Abstract
Background & Aims
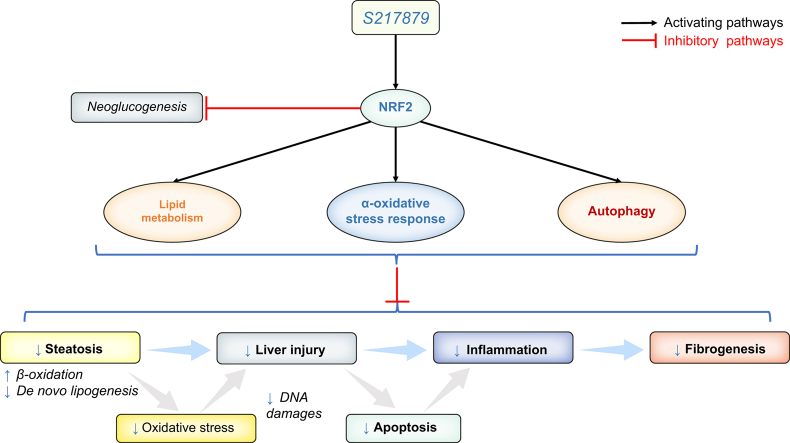
Oxidative stress triggers metabolic-associated fatty liver disease (MAFLD) and fibrosis. Previous animal studies demonstrated that the transcription factor nuclear factor (erythroid-derived 2)-like 2 (NRF2), the master regulator of antioxidant response, protects against MAFLD and fibrosis. S217879, a next generation NRF2 activator has been recently shown to trigger diet-induced steatohepatitis resolution and to reduce established fibrosis in rodents. Our aim was to evaluate the therapeutic potential of S217879 in human MAFLD and its underlying mechanisms using the relevant experimental 3D model of patient-derived precision cut liver slices (PCLS).
Methods
We treated PCLS from 12 patients with varying stages of MAFLD with S217879 or elafibranor (peroxisome proliferator-activated receptor [PPAR]α/δ agonist used as a referent molecule) for 2 days. Safety and efficacy profiles, steatosis, liver injury, inflammation, and fibrosis were assessed as well as mechanisms involved in MAFLD pathophysiology, namely antioxidant response, autophagy, and endoplasmic reticulum-stress.
Results
Neither elafibranor nor S217879 had toxic effects at the tested concentrations on human PCLS with MAFLD. PPARα/δ and NRF2 target genes (pyruvate dehydrogenase kinase 4 [PDK4], fibroblast growth factor 21 [FGF21], and NAD(P)H quinone dehydrogenase 1 [NQO1], heme oxygenase 1 [HMOX1], respectively) were strongly upregulated in PCLS in response to elafibranor and S217879, respectively. Compared with untreated PCLS, elafibranor and S217879-treated slices displayed lower triglycerides and reduced inflammation (IL-1β, IL-6, chemokine (C–C motif) ligand 2 [CCL2]). Additional inflammatory markers (chemokine (C–C motif) ligand 5 [CCL5], stimulator of interferon genes [STING], intercellular adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 [VCAM-1]) were downregulated by S217879. S217879 but not elafibranor lowered DNA damage (phospho-Histone H2A.X [p-H2A.X], RAD51, X-ray repair cross complementing 1 [XRCC1]) and apoptosis (cleaved caspase-3), and inhibited fibrogenesis markers expression (alpha smooth muscle actin [α-SMA], collagen 1 alpha 1 [COL1A1], collagen 1 alpha 2 [COL1A2]). Such effects were mediated through an improvement of lipid metabolism, activated antioxidant response and enhanced autophagy, without effect on endoplasmic reticulum-stress.
Conclusions
This study highlights the therapeutic potential of a new NRF2 activator for MAFLD using patient-derived PCLS supporting the evaluation of NRF2 activating strategies in clinical trials.
Impact and implications
Oxidative stress is a major driver of metabolic-associated fatty liver disease (MAFLD) development and progression. Nuclear factor (erythroid-derived 2)-like 2, the master regulator of the antioxidative stress response, is an attractive therapeutic target for the treatment of MAFLD. This study demonstrates that S217879, a new potent and selective nuclear factor (erythroid-derived 2)-like 2 activator, displays antisteatotic effects, lowers DNA damage, apoptosis, and inflammation and inhibits fibrogenesis in human PCLS in patients with MAFLD.
Keywords: NRF2, S217879, Elafibranor, MAFLD, NASH, Oxidative stress, PCLS, Inflammation, Fibrosis
Graphical abstract
Highlights
-
•
S217879, a new NRF2 activator, induces a potent antioxidative stress response in human PCLS with MAFLD.
-
•
S217879 displays antisteatotic effects by improving lipid metabolism in human PCLS with MAFLD.
-
•
S217879 has a therapeutic potential by preventing DNA damage, apoptosis, and inflammation in human PCLS with MAFLD.
-
•
S217879 inhibits fibrogenesis by preventing hepatic stellate cells activation in human PCLS with MAFLD.
Introduction
Metabolic-associated fatty liver disease (MAFLD) is an expanding health problem with an estimated global prevalence of 25%. MAFLD is associated with obesity, insulin resistance, or type 2 diabetes and other metabolic abnormalities collectively termed metabolic syndrome.[1], [2], [3], [4] MAFLD encompasses a spectrum of histological lesions ranging from simple steatosis to steatohepatitis which includes, in addition to steatosis, hepatocellular ballooning, and inflammation with varying degrees of fibrosis.[5], [6], [7] MAFLD can progress to cirrhosis and hepatocellular carcinoma and is projected to become the leading indication for liver transplantation in the next decades.[8], [9], [10] Despite its prevalence and severity, there is still no approved therapy for this condition11 and this can be attributed, at least in part, to the poor translational value of animal and in vitro models used in preclinical studies, supporting the critical need for more relevant experimental tools.
Oxidative stress is a key pathogenic event that promotes MAFLD progression from steatosis to steatohepatitis and fibrogenesis. During the course of the disease, lipotoxicity and increased levels of reactive oxygen species (ROS) lead to oxidative cellular and DNA damage and to the activation of apoptotic, inflammatory, and fibrotic pathways.5,12,13 Patients with MAFLD display increased levels of ROS and lipid peroxidation products, excessive systemic and hepatic oxidative stress, and decreased levels of antioxidant enzymes and compounds such as glutathione (GSH).[13], [14], [15], [16], [17] The nuclear factor (erythroid-derived 2)-like 2 (NRF2)/Kelch-like ECH-associated protein 1 (KEAP1) system is the master intracellular regulatory system of the antioxidant response to maintain cellular homeostasis.18 Under physiological conditions, KEAP1 binds and retains NRF2 in the cytoplasm leading to its ubiquitination and proteosomal degradation.19,20 Upon exposure to oxidative stress, KEAP1 releases NRF2 allowing its nuclear translocation and the induction of NRF2 target genes involved in oxidative stress-induced responses.21,22 NRF2 plays critical roles in various liver diseases including MAFLD,23,24 and its liver expression is downregulated in patients with MAFLD and in murine models.[25], [26], [27] Nrf2 deficient animals are more susceptible to diet-induced steatosis and inflammation.27,28 In contrast, genetic or pharmacological NRF2 activation attenuates diet-induced steatohepatitis and fibrosis,16,29,30 raising new hopes for MAFLD treatment through NRF2 pharmacological activators. Although encouraging results have been observed in experimental diet-induced steatohepatitis models, NRF2-based therapies have not been evaluated in a clinical setting to date.24
The availability of KEAP1 X-ray structure31 allowed the discovery of second generation NRF2 activators via the direct disruption of its interaction with KEAP132 but with limited oral bioavailability.33 We recently developed a new potent and selective small molecule disrupting KEAP1–NRF2 interaction, S217879, with good pharmacokinetic properties upon oral administration in rodents, with protective effects on diet-induced steatohepatitis and fibrosis murine models.34 Here, effects of S217879 were assessed in patients with MAFLD using the relevant human precision cut liver slices (PCLS) model, that maintains the morphological and biological organisation of the liver (cell heterogeneity and distribution, extracellular matrix) and all pathological features of the disease.35 Effects of S217879 were assessed and compared with those of the well-known peroxisome proliferator-activated receptor (PPAR)α/δ agonist, elafibranor.[36], [37], [38]
Materials and methods
Human liver samples
Fresh liver tissues were obtained from 12 patients with MAFLD undergoing liver resection at the digestive surgical department of Beaujon Hospital (Clichy, France) for primary or secondary liver cancer (11/12), or from an explanted liver (1/12). Only liver samples collected within 3 h after surgery were used to minimise ischaemic time and preserve hepatocellular viability. Liver specimens were examined by a pathologist, and samples were taken from the most distal nontumoral tissue. Indications for surgery, MAFLD evaluation and alcohol use are detailed in Table S1. Patients’ metabolic features are presented in Table S2. None of the patients had detectable anti-hepatitis C virus antibodies or HBs antigen. All patients gave a written consent to participate the study. This study was approved by the Institutional Review Board CPP SUD MEDITERRANEE V (NCT03634098). The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
PCLS generation and treatments
Fresh liver specimens were harvested and immediately kept in cold sterile University of Wisconsin solution (Belzer UW®, Bridge to Life, London, UK). Tissue cores were generated using 8-mm diameter biopsy punches, embedded into 5% low-gelling-temperature agarose (Sigma-Aldrich, Saint Quentin Fallavier, France) and mounted in a tissue slicer (Leica Biosystems VT1200S) filled with Hanks Balanced Salt Solution supplemented with 25 mM d-glucose (Sigma Aldrich, Saint Quentin Fallavier, France), 100 μg/ml streptomycin, 1 μg/ml amphotericin B (Gibco™, Thermo Fisher Scientific, Courtaboeuf, France). Samples of 250 μm thickness PCLS39 were generated using the following slicing parameters: speed 0.5 mm/s; thickness 250 μm; amplitude 3 mm. PCLS were transferred on 8-μm PET-tissue culture inserts (ThinCert™, Greiner bio-one, Courtaboeuf, France) in six-well plates containing 2 ml William’s E Medium (Gibco™, Thermo Fisher Scientific, Courtaboeuf, France) supplemented with 100 IU/ml penicillin and 100 μg/ml streptomycin, 1 μg/ml amphotericin B (Gibco™, Thermo Fisher Scientific, Courtaboeuf, France), and 25 mM d-glucose (Sigma-Aldrich, Saint Quentin Fallavier, France), to maintain the slice in an air–liquid interface and avoid tissue hypoxia.[39], [40], [41] After 2 h pre-incubation, medium was replaced with fresh culture medium in the presence or absence of 10 μM elafibranor42 (Selleckchem S3720, Planegg, Germany) or 3 μM S217879 (synthesised by Servier Medicinal Chemistry department), corresponding to the drug concentration reaching the liver upon oral administration in rodents (30 mg/kg)34 (dissolved in DMSO, final concentration 0.1%). PCLS were cultivated at 37 °C, 5% CO2, in normoxic conditions, under continuous orbital agitation (70 rpm) for 48 h. Culture medium was renewed daily. In another set of experiments, PCLS were treated with chloroquine (300 μmol/L, Sigma-Aldrich, Saint Quentin Fallavier, France) for 1 h before the end of the 48-h culture period, to assess autophagic flux. Patients’ characteristics for this set of experiments are detailed in Table S3. After 48 h of culture, PCLS were washed in cold phosphate-buffered saline and immediately snap-frozen in liquid nitrogen and stored at -80 °C until processing (for gene, protein, ATP, and triglycerides experiments) or fixed in 10% formalin and embedded in paraffin for histology analyses as described in the Supplementary information.
Statistics
Data are expressed as mean ± SEM. Comparisons were performed using the paired Wilcoxon t test. All tests were two-sided and a significance level of 0.05 was used. Statistical analyses and figures were performed/created using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) for Windows 11.
Results
Drug safety and efficacy
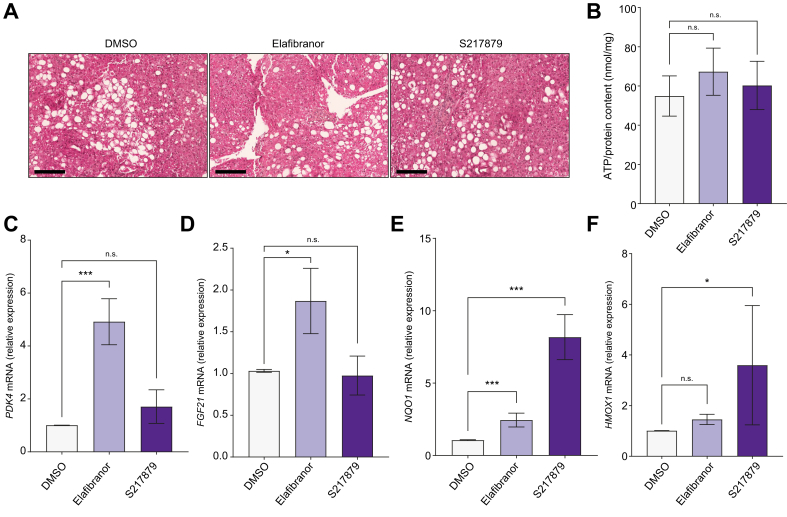
We first tested liver slices viability in response to 48 h of treatment with elafibranor (10 μM)42 or S217879 (3 μM) or vehicle (DMSO, 0.1%). No effects of elafibranor or S217879 on PCLS morphology were observed at histological examination (Fig. 1A). The ATP/protein content ratio, as a reflect of slices viability,39 was similar between elafibranor or S217879 and DMSO-treated PCLS (Fig. 1B). Of note, neither ATP nor protein contents were modulated by elafibranor or S217879 treatment (Fig. S1A and B). Furthermore, RNA content as well as aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) leakage in culture supernatants were not significantly affected by both treatments (Fig. S1C–E).
Fig. 1.
Drug safety and efficacy.
Human PCLS were generated from the liver of patients with MAFLD (n = 12) and treated with elafibranor (10 μM) or S217879 (3 μM) or vehicle (DMSO, 0.1%) for 2 days. (A) Representative images of H&E staining of human PCLS used for histological analysis of slice morphology. Scale bar: 200 μm. (B) Quantification of ATP content in human PCLS (ATP/protein content ratio). (C) qPCR analysis of PPARα/δ target genes expression PDK4 and (D) FGF21. (E) qPCR analysis of NRF2 target genes expression NQO1 and (F) HMOX1. Data are expressed as mean ± SEM. ∗p <0.05; ∗∗∗p <0.001; ns, not significant (Wilcoxon paired t test). FGF21, fibroblast growth factor 21; HMOX1, heme oxygenase 1, MAFLD, metabolic-associated fatty liver disease; NQO1, NAD(P)H quinone dehydrogenase 1; NRF2, nuclear factor (erythroid-derived 2)-like 2; PCLS, precision cut liver slices; PDK4, pyruvate dehydrogenase kinase 4; PPAR, peroxisome proliferator-activated receptor; qPCR, quantitative PCR.
We then confirmed target engagement induced by both molecules by assessing the expression of their respective target genes. PPARα/δ target genes pyruvate dehydrogenase kinase 4 (PDK4) and fibroblast growth factor 21 (FGF21) were upregulated in elafibranor but not in S217879-treated PCLS (Fig. 1C and D). Similarly, NRF2 target genes NAD(P)H quinone dehydrogenase 1 (NQO1) and heme oxygenase 1 (HMOX1) were strongly induced by S217879 (Fig. 1E and F). Of note, NQO1 expression, but not HMOX1 expression, was slightly induced by elafibranor (Fig. 1E and F). Collectively, these data indicate that elafibranor and S217879 have no hepatotoxic effects, and effectively induce their respective target genes in human PCLS with MAFLD at the tested concentrations.
S217879 improves features of steatohepatitis in human PCLS with MAFLD
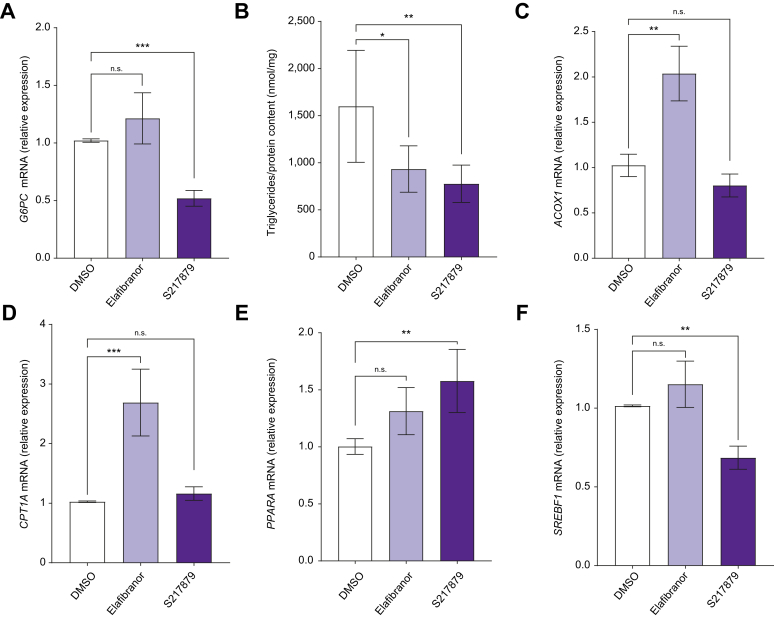
To assess effects of elafibranor and S217879 on liver metabolic features, we first evaluated glucose-6-phosphatase (G6PC) gene expression, a key enzyme of neoglucogenesis. Compared with vehicle-treated PCLS, G6PC expression was reduced by S217879 but not by elafibranor (Fig. 2A). In line with this, glucose concentration in culture supernatants was not affected by elafibranor and was reduced after S217879 treatment (Fig. S2A). We then assessed whether these treatments were able to modulate steatosis. No changes in steatosis using semiquantitative score could be evidenced at histological examination between elafibranor or S217879 and DMSO-treated PCLS (Fig. 1A). For a more precise analysis of steatosis, we measured triglycerides content in PCLS and observed that triglycerides on protein content ratio was significantly lower in both elafibranor and S217879-treated PCLS compared with the vehicle (Fig. 2B). We then sought to identify which metabolic pathways were involved in this improvement of triglycerides content by assessing gene expression of key players of lipid metabolism. Elafibranor significantly increased peroxisomal acyl-coenzyme A oxidase-1 (ACOX1) and carnitine palmitoyltransferase-1-alpha (CPT1A) gene expression (Fig. 2C and D), both enzymes involved in fatty acids beta-oxidation. However, S217879 significantly increased peroxisome proliferator-activated receptor-alpha (PPARA) gene expression, also involved in fatty acid beta-oxidation, and significantly downregulated sterol regulatory element-binding protein-1 (SREBF1) and fatty acid synthase (FASN) (Fig. 2E and F, Fig. S2B) gene expression, both involved in de novo lipogenesis. Elafibranor had no effect on PPARA, SREBF1, and FASN expression (Fig. 2E and F, Fig. S2B), and S217879 had no effect on ACOX1 and CPT1A (Fig. 2C and D) expression. These results indicate that both elafibranor and S217879 improve steatosis, whereas only S217879 improves glucose metabolism in human PCLS with MAFLD.
Fig. 2.
Effects of elafibranor and S217879 treatments on liver metabolic features.
Human PCLS were generated from the liver of patients with MAFLD (n = 12) and treated with elafibranor (10 μM) or S217879 (3 μM) or vehicle (DMSO, 0.1%) for 2 days. (A) qPCR analysis of G6PC expression in human PCLS. (B) Quantification of triglycerides content in human PCLS (triglycerides/protein content ratio). (C) qPCR analysis of ACOX1, (D) CPT1A, (E) PPARA and (F) SREBF1 expression in human PCLS. Data are expressed as mean ± SEM. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001; ns, not significant (Wilcoxon paired t test). ACOX1, peroxisomal acyl-coenzyme A oxidase 1; CPT1A, carnitine palmitoyl transferase 1 alpha; G6PC, glucose-6-phosphatase; MAFLD, metabolic-associated fatty liver disease; PCLS, precision cut liver slices; PPARA, peroxisome proliferator-activated receptor-alpha; qPCR, quantitative PCR; SREBF1, sterol regulatory element-binding protein-1.
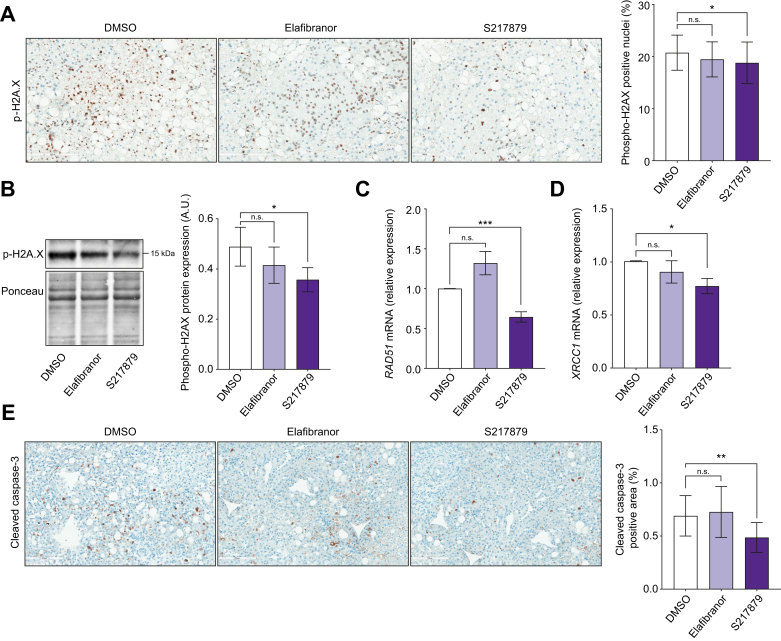
Oxidative stress promotes liver injury and DNA damage that initiate hepatic cell death in MAFLD.[43], [44], [45] We tested whether elafibranor and S217879 were able to lower liver injury in human PCLS with MAFLD. As mentioned above, AST and LDH levels in culture supernatants were not modulated neither by elafibranor nor by S217879 treatments (Fig. S1D and E). As oxidative stress initiates apoptosis by inducing DNA damage,44 we next investigated the expression of several markers of this pathway. Immunohistochemical and immunoblot analysis of phospho-Histone H2A.X (p-H2A.X) displayed less double-strand DNA damage in S217879-treated PCLS but not in elafibranor-treated slices (Fig. 3A and B). Consequently, liver expression of RAD51 (double-strand DNA damage repair enzyme), and X-ray repair cross complementing 1 (XRCC1; single-strand DNA damage repair enzyme), was significantly and specifically reduced in S217879-treated PCLS (Fig. 3C and D). Accordingly, immunohistochemical analysis of cleaved caspase-3 on PCLS sections revealed that only S217879 lowered cleaved caspase-3 area when compared with untreated slices (Fig. 3E). These results demonstrate that S217879, rather than elafibranor, reduces liver apoptosis by, at least in part, improving hepatic DNA damage.
Fig. 3.
S217879 but not elafibranor reduces liver DNA damage and apoptosis.
Human PCLS were generated from the liver of patients with MAFLD (n = 12) and treated with elafibranor (10 μM) or S217879 (3 μM) or vehicle (DMSO, 0.1%) for 2 days. (A) Immunohistochemical analysis of p-H2A.X-positive nuclei (%) on human PCLS sections. Scale bar: 200 μm. (B) Immunoblot analysis of liver p-H2A.X expression in human PCLS. (C) qPCR analysis of RAD51 and (D) XRCC1 expression in human PCLS. (E) Immunohistochemical analysis of cleaved Caspase-3 positive area (%) on human PCLS sections. Scale bar: 200 μm. Data are expressed as mean ± SEM. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001; ns, not significant (Wilcoxon paired t test). MAFLD, metabolic-associated fatty liver disease; PCLS, precision cut liver slices; p-H2A.X, phospho-Histone H2A.X; qPCR, quantitative PCR; XRCC1, X-ray repair cross complementing 1.
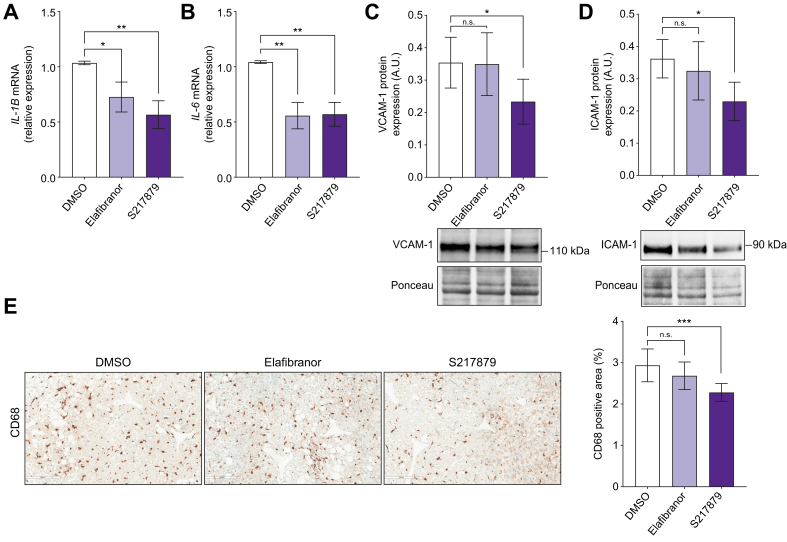
Having observed that S217879 reduces steatosis, liver DNA damage, and apoptosis, we hypothesised that this new NRF2 activator could also display anti-inflammatory properties in human PCLS with MAFLD. We first confirmed that elafibranor improved liver inflammation. As expected,36,37 elafibranor significantly reduced IL-1β, IL-6 (Fig. 4A and B) and chemokine (C–C motif) ligand 2 (CCL2) (Fig. S3A) liver gene expression. However, elafibranor had no effect on chemokine (C–C motif) ligand 5 (CCL5) gene expression (Fig. S3B), vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) protein expression (Fig. 4C and D), both markers of vascular inflammation, stimulator of interferon genes (STING) protein expression (Fig. S3C), a key player of the cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING) signalling pathway involved in innate immune response in MAFLD,46,47 and did not change macrophages markers expression as assessed on CD68 immunohistochemical analysis (Fig. 4E). Compared with vehicle-treated PCLS, S21879-treated slices had a significant lower gene expression of IL-1β, IL-6 (Fig. 4A and B), CCL2 and CCL5 (Fig. S3A and B). VCAM-1, ICAM-1 (Fig. 4C and D) and STING (Fig. S3C) protein expression was significantly reduced in S217879-treated PCLS compared with the vehicle. Moreover, the CD68-positive area was reduced in S217879-treated PCLS compared with untreated slices (Fig. 4E). Interestingly, similar effects of elafibranor and S217879 treatments were observed in PCLS from patients with advanced liver fibrosis (F3–4, n = 9) (Fig. S4A–H). These results indicate that both elafibranor and S217879 inhibit liver inflammatory pathways in human PCLS with MAFLD with the strongest anti-inflammatory response induced by S217879.
Fig. 4.
Elafibranor and S217879 reduce liver inflammation.
Human PCLS were generated from the liver of patients with MAFLD (n = 12) and treated with elafibranor (10 μM) or S217879 (3 μM) or vehicle (DMSO, 0.1%) for 2 days. (A) qPCR analysis of IL-1β and (B) IL-6 expression in human PCLS. (C) Immunoblot analysis of VCAM-1 and (D) ICAM-1 expression in human PCLS. (E) Immunohistochemical analysis of CD68 positive area (%) in human PCLS. Scale bar: 200 μm. Data are expressed as mean ± SEM. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001; ns, not significant (Wilcoxon paired t test). ICAM-1, intercellular adhesion molecule-1; MAFLD, metabolic-associated fatty liver disease; PCLS, precision cut liver slices; qPCR, quantitative PCR; VCAM-1, vascular cell adhesion molecule-1.
Collectively, these observations demonstrate that S217879 displays therapeutic properties against MAFLD in human liver samples.
S217879 reduces fibrosis markers expression in human PCLS with MAFLD
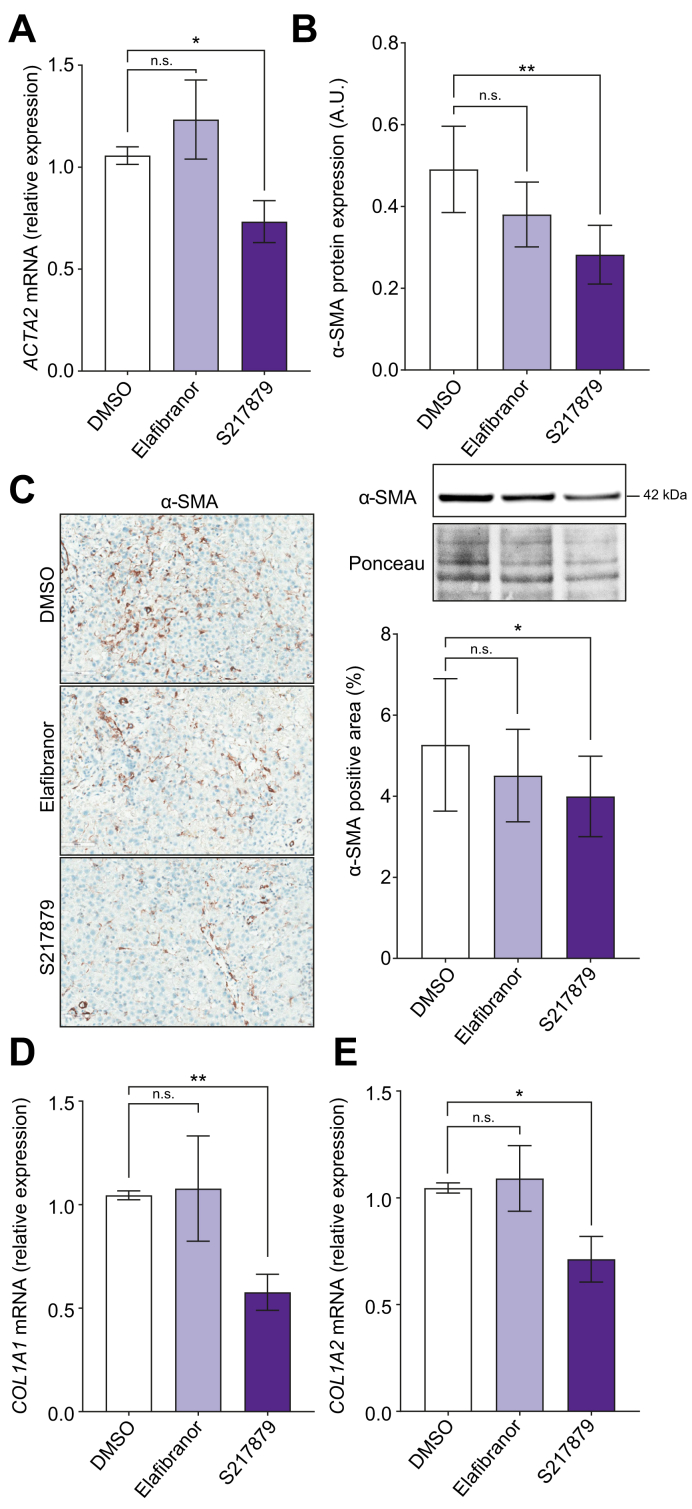
Sustained liver cell death and inflammation trigger hepatic stellate cells activation and fibrogenesis in MAFLD,5 so that prompted us to evaluate effects of both drugs on fibrogenesis. As expected,37 elafibranor had no effect on liver fibrogenesis markers expression in human PCLS with MAFLD (ACTA2, alpha smooth muscle actin [α-SMA], collagen 1 alpha 1 [COL1A1], and collagen 1 alpha 2 [COL1A2]) (Fig. 5). By contrast, qPCR, immunohistochemistry, and immunoblot analyses showed a reduction of α-SMA expression, a marker of hepatic stellate cells activation (Fig. 5A–C) in S217879-treated PCLS. Accordingly, this reduced hepatic stellate cell activation was associated with a decrease in COL1A1 and COL1A2 expression (Fig. 5D and E). Importantly, S217879 treatment was also effective to inhibit fibrogenesis markers expression specifically in PCLS from patients with advanced fibrosis (F3–4, n = 9) (Fig. S4I and J). Of note, fibrosis deposition evaluated on Sirius Red staining using quantitative approaches remained unchanged between elafibranor or S217879 and untreated PCLS, likely because of the short duration of treatment (Fig. S5).
Fig. 5.
S217879 but not elafibranor inhibits fibrogenesis.
Human PCLS were generated from the liver of patients with MAFLD (n = 12) and treated with elafibranor (10 μM) or S217879 (3 μM) or vehicle (DMSO, 0.1%) for 2 days. (A) qPCR analysis of ACTA2 expression in human PCLS. (B) Immunoblot analysis of α-SMA expression in human PCLS. (C) Immunohistochemical analysis of α-SMA-positive area (%) on human PCLS sections. Scale bar: 200 μm. (D) qPCR analysis of COL1A1 and (E) COL1A2 expression in human PCLS. Data are expressed as mean ± SEM. ∗p <0.05; ∗∗p <0.01; ns, not significant (Wilcoxon paired t test). α-SMA, alpha-smooth muscle actin; COL1A, collagen 1-alpha; MAFLD, metabolic-associated fatty liver disease; PCLS, precision cut liver slices; qPCR, quantitative PCR.
S217879 activates the antioxidant response and autophagy but does not modulate ER-stress in human PCLS with MAFLD
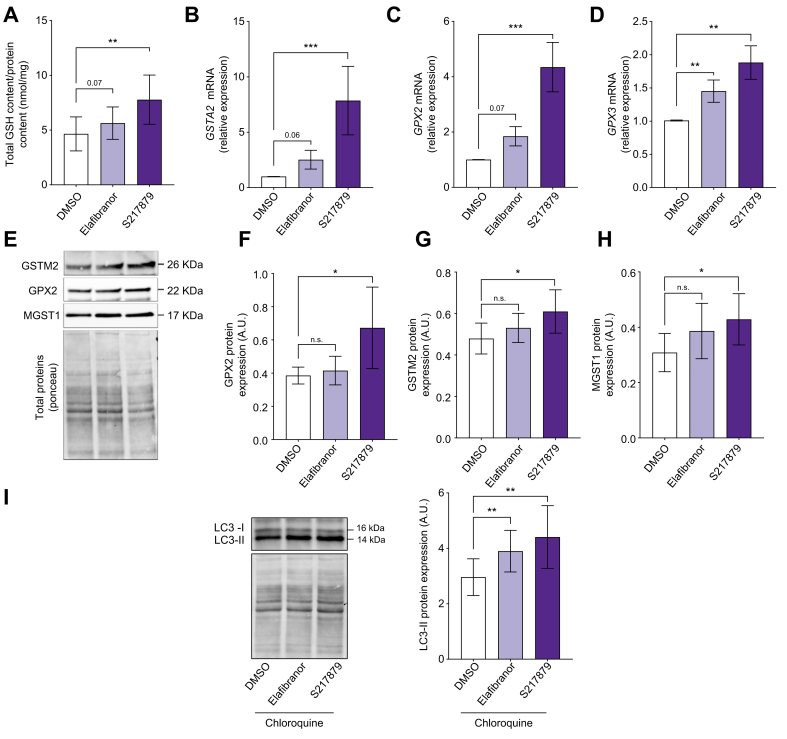
To dissect cellular mechanisms by which elafibranor and S217879 improve MAFLD features, we first evaluated the antioxidant response induced by both treatments. As expected,38,48 elafibranor treatment tended to increase total GSH content (Fig. 6A), glutathione S-transferase alpha-2 (GSTA2) and glutathione peroxidase 2 (GPX2) gene expression without reaching statistical significance, but significantly induced glutathione peroxidase-3 (GPX3) gene expression (Fig. 6B–D), all detoxifying enzymes involved in antioxidant response. At the protein level, elafibranor had no significant effect on detoxifying enzymes GPX2, glutathione S-transferase mu-2 (GSTM2), microsomal glutathione S-transferase-1 (MGST1), glutathione S-transferase theta-1 (GSTT1), and superoxide dismutase (SOD) expression (Fig. 6E–H; Fig. S6A–C). Compared with untreated PCLS, S217879-treated slices had a higher GSH content (Fig. 6A) and a strong overexpression of GSTA2, GPX2, and GPX3 (Fig. 6B–D). S217879 also significantly increased GPX2, GSTM2, and MGST1 protein expression and had no significant effect on GSTT1 and SOD protein expression (Fig. 6E–H; Fig. S6A–C). As expected, S217879 induced a more pronounced antioxidant response than elafibranor.
Fig. 6.
Elafibranor and S217879 induce antioxidative stress response and enhance autophagy.
Human PCLS were generated from the liver of patients with MAFLD (n = 12) and treated with elafibranor (10 μM) or S217879 (3 μM) or vehicle (DMSO, 0.1%) for 2 days. (A) Quantification of total GSH content in human PCLS (GSH/protein content ratio). (B) qPCR analysis of GSTA2, (C) GPX2 and (D) GPX3 expression in human PCLS. (E) Representative western blotting of GSTM2, GPX2 and MGST1 expression. (F) Quantification of GPX2, (G) GSTM2, and (H) MGST1 protein expression. (I) Immunoblot analysis of LC3-II expression in human PCLS (n = 11). Chloroquine (300 μM) was added for the last hour of the culture period. Data are expressed as mean ± SEM. ∗p <0.05; ∗∗p <0.01; ∗∗∗p <0.001; ns, not significant (Wilcoxon paired t test). GPX2, glutathione peroxidase 2; GPX3, glutathione peroxidase 3; GSH, glutatione; GSTA2, glutathione S-transferase alpha-2; GSTM2, glutathione S-transferase Mu 2; LC3, light chain-3; MAFLD, metabolic-associated fatty liver disease; MGST1, microsomal glutathione S-transferase 1; PCLS, precision cut liver slices; qPCR, quantitative PCR.
Reduced autophagy and increased ER-stress are two early events occurring in MAFLD promoting disease progression.5,[49], [50], [51] We hypothesised that beneficial effects of elafibranor and S217879 could be mediated, in addition to their well-known mechanisms of action, through autophagy activation and ER-stress improvement. Microtubule-associated protein light chain-3 (LC3, marker of autophagy) gene (MAP1LC3B) and protein (LC3-II) expression were both induced by elafibranor but not by S217879 under basal conditions (Fig. S6D-E). Because LC3 is post-translationally modified (lipidation of LC3-I to form LC3-II), and considering that a lack of effect or a change in LC3-II protein expression can reflect both unmodified or reduced or enhanced autophagic flux,52 we performed the same experiment by blocking late steps of autophagic flux with chloroquine. As shown in Fig. 6I, LC3-II protein expression was higher in PCLS treated with elafibranor and with S217879 compared with untreated PCLS, suggesting that both treatments activated autophagic flux. We also evaluated C/EBP homologous protein (CHOP) expression which plays an important role in ER-stress-mediated apoptosis and inflammation in MAFLD.51 Immunoblot analysis revealed that CHOP expression was not modulated by elafibranor or by S217879 (Fig. S6F), suggesting that both treatments do not act directly on ER-stress in human PCLS with MAFLD. Collectively, our results indicate that elafibranor and S217879 may work, in addition to their well-known mechanisms of action, via autophagy activation in the liver.
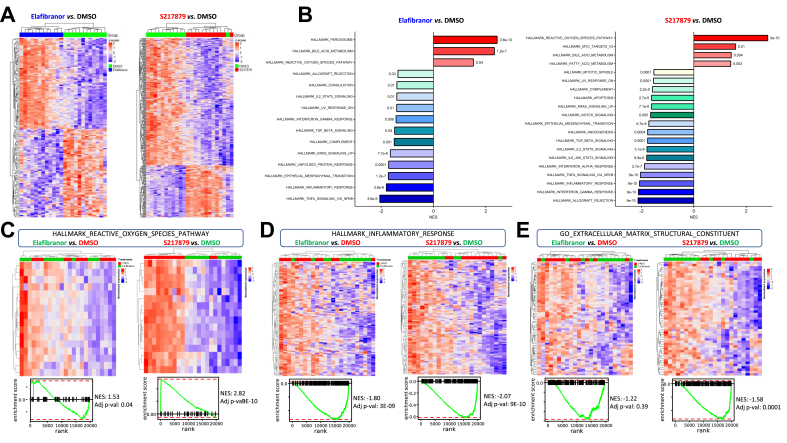
To broaden in the analysis of the mechanisms by which elafibranor and S217879 elicit their properties in human PCLS with MAFLD, we performed an RNA-sequencing analysis on liver total RNA. A total of 150 and 344 genes were significantly differentially expressed between elafibranor (76 up/74 down) and S217879 (100 up/244 down) vs. vehicle-treated PCLS (adjusted p value cut-off <0.05), respectively (Fig. 7A, Table S6). These transcriptional changes were further explored by gene set-enrichment analysis, which first confirmed the potent induction of the antioxidant response by S217879. Indeed, the ROS pathway was the most affected pathway in S217879-treated PCLS with a strong enrichment in antioxidant genes (Fig. 7B–C, Figs. S7A, S8, Table S6). As expected, elafibranor had a less pronounced effect on antioxidant pathways (Fig. 7B and C, Figs. S7A and S8, Table S6). Gene set-enrichment analysis also confirmed the protective properties of both treatments against steatohepatitis since inflammatory pathways such as ‘inflammatory response’ and ‘tumour necrosis factor alpha (TNFA) signalling’ were ranked among the most downregulated pathways upon elafibranor and S217879 treatments, with a more pronounced effect observed in S217879-treated PCLS (Fig. 7B and D, Fig. S8, Table S6). S217879 treatment additionally and specifically inhibited the apoptosis pathway, whereas elafibranor had no significant effect on this pathway (Figs. S7B and S8, Table S6). More interestingly, pathways and genes involved in fibrosis such as ‘extracellular matrix structural constituent’ were significantly downregulated by S217879, but not by elafibranor (Fig. 7 and Fig. S8, Table S6), thus confirming our previous observations (Fig. 5). The RNA-sequencing analysis also showed that S217879 but not elafibranor inhibited angiogenesis, that is, a key early event favouring MAFLD progression53 (Fig. S7C). Finally, the RNA sequencing analysis also confirmed the global more pronounced and additional effects of S217879 compared with those of elafibranor (Figs. S7 and S8, Table S6). A total of 368 genes were significantly differentially expressed between S217879 and elafibranor-treated slices (130 up/238 down; adjusted p value cut-off <0.05) (Fig. S9A, Table S6). As expected, antioxidant response was the most upregulated pathway in S217879-treated PCLS compared with elafibranor-treated slices (Fig. S9B–D). Pathways involved in MAFLD pathophysiology related to lipid metabolism (‘adipogenesis’), apoptosis, inflammation (‘inflammatory response’, ‘TNFA signalling’, ‘IL6 signalling’, ‘interferon gamma response’) and fibrosis (‘extracellular matrix structural constituent’, ‘transforming growth factor-β signalling’) were significantly lowered in S217879-treated PCLS when compared with elafibranor-treated slices (Fig. S9B,E–G).
Fig. 7.
S217879 treatment induces a potent antioxidant response and inhibits a wide spectrum of pathways involved in MAFLD progression.
Human PCLS were generated from the liver of patients with MAFLD (n = 12) and treated with elafibranor (10 μM) or S217879 (3 μM) or vehicle (DMSO, 0.1%) for 2 days. (A) Heatmap of significantly differentially expressed genes from elafibranor and S217879-treated PCLS with MAFLD vs. vehicle-treated PCLS (adjusted p value cut-off <0.05). (B) Gene set-enrichment analysis of most differentially modulated pathways in elafibranor and S217879-treated PCLS with MAFLD vs. vehicle-treated PCLS. (C) Heatmaps and enrichment plots of differentially expressed genes in reactive oxygen species, (D) inflammatory response, and (E) extracellular matrix structural constituent pathways in elafibranor and S217879-treated PCLS vs. vehicle treated-PCLS. MAFLD, metabolic-associated fatty liver disease; NES, normalised enrichment score; PCLS, precision cut liver slices.
Discussion
This study, based on human PCLS, a relevant ex vivo model of MAFLD, provides strong evidence for the therapeutic potential of a new NRF2 activator (S217879) by preventing steatosis, DNA damage, and apoptosis, and inhibiting inflammatory and fibrotic pathways. Such effects were mediated through the improvement of lipid metabolism, a potent induction of antioxidant response and enhanced autophagic flux. Our study extends the first description of S217879, a potent and selective small molecule disrupting KEAP1–NRF2 interaction with good safety and pharmacokinetic properties upon oral administration in two dietary-induced steatohepatitis murine models, that broadly affects key drivers of the disease.34
The strength of our study first comes from the use of the ex vivo PCLS model from patients with MAFLD to evaluate the early hepatic response to drug candidates. This model of ultra-thin liver slices culture constitutes to date the only tridimensional model that respects at the best the complex organisation of the liver, regarding its cellular heterogeneity and distribution, its micro-environment, and the pathological features of the human disease,54 overcoming the poor relevance of animal and in vitro models, especially in MAFLD. This allowed us the longitudinal study of human liver specimens obtained directly from patients with varying stages of MAFLD (from steatosis to steatohepatitis with and without fibrosis/cirrhosis). In addition, although sex differences are well-known in MAFLD,55 we included in this study both male and female patients, implying that the observed effects are not restricted to one sex. It should be also emphasised that we included in this study patients with and without type 2 diabetes and observed similar trends in drug responses whatever their diabetes status (data not shown), implying that S217879 also effectively works in patients with type 2 diabetes, a favouring condition for steatohepatitis and advanced fibrosis.6,56 A second strength of the study comes from the use of elafibranor as a reference molecule, which has shown beneficial effects in MAFLD,36,37 allowing us to validate the efficiency and the translational value of PCLS on the one hand, and to compare effects of S217879 in light of those of elafibranor on the other hand. Interestingly, S217879 exhibited more pronounced protective effects than elafibranor in this model.
The first major finding was the observation that S217879 treatment improved features of MAFLD in human livers through NRF2 pathway activation. First, S217879 lowered glucose concentration in culture supernatants by inhibiting neoglucogenesis, suggesting that this new molecule could have a potential for diabetes management in patients with MAFLD. Second, we confirmed the antisteatotic effect of elafibranor36,57 and observed the same effect of S217879 on liver triglycerides content. This antisteatotic effect was mediated by an activation of pathways involved in lipid catabolism, and S217879 additionally inhibited de novo lipogenesis pathways. This is in line with previous studies in mice showing that pharmacological and genetic NRF2 activation represses the expression of key factors of fatty acid synthesis with concomitant reduction of hepatic lipids levels.34,58,59 Besides lipid metabolism, S217879 and elafibranor enhanced autophagy in human PCLS (a prototypic catabolic process). We can yet speculate that the reduction of lipid content could be also attributed to the specific lipid degradation process by autophagy also known as lipophagy. By contrast, Mohs et al.16 reported that hepatic genetic activation of NRF2 by KEAP1 deletion in hepatocytes in mice60 did not modulate the expression of autophagy related genes. However, the authors analysed autophagy only at the gene expression level whereas autophagy proteins are highly post-translationally modified to modulate autophagic flux.52,61 Second, we demonstrated that S217879 treatment was able to reduce liver injury and inflammation. Levels of secreted common markers of liver injury (AST, LDH) were not modulated in culture supernatants in this model, whereas we observed a reduction of liver DNA damage that was associated with less apoptosis in S217879 treated slices. This result is in line with the recent description of the protective role of genetic NRF2 activation against oxidative stress-induced DNA damage and consequent apoptosis observed in the hepatocytes-NF-κB essential modulator (NEMO)-deficient genetic steatohepatitis model.16 A direct link can be made between oxidative stress, DNA damage, and apoptosis when considering that oxidative DNA damage occurs in MAFLD.43,62,63 and that apoptosis is induced by DNA damage44,45,64,65 Interestingly, implication of the cGAS-STING pathway in MAFLD recently emerged as a DNA damage sensing pathway recognising DNA leakage in the cytosol that initiates hepatic innate immune response through type-I interferon production.46,47,63 Our description of a decreased expression of STING in S217879-treated PCLS supports the observation of reduced DNA damage following pharmacological NRF2 activation by S217879. In addition, liver inflammation was also ameliorated in S217879-treated PCLS. This extends previous observations of anti-inflammatory effects of NRF2 activation in rodents28,29,34,66 but contrasts with recent results described by Mohs et al.16 in which immune cells infiltration and Il-1β expression were increased in the liver of hepatocyte-KEAP1-deficient mice in an MAFLD setting. This can be explained by the characteristics of the hepatocyte-NEMO deficient mice model steatohepatitis which displays a constitutive inactivation of the canonical nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway that regulates inflammation,60 and the crosstalk between the KEAP1–NRF2 axis and the NFκB pathway, that coordinately regulate cellular responses to resolve inflammation.67 Interestingly, we also observed a reduction of endothelial inflammation markers (ICAM-1, VCAM-1) in PCLS following NRF2 activator treatment, suggesting that S217879 may also improve liver sinusoidal endothelial cells homeostasis, which is of great importance in the pathophysiology of MAFLD because endothelial inflammation occurs in early steps of the disease and promotes its progression towards advanced steatohepatitis.50,53 The specific role of NRF2 activation in liver sinusoidal endothelial cells and other liver cell types deserves further studies.
The second major finding of this study was the observation that S217879 lowers fibrogenesis markers expression (Fig. 5), supporting the recent observations made in the amylin liver non-alcoholic steatohepatitis (AMLN) diet-induced steatohepatitis model,34 and suggesting an antifibrogenic potential of this treatment in humans, even with advanced stages of fibrosis. Importantly, we did not observe antifibrogenic effects of elafibranor, whereas this treatment has raised beneficial effects on liver fibrosis in mice,57 but failed to improve fibrosis in humans in a clinical trial.37 These observations obtained in our human PCLS model with MAFLD highlight the limited relevance of murine models and could be explained by the hyper-responsiveness of rodents to PPARα/δ agonism as illustrated by weight loss after elafibranor treatment in mice but not in humans.37,68 Meanwhile, protective effects of NRF2 activating strategies against liver fibrosis have been described in murine dietary-induced steatohepatitis models by suppressing TGF-β1 signalling and maintaining hepatic stellate cells quiescence.16,30,34,69,70 In this study, we report for the first time to our knowledge an antifibrotic effect of a pharmacological NRF2 activator in human livers with MAFLD.
All these therapeutic effects against MAFLD and fibrosis were first mediated, as expected, by the promotion of a potent antioxidant response as illustrated by the restoration of the GSH pool in PCLS from patients with MAFLD, and the induction of detoxifying enzymes.16,34 Of note, PPARα/δ agonism also activated antioxidant response in our model as in others,38,42,48 but effects of S217879 were obviously stronger than those of elafibranor. Autophagy was also enhanced upon S217879 treatment in human PCLS suggesting that S217879 could improve liver health by rescuing the defect of autophagy that occurs and promotes MAFLD progression.49,50
In conclusion, our observations provide for the first time new insights on the therapeutic potential of a new NRF2 activator in a human setting of MAFLD and support the use of NRF2 activating strategies in clinical trials.
Financial support
RHU QUID-NASH is funded by Agence Nationale de la Recherche (ANR-17-RHUS-0009); implemented by Inserm, Université Paris Cité, CNRS, CEA, Laboratoires Servier, Biopredictive, and AP-HP; and coordinated by Prof. Dominique Valla and Angélique Brzustowski.
Authors’ contributions
Designed the project and experiments and wrote the manuscript: AH, NP, PD, VP. Performed the experiments: AH, SL, NC. Performed the histological stainings and quantitative analyses: MA. Discussed and critically revised the manuscript: all authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of interest
AH was a post-doctoral researcher at Servier and Inserm. PD and NP are employees of Servier.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank members of the RHU QUID NASH consortium for their helpful discussions and their critical revision of the manuscript. We thank Agnès Dauvergne and Claude Hercend from the Clinical Biochemistry department of Beaujon Hospital, Clichy, France, for their help and advice on biochemical measurements in PCLS’ culture supernatants. We are thankful to the iGenSeq core facility, Institut du Cerveau (Paris, France), and JR Analytics technology (jr-analytics.fr) for sharing their expertise and helping us with RNA sequencing analyses.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100845.
Supplementary data
The following are the supplementary data to this article.
References
- 1.Karlsen T.H., Sheron N., Zelber-Sagi S., Carrieri P., Dusheiko G., Bugianesi E., et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet Lond Engl. 2022;399:61–116. doi: 10.1016/S0140-6736(21)01701-3. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eslam M., Sanyal A.J., George J., International Consensus Panel MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 5.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N., et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 8.Loomba R., Friedman S.L., Shulman G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Huang D.Q., El-Serag H.B., Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younossi Z.M. Non-alcoholic fatty liver disease – a global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Vuppalanchi R., Noureddin M., Alkhouri N., Sanyal A.J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2021;18:373–392. doi: 10.1038/s41575-020-00408-y. [DOI] [PubMed] [Google Scholar]
- 12.Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masarone M., Rosato V., Dallio M., Gravina A.G., Aglitti A., Loguercio C., et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cel Longev. 2018;2018 doi: 10.1155/2018/9547613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madan K., Bhardwaj P., Thareja S., Gupta S.D., Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD) J Clin Gastroenterol. 2006;40:930–935. doi: 10.1097/01.mcg.0000212608.59090.08. [DOI] [PubMed] [Google Scholar]
- 15.Palmieri V.O., Grattagliano I., Portincasa P., Palasciano G. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J Nutr. 2006;136:3022–3026. doi: 10.1093/jn/136.12.3022. [DOI] [PubMed] [Google Scholar]
- 16.Mohs A., Otto T., Schneider K.M., Peltzer M., Boekschoten M., Holland C.H., et al. Hepatocyte-specific NRF2 activation controls fibrogenesis and carcinogenesis in steatohepatitis. J Hepatol. 2021;74:638–648. doi: 10.1016/j.jhep.2020.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Videla L.A., Rodrigo R., Orellana M., Fernandez V., Tapia G., Quiñones L., et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci Lond Engl. 1979 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 18.Dodson M., de la Vega M.R., Cholanians A.B., Schmidlin C.J., Chapman E., Zhang D.D. Modulating NRF2 in disease: timing is everything. Annu Rev Pharmacol Toxicol. 2019;59:555–575. doi: 10.1146/annurev-pharmtox-010818-021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong K.I., Katoh Y., Kusunoki H., Itoh K., Tanaka T., Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong K.I., Padmanabhan B., Kobayashi A., Shang C., Hirotsu Y., Yokoyama S., et al. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellezza I., Giambanco I., Minelli A., Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Tang W., Jiang Y.-F., Ponnusamy M., Diallo M. Role of Nrf2 in chronic liver disease. World J Gastroenterol. 2014;20:13079–13087. doi: 10.3748/wjg.v20.i36.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu D., Xu M., Jeong S., Qian Y., Wu H., Xia Q., et al. The Role of Nrf2 in liver disease: novel molecular mechanisms and therapeutic approaches. Front Pharmacol. 2018;9:1428. doi: 10.3389/fphar.2018.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bricambert J., Alves-Guerra M.-C., Esteves P., Prip-Buus C., Bertrand-Michel J., Guillou H., et al. The histone demethylase Phf2 acts as a molecular checkpoint to prevent NAFLD progression during obesity. Nat Commun. 2018;9:2092. doi: 10.1038/s41467-018-04361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azzimato V., Jager J., Chen P., Morgantini C., Levi L., Barreby E., et al. Liver macrophages inhibit the endogenous antioxidant response in obesity-associated insulin resistance. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aaw9709. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y., Aleksunes L.M., Yeager R.L., Gyamfi M.A., Esterly N., Guo G.L., et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhry S., Nazmy M.H., Meakin P.J., Dinkova-Kostova A.T., Walsh S.V., Tsujita T., et al. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med. 2010;48:357–371. doi: 10.1016/j.freeradbiomed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Shimozono R., Asaoka Y., Yoshizawa Y., Aoki T., Noda H., Yamada M., et al. Nrf2 activators attenuate the progression of nonalcoholic steatohepatitis-related fibrosis in a dietary rat model. Mol Pharmacol. 2013;84:62–70. doi: 10.1124/mol.112.084269. [DOI] [PubMed] [Google Scholar]
- 30.Sharma R.S., Harrison D.J., Kisielewski D., Cassidy D.M., McNeilly A.D., Gallagher J.R., et al. Experimental nonalcoholic steatohepatitis and liver fibrosis are ameliorated by pharmacologic activation of Nrf2 (NF-E2 p45-Related Factor 2) Cell Mol Gastroenterol Hepatol. 2018;5:367–398. doi: 10.1016/j.jcmgh.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Zhang D., Hannink M., Beamer L.J. Crystal structure of the Kelch domain of human Keap1. J Biol Chem. 2004;279:54750–54758. doi: 10.1074/jbc.M410073200. [DOI] [PubMed] [Google Scholar]
- 32.Schmoll D., Engel C.K., Glombik H. The Keap1-Nrf2 protein-protein interaction: a suitable target for small molecules. Drug Discov Today Technol. 2017;24:11–17. doi: 10.1016/j.ddtec.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Davies T.G., Wixted W.E., Coyle J.E., Griffiths-Jones C., Hearn K., McMenamin R., et al. Monoacidic inhibitors of the Kelch-like ECH-associated protein 1: nuclear factor erythroid 2-related factor 2 (KEAP1:NRF2) protein-protein interaction with high cell potency identified by fragment-based discovery. J Med Chem. 2016;59:3991–4006. doi: 10.1021/acs.jmedchem.6b00228. [DOI] [PubMed] [Google Scholar]
- 34.Seedorf K., Weber C., Vinson C., Berger S., Vuillard L.-M., Kiss A., et al. Selective disruption of NRF2-KEAP1 interaction leads to NASH resolution and reduction of liver fibrosis in mice. JHEP Rep. 2022;5 doi: 10.1016/j.jhepr.2022.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palma E., Doornebal E.J., Chokshi S. Precision-cut liver slices: a versatile tool to advance liver research. Hepatol Int. 2019;13:51–57. doi: 10.1007/s12072-018-9913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staels B., Rubenstrunk A., Noel B., Rigou G., Delataille P., Millatt L.J., et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 37.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L., et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159.e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 38.Perakakis N., Stefanakis K., Feigh M., Veidal S.S., Mantzoros C.S. Elafibranor and liraglutide improve differentially liver health and metabolism in a mouse model of non-alcoholic steatohepatitis. Liver Int. 2021;41:1853–1866. doi: 10.1111/liv.14888. [DOI] [PubMed] [Google Scholar]
- 39.de Graaf I.A.M., Olinga P., de Jager M.H., Merema M.T., de Kanter R., van de Kerkhof E.G., et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat Protoc. 2010;5:1540–1551. doi: 10.1038/nprot.2010.111. [DOI] [PubMed] [Google Scholar]
- 40.Paish H.L., Reed L.H., Brown H., Bryan M.C., Govaere O., Leslie J., et al. A bioreactor technology for modeling fibrosis in human and rodent precision-cut liver slices. Hepatology. 2019;70:1377–1391. doi: 10.1002/hep.30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagaye S., Shen H., Saunier B., Nascimbeni M., Gaston J., Bourdoncle P., et al. Efficient replication of primary or culture hepatitis C virus isolates in human liver slices: a relevant ex vivo model of liver infection. Hepatology. 2012;56:861–872. doi: 10.1002/hep.25738. [DOI] [PubMed] [Google Scholar]
- 42.Boeckmans J., Buyl K., Natale A., Vandenbempt V., Branson S., De Boe V., et al. Transcriptomics data of a human in vitro model of non-alcoholic steatohepatitis exposed to elafibranor. Data Brief. 2019;25 doi: 10.1016/j.dib.2019.104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka S., Miyanishi K., Kobune M., Kawano Y., Hoki T., Kubo T., et al. Increased hepatic oxidative DNA damage in patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. J Gastroenterol. 2013;48:1249–1258. doi: 10.1007/s00535-012-0739-0. [DOI] [PubMed] [Google Scholar]
- 44.Clutton S. The importance of oxidative stress in apoptosis. Br Med Bull. 1997;53:662–668. doi: 10.1093/oxfordjournals.bmb.a011637. [DOI] [PubMed] [Google Scholar]
- 45.Wang J.Y. DNA damage and apoptosis. Cell Death Differ. 2001;8:1047–1048. doi: 10.1038/sj.cdd.4400938. [DOI] [PubMed] [Google Scholar]
- 46.Chen R., Du J., Zhu H., Ling Q. The role of cGAS-STING signalling in liver diseases. JHEP Rep Innov Hepatol. 2021;3 doi: 10.1016/j.jhepr.2021.100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo X., Li H., Ma L., Zhou J., Guo X., Woo S.-L., et al. Expression of STING is increased in liver tissues from patients with NAFLD and promotes macrophage-mediated hepatic inflammation and fibrosis in mice. Gastroenterology. 2018;155:1971–1984.e4. doi: 10.1053/j.gastro.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Risérus U., Sprecher D., Johnson T., Olson E., Hirschberg S., Liu A., et al. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 49.Allaire M., Rautou P.-E., Codogno P., Lotersztajn S. Autophagy in liver diseases: time for translation? J Hepatol. 2019;70:985–998. doi: 10.1016/j.jhep.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 50.Hammoutene A., Biquard L., Lasselin J., Kheloufi M., Tanguy M., Vion A.-C., et al. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J Hepatol. 2020;72:528–538. doi: 10.1016/j.jhep.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X.-Q., Xu C.-F., Yu C.-H., Chen W.-X., Li Y.-M. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:1768–1776. doi: 10.3748/wjg.v20.i7.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizushima N., Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 53.Hammoutene A., Rautou P.-E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J Hepatol. 2019;70:1278–1291. doi: 10.1016/j.jhep.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Dewyse L., Reynaert H., van Grunsven L.A. Best practices and progress in precision-cut liver slice cultures. Int J Mol Sci. 2021;22:7137. doi: 10.3390/ijms22137137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lonardo A., Nascimbeni F., Ballestri S., Fairweather D., Win S., Than T.A., et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70:1457–1469. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castera L., Laouenan C., Vallet-Pichard A., Vidal-Trécan T., Manchon P., Paradis V., et al. High prevalence of NASH and advanced fibrosis in type 2 diabetes: a prospective study of 330 outpatients undergoing liver biopsies for elevated ALT, using a low threshold. Diabetes Care. 2023;46:1354–1362. doi: 10.2337/dc22-2048. [DOI] [PubMed] [Google Scholar]
- 57.van den Hoek A.M., Verschuren L., Caspers M.P.M., Worms N., Menke A.L., Princen H.M.G. Beneficial effects of elafibranor on NASH in E3L.CETP mice and differences between mice and men. Sci Rep. 2021;11:5050. doi: 10.1038/s41598-021-83974-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yates M.S., Tran Q.T., Dolan P.M., Osburn W.O., Shin S., McCulloch C.C., et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chambel S.S., Santos-Gonçalves A., Duarte T.L. The dual role of Nrf2 in nonalcoholic fatty liver disease: regulation of antioxidant defenses and hepatic lipid metabolism. Biomed Res Int. 2015;2015 doi: 10.1155/2015/597134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luedde T., Beraza N., Kotsikoris V., van Loo G., Nenci A., De Vos R., et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Wani W.Y., Boyer-Guittaut M., Dodson M., Chatham J., Darley-Usmar V., Zhang J. Regulation of autophagy by protein post-translational modification. Lab Investig J Tech Methods Pathol. 2015;95:14–25. doi: 10.1038/labinvest.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishida N., Yada N., Hagiwara S., Sakurai T., Kitano M., Kudo M. Unique features associated with hepatic oxidative DNA damage and DNA methylation in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:1646–1653. doi: 10.1111/jgh.13318. [DOI] [PubMed] [Google Scholar]
- 63.Donne R., Saroul-Ainama M., Cordier P., Hammoutene A., Kabore C., Stadler M., et al. Replication stress triggered by nucleotide pool imbalance drives DNA damage and cGAS-STING pathway activation in NAFLD. Dev Cell. 2022;57:1728–1741. doi: 10.1016/j.devcel.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 64.Guicciardi M.E., Gores G.J. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaina B. DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem Pharmacol. 2003;66:1547–1554. doi: 10.1016/s0006-2952(03)00510-0. [DOI] [PubMed] [Google Scholar]
- 66.Wang P., Ni M., Tian Y., Wang H., Qiu J., You W., et al. Myeloid Nrf2 deficiency aggravates non-alcoholic steatohepatitis progression by regulating YAP-mediated NLRP3 inflammasome signaling. iScience. 2021;24 doi: 10.1016/j.isci.2021.102427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wardyn J.D., Ponsford A.H., Sanderson C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tølbøl K.S., Kristiansen M.N., Hansen H.H., Veidal S.S., Rigbolt K.T., Gillum M.P., et al. Metabolic and hepatic effects of liraglutide, obeticholic acid and elafibranor in diet-induced obese mouse models of biopsy-confirmed nonalcoholic steatohepatitis. World J Gastroenterol. 2018;24:179–194. doi: 10.3748/wjg.v24.i2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng H., Whitman S.A., Wu W., Wondrak G.T., Wong P.K., Fang D., et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prestigiacomo V., Suter-Dick L. Nrf2 protects stellate cells from Smad-dependent cell activation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.