Key Points
Question
Are there clinical or sociodemographic differences among older patients receiving immunotherapy for head and neck cancer (HNC)?
Findings
In this cohort study of 4860 patients 65 years and older with HNC, White patients had 80% increased odds of receiving immunotherapy as part of their treatment. Compared with patients with oropharyngeal HNC, those with nonoropharyngeal disease were more likely to receive immunotherapy.
Meaning
These findings suggest that White patients and patients with nonoropharyngeal cancer are more likely to receive immunotherapy for HNC.
This cohort study assesses clinical and nonclinical factors associated with receipt of immunotherapy among older patients with head and neck cancer.
Abstract
Importance
The US Food and Drug Administration approved immune checkpoint inhibitors (immunotherapy) for select cases of head and neck squamous cell carcinoma (HNSCC) in 2016. However, it is unclear whether there are clinical or sociodemographic differences among patients receiving immunotherapy as part of their care. Given the known disparities in head and neck cancer care, we hypothesized that there are differences in receipt of immunotherapy among patients with HNSCC based on clinical and nonclinical characteristics.
Objective
To characterize clinical and nonclinical factors associated with receipt of immunotherapy among older patients with HNSCC.
Design, Setting, and Participants
This retrospective cohort study included patients 65 years or older diagnosed with HNSCC (n = 4860) in a community oncology care setting. Electronic health records from Navigating Cancer were assessed from January 1, 2017, to April 30, 2022.
Main Outcomes and Measures
Multivariable logistic regression was used to characterize clinical (tumor stage [localized vs advanced] and anatomical subsite [oropharyngeal vs nonoropharyngeal]) and nonclinical (age, smoking history, race and ethnicity, sex, and marital status) factors associated with receipt of immunotherapy.
Results
In the study cohort of 4860 patients, 3593 (73.9%) were men; 4230 (87.0%) were White and 630 (13.0%) were of other races. A total of 552 patients (11.4%) had received immunotherapy. After adjusting for covariates, in the final model, White patients with HNSCC had 80% increased odds of receiving immunotherapy (adjusted odds ratio [AOR], 1.80 [95% CI, 1.30-2.48]) compared with patients of other races. There were no statistically significant differences in the odds of receiving immunotherapy based on age, sex, or smoking history. Patients with nonoropharyngeal disease were significantly more likely to receive immunotherapy than those with oropharyngeal cancer (AOR, 1.29 [95% CI, 1.05-1.59]), as were those with advanced compared with local disease (AOR, 2.39 [95% CI, 1.71-3.34]).
Conclusions and Relevance
The findings of this cohort study suggest that among older patients with HNSCC, White patients may be more likely to receive immunotherapy as part of their care. Equitable access to immunotherapy and other treatment options will reduce cancer-related health disparities and improve survival of patients with HNSCC.
Introduction
Head and neck cancer (HNC) represents approximately 4% of the cancer burden of the US, with an estimated 65 000 new cases in 2022.1 Outcomes of HNC are among the most disparate; there is a 25% point difference in 5-year relative survival of the disease, with the third-worst absolute survival difference for Black compared with White patients among all cancers.1 This survival difference is largely attributed to stage of presentation and treatment differences.1,2,3 Treatment of locally advanced HNC is traditionally multimodal, often requiring a combination of surgery, radiotherapy, and in some cases, chemotherapy.2 Most recently, immunotherapy has become a treatment option for select cases of HNC.4,5,6
In 2016, following landmark clinical trials,7,8,9 the US Food and Drug Administration approved immune checkpoint inhibitors (ICIs; interchangeably referred to as immunotherapy going forward) as an option for head and neck squamous cell carcinoma (HNSCC). These landmark trials, in addition to safety and efficacy, demonstrated improved survival for patients diagnosed with programmed cell death ligand 1–positive recurrent or metastatic HNSCC compared with treatment with platinum-based chemotherapy alone.10,11 Cytotoxic T-lymphocyte–associated antigen-4 and programmed cell death-1 are 2 immunomodulatory receptors expressed on T cells that are targeted by the available immunotherapy medications such as ipilimumab, nivolumab, and pembrolizumab.12 However, while there are several previous and ongoing clinical trials establishing the treatment efficacy of immunotherapy,5 there is very little clinical evidence of access to and receipt of immunotherapy among patients with HNC. This is a critical gap, as immunotherapy is a very expensive treatment option for patients with HNC.5 Disparate access to care remains a driver of outcomes in HNC3,13,14; however, it is unknown whether there are clinical and nonclinical differences among individuals receiving immunotherapy for HNC.
Previous literature has found racial disparities in HNC care, particularly for Black patients, who face worse outcomes.3,15,16 This may be partially attributed to factors related to socioeconomic differences and access to care.15 This is particularly relevant to immunotherapy care in HNC given the significant associated costs. Among racial minority group populations, Black patients with HNC have been shown to have the lowest socioeconomic status, which is associated with inadequate health insurance coverage.3,13,14 Based on our knowledge of the existing disparity in HNC care,1,3,15,16 we hypothesized that there are differences in receipt of immunotherapy, with more disparate access among racial minority group patients with HNC compared with White patients. To test this hypothesis, we characterized clinical and nonclinical factors associated with receipt of immunotherapy among patients with HNC. Characterizing these differences offers an opportunity to improve access to cancer therapies and reduce the health care disparities that may affect patient prognosis.
Methods
Data Source
For this cohort study, we used deidentified data from 37 different oncology clinics in the US that use the Navigating Cancer digital health solution. The Navigating Cancer database contains both source data through integration with clinic electronic health records, as well as data from Navigating Cancer’s digital health solution that includes electronic patient-reported outcomes, triage, and care management data. Navigating Cancer data consist of demographic variables (eg, age, race and ethnicity, sex, smoking history), clinical data (eg, cancer type, cancer site, cancer stage), and information on systemic therapy. Patient-level data are obtained from structured sources, and all electronic patient-reported outcomes data within Navigating Cancer’s digital health solution are collected in a user-friendly, Health Insurance Portability and Accountability Act–compliant manner for applicable patients who use the system. Approval for the study was received from the Duke University Institutional Review Board. This study was based on previously collected deidentified data; therefore, no additional request for consent was required. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Design
We identified a cohort of patients diagnosed with HNC based on the International Classification of Diseases, Ninth Revision, Clinical Modification or International Statistical Classification of Diseases, Tenth Revision, Clinical Modification between January 1, 2017, and April 30, 2022. To mitigate health care access concerns due to health insurance status, we only included patients 65 years or older in our analytic sample, as these patients typically are eligible for Medicare.17 Due to small sample sizes in individual race categories, race was categorized as White or racial minority group, the latter of which included American Indian or Alaska Native, Asian, Black, Native Hawaiian or Other Pacific Islander, and more than 1 or other race. Patients of Hispanic ethnicity were characterized based on self-identification as White or as a racial minority group individual. We categorized cancer stage as local (stages I and II) and advanced (stages III and IV). Anatomical subsites included were broadly grouped as oropharyngeal (codes C01, C02.4, C02.8, C02, any C09, C10, 141.0, 141.9, 146.0, 146.1, 146.2, 146.7, 146.8, 146.9, or 141.9), nonoropharyngeal (codes C00, C02.0, C02.1, C02.2, C02.3, C03, C04, C05, C06, C07, C08, C11, C12, C13, C30, C31, C32, 140.0, 140.1, 140.3, 140.4, 141.1, 141.2, 141.3, 141.4, 142.0, 142.9, 143.0, 143.1, 143.9, 144.0, 144.9, 145.0, 145.1, 145.2, 145.3, 145.4, 145.6, 145.9, 147.1, 147.2, 147.8, 147.9, 148.1, 148.2, 148.8, 148.9, 149.0, 161.0, 161.1, 161.3, 161.8, or 161.9), or unknown (C14, C76, or 195.0).
We studied the patients’ demographic (race, sex, smoking history [no vs yes or unknown]) and clinical (cancer site [oropharyngeal vs nonoropharyngeal or unknown] and cancer stage [local vs advanced or unknown]) factors associated with receipt of ICIs as part of their treatment. Immune checkpoint inhibitors included in this study were pembrolizumab, nivolumab, atezolizumab, durvalumab, dostarlimab, cemiplimab, and avelumab.
Statistical Analysis
We used a χ2 test to compare the categorical variables based on the receipt of immunotherapy. The mean with SD was used for continuous variables. Multivariable logistic regression models were constructed and used to identify baseline demographic (age, sex, race, marital status, and smoking history) and clinical (cancer site, cancer stage) characteristics associated with receipt of immunotherapy as part of treatment in patients with HNC. Variables included in the multivariable logistic regression were derived from statistically significant results of prior analyses.18,19 Results of logistic regression are expressed as odds ratios (ORs) with their 95% CIs. All data processing steps and analyses were performed in Python, version 3.8.5 (Python Software Foundation). R package ggplot2 (R Project for Statistical Computing) was used to visualize the output of logistic models in this study. All statistical tests were 2-tailed, and ORs at a 95% CI determined statistical significance.
Results
Baseline Demographic of the Patient Population
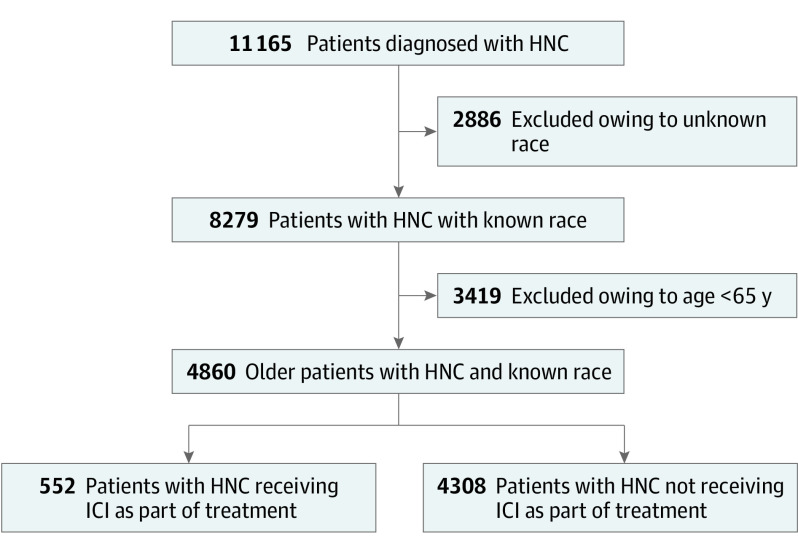
During the study period (January 1, 2017, to April 30, 2022), a total of 11 165 patients with HNC were identified within the Navigating Cancer database. Of these, 4860 patients met the study selection criteria (Figure 1). In the study cohort, most patients were men (3593 [73.9%] vs 1267 [26.1%] women) and had a reported history of smoking (3006 [61.9%]) (Table). Most patients were White (4230 [87.0%]); among the 630 racial minority group patients, 36 (5.7%) were American Indian or Alaska Native, 76 (12.1%) were Asian, 441 (70.0%) were Black, and 77 (12.2%) were of more than 1 or other race. The mean (SD) age of this cohort was 74.4 (7.1) years (Table). Patients with the nonoropharyngeal cancer subtype comprised slightly more than half (2448 [50.4%]) of the cohort. Among patients with HNC with known cancer stage, 1288 (26.5%) had an advanced cancer stage (stage III and stage IV). Additionally, 552 patients (11.4%) had received immunotherapy as treatment type (Figure 1).
Figure 1. Flow Diagram Showing the Selection of the Patient Cohort With Head and Neck Cancer (HNC).
ICI indicates immune checkpoint inhibitor.
Table. Baseline Characteristics of Patient Study Cohort.
| Characteristic | Patient groupa | |
|---|---|---|
| Without ICI (n = 4308) | With ICI (n = 552) | |
| Age, mean (SD), yb | 74.4 (7.4) | 75.0 (7.0) |
| Sexc | ||
| Women | 1130 (26.2) | 137 (24.8) |
| Men | 3178 (73.8) | 415 (75.2) |
| Racec | ||
| Racial minority group | 585 (13.6) | 45 (8.2) |
| American Indian or Alaska Native | 46 (7.9) | 4 (8.9) |
| Asian | 56 (9.6) | 5 (11.1) |
| Black | 451 (77.1) | 30 (66.7) |
| Native Hawaiian or Other Pacific Islander | 5 (0.8) | 2 (4.4) |
| >1 or Other race | 27 (4.6) | 4 (8.9) |
| White | 3723 (86.4) | 507 (91.8) |
| Smoking historyc | ||
| No | 1068 (24.8) | 142 (25.7) |
| Unknown | 577 (13.4) | 67 (12.1) |
| Yes | 2663 (61.8) | 343 (62.1) |
| Cancer sitec | ||
| Nonoropharyngeal | 2173 (50.4) | 275 (49.8) |
| Oropharyngeal | 1694 (39.3) | 170 (30.8) |
| Unknown | 441 (10.2) | 107 (19.4) |
| Cancer stagec | ||
| Local | 624 (14.5) | 47 (8.5) |
| Advanced | 1085 (25.2) | 203 (36.8) |
| Unknown | 2599 (60.3) | 302 (54.7) |
Abbreviation: ICI, immune checkpoint inhibitor therapy.
Unless otherwise indicated, data are expressed as No. (%) of patients. Percentages have been rounded and may not total 100.
Differences were statistically significant based on 2-tailed t test.
Differences were statistically significant based on χ2 test.
Factors Associated With Receipt of Immunotherapy
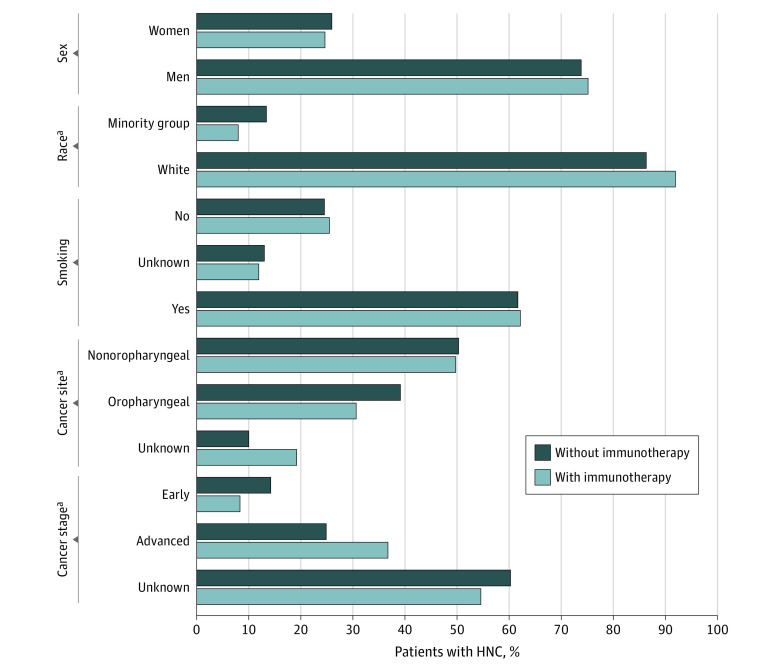
Next, we performed a χ2 test to study the association of receipt of ICIs with different demographic (race, sex, and smoking history) and clinical (cancer site, cancer stage) factors. In the overall cohort, 507 White patients (12.0%) received ICIs compared with 45 racial minority group patients (7.1%). Of the patients who received ICIs, the proportion of White patients with HNC was significantly higher (507 of 552 [91.8%]) than those who did not receive ICIs as part of their treatment (3723 of 4308 [86.4%]) (Figure 2 and the Table); however, no significant difference in receipt of ICIs was observed by sex (415 of 552 [75.2%] vs 3178 of 4308 [73.8%] men, respectively) and smoking history (343 of 552 [62.1%] vs 2663 of 4308 [61.8%] responding yes, respectively) (Figure 2). There were significant differences in the distribution of cancer sites and cancer stages between the 2 groups. As shown in Figure 2, more patients with HNC with advanced-stage disease received ICIs as part of treatment compared with those with local-stage disease (203 [36.8%] vs 1085 [25.2%], respectively). In terms of cancer site, fewer patients with oropharyngeal cancer received ICIs compared with those with nonoropharyngeal disease (170 [30.8%] vs 1694 [39.3%], respectively) (Figure 2).
Figure 2. Proportion of Patients With Head and Neck Cancer (HNC) With Different Factors Associated With Receipt of Immunotherapy.
The proportion of patients who received or did not receive immune checkpoint inhibitors (ICIs) as part of treatment with different demographic (sex, race, smoking status) and clinical (cancer site, cancer stage) variables were calculated. A χ2 test was used to study the association between ICI treatment received and different variables.
aDifference was statistically significant.
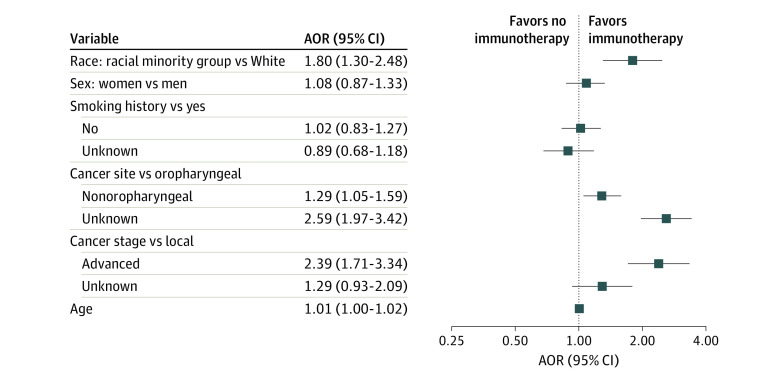
We performed multivariable logistic regression analysis for receipt of ICIs as part of treatment to control the demographic and clinical factor covariates in the data. After adjusting for covariates, there were no statistically significant differences in the odds of receiving immunotherapy based on age, sex, or smoking history. White patients with HNC had 80% increased odds of receiving immunotherapy as part of their treatment (adjusted OR [AOR], 1.80 [95% CI, 1.30-2.48]) compared with patients of racial minority groups (Figure 3). There were no meaningful differences in the odds of immunotherapy use based on sex (AOR, 1.08 [95% CI, 0.87-1.33]) or smoking history (AOR for yes vs no, 1.02 [95% CI, 0.83-1.27]; AOR for yes vs unknown, 0.89 [95% CI, 0.68-1.18]). However, patients with nonoropharyngeal disease (AOR, 1.29 [95% CI, 1.05-1.59]) and unknown disease site (AOR, 2.59 [95% CI, 1.97-3.42]) were more likely to receive immunotherapy than those with oropharyngeal cancer, as were those with advanced-stage disease (AOR, 2.39 [95% CI, 1.71-3.34]) compared with local-stage disease (Figure 3).
Figure 3. Forest Plot of the Association of Different Nonclinical and Clinical Factors With the Receipt of Immunotherapy in Patients With Head and Neck Cancer (HNC).
Logistic regression model was adjusted for race (White vs racial minority group), sex (women vs men), smoking history (yes vs no or unknown), cancer site (oropharyngeal vs nonoropharyngeal or unknown), cancer stage (local vs advanced or unknown), and age to identify the factors associated with the receipt of immunotherapy in older patients with HNC. AOR indicates adjusted odds ratio.
Discussion
The objective of this cohort study was to characterize the clinical and nonclinical factors associated with the receipt of immunotherapy among patients with HNC. Immunotherapy has recently become a successful treatment option for select cases of recurrent or metastatic HNSCC in the last decade, either as a stand-alone therapy or as part of a multimodal therapy. Although previous studies have found disparities in primary HNC treatment choice between surgery and radiotherapy,20 it is unclear whether there are any clinical or sociodemographic differences among patients receiving immunotherapy as part of their care. Our findings suggest that White patients with HNC were significantly more likely to receive immunotherapy treatment than racial minority group patients with HNC. Additionally, patients with advanced-stage cancer were more likely to receive ICIs compared to those with localized disease. By understanding the clinical and nonclinical factors associated with HNC, steps to provide appropriate cancer care—including reducing any disparities faced by communities—may be undertaken.
Our primary finding that White patients had increased odds of receiving immunotherapy compared with racial minority group patients supports our a priori hypothesis that racial minority group patients are more likely to have disparate access to receipt of immunotherapy for HNC. This is an important albeit unsurprising finding, as it mirrors the known disparate access to care in HNC. While previous studies have found racial and ethnic disparities in access to immunotherapy in other cancer sites,21,22,23,24,25 our study makes a novel contribution by describing a potential disparity specific to HNC using clinical data. Of all cancers, HNC has the third-worst survival disparity between Black and White patients, and the disparate receipt of immunotherapy reported in this study illustrates how new therapeutics, while potentially effective, may inadvertently exacerbate already existing survival disparity.24 In addition to access, there may be several other important factors beyond the scope of the present study, including health literacy, implicit bias, and distrust of clinicians, which might also contribute to the existing disparities in HNC care. Further analysis of these factors in relation to HNC will be necessary to understand and target areas of improvement. A previous study analyzing the association between race and primary treatment for human papillomavirus (HPV)–associated HNC20 found that Black patients were less likely to receive primary surgery compared with White patients. Our study adds important data in terms of HNC care with similar findings of disparities in access to care for patients who belong to racial minority groups.
Since up to 20% of patients with recurrent or metastatic HNSCC might benefit from receipt of immunotherapy,5 it is important to understand drivers of this racial disparity in access to this treatment modality. Most studies of cancer-related disparities have associated insurance status with access to care, and several HNC studies have pointed to disparate outcomes as primarily associated with being uninsured, or having Medicaid insurance, compared with private insurance.2,3,13,14,15,20 Interestingly, our present study attempted to account for this potential issue with access by restricting our study to include only older patients with HNC 65 years or older who are eligible and typically have access to Medicare.17 Our results therefore suggest that even after potentially accounting for issues related to health insurance, there may still be some disparities in receipt of immunotherapy in patients with HNC based on sociodemographic factors such as race. It is unknown whether these disparities are patient or health care system driven, and understanding the levels of influence driving this disparity will be critical to improving access to equitable care.24
An interesting finding of our study was the increased odds of immunotherapy received by patients diagnosed with nonoropharyngeal cancer compared with oropharyngeal cancer. It is currently unknown why this might be the case, and significant gaps in our understanding of immunotherapy in oropharyngeal cancer treatment exist. One such barrier described in the literature includes patient-specific resistance to immunotherapy, as indicated by low response rates.15 It is currently unclear why patients’ objective response rate for immunotherapy is rather low even among patients clinically indicated for immunotherapy; however, evidence suggests that there may be greater response in HPV-positive disease than HPV-negative HNSCC.4 Be that as it may, evidence also suggests that patients with HPV-negative disease may still benefit from immunotherapy.4,5,26 For patients with HNC who have HPV-negative disease, immunotherapy as a treatment option is particularly intriguing. For patients with locally advanced HPV-negative disease, who generally have the worst prognosis of all patients with HNC,5 immunotherapy may be an important option, especially following metastasis or recurrence after previous adjuvant treatment. For many advanced cases of HPV-negative disease, in addition to severe functional compromise, there are poor survival outcomes, with survival rate being less than 50% and significantly worse for Black patients, for whom the survival rate is 29%.1 However, it is important to note that only about 1 in 5 patients with HNC meeting the clinical indications for immunotherapy may actually benefit from it.5 Therefore, it is important that patient selection criteria are carefully established so that patients who may benefit from receipt of immunotherapy are able to do so and improve their survival rate significantly.
In our study, patients with advanced cancer had higher odds of receiving immunotherapy compared with patients with localized disease. Patients with worse prognoses despite multimodality approaches may benefit from a trial of neoadjuvant or adjuvant immunotherapy.5 While patients with local disease benefit from either radiotherapy and surgery,2 previous trials have examined immunotherapy as a monotherapy or a neoadjuvant treatment for local disease, in addition to some clinically indicated locally advanced cases.4,5,27 As immunotherapy becomes a mainstay treatment option for some cases of HNC, it will be interesting to examine whether there is a change in the association between disease stage and receipt of immunotherapy and whether there is a shift toward receiving immunotherapy for localized disease. As described below, it is also important that patients are carefully selected so that only those who may benefit from this treatment are selected to receive immunotherapy, which is not only associated with some toxic effects but is also expensive.5
Clinical and Public Health Implications
There are 3 main clinical and public health implications from this study, First, immunotherapy use in HNC care is relatively recent, and the mainstay immunotherapeutics, nivolumab and pembrolizumab, were approved by the US Food and Drug Administration for HNC only within the last decade. While several high-quality randomized trials7,8,9,10,11 have established safety and efficacy of these treatment options in HNC, there has been a paucity of clinical data describing the epidemiology of immunotherapy in HNC. Equitable access to care is a hallmark of high-quality cancer care,28,29 and more data are needed to describe differential access to immunotherapy in a highly disparate cancer such as HNC. As medicine and health care continue to address social determinants of health, it is critical that the drivers of disparities in access to immunotherapy and other treatment options for patients with HNC are understood and addressed.
Second, in addition to addressing disparities in access to care, more clinical immunotherapy data are needed beyond clinical trial data to truly understand factors associated with treatment response and survival outcomes following receipt of immunotherapy. As stated previously, only 1 in 5 patients clinically indicated for receipt of immunotherapy may benefit from this treatment option.5 In addition to its clinical relevance, this low rate of success brings up an issue of pharmacoequity.30 Knowing that the treatment itself is expensive, and knowing that HNC is one of the most expensive cancers to treat,31 it is critical that immunotherapy is targeted primarily toward only those who may benefit from the treatment to mitigate the financial toxicity generally associated with HNC and cancer care in general.
Third, it is critical to understand how to improve the efficacy of ICIs in HNC to increase the rate of success above 20%. Given that up to 50% of patients with locally advanced HNSCC may experience recurrence within 2 years of initial diagnosis and treatment,32,33 a more effective treatment option meeting the needs of patients with recurrent disease is desperately needed in the field of HNC. All 3 of these clinical and public health implications are beyond the scope of this study, and they remain critical gaps in the field of HNC.
Limitations
There are several limitations to this study. First, our data set only focused on patients 65 years and older, and there was a lack of data on patients’ comorbidity burden.34,35 Future studies describing disparate use and outcomes of immunotherapy in HNC should further investigate the association between disparity and overall comorbidity burden. Second, our data were not large enough to allow any meaningful analysis on racial and ethnic minority groups; hence, we grouped our cohort as White compared with racial minority groups. Grouping patients into 2 cohorts for race makes it difficult to draw conclusions about the racial minority individuals for whom public health intervention is most critical. Literature on HNC has shown differences in disease presentation and survival by race, and there are several differences in the health care experience of a patient by race that cannot be accounted for.36,37 There is an opportunity for future studies to verify these initial results with a larger data set that fully accounts for race and ethnicity and additional variables. Third, our study did not analyze variability in health insurance status, which may be independently associated with access to immunotherapy and/or mediate the association between race and receipt of immunotherapy. Fourth, while we controlled for stage of presentation as local vs advanced vs unknown, a large proportion of our patients were grouped as unknown stage due to data set limitations. Additionally, many patients were excluded from this study due to missing data on race, which may introduce bias in the analysis. Future studies should establish patterns of receipt of immunotherapy (monotherapy vs adjuvant treatment) based on more granular information on stage of presentation. Our study did not include survival outcomes based on receipt of immunotherapy. It will be interesting to examine potential differences in survival by race and ethnicity among patients with HNC matched for age, disease stage, and access to care.
Conclusions
In this cohort study, we identified clinical and nonclinical differences in the receipt of immunotherapy in a cohort of patients with HNC. Our results suggest that White patients were significantly more likely to receive immunotherapy as part of their care compared with racial minority group patients. Additionally, patients with advanced stages of cancer and diagnosis of nonoropharyngeal cancer were more likely to receive immunotherapy than patients diagnosed with localized disease or oropharyngeal cancer. Together, our findings suggest that there may be disparities in receipt of immunotherapy among patients with HNC. Equitable access to immunotherapy and other treatment options will reduce cancer-related health disparities and improve the survival of HNC.
Data Sharing Statement
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Osazuwa-Peters N, Christopher KM, Hussaini AS, Behera A, Walker RJ, Varvares MA. Predictors of stage at presentation and outcomes of head and neck cancers in a university hospital setting. Head Neck. 2016;38(suppl 1):E1826-E1832. doi: 10.1002/hed.24327 [DOI] [PubMed] [Google Scholar]
- 3.Taylor DB, Osazuwa-Peters OL, Okafor SI, et al. Differential outcomes among survivors of head and neck cancer belonging to racial and ethnic minority groups. JAMA Otolaryngol Head Neck Surg. 2022;148(2):119-127. doi: 10.1001/jamaoto.2021.3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: recent advances and future directions. Oral Oncol. 2019;99:104460. doi: 10.1016/j.oraloncology.2019.104460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata H, Saito S, Uppaluri R. Immunotherapy for head and neck cancer: a paradigm shift from induction chemotherapy to neoadjuvant immunotherapy. Front Oncol. 2021;11:727433. doi: 10.3389/fonc.2021.727433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forster MD, Devlin MJ. Immune checkpoint inhibition in head and neck cancer. Front Oncol. 2018;8:310. doi: 10.3389/fonc.2018.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. doi: 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 9.Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 Expansion Cohort. J Clin Oncol. 2016;34(32):3838-3845. doi: 10.1200/JCO.2016.68.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parameswaran J, Burtness B. Immune checkpoint inhibition in cancers that affect the head and neck. Int J Radiat Oncol Biol Phys. 2017;98(5):969-973. doi: 10.1016/j.ijrobp.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 11.Kao HF, Lou PJ. Immune checkpoint inhibitors for head and neck squamous cell carcinoma: current landscape and future directions. Head Neck. 2019;41(suppl 1):4-18. doi: 10.1002/hed.25930 [DOI] [PubMed] [Google Scholar]
- 12.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577-581. doi: 10.1038/nature13988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pannu JS, Simpson MC, Donovan CL, et al. Sociodemographic correlates of head and neck cancer survival among patients with metastatic disease. Head Neck. 2020;42(9):2505-2515. doi: 10.1002/hed.26284 [DOI] [PubMed] [Google Scholar]
- 14.Pannu JS, Simpson MC, Adjei Boakye E, et al. Survival outcomes for head and neck patients with Medicaid: a health insurance paradox. Head Neck. 2021;43(7):2136-2147. doi: 10.1002/hed.26682 [DOI] [PubMed] [Google Scholar]
- 15.Gaubatz ME, Bukatko AR, Simpson MC, et al. Racial and socioeconomic disparities associated with 90-day mortality among patients with head and neck cancer in the United States. Oral Oncol. 2019;89:95-101. doi: 10.1016/j.oraloncology.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 16.Osazuwa-Peters N, Massa ST, Christopher KM, Walker RJ, Varvares MA. Race and sex disparities in long-term survival of oral and oropharyngeal cancer in the United States. J Cancer Res Clin Oncol. 2016;142(2):521-528. doi: 10.1007/s00432-015-2061-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel DC, He H, Berry MF, et al. Cancer diagnoses and survival rise as 65-year-olds become Medicare-eligible. Cancer. 2021;127(13):2302-2310. doi: 10.1002/cncr.33498 [DOI] [PubMed] [Google Scholar]
- 18.Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113(10):2797-2806. doi: 10.1002/cncr.23889 [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Omeogu C, Islam JY, et al. Socioeconomic disparities in immunotherapy use among advanced-stage non–small cell lung cancer patients: analysis of the National Cancer Database. Sci Rep. 2023;13(1):8190. doi: 10.1038/s41598-023-35216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habib AM, Carey RM, Prasad A, et al. Impact of race and insurance status on primary treatment for HPV-associated oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2022;166(6):1062-1069. doi: 10.1177/01945998211029839 [DOI] [PubMed] [Google Scholar]
- 21.Verma V, Haque W, Cushman TR, et al. Racial and insurance-related disparities in delivery of immunotherapy-type compounds in the United States. J Immunother. 2019;42(2):55-64. doi: 10.1097/CJI.0000000000000253 [DOI] [PubMed] [Google Scholar]
- 22.Ermer T, Canavan ME, Maduka RC, et al. Association between Food and Drug Administration approval and disparities in immunotherapy use among patients with cancer in the US. JAMA Netw Open. 2022;5(6):e2219535. doi: 10.1001/jamanetworkopen.2022.19535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque W, Verma V, Butler EB, Teh BS. Racial and socioeconomic disparities in the delivery of immunotherapy for metastatic melanoma in the United States. J Immunother. 2019;42(6):228-235. doi: 10.1097/CJI.0000000000000264 [DOI] [PubMed] [Google Scholar]
- 24.Osarogiagbon RU, Sineshaw HM, Unger JM, Acuña-Villaorduña A, Goel S. Immune-based cancer treatment: addressing disparities in access and outcomes. Am Soc Clin Oncol Educ Book. 2021;41:1-13. doi: 10.1200/EDBK_323523 [DOI] [PubMed] [Google Scholar]
- 25.Ahn JC, Lauzon M, Luu M, et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022;76(6):1649-1659. doi: 10.1002/hep.32527 [DOI] [PubMed] [Google Scholar]
- 26.Botticelli A, Cirillo A, Strigari L, et al. Anti–PD-1 and anti–PD-L1 in head and neck cancer: a network meta-analysis. Front Immunol. 2021;12:705096. doi: 10.3389/fimmu.2021.705096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Economopoulou P, Agelaki S, Perisanidis C, Giotakis EI, Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma. Ann Oncol. 2016;27(9):1675-1685. doi: 10.1093/annonc/mdw226 [DOI] [PubMed] [Google Scholar]
- 28.Ayanian JZ, Markel H. Donabedian’s lasting framework for health care quality. N Engl J Med. 2016;375(3):205-207. doi: 10.1056/NEJMp1605101 [DOI] [PubMed] [Google Scholar]
- 29.Babar A, Montero AJ. Building quality from the ground up in a cancer center. In: Aljurf M, Majhail NS, Koh MBC, Kharfan-Dabaja MA, Chao NJ, eds. The Comprehensive Cancer Center: Development, Integration, and Implementation. Springer; 2022:135-143. doi: 10.1007/978-3-030-82052-7_14 [DOI] [PubMed] [Google Scholar]
- 30.Essien UR, Dusetzina SB, Gellad WF. A policy prescription for reducing health disparities-achieving pharmacoequity. JAMA. 2021;326(18):1793-1794. doi: 10.1001/jama.2021.17764 [DOI] [PubMed] [Google Scholar]
- 31.Jacobson JJ, Epstein JB, Eichmiller FC, et al. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: commercial insurance, Medicare, and Medicaid. Head Neck Oncol. 2012;4:15. doi: 10.1186/1758-3284-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denaro N, Merlano MC, Russi EG. Follow-up in head and neck cancer: do more does it mean do better? a systematic review and our proposal based on our experience. Clin Exp Otorhinolaryngol. 2016;9(4):287-297. doi: 10.21053/ceo.2015.00976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Jiang Y, Qiu B, Sun H, Wang J. Current radiotherapy for recurrent head and neck cancer in the modern era: a state-of-the-art review. J Transl Med. 2022;20(1):566. doi: 10.1186/s12967-022-03774-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110(4):593-602. doi: 10.1097/00005537-200004000-00011 [DOI] [PubMed] [Google Scholar]
- 35.Stordeur S, Schillemans V, Savoye I, et al. Comorbidity in head and neck cancer: is it associated with therapeutic delay, post-treatment mortality and survival in a population-based study? Oral Oncol. 2020;102:104561. doi: 10.1016/j.oraloncology.2019.104561 [DOI] [PubMed] [Google Scholar]
- 36.Ngo-Metzger Q, Legedza AT, Phillips RS. Asian Americans’ reports of their health care experiences: results of a national survey. J Gen Intern Med. 2004;19(2):111-119. doi: 10.1111/j.1525-1497.2004.30143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JD, Chang JT, Moghaddamjou A, et al. Asian and non-Asian disparities in outcomes of non-nasopharyngeal head and neck cancer. Laryngoscope. 2017;127(11):2528-2533. doi: 10.1002/lary.26603 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement