Abstract
AVID (Asymmetric ventriculomegaly, interhemispheric cyst, and dysgenesis of corpus callosum) spectrum is a rare phenomenon as such in its whole and the defects are not exclusive to the condition. Each may occur in isolation or together and have characteristic clinical and imaging findings. The vast array of mimics coexisting with the condition makes it a harder diagnosis to make and requires a great length of experience and observation which may explain the limited recordings of AVID. Sonography and fetal magnetic resonance imaging goes a long way and provide accurate diagnosis ruling out the mimics and aiding in prenatal visualization of the defects. Accurate diagnosis aids in effective management and counseling regarding outcomes and the potential timeline of the severity of the symptoms. In its rarity, this case report of AVID is one of the first report of its kind reported from Nepal.
Keywords: AVID, Sonography, MRI, Cyst, Callosum
Introduction
Asymmetric ventriculomegaly (AVM), interhemispheric cyst (IHC), and dysgenesis of the corpus callosum (DCC) collectively called (AVID) are rare distinct brain abnormalities that can occur separately or together in rare cases. These abnormalities can cause pressure on adjacent brain structures and lead to neurological problems, including developmental delay, seizures, and abnormal head growth. AVM is a condition in which one lateral ventricle is comparatively larger than the other, while IHCs are fluid-filled sacs that develop between the 2 hemispheres of the brain. DCC, on the other hand, refers to incomplete or abnormal development of the corpus callosum, which is the bundle of nerve fibers that connects the 2 hemispheres of the brain. The etiology of AVM, IHC, and DCC is not well understood, but mutations in genes involved in brain development, such as EMX2, have been identified in patients with AVM and DCC [1] and environmental such as maternal infection or exposure to toxins, may also contribute to the development of these abnormalities [2]. However, in most cases, the cause remains unknown.
Diagnoses are now made in-utero as routine prenatal sonography has helped to identify the anomalies earlier in fetuses who ultimately survive to delivery which would previously have been made clinically at birth or as a result of neurodevelopmental delays. Increasing experience and better technology have vastly improved the ability to diagnose such anomalies. Fetal magnetic resonance imaging (MRI) is often used to complement sonography, allowing for better prenatal diagnosis and counseling [[3], [4], [5],6].
Case discussion
A 34 years old female from a remote area of Nepal came to the neurology outpatient department with a chief complaint of seizures on and off since childhood which increased in frequency over the last 6 months and inability to perform her daily activities. During the general check-up, she did not have eye contact and produced inappropriate words, which we can't understand. She did not obey commands. Her vitals were within normal limits. Basic blood investigations like CBC, blood sugar level, LFT, and RFT were within normal limits.
Imaging findings
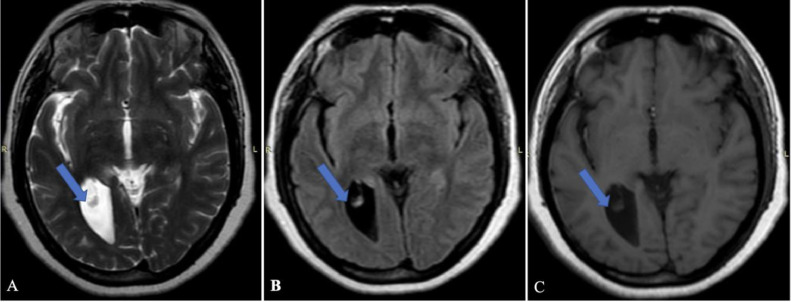
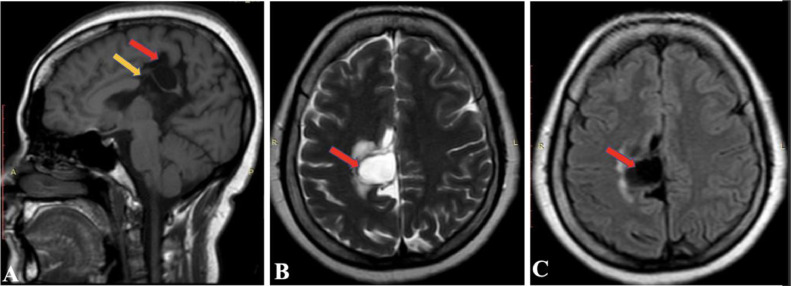
MRI was performed as a part of the imaging diagnosis. MRI demonstrates asymmetric dilatation of the right lateral ventricle. Fig. 1 and 2 demonstrate dilatation of the occipital and temporal horn of the right lateral ventricle respectively. There is also dysgenesis of the corpus callosum. Genu and anterior aspect of the body appear normal, while the posterior aspect of the body and splenium is absent (shown by the yellow arrow in Fig. 3(A). A well-defined cyst (CSF signal intensity on T1, T2, and FLAIR sequences) measuring 16 × 15 mm in the right paramedian location involving the posterior aspect of the cingulate gyrus with minimal perilesional edema (shown by the yellow arrow in Fig. 3A).
Fig. 1.
MRI axial (A) T2 weighted image, (B) FLAIR image, and (C) T1 weighted image demonstrating asymmetric dilatation of occipital horn of right lateral ventricle (showing by blue arrows in images).
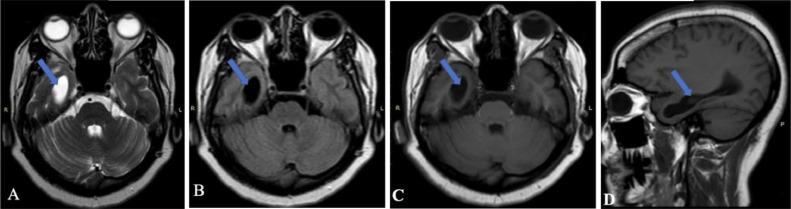
Fig. 2.
MRI (A) axial T2 weighted image, (B) axial FLAIR image, (C) axial T1 weighted image and (D) sagittal T1 weighted image, demonstrating asymmetric dilatation of temporal horn of right lateral ventricle (showing by blue arrows in images).
Fig. 3.
MRI (A) sagittal T1 weighted image, (B) axial T2 weighted image and (C) axial FLAIR image, demonstrating a well-defined cyst (CSF signal intensity on all sequences) in right paramedian location (showing by red arrows in images). There is dysgenesis of the corpus callosum with absent of part of body and splenium (showing by yellow arrow in image A).
Discussion
The key sonographic finding is usually asymmetric ventriculomegaly but recognition of an absent cavum septi pellucidi, callosal malformation, and an interhemispheric cyst requires experienced and detailed observation [7]. The other additional finding associated with these anomalies can be the presence of features of Chiari I malformation, possibly due to downward pressure on the tentorium and posterior fossa from the enlarging ventricles, all these findings eventually aiding to take an early decision of cesarean delivery based upon the degree of macrocephaly [7]. In a case series published by Oh et al. [7], they hypothesized that the process leading to incomplete or failed formation of the corpus callosum is attributed to the presence of the ventricular diverticulum or cyst that disrupts the normal cellular cues for directional migration of the commissural fibers in the developing brain. Similarly, it has been noted that the communicating cysts are formed after the embryologic detachment of ventricular tela choroidea from the thalamus on one side or both, which eventually expands [8]. The unexplained association of malformation of the corpus callosum with interhemispheric cysts [9] paved the way for more studies to acknowledge the triad imaging findings that are AVID as reported by Barkovich et al. [10,11]. They classified interhemispheric cysts as type 1 or 2 with subcategories in both based upon their study series that relied on pediatric imaging, in agreement with our case in point that exhibits type 1A cyst that is an intra-axial extension of the lateral ventricle.
Developmental and destructive processes form the differentials for cystic brain lesions which makes it equally important to distinguish these diagnoses from the 1 in question which is greatly achieved by sonography and fetal MRI [12]. Supratentorial developmental lesions like schizencephaly, holoprosencephaly, and arachnoid cysts mimic AVID [7,13]. Schizencephaly is a cortical defect characterized by the presence of linear clefts containing cerebrospinal fluid extending from the pia mater to the underlying ventricle and does not have a mass effect on adjacent brain parenchyma while interhemispheric cyst is a midline defect causing mass effect leading to the appearance of asymmetric ventricles [13]. The midline defect of the cyst is in constant analogy with that of holoprosencephaly but can be differentiated by the presence of falx and discontinuous midline parenchyma that is in contrast to the midline mono ventricle with a continuous cortical mantle in the latter [14]. Though the differentiation of cyst walls could be difficult with sonography, improved resolution with fetal MRI correlation can help delineate arachnoid cysts from the interhemispheric cysts, however, the extra-axial nature of the arachnoid cysts make it a rather clear distinction from other differentials [15]. Similarly, hydranencephaly, a condition of destructed parenchyma in the areas of anterior and middle cerebral arteries mimics cysts of AVID but with careful characterization and observance, it can easily be framed that with AVID cysts, supratentorial parenchyma is present bilaterally but compressed against the skull due to ventriculomegaly. Another destructive process called porencephaly is often the case of suspicion in the diagnosis of AVID but however, but that can be ruled out by noticing gray matter lining unlike in AVID where parenchymal destruction is not identified [16]. The various causes of hydrocephalus including asymmetric hydrocephalus, posthemorrhagic asymmetric hydrocephalus, and asymmetric lateral ventriculomegaly can be differentiated from AVID with fetal MRI that has the additional advantage of detecting the presence of blood products in contradiction to the AVID asymmetry. Additionally, the mentioned hydrocephalus may be associated with a normal cavum septum pellucidi in-utero unlike in AVID where the cavum is absent [7,14].
The basis of the prenatal diagnosis of AVID entirely falls on sonography and fetal MRI which are paramount at the least [17,18,19]. Asymmetric ventriculomegaly, absent cavum septi pellucidi, and porencephaly, as evidenced by the absent medial wall of the ventricle can all be detected by sonography [20]. These sonographic findings warrant the use of fetal MRI for definite identification of the cyst and also because the downside of the use of sonography in recording the findings of AVID includes the role of external factors that distort the findings that include varying fetal positions and unaccounted reverberated waves from the fetal skull [21]. Hence, fetal MRI gives us a reliable, sensitive, and specific option to better visualize the interhemispheric cysts and callosal malformation [6,19]. The added advantage of the MRI is that it shows us the midline ventricular cyst and preserved gray matter lining the margin of the cyst helping to exclude porencephaly due to an infarct or hemorrhage. Sagittal and coronal images illustrate the degree of presence of corpus callosum [6,17,18].
The prognosis of these patients varies markedly depending upon the presence of additional symptoms, especially hydrocephalus. The prognosis is less favorable if hydrocephalus is associated with other intracranial malformations as compared to isolated hydrocephalus [22,23]. Griebel et al. [24] showed mild to moderate developmental delays in association with AVID.
There are increasing works in this triad of imaging that are hugely based on postnatal diagnosis. The in-utero or prenatal diagnosis of the condition using sonography and fetal MRI can lead to unprecedented avenues for the management of AVID. Management of AVM, interhemispheric cyst, and DCC is largely supportive and focused on addressing the specific symptoms and complications that arise from these abnormalities, however, surgical intervention may be necessary to relieve pressure on the brain and prevent further damage [25]. Interhemispheric cysts may also require surgical removal or drainage to alleviate pressure and reduce the risk of neurological deficits [7,25]. DCC cannot be corrected, but early intervention and therapy can help to manage the associated symptoms and improve outcomes for affected individuals [7].
Conclusion
AVM, interhemispheric cyst, and DCC are rare brain abnormalities that can cause significant neurological problems and developmental delays. The exact causes of these abnormalities are not well understood, but genetic and environmental factors may play a role. Sonography and fetal MRI play a pivotal role in visualizing the relevant defects and ruling out others. Management of these conditions is largely supportive, with surgical intervention and early intervention therapy being the mainstay of treatment. Further research is needed to better understand the underlying mechanisms of these abnormalities and to develop more effective treatment options for affected individuals.
Patient consent
Written informed consent was obtained from the patient's parents/legal guardian for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Footnotes
Competing Interests: There is no conflict of interest among authors.
References
- 1.Cecchi C. Emx2: a gene responsible for cortical development, regionalization and area specification. Gene. 2002;291(1–2):1–9. doi: 10.1016/s0378-1119(02)00623-6. [DOI] [PubMed] [Google Scholar]
- 2.Hahn JS. Brain malformations and environmental factors. Arch Neurol. 2004;61(11):1734–1738. doi: 10.1001/archneur.61.11.1734. [DOI] [Google Scholar]
- 3.Hosny IA, Elghawabi HS. Ultrafast MRI of the fetus: an increasingly important tool in prenatal diagnosis of congenital anomalies. Magn Resonan Imaging. 2010;28(10):1431–1439. doi: 10.1016/j.mri.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Manfredi R, Tognolini A, Bruno C, Raffaelli R, Franchi M. Agenesis of the corpus callosum in fetuses with mild ventriculomegaly: role of MR imaging. La Radiol Med. 2009;115(2):301–312. doi: 10.1007/s11547-009-0474-7. [DOI] [PubMed] [Google Scholar]
- 5.Hetts SW, Sherr EH, Chao S, Gobuty S, Barkovich AJ. Anomalies of the corpus callosum: an MR analysis of the phenotypic spectrum of associated malformations. Am J Roentgenol-New Series. 2006;187(5):1343. doi: 10.2214/AJR.05.0146. [DOI] [PubMed] [Google Scholar]
- 6.Glenn OA, Goldstein RB, Li KC, Young SJ, Norton ME, Busse RF, et al. Fetal magnetic resonance imaging in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med. 2005;24(6):791–804. doi: 10.7863/jum.2005.24.6.791. [DOI] [PubMed] [Google Scholar]
- 7.Oh KY, Kennedy AM, Selden NR, McLean L, Sohaey R. Asymmetric ventriculomegaly, interhemispheric cyst, and dysgenesis of the corpus callosum (AVID): an imaging triad. J Ultrasound Med. 2012;31:1811–1820. doi: 10.7863/jum.2012.31.11.1811. [DOI] [PubMed] [Google Scholar]
- 8.Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology. 2010;52(6):447–477. doi: 10.1007/s00234-010-0696-3. [DOI] [PubMed] [Google Scholar]
- 9.Swett HA, Nixon GW. Agenesis of the corpus callosum with interhemispheric cyst. Radiology. 1975;114(3):641–645. doi: 10.1148/114.3.641. [DOI] [PubMed] [Google Scholar]
- 10.Barkovich AJ, Simon EM, Walsh CA. Callosal agenesis with cyst: a better understanding and new classification. Neurology. 2001;56(2):220–227. doi: 10.1212/wnl.56.2.220. [DOI] [PubMed] [Google Scholar]
- 11.Pavone P, Barone R, Baieli S, Parano E, Incorpora G, Ruggieri M. Callosal anomalies with interhemispheric cyst: expanding the phenotype. Acta Pædiatrica. 2005;94(8):1066–1072. doi: 10.1111/j.1651-2227.2005.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 12.Epelman M, Daneman A, Blaser SI, Ortiz-Neira C, Konen O, Jarrín J, et al. Differential diagnosis of intracranial cystic lesions at head US: correlation with CT and MR imaging. Radiographics. 2006;26(1):173–196. doi: 10.1148/rg.261055033. [DOI] [PubMed] [Google Scholar]
- 13.Pilliod RA, Pettersson DR, Gibson T, Gievers L, Kim A, Sohaey R, et al. Diagnostic accuracy and clinical outcomes associated with prenatal diagnosis of fetal absent cavum septi pellucidi. Prenat Diagn. 2018;38(6):395–401. doi: 10.1002/pd.5247. [DOI] [PubMed] [Google Scholar]
- 14.Limoges N, Ostrander B, Kennedy A, Woodward PJ, Bollo RJ. Neurological and clinical outcomes in infants and children with a fetal diagnosis of asymmetric ventriculomegaly, interhemispheric cyst, and dysgenesis of the corpus callosum. J Neurosurg. 2021;29(3):283–287. doi: 10.3171/2021.9.PEDS21252. [DOI] [PubMed] [Google Scholar]
- 15.Oh KY, Gibson TJ, Pinter JD, Pettersson D, Shaffer BL, Selden NR, et al. Clinical outcomes following prenatal diagnosis of asymmetric ventriculomegaly, interhemispheric cyst, and callosal dysgenesis (AVID) Prenat Diagn. 2019;39(1):26–32. doi: 10.1002/pd.5393. [DOI] [PubMed] [Google Scholar]
- 16.Eller KM, Kuller JA. Fetal porencephaly: a review of etiology, diagnosis, and prognosis. Obstet Gynecol Survey. 1995;50(9):684–687. doi: 10.1097/00006254-199509000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Hassan J, Sepulveda W, Teixeira J, PM Cox. Glioependymal and arachnoid cysts: unusual causes of early ventriculomegaly in utero. Prenat Diagn. 1996;16(8):729–733. doi: 10.1002/(SICI)1097-0223(199608)16:8<729::AID-PD901>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs F, Moutard ML, Blin G, Sonigo P, Mandelbrot L. Prenatal and postnatal follow-up of a fetal interhemispheric arachnoid cyst with partial corpus callosum agenesis, asymmetric ventriculomegaly and localized polymicrogyria. Fetal Diagn Ther. 2008;24(4):385–388. doi: 10.1159/000165511. [DOI] [PubMed] [Google Scholar]
- 19.Mühler MR, Hartmann C, Werner W, Meyer O, Bollmann R, Klingebiel R. Fetal MRI demonstrates glioependymal cyst in a case of sonographic unilateral ventriculomegaly. Pediatric Radiology. 2007;37:391–395. doi: 10.1007/s00247-007-0419-z. [DOI] [PubMed] [Google Scholar]
- 20.Sadan S, Malinger G, Schweiger A, Lev D, Lerman-Sagie T. Neuropsychological outcome of children with asymmetric ventricles or unilateral mild ventriculomegaly identified in utero. BJOG: Int J Obstet Gynaecol. 2007;114(5):596–602. doi: 10.1111/j.1471-0528.2007.01301.x. [DOI] [PubMed] [Google Scholar]
- 21.d'Ercole C, Girard N, Cravello L, Boubli L, Potier A, Raybaud C, et al. Prenatal diagnosis of fetal corpus callosum agenesis by ultrasonography and magnetic resonance imaging. Prenat Diagn. 1998;18(3):247–253. [PubMed] [Google Scholar]
- 22.Levitsky DB, Mack LA, Nyberg DA, Shurtleff DB, Shields LA, Nghiem HV, et al. Fetal aqueductal stenosis diagnosed sonographically: how grave is the prognosis? AJR: Am J Roentgenol. 1995;164(3):725–730. doi: 10.2214/ajr.164.3.7863902. [DOI] [PubMed] [Google Scholar]
- 23.Lee CS, Hong SH, Wang KC, Kim SK, Park JS, Jun JK, et al. Fetal ventriculomegaly: prognosis in cases in which prenatal neurosurgical consultation was sought. J Neurosurg. 2006;105(4):265–270. doi: 10.3171/ped.2006.105.4.265. [DOI] [PubMed] [Google Scholar]
- 24.Griebel ML, Williams JP, Russell SS, Spence GT, Glasier CM. Clinical and developmental findings in children with giant interhemispheric cysts and dysgenesis of the corpus callosum. Pediatr Neurol. 1995;13(2):119–124. doi: 10.1016/0887-8994(95)00144-5. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs F, Moutard ML, Blin G, Sonigo P, Mandelbrot L. Prenatal and postnatal follow-up of a fetal interhemispheric arachnoid cyst with partial corpus callosum agenesis, asymmetric ventriculomegaly, and localized polymicrogyria. Fetal Diagn Ther. 2008;24(4) doi: 10.1159/000165511. :385–8.s. [DOI] [PubMed] [Google Scholar]