Abstract
Cartilage homeostasis is essential for chondrocytes to maintain proper phenotype and metabolism. Because adult articular cartilage is avascular, chondrocytes must survive in low oxygen conditions, and changing oxygen tension can significantly affect metabolism and proteoglycan synthesis in these cells. However, whether long noncoding RNA participate in cartilage homeostasis under hypoxia has not been reported yet. Here, we first identified LncZFHX2 as a lncRNA upregulated under physiological hypoxia in cartilage, specifically by HIF‐1α. LncZFHX2 knockdown simultaneously accelerated cellular senescence, targeted multiple components of extracellular matrix metabolism, and increased DNA damage in chondrocytes. Through a series of in vitro and in vivo experiments, we identified that LncZFHX2 performed a novel function that regulated RIF1 expression through forming a transcription complex with KLF4 and promoting chondrocyte DNA repair. Moreover, chondrocyte-conditional knockout of LncZFHX2 accelerated injury-induced cartilage degeneration in vivo. In conclusion, we identified a hypoxia-activated DNA repair pathway that maintains matrix homeostasis in osteoarthritis cartilage.
Keywords: LncRNA, Hypoxia, DNA repair, Osteoarthritis, Senescence
1. Introduction
The most prevalent form of arthritis is osteoarthritis (OA), a type of aseptic inflammation mainly caused by articular cartilage degeneration, synovial inflammation, subchondral bone sclerosis, and osteophyte formation [1]. Though OA is a whole-joint disease, a hallmark of its development is progressive cartilage and extracellular matrix (ECM) loss from an imbalance in cartilage homeostasis [2]. Intracellular signaling of chondrocytes trigger interactions between anabolic and catabolic activities that govern cartilage homeostasis [3]. The ECM accumulation is promoted by the expression of collagen type 2 (COL2A1) and aggrecan (ACAN). The expression of COL2A1 and ACAN were regulated by SRY-Box Transcription Factor 9 (SOX9). In contrast, the degradation of ECM is enhanced by the expression of matrix metalloproteinases (MMPs), such as MMP13, and a disintegrin and metalloproteinase with thrombospondin motif (ADAMTS) proteins, such as ADAMTS5. Besides, cellular senescence also considered to be associated with osteoarthritis. In articular cartilage of healthy adults, resident chondrocytes are quiescent and exhibit low metabolic activity, with little turnover of matrix components. Because cartilage is avascular, chondrocytes exist in a relatively hypoxic environment and have limited capacity for repair or regeneration [4]. A growing body of evidence suggests that variation in precise oxygen tension (pO2, ranging from 6% O2 on the surface to <1% in deep regions) is critical to cartilage homeostasis [5], affecting wide-ranging processes such as chondrocyte metabolism, protein/proteoglycan synthesis, and cell senescence [6,7]. Chondrocytes have abundant mitochondria that produce sufficient ATP to maintain a suitable phenotype and metabolism despite the hypoxic environment [8]. However, pO2 below a certain threshold contributes to promoting ECM synthesis and inhibiting catabolism [9]. Given these conditions, chondrocytes are extremely sensitive to changes in pO2.
The mechanism of chondrocyte response to low pO2 is through hypoxia-inducible factors (HIFs). The HIF protein complex consists of three α-subunits (HIF‐1α, HIF‐2α, and HIF‐3α) and a constitutively expressed HIF-1β subunit; the latter is present in various tissues and is tightly regulated by pO2 variation [10,11]. The main isoform during a hypoxic response is HIF‐1α, which often serves as a hypoxia indicator [12,13]. At a low pO2 (2–3%), HIF‐1α is upregulated in chondrocytes, whereas higher pO2 decreases ATP production [7]. HIF‐1α is a critical component of chondrogenesis during early skeletal genesis, acting to regulate SOX9 expression in hypoxic prechondrogenic cells [14]. Through maintaining ECM integrity and inhibiting apoptosis, HIF‐1α is a survival factor in cartilage homeostasis [15], which is largely regulated via changes in relative expression of HIF-1α and HIF-2α. Chondrocyte survival improves with HIF-1α stimulation [16], whereas the protein's conditional deletion causes chondrocyte cell death [17].
Oxidative stress arises from reactive oxygen species (ROS) overwhelming cellular antioxidant capacity [18]. Since chondrocytes are extremely sensitive to changes in pO2, prolonged normoxia severely damages DNA in chondrocytes, as indicated by phosphorylation of H2A histone family member X (H2AX) and various senescence phenotypes [19]. Considering the senility of OA and the importance of hypoxia in senility, treatments can potentially be improved through a better understanding of chondrocyte homeostasis across various oxygen levels. One promising target of research is noncoding RNAs (ncRNAs), which are important to the regulation of cellular processes in cartilage [20,21]. Currently, little research has been done on how ncRNAs affect chondrocyte homeostasis under physiological hypoxia.
Approximately 60% of DNA transcripts are ncRNAs, classified according to length, biosynthesis, and mechanisms of action [22]. After transcription, ncRNAs may be highly conserved (e.g. microRNAs, miRNAs; circular RNAs, circRNAs) or relatively not conserved (e.g. long ncRNAs, lncRNAs) [23]. Regardless, their unique secondary and tertiary structures allow ncRNAs to participate in critical biological functions [24]. In particular, lncRNAs influence the translation of other RNAs and thus play a role in gene regulatory networks. The binding of lncRNAs to their RNA targets either creates substrates for protein function or removes access to miRNA and inhibitory protein effectors [25]. In this study, we identified a conserved lncRNA (LncZFHX2) that is upregulated by HIF‐1α in normal cartilage. LncZFHX2 drives DNA repair in chondrocytes via activating replication timing regulatory factor 1 (RIF1). Notably, LncZFHX2 conditional-knockout mice exhibited severe OA after surgical destabilization of the medial meniscus. Our data imply the existence of a novel lncRNA-dependent mechanism essential for maintaining cartilage homeostasis.
2. Materials and methods
2.1. Human participants
Human cartilage samples were collected from patients with primary OA (age-related, International Cartilage Repair Society grade 3–4) undergoing total knee arthroplasty. Written informed consent was obtained from all patients prior to the operative procedure, according to the protocol approved by the Ethics Committee of Sir Run Shaw Hospital in Zhejiang, China (Approval No 20211118–33). Patient information, including age, sex, height, weight and body mass index (BMI), was summarized in Table S1. We complied with all the relevant ethical regulations and approved study protocols. For each specimen, relatively healthy cartilage (with no apparent lesions) from the non-loading areas was harvested separately from the osteoarthritic cartilage (with cartilage lesions) from the loading areas. The harvested cartilage was either used for histological analysis, RNA extraction, or processed further to isolate chondrocytes. For isolating chondrocytes, cartilage was cut into small pieces and treated with 0.25% pronase (Gibco, Grand Island, NY, USA) for 30 min, followed by treatment with 0.2% collagenase type II (Invitrogen, Carlsbad, CA, USA) for 4 h at 37 °C. Then, the digested samples were filtered through a 0.075-mm cell strainer, and cells were cultured overnight in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) at 37 °C in 5% CO2. Cellular debris was then removed by washing with sterile phosphate-buffered saline (PBS). The chondrocytes were continuously cultured and used for experiments within three passages. The public or patients were not involved in the design, conduct, reporting, or dissemination of our research plans.
2.2. Cell stimulation
To induce apoptosis, human chondrocytes were treated with 100 μM Etoposide (Selleck Chemicals, Houston, TX, USA). For canonical inflammasome-induced pyroptosis, human chondrocytes were primed with 500 ng/mL lipopolysaccharides (LPS) (Sigma, St Louis, MO, USA) for 4 h in Opti-MEM (Gibco, Grand Island, NY, USA), and then stimulated for 30 min with 2 mM adenosine triphosphate (ATP) (Sigma, St Louis, MO, USA). To induce pyroptosis, human chondrocytes were treated with 10 μM Erastin (Selleck Chemicals, Houston, TX, USA). Human chondrocytes were treated with 400 μM CoCl2 (Selleck Chemicals, Houston, TX, USA) or 1 mM dimethyloxalylglycine (Selleck Chemicals, Houston, TX, USA) to activate or stabilize the HIF-1α. To induce oxidative stress in mouse chondrocytes (C57BL/6 mice), the cells were cultured in a growth medium without sodium pyruvate, and treated with H2O2 (200 μM) for a week. For long-term treatment of H2O2, the H2O2-containing medium was refreshed every 2 days.
2.3. Experimental OA in mice
Animal assays were approved by the Laboratory Animal Welfare and Ethics Committee of Zhejiang University (Zhejiang, China) (Approval number ZJU20220412). All transgenic mice were bred in a C57BL/6 background. All mice were housed in specific pathogen-free conditions and on a 12 h/12 h light/dark circle at approximately 22 °C. LncZFHX2 conditional knockout mice were purchased from Cyagen Biology Technology (USA). We bred mLncZFHX2fl/fl mice with Col2-CreERT2 mice (Jackson Laboratories, USA) or ACAN-CreERT2 mice (kindly gifted by Professor Wu Ximei, Zhejiang University School) to generate the inducible conditional mLncZFHX2 knockout mice. For the inducible deletion of mLncZFHX2, 2-month-old male mice (n = six to eight per group) were administered with five daily intraperitoneal injections of Tamoxifen (Sigma, St Louis, MO, USA) at a dosage of 100 μg/g body weight.
Post-traumatic OA was induced by destabilization of the medial meniscus (DMM) surgery in 12-week-old male mice. For the DMM model, the animals were anaesthetized with 75 mg/kg ketamine and 10 mg/kg xylazine and subjected to unilateral DMM procedures. Briefly, a skin incision was made to expose the patella, which was laterally dislocated using tweezers under a microscope. The knee was placed in full flexion to expose the medial meniscotibial ligament, which was then transected using micro scissors. The incision was sutured with 3–0 silk sutures. Control mice were sham-operated that underwent a skin incision and suturing without patellar dislocation or ligament transection.
For the spontaneous OA model, 40-week-old male spontaneous OA STR/Ort mice and their control CBA/CaCrl mice (kindly donated by the Research Centre for Regenerative Medicine, Guangxi Medical University) were sacrificed [26,27].
For the age-associated OA model, male C57BL/6 mice were fed normally for 6, 12, or 24 months. 12-month–old and 24-month–old mice were used for aging mice, and 6-month–old mice were used as controls.
For antisense oligonucleotides (ASOs) in vivo treatment experiment, male C57BL/6 mice were randomized into two groups (n = 8 per group). ASOs (RiboBio, Guangdong, China) were delivered by unilateral intra-articular injection every three days at a dose of 1 nmol each mouse (1 nmol ASO dissolved in 10 μl sterile PBS). After 12 weeks, the mice were euthanized.
For adeno-associated virus (AAV) treatment experiment, sham or DMM operated mice underwent intra-articular injections of AAV control or AAV expressing mLncZFHX2 and KLF4 (HANBIO TECH, Shanghai, China, 5 × 109 plaque-forming units (PFUs) in a total volume of 10 μL) with a 10 μL micro-syringe (Hamilton Company, Reno, NV, USA) as indicated or 1-week post-surgery.
2.4. Cell culture and cell lines
Harvesting of human chondrocytes was performed as previously described. For mouse chondrocytes harvesting, articular cartilage was carefully isolated from the femoral condyles and tibial plateaus of 5-day-old mice (mLncZFHX2 fl/fl or wild-type C57BL/6). Free auricular cartilage was washed three times by sterile PBS and digested with 0.2% collagenase type II (Invitrogen, Carlsbad, CA, USA). The digested samples were filtered through a 0.075 mm cell strainer, and the cells were cultured overnight in DMEM containing 10% FBS (Gibco, Grand Island, NY, USA) at 37 °C in a 5% CO2 atmosphere. The human HEK-293 cell line was obtained from the American Type Culture Collection (ATCC, VA, USA), and the mice ATDC5 cell line was obtained from the Cell Engineering Division (Tsukuba, Japan) and maintained in DMEM containing 10% FBS (Gibco, Grand Island, NY, USA) at 37 °C in a 5% CO2 atmosphere.
2.5. Animal praxeology assays
For hotplate nociception analysis, the mice were transferred to the procedure room for at least 30 min before the experiment. To assess thermal hyperalgesia, mice were individually placed on a hotplate (IITC) at 55 °C one at a time. The time elapsed before apparent hind limb licking, shaking or jumping was recorded as response latency. Each trial had a maximum duration of 45 s. The mice were removed from the hotplate immediately after a response was observed. The test was repeated three times in mice with a day break between the tests, and the mean values were calculated.
For the rotarod analysis, the mice were placed onto a rotarod (Panlab, Holliston, MA, USA) at a constant speed for training for at least 30 min to rule out differences in learning skills. After training, mice were placed onto an accelerating rotarod. The duration of time that each mouse stayed atop the rod was referred to as the first ride around time. Each trial lasted a maximum of 5 min [28].
2.6. MicroCT analysis
The knee joints of mice were dissected from the distal femur to the proximal tibia. The specimens were fixed in 4% paraformaldehyde for 48 h, washed, and stored in 70% ethanol. Computed tomography (CT) scanning was performed using a high-resolution μCT (Skyscan 1275, Skyscan, Aartselaar, Belgium) using the following settings: X-ray voltage, 45 kV; source current, 55 μA; and resolution, 9 μm. After 3-dimensional reconstruction, subchondral bone parameters were analyzed according to the standard protocol under the same conditions. Each specimen was evaluated by three researchers blinded to the experimental design.
2.7. Histology and immunohistochemistry
Cartilage specimens from humans and knee joints from mice were fixed with 4% paraformaldehyde for 48 h, decalcified in 10% ethylenediaminetetraacetic acid (EDTA), embedded in paraffin, and sectioned at 3 μm. Sections were stained with 1% fast green (Sigma, dissolve in 50% alcohol) for 5min, immersed in 1% acetic acid for 5 s, and stained with 1% Safranin O (Sigma, dissolved in 95% alcohol). Three blinded scorers assessed cartilage destruction according to the Osteoarthritis Research Society International (OARSI) score [29,30], subchondral bone plate (SBP) sickness and synovitis score [31] (See Table 1, Table 2). The measurements were averaged.
Table 1.
The recommended semi-quantitative scoring system.
| Grade | Associated criteria (tissue reaction) |
|---|---|
| 0 | Normal |
| 0.5 | Loss of Safranin-O without structural changes |
| 1 | Small fibrillations without loss of cartilage |
| 2 | Vertical clefts down to the layer immediately below the superficial layer and some loss of surface lamina |
| 3 | Vertical clefts/erosion to the calcified cartilage extending to <25% of the articular surface |
| 4 | Vertical clefts/erosion to the calcified cartilage extending to 25–50% of the articular surface |
| 5 | Vertical clefts/erosion to the calcified cartilage extending to 50–75% of the articular surface |
| 6 | Vertical clefts/erosion to the calcified cartilage extending >75% of the articular surface |
Table 2.
Scheme for the histopathological assessment of the three features of chronic synovitis.
| Points | Inflammatory infiltrate |
|---|---|
| 0 point | No inflammatory infiltrate |
| 1 point | Few mostly perivascular situated lymphocytes or plasma cells |
| 2 point | Numerous lymphocytes or plasma cells, sometimes forming follicle-like aggregates |
| 3 point | Dense band-like inflammatory infiltrate or numerous large follicle-like aggregates |
For immunohistochemistry, the sections were deparaffinized in xylene and hydrated with graded ethanol. After incubating with a sodium citrate antigen retrieval solution (Solarbio, Beijing, China), the sections were incubated successively with 3% H2O2 and 5% bovine serum albumin (BSA) (Fudebio, Zhejiang, China) at room temperature (20–25 °C) for 30 min. The sections were then incubated with antibodies against with the indicated antibodies overnight at 4 °C. On the following day, the sections were incubated with biotinylated linking and streptavidin-horseradish peroxidase (HRP) reagents (ZSGB-Bio, Beijing, China) for 10 min, followed by 3,30-diaminobenzidine tetrahydrochloride hydrate (DAB) (ZSGB-Bio) staining. Finally, the sections were stained with hematoxylin (Beyotime, Shanghai, China). Images were obtained using the KFBIO scan and analysis system (KFBIO, Zhejiang, China).
2.8. RNA fluorescent in situ hybridization (FISH)
Cy3-labeled specific probes to detect LncZFHX2 were designed and synthesized by RiboBio (Guangdong, China). FISH signals were detected using a FISH kit (GenePharma, Jiangsu, China), according to the manufacturer's guidelines. Briefly, human or C57BL/6 mice chondrocytes were incubated with FISH probes overnight at 37 °C. The sections were then washed three times with washing buffer at 42 °C. Nuclei were stained with 4, 6-diamidino-2-phenylindole (DAPI) (Beyotime, Shanghai, China) for 10 min at room temperature (20–25 °C). Fluorescence images were acquired using a fluorescence microscope (BX51TRF; Olympus, Tokyo, Japan). For in vivo FISH, sections were deparaffinized, rehydrated, and permeabilized with 0.8% pepsin treatment at 37 °C for 30 min before hybridization. The remaining procedures were the same as described above.
2.9. Immunocytochemistry (ICC)
Primary chondrocytes of mLncZFHX2fl/fl mice were cultured and infected with Cre adenovirus (HANBIO TECH) or control adenovirus (HANBIO TECH, Shanghai, China) and cultured for a week. Chondrocytes were fixed with 4% paraformaldehyde for 10 min and washed three times with PBS. The cells were permeabilized with 0.1% Triton X-100 for 5 min, washed three times with PBS, and blocked with 5% goat serum for 1 h. Cells were incubated with γH2AX (1:200; Proteintech, IL, USA) and KLF4 antibodies (1:200; Abcam, Cambridge, UK) overnight at 4 °C. After washing thrice with PBS, the cells were incubated with Alexa Fluor 594 or 488 goat anti-rabbit IgG (1:200; Fudebio, Zhejiang, China) in PBS for 1 h at room temperature (20–25 °C). The nuclei were stained with DAPI (Beyotime, Shanghai, China) for 10 min at room temperature. Fluorescence signals were detected using a fluorescence microscope (BX51TRF, Olympus, Tokyo, Japan).
2.10. ASOs, siRNAs and adenovirus overexpression
ASO and siRNAs targeting specific gene were designed and synthesized by RiboBio (Guangdong, China). SiRNAs were transfected into cells seeded in 6- or 24-well plates with lipofectamine iMax (Invitrogen, Carlsbad, CA, USA) (1 μL per 105 cells). After 72 h the experiments were performed.
For the adenovirus, a specific cDNA sequence was inserted into the pAdEasy-EF1-MCS-CMV vector (HANBIO TECH, Shanghai, China). The day before transfection, HEK293 cells were seeded in a 60 mm cell culture dish with a pre-treated surface at a density of 70%–80%. The cells were incubated for 6 h with 300 μL of Opti-MEM (Gibco, Grand Island, NY, USA) containing 15 μL lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) and 4 μg recombinant plasmid. After 48 h, the medium containing the adenovirus was purified using a 0.45-μm strainer (Millipore, Billerica, MA, USA). The adenovirus harvested from HEK293 cells was further purified and concentrated by cesium chloride centrifugation. Chondrocytes were cultured for 3 days, infected with an adenovirus (100–400 MOI) for 12 h, and cultured for 72 h before further analysis.
2.11. RNA extraction and quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted and purified from the cartilage or cultured chondrocytes (human, mLncZFHX2 fl/fl or C57BL/6 mice) using AG RNAex Pro Reagent (Accurate Biology, Hunan, China) reagent and an Ultrapure RNA kit (CWBIO, Beijing, China) according to the manufacturer's instructions. Nuclear/cytoplasmic RNA was purified using a Cytoplasmic and Nuclear RNA Purification Kit (Norgen Biotek, St. Catherine, Canada). Briefly, chondrocytes (human or C57BL/6 mouse) were lysed by lysis buffer J for 5 min. After centrifugation, the supernatant and lysate pellet were collected individually. Buffers SK and ethanol were added to the supernatant (cytoplasmic RNA fraction) and pellet (nuclear RNA fraction). The mixtures were individually applied to a spin column and washed three times. Then applied the mixture onto a spin column individually and washed 3 times. Nuclear/cytoplasmic RNA was eluted using nuclease-free water. cDNA was synthesized from 1 μg of RNA using the Evo M-MLV RT Kit Mix for qPCR (Accurate Biology, Hunan, China). RT-qPCR was performed using gene-specific primers (Supplementary Table S3) and Hieff® qPCR SYBR Green Master Mix (Yeasen, Shanghai, China) using an ABI QuantStudioTM 6 Flex Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer's instructions. The relative expression levels of the PCR products were calculated using the 2−ΔΔCt method. Individual gene expression levels were normalized to those of β-actin for mRNA analysis and U6 for miRNA analyses.
2.12. Western blotting
For total protein extraction, human, mLncZFHX2 fl/fl, or C57BL/6 mice chondrocytes were lysed with strong radioimmunoprecipitation assay (RIPA) lysis buffer (Fudebio, Zhejiang, China) supplemented with protease inhibitor cocktails (Fudebio, Zhejiang, China) and total protein was quantified using a bicinchoninic acid analysis kit (Fudebio, Zhejiang, China). Equal amounts of protein (20 μg per well) were loaded onto sodium dodecyl sulfate (SDS)-polyacrylamide gels for electrophoresis and the resolved bands were electroblotted onto 0.22-μm polyvinylidene fluoride membranes (Merck, New Jersey, USA) at 260 mA for 90 min. Membranes were blocked in 5% BSA and then incubated with specific antibodies overnight at 4 °C. The next day, the membranes were washed three times with tris-buffered saline (TBS) containing 0.1% Tween 20 and were then incubated with mouse or rabbit secondary antibody conjugated to HRP-conjugated secondary antibody (FDM007 and FDR007, Fudebio, Zhejiang, China, 1:5000) at room temperature (20–25 °C) for 1 h. Protein bands were detected using an Amersham Imager 600 (General Electric Company, Boston, MA, USA) with FDbio-Femto ECL substrates (Fudebio, Zhejiang, China).
The following antibodies were used in this study: anti-MMP13 (1:1000, ab39012, Abcam, Cambridge, UK), anti-ADAMTS5 (1:1000, ab41037, Abcam, Cambridge, UK), anti-SOX9 (1:1000, ab185966, Abcam, Cambridge, UK), anti-ACAN (1:1000, C8035, Millipore, Billerica, MA, USA), anti-COL2A1 (1:1000, sc-52658, Santa Cruz Biotechnology, Dallas, TX, USA), anti-P16INK4a (1:1000, ab211542, Abcam, Cambridge, UK), anti-P21 (1:1000, ab109199, Abcam, Cambridge, UK), anti-γ-H2AX (1:1000, 10856-1-AP, Proteintech, IL, USA), anti-replication timing regulatory factor 1 (RIF1) (1:1000, ab13422, Abcam, Cambridge, UK), anti-Kruppel-like factor 4 (KLF4) (1:1000, ab214666, Abcam, Cambridge, UK), anti-flag-tag (1:1000, ab205606, Abcam, Cambridge, UK), anti-caspase-3 (1:1000, ab32351, Abcam, Cambridge, UK), anti-cleaved N-terminal Gasdermin-D (GSDMD) (1:1000, ab215203, Abcam, Cambridge, UK), anti-glutathione peroxidase 4 (GPX4) (1:1000, 67763-1-Ig, Proteintech, IL, USA), anti-β-actin (1:1000; 3700, Cell Signaling Technology, MA, USA).
2.13. Luciferase reporter assays
HEK-293 cells were seeded in 24-well plates (1 × 105 cells/well) and transfected with specific genes luc (pGL3-Firefly-Luciferase, HANBIO TECH) and Renilla (pGL3-Renilla-Luciferase, HANBIO TECH, Shanghai, China). After 48 h, the firefly and Renilla luciferase activities were detected using a dual luciferase reporter assay kit (Beyotime, Shanghai, China) according to the manufacturer's protocol. Firefly luciferase activity was normalized to the Renilla luciferase activity to determine the ratio.
2.14. Flow cytometry
Human, mLncZFHX2 fl/fl, or C57BL/6 mice chondrocytes were seeded in 6-well plates (5 × 105 cells/well) and transfected with ASOs, adenovirus or siRNA. After 48 h, apoptosis was determined using a FITC Annexin V Kit (BD Biosciences, Franklin Lakes, NJ, USA). Briefly, after detaching with 0.05% trypsin and washing twice with PBS, the cells were incubated with annexin V-FITC and propidium iodide for 15 min prior to analysis using a flow cytometer (BD FACSCANTO II; BD Biosciences) and FlowJo software to evaluate apoptosis.
2.15. Non-homologous end joining (NHEJ) reporter assays
The DSB repair efficiency of NHEJ was measured in chondrocytes (mLncZFHX2 fl/fl or C57BL/6 mice) transfected with EJ5-green fluorescent protein (GFP) and I-SceI. The specific methods have been described before [32]. Briefly, chondrocytes were seeded in 6-well plates (5 × 105 cells/well) and transfected with adenovirus (400 MOI). After 48 h, cells transfected with 0.6 μg of EJ5-GFP expression plasmid (Vigene, Shandong, China) and either mock-transfected or transfected with 0.6 μg of I-SceI expression plasmid (Vigene, Shandong, China) using 5 μL of Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). 48 h after plasmid transfection, the cells were analyzed for GFP expression using flow cytometry (BD FACSCANTO II; BD Biosciences).
2.16. Three-dimensional chondrocyte cultures
Chondrocyte 3D agarose cultures were performed as previously described [33]. Briefly, before embedding, chondrocytes (mLncZFHX2 fl/fl or C57BL/6 mice) were transfected with adenovirus (400 MOI) or siRNA for 48h. Mixed 1 vol 5 × culture medium (10% FBS, 5% HEPES and 5% penicillin/streptomycin, Gibco, Grand Island, NY, USA) and 4 vol of the 2.5% low melting agarose solution to obtain a final concentration of 2% agarose and 1 × culture medium. Cells embedded in the agarose hydrogel were incubated in a 24-well plate (2 × 106 cells/well) for approximately 15 min until gelling occurred. Culture cell/agarose constructs for 1 week at 37 °C in 5% CO2 and change culture medium every day. Fixed with 4% paraformaldehyde, the samples were embedded in paraffin, sectioned at 7-μm thickness, and histology was analyzed by Alcian blue. Images of the sections were scanned using a KFBIO scan and analysis system (KFBIO, Zhejiang, China), and pericellular ECM thickness was quantified.
2.17. Senescence-associated β-galactosidase (SA-β-Gal) staining
Chondrocytes (mLncZFHX2 fl/fl or C57BL/6 mice) were seeded in 6-well plates (5 × 105 cells/well) and transfected with adenovirus or siRNA. SA-β-Gal staining was performed with the SA-β-Gal staining kit (Beyotime, Shanghai, China). Briefly, after washing twice with PBS, chondrocytes were fixed with 4% paraformaldehyde and 0.2% glutaraldehyde for 15 min at room temperature. Fixed cells were washed three times with PBS and incubated with 1 mL SA-β-Gal staining solution at 37 °C overnight. The next day, the cells were washed twice with PBS and 70% ethanol.
2.18. Chromatin immunoprecipitation assay (ChIP)
Chondrocytes (C57BL/6 mice) were cultured in 2.5% pO2 for 48 h. The ChIP assay was performed using the SimpleChIP® Plus Sonication Chromatin IP Kit (Cell Signaling Technology, MA, USA) according to the manufacturers’ protocols. Briefly, after washing with PBS, cells were incubated with 1% formaldehyde for 10 min at room temperature. The cells were incubated with glycine for 5 min at room temperature and washed twice with PBS. After incubation with Sonication Cell Lysis Buffer (1% SDS, 10 mM EDTA, 50 mM Tris, pH 8.1), the lysates were sonicated to make 300–500 bp chromatin fragments. Chromatin was fragmented by sonication and then incubated with specific antibody (1 μg per IP sample) overnight at 4 °C. The IP samples were then incubated with ChIP-Grade Protein G Magnetic Beads and washed with low- and high-salt buffers. Elute chromatin from the antibody/Protein G Magnetic Beads for 30 min at 65 °C with Elution Buffer and then reverse cross-links. DNA was purified using spin columns and used as a template for PCR and RT-qPCR assays.
2.19. Chromatin isolation by RNA purification (ChIRP) assay
CHIRP-qPCR and CHIRP-seq assays were performed as previously described [34,35]. The antisense DNA probe for mLncZFHX2 was designed by RiboBio (Supplementary Table S3). The probes were labeled with biotin at the 3’ end. Chondrocytes (from C57BL/6 mice) were cultured in 2.5% pO2 for 48 h. After washing with PBS, the cells were incubated with 1% formaldehyde for 10 min at room temperature (20–25 °C). Then cells were incubated with glycine for 5 min at room temperature and washed twice with PBS. After incubation with Sonication Cell Lysis Buffer (1% SDS, 10 mM EDTA, 50 mM Tris, pH 8.1), the lysates were sonicated to make 300–500 bp chromatin fragments. Chromatin was fragmented by sonication and diluted twice with the volume of hybridization buffer (750 mM NaCl, 1% SDS, 50 mM Tris, pH 7.0, 1 mM EDTA, 15% formamide, dithiothreitol, phenylmethanesulfonyl fluoride, protease inhibitor and RNase inhibitor). Biotin-labeled probes were added, and mixtures were rotated overnight at 4 °C. The IP samples were then incubated with Streptavidin-magnetic beads and washed with low- and high-salt buffers. Elute the DNA fragments and protein from the probe/streptavidin-magnetic beads with DNA Elution Buffer or protein loading buffer (Fudebio, Zhejiang, China). DNA was purified using spin columns and used as a template for PCR and RT-qPCR.
2.20. Silver staining
Silver staining was performed using a Fast Silver Stain Kit (Beyotime, Shanghai, China) according to the manufacturer's protocol. Briefly, the proteins obtained from the ChIRP assay denatured at 95 °C in 5 × loading buffer (Fudebio, Zhejiang, China) for 5 min and loaded onto SDS-polyacrylamide gel for electrophoresis. After fixing with a stationary liquid (50 mL ethanol, 10 mL acetic acid, and 40 mL double-distilled H2O) for 1 h, the gel was washed with 30% ethanol for 10 min and twice with double-distilled H2O. The gel was incubated with a silver staining buffer for 10 min and washed twice with double-distilled H2O. Gels were stained with a chromogenic agent until clear bands were visible, after which they were washed with the corresponding elimination agent.
2.21. Mass spectrometry analysis
The specific silver-stained bands were cut into pieces. For in-gel tryptic digestion, a gel piece with a matched molecular mass to the targeted protein was excised and destained with 50% acetonitrile in 50 mM ammonium bicarbonate (NH4HCO3). The destained gel pieces were dehydrated in 100% acetonitrile for 5 min, and incubated with 10 mM Tris (2-carboxyethyl) phosphine hydrochloride at 37 °C for 30 min. Then, the gel was again dehydrated with 100% acetonitrile and incubated with 25 mM iodoacetamide at room temperature for 30 min in the dark. Gel pieces were washed with 50 mM NH4HCO3 and dehydrated with 100% acetonitrile. Finally, the gel piece was rehydrated and digested with 2 μg trypsin in 50 mM NH4HCO3 at 37 °C overnight for protein in-gel digestion. After digestion, the peptides were extracted from the gel piece using 50% acetonitrile/0.1% formic acid. The extracted peptides were dried in a speed vacuum concentrator and resuspended in 0.1% formic acid for liquid chromatography tandem mass spectrometry (LC-MS/MS). Peptide samples were dissolved in mobile phase A (0.1% formic acid) and separated using an EASY nLC-1200 (Thermo Scientific, MA, USA). The nano-liquid chromatography gradient was maintained at a constant flow rate of 400 nL/min and comprised of an increase from 2% to 7% mobile phase B (0.1% formic acid in 80% acetonitrile) over 1 min, 7%–35% for 35 min, 35%–55% for 9 min, climbing to 100% in 7 min, and held at 100% for the last 8 min. The isolated peptides were subjected to a nano source, followed by Q Exactive HF-X mass spectrometry. The electrospray voltage applied was 2.0 kV, and intact peptides were detected in the Orbitrap at a resolution of 60,000. Peptides were then selected for MS/MS using an NCE setting of 27, and the fragments were detected using Orbitrap at a resolution of 30,000. Mass Spectra were acquired in data-dependent scan mode, including the selection of the 20 most abundant precursor ions in each MS spectrum for MS/MS analysis with 20s dynamic exclusion. Mass spectra were processed and searched against the UniProt protein database using Proteome Discoverer (version 2.4, Thermo Scientific, MA, USA). Trypsin/P (or other enzymes if any) was specified as the cleavage enzyme, allowing for up to two missing cleavages.
The mass tolerance allowed for the precursor ions was 10 ppm, whereas the mass tolerance of fragment ions was set to 0.01 Da. Carbamidomethyl on Cysteine was specified as a fixed modification, whereas oxidation on methionine and acetyl on the protein N-terminal was specified as a variable modification. The peptide confidence was set at high.
2.22. Biotinylated RNA pulldown assay
The PierceTM Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific) was used for this experiment. Briefly, 2 × 107 chondrocytes (C57BL/6 mice) were collected, lysed and sonicated. Beads were washed twice with 20 mM Tris before use. Biotinylated RNAs (GenePharma, Jiangsu, China) was incubated with 50 μL of streptavidin magnetic beads in RNA capture buffer at room temperature for 30 min. Then the samples were incubated with biotinylated RNA at 4 °C overnight. Beads were washed four times with wash buffer and complexes eluted with Biotin Elution Buffer for 15 min at 37 °C. The eluted proteins were analyzed using western blotting.
2.23. RNA immunoprecipitation assay (RIP)
In total, 2 × 107 chondrocytes (C57BL/6 mice) were transfected with Flag-KLF4 overexpressing plasmids. After 48 h, RIP was performed using the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) according to the manufacturer's protocol. Briefly, chondrocytes were lysed in complete RIP lysis buffer, with a part of the cell suspension set aside as the input. The resulting cell lysates were incubated with 5 μg of anti-Flag-tag antibody or control IgG antibody at 4 °C overnight. Total RNA was isolated for the detection of mLncZFHX2 by RT-qPCR, as described above.
2.24. RNA isolation and library construction for RNA-seq
A total of 5 × 105 chondrocytes (C57BL/6 mice) were collected and total RNA was purified as described above. RNA integrity was detected using a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) and a threshold of integrity >7.0 was used for library construction. Constructed from 1 μg of total RNA from individual sample using the fish TruSeqTM Stranded Total RNA Library Prep Kit (Illumina Inc, San Diego, CA, USA) according to the manufacturer's instructions, RNA libraries were amplified by PCR for 15 cycles. Standard cDNA libraries were constructed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and a high sensitivity DNA chip. Library concentrations were determined by qPCR using the KAPA Library Quantification kit (KAPA Biosystems, Foster City, CA, USA). The samples were adjusted to 20 pM concentration per library and sequenced using the MiSeq Reagent Kit v3 in an Illumina Miseq Sequencing System (Illumina Inc, San Diego, CA, USA) for 150 cycles. RNA library analysis was performed by Shanghai Origingene Bio-Pharm Biotechnology Co.,Ltd.
2.25. Molecular docking RNA
The crystal structures of KLF4 was obtained from the Protein Data Bank. The Amber14SB force field [36] was adopted for energy optimization. Docking between KLF4 and the nucleic acid interaction region was processed using Rosetta [37]. The protein-RNA docking tool in Rosetta primarily utilizes the Monte Carlo algorithm for conformational search in the entire space. During the low-resolution sampling stage, Rosetta constructs a model of the protein using the backbone atoms and amino acids side-chain centroids. The Monte Carlo algorithm was used for spatial search, and a low-resolution energy function was employed to determine whether a conformation was retained. In the high-resolution optimization stage, all heavy and polar hydrogen atoms were restored, and the Monte Carlo algorithm was used to optimize and insert the side chains. At this stage, a more complete and complex all-atom potential function is used for scoring. Finally, a composite scoring function and binding mode were used to select reasonable docking results.
2.26. Genome editing with Clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9
gRNAs were designed using the online tool CCTop-CRISPR/Cas9 as a target online predictor (Supplementary Table S3). We designed oligos for cloning gRNA targeting this site into a PEP-KO vector (Oligo1: 5′- ACCGNNNNNNNNNNNNNNNNNNNN-3′, Oligo2: 3′-CNNNNNNNNNNNNNNNNNNNNCAA-5′, N for gRNA sequence). Briefly, the two oligos were phosphorylated and annealed with T4 Ligation Buffer (Takara, Kyoto, Japan) and T4 PNK (Takara, Kyoto, Japan) according to the standard parameters. Annealed oligos were diluted 1:200 in nuclease-free water and ligated into the PEP-KO vector using T4 Ligation Buffer (Takara, Kyoto, Japan). After transfection of the vector into competent cells, 3–4 single colonies were picked into to 5 mL of Luria-Bertani (LB) broth containing carbenicillin. Plasmid DNA was isolated from amplified competent cells using a standard method and analyzed the plasmid DNA by Sanger sequencing. The plasmid was transfected into ATDC5 cells (500 ng PEP-KO, based on 6-well plate) following the standard infection protocol. After transfection for 48 h, select with puromycin (puro) and re-plated cells at a low density in a 10-cm dish (100 cells). Single colonies were expanded, and a portion of the cells was harvested, RT-qPCR, and western blotting analysis.
2.27. GST-protein purification and electrophoretic mobility shift assay (EMSA)
Biotin-labeled linearized LncZFHX2 was synthesized using T7 transcription in vitro (GenePharma, Jiangsu, China). To purify the of GST fusion protein, the KLF4 cDNA sequence was inserted into the pGEX-6P-2 GST Expression Vector (Addgene, USA) and transformed into BL21 competent cells. After culturing in 30 mL LB with appropriate antibiotics and 0.1 mM IPTG for 8 h, bacterial pellets were resuspended in lysis buffer and then sonicated for 30 min. The supernatant was collected after centrifugation and incubated with GSTSep Glutathione Magbeads (Yeasen, Shanghai, China). After eluting the protein with glutathione elution buffer, transferred the product to a microtube and kept in −80 °C in aliquots. Probes and KLF4 proteins were incubated in a binding buffer, and EMSA was performed using an RNA-EMSA Kit (Bersinbio, Guangdong, China) according to the manufacturer's protocol.
2.28. In vitro transcription and translation assay
In vitro transcription and translation were performed using TnT® Quick Coupled Transcription/Translation Systems (Promega, Madison, WI, USA) following the manufacturer's instructions. To use these systems, 1 μg of circular plasmid DNA containing a T7 promoter and LncZFHX2 or luciferase (positive control) sequence, were added to an aliquot of the TnT® Quick Master Mix, 20 μM methionine and TranscendTM Biotin-Lysyl-tRNA. Using the TranscendTM Systems, biotinylated lysine residues are incorporated into nascent proteins during translation. Then added nuclease-free water to a final volume of 50 μL and incubated for 90 min at 30 °C. The synthesized proteins were analyzed using SDS-polyacrylamide gel electrophoresis and detected.
2.29. Comet assay
Chondrocytes (mLncZFHX2 fl/fl or C57BL/6 mice) were transfected with adenovirus or siRNA and cultured under hypoxic conditions for 1 week. The comet assay was performed using an OxiSelectTM Comet Assay Kit (Cell Biolabs, San Diego, CA, USA). Briefly, heat the Comet Agarose bottle at 90–95 °C in a water bath and transfer the bottle to a 37 °C water bath. The cell samples were combined with Comet Agarose in a 1:10 ratio (v/v), mixed well by pipetting, and immediately transferred onto the top of the Comet Agarose Base Layer (75 μL per well). After solidification, immerse the slides in the Lysis Buffer for 30 min at 4 °C in the dark. The slides were then transferred to a pre-chilled tris-borate-EDTA (TBE) buffer for 10 min. A voltage was applied to the chamber for 10–20 min at 1 V/cm in the TBE buffer. The slides were then immersed in pre-chilled double distilled H2O for 2 min and repeated twice more. After replacing the slides with cold 70% ethanol for 5 min, the slides were stained with Vista Green DNA Dye for 15 min. Fluorescence images were acquired using a fluorescence microscope (BX51TRF; Olympus, Tokyo, Japan) and analyzed using the CaspLab software.
2.30. 5′ Rapid amplification of cDNA ends
The 5′ RACE was performed using a SMARTer RACE 5'/3′ Kit (Takara, Kyoto, Japan) according to the manufacturer's instructions. Briefly, total RNAs (∼5 μg) of chondrocytes were prepared for all of the 5′-RACE-Ready cDNA synthesis with 5X First-Strand Buffer, dithiothreitol (100 mM), and dNTPs (20 mM). To just the 5′-RACE cDNA synthesis reaction, add 1 μL of the SMARTer II A Oligonucleotide per reaction. Total RNA was converted into first-strand cDNA at 42 °C for 90 min. Rapid amplification of the cDNA ends was performed by PCR reactions using gene-specific primers. RACE products were electrophoresed on an agarose gel and DNA fragments of interest were extracted using the NuceloSpin Gel and PCR Clean-Up Kit (Takara, Kyoto, Japan). Gel-purified RACE products were cloned into linearized pRACE vector using an In-Fusion HD Cloning Kit (Takara, Kyoto, Japan) and transfected into competent cells. Plasmid DNA was isolated from amplified competent cells using a standard method and analyzed the plasmid DNA by Sanger sequencing.
2.31. Statistical analysis
Statistical analyses were performed using the GraphPad Prism software (version 9.0.0). Statistical significance was determined using unpaired Student's t-test, paired Student's t-test, one-way ANOVA, two-way ANOVA, Kruskal–Wallis test and Mann–Whitney U test. Results were considered statistically significant at P < 0.05.
3. Results
3.1. Expression and functional characterization of LncZFHX2 in human cartilage
The lncRNA microarray identified 162 lncRNAs that were differentially expressed between chondrocytes cultured in normoxia (pO2 = 21%) and hypoxia (pO2 = 2.5%) (Fig. 1A). We next used Ensembl (score >50) to examine the top eight upregulated or downregulated lncRNAs (Fig. 1B). Five of the lncRNAs investigated have conserved sequences between Homo sapiens and Mus musculus. Quantitative reverse transcription PCR (RT-qPCR) revealed that ENST00000553985 (hereafter LncZFHX2) and ENST00000522863 (hereafter LncHOXA11) were outliers based on fold change (hypoxia/normoxia) (Fig. S1A).
Fig. 1.
Identification and characterization of LncZFHX2 in human chondrocytes (HCs). (A) Flowchart illustrating screening criteria of potential regulatory lncRNAs enriched in HCs. (B) Heatmap of differentially expressed lncRNAs (eight upregulated and eight downregulated) in HCs. (C) RT-qPCR of LncZFHX2 levels in HCs transfected with ASO LncZFHX2 or ASO negative control (NC) (n = 6, mean ± SD). (D) Western blotting of MMP13, ADAMTS5, SOX9, ACAN, COL2A1, P16INK4a and P21 expression in HCs transfected with ASO LncZFHX2 or ASO NC. (E) Representative images of Safranin O/Fast Green and RNA FISH staining in human knee cartilage. Scale bars, 5 mm to 200 μm. (F) Percentage of LncZFHX2-positive cells in undamaged and damaged areas (n = 42). (G) RT-qPCR of LncZFHX2 levels in undamaged and damaged areas (n = 36 donors). (H) LncZFHX2 was transcribed and translated in vitro, then subjected to western blotting (lane 3). Biotinylated lysine residues are incorporated into nascent proteins during translation. Nascent proteins were analyzed by detecting biotin signal with streptavidin-HRP. The absence of protein product confirms LncZFHX2 as a noncoding RNA. An empty vector plasmid was used as NC (lane 1). Luciferase vectors were the positive controls (lane 2). The graphs show P values from one-way ANOVA (C) or paired two-tailed t-test (F, G).
We performed knockdown experiments with ASOs to understand the functions of LncZFHX2 and LncHOXA11, then validated the results with RT-qPCR (Fig. 1C and Fig. S1B). LncZFHX2 knockdown caused upregulation of MMP13, ADAMTS5, P16INK4a and P21, as well as downregulation of SOX9, COL2A1, and ACAN (Fig. 1D). However, LncHOXA11 knockdown did not significantly alter protein levels (Fig. S1C).
Next, we collected cartilage from the tibial plateau of OA patients treated with total knee arthroplasty. To minimise confounding effects of donor variations, we used normal cartilage from undamaged areas as individual controls. Safranin O and fast green staining identified the damaged and undamaged samples, and RNA fluorescent in situ hybridization (FISH) confirmed LncZFHX2 downregulation in damaged cartilage (Fig. 1E and F; n = 42). Similar results were obtained using RT-qPCR (Fig. 1G, n = 36).
The Coding Potential Calculator and Coding Potential Assessment Tool indicated that LncZFHX2 has low protein-coding potential (Fig. S1D). Using an in vitro transcription and translation assay with luciferase as a positive control, we confirmed LncZFHX2 was not translated to protein (Fig. 1H). Since cell death was observed after LncZFHX2 knockdown in human chondrocytes (HCs), we investigated whether apoptosis, ferroptosis, or pyroptosis occurred. Western blotting confirmed that HCs experienced apoptosis, as evidenced by an increase in cleaved-caspase3 without changes to GPX4 or GSDMD (Fig. S1E). Flow cytometry then verified that chondrocyte apoptosis increased with LncZFHX2 knockdown (Fig. S1F). Furthermore, RNA FISH in HCs and RT-qPCR of cellular fractions showed that LncZFHX2 localized mostly in the nucleus (Figs. S1G and H).
3.2. mLncZFHX2 expression correlated with hypoxia and senescence
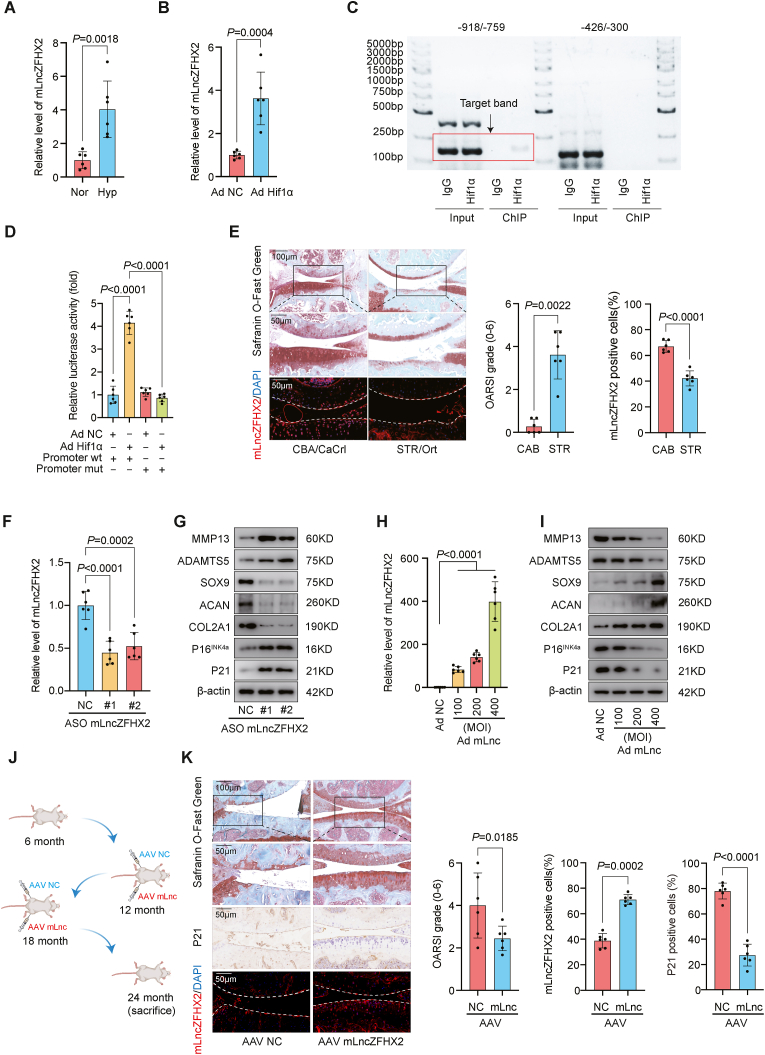
Ensembl data revealed that LncZFHX2 and ENSMUST00000183822 (hereafter mLncZFHX2) were highly conserved. We validated the sequence of full-length mLncZFHX2 through 5′ rapid amplification of cDNA ends (RACE) and DNA sequencing (Figs. S2A and B). We used RT-qPCR to measure mLncZFHX2 expression across mouse tissues and found specific mLncZFHX2 expression in cartilage (Fig. S2c). Notably, mLncZFHX2 also increased under hypoxic conditions in mouse chondrocytes (MCs) (Fig. 2A).
Fig. 2.
mLncZFHX2 regulates osteoarthritis (OA) in vivo and in vitro. (A) RT-qPCR of mLncZFHX2 levels in mouse chondrocytes (MCs) under normoxia or hypoxia (n = 6, mean ± SD). (B) RT-qPCR of mLncZFHX2 levels in MCs infected with adenovirus (Ad) NC or Ad HIF‐1α (400 MOI) (n = 6, mean ± SD). (C) HIF‐1α ChIP assay of mLncZFHX2 promoter region. (D) Luciferase activities of HEK-293 cells co-transfected with a luciferase reporter construct containing wild-type or mutant mLncZFHX2 promoter region and Ad NC or Ad HIF‐1α (400 MOI) (n = 6, mean ± SD). (E) Representative images of Safranin O/Fast Green and RNA FISH staining of knee joints in CBA/CaCrl and SRT/Ort mice. Degree of OA was determined with OARSI grade (n = 6 per group, mean ± SD). Scale bar, 50 μm and 100 μm. (F) RT-qPCR of mLncZFHX2 levels in MCs transfected with ASO mLncZFHX2 or ASO NC (n = 6, mean ± SD). (G) Western blotting of MMP13, ADAMTS5, SOX9, ACAN, COL2A1, P16INK4a and P21 expression in HCs transfected with ASO LncZFHX2 or ASO NC. (H) RT-qPCR of mLncZFHX2 (mLnc) levels in MCs transfected with Ad NC (400 MOI) or Ad mLnc (100–400 MOI) (n = 6, mean ± SD). (I) Western blotting of MMP13, ADAMTS5, SOX9, ACAN, COL2A1, P16INK4a and P21 expression in MCs transfected Ad NC (400 MOI) or Ad mLnc (100–400 MOI). (J) Schematic of experimental design. Mice (12 and 18 months old) were subjected to intra-articular injections of mLncZFHX2 AAV or control AAV and sacrificed at 24 months. (K) Representative images of Safranin O/Fast Green, immunohistochemistry (P21), and RNA FISH (mLncZFHX2) staining of knee joints in aged mouse model. Degree of OA was evaluated with OARSI grade (n = 6 per group, mean ± SD). Scale bar, 50 μm and 100 μm. Graphs show P values from unpaired two-tailed t-test (A, B), one-way ANOVA (D, F, H). OARSI grade was analyzed with Mann-Whitney U tests (E, K), and positive cells were compared with unpaired two-tailed t-test (E), or paired two-tailed t-test (K).
We then investigated the influence of HIF‐1α, HIF‐2α, and HIF‐3α on mLncZFHX2. Only overexpression of HIF‐1α induced a significant increase of mLncZFHX2 (Fig. 2B and Fig. S2D). Additionally, CoCl2 and dimethyloxalylglycine (DMOG) both promoted HIF‐1α stabilisation and accumulation, significantly inducing mLncZFHX2 expression in MCs (Fig. S2E). Exposure to prolonged normoxia decreased mLncZFHX2 content (Fig. S2F). The JASPAR database predicted that the mLncZFHX2 promoter region has two HIF‐1α binding sites [38]. Based on this finding, we generated two constructs derived from the promoter fragment of mLncZFHX2, each containing a putative transcriptional binding site. ChIP assay and electrophoresis revealed that HIF‐1α was bound to the segment from −918 to −759 bp (Fig. 2C). Next, we constructed a luciferase reporter plasmid containing the mLncZFHX2 promoter and a mutant plasmid (Fig. S2G). The luciferase assay further validated mLncZFHX2 involvement in regulation of HIF‐1α and thus in hypoxia response (Fig. 2D).
3.3. mLncZFHX2 regulated OA progress in vivo and in vitro
To investigate the relationship between mLncZFHX2 and OA, we detected mLncZFHX2 in various OA models using RNA FISH assay. In naturally aged mice (12 and 24 months), mLncZFHX2 decreased from control (6 months old mice) levels in an age-dependent manner (Fig. S2H). Safranin O/Fast Green staining and OARSI scores confirmed OA development in experimental mice. Moreover, immunohistochemistry performed on joints showed that P21 increased with age (Fig. S2H). As further evidence, mLncZFHX2 decreased significantly in STR/Ort mice with spontaneous OA compared with levels in CBA/CaCrl controls (Fig. 2E). These data all suggested that mLncZFHX2 is important in OA.
Western blotting showed that mLncZFHX2 knockdown upregulated MMP13, ADAMTS5, P16INK4a and P21 in MCs, but downregulated SOX9, COL2A1, and ACAN (Fig. 2F and G). To further validate whether mLncZFHX2 loss accelerated ECM loss, we completely abolished mLncZFHX2 in ATDC5 cells using CRISPR/Cas9. We verified gene editing success with PCR and selected donor 7 as the mLncZFHX2 knockout (KO) cell line (Fig. S3A). KO status of donor 7 was then confirmed (Fig. S3B). Next, we overexpressed mLncZFHX2 in KO ATDC5 cells and verified efficiency with RT-qPCR (Fig. S3C). The expression of anabolic factors SOX9, COL2A1, and ACAN was significantly lower in KO ATDC5 cells than in wild-type cells. Overexpressing mLncZFHX2 rescued ECM loss, suggesting that mLncZFHX2 performs valuable functions in chondrocyte homeostasis (Fig. S3D). Additionally, flow cytometry revealed that MC apoptosis increased with mLncZFHX2 knockdown (Figs. S3E and F). For in vivo experiments, male C57BL/6 mice were administered an articular injection of ASO mLncZFHX2 or the ASO control for 12 weeks. Knockdown of mLncZFHX2 caused mild articular surface abrasion, synovitis, and thickening of the subchondral bone plate (Fig. S3G).
Furthermore, adenoviral overexpression of mLncZFHX2 (Ad mLnc) upregulated SOX9, COL2A1, and ACAN while downregulating MMP13, ADAMTS5, P16INK4a and P21 (Fig. 2H and I). Next, we performed DMM surgery on male C57BL/6 mice, followed by intra-articular injections of mLncZFHX2 AAV or control AAV. The mLncZFHX2 AAV treatment partially inhibited symptoms of DMM-induced OA but did not improve synovitis (Fig. S3H). Finally, we used a mouse model of knee OA to investigate whether mLncZFHX2 can inhibit aging-related degeneration. For all mice, one knee joint was injected with mLncZFHX2 AAV and the other with control AAV (Fig. 2J). The results indicated that mLncZFHX2 markedly attenuated cartilage destruction in old mice (Fig. 2K).
3.4. mLncZFHX2 forms a transcription complex with KLF4 to regulate RIF1 expression
Because mLncZFHX2 is largely localized in the nucleus of MCs, we hypothesized that mLncZFHX2 might function in combination with transcription factors (TFs) to regulate gene transcription and influence OA progression (Fig. S4A). We performed ChIRP assay in MCs to investigate mLncZFHX2-interacting proteins and their locations in the genome. The analysis revealed 1451 mLncZFHX2 binding sites that were also promoter regions (fold enrichment ≥7).
To explore whether mLncZFHX2 affects gene expression, we extracted total RNA from MCs (n = 3 donors per group) treated with Ad mLnc or control adenovirus (Ad NC). After constructing RNA-seq libraries, we identified 533 potential genes that met the significance threshold (fold change ≥2 and P < 0.05). Of these, 10 were regulated directly by mLncZFHX2 binding to their promoters (Fig. 3A and B). These results were validated with RT-qPCR of MCs overexpressing mLncZFHX2 (Fig. 3C). We also detected the 10 genes in WT and KO ATDC5 cells with RT-qPCR (Fig. S4B). RIF1 demonstrated the most discrepancies between the two experiments, indicating that RIF1 is the most likely downstream target directly regulated by mLncZFHX2. In addition, RT-qPCR of DNA fragments in the ChIRP assay revealed that mLncZFHX2 was enriched in the promoter region (−1800 to −1400) of RIF1 (Fig. 3D). Through sequence alignment, we noticed that a pairing complementary bases existed between RIF1 promoter (−1323 to −1313) and mLncZFHX2 (82 to −92). Therefore, we concluded that mLncZFHX2 only affects transcription of RIF1 rather than other KLF4-target genes by binding to this sequence.
Fig. 3.
mLncZFHX2 regulates RIF1 expression via recruiting KLF4 onto RIF1 promoter. (A) Venn diagram of overlaps between ChIRP-seq (probe mLnc/input) and RNA-seq (Ad mLnc/Ad NC). (B) Heatmap of 10 genes in MCs treated with Ad mLnc or Ad NC. (C) RT-qPCR of mRNA levels of 10 genes in MCs treated with Ad mLnc or Ad NC (n = 6, mean ± SD). (D) MCs cultured in hypoxia for 48 h and subjected to ChIRP assay using biotin-labeled probes against mLncZFHX2. Precipitated DNA fragments were extracted for RT-qPCR to determine mLncZFHX2 enrichment on the RIF1 promoter (n = 6, mean ± SD). (E) Complementarity between the RIF1 promoter (−1323 to −1313) with the mLncZFHX2 sequence (82 to −92). (F) Venn diagram of overlap between probe mLnc specifically conjugated proteins and mouse transcription factors from CIS-BP. (G) RNA pulldown assay on biotin-labeled fragments of mLncZFHX2 from MC lysates. (H) Computed electrostatic potentials of mLncZFHX2 and KLF4 from molecular docking experiments. (I) A RIP assay of KLF4 and its mutants interacting with mLncZFHX2 was conducted using anti-Flag (n = 6, mean ± SD). (J) EMSA using biotin-labeled mLncZFHX2 and recombinant KLF4 with or without anti-KLF4. (K) Schematic of putative KLF4-binding sites within the RIF1 promoter region (2000 bp). KLF4 ChIP assay of the RIF1 promoter. (L) Luciferase reporter assay using RIF1 promoters with mLncZFHX2 or KLF4 overexpression in HEK-293 cells (n = 6, mean ± SD). (M) Luciferase assay of RIF1 wild-type and mutant promoter activity under mLncZFHX2 and KLF4 co-overexpression in HEK293 cells. (N) MCs were transfected with Ad NC or Ad mLnc and then subjected to KLF4 ChIP assay targeting the RIF1 promoter. (O) Schematic of mLncZFHX2 functional mechanisms. Graphs show P values from unpaired two-tailed t-test (C, D), one-way ANOVA (I, L, M) and two-way ANOVA (N).
We then subjected all interacting proteins in the ChIRP assay to silver staining and mass spectrometry, identifying 433 specifically conjugated proteins (Fig. S4C). A search against CIS-BP (online library of TFs and their DNA binding motifs) revealed 17 TFs from the 433 proteins (Fig. 3F and Fig. S4D). Screening in JASPAR revealed that the RIF1 promoter region contained putative binding sites for PITX1, SOX6, STAT1, JUNB, RUNX1, and KLF4 (relative score >0.9). Knockdown of these six TFs using small inhibitory RNA (siRNA) and RT-qPCR revealed that RIF1 was downregulated following KLF4 knockdown (Fig. S4E).
We validated the binding of mLncZFHX2 to KLF4 with four experiments. First, ICC and RNA FISH confirmed the signals of mLncZFHX2 and KLF4 signals were nuclear in MCs (Fig. S4F). Second, we predicted the loop structure of mLncZFHX2 with secondary structures of single-stranded RNA or DNA sequence prediction tools (RNAfold web server) (Fig. S4G). Since mLncZFHX2 harbors three stem-loop structures (1–190, 191–440, 441–670), LncZFHX2 may form tertiary topological structures that may mediate the binding of mLncZFHX2 to KLF4. A biotinylated RNA pulldown assay then showed that a specific fragment (nt 1–190) of mLncZFHX2 was necessary for binding to KLF4 (Fig. 3G). Third, molecular docking experiments revealed that KLF4 domains bind to the double-helix hairpin structure of mLncZFHX2 (Fig. S4H). The 3D structure of KLF4 used in docking procedures was obtained from the RCSB Protein Data Bank. Various hydrogen-bond-forming sites in KLF4 (threonine, T; aspartic acid, D; lysine, L; cysteine, C; tryptophan, W; glycine, G; and arginine, R) were found to be important for mLncZFHX2 binding: T401, D403, L418, L428, C432, G438, W439 and L440 on surface 1; L464, R467 and R481 on surface 2 (Fig. 3H). To block hydrogen bond formation, we mutated T401, D403, L418, L428, C432, G438, W439, and L440 on surface1 (mut1) to alanine and mutated L464, R467, and R481 on surface 2 (mut2) to alanine. A RIP assay confirmed the direct interaction between mLncZFHX2 and KLF4 on surface 1 (Fig. 3I). Fourth, we further verified this interaction using an RNA EMSA (Fig. 3J).
We next determined whether KLF4 participates in regulating RIF1 expression of MCs. JASPAR predicted that the RIF1 promoter region contains six putative binding sites for KLF4 (Fig. 3K). We considered sites 4–6 as one site because of their close location and overlap. The results of ChIP assays showed that KLF4 binds to nt-1567–1352 (site 2) and nt-221–53 (sites 4–6) of the RIF1 promoter. We then constructed luciferase reporter plasmids containing the RIF1 promoter and mutated reporter plasmids at sites 2 (mut1) and sites 4–6 (mut2) (Fig. S4I). Luciferase assays confirmed that concomitant mLncZFHX2 and KLF4 overexpression promoted RIF1 transcription in HEK-293 cells (Fig. 3L). Furthermore, we observed that neither mLncZFHX2 nor KLF4 induced luciferase activity of reporter plasmids containing mut1 or co-mut binding sites, indicating that site 2 was the active promoter fragment (Fig. 3M). Through ChIP assay, we validated that the binding of KLF4 to RIF1 promoter was increased with mLncZFHX2 overexpression (Fig. 3N). Taken together, these data demonstrated that mLncZFHX2 is a scaffold for KLF4 recruitment to regulate RIF1 transcription (Fig. 3O).
3.5. Conditional knockout of mLncZFHX2 in chondrocytes accelerated OA progression
We established mLncZFHX2fl/fl mice to further investigate the role of mLncZFHX2 in OA pathogenesis. Primary chondrocytes of mLncZFHX2fl/fl were infected with Cre or control adenovirus and cultured for a week. Knockout efficiency was confirmed using RT-qPCR (Fig. 4A). We then assessed NHEJ of mLncZFHX2fl/fl MCs; NHEJ is the mechanism underlying RIF1 repair of DNA double-strand breaks (DSB) [39]. We used an established GFP-based chromosomal reporter containing a promoter separated by the puro gene from a GFP coding cassette. The puro gene itself was flanked by two I-SceI sites in the same orientation, generating DSBs that can be repaired through NHEJ. This repair excises the puro gene and ligates the promoter to the expression cassette, restoring the GFP+ gene (Fig. 4B) [32]. Flow cytometry identified a defect in NHEJ-mediated repair after Cre knockdown of mLncZFHX2 (Fig. 4C). Additionally, we designed RIF1-depleted control experiments to verify the effects of mLncZFHX2 overexpression on chondrogenic phenotypes. Adenoviral overexpression of mLncZFHX2 showed increased NHEJ-mediated DNA repair and RIF1 depletion suppressed the effects of mLncZFHX2 overexpression (Fig. S6A). A comet assay evaluating DNA damage in mLncZFHX2fl/fl MCs indicated that Cre-treated MCs had markedly increased tail moments (Fig. 4D and Fig. S5A). Overexpression of mLncZFHX2 resulted in a reduction in tail moments, and this reduction was reversed upon depletion of RIF1 (Fig. S6B). Immunocytochemistry further detected an increase in γH2AX-positive cells among Cre-treated mLncZFHX2fl/fl MCs (Fig. 4E and Fig. S5B). In contrast, γH2AX-positive cells were decreased with adenoviral overexpression of mLncZFHX2 and this reduction was reversed upon depletion of RIF1 (Fig. S6C). RIF1 downregulation and increased levels of phosphorylated H2AX upregulated matrix-degrading enzymes MMP13 and ADAMTS5, as well as cellular senescence markers P16INK4a and P21, while downregulating SOX9, COL2A1, and ACAN expression (Fig. 4F and Fig. S6D). Furthermore, we observed high proteoglycan abundance in mLncZFHX2fl/fl MCs transfected with control adenovirus. In contrast, Cre-transfected MCs exhibited lower ECM deposition (Fig. 4G and Fig. S5C). Adenoviral overexpression of mLncZFHX2 showed proteoglycan abundance and RIF1 depletion suppressed the effects of mLncZFHX2 overexpression (Fig. S6E). To confirm that mLncZFHX2 is a driver of cellular senescence in MCs, we performed senescence-associated β-galactosidase (SA-β-Gal) staining (Fig. 4H and Fig. S5D). In contrast, RIF1 depletion suppressed the effects of mLncZFHX2 (Fig. S6F).
Fig. 4.
Conditional knockout of mLncZFHX2 in chondrocytes accelerated cartilage degeneration. (A) RT-qPCR of mLncZFHX2 levels in mLncZFHX2fl/fl MCs infected with Cre or control adenovirus (n = 6, mean ± SD). (B) Schematic of experimental design. EJ5-GFP is shown along with a promoter separated from a GFP coding cassette by a puro gene with two flanking I-SceI sites. (C) Measurement of NHEJ proficiency in mLncZFHX2fl/fl MCs infected with Cre or control adenovirus (n = 6, mean ± SD). (D–H) mLncZFHX2fl/fl MCs were transfected with adenovirus and cultured under normoxia for 1 week. (D) Comet assay measuring DNA damage accumulation. Scale bar, 5 μm. (E) Immunocytochemistry detection of γH2AX. The arrows indicated the ɣH2AX foci. Scale bar, 5 μm. (F) Western blotting for RIF1, γH2AX, MMP13, ADAMTS5, SOX9, ACAN, COL2A1, P16INK4a and P21 detection. (G) Alcian blue staining of MCs grown in 3D matrices for 7 days. Scale bar, 100 and 200 μm. (H) SA-β-Gal staining of MCs. Scale bar, 200 μm. (I) Micro-CT images of knee joints from mLncZFHX2fl/fl and mLncZFHX2fl/fl/Col2-CreERT2 mice. Osteophytes are highlighted in red. (J) Calculated BV/TV, TB.Th, and TB.Sp of subchondral bone across groups (n = 6, mean ± SD). (K) Representative images of Safranin O/Fast Green staining of knee joints in mLncZFHX2fl/fl and mLncZFHX2fl/fl/Col2-CreERT2 mice that underwent sham or DMM surgery (n = 8, mean ± SD). Scale bar, 50 and 100 μm. Experimental OA was evaluated with OARSI grade, SBP thickness, and synovitis. (L) Representative images of RNA FISH (mLncZFHX2), immunofluorescence (RIF1), and immunohistochemistry (γH2AX) of knee joints in mLncZFHX2fl/fl and mLncZFHX2fl/fl/Col2-CreERT2 mice that underwent sham or DMM surgery (n = 8 per group, mean ± SD). Mann-Whitney U test was performed for OARSI grade and synovitis; two-way ANOVA was performed for SBP thickness (K).
Next, we crossed mLncZFHX2fl/fl mice with Col2-CreERT2 and ACAN-CreERT2 mice to generate mLncZFHX2 conditional-knockout (cKO) mice. Reconstructed 3D coronal and sagittal micro-CT images showed increased osteophyte formation in mLncZFHX2 cKO mice with DMM-induced OA (Fig. 4I and Fig. S5E). Subchondral bone remodelling was more severe in both mLncZFHX2fl/fl/Col2-CreERT2 and mLncZFHX2fl/fl/ACAN-CreERT2 mice after DMM surgery (Fig. 4J and Fig. S5F). Additionally, while sham operations did not alter morphology in mLncZFHX2 cKO mice, DMM surgery caused more severe cartilage destruction in cKO mice (Fig. 4K and Fig. S5G). The latter group also exhibited higher OARSI scores and a greater degree of SBP sclerosis; synovitis was not observed. Results from hot-plate and rotarod assays indicated that mLncZFHX2 cKO mice were more sensitive to DMM-induced OA pain and exhibited locomotor disability, respectively (Figs. S5H and I). Though effects on pathophysiology in the osteoarthritis models were modest, our results effectively demonstrate that the effect of lncZFXH2 is statistically significant. Immunofluorescence and RNA FISH performed on knee joints of cKO mice revealed lower RIF1 and mLncZFHX2 levels after DMM-induced OA. Furthermore, immunohistochemistry demonstrated that phosphorylation of H2AX was significantly upregulated during OA progression in cKO mice (Fig. 4L and Fig. S5J).
Additionally, we observed that RIF1 protein increased under adenoviral overexpression of HIF-1α in mouse chondrocytes. Simultaneously, RIF1 protein decreased following mLncZFHX2 or KLF4 knockdown. Overexpression of HIF-1α upregulated SOX9, COL2A1, and ACAN, while downregulated cellular senescence markers P16INK4a and P21. MMP13 and ADAMTS5 were not altered significantly with HIF-1α increased. The decrease of P16INK4a and P21 can be blocked by mLncZFHX2 or KLF4 depletion but SOX9, COL2A1, and ACAN levels were not blunted evidently (Fig. S6G).
3.6. mLncZFHX2 and KLF4 synergistically attenuated post-injury OA development
To validate the synergistic action of mLncZFHX2 and KLF4, the synergism between lncZFHX2 and KLF4 for induction of RIF1, mLncZFHX2fl/fl primary chondrocytes were transfected with Cre adenovirus, then co-transfected mLncZFHX2 with KLF4 adenovirus. We then used RT-qPCR to verify mLncZFHX2 and RIF1 overexpression (Fig. S7A and Fig. S7B). We also observe d that RIF1 protein increased under adenoviral overexpression of mLncZFHX2 and KLF4, but that the effect on DNA damage and senescence markers was not observed. Then we constructed mutant mLncZFHX2 adenovirus that without the KLF4 binding domain (nt 1–190). After transfection with Cre adenovirus, we co-transfected mutant or WT mLncZFHX2 with KLF4 adenovirus into mLncZFHX2fl/fl primary chondrocytes, followed by H2O2 treatment.
We then used RT-qPCR to verify mLncZFHX2 and RIF1 overexpressio n (Fig. S7D and Fig. S7E). Through peroxide, we confirmed that KLF4+mLnc WT still effectively plays a role in DNA damage repair during high levels of DNA damage, in turn downregulating H2AX phosphorylation and senescence markers P16INK4a and P21 (Fig. S7F).
We then verified in vitro results with in vivo experiments, injecting mutant or WT mLncZFHX2 with KLF4 AAV into the knee joint of mLncZFHX2fl/fl/Col2-CreERT2 mice after DMM surgery. KLF4 AAV failed to reverse post-traumatic OA with mutant mLncZFHX2. However, WT mLncZFHX2 combined with KLF4 synergistically inhibited OA progression (Fig. S7D). OARSI scores and SBP thickness validated the attenuated cartilage degeneration in the co-injection group. Finally, immunofluorescence, immunohistochemistry, and RNA FISH confirmed RIF1, phosphorylation of H2AX, and mLncZFHX2 expression in vivo (Fig. S7E). Combined, these data imply that mLncZFHX2 is important for OA development.
4. Discussion
Low oxygen tension has been reported to increase ECM abundance (as measured via the marker COL2A1) and provides favourable conditions for maintaining cartilage homeostasis [40]. In this study, we clarified the mechanism underlying physiological hypoxia regulation of chondrocytes. Specifically, we investigated the involvement of HIF-1α in inducing the expression of LncZFHX2, which in turn affected the levels of RIF1, ECM synthesis, and chondrocyte senescence. Previous studies have demonstrated that HIF-1α regulates ECM synthesis in different ways [15,41]. For example, HIF-1 binds to SOX9 and ACAN promoter to regulates the transcription of SOX9 and ACAN. And SOX9 is an upstream regulator of COL2A1. As a result, ECM synthesis was not blunted by LncZFHX2 or KLF4 depletion.
Our experiments further showed that LncZFHX2 regulates senescence-related cellular pathway. Senescence is a complex process associated with multiple stress stimuli, including telomere dysfunction, oxidative stress, chromatin alterations, active oncogenes, and DNA damage [42]. A hallmark of senescence is mitochondrial dysfunction, resulting in elevated cellular ROS and oxidative stress [43]. Aberrant accumulation of DNA damage and DNA replication can also induce senescence [44,45]. Osteoarthritis is characterized by the imbalanced homeostasis between anabolism (marked by SOX9, COL2A1, ACAN, etc.) and catabolism (marked by MMP13, ADAMTS5, etc.). This imbalance could be secondary effect caused by the increased senescence observed in LncZFHX2-depleted cells [19]. Previous work from others has demonstrated that chondrocyte senescence is considered a crucial cellular event contributing to matrix catabolism during osteoarthritis development. Moreover, senescent chondrocytes expressing high levels of P16 also displayed lower expression of cartilage-related ECM proteins, such as ACAN, but increased expression of ECM-degrading factors, such as MMP13 [46]. Enzymes linked to OA have been identified as senescence-associated secretory phenotype (SASP) factors, and a branch of therapy called senomorphics focuses on their selective inhibition [46]. Future research should investigate whether lncRNAs can be targeted in novel therapies for aging-associated OA, similar to SASP factors.
Here, we validated the enriched expression of LncZFHX2 in the cartilage. Exposure to prolonged normoxia decreased mLncZFHX2 content, which is likely a partial explanation for the DNA damage response and senescence phenotypes in MCs under normoxia [19]. In vitro knockdown of LncZFHX2 aggravated cell senescence and the OA phenotype of chondrocytes. In vivo assay showed reduction of LncZFHX2-positive cells in the OA models. Since blood vessel formation induced by osteoclasts in subchondral bone in early stage of OA alters the joint hypoxia environment and contributes to sustained cartilage degeneration [47]. The disrupted joint hypoxia environment with elevated oxygen partial pressure promote the degradation of HIF-1α. With the absence of HIF-1α, the expression of LncZFHX2 also decreased. The reduction of LncZFHX2 further promoted the progress of OA. There may be a mutually reinforcing relationship between OA and LncZFHX2 reduction. As a feature of post-traumatic OA, joint injury can stimulate cartilage degradation and accelerate chondrocyte senescence [48]. Senescence from DNA damage then expedites OA progression. Previous research has found that stochastic DNA damage diversifies gene expression and is a core mechanism that induces functional failure and phenotypic variation in aged chondrocytes [49]. Thus, OA chondrocytes exhibit progressive stress-induced senescence. In patients with OA, chondrocyte DNA damage linearly increases with age, particularly upon reaching end-stage OA [50]. Research in porcine cartilage has also provided some evidence for the essential role of DNA damage in OA chondrocytes [51].
RIF1 is a macromolecular protein comprising 2472-residue proteins with a highly structured N-terminal domain [52,53], and is part of the telomeric complex that evolved in complex eukaryotes [54]. The protein plays a critical role in genome maintenance and is involved in the regulation of replication timing, telomere homeostasis, and DSB repair pathway choice [55]. A study that induced DNA damage through hydroxyurea, ultraviolet light, and etoposide detected RIF1 foci formation, suggesting that the marker plays a role in DNA-damage-response signaling [56]. Together with our results, the available data suggest that RIF1 is a promising therapeutic target.
Here, we propose that LncZFHX2 acts through recruiting KLF4 to the promoter region of RIF1 and stimulating its transcription. LncRNAs can regulate gene expression via chromatin modification, transcriptional and post-transcriptional processing, including pairing with other RNAs, co-transcriptional regulation and scaffolding of cytoplasmic complexes [57,58]. Whether lncRNAs could directly promote transcription via binding to promoter regions in OA remains undefined in previous study. In conclusion, we highlighted the paramount importance of LncZFHX2 in DNA damage response during OA progression and demonstrated its potential as a therapeutic target for OA and other degenerative diseases.
Availability of data and materials
All data associated with this study are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Author contributions
Conceptualization: F.S, S.S, H.Z. Methodology: W.Y, S.Y. Investigation: L.Y, Z.H, Y.W, G.T. Visualization: N.W, Z.H. Funding acquisition: X.W, M.Y, S.S, H.Z. Project administration: F.S, S.S, H.Z. Supervision: N.W, Z.H. Writing – original draft: N.W, Z.H, M.Z. Writing – review & editing: N.W, Z.H, M.Z.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr. An Qin and Prof. Ximei Wu for assistance on the project. This study was sponsored by the National Natural Science Foundation of China (82101647, 82001462, 82171560 and 82272522), Natural Science Foundation of Zhejiang Province of China (Y23H060039), and Distinguished Young Scholars of Zhejiang Province (LR22H060001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102858.
Contributor Information
Shunwu Fan, Email: shunwu_fan@zju.edu.cn.
Shuying Shen, Email: 11207057@zju.edu.cn.
Ziang Hu, Email: zianghusrrsh@zju.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Hunter D., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014;10:437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 2.Wu X., et al. Kindlin-2 preserves integrity of the articular cartilage to protect against osteoarthritis. Nature Aging. 2022;2:332–347. doi: 10.1038/s43587-021-00165-w. [DOI] [PubMed] [Google Scholar]
- 3.Han S. Osteoarthritis year in review 2022: biology. Osteoarthritis Cartilage. 2022;30:1575–1582. doi: 10.1016/j.joca.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Bolduc J.A., Collins J.A., Loeser R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019;132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fermor B., et al. Oxygen, nitric oxide and articular cartilage. Eur. Cell. Mater. 2007;13:56–65. doi: 10.22203/ecm.v013a06. discussion 65. [DOI] [PubMed] [Google Scholar]
- 6.Grimshaw M.J., Mason R.M. Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteoarthritis Cartilage. 2000;8:386–392. doi: 10.1053/joca.1999.0314. [DOI] [PubMed] [Google Scholar]
- 7.Grimshaw M.J., Mason R.M. Modulation of bovine articular chondrocyte gene expression in vitro by oxygen tension. Osteoarthritis Cartilage. 2001;9:357–364. doi: 10.1053/joca.2000.0396. [DOI] [PubMed] [Google Scholar]
- 8.Terkeltaub R., Johnson K., Murphy A., Ghosh S. Invited review: the mitochondrion in osteoarthritis. Mitochondrion. 2002;1:301–319. doi: 10.1016/s1567-7249(01)00037-x. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y., et al. Hypoxia combined with spheroid culture improves cartilage specific function in chondrocytes. Integr. Biol. 2015;7:289–297. doi: 10.1039/c4ib00273c. [DOI] [PubMed] [Google Scholar]
- 10.Majmundar A.J., Wong W.J., Simon M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serocki M., et al. miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis. 2018;21:183–202. doi: 10.1007/s10456-018-9600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blancher C., Moore J.W., Talks K.L., Houlbrook S., Harris A.L. Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res. 2000;60:7106–7113. [PubMed] [Google Scholar]
- 13.Dengler V.L., Galbraith M., Espinosa J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014;49:1–15. doi: 10.3109/10409238.2013.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amarilio R., et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 15.Duval E., et al. Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis Rheum. 2009;60:3038–3048. doi: 10.1002/art.24851. [DOI] [PubMed] [Google Scholar]
- 16.Bohensky J., et al. HIF-1 regulation of chondrocyte apoptosis: induction of the autophagic pathway. Autophagy. 2007;3:207–214. doi: 10.4161/auto.3708. [DOI] [PubMed] [Google Scholar]
- 17.Schipani E., et al. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui W., et al. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann. Rheum. Dis. 2016;75:449–458. doi: 10.1136/annrheumdis-2014-206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang D., et al. Stress-activated miR-204 governs senescent phenotypes of chondrocytes to promote osteoarthritis development. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aar6659. [DOI] [PubMed] [Google Scholar]
- 20.Ali S.A., Peffers M.J., Ormseth M.J., Jurisica I., Kapoor M. The non-coding RNA interactome in joint health and disease. Nat. Rev. Rheumatol. 2021;17:692–705. doi: 10.1038/s41584-021-00687-y. [DOI] [PubMed] [Google Scholar]
- 21.van Hoolwerff M., et al. Elucidating epigenetic regulation by identifying functional cis-acting long noncoding RNAs and their targets in osteoarthritic articular cartilage. Arthritis Rheumatol. 2020;72:1845–1854. doi: 10.1002/art.41396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djebali S., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnsson P., Lipovich L., Grandér D., Morris K.V. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim. Biophys. Acta. 2014;1840:1063–1071. doi: 10.1016/j.bbagen.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujii Y., et al. Cartilage homeostasis and osteoarthritis. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anastasiadou E., Jacob L.S., Slack F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer. 2018;18:5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staines K.A., Poulet B., Wentworth D.N., Pitsillides A.A. The STR/ort mouse model of spontaneous osteoarthritis - an update. Osteoarthritis Cartilage. 2017;25:802–808. doi: 10.1016/j.joca.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers M.G., et al. Matrix metalloproteinases and aggrecanases cleave aggrecan in different zones of normal cartilage but colocalize in the development of osteoarthritic lesions in STR/ort mice. Arthritis Rheum. 2001;44:1455–1465. doi: 10.1002/1529-0131(200106)44:6<1455::Aid-art241>3.0.Co;2-j. [DOI] [PubMed] [Google Scholar]
- 28.Ruan M.Z., Patel R.M., Dawson B.C., Jiang M.M., Lee B.H. Pain, motor and gait assessment of murine osteoarthritis in a cruciate ligament transection model. Osteoarthritis Cartilage. 2013;21:1355–1364. doi: 10.1016/j.joca.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritzker K.P., et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Glasson S.S., Chambers M.G., Van Den Berg W.B., Little C.B. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Krenn V., et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 32.Bennardo N., Cheng A., Huang N., Stark J.M. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bougault C., Paumier A., Aubert-Foucher E., Mallein-Gerin F. Investigating conversion of mechanical force into biochemical signaling in three-dimensional chondrocyte cultures. Nat. Protoc. 2009;4:928–938. doi: 10.1038/nprot.2009.63. [DOI] [PubMed] [Google Scholar]
- 34.Chu C., Qu K., Zhong F.L., Artandi S.E., Chang H.Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell. 2011;44:667–678. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu B., et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat. Immunol. 2017;18:499–508. doi: 10.1038/ni.3712. [DOI] [PubMed] [Google Scholar]
- 36.Maier J.A., et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theor. Comput. 2015;11:3696–3713. doi: 10.1021/acs.jctc.5b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleishman S.J., et al. Rosetta in CAPRI rounds 13-19. Proteins. 2010;78:3212–3218. doi: 10.1002/prot.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro-Mondragon J.A., et al. Jaspar 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022;50:D165–d173. doi: 10.1093/nar/gkab1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman J.R., et al. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell. 2013;49:858–871. doi: 10.1016/j.molcel.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ströbel S., et al. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res. Ther. 2010;12:R34. doi: 10.1186/ar2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stegen S., et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565:511–515. doi: 10.1038/s41586-019-0874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Y., et al. Cycloastragenol prevents age-related bone loss: evidence in d-galactose-treated and aged rats. Biomed. Pharmacother. 2020;128 doi: 10.1016/j.biopha.2020.110304. [DOI] [PubMed] [Google Scholar]
- 43.Venkataraman K., Khurana S., Tai T.C. Oxidative stress in aging--matters of the heart and mind. Int. J. Mol. Sci. 2013;14:17897–17925. doi: 10.3390/ijms140917897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Micco R., et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 45.Bartkova J., et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 46.Coryell P.R., Diekman B.O., Loeser R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021;17:47–57. doi: 10.1038/s41584-020-00533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H., et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci. Adv. 2023;9 doi: 10.1126/sciadv.abo7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon O.H., et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose J., et al. DNA damage, discoordinated gene expression and cellular senescence in osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2012;20:1020–1028. doi: 10.1016/j.joca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Copp M.E., et al. Comet assay for quantification of the increased DNA damage burden in primary human chondrocytes with aging and osteoarthritis. Aging Cell. 2022;21 doi: 10.1111/acel.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen A.F., Davies C.M., De Lin M., Fermor B. Oxidative DNA damage in osteoarthritic porcine articular cartilage. J. Cell. Physiol. 2008;217:828–833. doi: 10.1002/jcp.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buonomo S.B., Wu Y., Ferguson D., de Lange T. Mammalian Rif1 contributes to replication stress survival and homology-directed repair. J. Cell Biol. 2009;187:385–398. doi: 10.1083/jcb.200902039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu L., Blackburn E.H. Human Rif1 protein binds aberrant telomeres and aligns along anaphase midzone microtubules. J. Cell Biol. 2004;167:819–830. doi: 10.1083/jcb.200408181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams I.R., McLaren A. Identification and characterisation of mRif1: a mouse telomere-associated protein highly expressed in germ cells and embryo-derived pluripotent stem cells. Dev. Dynam. 2004;229:733–744. doi: 10.1002/dvdy.10471. [DOI] [PubMed] [Google Scholar]
- 55.Fontana G.A., Reinert J.K., Thomä N.H., Rass U. Shepherding DNA ends: rif1 protects telomeres and chromosome breaks. Microb Cell. 2018;5:327–343. doi: 10.15698/mic2018.07.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar R., Cheok C.F. RIF1: a novel regulatory factor for DNA replication and DNA damage response signaling. DNA Repair. 2014;15:54–59. doi: 10.1016/j.dnarep.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 57.He R.Z., Luo D.X., Mo Y.Y. Emerging roles of lncRNAs in the post-transcriptional regulation in cancer. Genes Dis. 2019;6:6–15. doi: 10.1016/j.gendis.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ulitsky I., Bartel D.P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Data will be made available on request.