Abstract
Backgroud
We aimed to explore the prognostic features of ligand and receptor genes associated with disulfidoptosis in hepatocellular carcinoma (HCC) and establish a risk signature utilizing these genes to predict the prognosis of HCC patients.
Methods
We used scRNA-seq data from GSE166635 to differentiate malignant cells from normal cells using “copykat”.The study thoroughly examined the disparities in disulfidoptosis scores and the associated gene expressions between malignant and normal cells.We identified key ligand and receptor genes that are specific to HCC cells.Subsequently, Correlation analysis was conducted to ascertain the ligand and receptor genes associated with disulfidoptosis.We performed univariate Cox regression analysis to identify prognostic ligand and receptor genes associated with disulfidoptosis.We employed LASSO to construct a risk signature using prognostic ligand and receptor genes associated with disulfidoptosis.Lastly, we developed a nomogram model that integrates the risk signature with clinicopathological characteristics.
Results
Malignant cells displayed a marked increase in disulfidoptosis scores and the expression of associated genes compared to normal cells.We identified 110 receptor and ligand genes significantly associated with disulfidoptosis, and narrowed them down to create a risk signature comprising eight genes.Multivariate analysis confirmed the risk signature as an independent prognostic factor for HCC and validated its predictive value for immunotherapy outcomes.A novel nomogram was developed, incorporating stage information and the risk signature derived from disulfidoptosis-related receptor and ligand genes, demonstrating excellent predictive accuracy and reliability in HCC prognosis prediction.
Conclusion
Risk signatures based on disulfidoptosis-associated ligand and receptor genes can effectively predict HCC prognosis and may inform immunotherapy strategies.
Keywords: Hepatocellular carcinoma, Disulfidoptosis, Ligand and receptor genes, Single cell RNA sequencing, Bulk RNA sequencing
1. Introduction
Hepatocellular Carcinoma is the predominant histological type of liver cancer, which is one of the major cancer burdens worldwide [1]. The incidence of this disease has been on the rise in many countries over the past few decades. According to the latest "Global Cancer Statistics 2020″ report released by the World Health Organization (WHO), there were 905,677 new cases of primary HCC and 830,180 deaths globally in 2020, making it the third leading cause of cancer-related deaths worldwide. HCC accounts for the vast majority of diagnosed and fatal cases of liver cancer [2].In China, the number of new cases of primary HCC accounts for 45.3% of the world's total, and the number of deaths accounts for 47.1% of the world's total [3].
Cell death is an essential physiological process for maintaining the development and internal environment of organisms. Dysregulation of cell death is associated with various diseases, including cancer. Targeting cell death-related pathways to kill cancer cells is a major direction in cancer therapy [4].In 2020, Professor Gan Boyi and his research team at MD Anderson Cancer Center, University of Texas, made a significant discovery. They found that the conversion of ingested cystine to cysteine, mediated by SLC7A11, critically relies on the production of reduced nicotinamide adenine dinucleotide phosphate (NADPH) through the glucose-pentose phosphate pathway.Consequently, in situations of glucose deprivation, cells exhibiting elevated SLC7A11 expression experience a rapid depletion of intracellular NADPH [5]. This depletion results in the abnormal accumulation of disulfides, including cystine, which induces disulfide stress and prompts rapid cell death.Subsequent investigations have unveiled that the upregulation of SLC7A11 induced by glucose deprivation, which ultimately results in cancer cell death, does not align with any established cell death mechanisms. This newly identified mode of cell death remains unresponsive to conventional inhibitors of cell death or the ablation of pivotal genes implicated in ferroptosis and apoptosis. However, the presence of disulfide-generating agents, including dithiothreitol and dimethyl fumarate, significantly intensifies this particular form of cell death. Therefore, this unique cellular demise has been designated as disulfidoptosis.
The tumor microenvironment's potential intercellular communication plays a critical role in HCC development.Exploring ligand and receptor genes related to disulfidptosis is essential in guiding research and clinical applications.In this study, we analyzed the expression of ligand and receptor genes related to disulfidptosis from multiple omics perspectives using bioinformatics methods. We investigated their correlations with HCC survival, prognosis, immunity, and tumor microenvironment.Based on our analysis, we identified potential ligand and receptor genes that may regulate disulfidptosis.
2. Method
2.1. Data download and preprocessing
We retrieved transcriptomic and clinical data related to HCC from the UCSC Xena database (https://xena.ucsc.edu/). For further research, 310 tumor samples having both expression and survival information were saved. We retrieved three validation datasets for HCC: GSE116174 from the GEO database with 115 tumor samples, GSE14520 from the GEO database with 488 tumor samples, and a dataset from the ICGC database with 445 tumor samples.Moreover, HCC scRNA-seq data were obtained from the GEO database under the dataset number GSE166635, containing two tumor samples. Expression in all five datasets was derived from human liver tissue.
2.2. Single-cell analysis and cell-cell interaction analysis
The Seurat packagewas [6] applied to examine the scRNA-seq data, and 11,618 cells in total were obtained by excluding low-quality cells having less than 200 expressed genes, unique molecular identifiers (UMIs) greater than 8000 and mitochondrial-derived UMI counts greater than 20% during quality control. The samples’ batch effect was eliminated by employing the harmony algorithm before further searching for high-variant genes. Following normalization and principal component analysis (PCA) of the genes with high variability, we chose the first 40 principal components (PCs) with a resolution level of 0.5 to cluster all cells, and the annotation of major cell types was based on their highly expressed marker genes or known functional genes for cell subtypes. Then, we utilized the "copykat" package [7] in R to predict copy number alterations in each cell and infer the ploidy status, distinguishing between diploid (normal cells) and aneuploid (tumor cells). Subsequently, we downloaded and curated 50 hallmark and disulfidptosis pathways from the Molecular Signatures Database (MsigDB, http://www.gsea-msigdb.org/gsea/index.jsp) and Nature. Enrichment scores for each pathway were obtained through ssGSEA analysis of tumor and normal cells within each sample. A heatmap was used to display the differences in pathway enrichment scores and expression of genes associated with disulfidptosis between diploid (normal cells) and non-diploid (tumor cells).The CellChat R package [8] was used to infer, analyze and visualize intercellular communication between various cell types based on scRNA-seq data, and to identify different ligand-receptor interactions and specific signaling pathways.
2.3. Identification of key ligands and receptors involved in disulfidptosis in tumor cells
We conducted a differential gene expression analysis using TCGA expression data between two types of data sets: disulfidptosis and key receptors and ligands of hepatocellular carcinoma cells. First, we merged the TCGA expression data with each type of data set separately. Then, a correlation test was performed between each gene in the key receptors and ligands in hepatocellular carcinoma data and each gene in thedisulfidptosis data, using a correlation threshold of 0.3 and a p-value threshold of 0.05. Genes with a correlation coefficient greater than 0.3 and a p-value less than 0.05 were considered positively regulated, while genes with a correlation coefficient less than −0.3 and a p-value less than 0.05 were considered negatively regulated.
2.4. Construction and efficacy assessment of prognostic signatures
The hazard ratio (HR) and prognostic significance of key ligand and receptor genes related to disulfidptosis were evaluated using univariate Cox regression analysis, and genes with p < 0.05 were identified as prognostic-related genes. We further carried out Least Absolute Shrinkage and Selection Operator (LASSO)-Cox regression analysis based on these genes. Moreover, the model's penalty parameter (λ) was evaluated via 10-fold cross-validation under ideal conditions, and then the most predictive genes that affected the patients survival were identified. We were able to compute the prognostic risk score for every patient using the expression pattern of these genes and the linear combination of the associated weights. Based on the prognostic risk score of each patient, the patients were further classified into high- and low-risk groups. The expression of each gene in the model was also analyzed in both tumor risk groups. Furthermore, the model prediction performance was evaluated using time-dependent subject workup curves. KM survival curves and Log-rank tests had also been conducted to examine patients' survival differences, and Cox regression was applied to assess the prognostic impact of each clinicopathological characteristic, including model risk scores. Nomograms were constructed using the "rms" package [9]to determine overall survival (OS) at 1, 3, and 5 years based on the multivariate Cox proportional hazards analysis outcomes. The nomograms provide a graphical depiction of these characteristics, allowing the prognostic risk of every patient to be determined based on the points linked with the individual risk factors.
2.5. Prediction of immunotherapy response and tumor immune microenvironment using ligand and receptor genes related to disulfidptosis models
In the TCGA cancer cohort, the ratio of different immune cell infiltration was estimated based on the CIBERSORT method [10], Spearman's correlation was then calculated for different immune cell infiltration rates in the high-risk group versus the low-risk group; immune checkpoints are a series of components expressed on immune cells that are associated with regulating the level of immunological activation and preventing the immune system from becoming overly active. The expression or malfunction of immune checkpoints is one of the major causes of many diseases. Furthermore, the spearman correlations were calculated between the two risk groups with different immune checkpoint expression levels in the TCGA cancer cohort. Higher tumor immune dysfunction and exclusion (TIDE) scores are widely known to be related to inferior immune checkpoint blockade therapy and shorter survival. TIDE scores can assist clinicians in selecting patients who will benefit from immune checkpoint therapy [11].Therefore, we utilized the TIDE database to estimate TIDE scores for HCC patients in TCGA. Finally, we confirmed the predictive efficacy of this model in immunotherapy cohorts IMVIGOR210.
3. Results
3.1. Single-cell analysis and cell-cell interaction analysis
Firstly, we filtered the single-cell data by requiring each gene to be expressed in at least three cells and each cell to express at least 250 genes, ensuring that each cell expressed more than 100 and less than 8000 genes, with each cell having at least 100 unique molecular identifiers (UMIs). This filtering process resulted in 10,293 cells. Fig. 1A shows the correlation between the filtered data, and the PercentageFeatureSet function was used to calculate the proportion of mitochondrial and ribosomal RNA genes. Fig. 1B and C displays the statistics of cells before and after filtering, respectively. Next, log-normalization was performed separately on the data from the two samples. The FindVariableFeatures function was used to identify highly variable genes based on variance stabilizing transformation (VST), followed by scaling of all genes using the ScaleData function. PCA was then performed using the RunPCA function to find anchor points (as shown in Fig. 1D), with dim = 40 selected. The cells were then clustered using the FindNeighbors and FindClusters functions (with a resolution of 0.5), resulting in 14 subgroups. The RunTSNE function was used for t-distributed stochastic neighbor embedding (t-SNE) analysis, and the distribution of cells from different samples was shown in a t-SNE plot. The classical marker analysis was then employed to annotate the 14 subgroups, resulting in nine distinct cell subtypes (Fig. 2A). We identified marker genes for each cell subtype using the FindAllMarkers function, with logfc = 0.5 (fold change) and Minpct = 0.35 (minimum expression proportion of differentially expressed genes), and visualized the top 5 differentially expressed genes in each subtype using bubble plots (Fig. 2B). Additionally, we utilized the "copykat" package to examine copy number variations (CNVs) in the single-cell data, revealing 1149 tumor cells and 9144 normal cells. Finally, we evaluated the ratio of tumor cells to normal cells in each sample, as described in Fig. 2C, which showed that in most OS samples, the percentage of normal cells was significantly higher than that of malignant cells.
Fig. 1.
(A)Correlation among mitochondrial gene expression, expression of all genes, and cell number of filtered samples; (B–C)Statistical analysis of the number of detected genes and mitochondrial content before and after sample filtering (D) Dimensionality reduction by principal component analysis and selection of appropriate inflection points.
Fig. 2.
(A)t-SNE plots depicting the distribution of 14 cell subgroups prior to and following annotation; (B)Bubble plots depicting the expression of the top 5 marker genes in annotated cell subgroups.(C)t-SNE plots of tumor and normal cells in HCC samples, along with the proportion of tumor and normal cells in each HCC sample.
After distinguishing between tumor cells and normal tissue, we utilized ssGSEA to calculate the enrichment scores of HALLMARK and disulfidptosis-related pathways at the single-cell level. Our results demonstrated that the enrichment score of the disulfidptosis-related pathway in tumor cells was significantly higher than in normal cells, suggesting that the tumor might evade disulfidptosis-related processes to protect itself and survive (Fig. 3A and B). Targeted intervention of disulfidptosis may lead to tumor cell death and potentially improve patient prognosis. In addition, we observed higher expression levels of most disulfidptosis-related genes in tumor cells compared to normal cells (Fig. 3C and D).
Fig. 3.
(A)Differences in the activation of biological pathways between tumor and normal cells in HCC samples; (B) Differences in the expression of biological pathways between tumor and normal cells in HCC samples; (C)Enrichment scores of different signaling pathways in normal and malignant cells of each HCC sample; (D)Expression of disulfidptosis-related genes in normal and malignant cells of each HCC sample.
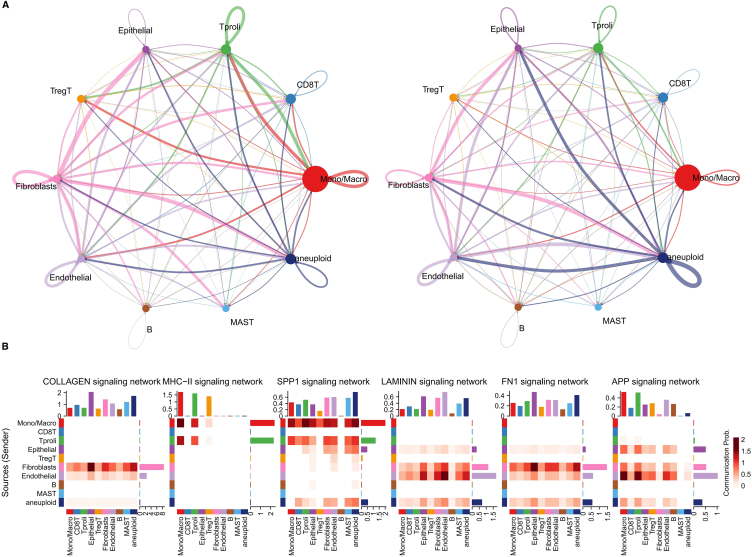
In order to identify key ligands and receptors in tumor cells, we conducted an analysis on two HCC samples using the CellChat package, as demonstrated in Fig. 4A. Our analysis revealed complex interactions between tumor cells and other cell subpopulations. Subsequently, we compared the contributions of key ligand-receptor pathways in tumor cells with those in other cells, and observed that pathways such as COLLAGEN, SPP1, KAMININ, and FN1 were significantly more important in tumor cells (Fig. 4B).
Fig. 4.
(A) Identification of important efferent or afferent signals between different cell subpopulations. (B) Heatmap showing pathway contribution score differences across cell types in the tumor microenvironment of HCC.
3.2. Construction of prognostic signatures
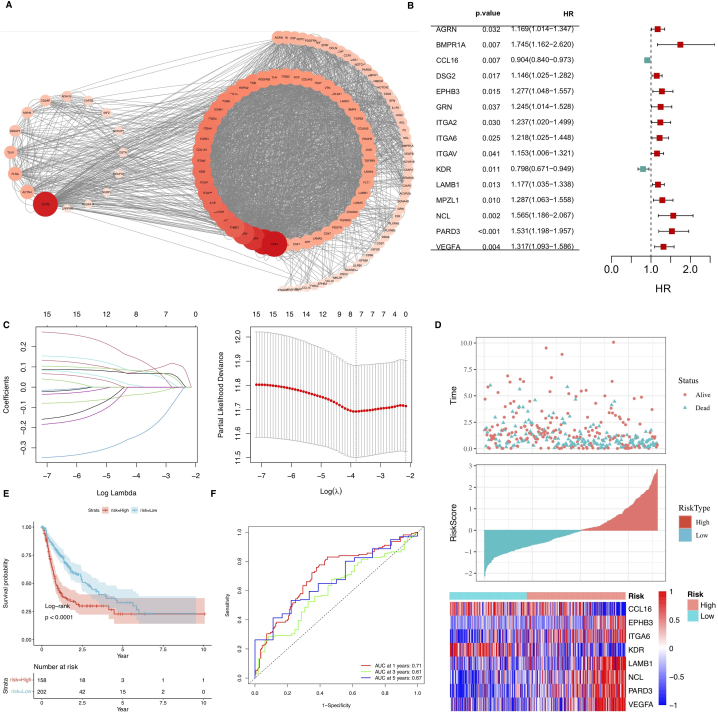
In order to further identify prognostically relevant ligand-receptor pairs, we first screened for key ligands and receptors involved in intercellular communication between tumor cells and various cells in HCC samples. We then identified 110 genes associated with disulfidptosis in tumor cells through correlation analysis (Fig. 5A).
Fig. 5.
(A) The correlation between disulfidptosis-related genes and ligand and receptor genes of tumor cell, Y axes represent time in years.(B)Multivariate Cox regression forest plot.(C) Eight genes were selected using Lasso regression to develop the risk model. (D) Risk maps of the high- and low-risk groups in the TCGA cohort and the expression of the eight model genes in both groups. (E) KM curves of the models in the TCGA cohort. Subjects in the group with high risk had considerably lower survival rates in comparison with the other group (p < 0.01). (F) ROC curves of 1-, 3- and 5-year survival rates in the TCGA cohort.
The univariate Cox regression analysis was carried out on the 110 genes aassociated with disulfidptosis in tumor cells through correlation analysis to find disulfidptosis-related prognostic mRNAs in the TCGA dataset. Finally, 16 prognosis-related mRNAs were identified (Fig. 5B). The expression matrix LASSO-Cox analysis of the 16 mRNAs was performed on the TCGA dataset, and eight genes associated with disulfidptosis were identified(Fig. 5C). Furthermore, the RiskScore was calculated for every patient based on the Cox regression coefficients, and the expression values of individual genes were estimated using the formula given below: RSRiskScore = (−0.049 × CCL16 expression) + (0.032 × EPHB3 expression) + (0.203 × ITGA6 expression) + (−0.306 × KDR expression) + (0.099 × LAMB1 expression) + (0.067 × NCL expression) + (0.055 × PARD3 expression)+ (0.088 × VEGFA expression), and patients were categorized into high- and low-risk subgroups based on median values (Fig. 5D). KM survival curves demonstrated that patients in the high-risk subgroup had inferior clinical outcomes in contrast with those belonging to the other subgroup (Fig. 5E). The ROC curves assessed the predictive ability of the prognostic model for OS, with the area under the curve (AUC) reaching 0.71 at 1 year, 0.61 at 3 year and 0.67 at 5 years (Fig. 5F).
3.3. Clinical correlation of ligand and receptor genes related to disulfidptosis prognostic signatures
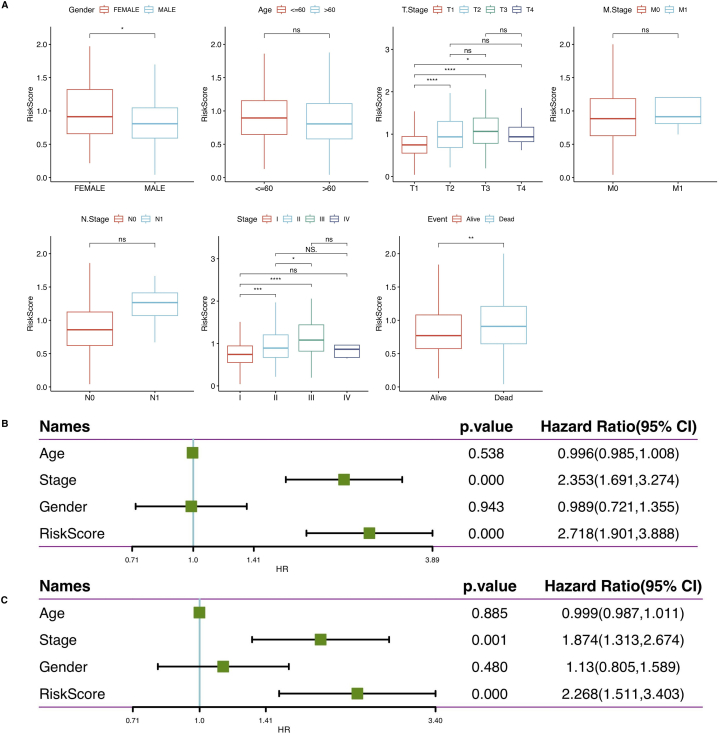
The risk score corresponded to the age, pathological TNM stage, grade, and primary treatment result of people suffering from HCC (Fig. 6A). Femal HCC patients had lower risk scores than male HCC patients. Furthermore, the risk scores for advanced HCC were higher than those for early stages of HCC.
Fig. 6.
(A) The model could distinguish between the two risk groups by T-stage, M-stage, N-stage, staging, age, and final disease event.(B) Using univariate, the independence of clinical application of the 8-gene model was evaluated. (C) Using multivariate, Cox regression analysis of clinical information and risk scores.
3.4. Establishment of prognostic signature nomogram and efficacy assessment
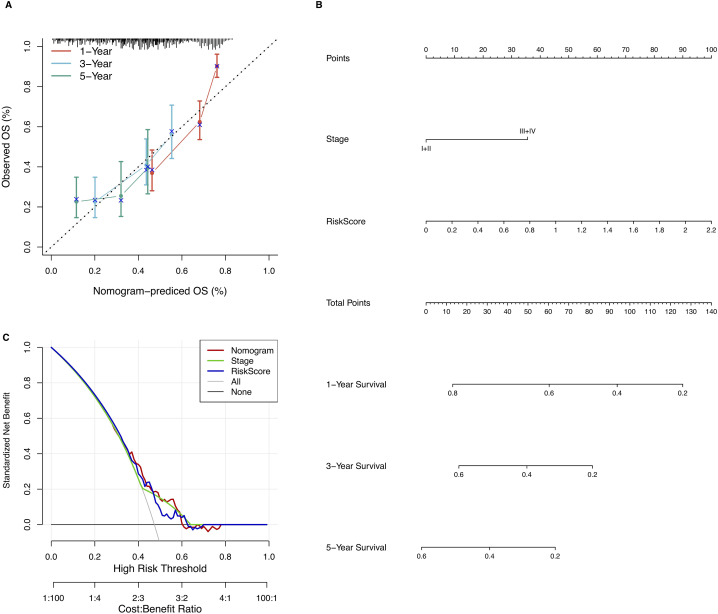
Univariate and multivariate Cox regression analyses were performed in the TCGA cohort to investigate the independent prognostic effect of the model. Univariate Cox regression analysis revealed a substantial correlation among RiskScore, disease stage, and prognosis (Fig. 6B), while the corresponding multivariate Cox regression analysis revealed a substantial correlation between RiskScore, disease stage, and survival (Fig. 6C). Based on the multivariate Cox regression analysis outcomes, disease stage and risk score were included for constructing the nomogram. Furthermore, the R package “rms” was utilized to integrate data on survival time, survival status, and two characteristics to create a nomogram that evaluated the prognostic significance of these characteristics in a sample of 310 (Fig. 7B), and the overall C-index of the model was: 0.70, 95% CI (0.65–0.76), pvalue = 1.43e-11. The calibration curves demonstrated that the actual 1-, 3- and 5-year survival probabilities were consistent with the survival probabilities predicted by the nomogram model (Fig. 7A),. The decision curve analysis revealed that the nomogram model was superior to other factors in predicting prognosis in HCC (Fig. 7C).The above results indicated that the nomogram had the best performance in predicting prognosis, which was helpful for clinical decision-making and personalized treatment.
Fig. 7.
(A) Calibration curves for nomogram; (B) Nomogram based on risk scores and clinical characteristics. (C) Decision curve analysis for nomogram.
3.5. Prognostic signature estimation of tumor immune microenvironment and immunotherapy response
The relationship of RiskScore with immune cell abundance was assessed by employing the CIBERSORT algorithm. Fig. 8A illustrates a positive correlation of RiskScore with infiltration of regulatory T cells, follicular helper T cells (Tfh), and M0 macrophages, whereas a negative correlation of RiskScore with activated mast cells, Eosinophils, activated NK cells, and M1 macrophages. In addition, the RiskScore exhibited a negative link with the matrix score (Fig. 8B). Furthermore, the link between immune checkpoints and this prognostic feature was evaluated. Fig. 8C shows the differential representation of 42 immune checkpoints in both risk subgroups, and that chemotherapy, targeted therapy, and immunotherapy may inhibit tumor growth and improve prognosis in people suffering from HCC. To examine the immune response in people with HCC, the TIDE score was estimated to predict the responsiveness of patients. As illustrated in Fig. 8D, The low-risk group exhibited a low TIDE score, indicating the higher sensitivity of low-risk patients towards immunotherapy。
Fig. 8.
(A) Box plots of the difference in the proportion of tumor-infiltrating immune cells in both groups. (B) Box plots of immune and matrix scores in both groups (higher tumor immunoreactivity in the low-risk group and higher cellular stromal content in the high-risk group); (C) Box plots of the differences in immune check scores between the two groups; (D) Box plots of the differences in TIDE score between the two groups; (*p < 0.05; **p < 0.01; ***p < 0.001).
3.6. Anti-PD-1 therapy response
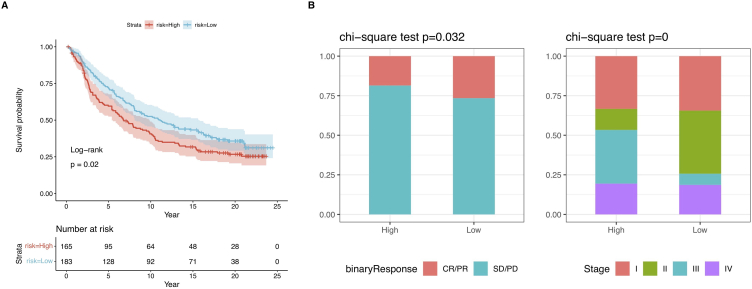
Furthermore, we used the immunotherapy cohort (IMvigor210) to investigate whether prognostic models could predict PD-1 treatment outcomes. The KM survival analysis demonstrated a poor prognosis in patients from the high-risk group (Fig. 9A). The bar chart in Fig. 9B showed a greater proportion of CR/PR patients in the low-risk group in comparison with the other group. Therefore, patients in this group showed an improved response to PD-1 therapy. Remarkably, within the IMvigor210 cohort, patients possessing a low-risk score demonstrated elevated response rates to PD-L1 immune therapy and longer overall survival.Despite our predictive model being built using LIHC data, it performed well across different cancer types. This further demonstrates the stability and reliability of our model, highlighting its broad applicability in various cancer types. Taken together, these data suggest that the risk score model is a robust tool that may aid HCC patients in making treatment decisions. Patients with a low-risk score may potentially benefit more from immune therapy and experience better survival outcomes.
Fig. 9.
(A) KM curves for patients having high and low ICI scores in the IMvigor210 cohort. Log-rank test, p < 0.05. (B) Clinical response rates (complete response [CR]/partial response [PR] and stable disease [SD]/progressive disease [PD]) to anti-PD-L1 immunotherapy in the high or low ICI score groups in the IMvigor210 cohort.
3.7. Validation of the prognostic signature
In the validation datasets GSE116174, GSE14520, and ICGC, we computed the risk values of patients using the same formula as that of the training set, and then categorized them into high- and low-risk groups (Fig. 10A, B, and 10C, respectively). Consistent with the findings of the TCGA cohort, patients with high-risk scores exhibited shorter overall survival (OS) compared to those with low risk in the GEO and ICGC datasets (Fig. 10D, E, and 10F). The prognostic model demonstrated good predictive ability for OS in the GEO and ICGC cohorts, as evidenced by the area under the curve (AUC) values of 0.73 at 1 year, 0.65 at 3 years, and 0.77 at 5 years for the GEO cohort (Fig. 10G and H) and 0.64 at 1 year, 0.69 at 3 years, and 0.70 at 5 years for the ICGC cohort (Fig. 10I).
Fig. 10.
A–C) Risk maps of both risk groups in the GSE cohort and the expression of eight prognosis-related genes in both groups, Y axes represent time in years. (D–F) KM curves of the model in the GSE and ICGC cohort. Patients in the high-risk group had considerably shorter survival time in comparison with the other group (p < 0.05). (G–I) ROC curves of 1-, 3- and 5-year survival in the GSE and ICGC cohortd.
4. Discussion
In our study, after conducting quality control and normalization on the data obtained from single-cell RNA sequencing (scRNA-seq), we observed significantly higher disulfidoptosis scores and upregulation of genes related to disulfidoptosis in aneuploid cells. However, it is important to clarify that this observation does not necessarily imply immediate onset of disulfidoptosis, as additional external conditions, such as glucose starvation, are required to trigger this process. The phenomenon of metabolic reprogramming empowers cancer cells to augment the uptake of crucial nutrients essential for biosynthesis and bioenergetics, such as glucose and amino acids, including glutamine [12]. This enhanced nutrient acquisition is primarily achieved by upregulating transporters responsible for the uptake of glucose and amino acids. Consequently, certain cancer cells may undergo cell death when glucose or amino acids are limited, whereas normal cells can survive under such conditions. Exploiting this nutrient dependency provides promising metabolic vulnerabilities that could be targeted for cancer therapy [13].The work conducted by Gan Boyi et al. suggests that targeting the metabolic vulnerabilities that lead to disulfidoptosis, mediated by high SLC7A11 expression, could be an effective strategy against cancer [14]. Notably, recent research has revealed that glucose transporter (GLUT) inhibitors induce disulfidoptosis more effectively in cancer cells with high SLC7A11 expression compared to those with low SLC7A11 expression [5]. Additionally, the use of KL-11743, a potent pan-inhibitor of GLUT1 and GLUT3, selectively suppressed the growth of lung cancer tumors with high SLC7A11 expression in both cell line xenografts and patient-derived xenografts [15].Moreover, cancers with high SLC7A11 expression tend to rely heavily on glutamine. Furthermore, cancers with KEAP1 mutations exhibit NRF2 hyperactivation and high SLC7A11 expression. Notably, the glutaminase inhibitor CB-839 demonstrated more significant inhibition of tumor growth against KEAP1 mutant cell lines or patient-derived xenografts compared to KEAP1 wild-type counterparts [16]. Additionally, high SLC7A11 expression could serve as a potential biomarker for the selection of cancer patients who might benefit from treatment with glutaminase or GLUT inhibitors. The discovery of disulfidoptosis holds promise for targeting the metabolic vulnerabilities of cancer cells, offering new possibilities for metabolic cancer therapy.
Due to the communication between different cells in the tumor microenvironment primarily occurring through interactions between ligands and receptors in soluble or membrane-bound forms, checkpoint inhibitors based on ligand-receptor interactions have emerged as potent tools for disease treatment. Given their crucial roles in tumor development, we conducted correlation analysis to identify 110 ligand and receptor genes associated with disulfidoptosis. Ultimately, we established a risk signature based on disulfidoptosis-related ligand and receptor genes, comprising eight genes. This signature consists of two protective genes (CCL16 and KDR) and six risk genes (EPHB3, ITGA6, LAMB1, NCL, PARD3, and VEGFA). The chemokine CCL16 promotes immune cell infiltration [17]. EPHB3 activate PI3K/AKT, and RAS/MAPK signaling to drive proliferation and invasion [18,19]. ITGA6 encodes the α6 integrin subunit, forming the laminin receptor with β1. ITGA6 activates Wnt/β-catenin signaling [20]. KDR encodes VEGFR2, an important VEGF receptor that signals via PI3K/AKT [21]. LAMB1 regulates adhesion and metastasis [22]. NCL alters tumor metabolism. PARD3 regulates polarity and invasion [23].VEGFA promotes angiogenesis via receptor signaling [24]. In summary, these genes promote tumor progression through effects on the microenvironment, adhesion, metabolism, proliferation, and angiogenesis.
Immunotherapy has significantly broadened the treatment options for advanced hepatocellular carcinoma (HCC). Immune checkpoint inhibitors (ICIs) play a crucial role in blocking immune checkpoints, restoring T cell function, and alleviating immunosuppression, thereby exerting anti-tumor activity [25]. The MOUSEION-03 study represents the first large-scale and up-to-date meta-analysis of existing randomized trials, systematically investigating the potential for complete response (CR) in patients with solid tumors who received immunotherapy or combination therapy involving immunooncology agents. The analysis encompassed 12,130 potentially relevant trials, among which 85 randomized controlled trials were included. The most common malignancies assessed were non-small cell lung cancer, urothelial carcinoma, renal cell carcinoma, and melanoma.The study revealed that the use of ICIs may significantly increase the likelihood of achieving CR in cancer patients compared to control treatments [26].Notably, antibodies targeting the programmed cell death protein-1 (PD-1)/programmed death ligand-1 (PD-L1) pathway have emerged as a major breakthrough in oncology drug development during the past decade. PD-L1 expression is widely observed on the surface of tumor cells, antigen presenting cells, and other immune cells. However, in HCC, PD-L1 expression is generally low (approximately 10% of tumor cells) and has been associated with cancer recurrence and shorter overall survival (OS) [27].Interestingly, anti-PD-1 monotherapy in first-line (nivolumab vs. sorafenib) or second-line (pembrolizumab vs. placebo) randomized trials for HCC did not demonstrate statistically significant OS benefits, despite initial high expectations. In contrast, combination immune-based therapies have shown more remarkable outcomes. Notably, the phase III IMbrave150 trial evaluated the combination of the antiangiogenic agent bevacizumab and the PD-L1 inhibitor atezolizumab compared to sorafenib monotherapy, successfully establishing a new standard-of-care regimen for patients with advanced disease. Based on the IMbrave150 trial, atezolizumab-bevacizumab demonstrated statistically significant and clinically meaningful benefits across several clinical endpoints, including objective response rate (ORR), progression-free survival (PFS), and OS compared to sorafenib. Updated results from the trial confirmed these advantages, showing a median OS of over 19 months in HCC patients receiving the immunotherapy combination [28,29].
Our data suggest that risk scoring can serve as a predictor of response to PD-L1 immunotherapy, wherein immune evasion by tumor cells emerges as a major resistance mechanism. The tumor microenvironment (TME) plays a critical role, with immune cell infiltration being a key determinant. Low-risk patients demonstrated higher immunological scores, increased immune cell infiltration, and stronger anti-tumor immunity, displaying an immune-activated "hot" phenotype. Conversely, high-risk patients exhibited more immunosuppressive cells, particularly regulatory T cells (Tregs), leading to an immune-suppressed "cold" tumor phenotype. Existing research indicates that Tregs can inhibit the functions of CD4+ and CD8+ effector T cells, NK cells, and antigen-presenting cells through various mechanisms, resulting in ineffective immune responses and poor prognosis.In summary, our findings suggest that the TME and immune landscape, including Treg infiltration, significantly influence the response to PD-L1 immunotherapy. Patients with "hot," T cell-inflamed tumors tend to respond better compared to those with "cold," immunosuppressed tumors. Further endeavors to modify immunosuppressive TMEs may hold promise for improving immunotherapy outcomes.
Remarkably, our investigation unveiled a pronounced abundance of M0 macrophages within the tumor microenvironment of the high-risk group. Tumor-associated macrophages (TAMs) exhibit diverse functional phenotypes, with the extensively studied subsets being M1 macrophages, induced by lipopolysaccharide, and M2 macrophages, stimulated by IL-4. Classically activated M1 macrophages can galvanize anti-tumor immune responses by presenting antigens to adaptive immune cells, generating pro-inflammatory cytokines, and engaging in the phagocytosis of tumor cells [30,31]. Conversely, M2-polarized TAMs can propel HCC progression by orchestrating an upregulation in cytokine secretion and protein expression. Resting macrophages (M0), originating from bone marrow, are conventionally conceived as precursors to polarized macrophages. The prevailing paradigm contends that both M1 and M2 macrophages emanate from M0, constituting the quiescent state of macrophages before polarization, without distinct functions. Notably, while the macrophage polarization model has been widely employed in immunological inquiries across various cancers, the proposition of this mutually exclusive activation theory was rooted in in vitro conditions and has grappled with multiple challenges in explaining the intricate in vivo milieu of tumor immunity [31]. Recent investigations have divulged that macrophages infiltrating glioblastoma tissues linger in an intermediate state reminiscent of both M1 and M2 phenotypes, akin to M0 macrophages [32]. Moreover, mRNA expression profiles of glioblastoma-associated microglia and macrophages have showcased that a significant proportion of the differentially expressed genes in TAMs fail to align with any classical polarized phenotypes [33]. Rigorous scrutiny of HCC data sourced from The Cancer Genome Atlas (TCGA) has lent credence to the correlation between the differentiation of M0-like macrophages and unfavorable prognosis in HCC cases [34]. These cumulative insights collectively suggest that the phenotype of macrophages associated with HCC might markedly diverge from those observed in other solid malignancies, displaying a heightened propensity towards adopting an M0-like phenotype.
In summary, our study demonstrates that disulfidoptosis is closely associated with the immune microenvironment and prognosis in HCC patients. The disulfidoptosis-related gene signature established in this study may serve as a predictor of survival for patients with HCC.
5. Conclusions
Through single-cell RNA sequencing technology, we found significant differences in the expression of genes associated with disulfidptosis between tumor cells and non-tumor cells.We obtained the ligand and receptor genes related to tumor cells through intercellular communication and analyzed their correlation with genes associated with disulfidptosis.Furthermore, we developed prognostic and risk models using machine learning and comprehensively assessed the correlation between different risk models and the infiltration of cells in the tumor microenvironment, as well as the effectiveness of immunotherapy for HCC.Our study uncovered potential mechanisms of intercellular interaction involving disulfidptosis in the tumor microenvironment of HCC, and could lead to the identification of new biomarkers.
Declarations
Author contribution statement
Chong Fu: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Chang Cheng, Yanping Zhang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Scientific Research Foundation of Anqing City {2022Z2001} and Wannan Medical College {JXYY202233}
Data availability statement
Data included in article/supp. material/referenced in article.
Additional information
No additional information is available for this paper.
Ethics approval and consent to participate
Not applicable.
Availability of data and materials
This study analyzed publicly available datasets from the NCBI Gene Expression Omnibus database (GSE116174, GSE14520, GSE166635) and the ICGC.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We gratefully acknowledge Dr.mumdark (Nucleobase translocation in bioinformatics) and all members of his bioinformatics team, biotrainee, for generously sharing their experience and codes.
References
- 1.Rumgay H., Ferlay J., de Martel C., Georges D., Ibrahim A.S., Zheng R., Wei W., Lemmens V.E., Soerjomataram I. Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer. 2022;161:108–118. doi: 10.1016/j.ejca.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Xie D., Shi J., Zhou J., Fan J., Gao Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: a Chinese perspective. Clin. Mol. Hepatol. 2023;29:206. doi: 10.3350/cmh.2022.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai Y., Zeng T., Liang X., Wu W., Zhong F., Wu W. Cell death-related molecules and biomarkers for renal cell carcinoma targeted therapy. Cancer Cell Int. 2019;19:1–15. doi: 10.1186/s12935-019-0939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X., Olszewski K., Zhang Y., Lim E.W., Shi J., Zhang X., Zhang J., Lee H., Koppula P., Lei G. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 2020;22:476–486. doi: 10.1038/s41556-020-0496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573. doi: 10.1016/j.cell.2021.04.048. 3587. e3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Falco A., Caruso F., Su X.-D., Iavarone A., Ceccarelli M. A fast variational algorithm to detect the clonal copy number substructure of tumors from single-cell data. bioRxiv. 2021;11:1–13. doi: 10.1038/s41467-023-36790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin S., Guerrero-Juarez C.F., Zhang L., Chang I., Ramos R., Kuan C.-H., Myung P., Plikus M.V., Nie Q. Inference and analysis of cell-cell communication using cellchat. Nat. Commun. 2021;12:1088. doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iasonos A., Schrag D., Raj G.V., Panageas K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 10.Chen B., Khodadoust M.S., Liu C.L., Newman A.M., Alizadeh A.A. Profiling tumor infiltrating immune cells with cibersort. Cancer Systems Biology: Methods and Protocols. 2018:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang P., Gu S., Pan D., Fu J., Sahu A., Hu X., Li Z., Traugh N., Bu X., Li B. Signatures of t cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 2018;24:1550–1558. doi: 10.1038/s41591-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng P., Zhou C., Ding Y., Duan S. Disulfidptosis: a new target for metabolic cancer therapy. J. Exp. Clin. Cancer Res. 2023;42:103. doi: 10.1186/s13046-023-02675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koppula P., Zhang Y., Zhuang L., Gan B. Amino acid transporter slc7a11/xct at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018;38:1–13. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koppula P., Zhuang L., Gan B. Cystine transporter slc7a11/xct in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein & cell. 2021;12:599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang L., Kon N., Li T., Wang S.-J., Su T., Hibshoosh H., Baer R., Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R., Sayin V.I., Davidson S.M., Bauer M.R., Singh S.X., LeBoeuf S.E., Karakousi T.R., Ellis D.C., Bhutkar A., Sanchez-Rivera F.J. Keap1 loss promotes kras-driven lung cancer and results in dependence on glutaminolysis. Nat. Med. 2017;23:1362–1368. doi: 10.1038/nm.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappello P., Caorsi C., Bosticardo M., De Angelis S., Novelli F., Forni G., Giovarelli M. Ccl16/lec powerfully triggers effector and antigen-presenting functions of macrophages and enhances t cell cytotoxicity. Journal of Leucocyte Biology. 2004;75:135–142. doi: 10.1189/jlb.0403146. [DOI] [PubMed] [Google Scholar]
- 18.Wang A.L., Li Y., Zhao Q., Fan L.Q. Formononetin inhibits colon carcinoma cell growth and invasion by microrna-149-mediated ephb3 downregulation and inhibition of pi3k/akt and stat3 signaling pathways. Mol. Med. Rep. 2018;17:7721–7729. doi: 10.3892/mmr.2018.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao H., Wei B.-R., Peehl D.M., Li Q., Alexandrou T., Schelling J.R., Rhim J.S., Sedor J.R., Burnett E., Wang B. Activation of epha receptor tyrosine kinase inhibits the ras/mapk pathway. Nat. Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- 20.Biswas S., Shahriar S., Giangreco N.P., Arvanitis P., Winkler M., Tatonetti N.P., Brunken W.J., Cutforth T., Agalliu D. Mural wnt/β-catenin signaling regulates lama2 expression to promote neurovascular unit maturation. Development. 2022;149 doi: 10.1242/dev.200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantoni S., Cavallini C., Bianchi F., Bonavita F., Vaccari V., Olivi E., Frascari I., Tassinari R., Valente S., Lionetti V. Rosuvastatin elicits kdr-dependent vasculogenic response of human placental stem cells through pi3k/akt pathway. Pharmacol. Res. 2012;65:275–284. doi: 10.1016/j.phrs.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Lin Q., Lim H.S., Lin H.L., Tan H.T., Lim T.K., Cheong W.K., Cheah P.Y., Tang C.L., Chow P.K., Chung M.C. Analysis of colorectal cancer glyco‐secretome identifies laminin β‐1 (lamb1) as a potential serological biomarker for colorectal cancer. Proteomics. 2015;15:3905–3920. doi: 10.1002/pmic.201500236. [DOI] [PubMed] [Google Scholar]
- 23.Wang L., Zhang H., Hasim A., Tuerhong A., Hou Z., Abdurahmam A., Sheyhidin I. Partition-defective 3 (pard3) regulates proliferation, apoptosis, migration, and invasion in esophageal squamous cell carcinoma cells. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res.: International Medical Journal of Experimental and Clinical Research. 2017;23:2382. doi: 10.12659/MSM.903380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claesson‐Welsh L., Welsh M. Vegfa and tumour angiogenesis. Journal of internal medicine. 2013;273:114–127. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo A., Cusmai A., Gadaleta-Caldarola G., Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expet Rev. Gastroenterol. Hepatol. 2022;16:333–339. doi: 10.1080/17474124.2022.2064273. [DOI] [PubMed] [Google Scholar]
- 26.Santoni M., Rizzo A., Kucharz J., Mollica V., Rosellini M., Marchetti A., Tassinari E., Monteiro F.S.M., Soares A., Molina-Cerrillo J. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the mouseion-03 meta-analysis, Cancer Immunology. Immunotherapy. 2023:1–15. doi: 10.1007/s00262-022-03349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizzo A., Ricci A.D., Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Medicine. 2020;16:2587–2589. doi: 10.2217/fon-2020-0669. [DOI] [PubMed] [Google Scholar]
- 28.Cheng A.-L., Qin S., Ikeda M., Galle P., Ducreux M., Zhu A., Kim T.-Y., Kudo M., Breder V., Merle P. Imbrave150: efficacy and safety results from a ph iii study evaluating atezolizumab (atezo)+ bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (hcc) Ann. Oncol. 2019;30:ix186–ix187. [Google Scholar]
- 29.Di Federico A., Rizzo A., Carloni R., De Giglio A., Bruno R., Ricci D., Brandi G. Atezolizumab-bevacizumab plus y-90 tare for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expet Opin. Invest. Drugs. 2022;31:361–369. doi: 10.1080/13543784.2022.2009455. [DOI] [PubMed] [Google Scholar]
- 30.Newman A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., Hoang C.D., Diehn M., Alizadeh A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aran D., Hu Z., Butte A.J. Xcell: digitally portraying the tissue cellular heterogeneity landscape. Genome biology. 2017;18:1–14. doi: 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabrusiewicz K., Rodriguez B., Wei J., Hashimoto Y., Healy L.M., Maiti S.N., Thomas G., Zhou S., Wang Q., Elakkad A. Glioblastoma-infiltrated innate immune cells resemble m0 macrophage phenotype. JCI insight. 2016;1 doi: 10.1172/jci.insight.85841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szulzewsky F., Pelz A., Feng X., Synowitz M., Markovic D., Langmann T., Holtman I.R., Wang X., Eggen B.J., Boddeke H.W. Glioma-associated microglia/macrophages display an expression profile different from m1 and m2 polarization and highly express gpnmb and spp1. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Zou J., Chen R. An m0 macrophage-related prognostic model for hepatocellular carcinoma. BMC Cancer. 2022;22:791. doi: 10.1186/s12885-022-09872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.
Additional information
No additional information is available for this paper.
This study analyzed publicly available datasets from the NCBI Gene Expression Omnibus database (GSE116174, GSE14520, GSE166635) and the ICGC.