Abstract
BACKGROUND
Polycystic ovarian syndrome and endometriosis are 2 of the most common reproductive disorders among women but are thought to be unrelated.
OBJECTIVE
This study aimed to examine the overlap and common symptoms of polycystic ovarian syndrome and endometriosis.
STUDY DESIGN
The study population included the Endometriosis, Natural History, Diagnosis, and Outcomes Study (2007–2009) operative cohort: 473 women, aged 18 to 44 years, who underwent a diagnostic and/or therapeutic laparoscopy or laparotomy at 1 of 14 surgical centers located in Salt Lake City, Utah, or San Francisco, California, in addition to a population cohort composed of 127 women from the surgical centers’ catchment areas. Age and site-adjusted multinomial regression models were used to estimate adjusted prevalence ratios and 95% confidence intervals of reproductive history characteristics among women with endometriosis only, women with polycystic ovarian syndrome only, and women with both endometriosis and polycystic ovarian syndrome.
RESULTS
Among the operative cohort, 35% had endometriosis only, 9% had polycystic ovarian syndrome only, and 5% had endometriosis and polycystic ovarian syndrome. Among the population cohort, 10% had endometriosis only, 8% had polycystic ovarian syndrome only, and 2% had endometriosis and polycystic ovarian syndrome. In the operative cohort, a history of subfertility was associated with a higher adjusted probability of having both conditions (adjusted prevalence ratio, 10.33; 95% confidence interval, 3.94–27.08), followed by having endometriosis only (adjusted prevalence ratio, 2.45; 95% confidence interval, 1.56–3.84) or polycystic ovarian syndrome only (adjusted prevalence ratio, 1.15; 95% confidence interval, 0.51–2.61), than having neither condition. In addition, experiencing chronic pelvic pain within the past 12 months was associated with a higher probability of having both conditions (adjusted prevalence ratio, 2.53; 95% confidence interval, 1.07–6.00) than having neither condition.
CONCLUSION
Among a cohort of women undergoing gynecologic laparoscopy or laparotomy, our study found that nearly 1 in 20 women had both an incident endometriosis diagnosis and symptoms consistent with polycystic ovarian syndrome. Among a population cohort of women not seeking gynecologic care, polycystic ovarian syndrome and endometriosis overlap prevalence was approximately 1 in 50 women.
Key words: endometriosis, epidemiology, pelvic pain, polycystic ovarian syndrome, subfertility
AJOG MFM at a Glance.
Why was this study conducted?
Understanding the co-occurrence of endometriosis and polycystic ovarian syndrome (PCOS) and shared symptoms in a well-characterized population may help elucidate the etiology of these gynecologic disorders.
Key findings
In this cohort of women without a previous endometriosis diagnosis undergoing laparoscopy or laparotomy for various indications, the prevalence rates of incident endometriosis + PCOS were 5% in the operative cohort and 2% in the population cohort, with subfertility history having a 10-fold higher prevalence in women with endometriosis + PCOS than in women with neither condition.
What does this add to what is known?
Our study informs the clinical enigma of sustained subfertility among women with PCOS once ovulatory status has been restored; the coexistence of endometriosis may be a common contributing factor.
Introduction
Endometriosis, defined classically as the presence of endometrial glands and stroma outside the endometrium, including, but not limited to, the pelvic peritoneum, ovaries, and rectovaginal septum,1 affects up to 11% of women in population-based samples.2 Endometriosis is a common cause of subfertility, with an estimated 30% to 50% of women with endometriosis being diagnosed with subfertility,3 and has been linked to several chronic diseases, including cardiovascular disease.4 Endometriosis requires laparoscopic surgery or a laparotomy for a formal diagnosis to be made.5
Polycystic ovarian syndrome (PCOS) is another common gynecologic disorder, estimated to affect 6% to 15% of community-based samples.6, 7, 8 PCOS, similar to endometriosis, is one of the leading causes of female subfertility9 and increases the likelihood of other serious health problems in the life span, including type 2 diabetes mellitus and cardiovascular disease.10, 11, 12, 13 There are 3 different diagnostic criteria that are commonly used for the diagnosis of PCOS, including the Rotterdam criteria, the National Institutes of Health criteria, and the Androgen Excess and PCOS Society (AE-PCOS) criteria.14 As per the AE-PCOS criteria, there is no single test to diagnose PCOS. Preferably, after other conditions are ruled out, a woman who has at least 2 of the following criteria may be diagnosed with PCOS: (1) infrequent or prolonged periods; (2) higher than normal blood levels of androgens; (3) excess facial and body hair (hirsutism), acne, or thinning of scalp hair; and (4) many small cysts (follicles) on 1 or both ovaries.15
Because of contributing factors that affect both conditions, including alterations in sex steroid synthesis, inflammation, and/or toxic exposures, women with endometriosis might exhibit an increased risk of PCOS.16,17 A study evaluating the hormonal and metabolic profiles of women with PCOS found that a higher level of estrogen relative to progesterone rather than progesterone in itself is associated with failure of follicular maturation that presents as irregular menstrual cycles and subfertility.18 Moreover, both disorders are thought to involve alterations to prenatal testosterone levels and atypical functioning of the hypothalamic-pituitary-gonadal (HPG) axis19; however, whether the alterations are in opposite directions resulting in diametric and distinct diseases is under debate. Despite shared risk factors, chronic disease outcomes, and pathophysiology between endometriosis and PCOS, there is limited research on the overlapping prevalence of both conditions. To date, research has sampled relatively small, clinical populations among mostly women with infertility.20, 21, 22, 23, 24, 25 An accurate understanding of overlapping prevalence between the 2 conditions will help inform gynecological health researchers as to whether there may be shared etiology and, if so, exposures that could contribute to the pathophysiology of both and may help guide clinicians as to the level of evaluation that is clinically relevant for a potential additional disorder in a patient that is known to have 1 of 2 conditions.
Here, we set out to examine the overlap between PCOS and endometriosis among the operative and population cohort of women who participated in the Endometriosis, Natural History, Diagnosis, and Outcomes (ENDO) Study. In addition, we assessed symptoms for women with both endometriosis and PCOS and compared them to women who have only 1 of 2 conditions or neither of the conditions.
Materials and Methods
Study population
This was a secondary data analysis of the ENDO Study, whose original purpose was (1) to estimate the scope and magnitude of endometriosis at both the clinical and population level by diagnostic method and choice of comparison group and (2) to assess the relation of endocrine disrupting chemicals and risk of gynecologic pathology, including endometriosis.2 Women who were currently menstruating, aged 18 to 44 years, and scheduled to undergo a diagnostic and/or therapeutic laparoscopy or laparotomy regardless of clinical indication at 1 of 14 participating clinical sites in Utah and California (2007–2009) were eligible for the operative cohort study enrollment.2 A population cohort, matched to the operative cohort by age and residence within the geographic catchment areas for the participating surgical centers, that is, approximately a 50-mile radius that captured approximately 90% of pelvic surgeries based on residential zip codes at the time of surgery. For both cohorts, women with a history of surgically confirmed endometriosis were excluded, as were women who breastfed within the past 6 months, women who had injectable hormonal treatment within the past 2 years, or women who had a history of cancer. Human subjects approval was obtained by all participating research institutions, and all women provided informed consent before any data collection.2
Data collection
Women completed a baseline interview before surgery via computer-assisted personal interviews, capturing sociodemographic, lifestyle, and psychosocial factors along with health and detailed reproductive history.2
Endometriosis diagnosis
Operative cohort
Surgeons completed a standardized operative report immediately after laparoscopy or laparotomy to record postoperative diagnoses and to complete the Revised American Society for Reproductive Medicine (rASRM) classification5 staging of endometriosis. In addition to endometriosis, surgeons also recorded on the operative report any additional gynecologic pathology, including uterine fibroids, pelvic adhesions, benign ovarian cysts, neoplasms, and/or congenital Müllerian anomalies.2 Our previous work showed high agreement for endometriosis diagnosis among 8 expert surgeons viewing operative digital images (κ=0.69) or digital images in addition to operative reports (κ=0.88).26,27
Population cohort
Participation required a willingness to undergo a pelvic magnetic resonance imaging to identify endometriosis. All images were double read, first by the initial and subsequently by a second radiologist.2
Polycystic ovarian syndrome
During the baseline personal interview, women were asked whether they had been diagnosed by a doctor with “polycystic ovarian syndrome.” If they checked “yes,” the women were asked at what age were they diagnosed. In addition, women answered detailed questions, informed by diagrams, regarding several PCOS features. Specifically, women were asked to note the natural distribution of hair on their upper lip, chin, chest, and pubis, reported as categories 0 to 4, with illustration (ie, the Ferriman-Gallwey scoring system). Moreover, women were asked to report the number of menstrual cycles that they had in the previous year and whether they had fewer periods as a result of any medication that they were taking. For our study, in which we were not able to assess biochemical hyperandrogenism via blood draws or polycystic ovaries via ultrasounds, we relied on clinical measures. Here, the women were considered to have PCOS if they reported being diagnosed with PCOS by a doctor or reported having ≤9 cycles per year (not because of medication) and scoring ≥5 on the modified Ferriman-Gallwey scoring system for hirsutism.28
Symptoms
To identify subfertility, women were asked whether they had either ever tried for >6 months to get pregnant or had used fertility treatment to get pregnant. Women who reported trying for >6 months to get pregnant were also asked to report the number of months they had attempted to get pregnant. Here, the women who tried longer than 12 months if <35 years old or longer than 6 months if >35 years old were considered infertile. To assess pelvic pain, the women reported whether they experienced pain lasting >6 months that was either cyclic or chronic.29 Women were queried about lifetime and current acne that was medically diagnosed, including age at onset, severity, and location. Finally, in addition to reporting the number of menstrual cycles that they had in the previous year, women also reported the number of days between their periods, the length of their shortest periods, and the length of their longest periods.
Statistical analysis
We assessed the distribution of endometriosis and PCOS as either singularly or co-occurring gynecologic diseases in both the operative and population cohorts using proportional Venn diagrams30 and descriptive statistics to report differences in women's characteristics by diagnosis (counts were too small to assess differences in the population cohort). We used multinomial regression models21 to estimate adjusted prevalence ratios (aPRs) and 95% confidence intervals (CIs) for sociodemographic, lifestyle, and reproductive factors among women with endometriosis only, PCOS only, both endometriosis and PCOS, and neither condition, with neither condition being our reference.31 We performed a sensitivity analysis in which we restricted our reference category to women who had neither endometriosis, PCOS, nor any other gynecologic pathology. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC).
Results
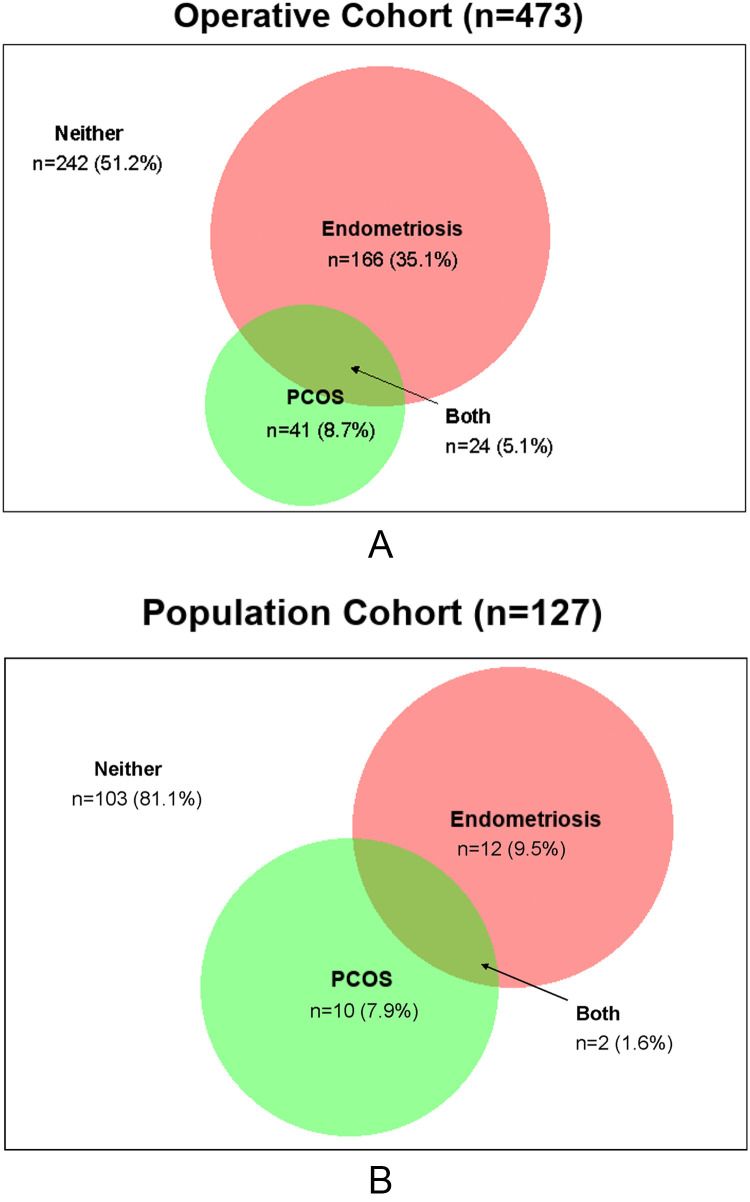
Among the operative cohort, 35% had endometriosis only, 9% had PCOS only, and 5% had endometriosis and PCOS (Figure, A). Among the population cohort, 10% had endometriosis only, 8% had PCOS only, and 2% had endometriosis and PCOS (Figure, B).
Figure.
Venn diagram illustrating prevalence of endometriosis and/or PCOS
A, The ENDO operative cohort. B, The ENDO population cohort.
ENDO, endometriosis, natural history, diagnosis, and outcomes; PCOS, polycystic ovarian syndrome.
Schliep. Endometriosis and polycystic ovarian syndrome overlap. Am J Obstet Gynecol Glob Rep 2023.
In the operative cohort, women who had both endometriosis and PCOS were more likely to be White or non-Hispanic White, be above the poverty level, and be college educated and less likely to be a current smoker, be an alcohol consumer, and have moderate to high physical activity levels than women who have neither condition (Table 1). Moreover, they were more likely to have previously used oral contraception, report subfertility, be nulligravid or nulliparous, have ≤9 periods over the past year, and have experienced chronic pelvic pain and/or cyclic pain (Table 2).
Table 1.
Characteristics of women in the operative cohort of the Endometriosis, Natural History, Diagnosis, and Outcomes Study (2007–2009) (N=473)
| Characteristic | Total (N=473 [100%]) | Endometriosis (n=166 [35%]) | PCOS (n=41 [9%]) | Endometriosis + PCOS (n=24 [5%]) | Neither (n=242 [51%]) | P value |
|---|---|---|---|---|---|---|
| Sociodemographic or lifestyle | ||||||
| Age (y), mean±SD | 33.0±7.0 | 32.1±6.9 | 31.5±6.9 | 31.3±6.0 | 34.0±7.1 | .01 |
| Study site | .15 | |||||
| Utah | 412 (87) | 151 (91) | 35 (85) | 22 (92) | 204 (84) | |
| California | 61 (13) | 15 (9) | 6 (15) | 2 (8) | 38 (16) | |
| Race and ethnicity | .63 | |||||
| Non-Hispanic or White | 354 (75) | 123 (74) | 32 (78) | 19 (79) | 180 (74) | |
| Hispanic White | 63 (13) | 23 (14) | 7 (17) | 1 (4) | 32 (13) | |
| Other | 56 (12) | 20 (12) | 2 (5) | 4 (17) | 30 (12) | |
| BMI (kg/m2), mean±SD | 28.0±8.0 | 26.0±7.1 | 31.9±9.2 | 28.7±7.8 | 28.8±8.2 | <.001 |
| Percentage above poverty level | 412 (88) | 147 (90) | 32 (78) | 23 (100) | 210 (88) | .06 |
| College graduate or higher | 189 (40) | 70 (42) | 14 (34) | 13 (54) | 92 (38) | .35 |
| Current smoker | 67 (14) | 19 (12) | 8 (20) | 1 (4) | 39 (16) | .19 |
| Current alcohol consumer | 188 (41) | 60 (37) | 16 (39) | 8 (33) | 104 (45) | .36 |
| Physical activity level | .61 | |||||
| Low | 75 (18) | 23 (16) | 6 (16) | 3 (13) | 43 (20) | |
| Moderate | 152 (36) | 53 (37) | 12 (32) | 12 (52) | 75 (35) | |
| High | 191 (46) | 69 (48) | 20 (53) | 6 (35) | 94 (44) | |
| Reproductive history | ||||||
| Ever sexually active | 407 (86) | 140 (84) | 33 (80) | 23 (96) | 211 (88) | .25 |
| Age of intercourse (y), mean±SD | 18.7±4.0 | 19.1±4.4 | 19.0±4.4 | 19.7±3.2 | 18.2±3.7 | .12 |
| Ever oral contraceptives | 403 (85) | 145 (87) | 33 (80) | 23 (96) | 202 (83) | .26 |
| History of STIs | 95 (20) | 23 (14) | 10 (24) | 7 (29) | 55 (23) | .08 |
| Subfertility (tried >12 mo) | 140 (30) | 64 (39) | 9 (22) | 17 (71) | 50 (21) | <.001 |
| Infertility treatment | 87 (18) | 38 (23) | 4 (10) | 14 (58) | 32 (13) | <.001 |
| Parity | .005 | |||||
| Never pregnant | 155 (33) | 71 (43) | 12 (29) | 10 (42) | 62 (26) | |
| Pregnant but no live birth | 46 (10) | 17 (10) | 5 (12) | 4 (17) | 20 (8) | |
| ≥1 live birth | 269 (57) | 77 (47) | 24 (59) | 10 (42) | 158 (66) |
Data are presented as number (percentage), unless otherwise indicated. To assess the differences among diagnosis of endometriosis, PCOS, endometriosis + PCOS, and neither, the analysis of variance or Kruskal-Wallis test was used for continuous variables, and the chi-square or Fisher exact test was used for categorical variables. There are missing data for age (n=0), race and ethnicity (n=0), BMI (n=5), poverty level (n=7), education (n=3), smoking (n=2), alcohol (n=13), and physical activity (n=55).
BMI, body mass index; PCOS, polycystic ovarian syndrome; SD, standard deviation; STI, sexually transmitted infection.
Schliep. Endometriosis and polycystic ovarian syndrome overlap. Am J Obstet Gynecol Glob Rep 2023.
Table 2.
Characteristics of menarche and menstruation history of the Endometriosis, Natural History, Diagnosis, and Outcomes Study (2007–2009) (N=473)
| Characteristic | Total (n=473 [100.0%]) | Endometriosis (n=166 [35.0%]) | PCOS (n=41 [9.0%]) | Endometriosis + PCOS (n=24 [5.0%]) | Neither (n=242 [51.0%]) | P value |
|---|---|---|---|---|---|---|
| Age at menarche (y) | .33 | |||||
| ≤11 | 90 (19.1) | 32 (19.3) | 4 (9.8) | 8 (33.3) | 46 (19.1) | |
| 12–13 | 236 (50.0) | 80 (48.2) | 24 (58.5) | 8 (33.3) | 124 (51.4) | |
| ≥14 | 146 (30.9) | 54 (33.5) | 13 (31.7) | 8 (33.3) | 71 (29.5) | |
| No. of menstrual cyclesa | <.001 | |||||
| None | 18 (3.8) | 3 (1.8) | 2 (4.9) | 3 (8.3) | 11 (4.6) | |
| 1–3 | 25 (5.3) | 2 (1.2) | 7 (17.1) | 3 (12.5) | 13 (5.4) | |
| 4–6 | 31 (6.6) | 6 (3.6) | 13 (31.7) | 2 (8.3) | 10 (4.2) | |
| 7–9 | 37 (7.9) | 14 (8.5) | 8 (19.5) | 6 (25) | 9 (3.8) | |
| 10–12 | 286 (60.8) | 118 (71.5) | 9 (22.0) | 10 (41.7) | 149 (62.1) | |
| ≥13 | 73 (15.5) | 22 (12.3) | 2 (4.9) | 1 (4.2) | 48 (20) | |
| Average cycle lengtha | <.001 | |||||
| <22 | 89 (19.4) | 23 (14.1) | 6 (15.4) | 4 (18.2) | 56 (23.7) | |
| 22–24 | 15 (3.3) | 5 (3.1) | 0 (0) | 0 (0) | 10 (4.2) | |
| 25–27 | 49 (10.6) | 20 (12.3) | 1 (2.6) | 2 (9.1) | 26 (11.0) | |
| 28–30 | 244 (53.0) | 96 (58.9) | 17 (43.6) | 9 (40.9) | 122 (51.7) | |
| 31–33 | 22 (4.8) | 11 (6.8) | 3 (7.7) | 1 (4.6) | 7 (3.0) | |
| ≥34 | 40 (8.9) | 8 (4.9) | 12 (30.8) | 6 (27.3) | 15 (6.4) | |
| Mean±SD | 29.5±25.2 | 27.1±5.9 | 48.7±64.9 | 32.1±21.7 | 27.7±20.6 | <.001 |
| Length of shortest cycle (d)a | 19.5±23.7 | 17.8±10.3 | 36.4±67.8 | 18.0±13.9 | 17.9±14.3 | <0.001 |
| Length of longest cycle (d)a | 31.2±33.6 | 25.5±15.4 | 58.8±73.7 | 50.0±46.7 | 28.8±27.9 | <.001 |
| Periods in the past 12 mo typical of the last 5 y | ||||||
| Yes | 178 (37.9) | 72 (43.4) | 13 (32.5) | 11 (47.8) | 82 (34.2) | .17 |
| No, specify | ||||||
| More frequent | 83 (28.5) | 26 (27.7) | 6 (22.2) | 3 (25.0) | 48 (30.4) | .83 |
| Less frequent | 49 (16.8) | 13 (13.8) | 10 (37.0) | 4 (33.3) | 22 (13.9) | .008 |
| Heavier bleeding | 174 (59.8) | 63 (67.0) | 7 (25.9) | 9 (75.0) | 95 (60.1) | .001 |
| Lighter bleeding | 77 (26.5) | 21 (22.3) | 12 (44.4) | 3 (25.0) | 41 (26.0) | .15 |
| Bleeding more days | 143 (49.1) | 50 (53.2) | 8 (29.6) | 6 (50.0) | 79 (50.0) | .19 |
| Bleeding fewer days | 56 (19.2) | 18 (19.2) | 7 (25.9) | 2 (16.7) | 29 (18.4) | .82 |
| Pelvic pain of >6 mo affecting normal function | .06 | |||||
| No | 290 (61.4) | 96 (57.8) | 24 (58.5) | 10 (41.7) | 160 (66.4) | |
| Yes | 182 (38.6) | 70 (42.2) | 17 (41.5) | 14 (58.3) | 81 (33.6) | |
| Painful menstrual cramps of >6 mo | <.001 | |||||
| No | 287 (60.9) | 81 (48.8) | 32 (78.0) | 15 (62.5) | 159 (66.3) | |
| Yes, specify duration (mo) | ||||||
| <6 | 15 (5.9) | 8 (7.6) | 1 (5.3) | 1 (6.7) | 5 (4.4) | |
| 6–12 | 55 (21.6) | 21 (19.8) | 8 (42.1) | 3 (20.0) | 23 (20.2) | |
| 13–24 | 46 (18.1) | 24 (22.6) | 1 (5.3) | 2 (13.3) | 19 (16.7) | |
| >24 | 138 (54.3) | 53 (50.0) | 9 (47.4) | 9 (60.0) | 67 (58.8) | |
Data are presented as number (percentage), unless otherwise indicated. To assess differences among the diagnosis of endometriosis, PCOS, endometriosis + PCOS, and neither, the analysis of variance or Kruskal-Wallis test was used for continuous variables, and the chi-square or Fisher exact test was used for categorical variables.
PCOS, polycystic ovarian syndrome; SD, standard deviation.
aLast 12 months.
Schliep. Endometriosis and polycystic ovarian syndrome overlap. Am J Obstet Gynecol Glob Rep 2023.
pt?>After adjusting for age at enrollment and study site (Utah vs California), no clear association was found between having endometriosis and/or PCOS, compared with neither condition, and sociodemographic factors except body mass index (BMI; kg/m2), which was lower in women with endometriosis (aPR, 0.95; 95% CI, 0.92–0.98), higher in women with PCOS (aPR, 1.04; 95% CI, 1.01–1.08), and null in women with both conditions (aPR, 1.00; 95% CI, 0.95–1.00), compared with women with neither condition (Table 3). A history of subfertility (women who tried longer than 12 months if <35 years old or longer than 6 months if >35 old were considered infertile) was associated with a higher adjusted probability of having both conditions (aPR, 10.33; 95% CI, 3.94–27.08), followed by having endometriosis only (aPR, 2.45; 95% CI, 1.56–3.84) or PCOS only (aPR, 1.15; 95% CI, 0.51–2.61), than having neither condition (Table 3). Experiencing chronic pelvic pain within the past year was associated with a higher probability of having both conditions (aPR, 2.53; 95% CI, 1.07–6.00), followed by having endometriosis (aPR, 1.34; 95% CI, 0.89–2.03) or PCOS (aPR 1.31, 95% CI, 0.66–2.60) than having neither condition. In our sensitivity analyses, we found that estimates were similar in magnitude and precision when women without any noted gynecologic disorders on their operative report were considered the reference group (Supplemental Table).
Table 3.
PRs of the characteristics and menstruation history of the Endometriosis, Natural History, Diagnosis, and Outcomes Study, adjusted for age at enrollment and study site (Utah or California) (2007–2009) (N=473; the referent group is neither disease)
| Characteristic | Endometriosis | PCOS | Endometriosis + PCOS | Neither |
|---|---|---|---|---|
| aPR (95% CI) | ||||
| Sociodemographics or lifestyle | ||||
| Percentage above poverty level (yes or no) | 1.43 (0.74–2.76) | 0.56 (0.24–1.35) | NA | 1.00 |
| College educated (yes or no) | 1.35 (0.89–2.06) | 0.88 (0.43–1.81) | 2.27 (0.96–5.42) | 1.00 |
| White or non-Hispanic White | 0.95 (0.53–1.72) | 0.81 (0.32–2.02) | 3.71 (0.48–28.84) | 1.00 |
| Current smoker | 0.62 (0.34–1.12) | 1.17 (0.50–2.74) | 0.20 (0.03–1.55) | 1.00 |
| Body mass index (kg/m2) | 0.95 (0.92–0.98) | 1.04 (1.01–1.08) | 1.00 (0.95–1.06) | 1.00 |
| Reproductive history | ||||
| Gravid (vs nulligravid) | 0.96 (0.44–2.08) | 1.80 (0.53–6.09) | 1.86 (0.48–7.13) | 1.00 |
| Parous (vs nulligravid) | 0.43 (0.26–0.69) | 1.05 (0.46–2.40) | 0.43 (0.16–1.19) | 1.00 |
| Subfertility (yes or no) | 2.45 (1.56–3.84) | 1.15 (0.51–2.61) | 10.33 (3.94–27.08) | 1.00 |
| Subfertility treatment | 1.97 (1.16–3.34) | 0.74 (0.25–2.24) | 9.53 (3.84–23.64) | 1.00 |
| Age at first consenting sex (y) | 1.06 (1.01–1.12) | 1.06 (0.96–1.16) | 1.11 (1.00–1.22) | 1.00 |
| Age at menarche (y) | 1.05 (0.94–1.19) | 1.04 (0.85–1.26) | 0.98 (0.76–1.26) | 1.00 |
| Mean no. of periods | 0.99 (0.96–1.02) | 0.80 (0.75–0.86) | 0.85 (0.78–0.93) | 1.00 |
| Mean cycle length (d) | 1.00 (0.98–1.01) | 1.02 (1.01–1.03) | 1.01 (0.99–1.03) | 1.00 |
| Mean shortest cycle (d) | 1.00 (0.99–1.02) | 1.02 (1.00–1.04) | 1.00 (0.97–1.03) | 1.00 |
| Mean longest cycle (d) | 0.99 (0.98–1.00) | 1.02 (1.01–1.03) | 1.01 (1.00–1.02) | 1.00 |
| Chronic cyclic pain (yes or no) | 1.94 (1.29–2.93) | 0.51 (0.23–1.13) | 1.07 (0.45–2.58) | 1.00 |
| Chronic pelvic pain (yes or no) | 1.34 (0.89–2.03) | 1.31 (0.66–2.60) | 2.53 (1.07–6.00) | 1.00 |
aPR, adjusted prevalence ratio; CI, confidence interval; NA, not available; PCOS, polycystic ovarian syndrome; PR, prevalence ratio.
Schliep. Endometriosis and polycystic ovarian syndrome overlap. Am J Obstet Gynecol Glob Rep 2023.
Discussion
Principal findings of the study
Among a cohort of women undergoing diagnostic and/or therapeutic laparoscopy or laparotomy, regardless of clinical indication, our findings suggested that endometriosis may co-occur with PCOS in 1 in 20 women with newly diagnosed endometriosis. Among a population cohort of women not seeking gynecologic care, PCOS and endometriosis overlap prevalence was 1 in 50 women. Our findings showed that the co-occurrence of endometriosis and PCOS, within the operative cohort, was associated with a 10-fold higher probability of subfertility, followed by a 3-fold higher probability among women with endometriosis and a 1.2-fold higher probability among women with PCOS only, than having neither condition.
Results in the context of what is known
Prevalence of co-occurrence of endometriosis and polycystic ovarian syndrome
Few studies have assessed the prevalence of endometriosis with PCOS,20, 21, 22, 23, 24, 25,32, 33, 34 especially in cohorts of women with surgically visualized disease. Our finding of 5% of women with both conditions in the operative cohort is in line with the 7% to 8% co-occurrence found in previous studies among asymptomatic women with minimal to mild endometriosis,32,33 which made up 71% of endometriosis staging in the ENDO operative cohort. Higher rates of endometriosis among women with PCOS (up to 70%) have been reported among women hospitalized for PCOS34 or with self-reported infertility and/or pelvic pain,21 mirroring our results showing a 10-fold higher probability of subfertility and a 2.5-fold higher probability of chronic pelvic pain in women with both conditions than with neither condition. Our overlapping prevalence is lower than most studies conducted in the 1990s or previously.20,22, 23, 24 Specifically, in a 1994 report of 192 women undergoing laparoscopies over a 2-year period (mean age, 30.9 years), 10% had both PCOS (as per abnormal sex steroid profile and polycystic ovaries) and visualized endometriosis.20 Our study is unique in capturing overlapping prevalence in a population-based sample of women not seeking gynecologic care. Our 2% estimate should be corroborated in future research before definitive conclusions are made.
Although study comparisons are difficult because of various criteria used to diagnose PCOS, our 2% to 5% overlap prevalence of endometriosis and PCOS, which is the lowest reported to date, is biologically what may be expected. Backed by experimental and epidemiologic evidence,19,35 an emerging theory, known as the diametric disorder hypothesis for endometriosis and PCOS, proposes that opposite levels of prenatal testosterone exposure (low for endometriosis and high for PCOS) program the developing HPG axis resulting in underproduction (in endometriosis) and overproduction (in PCOS) of adult ovarian testosterone relative to estradiol.19 Further research that takes into account prenatal alterations of sex hormone exposure and causes for such alterations19,33 will help in better understanding the pathophysiology of endometriosis and PCOS and whether women with both conditions have a distinct phenotype.
Shared symptoms and risk factors for endometriosis and polycystic ovarian syndrome
Our findings are consistent with previous research showing the positive associations between endometriosis and history of subfertility and dysmenorrhea and the negative associations between gravidity and parity.36, 37, 38
Because of small counts in certain categories, we were limited in our ability to detect differences in sociodemographic characteristics. For BMI on the continuous scale, we found an inverse relationship with endometriosis (aPR, 0.95; 95% CI, 0.92–0.98) and a positive association with PCOS (aPR, 1.04; 95% CI, 1.01–1.08), which tracks with previous research.37,39 Given the contrasting relationship, it was not surprising to find a null relationship with endometriosis comorbid with PCOS (aPR, 1.00; 95% CI, 0.95–1.06). Given our and other's previous research indicating that women with deep infiltrating endometriosis and endometriomas have the lowest adiposity indicators40 than women with superficial endometriosis, taking into consideration endometriosis typology when looking at potential shared risk factors with PCOS is warranted.
Of notable interest is the 9- to 10-fold increased probability of reporting subfertility or previous subfertility treatment and significantly reduced gravidity and parity among women with both PCOS and endometriosis compared with women with neither condition. The multifactorial nature of female subfertility is well established,41,42 supporting the findings from this study. Although mechanisms for how endometriosis or PCOS cause female subfertility are well established,38,43 what is not clear is whether women who suffer from both endometriosis and PCOS have unique risk profiles that may additively or multiplicatively increase their risk of subfertility. If this is the case, increased surveillance and tailored treatment protocols are warranted.
In addition, although chronic pelvic pain was higher in women with endometriosis and/or PCOS than in women with neither condition, painful menstrual cramps (dysmenorrhea) showed the strongest relationship with having both conditions. Previous research has reported that 74% of women with PCOS experiencing chronic or cyclic pelvic pain and/or subfertility also have endometriosis lesions.21 Although most women's health clinicians and researchers associate endometriosis with chronic pelvic pain and dysmenorrhea, women with PCOS are also at increased risk of dysmenorrhea.44,45
Clinical and research implications
Given that endometriosis and PCOS are the 2 most common reasons for female subfertility and our finding that 1 in 20 individuals undergoing laparoscopy or laparotomy for any indication may have both conditions, with increased risk of subfertility and dysmenorrhea, clinicians are advised to consider endometriosis as a potential risk factor among women with subfertility whose subfertility is not corrected after ovulation is restored.21 As both endometriosis and PCOS may share a common inflammatory pathway, future research that can look at shared inflammatory biomarkers (eg, C-reactive protein) and their potential additive effect on the risk of subfertility is warranted. Although PCOS is clinically apparent, mild to moderate endometriosis, at present, currently requires laparoscopy for diagnosis. In the future, we hope that a clinical or biomarker diagnosis of endometriosis will become available to further clarify appropriate therapeutic alternatives in women with PCOS who remain subfertile despite the correction of anovulation.46 Because women with PCOS often have less frequent menstrual cycles, the symptom of dysmenorrhea could be attenuated,47 making it less likely to consider endometriosis as a comorbidity.
Strengths and limitations
Our study had several strengths, including a gold standard assessment of incident endometriosis5 among a relatively large sample of women undergoing diagnostic and/or therapeutic laparoscopy or laparotomy, regardless of clinical indication. Furthermore, our study is unique in capturing the overlapping prevalence of PCOS and endometriosis within a population-based sample not seeking gynecologic care. In addition, we used patient reports of physician-diagnosed PCOS and PCOS self-reported symptomology to identify prevalent PCOS within our sample.15 Finally, in our endometriosis prospective assessment, we were able to assess many sociodemographic, lifestyle, and reproductive history factors, including PCOS, before endometriosis assessment, limiting potential recall bias. Nevertheless, our study had some limitations. We are not able to confirm whether or not a clinician with expertise in PCOS made the “physician-diagnosed” PCOS assessment. Given that PCOS diagnostic criteria have evolved significantly over the past 2 decades,15 we lack diagnostic precision within our study. In addition, our sample included women only from Utah and California, which limits generalizability, especially concerning looking at risk factors for endometriosis and/or PCOS. Finally, given that we relied on capturing PCOS via questionnaires rather than biomarkers of hyperandrogenism, we may have unidentified PCOS cases, leading to misclassification bias. However, it should be also noted that the prevalence of PCOS (with or without endometriosis) was 14% in the operative cohort and 10% in the population cohort, which is in line with the 6% to 15% prevalence estimate found in before community-based samples.6, 7, 8 Nevertheless, with only 65 women with PCOS, we may have been underpowered to ascertain differences in women's symptoms by diagnostic category. Future studies that can include larger population-based samples and additionally use gold standard methods for PCOS diagnosis will better quantify the actual extent of the overlap.
Conclusions
Individuals with multiple gynecologic conditions may have difficulty conceiving; however, there is limited research on the overlapping prevalence and risk factors of various gynecologic pathologies, including PCOS and endometriosis. Many clinicians and researchers consider PCOS and endometriosis to be diametric disorders. However, despite a paucity of research to date, previous hospital-based studies indicate anywhere from a 10% to 70% overlapping prevalence depending on the severity of PCOS and/or endometriosis among the enrolled patients.
In this cohort of relatively healthy women without a previous endometriosis diagnosis undergoing gynecologic laparoscopy for various indications, including tubal ligation, 5% were found to have both PCOS and an incident endometriosis diagnosis. Among a population sample within the same geographic catchment area but not seeking care, 2% were found to have both PCOS and an incident endometriosis diagnosis. Women having both conditions had a 10-fold higher prevalence of being subfertile than women having neither PCOS nor endometriosis. Our study is novel in assessing PCOS and endometriosis overlap among both women seeking gynecologic care for multiple indications and women in the population not seeking gynecologic care. Both cohorts had few exclusion criteria, thus enhancing representativeness. Our study, in combination with previous studies, may inform the clinical enigma of sustained subfertility among women with PCOS once ovulatory status has been restored; the coexistence of endometriosis may be a contributing factor. Future studies that can better predict the risk of having both PCOS and endometriosis, based on sociodemographic and health history factors among hospital and population-based samples, are needed for improved surveillance and care.
Footnotes
The authors report no conflict of interest.
This study was supported by the Intramural Research Program, Eunice Kennedy ShriverNational Institute of Child Health and Human Development, National Institutes of Health (contract numbers: NO1-DK-63428, NO1-DK-6-3427, and 10001406-02). In addition, research was supported by the National Institute on Aging (NIA) (project number: K01AG058781; principal investigator: K.C.S.)
A portion of this research was presented at the 15th World Congress on Endometriosis, Edinburgh, Scotland, May 3–6, 2023.
All participants provided informed consent before any data collection under the University of Utah Institutional Review Board (approval number: 00021732).
Cite this article as: Schliep KC, Ghabayen L, Shaaban M, et al. Examining the co-occurrence of endometriosis and polycystic ovarian syndrome. Am J Obstet Gynecol Glob Rep 2023;XX:x.ex–x.ex.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2023.100259.
Appendix. Supplementary materials
References
- 1.Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244–1256. doi: 10.1056/NEJMra1810764. [DOI] [PubMed] [Google Scholar]
- 2.Buck Louis GM, Hediger ML, Peterson CM, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96:360–365. doi: 10.1016/j.fertnstert.2011.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Practice Committee of the American Society for Reproductive Medicine Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98:591–598. doi: 10.1016/j.fertnstert.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Kvaskoff M, Mu F, Terry KL, et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. 2015;21:500–516. doi: 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 6.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 7.Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P T. 2013;38:336–355. [PMC free article] [PubMed] [Google Scholar]
- 8.Skiba MA, Bell RJ, Herbert D, Garcia AM, Islam RM, Davis SR. Use of community-based reference ranges to estimate the prevalence of polycystic ovary syndrome by the recognised diagnostic criteria, a cross-sectional study. Hum Reprod. 2021;36:1611–1620. doi: 10.1093/humrep/deab069. [DOI] [PubMed] [Google Scholar]
- 9.Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril. 2018;110:794–809. doi: 10.1016/j.fertnstert.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Scicchitano P, Dentamaro I, Carbonara R, et al. Cardiovascular risk in women with PCOS. Int J Endocrinol Metab. 2012;10:611–618. doi: 10.5812/ijem.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz LB, Wild RA. Polycystic ovarian syndrome: an evidence-based approach to evaluation and management of diabetes and cardiovascular risks for today's clinician. Clin Obstet Gynecol. 2007;50:226–243. doi: 10.1097/GRF.0b013e31802f5197. [DOI] [PubMed] [Google Scholar]
- 12.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:618–637. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 13.Bentley-Lewis R, Seely E, Dunaif A. Ovarian hypertension: polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2011;40:433–449. doi: 10.1016/j.ecl.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christ JP, Cedars MI. Current guidelines for diagnosing PCOS. Diagnostics (Basel) 2023;13:1113. doi: 10.3390/diagnostics13061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome–part 1. Endocr Pract. 2015;21:1291–1300. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- 16.Palioura E, Diamanti-Kandarakis E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs) Rev Endocr Metab Disord. 2015;16:365–371. doi: 10.1007/s11154-016-9326-7. [DOI] [PubMed] [Google Scholar]
- 17.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 18.Doi SA, Al-Zaid M, Towers PA, Scott CJ, Al-Shoumer KA. Irregular cycles and steroid hormones in polycystic ovary syndrome. Hum Reprod. 2005;20:2402–2408. doi: 10.1093/humrep/dei093. [DOI] [PubMed] [Google Scholar]
- 19.Dinsdale NL, Crespi BJ. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol Appl. 2021;14:1693–1715. doi: 10.1111/eva.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brincat M, Galea R, Buhagiar A. Polycystic ovaries and endometriosis: a possible connection. Br J Obstet Gynaecol. 1994;101:346–348. doi: 10.1111/j.1471-0528.1994.tb13625.x. [DOI] [PubMed] [Google Scholar]
- 21.Holoch KJ, Savaris RF, Forstein DA, et al. Coexistence of polycystic ovary syndrome and endometriosis in women with infertility. J Endometriosis Pelvic Pain Disord. 2014;6:79–83. [Google Scholar]
- 22.Singh KB, Patel YC, Wortsman J. Coexistence of polycystic ovary syndrome and pelvic endometriosis. Obstet Gynecol. 1989;74:650–652. [PubMed] [Google Scholar]
- 23.Kichukova D. Polycystic ovaries in association with pelvic endometriosis in infertile women diagnosed by laparoscopy. Folia Med (Plovdiv) 1996;38:71–73. [PubMed] [Google Scholar]
- 24.Zarcone R, Longo M, Ferrara G, Cardone A. Association between inguinal endometriosis and polycystic ovary. Minerva Ginecol. 1992;44:197–200. [PubMed] [Google Scholar]
- 25.Soules MR, Makinak LR, Bury R, Poindexter A. Endometriosis and anovulation: a coexisting problem in the infertile female. Am J Obstet Gynecol. 1976;125:412–417. doi: 10.1016/0002-9378(76)90578-0. [DOI] [PubMed] [Google Scholar]
- 26.Schliep KC, Stanford JB, Chen Z, et al. Interrater and intrarater reliability in the diagnosis and staging of endometriosis. Obstet Gynecol. 2012;120:104–112. doi: 10.1097/AOG.0b013e31825bc6cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schliep KC, Chen Z, Stanford JB, et al. Endometriosis diagnosis and staging by operating surgeon and expert review using multiple diagnostic tools: an inter-rater agreement study. BJOG. 2017;124:220–229. doi: 10.1111/1471-0528.13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Han Y, Wang W, et al. Assessing new terminal body and facial hair growth during pregnancy: toward developing a simplified visual scoring system for hirsutism. Fertil Steril. 2016;105:494–500. doi: 10.1016/j.fertnstert.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 29.Schliep KC, Mumford SL, Peterson CM, et al. Pain typology and incident endometriosis. Hum Reprod. 2015;30:2427–2438. doi: 10.1093/humrep/dev147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulsen T, de Vlieg J, Alkema W. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics. 2008;9:488. doi: 10.1186/1471-2164-9-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blizzard L, Hosmer DW. The log multinomial regression model for nominal outcomes with more than two attributes. Biom J. 2007;49:889–902. doi: 10.1002/bimj.200610377. [DOI] [PubMed] [Google Scholar]
- 32.Hager M, Wenzl R, Riesenhuber S, et al. The prevalence of incidental endometriosis in women undergoing laparoscopic ovarian drilling for clomiphene-resistant polycystic ovary syndrome: a retrospective cohort study and meta-analysis. J Clin Med. 2019;8:1210. doi: 10.3390/jcm8081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghadami-Tabrizi NA, Mohammad K, Dabirashrafi H, Zandinejad K, Zomorrodian S. The occurrence of endometriosis among infertile patients with uterine myoma and those with polycystic ovarian disease: comparison with infertile control cases. MJIRI. 1998;11:315–317. [Google Scholar]
- 34.Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman's long-term health using data linkage. J Clin Endocrinol Metab. 2015;100:911–919. doi: 10.1210/jc.2014-3886. [DOI] [PubMed] [Google Scholar]
- 35.Pan Z, Zhu F, Zhou K. A systematic review of anogenital distance and gynecological disorders: endometriosis and polycystic ovary syndrome. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.696879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson CM, Johnstone EB, Hammoud AO, et al. Risk factors associated with endometriosis: importance of study population for characterizing disease in the Endo Study. Am J Obstet Gynecol. 2013;208 doi: 10.1016/j.ajog.2013.02.040. 451.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemmings R, Rivard M, Olive DL, et al. Evaluation of risk factors associated with endometriosis. Fertil Steril. 2004;81:1513–1521. doi: 10.1016/j.fertnstert.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 38.Evans MB, Decherney AH. Fertility and endometriosis. Clin Obstet Gynecol. 2017;60:497–502. doi: 10.1097/GRF.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 39.Backonja U, Buck Louis GM, Lauver DR. Overall adiposity, adipose tissue distribution, and endometriosis: a systematic review. Nurs Res. 2016;65:151–166. doi: 10.1097/NNR.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byun J, Peterson CM, Backonja U, et al. Adiposity and endometriosis severity and typology. J Minim Invasive Gynecol. 2020;27:1516–1523. doi: 10.1016/j.jmig.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Infertility Workup for the Women's Health Specialist: ACOG Committee Opinion Summary, Number 781. Obstet Gynecol. 2019;133:1294–1295. doi: 10.1097/AOG.0000000000003272. [DOI] [PubMed] [Google Scholar]
- 42.Stanford JB, Carpentier PA, Meier BL, Rollo M, Tingey B. Restorative reproductive medicine for infertility in two family medicine clinics in New England, an observational study. BMC Pregnancy Childbirth. 2021;21:495. doi: 10.1186/s12884-021-03946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132:321–336. doi: 10.1097/AOG.0000000000002698. [DOI] [PubMed] [Google Scholar]
- 44.Tomás-Rodríguez MI, Palazón-Bru A, Martínez-St John DR, Navarro-Cremades F, Toledo-Marhuenda JV, Gil-Guillén VF. Factors associated with increased pain in primary dysmenorrhea: analysis using a multivariate ordered logistic regression model. J Pediatr Adolesc Gynecol. 2017;30:199–202. doi: 10.1016/j.jpag.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Jeong JY, Kim MK, Lee I, et al. Polycystic ovarian morphology is associated with primary dysmenorrhea in young Korean women. Obstet Gynecol Sci. 2019;62:329–334. doi: 10.5468/ogs.2019.62.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans-Hoeker E, Lessey BA, Jeong JW, et al. Endometrial BCL6 overexpression in eutopic endometrium of women with endometriosis. Reprod Sci. 2016;23:1234–1241. doi: 10.1177/1933719116649711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jukic AM, Weinberg CR, Baird DD, Hornsby PP, Wilcox AJ. Measuring menstrual discomfort: a comparison of interview and diary data. Epidemiology. 2008;19:846–850. doi: 10.1097/EDE.0b013e318187ac9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.