Abstract
Objective
The Italian National Action Plan to contrast AMR identified among its objectives the development and implementation of a national Healthcare-Associated Infection (HAI) surveillance system based on European Centre for Disease Prevention and Control (ECDC) indications, through point prevalence surveys (PPS) of HAIs and antibiotic use in acute-care hospitals and long-term care facilities (LTCFs). We aimed to assess feasibility and appropriateness of proposed tools for a national surveillance system of HAIs and antibiotic use in LTCFs.
Study design
Point prevalence survey.
Methods
A pilot PPS was conducted between May–June 2022, among 15 LTCFs of 7 Italian regions. Data were collected in a single day in each LTCF, at the LTCF, ward, and resident levels, using a web-based data collection tool developed ad hoc. Data collector teams of each facility were invited to complete a questionnaire investigating opinions on the proposed tools.
Results
Among 1025 included residents, the prevalence of residents with at least one HAI was 2.5% (95% CI 1.7%-3.7%) considering all HAIs and 2.2% (95% CI 1.3%–3%) without considering SARS-CoV-2 infections. The prevalence of antimicrobial use was 3% (95% CI 0.2%–4.3%). Overall, most respondents were satisfied with the web-based software, training and protocol, even though some difficulties were reported.
Conclusions
A national surveillance network was established, which will facilitate future surveillance efforts. Further studies are necessary to evaluate the impact of the pandemic on HAI transmission and antibiotic use in LTCFs.
Keywords: Healthcare associated infections, Surveillance, Long term care facilities, Nursing homes, Italy
Author contributions
Conception and design: CV, CMZ; Coordination of surveillance: FDA, AA, MB, SB, EF, SF, ER, GR, CS, CMZ; Data collection: AR, ER, VB, AA, MB, SB, EF, SF, ER, GR, CS, collaborating group; Software: DG; Data management and statistical analysis: SB; Writing – original draft: CV, AR; Writing – review and editing: FDA, CMZ, AA, MB, SB, EF, SF, ER, GR, CS; Project coordination: FDA, CMZ.
1. Background
Elderly people in long-term care facilities (LTCFs) are at increased risk of healthcare-acquired infections (HAIs), which are associated with a significant clinical and economic burden, from both the patient and health-system perspectives [1]. The most frequent HAIs in the LTCF setting are respiratory tract infections [2], urinary tract infections (UTIs) [3], gastro-intestinal infections [4], including infections caused by Norovirus [5] and Clostridioides difficile [6], and skin and soft tissue infections [7]. These infections, if not adequately recognized and treated, can evolve into sepsis, leading to patient hospitalization and even death [8]. Another important issue is antibiotic consumption associated with HAIs, and the consequent risk of development of antimicrobial resistance (AMR) with inappropriate therapy [9,10]. Furthermore, the impact of the COVID-19 pandemic on HAIs, antibiotic consumption, and infection prevention and control (IPC) activities remains to be determined. Some studies have highlighted an association with increased HAI incidence [11] and higher AMR rates [12]; while results of other reports suggest the increased attention to IPC measures adopted during the pandemic in healthcare settings could have led to reduced HAI transmission [13].
In 2008, the European Centre for Disease Prevention and Control (ECDC) began the surveillance of HAIs and antibiotic use in European LTCFs through the “Healthcare-associated infections in long-term care facilities” (HALT) project [14]. Italy has participated with increasing commitment in all three previous point prevalence surveys (PPS) promoted by the ECDC: in 2010 (HALT-1), 2013 (HALT-2) and 2017 (HALT-3), obtaining results in line with those in Europe, both in terms of antibiotic use and HAI prevalence [[15], [16], [17], [18]]. The previous editions of the PPS have made it possible to test the feasibility of surveillance activities in LTCFs in our country. So far, in the absence of precise national indications, regions and individual hospitals have participated in the project on a voluntary basis, and surveillance activities are not undertaken in all regions at a similar level [19].
The Italian Ministry of Health promoted a National action plan to contrast AMR (Piano Nazionale di Contrasto dell’Antimicrobico-Resistenza, PNCAR) which was approved in November 2017, and agreed upon between national government, regions and autonomous provinces [20]. The PNCAR has identified among its objectives the development and implementation of a national HAI surveillance system, based on ECDC indications [21]. The establishment of the national HAI surveillance network was assigned to the National health institute (Istituto Superiore di Sanità, ISS) through a project financed by the Italian Ministry of Health [22]. The objectives of the project are to progressively expand already existing surveillance activities at the national level, and to establish new surveillances, with the final aim of developing a stable and integrated national system coordinated by the ISS. Considering the high level of devolution of the Italian National Health System, with Regional health authorities responsible for the provision of healthcare, developing a national surveillance network, standardizing and coordinating data collection, and mandating participation in each region are essential to guarantee representativeness and ensure equal quality of care across regions [23].
As an institution involved in this project, the University of Turin was assigned the coordination of PPS surveillance activities at the national level, both in acute-care hospitals and LTCFs. A surveillance network was established among regional authorities, and new web-based data collection tools were developed. In order to test the proposed instruments, a pilot PPS was conducted among LTCFs. The specific aims of the pilot study were to assess the feasibility of a national surveillance system and the appropriateness of the proposed tools (protocol, definitions, data collection software, and training). Here, we present the main results of the pilot study.
2. Methods
2.1. Study design
A PPS in LTCFs was conducted between May 15 and June 15, 2022, in 7 Italian regions, which participated on a voluntary basis: Emilia-Romagna, Liguria, Molise, Piedmont, Sicily, Tuscany, and Veneto. Each region was tasked with enrolling a minimum of two LTCFs or 100 residents each, using convenience sampling.
The protocol of this study is an updated version of the HALT-3 protocol (the HALT-4 protocol was not yet available); as such, it uses the same definitions provided by the ECDC for the recognition of active HAIs [24]. The methodology for data collection has been previously described [17,18]. Briefly, data on all active HAIs and antimicrobial use on the day of the PPS are collected. An HAI is defined as active when the signs/symptoms of infection are present on the day of the survey or signs/symptoms were present in the past and the resident is still receiving treatment for infection on the day of the PPS. In line with the ECDC protocol, patient-level data were only collected for patients with an active HAI or ongoing antibiotic therapy on the day of the PPS.
The main change to the ECDC protocol at the patient level was the addition of COVID-19 among HAIs (with the following classification for infection severity: asymptomatic, mild/moderate, severe, based on the presence of symptoms, the need for oxygen therapy and level of O2 saturation). At the LTCF level, the following data were added: number of COVID-19 cases and outbreaks in the previous two years (2020–2021), and information on SARS-CoV-2 and influenza vaccinations among healthcare workers and vaccination coverage among residents.
2.2. Study population
LTCFs were considered eligible if they provided continuous supervision and nursing care, and if residents did not require constant specialist medical assistance. The following types of facilities were eligible for inclusion: general nursing homes, residential homes, specialized LTCFs, and mixed LTCFs. Facilities were classified as general nursing homes if residents required medical or skilled nursing assistance and supervision 24 h per day. Residential homes were defined as facilities hosting residents unable to live independently and requiring assistance for daily living activities. If facilities provided one specific type of care (e.g. for patients with chronic diseases, or requiring rehabilitation) they were classified as specialized LTCFs. If facilities provided different types of care, they were classified as mixed LTCFs. Regardless of facility type, LTCFs in Italy can have their own physician on-site, or medical assistance can be shared among several facilities. Each Italian Region has a different organization in terms of medical care in LTCFs; in some Regions assistance is provided through external general practitioners (similarly to the general population).
The following residents were considered eligible to be included in the study: full-time residents, residents present at 8:00 a.m. on the day of the PPS, residents not discharged from the LTCF at the time of study.
2.3. Data collection
Data were collected by internal or external surveyors (mainly nursing and medical personnel) in a single day in each facility, at the LTCF, ward, and resident levels. A web-based software was employed for data collection. The software was designed by the national coordinators, and developed in collaboration with software engineers. In order to comply with the EU General data protection regulation (GDPR), only authorized users could access the software. To obtain authorization, personnel involved in data entry were required to follow a course on the GDPR. Only pseudononimized data were collected, both at the patient and LTCF levels.
In addition to the GDPR course, the national coordinating centre provided training on the protocol, definitions, and on how to access and use the online software through on-line sessions conducted in the weeks prior to the study. A manual with instructions on how to use the data collection software specifically developed for the study was also provided.
Data collector teams of each facility were invited to complete a questionnaire investigating opinions on the proposed tools (one response for each LTCF). The questionnaire was also administered via the same data collection tool. Responders were asked to rate user experience and the appropriateness of the user manual through 10-point scales. The training sessions and the study protocol were evaluated through multiple choice questions. Suggestions for improvement could also be added in a free-text field. Difficulties and strengths of the study were discussed during an additional on-line session, where the main results of the study were also presented.
All on-line sessions (training and feedback) were not mandatory. Live participation was encouraged, however training sessions were recorded and made available to study participants prior to the study.
2.4. Statistical analysis
HAI and antibiotic use prevalence in each LTCF were calculated as the number of residents with at least one HAI or receiving at least one antimicrobial agent, divided by the total number of eligible residents on the day of the survey. Crude, pooled prevalence estimates and 95% confidence intervals (CI) are provided. Descriptive statistics were used to summarize LTCF and patient-level characteristics, including LTCF type, type of provided assistance, average length of stay, IPC practices, antibiotic stewardship (AMS) activities, vaccination coverage, characteristics of residents with active HAIs or ongoing antibiotic therapy.
Spearman's correlation was used to investigate the relation between HAI prevalence and antibiotic use prevalence. The association between number of IPC practices and HAI prevalence, and between number of AMS activities and antibiotic use prevalence was assessed using linear regression. Analyses were performed considering LTCF-level data. Significance was set at two-tailed p < 0.05. Statistical analyses were conducted using MATLAB v. 9.13 (R2022b), Natick, Massachusetts: The MathWorks Inc.
3. Results
3.1. LTCF-level data
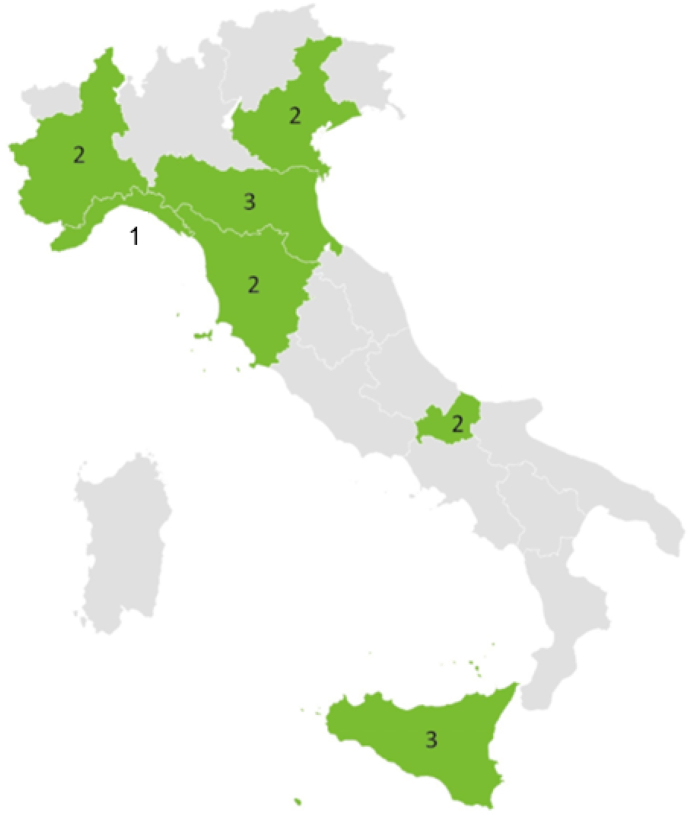
Fifteen LTCFs participated in the pilot PPS (Fig. 1): of these, 5/15 were classified residential homes (33.3%), 6/15 general nursing homes (40%) and 4/15 mixed, specialized or other (26.7%). As for the ownership type, 3/15 were public (20%), 8/15 were private (53.3%) and 4/15 were not-for-profit (26.7%). The recorded bed occupancy rate was 76.1%. In total, 1025 residents were considered eligible and included in the study.
Fig. 1.
Number of long-term care facilities participating in the LTCF PPS survey by regions, Italy, May–June 2022 (N = 15).
Table 1 provides a summary of IPC practices, AMS activities, and vaccination policy and coverage at the LTCF level.
Table 1.
Number and percentage of infection prevention and control (IPC) practices, antimicrobial stewardship (AMS) activities, and vaccination policy and coverage among residents at the long-term care facility (LTCF) level (N = 15).
| IPC practices | N = 15 |
|---|---|
| Trained staff working in the LTCF | 9 (60%) |
| LTCFs offering regular IPC training | 11 (73.3%) |
| Internal or external IPC committee | 10 (66.7%) |
| Availability of IPC protocols | 15 (100%) |
| Regular surveillance of HAIs | 8 (53.3%) |
| Feedback of surveillance results | 3 (20%) |
| Regular auditing of hand hygiene practices | 4 (26.7%) |
| AMS activities | N = 15 |
| LTCF offering regular training on appropriate antibiotic use to staff | 0 |
| Internal committee on appropriate antibiotic use | 3 (20%) |
| Availability of an antibiotic therapy manual | 8 (53.3%) |
| Restriction of specific antibiotic agents/classes | 8 (53.3%) |
| Authorization required for the prescription of restricted antibiotics | 2 (13.3%) |
| Surveillance and feedback of annual antibiotic consumption | 3 (20%) |
| Surveillance and feedback of antimicrobial resistant organisms | 6 (40%) |
| Availability of point-of-care tests (e.g. dipstick for urinary tract infections) | 2 (13.3%) |
| Vaccination policy and coverage among residents | N = 14 |
| LTCF offering on-site annual influenza vaccination, n (%) | 13 (92.9%) |
| Vaccination coverage against influenza in 2021, % median (IQR) | 84% (0–100) |
| Vaccination coverage against SARS-CoV-2, % median (IQR) | |
| first dose | 100% (98–100) |
| second dose | 100% (97–100) |
| additional (booster) dose | 97% (92–100) |
| second additional (booster) dose | 15% (0–32) |
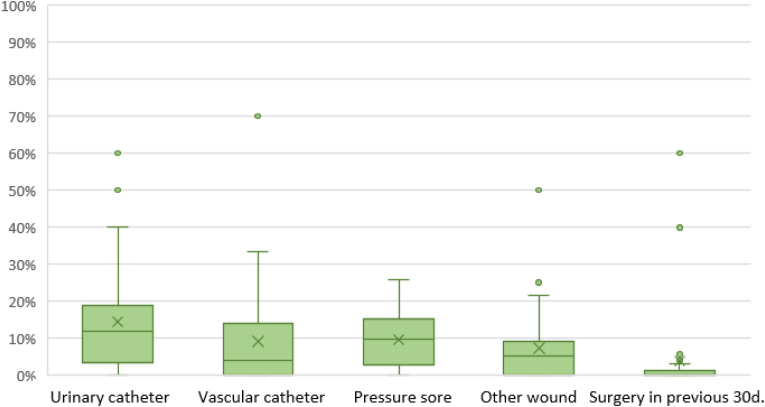
Concerning available IPC protocols, all LTCFs had a written protocol on hand hygiene, 12 (80%) on the management of urinary catheters, 11 (73.3%) on the management of vascular catheters and enteral feeding procedures, and 8 (53.3%) on isolation practices for outbreaks of methicillin-resistant Staphylococcus aureus or other multidrug-resistant pathogens.
The antibiotic agents or classes most frequently subject to restrictions were: carbapenems (in 40% of LTCFs, n = 6), third generation cephalosporins (33.3%, n = 5), vancomycin (33.3%, n = 5), fluoroquinolones (26.7%, n = 4), and glycopeptides (26.7%, n = 4). Guidelines for appropriate antibiotic use were available for the following infections: respiratory tract infections (in 13.3% of LTCFs, n = 2), UTIs (20%, n = 3), and wound and soft tissue infections (13.3%, n = 2).
3.2. Patient-level data
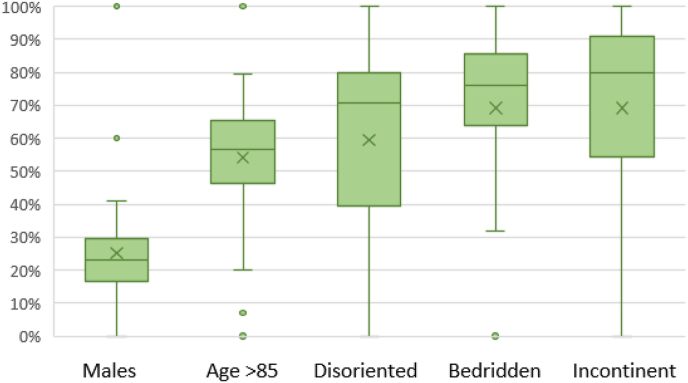
Of the 1025 included patients, 37 presented an active HAI or received an antibiotic on the day of the survey: 6 residents had an HAI, 11 received an antibiotic and 20 patients had both. Demographic and clinical characteristics of residents with an active HAI and/or receiving antibiotic therapy are summarized in Fig. 2, Fig. 3.
Fig. 2.
Demographic and clinical characteristics of residents with an active healthcare-associated infection (HAI) and/or receiving antibiotic therapy on the day of the survey (N = 37). Legend for Fig. 2: Box plots depicting median and interquartile range of the distribution of each variable. The X indicates the mean for each variable.
Fig. 3.
Risk factors for healthcare associated infections (HAIs) among residents with an active healthcare-associated infection (HAI) and/or receiving antibiotic therapy on the day of the survey (N = 37).
Among all eligible residents, 26 had at least one active HAI on the day of the survey, whereas the total number of HAIs was 32. The prevalence of residents with at least one HAI was 2.5% (95% CI 1.7%–3.7%) considering all HAIs and 2.2% (95% CI 1.3%–3%) without considering SARS-CoV-2 infections. Six residents had more than one HAI (0.6% of eligible patients and 23.1% of patients with at least one HAI), all six cases were SARS-CoV2 co-infections. Overall, 11 HAIs (34.4%) were acquired in the current LTCF, 10 (31.3%) were acquired in an acute-care hospital, and 11 (34.4%) had an unknown origin.
The most frequent HAI types were: respiratory tract infections (n = 13, 40.6% of active HAIs), UTIs (n = 9, 28.1%), and skin/wound infections (n = 2, 6.3%). Concerning respiratory tract infections, 4 (12.5%) were asymptomatic SARS-CoV-2 infections, 6 (18.8%) mild/moderate SARS-CoV-2 infections, and 3 (9.4%) other lower respiratory tract infections. No severe SARS-CoV-2 infections were recorded.
Of 32 HAIs, 9 (28.1%) were not investigated and in 5 cases (15.6%) microbiology results were not available at the time of the survey. In total, 19 microorganisms were isolated from HAIs: SARS-CoV-2 (n = 10, 52.6% of identified micro-organisms), Escherichia coli (n = 5, 26.3%), Staphylococcus aureus (n = 2, 10.5%), Proteus mirabilis (n = 1, 5.3%), and Acinetobacter baumannii (n = 1, 5.3%).
Concerning antibiotic use, 31 residents were recorded as receiving one antimicrobial agent on the day of the survey (no residents received more than one antibiotic). The prevalence of residents receiving at least one antimicrobial was 3% (95% CI 0.2%-4,3%). Overall, 25 (80.7%) antimicrobials were prescribed in the same LTCF and 3 (9.7%) in an acute-care hospital. The majority (n = 23, 74.2%) of antibiotic were administered parenterally, and 8 (25.8%) were administered orally. Antibiotic use was indicated for the treatment of infections in 27 cases (87.1%), and in 4 cases (12.9%) for prophylaxis. Concerning treatment indications, the most frequent sites of infection were: UTIs (n = 9, 33.3% of treatment indications), respiratory tract infections (n = 7, 25.9%), and skin/wound infections (n = 3, 11.1%). The most prescribed classes of antibiotics were third generation cephalosporins (n = 16, 51.6% of all agents), beta lactam/beta lactamase inhibitor combinations (n = 2, 6.5%), and fluoroquinolones (n = 2, 6.5%). The most prescribed agent was ceftriaxone (n = 15, 48.4% of all agents). Considering the WHO 2021 AWaRe classification [25], the majority of administered agents belonged to the Watch class (n = 25, 80.7%).
3.3. Correlation and regression analyses
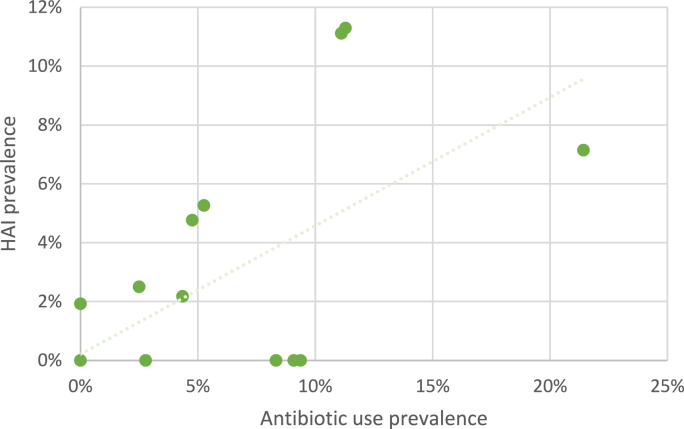
As shown in the scatterplot depicted in Fig. 4, a weak but significant positive correlation was identified between LTCF-level HAI prevalence and prevalence of residents receiving at least one antibiotic (Spearman's ρ 0.395, p 0.0253). No significant correlation was found between number of IPC practices (as defined in Table 1) and HAI prevalence (R2 0.0128; p 0.71), or between number of AMS activities practices (as defined in Table 1) and antibiotic use prevalence (R2 0.1344; p 0.21).
Fig. 4.
Healthcare associated infection (HAI) prevalence plotted against prevalence of residents receiving at least one antibiotic (facility-level data, N = 15).
3.4. Data collector survey
Fourteen out of fifteen invited data collector teams completed the survey (93.3% response rate). Table 2 summarizes the questionnaire and responses. In addition to the multiple-choice questionnaire, respondents had the possibility to provide comments or suggestions in a free field. Three responders highlighted difficulties in obtaining an active account on the web-based platform, in particular due to the mandatory GDPR course (which was in English). Two responders suggested that more free fields should be available to be able to add specifications on reported data, in particular concerning LTCF-level data.
Table 2.
Questionnaire investigating opinions on the proposed tools, and responses of data collectors (N = 14).
| Web-based data collection software | Responses, median score out of 10 (IQR) |
|---|---|
| User experience in completing the online data collection form | 7 (5–9) |
| Appropriateness of the user manual | 8 (5–9) |
| Training for data collectors | Responses, n (%) |
| Were the chosen topics appropriate? | |
| Not at all | 0 |
| Insufficiently | 0 |
| Sufficiently | 9 (64.3) |
| Completely | 5 (35.7) |
| Was the course easy to understand? | |
| Not at all | 0 |
| Insufficiently | 0 |
| Sufficiently | 9 (64.3) |
| Completely | 5 (35.7) |
| Study protocol | Responses, n (%) |
| Were the definitions clear? | |
| Not at all | 0 |
| Insufficiently | 0 |
| Sufficiently | 6 (42.9) |
| Completely | 8 (57.1) |
| Were the study objectives and design clearly described? | |
| Not at all | 0 |
| Insufficiently | 0 |
| Sufficiently | 8 (57.1) |
| Completely | 6 (42.9) |
| Were data collection forms sufficiently detailed? | |
| Not at all | 0 |
| Insufficiently | 1 (7.1) |
| Sufficiently | 7 (50) |
| Completely | 6 (42.9) |
4. Discussion
The main objectives of the study were establishing a centralized surveillance network and assessing the appropriateness of proposed tools. Overall, most respondents were satisfied with the web-based software, training and protocol, even though some difficulties were reported (in particular concerning the mandatory GDPR course). From an organizational perspective, using an online software for data collection required an additional degree of work prior to the study (in terms of providing training, authorizing personnel, and assisting in the accreditation process), however very few difficulties were encountered during the study and in the collation and analysis of collected data. Further, the process ensured compliance with GDPR regulations, which facilitated the enrolment of LTCFs.
Results from both the questionnaire investigating opinions on the proposed tools and the final feedback session showed an overall positive evaluation. Suggestions were important to inform the development of a similar web-based data collection tool that was used in November 2022 to conduct the national PPS of HAIs and antibiotic use in acute-care hospitals, which was led by the same national coordinating team and within the same project financed by the Ministry of Health. In particular, free-text fields were added to each data collection form (investigating structure, ward, and patient-level variables), which allowed surveyors to add important local context that will facilitate the interpretation of results. Finally, a PPS among LTCFs at the national level using an updated version of the tools developed for the pilot study will be conducted in parallel with the European HALT-4 survey.Even though the aim of this study was not to achieve national representativeness, this study provides prevalence results for HAIs and antibiotic use among residents of a convenience sample of Italian LTCFs. Considering case-mix, the majority of residents with an HAI or an antibiotic course were over 85 years old, disoriented, incontinent, or bedridden, highlighting a high care burden in these facilities.
The overall HAI prevalence was 2.5% (95% CI 1.7%–3.7%) and 2.2% (95% CI 1.3%–3%) without considering SARS-CoV-2 infections. Both estimates were lower than that the HAI prevalence measured by the Italian HALT-2 and HALT-3 studies conducted in 2013 and 2017 (3.3% and 3.9% respectively) [19], despite the addition of COVID-19 among measured HAIs. Further, the most frequently reported HAIs were the same in the present study as in HALT-3: respiratory tract infections, UTIs, and skin/wound infections. Notwithstanding the limited comparability due to relatively smaller sample size and objectives of the current survey, these findings could indicate a reduction in HAI transmission. The reduction in HAI prevalence could be due to an increased awareness to HAIs and IPC related to the COVID-19 pandemic [26], and to non-pharmaceutical interventions still in place at the time of the survey, such as restricted access to visitors. Some improvements in IPC activities were reported compared to 2017, in particular in regards to hand hygiene practices and IPC training [19]. A recent study conducted among LTCFs in the US also did not identify a significant increase in HAIs during to the pandemic, possibly due to heightened vigilance and optimization of IPC practices [27].
Among patients with healthcare-acquired SARS-CoV-2 infections, 60% had a co-infection with another HAI, providing support to previous findings on the high clinical burden due to secondary infections in patients infected with SARS-CoV-2 [27].
The prevalence of antibiotic use was 3% (95% CI 0.2%–4.3%). This estimate was also lower compared to the previous Italian studies (HALT-2: 3.9% and HALT-3:4.2%), even though no improvements in AMS activities were highlighted [19]. The most frequently prescribed agents were also unchanged in comparison to HALT-3 [19]. Previous reports have highlighted difficulties in maintaining AMS programs during the pandemic, due to time constraints and to the diversion of resources [28]. The high proportion of “Watch class” antibiotics and of parenterally administered agents is nonetheless cause for concern.
Concerning vaccination coverage, good adherence to the COVID-19 vaccination campaign was found; the relatively lower adherence to the second booster dose was due to the timing of the study which coincided with the vaccination campaign: several facilities had already scheduled the administration of the second booster dose in the days/weeks following the day chosen for data collection. Even though a causal relation cannot be inferred due to the study design, no severe COVID-19 cases were recorded among our highly vaccinated sample. The median influenza vaccination coverage rate among included LTCFs was 84%, which is relatively high compared to the vaccination coverage among elderly Italians (65.3% in 2021/2022 according to Ministry of Health data), and to international comparisons among LTCF residents [29,30]. These findings could indicate LTCF residents (or the structure managers) have an increased propensity to vaccination, which could be due to the high prevalence of characteristics associated with influenza-related morbidity [30], and suggest providing on-site influenza vaccination could be an effective strategy to achieve high coverage rates.
This study has several limitations, mainly due to the study design and sampling strategy. However, both were chosen as LTCFs are settings with relatively limited resources for surveillance and IPC activities, in particular during the pandemic. The limited sample size reduces generalizability of results. Further, no validation study was performed on collected data and concordance between internal and external surveyors was not assessed.
5. Conclusion
In conclusion, this study aimed to assess the feasibility of a national surveillance system and the appropriateness of new data-collection tools, in view of the ECDC HALT-4 study that will take place in 2023. A national surveillance network was established, which will facilitate future surveillance efforts. The decrease in HAI rates found in this study could indicate improved IPC practices, however further studies are necessary to evaluate the impact of the pandemic on HAI transmission in LTCFs. Further efforts should be directed to developing and sustaining IPC and AMS practices in the LTCF setting.
Ethical approval
This study obtained ethical approvals from the following Institutional review boards: “Comitato di bioetica d’Ateneo, University of Turin” (protocol code n. 0169,983, March 14, 2022), “Azienda Ospedaliero Universitaria San Luigi Gonzaga, Orbassano” (protocol code n. 4611, March 21, 2022) and “Comitato Etico Nazionale per le sperimentazioni degli Enti Pubblici di Ricerca e altri Enti Pubblici a carattere nazionale, Istituto Superiore di Sanità” (protocol code n.0015,064, April 19, 2022), in addition to approvals by each appropriate local Institutional review board.
Availability of data and materials
All data collected within the CCM project “Sostegno alla Sorveglianza delle infezioni correlate all'assistenza anche a supporto del PNCAR”, including this study, are owned by the Italian Ministry of Health. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding statement
This study was funded by the Italian Ministry of Health within the CCM project “Sostegno alla Sorveglianza delle infezioni correlate all'assistenza anche a supporto del PNCAR”.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The Authors gratefully acknowledge the LTCF personnel involved in data collection. Members of the working group: Carmen Adesso, Monica Andreani, Paolo Balbo, Claudio Baldacci, Lorenzo Bandini, Giacomo Bastianelli, Sergio Bernabè, Angela Bertorelle, Emanuela Bolamperti, Guglielmo Bonaccorsi, Maria Giovanna Bottin, Mario Bruschi, Elisabetta Campisi, Michela Canton, Francesca Collini, Antonio D'Amico, Roberta De Dona, Michela Anna Di Palma, Giulia Fadda, Giuliana Favara, Fabrizio Gemmi, Federico Grammatico, Adriano Grossi, Claudia Isonne, Claudia La Mastra, Maria Clara La Rosa, Roberta Magnano San Lio, Andrea Maugeri, Anna Natale, Crosio Pizzorni, Michela Rigon, Gigliola Scattolin, Nicandro Samprati, Arturo Santagata, Margherita Tancredi, Manuela Tamburro, Vittorio Viccione, Paola Vivani.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhip.2023.100421.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Bordino V., Vicentini C., D'Ambrosio A., Quattrocolo F., Novati R., Sticchi C., et al. Burden of healthcare-associated infections in Italy: incidence, attributable mortality and disability-adjusted life years (DALYs) from a nationwide study, 2016. J. Hosp. Infect. 2021;113:164–171. doi: 10.1016/j.jhin.2021.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Henig O., Kaye K.S. Bacterial pneumonia in older adults. Infect. Dis. Clin. 2017 Dec;31(4):689–713. doi: 10.1016/j.idc.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolle L.E. Urinary tract infections in long-term-care facilities. Infect. Control Hosp. Epidemiol. 2001 Mar;22(3):167–175. doi: 10.1086/501886. [DOI] [PubMed] [Google Scholar]
- 4.Greig J.D., Lee M.B. Enteric outbreaks in long-term care facilities and recommendations for prevention: a review. Epidemiol. Infect. 2009;137(2):145–155. doi: 10.1017/S0950268808000757. [DOI] [PubMed] [Google Scholar]
- 5.Rajagopalan S., Yoshikawa T.T. Norovirus infections in long-term care facilities. J. Am. Geriatr. Soc. 2016 May;64(5):1097–1103. doi: 10.1111/jgs.14085. [DOI] [PubMed] [Google Scholar]
- 6.Simor A.E. Diagnosis, management, and prevention of Clostridium difficile infection in long-term care facilities: a review. J. Am. Geriatr. Soc. 2010 Aug;58(8):1556–1564. doi: 10.1111/j.1532-5415.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 7.Kish T.D., Chang M.H., Fung H.B. Treatment of skin and soft tissue infections in the elderly: a review. Am. J. Geriatr. Pharmacother. 2010;8(6):485–513. doi: 10.1016/S1543-5946(10)80002-9. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa T.T., Reyes B.J., Ouslander J.G. Sepsis in older adults in long-term care facilities: challenges in diagnosis and management. J. Am. Geriatr. Soc. 2019 Nov;67(11):2234–2239. doi: 10.1111/jgs.16194. [DOI] [PubMed] [Google Scholar]
- 9.van Buul L.W., van der Steen J.T., Veenhuizen R.B., Achterberg W.P., Schellevis F.G., Essink R.T.G.M., et al. Antibiotic use and resistance in long term care facilities. J. Am. Med. Dir. Assoc. 2012;13(6):568.e1–568.e13. doi: 10.1016/j.jamda.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Vicentini C., Quattrocolo F., D'Ambrosio A., Corcione S., Ricchizzi E., Moro M.L., et al. Point prevalence data on antimicrobial usage in Italian acute-care hospitals: evaluation and comparison of results from two national surveys (2011-2016) Infect. Control Hosp. Epidemiol. 2020;41(5):579–584. doi: 10.1017/ice.2020.18. [DOI] [PubMed] [Google Scholar]
- 11.O'Toole R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin. Microbiol. Infect. 2021 Dec;27(12):1772–1776. doi: 10.1016/j.cmi.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai C.C., Chen S.Y., Ko W.C., Hsueh P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents. 2021 Apr;57(4) doi: 10.1016/j.ijantimicag.2021.106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wee L.E.I., Conceicao E.P., Tan J.Y., Magesparan K.D., Amin I.B.M., Ismail B.B.S., et al. Unintended consequences of infection prevention and control measures during COVID-19 pandemic. Am. J. Infect. Control. 2021 Apr;49(4):469–477. doi: 10.1016/j.ajic.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control About the point prevalence survey of healthcare-associated infections and antimicrobial use in European long-term care facilities. https://www.ecdc.europa.eu/en/infectious-diseases-public-health/healthcare-associated-infections-long-term-care-facilities-1
- 15.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2023. Point Prevalence Survey of Healthcareassociated Infections and Antimicrobial Use in European Long-Term Care Facilities: 2016–2017. [Google Scholar]
- 16.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2014. Point Prevalence Survey of Healthcareassociated Infections and Antimicrobial Use in European Long-Term Care Facilities. April–May 2013. [Google Scholar]
- 17.Ricchizzi E., Latour K., Kärki T., Buttazzi R., Jans B., Moro M.L., et al. Antimicrobial use in european long-term care facilities: results from the third point prevalence survey of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill. 2018;23(46) doi: 10.2807/1560-7917.ES.2018.23.46.1800394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suetens C., Latour K., Kärki T., Ricchizzi E., Kinross P., Moro M.L., et al. 2017. Prevalence of Healthcare-Associated Infections , Estimated Incidence and Composite Antimicrobial Resistance Index in Acute Care Hospitals and Long-Term Care Facilities : Results from Two European Point Prevalence Surveys; pp. 1–17. 2016 to 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furmenti M.F., Rossello P., Bianco S., Olivero E., Thomas R., Emelurumonye I.N., et al. Healthcare-associated infections and antimicrobial use in long-term care facilities (HALT3): an overview of the Italian situation. J. Hosp. Infect. 2019;102(4):425–430. doi: 10.1016/j.jhin.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Ministero della Salute Piano nazionale di Contrasto dell'Antimicrobico-resistenza (PNCAR) 2017-2020. https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?id=2660 October 20, 2022)
- 21.European Centre for Disease Prevention and Control Healthcare-associated infections surveillance network (HAI-Net) https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/hai-net
- 22.Centro nazionale per la prevenzione e il controllo delle malattie (CCM) Sostegno alla Sorveglianza delle infezioni correlate all’assistenza anche a supporto del PNCAR. https://www.ccm-network.it/progetto.jsp?id=node/2042&idP=740
- 23.OECD/European Observatory on Health Systems and Policies . OECD Publishing, Paris/European Observatory on Health Systems and Policies; Brussels: 2021. Italy: Country Health Profile 2021, State of Health in the EU. [Google Scholar]
- 24.EpiCentro Sorveglianza nazionale delle ICA nelle strutture residenziali per anziani. https://www.epicentro.iss.it/sorveglianza-ica/sorveglianza-strutture-residenziali-anziani
- 25.World Health Organization . WHO; Geneva, Switzerland: 2021. WHO Access, Watch, Reserve, Classification of Antibiotics for Evaluation and Monitoring of Use. 2021. [Google Scholar]
- 26.Stevens M.P., Doll M., Pryor R., Godbout E., Cooper K., Bearman G. Impact of COVID-19 on traditional healthcare-associated infection prevention efforts. Infect. Control Hosp. Epidemiol. 2020;41(8):946–947. doi: 10.1017/ice.2020.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans M.E., Simbartl L.A., Kralovic S.M., Clifton M., Deroos K., McCauley B.P., et al. Healthcare-associated infections in veterans affairs acute and long-term healthcare facilities during the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control Hosp. Epidemiol. 2022 doi: 10.1017/ice.2022.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monnet D.L., Harbarth S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Euro Surveill. 2020;25(45):1–6. doi: 10.2807/1560-7917.ES.2020.25.45.2001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Italian Ministry of Health Vaccine coverage data for influenza. https://www.salute.gov.it/portale/influenza/dettaglioContenutiInfluenza.jsp?lingua=italiano&id=679&area=influenza&menu=vuoto
- 30.Mulla R.T., Turcotte L.A., Wellens N.I.H., Angevaare M.J., Weir J., Jantzi M., et al. Prevalence and predictors of influenza vaccination in long-term care homes: a cross-national retrospective observational study. BMJ Open. 2022 Apr;12(4) doi: 10.1136/bmjopen-2021-057517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data collected within the CCM project “Sostegno alla Sorveglianza delle infezioni correlate all'assistenza anche a supporto del PNCAR”, including this study, are owned by the Italian Ministry of Health. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.