population of the study, the subgroup of patients with RD, and the populations for which RCB, RD-TILs, and their integrated evaluation were available. BC, breast cancer.
Table 1.
Clinicopathologic characteristics of patients with RD after surgery.
| Characteristics | N (%) | Median (Q1–Q3) | |
|---|---|---|---|
| Gender | Female | 195 (100) | |
| Male | 0 (0) | ||
| Age (years) | 50.4 (43.7–60.3) | ||
| Histology | Ductal carcinoma | 181 (92.8) | |
| Lobular carcinoma | 7 (3.6) | ||
| Other/NA | 7 (3.6) | ||
| Grading | 1 | 0 (0.0) | |
| 2 | 75 (38.5) | ||
| 3 | 104 (53.3) | ||
| NA | 16 (8.2) | ||
| Clinical stage - cT | 1 | 11 (5.6) | |
| 2 | 133 (68.2) | ||
| 3 | 34 (17.4) | ||
| 4 | 16 (8.2) | ||
| NA | 1 (0.6) | ||
| Clinical stage - cN | Negative | 81 (41.5) | |
| Positive | 114 (58.5) | ||
| Clinical TNM | I | 7 (3.6) | |
| II | 137 (70.2) | ||
| III | 51 (26.2) | ||
| Estrogen expression (%) | 50 (0–90) | ||
| Progesterone expression (%) | 5 (0–50) | ||
| Hormone receptor status | Negative (<1%) | 58 (29.7) | |
| Positive (≥1%) | 137 (70.3) | ||
| Ki67 expression (%) | 30 (20–45) | ||
| Neoadjuvant treatment | Anti-HER2 | 195 (100) | |
| Anthracycline+taxane CT | 165 (84.6) | ||
| Taxane-based CT | 28 (14.3) | ||
| Other CT | 2 (1.1) | ||
| Post-neoadjuvant nodal stage | Negative | 116 (59.5) | |
| Positive | 76 (39.0) | ||
| NA | 3 (1.5) | ||
| Adjuvant CT | Yes | 53 (15.6) | |
| Adjuvant anti-HER2 treatment | Trastuzumab | 157 (80.5) | |
| T-DM1 | 24 (12.3) | ||
| None | 14 (7.2) | ||
| Adjuvant ET | Yes | 125 (64.1) | |
Abbreviations: CT, chemotherapy; ET, endocrine therapy; NA, not available; Q1–Q3, interquartile range.
Most patients had ductal histology, poor differentiation, and a clinically negative nodal status. In addition, almost two-thirds of patients had hormone receptor–positive disease. All patients underwent anti-HER2 plus CT-based NAT. Neoadjuvant CT consisted of anthracycline+taxane in more than 80% of patients. After NAT, the nodal status was negative in most cases. In the adjuvant setting, the majority of patients received trastuzumab and ≈12% received escalated treatment with T-DM1. Almost two-thirds of the population was exposed to adjuvant endocrine treatment.
RCB and TIL evaluation
RCB and RD-TILs were available for 180 and 159 patients, respectively. The median RCB score was 1.7 (Q1–Q3, 1.4–2.9). RCB class distribution was 21.7% (n = 39) class I, 62.2% (n = 112) class II, and 16.1% (n = 29) class III. No differences in the RCB class distribution were observed according to hormone receptor status (P = 0.23).
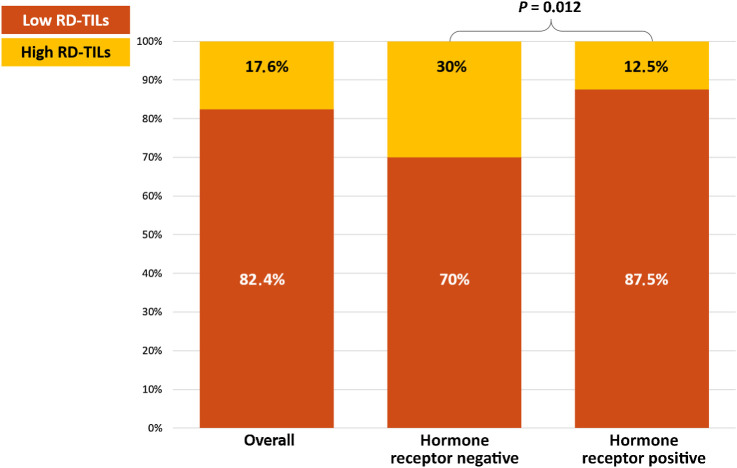
Median RD-TILs were 5.0% (Q1–Q3, 1.0%–10.0%). Fifteen percent of RD-TILs were identified as the optimal prognostic cutoff for OS, with TILs <15% defined as low-RD-TILs and ≥15% as high RD-TILs. The distribution of low RD-TIL and high RD-TIL categories was 82.4% and 17.6%, respectively (Fig. 2). RD-TILs were significantly associated with hormone receptor status, with hormone receptor–negative patients having a significantly higher likelihood of exhibiting high levels of RD-TILs (P = 0.012; Fig. 2).
Figure 2.
Distribution of RD-TILs. Distribution of RD-TILs in the overall cohort and according to hormone receptor status. RD-TILs, tumor-infiltrating lymphocytes on residual disease.
RCB and RD-TILs were not significantly correlated with each other (Pearson correlation, P = 0.360). In addition, the distribution of median RD-TILs was not significantly different according to RCB classes, with numerically lower RD-TILs in RCB I and no difference between RCB II and RCB III (median RD-TILs in RCB I, II, III: 2% vs. 5% vs. 5%, P = 0.054).
Prognostic role of RCB and RD-TILs
At a median follow-up of approximately 84 months, 37 patients died. Of these events, 91.9% (n = 34) were deaths related to breast cancer and 8.1% (n = 3) were deaths from other causes.
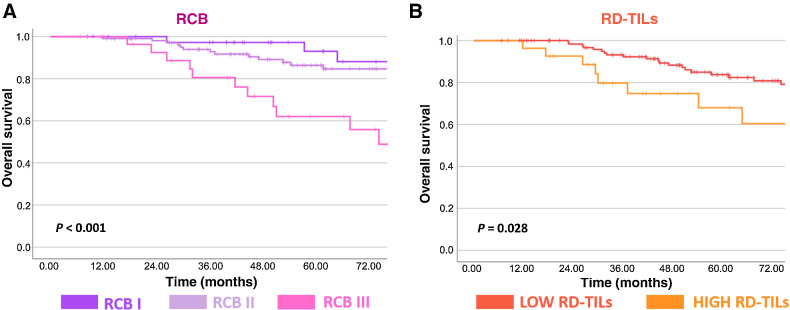
RCB was significantly associated with OS both when considered as a continuous (HR, 1.97; 95% CI, 1.41–2.74; P < 0.001; Fig. 3A) and a categorical variable (P < 0.001; Fig. 3A). RD-TILs were significantly associated with OS, with patients exhibiting high RD-TILs experiencing significantly poorer OS as compared to those with low RD-TILs (HR 2.32; 95% CI, 1.07–5.03; P = 0.028; Fig. 3B).
Figure 3.
Kaplan–Meier curves for OS. Kaplan–Meier curves for OS according to RCB class (I–III; A) and RD-TILs (B). RCB, residual cancer burden; RD-TILs, tumor-infiltrating lymphocytes on residual disease.
The significant prognostic role of RD-TILs was driven by the RCB II subgroup (n = 103; HR, 3.25; 95% CI, 1.1–9.7; P = 0.036), whereas the prognostic association between RD-TILs and OS was not significant within RCB I and III.
Given the enrichment of our cohort for hormone receptor–positive cases, we assessed the prognostic role of RD-TILs specifically in this subgroup, confirming the significant association with OS (HR, 3.65; 95% CI, 1.4–9.4).
In multivariate analysis, both RCB (index) and RD-TIL categories maintained their independent prognostic value for OS [RCB: HR, 1.90 (95% CI, 1.35–2.67), P < 0.001; RD-TILs: HR, 2.30 (95% CI, 1.06–5.01), P = 0.036; Table 2].
Table 2.
Multivariate Cox analysis for OS in patients with residual disease after surgery.
| Factors | HR (95% CI) | P |
|---|---|---|
| RCB, continuous | 1.90 (1.35–2.67) | <0.001 |
| RD-TILs (high vs. low) | 2.30 (1.06–5.01) | 0.036 |
Note: RD-TIL cut-off high (≥15%) and low (<15%).
Abbreviations: HR, Hazards ratio; RCB, residual cancer burden; RD-TILs, tumor-infiltrating lymphocytes on residual disease.
For descriptive purposes, the association between RCB and RD-TILs with DFS was also explored. Supplementary Table S3 shows the multivariate regression model including RCB and RD-TILs. Both RCB and RD-TILs were independently associated with DFS.
We then prognostically stratified patients according to the integrated evaluation of RD-TIL categories and RCB classes, focusing on the intermediate and extensive RD burden categories (RCB II – TIL low, RCB II – TIL high, RCB III – TIL low, RCB III – TIL high). We observed that RD-TILs were capable of refining the risk assessment, with high RD-TILs determining a detrimental effect within the same RCB class, as shown in Supplementary Fig. S1.
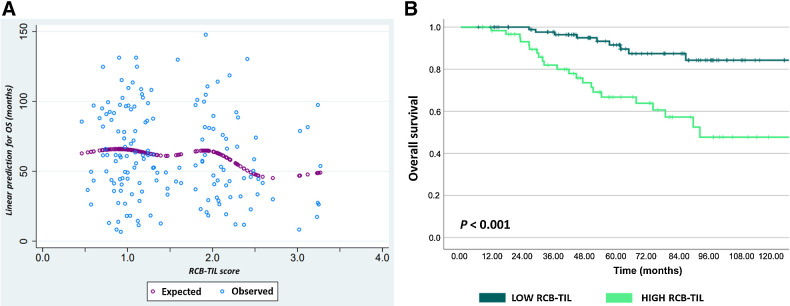
A combined score, RCB+TIL, was calculated from the estimated coefficient of RD-TILs (dichotomous, high vs. low) and the RCB index (continuous) in a bivariate logistic model for OS: TILs (0 = low/1 = high) × 0.83 + RCB (index) × 0.64. RCB+TIL score was significantly associated with OS (HR, 2.72; 95% CI, 1.72–4.31; P < 0.001), with a decreasing probability of OS with increasing RCB+TIL levels, as shown in Fig. 4A.
Figure 4.
Association between RCB+TIL and OS. A, Predicted correlation between composite RCB+TIL score and OS (months). B, Kaplan–Meier curves for OS according to dichotomic RCB+TIL.
By applying the Harrel C test for all RCB-TIL values, we identified 1.72 as the optimal prognostic cutoff for OS. We found a highly significant association between the dichotomous RCB-TILs and OS (HR, 4.36; 95% CI, 2.01–9.48; P < 0.001), as shown in Fig. 4B.
The C-index for OS of the RCB+TIL group was numerically higher than that of the RCB (0.73 vs. 0.68, P = 0.08) and significantly higher than that of the RD-TILs (0.73 vs. 0.58, P = 0.007).
For descriptive purposes we also developed the RCB+TIL combined score calculated from the estimated coefficient of RD-TILs (dichotomous, high vs. low) and the RCB index (continuous) in a bivariate logistic model for DFS (Supplementary Results). The combined score (continuous) was significantly associated with DFS.
Exploratory analysis
Baseline TILs
Baseline TILs were available for 156 patients. Baseline TILs were significantly associated with pCR both when considered as a continuous (P < 0.001) and a categorical variable by considering the already validated cutoffs (pCR rates in high vs. low baseline TILs: 64% vs. 33%, P < 0.001; ref. 22), as shown in Supplementary Fig. S2A. This association was confirmed and was particularly pronounced in patients receiving neoadjuvant anti-HER2 treatment in association with taxane-only chemotherapy (N = 27; pCR rates in high vs. low baseline TILs: 82% vs. 44%, P < 0.001; Supplementary Fig. S2B).
TIL dynamics during NAT
A matched evaluation of baseline TILs was available for 84 patients. In the overall cohort of patients with RD, TIL levels did not significantly differ between baseline biopsy and RD (median baseline TILs and RD-TILs: 13.9% vs. 9.8%, P = 0.300; Supplementary Fig. S3A). However, when differentiating between patients with high RD-TILs versus low RD-TILs, an inverse dynamic emerged. In particular, although a significant increase in TILs after NAT exposure was observed in patients with high RD-TILs (median baseline TILs vs. RD-TILs: 13.3% vs. 38.2%, P < 0.001), with all cases showing some degree of TIL increase (Supplementary Fig. S3B), a numerical decrease in TILs was instead seen in patients with low RD-TILs (median baseline TILs vs. RD-TILs: 11.8% vs. 3.8%, P = 0.113; Supplementary Fig. S3C).
TIL-delta between baseline and RD (continuous) did not significantly differ across RCB classes (P = 0.257). In addition, among patients with low RD-TILs, we did not observe any significant association between TIL-delta and RCB class distribution (P = 0.161).
Discussion
In this study, we investigated the prognostic role of TIL levels on RD in a cohort of retrospectively selected patients with HER2+ breast cancer who failed to achieve pCR after anti-HER2 plus taxane±anthracycline-based CT NAT.
We reported a significant inverse association between TILs on RD and OS, with patients exhibiting more than 15% of RD-TIL levels experiencing significantly shorter OS as compared with those with lower RD-TILs. Interestingly, although TIL levels evaluated on pretreatment biopsies have been consistently correlated with higher pCR rates and improved survival in patients with HER2+ breast cancer patients (23–26), the evaluation of TILs on RD in patients failing to achieve pCR has provided contradictory results. In fact, some studies have found higher RD-TILs to retain a positive prognostic value (11), whereas other groups reported no prognostic association at all (14–17). In line with our evaluation, a worse prognostic significance has also been suggested (12, 13). In this latter regard, similarly to our study, Hamy and colleagues reported a negative prognostic association between high RD-TILs and DFS in a cohort of 175 patients with HER2+ breast cancer treated with NAT (12, 13), which was confirmed by multivariate analysis (13). The cutoff for defining high TILs in Hamy's and our study slightly differed (25% and 15%, respectively), thus making results not entirely comparable. However, although the retrospective nature of the data produced so far, as well as analytical challenges, could limit the interpretation of available evidence, these data remain in direct contrast to what has been observed in triple-negative breast cancer (TNBC), where high RD-TILs have been consistently associated with substantially better outcomes (27, 28). Interestingly, the negative prognostic role of RD-TILs was driven by the moderate RD burden (RCB II) cohort, whereas no significant association was found within the minimal and extensive RD burden categories (RCB I and III, respectively). A similar observation—albeit in the opposite direction—has been made for the prognostic role of RD-TILs in TNBC, where the magnitude of the positive prognostic impact of RD-TILs was greater within the RCB II subgroup with no effect within the RCB III category (28). Although this analogy appears intriguing, the under-representation, in our cohort, of patients with RCB I and III precludes the possibility to solidly interpret our results, which may reflect either a true differential prognostic effect of RD-TILs according to RD burden or a limitation in terms of statistical power.
The apparently counterintuitive behavior of RD-TILs in HER2+ breast cancer as compared with TNBC could be interpreted as the result of the unique immune–cancer cell interaction occurring within HER2+ disease, where the simultaneous evidence of incomplete eradication of cancer cells by chemotherapy plus anti-HER2 targeted treatment and high levels of immune infiltrate may reflect a self-sustaining vicious circle of detrimental mutual relationship between cancer cells and immune cells, unbalanced towards an immunosuppressive/protumorigenic polarization. In fact, RD-TIL evaluation constitutes the bulk measurement of different lymphocyte subpopulations, including T cells, B cells, and NK cells, possessing different relative densities, phenotypes (29, 30), spatial distributions, and complex interactions with their surroundings (29), which can influence their functional status and ultimately converge to determine their clinical significance in terms of treatment response and prognosis (30–34). Our results integrate well within this framework, where the significant negative impact of RD-TILs on prognosis may be interpreted as the direct and deleterious result of these complex immune–tumor interactions. Of course, future research should focus on better characterizing the composition of the RD immune infiltrate, by including markers of antibody-dependent cellular cytotoxicity/phagocytosis (e.g., NK cells; refs. 31, 32) or informing regarding the polarization either in immunosuppressive or cytotoxic direction (e.g., CD8/FOXP3 ratio; ref. 31).
We also assessed the association between the RCB index and OS in our cohort, confirming and strengthening the strong prognostic value of RCB in patients with HER2+ breast cancer treated with taxane ± anthracycline-based CT and anti-HER2 treatment (8). Importantly, RD-TILs and RCB were not significantly correlated with each other, and both preserved their independent association with OS in multivariate analysis. These findings suggest the complementarity of these two biomarkers from a prognostic point of view. Intriguingly, when prognostically stratifying patients according to the integrated evaluation of RD-TILs and RCB classes, we observed that RD-TILs were capable of refining the risk assessment of patients with HER2+ breast cancer failing to achieve pCR. In particular, by focusing on moderate and extensive RD burden categories, we observed that within the same RCB class, RD-TILs were capable of “up/down-staging” the risk category determined by the sole evaluation of RD burden. This observation is appealingly similar—albeit in opposite sense—to what has already been validated within TNBC (35), and further emphasizes the importance of validating RD-TILs in patients with HER2+ breast cancer undergoing NAT, by integrating their use within the framework of risk assessment in the experimental scenario and, in a close future, even in the daily practice setting. These observations lay the foundation for the development of a novel composite prognostic score incorporating both RD-TILs and RCB index (the so-called RCB+TIL). Importantly, the RCB+TIL composite score was significantly associated with OS, outperforming the prognostic performance of RD-TILs alone, and providing numerically stronger prognostic information than the RCB index. These findings provided proof of principle that the incorporation of data reflecting both the burden and immune biology of RD in patients with HER2+ breast cancer failing to achieve pCR after standard NAT may be more informative than the isolated evaluation of the two single biomarkers, thus adding a further layer of knowledge regarding the complex interaction between cancer, immunity, and systemic treatments. A subtler consideration regarding the association between this novel composite prognostic score and the endpoint OS suggests that the co-existence of a high burden of RD and fraudulent immune infiltration may actually affect the entire natural history of HER2+ breast cancer.
To our knowledge, this is the first attempt to combine the prognostic information provided by RCB with RD-TILs in patients with HER2+ breast cancer. Previous studies investigated the prognostic value of composite models integrating residual cellularity/tumor with immune information; however, they focused on TILs measured at baseline or early during NAT, thus making their results not comparable to ours (25, 36). If validated in larger prospective cohorts, the new prognostic model provided in this study may contribute to a more reliable prediction of the outcome of the heterogeneous population of patients with HER2+ breast cancer failing to achieve complete clearance of cancer cells from the breast and axilla after standard neoadjuvant therapy.
The value of our observations is further appealing considering the results of our exploratory analysis aimed at investigating TIL dynamics under NAT exposure. Interestingly, although this analysis involved only a subgroup of patients and should therefore be interpreted with caution, it provided the insight of a diametrically opposed behavior of TIL evolution in patients with high versus low RD-TILs, with the first experiencing a significant increase from baseline and the latter a numerical decrease from baseline, revealing that the immune microenvironment of RD reflects acquired mechanisms possibly shaped by NAT exposure. This ultimately generates the hypothesis that although baseline TILs may reflect the intrinsic immunogenicity features of HER2+ breast cancer, RD-TILs could more comprehensively capture the complex interaction between tumor, host, and treatments. Importantly, although previous studies consistently demonstrated a highly dynamic behavior of immune infiltration under neoadjuvant chemotherapy + anti-HER2 treatment (9, 25, 37), none of these studies assessed TIL dynamics using our highly reproducible approach (18).
Overall, our findings may provide the rationale for redefining the endpoints for neoadjuvant trials, switching from a purely quantitative and punctiform perspective towards a more qualitative, dynamic, and integrated appraisal of RD, thus well integrating with accumulating evidence highlighting the suboptimality of pCR, regarded in isolation, as a prognostic biomarker in HER2+ breast cancer (38, 39). Within this specific framework, the RCB+TIL composite model may assist in the identification of patients at high risk of relapse, who may potentially benefit, in experimental platforms, from integrated systemic approaches in the post-neoadjuvant setting, with the aim of disrupting the vicious loop established between micro-metastatic cancer cells and host immunity, sustaining both anti-HER2 treatment resistance and antitumor immune anergy. Indeed, it can be speculated that the administration of post-neoadjuvant strategies exclusively focused on targeting HER2 might not be sufficient in patients failing to achieve pCR after CT+anti-HER2 NAT and exhibiting high RD-TILs/RCB+TIL scores. In this context, although results from trials investigating immunotherapy activity and efficacy in unselected HER2+ breast cancer have been unsatisfactory so far (40), the contextual evaluation of TILs and RCB into the RCB+TIL composite model may allow the identification of patients with suboptimal responses to anti-HER2-based NAT and an enhanced immune infiltration, thus potentially serving as a reproducible and easily obtainable tool to guide patient selection in the future development of anti-HER2 plus immunologic integrated treatment strategies for the post-neoadjuvant management of high-risk patients with HER2+ breast cancer.
Finally, we also performed an exploratory analysis aimed at assessing the role of baseline TILs in pCR prediction. We confirmed in our cohort of patients with HER2+ breast cancer the strong association between more than 10% of baseline TILs and the subsequent achievement of pCR, as demonstrated previously (22), thus solidifying the solid role of baseline TILs in the identification of patients with a higher likelihood of achieving pCR after standard NAT. Interestingly, this association was confirmed and was numerically even more pronounced in the subgroup of patients receiving anthracycline-free chemotherapy backbone, with more than 80% of patients with intermediate/high TILs achieving pCR after taxane-only chemotherapy (+HER2 blockade). This observation is particularly timed given the great enthusiasm regarding the possibility of omitting anthracycline from the neoadjuvant chemotherapy backbone of patients with HER2+ breast cancer (41) fueled by preliminary data suggesting a more favorable risk/benefit ratio of anthracycline-free neoadjuvant regimens (42, 43). Within this framework, our results, if validated, suggests that TILs may find a positioning in guiding the selection of patients capable of achieving pCR with a de-escalated neoadjuvant approach.
This study has several strengths. First, it constitutes one of the largest multi-institutional evaluations of RD-TILs in a cohort of patients with HER2+ breast cancer treated with neoadjuvant anti-HER2 treatment plus taxane±anthracycline chemotherapy. In addition, RD-TILs were assessed according to standardized guidelines (18). Moreover, the adoption of OS as a prognostic endpoint allowed us to appraise the impact of the proposed prognostic model on the evolution of the disease in HER2+ breast cancer with RD after NAT.
We acknowledge some limitations, including the retrospective design of our study and the inclusion of a subpopulation of patients not receiving anti-HER2 escalated therapy, such as neoadjuvant pertuzumab or post-neoadjuvant T-DM1, which could potentially have different interactions with the RD immune compartment and therefore impact the prognostic information derived from RD-TILs. Moreover, our results do not envisage the possible differential impact of different chemotherapy regimens on shaping the RD immune microenvironment (44). However, since approximately 85% of patients received anthracycline+taxane-based neoadjuvant chemotherapy, the possible confounding impact of this bias appears resized. In addition, although the investigation of matched baseline TILs provided highly informative insights, it involved a relatively small subgroup of patients, and thus requires further investigation. Moreover, although the prognostic value of the RCB+TIL composite model was not affected by hormone receptor status, our HER2+ cohort was enriched in patients with hormone receptor–positive breast cancer, which may be associated with a different immune milieu (45). As we did not characterize gene expression profiling (GEP) regarding intrinsic molecular subtypes, we cannot exclude the influence of this variable in the interpretation of our data. Although in HER2+ breast cancer, HER2-enriched is the most predominant intrinsic subtype, all the different intrinsic molecular subtypes can be represented within HER2+ disease (46, 47). Finally, our population was enriched for RCB class II cases, thus limiting the statistical power to properly interpret the possibly differential prognostic significance of RD-TILs across different RCB classes.
In conclusion, we have reported an independent negative prognostic impact of higher RD-TILs after anti-HER2+CT-based NAT, which might underlie an imbalance of the RD immune microenvironment towards immunosuppressive features. We also provided a new composite prognostic score based on RCB+TIL, which was significantly associated with OS, proving to be more informative than the isolated evaluation of RCB and RD-TILs.
Supplementary Material
Supplementary Figure 1 - Kaplan–Meier curves of overall survival according to RCB classes and RD-TILs.
Supplementary Figure 2 – Association between baseline TILs and pCR in: 2a) the overall cohort and 2b) the cohort of patients receiving taxane-only neoadjuvant chemotherapy (plus anti-HER2 treatment).
Supplementary Figure 3 – Matched evaluation of baseline TIL (b-TILs) and residual (RD-TILs) in the overall RD cohort (Fig. 3a), the high RD-TILs cohort (Fig. 3b) and in the low RD-TILs cohort (Fig. 3c).
Supplementary Table 1 - Clinico-pathological characteristics of the overall population.
Supplementary Table 2 - Representativeness of Study Participants
Supplementary Table 3 – Multivariate Cox analysis for disease-free survival (DFS) in patients with residual disease (RD) after surgery
Acknowledgments
The publication costs of this article have been covered by the following funding: 5×1000/2016 – Approccio globale alle problematiche oncologiche (AGON) (to V. Guarneri).
The authors also acknowledge the following fundings: Fondazione AIRC under 5 per mille 2019 - ID. 22759 program (to V. Guarneri); Veneto Institute of Oncology IOV-IRCCS (to M.V. Dieci and V. Guarneri); and DOR funding from the University of Padova (to G. Griguolo, M.V. Dieci, V. Guarneri).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
F. Miglietta reports personal fees from Roche, Novartis, and Gilead outside the submitted work. G. Griguolo reports personal fees from Novartis, Eli Lilly, and Gilead outside the submitted work. D. Massa reports personal fees from Eli Lilly outside the submitted work. F. Girardi reports personal fees from AstraZeneca and Eli Lilly outside the submitted work. M. Fassan reports grants and personal fees from Astellas; personal fees from GSK, MSD, Pierre Fabre, Lilly, Novartis, Roche, and AstraZeneca; and grants from Diaceutics, QED, and Macrophage Pharma outside the submitted work. R. De Sanctis reports personal fees from Istituto Clinico Gentili, Novartis, and Amgen outside the submitted work. A. Zambelli reports personal fees from Roche, Lilly, AstraZeneca, Seagen, and MSD; personal fees and nonfinancial support from Novartis, Daiichi Sankyo, and Exact Sciences; and grants and personal fees from Pfizer outside the submitted work. V. Guarneri reports personal fees from Eisai, Eli Lilly, Exact Sciences, Gilead, Merck Serono, MSD, Novartis, Olema Oncology, Sanofi, Amgen, GSK, and AstraZeneca outside the submitted work; in addition, V. Guarneri has a patent for HER2DX licensed to Reveal Genomics. M.V. Dieci reports personal fees from Eli Lilly, MSD, Exact Sciences, Novartis, Pfizer, and Seagen outside the submitted work; in addition, M.V. Dieci has a patent for HER2DX pending to Reveal Genomics. No disclosures were reported by the other authors.
Authors' Contributions
F. Miglietta: Conceptualization, data curation, formal analysis, investigation, visualization, methodology, writing–original draft. M. Ragazzi: Investigation, writing–review and editing. B. Fernandes: Investigation, writing–review and editing. G. Griguolo: Investigation, methodology, writing–review and editing. D. Massa: Writing–original draft. F. Girardi: Methodology, writing–review and editing. M. Bottosso: Writing–review and editing. A. Bisagni: Investigation, writing–review and editing. G. Zarrilli: Writing–review and editing. F. Porra: Investigation, writing–review and editing. D. Iannaccone: Investigation, writing–review and editing. L. Dore: Investigation, writing–review and editing. M. Gaudio: Investigation, writing–review and editing. G. Santandrea: Investigation, writing–review and editing. M. Fassan: Writing–review and editing. M. Lo Mele: Writing–review and editing. R. De Sanctis: Writing–review and editing. A. Zambelli: Investigation, writing–review and editing. G. Bisagni: Investigation, writing–review and editing. V. Guarneri: Supervision, funding acquisition, writing–review and editing. M.V. Dieci: Conceptualization, formal analysis, supervision, funding acquisition, writing–review and editing.
References
- 1. Miglietta F, Dieci MV, Griguolo G, Guarneri V. Neoadjuvant approach as a platform for treatment personalization: focus on HER2-positive and triple-negative breast cancer. Cancer Treat Rev 2021;98:102222. [DOI] [PubMed] [Google Scholar]
- 2. Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol 2016;2:751–60. [DOI] [PubMed] [Google Scholar]
- 3. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–72. [DOI] [PubMed] [Google Scholar]
- 4. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res 2020;26:2838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019;380:617–28. [DOI] [PubMed] [Google Scholar]
- 6. Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414–22. [DOI] [PubMed] [Google Scholar]
- 7. Peintinger F, Sinn B, Hatzis C, Albarracin C, Downs-Kelly E, Morkowski J, et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod Pathol 2015;28:913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 2017;35:1049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Griguolo G, Pascual T, Dieci MV, Guarneri V, Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J Immunother Cancer 2019;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Solinas C, Ceppi M, Lambertini M, Scartozzi M, Garaud S, Fumagalli D, et al. Tumor infiltrating lymphocytes in HER2-positive breast cancer patients treated with neoadjuvant chemotherapy plus trastuzumab, lapatinib or their combination: a meta-analysis of published randomized clinical trials. Ann Oncol 2017;28. [DOI] [PubMed] [Google Scholar]
- 11. Kurozumi S, Inoue K, Matsumoto H, Fujii T, Horiguchi J, Oyama T, et al. Prognostic utility of tumor-infiltrating lymphocytes in residual tumor after neoadjuvant chemotherapy with trastuzumab for HER2-positive breast cancer. Sci Rep 2019;9:1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamy AS, Pierga JY, Sabaila A, Laas E, Bonsang-Kitzis H, Laurent C, et al. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann Oncol 2017;28:2233–40. [DOI] [PubMed] [Google Scholar]
- 13. Hamy AS, Bonsang-Kitzis H, De Croze D, Laas E, Darrigues L, Topciu L, et al. Interaction between molecular subtypes and stromal immune infiltration before and after treatment in breast cancer patients treated with neoadjuvant chemotherapy. Clin Cancer Res 2019;25:6731–41. [DOI] [PubMed] [Google Scholar]
- 14. Bianchini G, Pienkowski T, Im Y-H, Bianchi GV, Tseng L-M, Liu M-C, et al. Residual disease after HER2-directed therapies in the neosphere study: modulation of tumor lymphocyte infiltration (TIL) and prognosis. J Clin Oncol 2016;34:517.26729440 [Google Scholar]
- 15. Force J, Howie LJ, Abbott SE, Bentley R, Marcom PK, Kimmick G, et al. Early stage HER2-positive breast cancers not achieving a pCR from neoadjuvant trastuzumab- or pertuzumab-based regimens have an immunosuppressive phenotype. Clin Breast Cancer 2018;18:410–7. [DOI] [PubMed] [Google Scholar]
- 16. Griguolo G, Serna G, Pascual T, Fasani R, Guardia X, Chic N, et al. Immune microenvironment characterisation and dynamics during anti-HER2-based neoadjuvant treatment in HER2-positive breast cancer. NPJ Precis Oncol 2021;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ignatiadis M, Van Den Eynden G, Roberto S, Fornili M, Bareche Y, Desmedt C, et al. Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: A TRYPHAENA Substudy. J Natl Cancer Inst 2019;111:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dieci MV, Radosevic-Robin N, Fineberg S, van den Eynden G, Ternes N, Penault-Llorca F, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the international immuno-oncology biomarker working group on breast cancer. Semin Cancer Biol 2018;52:16–25. [DOI] [PubMed] [Google Scholar]
- 19. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs working group 2014. Ann Oncol 2015;26:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543–6. [PubMed] [Google Scholar]
- 21. Schmid M, Wright MN, Ziegler A. On the use of Harrell's C for clinical risk prediction via random survival forests. Expert Syst Appl 2016;63. [Google Scholar]
- 22. Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40–50. [DOI] [PubMed] [Google Scholar]
- 23. Denkert C, Von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33:983–91. [DOI] [PubMed] [Google Scholar]
- 24. Heppner BI, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt W, et al. Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res 2016;22:5747–54. [DOI] [PubMed] [Google Scholar]
- 25. Nuciforo P, Pascual T, Cortés J, Llombart-Cussac A, Fasani R, Paré L, et al. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann Oncol 2018;29:170–7. [DOI] [PubMed] [Google Scholar]
- 26. Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol 2015;1:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 2014;25:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luen SJ, Salgado R, Dieci MV, Vingiani A, Curigliano G, Gould RE, et al. Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann Oncol 2019;30:236–42. [DOI] [PubMed] [Google Scholar]
- 29. Massa D, Tosi A, Rosato A, Guarneri V, Dieci MV. Multiplexed in situ spatial protein profiling in the pursuit of precision immuno-oncology for patients with breast cancer. Cancers 2022;14:4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res 2008;14:2413–20. [DOI] [PubMed] [Google Scholar]
- 31. Hurvitz SA, Caswell-Jin JL, McNamara KL, Zoeller JJ, Bean GR, Dichmann R, et al. Pathologic and molecular responses to neoadjuvant trastuzumab and/or lapatinib from a phase II randomized trial in HER2-positive breast cancer (TRIO-US B07). Nat Commun 2020;11:5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McNamara KL, Caswell-Jin JL, Joshi R, Ma Z, Kotler E, Bean GR, et al. Spatial proteomic characterization of HER2-positive breast tumors through neoadjuvant therapy predicts response. Nat Cancer 2021;2:400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muntasell A, Rojo F, Servitja S, Rubio-Perez C, Cabo M, Tamborero D, et al. NK cell infiltrates and HLA class I expression in primary HER2+ breast cancer predict and uncouple pathological response and disease-free survival. Clin Cancer Res 2019;25:1535–45. [DOI] [PubMed] [Google Scholar]
- 34. Wolf NK, Kissiov DU, Raulet DH. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat Rev Immunol 2023;23:90–105. [DOI] [PubMed] [Google Scholar]
- 35. Loi S, Salgado R, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer 2022;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chic N, Luen SJ, Nuciforo P, Salgado R, Fumagalli D, Hilbers F, et al. Tumor cellularity and infiltrating lymphocytes as a survival surrogate in HER2-positive breast cancer. J Natl Cancer Inst 2022;114:467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shang M, Chi Y, Zhang J, Chang J, Yang H, Yin S, et al. The therapeutic effectiveness of neoadjuvant trastuzumab plus chemotherapy for HER2-positive breast cancer can be predicted by tumor-infiltrating lymphocytes and PD-L1 expression. Front Oncol 2022;11:706606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Mackelenbergh MT, Loibl S, Untch M, Buyse M, Geyer CE, Gianni L, et al. Pathologic complete response and individual patient prognosis after neoadjuvant chemotherapy plus anti–human epidermal growth factor receptor 2 therapy of human epidermal growth factor receptor 2–positive early breast cancer. J Clin Oncol 2023;41:2998–3008. [DOI] [PubMed] [Google Scholar]
- 39. Squifflet P, Saad ED, Loibl S, van MMT, Untch M, Rastogi P, et al. Re-evaluation of pathologic complete response as a surrogate for event-free and overall survival in human epidermal growth factor receptor 2–positive, early breast cancer treated with neoadjuvant therapy including anti–human epidermal growth factor receptor 2 therapy. J Clin Oncol 2023;41:2988–97. [DOI] [PubMed] [Google Scholar]
- 40. Huober J, Barrios CH, Niikura N, Jarząb M, Chang Y-C, Huggins-Puhalla SL, et al. Atezolizumab with neoadjuvant anti–human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2–positive early breast cancer: primary results of the randomized phase III IMpassion050 trial. J Clin Oncol 2022;40:2946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guarneri V, de Azambuja E. Anthracyclines in the treatment of patients with early breast cancer. ESMO Open 2022;7:100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278–84. [DOI] [PubMed] [Google Scholar]
- 43. van Ramshorst MS, van der Voort A, van Werkhoven ED, Mandjes IA, Kemper I, Dezentjé VO, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018;19:1630–40. [DOI] [PubMed] [Google Scholar]
- 44. Galluzzi L, Zitvogel L, Kroemer G. Immunological mechanisms underneath the efficacy of cancer therapy. Cancer Immunol Res 2016;4:895–902. [DOI] [PubMed] [Google Scholar]
- 45. Dieci MV, Miglietta F, Guarneri V. Immune infiltrates in breast cancer: recent updates and clinical implications. Cells 2021;10:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prat A, Carey LA, Adamo B, Vidal M, Tabernero J, Cortés J, et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst 2014;106:dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schettini F, Pascual T, Conte B, Chic N, Brasó-Maristany F, Galván P, et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: a systematic review and meta-analysis. Cancer Treat Rev 2020;84:101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 - Kaplan–Meier curves of overall survival according to RCB classes and RD-TILs.
Supplementary Figure 2 – Association between baseline TILs and pCR in: 2a) the overall cohort and 2b) the cohort of patients receiving taxane-only neoadjuvant chemotherapy (plus anti-HER2 treatment).
Supplementary Figure 3 – Matched evaluation of baseline TIL (b-TILs) and residual (RD-TILs) in the overall RD cohort (Fig. 3a), the high RD-TILs cohort (Fig. 3b) and in the low RD-TILs cohort (Fig. 3c).
Supplementary Table 1 - Clinico-pathological characteristics of the overall population.
Supplementary Table 2 - Representativeness of Study Participants
Supplementary Table 3 – Multivariate Cox analysis for disease-free survival (DFS) in patients with residual disease (RD) after surgery
Data Availability Statement
The datasets that support the findings of this study are not publicly available to protect patient privacy. The data will be available on reasonable request from the corresponding author.