Abstract
We present a unique case of neurosarcoidosis diagnosed based on thyroid biopsy and FDG PET (Fluorodeoxyglucose positron emission tomography) imaging. A patient presented for a second opinion after being placed in hospice for rapidly progressing dementia, presumed to be due to Creutzfeldt Jakob disease despite negative workup and was unable to perform activities of daily life or communicate with his wife. The patient underwent a workup including whole-body FDG PET, which showed hypermetabolic lymph nodes as well as a hypermetabolic nodule in the thyroid. Biopsy of the lymph nodes was nondiagnostic, but the thyroid biopsy tissue yielded a diagnosis of sarcoid. After ruling out other causes and reviewing the tissue pathology, the patient was diagnosed with systemic sarcoidosis with neurological involvement and started on infliximab with rapid improvement.
Keywords: Neurosarcoidosis, FDG-PET, Systemic sarcoid
Introduction
Sarcoidosis is the presence of noncaseating granulomatous disease, most commonly in the lungs, lymph nodes, skin, and liver [1]. Less commonly, sarcoid can affect the central or peripheral nervous system resulting in a wide range of clinical manifestations including cranial neuropathies, neuroendocrine dysfunction, meningitis, seizures, encephalopathy, myelopathy, hydrocephalus, and myopathy [2]. Neurologic sarcoidosis can accompany systemic sarcoidosis or, more rarely, may occur in isolation. Imaging of the lungs and lymph nodes may suggest the diagnosis of sarcoidosis and can help identify suitable targets for confirmatory tissue biopsy.
Case report
A 65-year-old male initially presented with slow, shuffling gait. Past medical history was unremarkable, excluding recently stopping adalimumab for psoriatic arthritis due to insurance coverage changes. Over the next months, the patient developed confusion and memory issues affecting daily life, urinary incontinence, poor appetite, and repeated falls. Three months after initial presentation, the patient was hospitalized for urinary tract infections and was found to have second degree heart block, which was treated with pacemaker placement. Four months after initial symptoms, the patient had lost 50 pounds and was bed- and wheelchair-bound with bowel and bladder incontinence and alogia, and he required assistance with activities of daily living (e.g., dressing and brushing teeth). At this point, the patient was presumed to have rapidly progressive dementia, believed to be due to Creutzfeldt-Jakob disease based on outside interpretation of brain MRI, and was placed in hospice.
The patient sought a second opinion and underwent whole-body imaging with [¹⁸F]Fluorodeoxyglucose positron emission tomography with computed tomography (FDG PET/CT) to rule out possible neoplastic process and paraneoplastic syndrome as the cause of his symptoms. The FDG PET/CT demonstrated multiple markedly hypermetabolic lymph nodes throughout the body, an atypical pattern of myocardial uptake, a hypermetabolic 2.0 × 1.4 cm thyroid nodule with 20 SUV max, and multiple hypermetabolic nodules throughout the skeletal muscular fascia and subcutaneous fat (Fig. 1). Additionally, marked global hypometabolism was seen in the brain, with preservation of the sensorimotor cortex and occipital lobe uptake. MRI brain showed nonspecific confluent foci of T2 hyperintensity in the periventricular and subcortical white matter in both cerebral hemispheres and enlarged ventricles (Fig. 2). While no focal hypermetabolic foci were seen in the brain or cerebral meninges to suggest neurosarcoidosis, there were scattered hypermetabolic foci throughout the spinal canal. MRI spine showed radicular enhancement of the cauda equina and T2 hyperintensity of the paraspinal musculature.
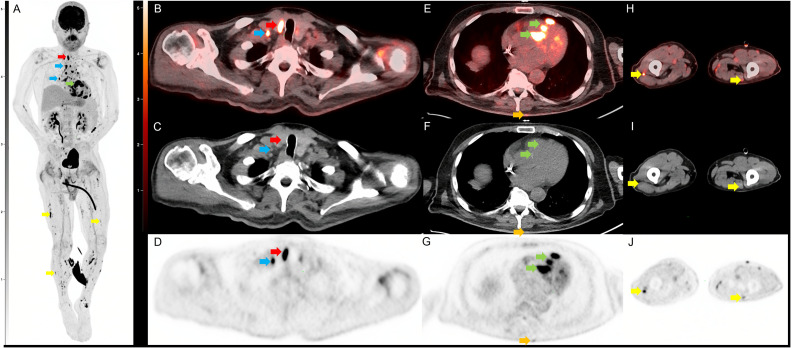
Fig. 1.
Selected images from the initial FDG PET/CT. Coronal FDG PET maximum intensity projection (MIP; A) demonstrates multifocal FDG avid foci including a right thyroid nodule (red arrow), multiple supraclavicular, mediastinal, hilar, and mesenteric lymph nodes (blue arrow), multifocal nodular cardiac uptake (green arrow), and multiple nodular soft tissue deposits in the intramuscular fascia and skin (yellow arrow). Transaxial FDG PET/CT (B) CT (C) and PET (D) images show a hypermetabolic nodule along the right thyroid gland which was a pathology proven to be sarcoidosis. Adjacent hypermetabolic right supraclavicular lymph node (blue arrow) was also noted and likely nodal sarcoid involvement. Transaxial FDG PET/CT (E) CT (F) and PET (G) images through the thorax demonstrate nodular intense FDG uptake along the cardiac intraventricular septum (green arrow) and a nodular focus of cutaneous uptake along the back (orange arrow) which was found to be inflamed seborrheic keratoses. Transaxial FDG PET/CT (H) CT (I) and PET (J) images through the legs demonstrate multinodular hypermetabolic focal (yellow arrow) along the intramuscular fascia and subcutaneous fat which is also likely related to the patient sarcoid.
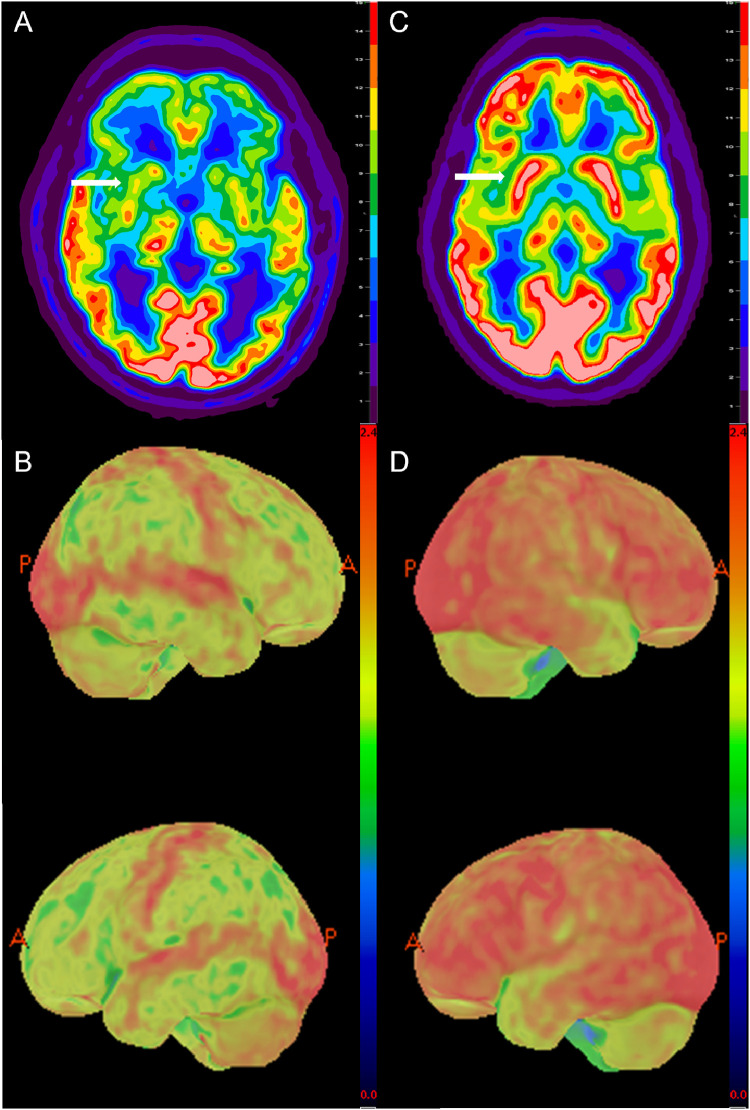
Fig. 2.
Transaxial 18F-FDG PET image (A) of the patient's brain through the basal ganglia (white arrow) and 3-D surface rendering (B) clearly demonstrates the marked global hypometabolism including deep gray matter (white arrow) when compared to age-normalized standard with relative preservation of the sensorimotor cortex and occipital lobe. For comparison, transaxial 18F-FDG PET of a 58-year-old man with suspected lymphoma and paraneoplastic syndrome (C) demonstrates normal intense physiologic FDG uptake in the brain and basal ganglia (white arrow) and 3-D surface rendering (2D) demonstrate the expected physiologic uptake with basal ganglia comparable to gray matter.
To assess for myocardial sarcoid involvement, the patient underwent cardiac MR, but imaging was nondiagnostic due to the patient being unable to control his breathing. Echocardiogram showed an ejection fraction of 26%. Due to the patient's clinical status, myocardial biopsy was deferred, and goal-directed medical management for heart failure was initiated. Subsequent thyroid ultrasound showed an ill-defined hypoechoic nodule in the left lobe (Fig. 3) and an abnormal cervical lymph node. Initially, thyroid fine needle aspiration was attempted but yielded no cells after several passes. In the setting of the patient's clinical status and need for tissue diagnosis, a thyroid core biopsy was performed and yielded granulomatous inflammation and was negative for acid-fast bacilli, fungal colonization, or malignancy. Of note, the patient's thyroid stimulating hormone (TSH) was within normal limits. This result coupled with the PET/CT findings suggested the diagnosis of sarcoid with neurologic, thyroid, and likely cardiac involvement.
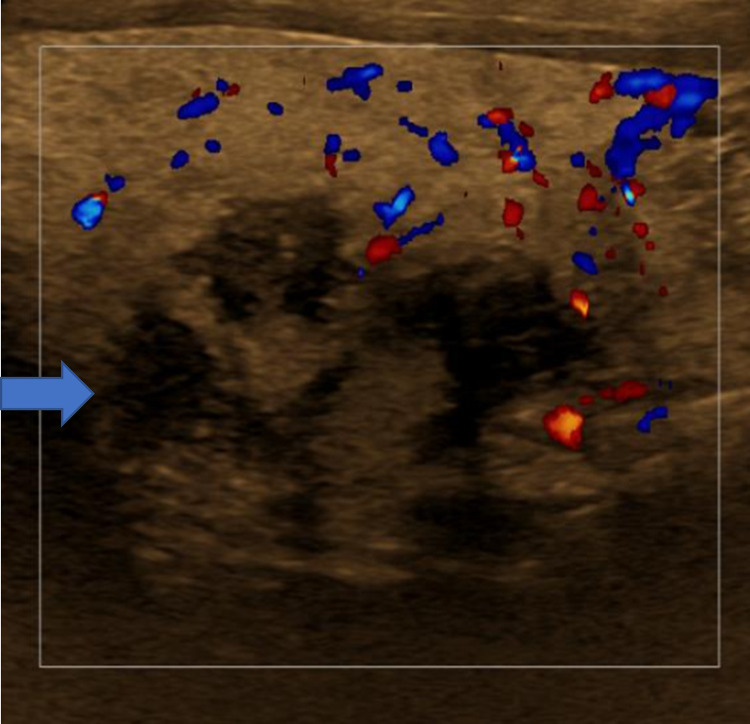
Fig. 3.
Color Doppler images of thyroid region of concern showing mixed echogenicity, part solid, poorly defined mass. After fine needle aspiration yielded no cells, a core biopsy was performed which showed noncaseating granulomas.
The patient was started on intravenous methylprednisolone with rapid and dramatic clinical improvement. A day later, he was able to feed himself and have conversations with his family. He continued to improve, was placed on infliximab, attended short-term rehabilitation, and then returned home. The patient regained enough strength to perform transfers, walk with an assistive device, participate in physical therapy, and could have prolonged, complex conversations with his wife.
Unfortunately, the patient contracted Coronavirus disease 2019 (COVID-19) the following year, after which he developed weakness, mental fatigue, and worsening heart failure with an ejection fraction of 20%. The patient's infliximab was held due to congestive heart failure. After some improvement in his ejection fraction, infliximab was restarted, but the patient did not have a favorable response. The patient passed away 29 months after his diagnosis.
Discussion
FDG PET-CT and thyroid biopsy ultimately led to this patient's diagnosis. Before undergoing this evaluation, the patient was given a terminal diagnosis and recommended hospice. The thyroid biopsy yielded a diagnosis of sarcoid, which could be treated with immunosuppressants, providing the patient with immediate improvement in quality of life. The patient continued to do well until developing complications from COVID infection. The patient, initially given less than 6 months to live and bed-bound, was given at least a year of improved quality of life and was able to walk with assistive devices and converse with his family.
FDG PET-CT is nonspecific for sarcoid diagnosis as it can detect malignancy, infectious, and inflammatory conditions. It can, however, give valuable information about active sarcoid disease when combined with tissue samples [3]. Sarcoid presents most commonly as hypermetabolic mediastinal and upper abdominal lymph nodes and can less frequently present in the cervical nodes, spleen, or in skeleton, typically as lytic lesions. As the appearance of sarcoid on FDG PET is indicated by metabolic activity, it can be mistaken for lymphoma or other malignancies that may lead to hypermetabolic lymph nodes. FDG-PET (with the proper dietary preparation to prevent false positive metabolism) can also detect and monitor therapy of cardiac sarcoid involvement, presenting as hypermetabolic foci [4]; however, cardiac MR and myocardial biopsy remain the gold standard for diagnostic cardiac involvement. In both systemic sarcoid and cardiac sarcoid, FDG-PET has been investigated as a means of evaluating response to immunosuppressive therapy [3,4]. Active neurosarcoidosis can also be seen as abnormal hypermetabolic areas on FDG-PET [5]. While FDG-PET in isolation cannot diagnose neurosarcoidosis, it can elicit typical patterns of sarcoidosis throughout the body and provide targets for biopsy and tissue diagnosis [6].
Previous cases of neurosarcoidosis have been diagnosed with the aid of thyroid biopsy. In 1 such case, a patient presented with rapid functional decline and memory loss; a workup showed lymphadenopathy and thyroid goiter on chest CT as well as laboratory evidence of hyperthyroidism [2]. Thyroid core biopsy yielded noncaseating granulomas, which along with mediastinal lymphadenopathy and neurological symptoms led to the diagnosis of systemic sarcoidosis with thyroid, pulmonary, and CNS involvement. An FDG-PET was not reported.
Another interesting aspect of our case is that the patient had become symptomatic after stopping therapy with adalimumab for psoriatic arthritis. Adalimumab, a fully human monoclonal antibody against tumor necrosis factor-alpha (TNF-alpha), has been used to treat both cardiac sarcoid and systemic sarcoidosis [7,8] as an alternative to infliximab. There have also been reports of adalimumab causing sarcoidosis-like reactions in patients who recently started the therapy, and the reaction is seen in approximately 1/2800 patients treated with TNF-alpha antagonists [9]. The sarcoidosis-like reaction to TNF-alpha antagonists resolves with the cessation of inciting medication and anti-inflammatory treatment. In our case, the patient had recently stopped the medication when he began developing symptoms, and symptoms continued to progress while off adalimumab therapy, arguing against sarcoidosis-like reaction due to TNF-alpha antagonist therapy. It is unknown, therefore, if the patient's adalimumab was treating pre-existing sarcoidosis which was revealed upon cessation, or if the patient developed sarcoidosis due to immune dysregulation.
Conclusions
Our case emphasizes the role of FDG-PET in diagnosing sarcoidosis as a means of providing initial hints to a systemic process as well as providing targets for tissue confirmation biopsy. The utilization of FDG-PET in this case and the subsequent thyroid biopsy allowed physicians to determine systemic sarcoidosis with involvement of the nervous system and thyroid along with probable involvement of the heart. The patient, initially given less than 6 months to live, started immune therapy with instant improvement, giving him a better quality of life for at least a year after his diagnosis.
Patient consent
Written, informed consent was obtained from the patient's representative, given that the patient had passed at the time of the manuscript being written.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Judson MA. Environmental risk factors for sarcoidosis. Front Immunol. 2020;11:1340. doi: 10.3389/fimmu.2020.01340. Epub 20200626PubMed PMID: 32676081; PubMed Central PMCID: PMC7333358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quertermous BP, Kavuri S, Walsh DW. An old disease with a new twist: CNS and thyroid sarcoidosis presenting as subacute dementia. Clin Case Rep. 2021;9(10):e04829. doi: 10.1002/ccr3.4829. Epub 20211006PubMed PMID: 34631064; PubMed Central PMCID: PMC8493448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakar HB, Rabinowitz CB, Gibbons FK, O'Donnell WJ, Shepard J-AO, Aquino SL. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. Am J Roentgenol. 2008;190(3_supplement):S1–S6. doi: 10.2214/AJR.07.7001. [DOI] [PubMed] [Google Scholar]
- 4.Sze Jia N, Hui Chong L, William R, Thomas W, Babak S, Mona-Elisabeth R, et al. Diagnosis and evaluation of cardiac sarcoidosis with FDG PET/CT and PET/MR. J Nucl Med. 2022;63(supplement 2):2641. [Google Scholar]
- 5.Wang Y, Andrews J, Jenkins Colon P, Wundes A. FDG-PET abnormalities leading to the diagnosis of an unusual case of probable neurosarcoidosis. Neurol - Neuroimmunol Neuroinflamm. 2018;5(6):e506. doi: 10.1212/nxi.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartels S, Kyavar L, Blumstein N, Görg T, Glöckner SC, Zimmermann R, et al. FDG PET findings leading to diagnosis of neurosarcoidosis. Clin Neurol Neurosurg. 2013;115(1):85–88. doi: 10.1016/j.clineuro.2012.03.042. Epub 20120413PubMed PMID: 22503061. [DOI] [PubMed] [Google Scholar]
- 7.Crommelin HA, van der Burg LM, Vorselaars AD, Drent M, van Moorsel CH, Rijkers GT, et al. Efficacy of adalimumab in sarcoidosis patients who developed intolerance to infliximab. Respir Med. 2016;115:72–77. doi: 10.1016/j.rmed.2016.04.011. Epub 20160423PubMed PMID: 27215507. [DOI] [PubMed] [Google Scholar]
- 8.Sweis JJG, Sweis NWG, Ascoli C, Levin B, Avitall B, Rubinstein I, et al. Adalimumab in the treatment of cardiac sarcoidosis: single center case series and narrative literature review. Respir Med Case Rep. 2022;40 doi: 10.1016/j.rmcr.2022.101766. Epub 20221025PubMed PMID: 36340865; PubMed Central PMCID: PMC9627097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhargava S, Perlman DM, Allen TL, Ritter JH, Bhargava M. Adalimumab induced pulmonary sarcoid reaction. Respir Med Case Rep. 2013;10:53–55. doi: 10.1016/j.rmcr.2013.07.002. Epub 20131026PubMed PMID: 26029514; PubMed Central PMCID: PMC3920421. [DOI] [PMC free article] [PubMed] [Google Scholar]