Abstract
Life-prolonging central nervous system active systemic therapies for metastatic NSCLC have increased the complexity of managing brain metastases (BMs). Australian medical oncologists, radiation oncologists, and neurosurgeons discussed the evidence guiding the diverse clinical approaches to the management of BM in NSCLC. The Australian context is broadly applicable to other jurisdictions; therefore, we have documented these discussions as principles with broader applications. Patient management was stratified according to clinical and radiologic factors under two broad classifications of newly diagnosed BMs: symptomatic and asymptomatic. Other important considerations include the number and location of metastases, tumor histotypes, molecular subtype, and treatment purpose. Careful consideration of the pace and burden of symptoms, risk of worsening neurologic function at a short interval, and extracranial disease burden should determine whether central nervous system active systemic therapies are used alone or in combination with local therapies (surgery with or without radiation therapy). Most clinical trial evidence currently focuses on historical treatment options or a single treatment modality rather than the optimal sequencing of multiple modern therapies; therefore, an individualized approach is key in a rapidly changing therapeutic landscape.
Keywords: Non–small cell lung cancer, Brain metastases, Neurosurgical procedures, Radiotherapy, Molecular targeted therapy
Introduction
Between 25% and 50% of patients with advanced NSCLC will develop brain metastases (BMs).1,2 As systemic therapies become more effective in prolonging survival, the management of BM has become increasingly important.3,4 Although multiple practice guidelines5,6 are available for the management of BM in NSCLC, the real-world case scenarios may not perfectly meet specific guideline criteria, and in many cases, the quality of the evidence supporting recommendations is low.5 Therefore, a multidisciplinary discussion is encouraged for the treatment of BM in patients with NSCLC.4
A series of expert meetings attended by medical oncologists, radiation oncologists, neurosurgeons, and lung cancer nurses were conducted in various major Australian cities in 2020 and 2021 to discuss the evidence guiding the diverse clinical practices within Australia. Discussions focused on three case scenarios: a patient with EGFR-mutant NSCLC treated with a tyrosine kinase inhibitor (TKI) who develops BM, a patient with NSCLC with no known driver mutations with BM at baseline, and a patient with ALK-positive NSCLC with multiple BM at baseline. This review is based on these discussions. It is broadly applicable to other jurisdictions with similar health care models and access to therapies.
Importance of Multidisciplinary Involvement
Presentation of cases to a multidisciplinary team meeting (MDT) has been reported to increase the survival of patients with inoperable NSCLC from 205 to 280 days.7 However, currently not all cases are presented to MDT meetings because of limitations in MDT availability, funding, and time constraints. As some MDT meetings are tumor-site specific, both lung cancer and neuro-oncology expertise may not be present. This remains a significant barrier to optimal care.
Recommendation: Multidisciplinary team meetings
All patients with NSCLC who have BM should be discussed at Thoracic Oncology and Neurooncology MDT meetings.
Stratification of Patients With BM According to Clinical and Radiologic Features
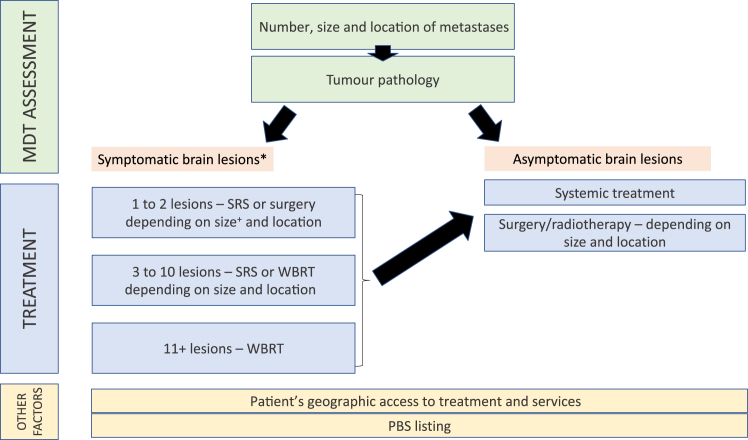
Management of patients is stratified according to clinical and radiological features (Figure 1) into1 patients with symptomatic BM (further subclassified into those with one to two lesions, three to 10 lesions, and 11 or more lesions) and those with asymptomatic BM.5
Figure 1.
Considerations in the management and treatment of CNS metastases in NSCLC in an Australian setting. ∗Patients with poor performance status who are unfit for anticancer therapy may be considered for best supportive care. Earlier initiation of CNS active systemic therapies may be considered for eligible patients with high extracranial disease burden and mildly symptomatic brain lesions. +Small tumors <30 mm are treated with SRS, large tumors ≥30 mm are treated with surgery.6 CNS, central nervous system; MDT, multidisciplinary team; PBS, pharmaceutical benefits scheme; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Symptomatic BM
Patients with symptomatic BM, especially when life-threatening symptoms or limited response to corticosteroids should be considered for local therapy (radiosurgery, radiation therapy or neurosurgery). For patients with known driver mutations and not at risk of local crisis such as cerebral herniation, the use of highly penetrant TKIs instead of local therapy is an option. Patients with poor performance status that will not be improved by local therapy and who are not fit to receive systemic anticancer therapies should be provided with best supportive care.
Asymptomatic BM
Patients with asymptomatic BM may have local therapy deferred, especially when small volume, the extracranial disease requires urgent treatment, or there is an option to use a central nervous system (CNS)–penetrant targeted therapy unless otherwise recommended by the MDT.5 Molecularly targeted therapy should be considered when appropriate (see the section on intracranial activity of established molecularly targeted therapies below).
Indications for neurosurgery
Surgery is a first-line treatment option for patients with good performance status, one or few accessible BM, and well-controlled extracranial disease for those with recurrent disease.8 Resection has the advantage of rapid improvement of CNS symptoms and procures tissue for diagnostic confirmation and molecular profiling.9, 10, 11 For those with single lesions, there is evidence that surgery plus whole-brain radiation therapy (WBRT) improves overall survival (OS) compared with WBRT alone.12 There is also evidence that surgery is more effective at reducing edema secondary to tumors than stereotactic radiosurgery (SRS) alone.13 The importance of en bloc or microscopic total resection is increasingly being recognized for reducing local recurrence without increasing morbidity.14, 15, 16 Surgical resection is indicated for the immediate management of mass effect or brain herniation,17 a neurologic emergency, with the potential for significant and catastrophic deficits.
Recommendations: Neurosurgery
Brain Multidisciplinary Meeting: Patients should be referred to, or discussed with, a neurosurgeon regarding the possibility of resection. Neurosurgery is recommended in the following situations:
-
1.
Impending crisis (mass effect or herniation)
-
2.
Limited number of lesions (1-2) - not eligible for CNS-penetrant TKI; maximum diameter of a lesion > 4 cm
-
3.
When histologic diagnosis is required
Indications for Stereotactic Radiation Therapy
WBRT is a historical standard that treats the entire brain to a homogeneous lower dose, typically delivering 20 Gy in five daily fractions or 30 Gy in 10 daily fractions. In contrast, SRS involves the precise delivery of high or ablative doses of ionizing radiation to defined targets within the brain. SRS is associated with high rates of local control but is not expected to reduce the risk of developing subsequent metastases elsewhere in the brain. SRS is accepted as a reference standard treatment for patients with limited BM, because of its efficacy and minimally invasive nature.1 SRS has been found to be effective even in patients with higher tumor burden, with one Japanese study reporting noninferior OS when comparing outcomes of patients with two to four BM compared with five to 10 BM.18 This Japanese study considered limited number of BM as not more than 10 BM, and small size as less than 3 cm in longest diameter. Results from a cohort of patients treated with SRS in Australia revealed that the total tumor volume may be more important in predicting survival outcomes than the number of metastases, further recommending the inclusion of a tumor volume index in future studies involving patients with BM.19
There has been increasing use of SRS as a means of delaying or avoiding WBRT. SRS has fewer neurocognitive adverse effects than WBRT.18 The addition of SRS to WBRT prolongs survival in patients with a single BM and those with up to three BM and no active extracranial disease.20 Randomized controlled trials have reported that the addition of WBRT to SRS is associated with reduced neurocognitive function, increased fatigue, and decreased quality of life.21 For this reason, the use of WBRT across Australia has reduced over the past decade and is no longer routinely recommended. A study of Australian practice between 2013 and 2017 found that, of 1049 patients who received SRS for BM, only 2% had received adjuvant WBRT.22 Notably, this decreased from 4% in 2013 to 0.7% in 2017 (p = 0.02).22
The 12-month cumulative incidence of radiation necrosis was found to be significantly increased in patients who had received systemic therapy in addition to upfront SRS and WBRT compared with those who had received only SRS and WBRT (8.8% versus 5.3%, p < 0.01) in a study of patients with newly diagnosed BM undergoing SRS.23 Systemic therapies associated with a significant increase in rates of radiation necrosis were vascular epithelial growth factor receptor TKIs and EGFR TKIs.
Complications after SRS, such as radiation necrosis, are rare but may occur many months to years after treatment.24 Risk factors for radiation necrosis include tumor volume, prescribed radiation dose, fraction size, the volume of normal brain irradiated, previous use of CNS radiation, and use of concurrent systemic therapy. Having a short period of systemic therapy before SRS may be considered when there are concerns about increased risk of SRS-related complications. However, this decision needs to be individualized considering the burden of intracranial disease and the risk of extracranial disease progression.
Whole-Brain Radiation Therapy
WBRT is a historic treatment option for patients with multiple BMs secondary to NSCLC.25 Nowadays, WBRT is only used in highly selected patients, for example, patients with BM ineligible for surgery or SRS but with otherwise favorable prognostic characteristics.6 Those ineligible patients for SRS may have more than 10 BM on the basis of a Japanese prospective cohort study, which reported that selected patients with five to 10 BM treated by SRS may have similar OS compared with those with two to four BM.18 Symptomatic and radiologic response rates are in the order of 50% but typically not durable beyond 6 to 12 months. WBRT can be associated with moderate neurotoxicity and memory impairment over time.26,27 The trend to omit WBRT is supported by recent data from the QUARTZ trial (NCT00403065) reporting no significant improvement in survival or quality of life in older patients with poor performance status and limited prognosis compared with best supportive care.25
Another approach to maintaining longer-term neurocognitive function is hippocampal avoidance (HA)-WBRT, which may be appropriate for patients with large-volume BM not suitable for neurosurgery.6,28 A reduction in radiation to the hippocampal regions using HA-WBRT has been found to reduce neurocognitive decline as measured by the Hopkins Verbal Learning Test–Revised, a delayed recall test, to 7% at 4 months compared with 30% with WBRT (historical control).29
A recent trial in adult patients with BM found that the risk of cognitive failure was significantly lower after HA-WBRT plus memantine versus WBRT plus memantine (adjusted hazard ratio [HR] = 0.74, 95% confidence interval [CI]: 0.58–0.95, p = 0.02), with no difference in intracranial progression-free survival (PFS) and OS. HA-WBRT should, therefore, be considered a standard of care for patients with good performance status who plan to receive WBRT for BM with no metastases in the HA region.29
Postoperative Radiotherapy (SRS to a Surgical Cavity or WBRT)
Historically, WBRT was added after local therapy (surgery or SRS) to improve intracranial control. A meta-analysis of five randomized clinical trials (663 patients) with one to four BM found that adding WBRT decreased the relative risk of any intracranial disease progression at 1 year by 53% (relative risk = 0.47, 95% CI: 0.34–0.66, p < 0.0001) but did not improve OS (HR = 1.11, 95% CI: 0.83–1.48, p = 0.47).30 In two subsequent randomized clinical trials, the addition of WBRT after local therapy was associated with worse neurocognitive outcomes than cavity SRS despite an increased reliance on salvage therapy, suggesting that the overall morbidity associated with adjuvant WBRT to reduce the risk of intracranial recurrence was greater in longer-term survivors than the morbidity associated with the recurrence itself and subsequent therapy.31,32 Thus, although adjuvant WBRT can improve intracranial control after local therapy, the clinical benefit is uncertain, and it is now not recommended as routine therapy.21 Another caveat is whether surgical cavity SRS is still warranted in the era of newer generation TKIs with impressive brain penetration.
Recommendations: Cranial Radiation Therapy
SRS or SRT is recommended for selective patients with a limited number of BM. SRS may be performed in combination with surgery for larger or symptomatic BM. WBRT is generally recommended only in selective patients with a high burden of BM or leptomeningeal disease and limited or no other treatment options. HA-WBRT can be used to reduce WBRT-related cognitive deficits.
Intracranial Activity of Established Molecularly Targeted Therapies and Immune Checkpoint Inhibitors in NSCLC
EGFR TKI
Approximately 10% to 15% of white and 50% of Asian people with NSCLC adenocarcinoma have an EGFR-activating mutation.1 Among patients with EGFR-positive disease, CNS metastases are present at diagnosis in about 24% of patients.1 First- and second-generation EGFR TKIs (i.e., gefitinib, erlotinib, afatinib) generally have poor biopharmaceutical properties for blood-brain barrier penetration, and acquired resistance develops in most patients.33
Osimertinib is a third-generation, irreversible EGFR TKI that selectively inhibits EGFR TKI–sensitizing and EGFR T790M–resistant mutations (T790M-positive).1,34 Moreover, it has been found to cross the blood-brain barrier.35 Osimertinib was approved in Australia as first-line therapy for patients with EGFR-mutated NSCLC on the basis of the FLAURA trial (NCT02296125),34 which exhibited superior CNS efficacy compared with first-generation EGFR TKIs.1
The optimal sequencing of treatments needs to be carefully considered, particularly with respect to targeted treatment versus SRS or WBRT.36 A multi-institutional analysis revealed that SRS followed by first-generation EGFR TKI resulted in the longest OS and prevented any potential neurocognitive sequelae of WBRT.36 The impact of osimertinib on PFS with or without upfront SRS is currently being investigated in two randomized studies: the Trans-Tasman Radiation Oncology Group OUTRUN trial (NCT03497767) and the British Columbia Cancer Agency trial (NCT03769103). Results are expected in 2024 and 2025, respectively.
Despite the initial benefit in most patients, progression on osimertinib invariably occurs.37 Combination therapy, such as with savolitinib (a MET inhibitor) is being investigated in the TATTON phase 1B study (NCT02143466), SAVANNAH (NCT03778229), and ORCHARD (NCT03944772).38 To date, an acceptable risk-benefit profile and encouraging antitumor activity have been reported in patients who experienced disease progression on a previous EGFR TKI. The impact of combination targeted therapies on intracranial metastases is also unknown and an area of ongoing research.
ALK-Rearranged NSCLC
Approximately 5% of NSCLC tumors are ALK-positive adenocarcinomas, with EML4-ALK translocations as the most common rearrangement.39 Patients with ALK-positive tumors tend to be younger at disease onset and never- or light smokers; they almost never have concurrent EGFR or KRAS mutations.40 At the time of diagnosis, 30% to 50% of patients with ALK-rearranged NSCLC have BM1 and the CNS is also the most common site of disease progression.
Treatment of ALK-positive NSCLC is currently initiated with the second-generation TKIs alectinib or brigatinib. The first-generation ALK and ROS1 TKI, crizotinib, is no longer preferred for the treatment of ALK-positive NSCLC because of its poor CNS penetration, particularly when patients have a BM at diagnosis.1 Alectinib has the longest follow-up in clinical trials of the ALK TKIs, with OS data up to 5 years.41 It is used as a first-line therapy in patients with NSCLC and ALK rearrangements and has activity against the most common crizotinib-resistant mutations. It does not have activity against ROS1. In the phase III ALEX trial of alectinib versus crizotinib in treatment-naive ALK-positive advanced NSCLC (NCT02075840), the overall CNS response rates were 81% and 50%, and the duration of response for the CNS was 17.3 months and 5.5 months, respectively.42 The 5-year OS rate was 62.5% (95% CI: 54.3%–70.8%) with alectinib and 45.5% (95% CI: 33.6%–57.4%) with crizotinib.41 Alectinib was also better tolerated than crizotinib.
Brigatinib is also approved in Australia for crizotinib-refractory and treatment-naive patients. The phase 3 ALTA-1L study investigated brigatinib versus crizotinib in patients with treatment-naive ALK-positive advanced NSCLC (NCT02737501). In the second interim analysis, PFS was longer with brigatinib than crizotinib (median PFS 24.0 versus 11.0 mo, HR = 0.49 [95% CI: 0.35–0.68], log-rank p < 0.001).43 The confirmed rate of intracranial response among patients with measurable lesions was 78% (95% CI: 52%–78%) for brigatinib-treated patients and 29% (95% CI: 11%–52%) for those receiving crizotinib.44
Another second-generation TKI, ceritinib, has activity against ALK and ROS1 and is about 20 times more potent than crizotinib. It is approved in Australia for use in metastatic NSCLC with ALK rearrangement; however, despite its CNS efficacy, it is used less frequently because of a higher rate of gastrointestinal adverse effects.1
Lorlatinib, a third-generation ALK TKI, approved in Australia for the treatment of patients with metastatic ALK-positive NSCLC is highly potent and CNS penetrating, and has activity against most of the ALK inhibitor resistance mutations, including G1202R. In ALK-positive patients with at least one previous ALK TKI, objective responses were achieved in 93 of 198 patients (47.0% [95% CI: 39.9%–54.2%]) and objective intracranial responses in those with measurable baseline CNS lesions in 51 of 81 patients (63.0% [95% CI: 51.5%–73.4%]).45 It is the preferred agent for CNS progression with a second-generation ALK TKI.
The interim data from the phase 3, open-label randomized, multicenter CROWN study (NCT03052608), comparing lorlatinib to crizotinib in patients with untreated ALK-positive NSCLC reported statistically significant and clinically meaningful improvement in PFS with lorlatinib compared with crizotinib.46,47 A post hoc exploratory analysis of PFS to assess the impact of BM and previous brain radiotherapy (RT) on efficacy found that lorlatinib had potent and durable efficacy in patients with and without BM at baseline, regardless of previous brain RT use.46 The 12-month PFS rates for lorlatinib versus crizotinib were 78% versus 22% in patients with BM at baseline and 78% versus 45% in those without. On the basis of the findings from the CROWN study, some recommend lorlatinib be considered a new first-line treatment option for patients with ALK-positive NSCLC.48 However, others argue that lorlatinib should be reserved for salvage therapy because of its toxicity profile.49 Currently, lorlatinib is only available in the second-line setting in Australia but this is set to change on the basis of the CROWN results.
ROS1-Rearranged NSCLC
Approximately 1% to 2% of patients with NSCLC have ROS1-positive disease.1 In this population, the incidence of BM is between 19% and 36%.50 TKI therapy is a first-line standard of care for patients with ROS1 gene fusions or rearrangements, including those with advanced disease.50,51
Crizotinib is a beneficial first-line treatment option for patients with ROS1-positive disease, with or without BM at presentation.51 There is also emerging evidence of intracranial response with entrectinib, a multikinase inhibitor with additional activity against ROS1.50 Both crizotinib and entrectinib lead to high objective response rates and have manageable adverse effects, but entrectinib has more potent intracranial efficacy52 and is generally the agent of choice for patients with de novo CNS disease because of its superior CNS penetration.52
Lorlatinib exhibits activity in both TKI-naive and some crizotinib-resistant settings.52 Lorlatinib has exhibited efficacy in heavily pretreated patients53; however, it has limited potency against the crizotinib-, entrectinib-resistant ROS1-G2032R mutation.52 Adverse events are common with lorlatinib, and most patients require treatment with a lipid-lowering agent.52 Currently, lorlatinib is unavailable on the Australian Government Pharmaceutical Benefits Scheme (PBS) for this indication.
Repotrectinib is a novel, next-generation ROS1 TKI designed to overcome refractory G2032R solvent-front mutations and has exhibited excellent antitumor activity in ROS1-rearranged tumors in preclinical studies, with good CNS penetration.54 The clinical activity of repotrectinib is being further investigated in a phase 1-2 trial (NCT03093116).
Programmed Cell Death Protein 1 and Programmed Death-Ligand 1 Inhibitors
Treatment with programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors has become a standard of care for patients with NSCLC and BM who have no targetable oncogenic driver alterations. Single-agent pembrolizumab is the standard first-line therapy for patients with greater than 50% PD-L1 expression and in combination with platinum-based chemotherapy irrespective of PD-L1 expression.55,56 Pembrolizumab has been found to have activity in the brain in patients with NSCLC, with one study reporting CNS response from 6 of 18 patients (33% [95% CI: 14–59]).57 Patients with asymptomatic or treated BMs included in the pembrolizumab phase 3 trials exhibited a similar benefit to the overall populations55,57; however, in clinical practice, the response may not be as high, and thus, brain-directed therapies, including surgery and RT, are still needed in this subgroup.
A combination of nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor) plus two cycles of chemotherapy was compared with chemotherapy alone in patients with previously untreated advanced NSCLC in the CheckMate 9LA study (NCT03215706).58 This study found a significant OS benefit in the nivolumab and ipilimumab arm compared with chemotherapy alone. In a post hoc subgroup analysis of efficacy and safety in patients with and without baseline BM, patients with BM receiving nivolumab and ipilimumab obtained durable survival benefits compared with those receiving chemotherapy, consistent with the overall population. OS was superior in the nivolumab and ipilimumab plus chemotherapy arm compared with the chemotherapy alone arm with an HR of 0.43 (95% CI: 0.27–0.67) and a 2-year OS of 35% (95% CI: 22.6%–48.2%).59 Similarly, a post hoc subgroup analysis of the CheckMate-227 (NCT02477826) of nivolumab and ipilimumab compared with chemotherapy alone also found a benefit in patients with treated BM (OS HR = 0.63 [95% CI: 0.43–0.92]).60
Atezolizumab plus carboplatin and pemetrexed is being studied in 40 patients with advanced nonsquamous NSCLC and untreated asymptomatic BM (ATEZO-BRAIN study; NCT03526900).61 PD-L1 expression was high in 50% of patients. Interestingly, 40% of patients had an intracranial objective response and 48% achieved a systemic objective response, with 10% of patients having discordant results between the brain and body. There were no differences observed in the overall response rate by PD-L1 expression. The estimated 2-year OS rate was 31% (95% CI: 18%–50%).
Recommendations: Targeted Therapy
-
1.
Comprehensive molecular testing, including PD-L1 status, should be performed.
-
2.
Later-generation TKIs are preferred for patients with CNS disease given greater intracranial efficacy in NSCLC with EGFR (e.g., osimertinib), ALK (e.g., lorlatinib), and ROS1 (e.g., entrectinib).
-
3.
In asymptomatic or minimally symptomatic BM, systemic therapies could be considered as primary therapies. Immunotherapy has activity in BM but may need to be used in conjunction with local therapies as response rates are limited compared with oncogene-driven tumors.
-
4.
Systemic therapies may be used as a single modality initially in select cases of symptomatic CNS disease.
Molecular Testing in NSCLC
Given that CNS-penetrant targeted therapies can inform the management of both intracranial and extracranial disease, knowledge of mutational status is critical in determining the optimum management strategy. EGFR-mutation and T790M testing can take 2 weeks, which can therefore impact the commencement of systemic treatment and affect treatment decisions regarding other modalities. ALK and ROS1 screening are routinely performed using immunohistochemistry; however, access to PBS-subsidized therapy requires fluorescence in situ hybridization testing. Ideally, comprehensive genomic profiling using next-generation sequencing to analyze a broad panel of genes would be performed but this is not readily available in Australia except in a clinical trial setting.
Leptomeningeal Metastases
Leptomeningeal metastases (LMs) develop in 10% of people with NSCLC.62 Even with treatment, LMs offer a poor prognosis and OS between 2 and 4 months.63 Symptoms such as headaches, changed mental status, difficulty walking, seizures, and vomiting reflect the neuraxial involvement.63,64
The risk of leptomeningeal spread after surgery, SRS and WBRT is contentious, with some claiming each differs in recurrence,65, 66, 67 whereas others find no difference.32,68, 69, 70 Overall, the pattern of leptomeningeal disease after resection and SRS is associated with a more favorable OS than what is observed from classic leptomeningeal relapse,70 suggesting the benefits of treatment outweigh the risks of local recurrence.
In patients with advanced NSCLC, the risk of developing LMs may be decreased by osimertinib exposure.71 Furthermore, osimertinib 160 mg daily has exhibited CNS efficacy and manageable safety in patients with LMs after they have progressed on an EGFR TKI (BLOOM study; NCT02228369).72 Despite current guideline recommendations for patients with LMs, osimertinib 160 mg daily is not available on the PBS, thus, limiting its use in the Australian context. Further investigation into therapies and prognostic variables is warranted, especially considering the rising prevalence of LM in NSCLC.73
Surveillance Imaging After Treatment of BM or for Those at Risk of BM
Whereas contrast-enhanced magnetic resonance imaging (MRI) is the imaging modality of choice for surveillance of CNS metastases, access to services can be an issue. It is recognized that patients treated with neurosurgery or SRS for BM are at higher risk of developing subsequent BM elsewhere in the brain, highlighting the importance of ongoing cranial imaging surveillance.21 Similar to follow-up schedules in clinical trials comparing SRS with or without WBRT in patients with newly diagnosed BM, the frequency of surveillance MRIs in the authors’ clinical practice is approximately once every 2 to 3 months for the first 2 years, and 4 to 6 months thereafter.74,75 However, the timing and interval of post-treatment imaging need to be individualized.
Recommendations: Imaging for BM
-
1.
Imaging should include intracranial imaging and chest imaging.
-
2.
Regular MRI imaging is recommended to monitor disease status and treatment response. The timing and interval of imaging needs to be individualized.
In summary, this article provides general guidance for the management paradigm of NSCLC with BM in an Australian setting. While the genomic makeup of the tumor will help decide the treatment pathway, broader socioeconomic factors also come into play. These determine the patient’s access to both treatments (cost, when not listed on the PBS) and services (geography, where the patient lives in relation to the specialist treatment center) (Figure 1). Clinical trial evidence focuses on traditional treatment options or a single therapeutic area, for example, surgery versus no surgery, or WBRT versus no WBRT, which does not take into consideration a multifaceted approach to the care of this patient population. This article highlights the complexity of developing treatment guidelines in a rapidly changing therapeutic landscape and one in which individualized therapy is key.
CRediT Authorship Contribution Statement
Chee Khoon Lee: Conceptualization; Writing - original draft; Writing - review & editing.
Yu Yang Soon: Conceptualization; Writing - original draft; Writing - review & editing.
Rosalind Jeffree: Writing - review & editing.
Rohit Joshi: Writing - review & editing.
Eng-Siew Koh: Writing - review & editing.
Wei-Sen Lam: Writing - review & editing.
Hien Le: Writing - review & editing.
Zarnie Lwin: Writing - review & editing.
Mark B. Pinkham: Writing - review & editing.
Shankar Siva: Writing - review & editing.
Evan Ng: Writing - review & editing.
Thomas John: Conceptualization; Writing - original draft; Writing - review & editing.
Acknowledgments
AstraZeneca, Sydney, Australia sponsored the educational roundtable and funded medical writing support for the publication in accordance with Good Publication Practice (GPP2022) guidelines (https://www.ismpp.org/gpp-2022). The authors thank Pippa Burns, PhD, CMPP and Sophie Gibb, PhD, CMPP of WriteSource Medical Pty Ltd., Sydney, Australia, for providing medical writing support.
Footnotes
Disclosure: Dr. Lam reports receiving travel/education support from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, Pfizer, Novartis, and AstraZeneca; and honoraria from Bristol-Myers Squibb, AstraZeneca, Novartis, and Roche. Dr. Pinkham has received speaker honoraria from Elekta, Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, and AstraZeneca. Dr. Lwin has received consulting fees from Merck Sharp & Dohme, honoraria from AstraZeneca, Pfizer, and Merck Sharp & Dohme; travel support from Pfizer and Bristol-Myers Squibb; and participated in an advisory board for Merck Sharp & Dohme and Pfizer. Dr. Le has received honorarium from AstraZeneca. Dr. Lee has received honoraria from AstraZeneca, Roche, Amgen, GlaxoSmithKline, Merck KGA, Novartis, Pfizer, and Takeda; and travel support from AstraZeneca. Dr. Ng holds stock in GenesisCare. Dr. Jeffree received honoraria from Gilean, Novartis and Merck Sharp & Dohme. Dr. Siva received grants from Varian Medical Systems and Reflexion; consulting fees from AstraZeneca and Janssen; received honoraria from AstraZeneca, Varian Medical Systems, Roche and Bristol-Myers Squibb; and travel support from AstraZeneca. The remaining authors declare no conflict of interest.
Cite this article as: Lee CK, Soon YY, Jeffree RL, et al. Management paradigm of central nervous system metastases in NSCLC: an Australian perspective. JTO Clin Res Rep. 2023;4:100553.
References
- 1.Ernani V., Stinchcombe T.E. Management of brain metastases in non-small-cell lung cancer. J Oncol Pract. 2019;15:563–570. doi: 10.1200/JOP.19.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang Y., Jin Y., Li Q., Yuan X. Advances in lung cancer driver genes associated with brain metastasis. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.606300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar J., Urban D., Amit U., et al. Long-term survival of patients with metastatic non-small-cell lung cancer over five decades. J Oncol. 2021;2021 doi: 10.1155/2021/7836264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf A., Kvint S., Chachoua A., et al. Toward the complete control of brain metastases using surveillance screening and stereotactic radiosurgery. J Neurol Surg. 2018;128:23–31. doi: 10.3171/2016.10.JNS161036. [DOI] [PubMed] [Google Scholar]
- 5.Vogelbaum M.A., Brown P.D., Messersmith H., et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J Clin Oncol. 2022;40:492–516. doi: 10.1200/JCO.21.02314. [DOI] [PubMed] [Google Scholar]
- 6.Gondi V., Bauman G., Bradfield L., et al. Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2022;12:265–282. doi: 10.1016/j.prro.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Rajpurohit A., Purandare N., Moiyadi A., et al. Multidisciplinary brain metastasis clinic: is it effective and worthwhile? Ecancermedicalscience. 2020;14:1136. doi: 10.3332/ecancer.2020.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soffietti R., Abacioglu U., Baumert B., et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO) Neurooncol. 2017;19:162–174. doi: 10.1093/neuonc/now241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarria P., Pessina F., Cozzi L., et al. Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol. 2016;11:76. doi: 10.1186/s13014-016-0653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattozzi D.A., Alvarado A., Kitzerow C., et al. Very large metastases to the brain: retrospective study on outcomes of surgical management. World Neurosurg. 2018;116:e874–e881. doi: 10.1016/j.wneu.2018.05.120. [DOI] [PubMed] [Google Scholar]
- 11.Salvati M., Tropeano M.P., Maiola V., et al. Multiple brain metastases: a surgical series and neurosurgical perspective. Neurol Sci. 2018;39:671–677. doi: 10.1007/s10072-017-3220-2. [DOI] [PubMed] [Google Scholar]
- 12.Patchell R.A., Tibbs P.A., Walsh J.W., et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 13.Shimony N., Shofty B., Harosh C.B., Sitt R., Ram Z., Grossman R. Surgical resection of cerebral metastases leads to faster resolution of peritumoral edema than stereotactic radiosurgery: a volumetric analysis. Ann Surg Oncol. 2017;24:1392–1398. doi: 10.1245/s10434-016-5709-y. [DOI] [PubMed] [Google Scholar]
- 14.Yoo H., Kim Y.Z., Nam B.H., et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurol Surg. 2009;110:730–736. doi: 10.3171/2008.8.JNS08448. [DOI] [PubMed] [Google Scholar]
- 15.Patel A.J., Suki D., Hatiboglu M.A., et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurol Surg. 2010;113:181–189. doi: 10.3171/2009.11.JNS09659. [DOI] [PubMed] [Google Scholar]
- 16.Patel A.J., Suki D., Hatiboglu M.A., Rao V.Y., Fox B.D., Sawaya R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurol Surg. 2015;122:1132–1143. doi: 10.3171/2014.9.JNS13939. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network® (NCCN®). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Central Nervous System Cancers. version 2.2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425. Accessed December 8, 2021.
- 18.Yamamoto M., Serizawa T., Shuto T., et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 19.Izard M.A., Moutrie V., Rogers J.M., et al. Volume not number of metastases: gamma Knife radiosurgery management of intracranial lesions from an Australian perspective. Radiother Oncol. 2019;133:43–49. doi: 10.1016/j.radonc.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Andrews D.W., Scott C.B., Sperduto P.W., et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 21.The Royal Australian and New Zealand College of Radiologists Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. https://www.choosingwisely.org.au/recommendations/ranzcr10 Accessed December 8, 2021.
- 22.Ong W.L., Wada M., Ruben J., Foroudi F., Millar J. Contemporary practice patterns of stereotactic radiosurgery for brain metastasis: a review of published Australian literature. J Med Imaging Radiat Oncol. 2019;63:711–720. doi: 10.1111/1754-9485.12942. [DOI] [PubMed] [Google Scholar]
- 23.Kim J.M., Miller J.A., Kotecha R., et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol. 2017;133:357–368. doi: 10.1007/s11060-017-2442-8. [DOI] [PubMed] [Google Scholar]
- 24.Vellayappan B., Tan C.L., Yong C., et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol. 2018;8:395. doi: 10.3389/fonc.2018.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glatzer M., Faivre-Finn C., De Ruysscher D., et al. Role of radiotherapy in the management of brain metastases of NSCLC - decision criteria in clinical routine. Radiother Oncol. 2021;154:269–273. doi: 10.1016/j.radonc.2020.10.043. [DOI] [PubMed] [Google Scholar]
- 26.Lamba N., Mehanna E., Kearney R.B., et al. Prescription of memantine during non-stereotactic, brain-directed radiation among patients with brain metastases: a population-based study. J Neurooncol. 2020;148:509–517. doi: 10.1007/s11060-020-03542-4. [DOI] [PubMed] [Google Scholar]
- 27.Meyers C.A., Smith J.A., Bezjak A., et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 28.Wang B., Fu S., Huang Y., et al. The effect of hippocampal avoidance whole brain radiotherapy on the preservation of long-term neurocognitive function in non-small cell lung cancer patients with brain metastasis. Technol Cancer Res Treat. 2021;20 doi: 10.1177/15330338211034269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown P.D., Gondi V., Pugh S., et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020;38:1019–1029. doi: 10.1200/JCO.19.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soon Y.Y., Tham I.W., Lim K.H., Koh W.Y., Lu J.J. Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev. 2014;2014:CD009454. doi: 10.1002/14651858.CD009454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown P.D., Jaeckle K., Ballman K.V., et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown P.D., Ballman K.V., Cerhan J.H., et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahluwalia M.S., Becker K., Levy B.P. Epidermal growth factor receptor tyrosine kinase inhibitors for central nervous system metastases from non-small cell lung cancer. Oncologist. 2018;23:1199–1209. doi: 10.1634/theoncologist.2017-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soria J.C., Tan D.S.W., Chiari R., et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK -rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 35.Colclough N., Chen K., Johnström P., et al. Preclinical Comparison of the blood-brain barrier Permeability of Osimertinib with Other EGFR TKIs. Clin Cancer Res. 2021;27:189–201. doi: 10.1158/1078-0432.CCR-19-1871. [DOI] [PubMed] [Google Scholar]
- 36.Magnuson W.J., Lester-Coll N.H., Wu A.J., et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. doi: 10.1200/JCO.2016.69.7144. [DOI] [PubMed] [Google Scholar]
- 37.Harada D., Takigawa N. Oligoprogression in non-small cell lung cancer. Cancers (Basel) 2021;13(22):5823. doi: 10.3390/cancers13225823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sequist L.V., Han J.Y., Ahn M.J., et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21:373–386. doi: 10.1016/S1470-2045(19)30785-5. [DOI] [PubMed] [Google Scholar]
- 39.Pikor L.A., Ramnarine V.R., Lam S., Lam W.L. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer. 2013;82:179–189. doi: 10.1016/j.lungcan.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 40.Shaw A.T., Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17:2081–2086. doi: 10.1158/1078-0432.CCR-10-1591. [DOI] [PubMed] [Google Scholar]
- 41.Mok T., Camidge D.R., Gadgeel S.M., et al. Updated OS and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31:1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- 42.Gadgeel S., Peters S., Mok T., et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29:2214–2222. doi: 10.1093/annonc/mdy405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camidge D.R., Kim H.R., Ahn M.J., et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J Clin Oncol. 2020;38:3592–3603. doi: 10.1200/JCO.20.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camidge D.R., Kim H.R., Ahn M.J., et al. Brigatinib versus crizotinib in ALK-Positive non-small-Cell Lung Cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 45.Solomon B.J., Besse B., Bauer T.M., et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 46.Solomon B.J., Bauer T.M., Ou S.H.I., et al. Post hoc analysis of lorlatinib intracranial efficacy and safety in patients with ALK-positive advanced non–small-cell lung cancer from the phase III CROWN study. J Clin Oncol. 2022;40(31):3593–3602. doi: 10.1200/JCO.21.02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw A.T., Bauer T.M., de Marinis F., et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 48.Solomon B., Bauer T.M., De Marinis F., et al. LBA2 Lorlatinib vs crizotinib in the first-line treatment of patients (pts) with advanced ALK-positive non-small cell lung cancer (NSCLC): results of the phase III crown study. Ann Oncol. 2020;31(suppl 4):S1180–S1181. [Google Scholar]
- 49.Camidge D.R. Lorlatinib should not be considered as the preferred first-line option in patients with advanced ALK rearranged NSCLC. J Thorac Oncol. 2021;16:528–531. doi: 10.1016/j.jtho.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Tan A.C., Itchins M., Khasraw M. Brain metastases in lung cancers with emerging targetable fusion drivers. Int J Mol Sci. 2020;21(4):1416. doi: 10.3390/ijms21041416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y., Zhang X., Zhang R., et al. Clinical and molecular factors that impact the efficacy of first-line crizotinib in ROS1-rearranged non-small-cell lung cancer: a large multicenter retrospective study. BMC Med. 2021;19:206. doi: 10.1186/s12916-021-02082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sehgal K., Piper-Vallillo A.J., Viray H., Khan A.M., Rangachari D., Costa D.B. Cases of ROS1-rearranged lung cancer: when to use crizotinib, entrectinib, lorlatinib, and beyond? Precis. Cancer Med. 2020;3:17. doi: 10.21037/pcm-2020-potb-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frost N., Christopoulos P., Kauffmann-Guerrero D., et al. Lorlatinib in pretreated ALK- or ROS1-positive lung cancer and impact of TP53 co-mutations: results from the German early access program. Ther Adv Med Oncol. 2021;13 doi: 10.1177/1758835920980558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yun M.R., Kim D.H., Kim S.Y., et al. Repotrectinib exhibits potent antitumor activity in treatment-naive and solvent-front-mutant ROS1-rearranged non-small cell lung cancer. Clin Cancer Res. 2020;26:3287–3295. doi: 10.1158/1078-0432.CCR-19-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive non-small-Cell Lung Cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 56.Gandhi L., Rodriguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg S.B., Gettinger S.N., Mahajan A., et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 59.Carbone D., Ciuleanu T., Cobo M., et al. OA09.01 first-line nivolumab + ipilimumab + chemo in patients with advanced NSCLC and brain metastases: results from CheckMate 9LA. J Thorac Oncol. 2021;16(10 suppl):S862. [Google Scholar]
- 60.Reck M., Ciuleanu T.E., Pluzanski A., et al. 122MO Nivolumab (NIVO) + ipilimumab (IPI) as first-line (1L) treatment (tx) for patients (pts) with advanced NSCLC (aNSCLC) and baseline (BL) brain metastases (mets): intracranial and systemic outcomes from CheckMate 227 Part 1. Ann Oncol. 2021;32(suppl 7):S1430–S1431. [Google Scholar]
- 61.Nadal E., Rodriguez-Abreu D., Massuti B., et al. Updated analysis from the ATEZO-BRAIN trial: atezolizumab plus carboplatin and pemetrexed in patients with advanced nonsquamous non–small cell lung cancer with untreated brain metastases. J Clin Oncol. 2022;40(suppl 16) doi: 10.1200/JCO.22.02561. 9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seute T., Leffers P., ten Velde G.P., Twijnstra A. Leptomeningeal metastases from small cell lung carcinoma. Cancer. 2005;104:1700–1705. doi: 10.1002/cncr.21322. [DOI] [PubMed] [Google Scholar]
- 63.Buszek S.M., Chung C. Radiotherapy in leptomeningeal disease: a systematic review of randomized and non-randomized trials. Front Oncol. 2019;9:1224. doi: 10.3389/fonc.2019.01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng H., Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:e43–e55. doi: 10.1016/S1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- 65.Foreman P.M., Jackson B.E., Singh K.P., et al. Postoperative radiosurgery for the treatment of metastatic brain tumor: evaluation of local failure and leptomeningeal disease. J Clin Neurosci. 2018;49:48–55. doi: 10.1016/j.jocn.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson M.D., Avkshtol V., Baschnagel A.M., et al. Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94:537–543. doi: 10.1016/j.ijrobp.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 67.Ma R., Levy M., Gui B., et al. Risk of leptomeningeal carcinomatosis in patients with brain metastases treated with stereotactic radiosurgery. J Neurooncol. 2018;136:395–401. doi: 10.1007/s11060-017-2666-7. [DOI] [PubMed] [Google Scholar]
- 68.Mahajan A., Ahmed S., McAleer M.F., et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang A.J., Huang K.E., Page B.R., et al. Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J Neurooncol. 2014;120:163–169. doi: 10.1007/s11060-014-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prabhu R.S., Turner B.E., Asher A.L., et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neurooncol. 2019;21:1049–1059. doi: 10.1093/neuonc/noz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X., Cai J., Zeng Z., Liu A. Efficacy of osimertinib for preventing leptomeningeal metastasis derived from advanced EGFR-mutated non-small cell lung cancer: a propensity-matched retrospective study. BMC Cancer. 2021;21:873. doi: 10.1186/s12885-021-08581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang J.C.H., Kim S.W., Kim D.W., et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38:538–547. doi: 10.1200/JCO.19.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turkaj A., Morelli A.M., Vavalà T., Novello S. Management of leptomeningeal metastases in non-oncogene addicted non-small cell lung cancer. Front Oncol. 2018;8:278. doi: 10.3389/fonc.2018.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aoyama H., Shirato H., Tago M., et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain Metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 75.Kocher M., Soffietti R., Abacioglu U., et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]