Abstract
Objective
Despite strong evidence on the safety and tolerability of the COVID‐19 vaccine, data on vaccination in children with epilepsy, particular younger children with specific epilepsy syndromes, are limited. The protective effects of vaccination against seizure increase upon COVID‐19 infection also remain to be elucidated.
Methods
Questionnaire surveys were distributed online via an established WeChat group for patient management as well as in our outpatient clinic. The data collected included demographics and clinical information related to COVID‐19 vaccination and infection. Detailed information related to epilepsy diagnosis and treatment was also collected from our patient database. Logistic regression analysis was performed to determine the factors associated with non‐vaccination. The characteristics of seizures following COVID‐19 infection were described.
Results
In total, 354 suitable questionnaires were included in the study. The median age at survey was 6 years (interquartile range 4, 9). The most common epilepsy syndrome was self‐limited epilepsy (n = 153, 43.2%), followed by developmental and/or epileptic encephalopathy (D/EE, n = 81, 22.9%) and genetic generalized epilepsy (n = 59, 16.7%). The vaccine uptake rate was 43.8% (n = 155), and all related side‐effects (n = 11, 7.1%) remitted spontaneously. Younger age (odds ratio [OR] = 0.877, P = 0.001), D/EE (OR = 5.096, P = 0.008), and less than six months seizure‐freedom before vaccination (OR = 3.026, P = 0.005) were associated with unwillingness to be vaccinated. There were no significant differences in the rate of COVID‐19 infection (33.7% vs 32.7%, P = 0.879) and resultant increased seizure activity following infection between the vaccinated and unvaccinated groups after propensity score matching (9.1% vs 15.6%, P = 0.428).Three unvaccinated cases of Dravet syndrome developed status epilepticus following COVID‐19 infection.
Significance
Vaccination against COVID‐19 is safe and well tolerated in children, even in younger patients with D/EE. Although the risk of worsening seizures following COVID‐19 infection may not be reduced by immunization, education focused on increased vaccination in pediatric epilepsy is still warranted.
Keywords: children, COVID‐19, epilepsy, vaccine
Key Points.
Vaccination against COVID‐19 was safe and tolerated in children with variant epilepsy and epilepsy syndromes.
Vaccination against COVID‐19 infection may not reduce the risk of worsening seizures when being infected.
Measures and education should target the younger children, cases with active epilepsy and DEE to advance the vaccine take‐up.
1. INTRODUCTION
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) represents the most effective tool in combatting coronavirus disease 2019 (COVID‐19). 1 Accumulating evidence has suggested that increases in post‐vaccination seizures are infrequent and vaccination is generally well tolerated in adult individual with epilepsy. 2 , 3 Vaccination uptake in the adult epilepsy population varies across districts and samples, with reported rates of 11%, 4 42%, 2 , 5 and 91.3%. 6 Vaccination hesitancy concerns include potential adverse effects, fear of losing seizure control, 2 and discouragement from healthcare providers. 4
In contrast, the impact of COVID‐19 vaccination on seizures in children with epilepsy and the potential factors associated with vaccine hesitancy from the caregiver are less known. A previous study on patients with Dravet syndrome (DS) (aged ≥12 years) revealed a vaccination rate of 43.1%, with 13% (16/120) reporting an increase in seizures after COVID‐19 vaccination. 7 Another cross‐sectional study 8 involving self‐limited epilepsy with centrotemporal spikes (SeLECTS) reported a COVID‐19 vaccination rate of 64.2%, with no increase in the frequency or duration of new seizures after vaccination. Therefore, it appears that the occurrence of epilepsy syndrome and its underlying etiologies in pediatric populations may impact the vaccination choices made by their caregivers, as well as the subsequent occurrence of seizures following vaccination. Despite epilepsy sufferers facing a greater risk of harm than the general population following SARS‐CoV‐2 infection, 3 our understanding regarding the impact of vaccination on seizure occurrences in children following infection remains limited.
Few reports on the safety of vaccines in younger children with epilepsy have been documented, with little known regarding the factors contributing to hesitancy towards COVID‐19 vaccination. In China, the CoronaVac inactivated SARS‐CoV‐2 vaccine is considered to be well tolerated, safe, and effective in children and adolescents aged 3‐17 years. 9 Consequently, it is the nationally recommended vaccine, with guidance specifying the administration of a 3.0 g dose on a two‐immunization schedule, targeting all eligible children aged ≥3 years, subject to parental consent.
In this cross‐sectional study, we collected data on vaccine uptake in a cohort of children with epilepsy and related complications. Seizure outcomes after infection in children with or without vaccination were also compared. The safety and protective effects post‐vaccination, as well as the factors associated with vaccine hesitancy, particularly in younger children, were analyzed to enhance decision‐making for caregivers and healthcare providers.
2. MATERIALS AND METHODS
We conducted a cross‐sectional survey from 14 December 2022 to 2 January 2023 using online questionnaires or face‐to‐face outpatient interviews at the Department of Neurology in the Children's Hospital of Chongqing Medical University (CHCMU). All patients included in the survey had a minimum follow‐up period of 6 months at the time of the study, some of whom were previously published cases. 10 , 11 Caregivers were invited to participate in the survey via WeChat groups and were contacted five times throughout the study period. Incomplete questionnaires were carefully reviewed, and if needed, additional information, such as seizure duration, frequency, and in‐hospital treatment, was obtained through telephone interviews. Caregiver consent was obtained prior to the survey.
The study inclusion criteria were as the follows: completed the scheduled two immunizations of inactive CoronaVac vaccine 9 ; followed up for at least 1 month after the last vaccination; and minimal 6 months follow‐up since the establishment of diagnosis at the time of survey. Patients with incomplete questionnaires, caregivers who refused to participate, and individuals with insufficient information regarding their diagnostic framework were excluded from the study.
The questionnaire was divided into two parts. The first part included the collection of demographic data, such as name, CHCMU‐registered identity number, residency, and level of education (caregiver). Details concerning epilepsy diagnosis and treatment regimens were extracted from our existing database. The second part included the collection of vaccination‐related information, such as date of vaccination, type of vaccine received, seizure outcomes, and any adverse events experienced within 7 days following vaccination (eg, fever, muscle soreness, headache, vomiting, and diarrhea). Additional comprehensive information regarding seizures after vaccination and/or infection was collected, including the frequency of seizures recorded after each injection for 1 week, seizure duration, and any treatment administered during the acute period. Almost all items in the questionnaire were presented as choice questions (Appendix S1). Seizure freedom was defined as the absence of observed seizures for a continuous period of 6 months.
This study and all protocols were approved by the Ethics Committee of CHCMU and adhered to all principles of the Declaration of Helsinki.
2.1. Statistical analysis
All data were analyzed using IBM SPSS v27.0 and GraphPad Prism v9. Descriptive statistics were used to show demographic variables by frequency, percentage, and quartile interval. Categorical data were determined by chi‐square test (χ2) and statistically significant variables were analyzed by multiple logistic regression, with P < 0.05 considered significant. Propensity score matching was performed between the vaccinated and unvaccinated groups according to sex, age, type of epilepsy, and number of antiseizure medications (ASMs) being used.
3. RESULTS
3.1. Demographic and baseline data
There were 542 questionnaires distributed online and 35 questionnaires distributed in the outpatient clinic. In total, 396 recipients completed the survey, with 354 questionnaires included in the study after removing disqualified responses. Among the 354 cases, 43.8% (n = 155) finished the two scheduled immunizations of the inactivated SARS‐CoV‐2 vaccine. The median age at survey was 6 years (interquartile range [IQR] 4, 9) across all cases, 5 years (IQR 3, 7.8) in the vaccinated group, and 8 years (IQR 5.1, 11) in the unvaccinated group. Within the entire cohort, cases aged 3‐12 years accounted for 80.5% (n = 285) of the total, with 82.3% (128/155) in the vaccinated group and 79.4% (157/199) in the unvaccinated group. Among caregivers, 61.3% had attained an educational level of senior high school or below. All demographic data are shown in Table 1.
TABLE 1.
Demographic and clinical data between vaccinated and unvaccinated groups.
| Characteristic | Vaccinated, n = 155 | Unvaccinated, n = 199 | Total, n = 354 | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|---|
| Age, Median (IQR) | 5 (3, 7.8) | 8 (5.1, 11) | 6 (4, 9) | 0.835 (0.776‐0.898) | <0.001 |
| Sex, n (%) | |||||
| Male | 92 (59.4) | 102 (51.3) | 194 (54.8) | 1.340 (0.860‐2.089) | 0.196 |
| Female | 63 (40.6) | 97 (48.7) | 160 (45.2) | ||
| Caregiver's education, n (%) | |||||
| Senior high school and below | 99 (63.9) | 118 (59.3) | 217 (61.3) | 1.143 (0.726‐1.800) | 0.564 |
| University or above | 56 (36.1) | 81 (40.7) | 137 (38.7) | ||
| Number of ASMs, n (%) | |||||
| 0 | 23 (14.8) | 15 (7.5) | 38 (10.7) | 1.634 (1.230‐2.172) | <0.001 |
| 1 | 103 (66.5) | 112 (56.3) | 215 (60.7) | ||
| 2 | 19 (12.3) | 43 (21.6) | 62 (17.5) | ||
| 3 | 8 (5.2) | 27 (13.6) | 35 (9.9) | ||
| 4 | 2 (1.3) | 2 (1.0) | 4 (1.1) | ||
| Epilepsy classification | |||||
| Focal epilepsies, n (%) | 100 (64.5) | 94 (47.2) | 194 (54.8) | 0.492 (0.314‐0.773) | 0.002 |
| SeLIE | 9 (9.0) | 40 (42.6) | 49 (25.3) | ||
| SeLFNIE | 1 (1.0) | 3 (3.2) | 4 (2.1) | ||
| SeLECTS | 61 (61.0) | 34 (36.2) | 95 (49.0) | ||
| SeLEAS | 3 (3.0) | 2 (2.1) | 5 (2.6) | ||
| COVE | 2 (2.0) | 0 | 2 (1.0) | ||
| SHE | 2 (2.0) | 5 (5.3) | 7 (3.6) | ||
| Unclassifiable | 22 (22.0) | 10 (10.6) | 32 (16.5) | ||
| GGEs, n (%) | 38 (24.5) | 21 (10.6) | 59 (16.7) | 0.382 (0.207‐0.704) | 0.002 |
| IGEs | 21 (55.3) | 5 (23.8) | 26 (44.1) | ||
| Others | 17 (44.7) | 16 (76.2) | 33 (55.9) | ||
| DEE, n (%) | 9 (5.8) | 72 (36.2) | 81 (22.9) | 8.961 (4.251‐18.887) | <0.001 |
| IESS | 1 (11.1) | 24 (33.3) | 25 (30.9) | ||
| DS | 1 (11.1) | 14 (19.4) | 15 (18.5) | ||
| LGS | 1 (11.1) | 4 (5.6) | 5 (6.2) | ||
| EMAtS | 0 | 1 (1.4) | 1 (1.2) | ||
| FIRES | 1 (11.1) | 0 | 1 (1.2) | ||
| DEE‐SWAS | 2 (22.2) | 7 (9.7) | 9 (11.1) | ||
| PCDH19 clustering epilepsy | 1 (11.1) | 0 | 1 (1.2) | ||
| CDKL5‐DEE | 0 | 1 (1.4) | 1 (1.2) | ||
| GS | 1 (11.1) | 0 | 1 (1.2) | ||
| Unclassifiable | 1 (11.1) | 21 (29.2) | 22 (27.2) | ||
| NSE | 8 (5.2) | 12 (6.0) | 20 (5.6) | 1.201 (0.461‐3.127) | 0.708 |
| Seizure freedom, n (%) | |||||
| Yes | 143 (92.3) | 155 (77.9) | 298 (84.2) | 3.258 (1.622‐6.547) | <0.001 |
| No | 12 (7.7) | 44 (22.1) | 56 (15.8) | ||
| Infection, n (%) | 62 (40.0) | 81 (40.7) | 143 (40.4) | 1.030 (0.671‐1.580) | 0.894 a |
| Infection, n (%) a | 33 (33.7) | 32 (32.7) | 65 (33.2) | 0.955 (0.527‐1.731) | 0.879 |
Abbreviations: ASMs, anti‐seizure medications; COVE, childhood occipital visual epilepsy; DEE, developmental and/or epileptic encephalopathy; DEE‐SWAS, developmental epileptic encephalopathy with spike‐ and wave‐ activation in sleep; DS, Dravet syndrome; EMAtS, epilepsy with myoclonic atonic seizures; FIRES, febrile infection‐related epilepsy syndrome; GGEs, genetic generalized epilepsies; GS, gelastic seizures; IESS, infantile epileptic spasms syndrome; IGEs, idiopathic generalized epilepsy syndrome; LGS, Lennox–Gastaut syndrome; NSE, non‐syndromic epilepsy; SeLEAS, self‐limited epilepsy with autonomic seizures; SeLECTS, self‐limited epilepsy with centrotemporal spikes; SeLIE, self‐limited (familial) infantile epilepsy; SeLFNIE, self‐limited familial neonatal‐infantile epilepsy; SHE, Sleep‐ related hypermotor (hyperkinetic) epilepsy.
Vaccinated and unvaccinated groups were subjected to propensity score matching (PSM), with 196 cases matched successfully.
Within the cohort, 89.3% of enrolled patients were on ASMs at the time of survey, with most (60.7%) receiving monotherapy. In terms of epilepsy syndrome classification, a total of 85.3% (n = 302) of cases were categorized with a specific syndrome, including self‐limited epilepsy (43.2%, n = 153), childhood occipital visual epilepsy (0.06%, n = 2), sleep‐related hypermotor (hyperkinetic) epilepsy (2.0%, n = 7), developmental and/or epileptic encephalopathy (22.9%, n = 81), and genetic generalized epilepsy (16.7%, n = 59).Among the remaining 52 cases as non‐syndromic epilepsy, 32 cases were focal epilepsy and the other 20 cases showed unknown seizure origin. In total, 298 cases (84.2%) had achieved seizure freedom before vaccination. In total, 298 cases (84.2%) had achieved seizure freedom before vaccination.
3.2. Vaccination rate and associated factors
As seen in Table 1, univariate analysis showed that age at survey (odds ratio (OR): 0.835, 95% confidence interval (CI): 0.776‐0.898, P < 0.001), number of ASMs (OR: 1.634, 95% CI: 1.230‐2.172, P < 0.001), focal epilepsy syndrome (OR: 0.492, 95% CI: 0.314‐0.773, P = 0.002), generalized epilepsy syndrome (OR: 0.382, 95% CI: 0.207‐0.704, P = 0.002), developmental and/or epileptic encephalopathy (D/EE) (OR: 8.961, 95% CI: 4.251‐18.887, P < 0.001), and sustained seizure‐freedom for 6 months before vaccination (OR: 3.258, 95% CI: 1.622‐6.547, P < 0.001) differed significantly between the vaccinated and unvaccinated groups. Following binary multivariate logistic regression analysis, D/EE (OR: 5.096, 95% CI: 1.529‐16.987, P = 0.008), <6‐month seizure‐freedom before vaccination (OR: 3.026, 95% CI: 1.386‐6.609, P = 0.005), and younger age (OR: 0.877, 95% CI: 0.812‐0.949, P = 0.001) remained significant reasons for non‐vaccination (Figure 1).
FIGURE 1.
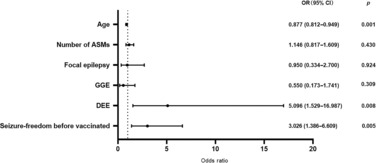
Reasons for unvaccinated.
Based on the questionnaires, a total of 11 (7.1%) vaccinated patients experienced post‐vaccination side‐effects from days 1 to 3, including muscle pain (n = 6, 46.2%), fever (n = 4, 30.8%), rash (n = 1, 7.7%), fatigue (n = 1, 7.7%), and nausea (n = 1, 7.7%), which were reported as tolerable. Only two patients experienced seizure recurrence lasting <5 min on day 1 of the second vaccination, and the diagnoses were non‐syndromic epilepsy and sleep‐related hypermotor epilepsy, respectively.
3.3. Seizure outcomes after COVID‐19 infection
We matched the propensity scores of the vaccinated and unvaccinated groups by sex, age, type of epilepsy/epilepsy syndrome, and number of ASMs being used. Among the enrolled cases, there were no significant differences in the percentage of infection between the vaccinated and unvaccinated groups (33.7% vs 32.7%, OR: 0.955, 95% CI: 0.527‐1.731, P = 0.879, Table 1). Furthermore, after propensity score matching, there were no significant differences in terms of seizure increase between the vaccinated and unvaccinated groups for the seven consecutive days after COVID‐19 infection (9.1% vs 15.6%, OR: 1.852, 95% CI: 0.404‐8.491, P = 0.428, Table 2). The clinical characteristics of infected children who presented with seizure recurrence are listed in Table 2. More than half were diagnosed with D/EE, which has been identified as an independent factor associated with non‐vaccination. A total of 18 (12.3%) children experienced an increase in seizure frequency following COVID‐19 infection, the majority of which (83%, 15/18) experienced single or cluster seizures, each lasting less than 5 min. Among them, eight (44.4%) children were hospitalized due to increased seizures, including three cases in the vaccinated group and five cases in the unvaccinated group. Of note, three unvaccinated children (3.7%) with DS had status convulsions during the acute period of COVID‐19 infection, with two receiving mechanical ventilation.
TABLE 2.
Demographic and clinical data related to increased seizures after infection.
| Characteristic | Vaccinated, n = 5 | Unvaccinated, n = 13 | Total, n = 18 | Odds ratio (95% CI) | P‐value |
|---|---|---|---|---|---|
| Percentage of group, n (%) | 8.1 (5/62) | 16.0 (13/81) | 2.179 (0.733‐6.481) | 0.161 | |
| Percentage of group a , n (%) | 9.1 (3/33) | 15.6 (5/32) | 1.852 (0.404‐8.491) | 0.428 | |
| Age, Median (Q1, Q3) | 8 (7, 9.9) | 3.5 (1.6, 5) | 6.5 (4.1, 12) | ||
| Sex, n (%) | |||||
| Male | 4 (80) | 5 (38.5) | 9 (50) | N/A | |
| Female | 1 (20) | 8 (61.5) | 9 (50) | ||
| Number of ASMs, n (%) | |||||
| 0 | 1 (12.5) | — | 1 (5.6) | N/A | |
| 1 | — | 4 (30.8) | 4 (22.2) | ||
| 2 | 2 (25) | 2 (15.4) | 4 (22.2) | ||
| 3 | — | 6 (46.2) | 6 (33.3) | ||
| 4 | 2 (25) | 1 (7.7) | 3 (16.7) | ||
| Epilepsy classification | |||||
| Focal epilepsies, n (%) | 7 (38.9) | N/A | |||
| SeLIE | — | 1 (7.7) | 1 (5.6) | ||
| SeLECTS | 1 (20) | 2 (15.4) | 3 (16.7) | ||
| SHE | — | 1 (7.7) | 1 (5.6) | ||
| Unclassifiable | 2 (40) | — | 2 (11.1) | ||
| GGEs, n (%) | 1 (5.6) | N/A | |||
| GTCA | 1 (20) | — | 1 (5.6) | ||
| DEE, n (%) | 10 (55.5) | ||||
| IESS | — | 1 (7.7) | 1 (5.6) | ||
| FIRES | 1 (20) | — | 1 (5.6) | ||
| DS | — | 7 (53.8) | 7 (38.9) | ||
| LGS | — | 1 (7.7) | 1 (5.6) | ||
| Seizure‐freedom before infection, n (%) | |||||
| Yes | 4 (80) | 6 (46.2) | 10 (55.6) | N/A | |
| No | 1 (20) | 7 (53.8) | 8 (44.4) | ||
Abbreviations: ASMs, anti‐seizure medicines; DS, Dravet syndrome; FIRES, febrile infection‐related epilepsy syndrome; GTCA, epilepsy generalized tonic–clonic seizures alone; IESS, infantile epileptic spasms syndrome; IGEs, idiopathic generalized epilepsy syndrome; LGS, Lennox–Gastaut syndrome; N/A: not applicable; SeLECTS, self‐ limited epilepsy with centrotemporal spikes; SeLIE, self‐limited (familial) infantile epilepsy; SHE, sleep‐related hypermotor (hyperkinetic) epilepsy.
Vaccinated and unvaccinated groups were subjected to propensity score matching (PSM), with 196 cases matched successfully.
3.4. Hesitancy and barriers for vaccination
Based on the questionnaires completed by 199 parents of unvaccinated children, the primary reasons for parental hesitation included: concerns about potential exacerbation of seizures associated with vaccination (156, 78.4%), recent occurrence of active seizures within the past 6 months (n = 45, 22.6%), concern about potential interactions between ASMs and vaccines (n = 43, 21.6%), non‐recommendation of vaccination by healthcare practitioners (n = 40, 20.1%), abnormal EEG recordings (n = 37, 18.6%), young age of the child (n = 18, 9.0%), and presence of comorbidities (n = 6, 3.0%). Interestingly, 153 (76.9%) respondents expressed their willingness for the child to be vaccinated but had not yet decided due to the aforementioned concerns. Furthermore, 34 (17.1%) respondents explicitly stated that they refused to have their child vaccinated against COVID‐19 due to seizure recurrence following non‐COVID‐19 vaccines (n = 2), fear of seizure impact (n = 7), and unidentified reasons. The remaining six (3.0%) questionnaires did not provide a clear answer.
In addition, 354 caregivers completed the questionnaire regarding concerns during the pandemic, yielding the following results: potential seizure aggravation associated with COVID‐19 infection (n = 250, 70.6%), disruption or delay in scheduled follow‐up plans (n = 168, 47.5%), difficulties in accessing ASMs (59, 16.7%), and limited availability or accessibility to professional consultation and treatment (36, 10.2%).
4. DISCUSSION
This study concluded that vaccination against COVID‐19 was safe and tolerable, even in younger children with D/EE. However, the protective effect of immunization against recurrence of seizures upon COVID‐19 infection was not obvious. Importantly, younger age at survey, D/EE and non‐seizure‐freedom at vaccination were independent factors associated with non‐vaccination. These findings have important implications for both caregivers and practitioners in terms of providing practical guidance on immunization against COVID‐19.
The vaccination rate in our cohort was 43.8%, which is comparable to that reported in adults 7 and DS patients aged 12 years or older, 2 but considerably lower than that reported in patients with SeLECTS (75.3%). 8 This discrepancy is likely related to epilepsy syndrome, age at survey, and potential causes of epilepsy. Indeed, in our study, younger age, D/EE, and non‐seizure freedom were associated with a higher likelihood of vaccine hesitancy. In China, the current lower age limit for inactivated COVID‐19 vaccination is >3 years. The efficacy and safety of the inactivated CoronaVac vaccine has also been confirmed in this population in other countries beyond China. 12 , 13 Despite the demonstrated safety, immunogenicity, and effectiveness of the BNT162b2 COVID‐19 vaccine in children aged 6 months to 4 years, 14 there is a need for further exploration regarding the possibility of extending the lower age limit for inactivated vaccines and conducting large‐scale comparative studies involving different vaccine formulations.
While vaccinations are not implicated in epileptic encephalopathies such as DS, they may act as non‐specific triggers of seizures in individuals with underlying structural or genetic etiologies. 15 At present, data on the safety of vaccination against COVID‐19 in D/EE cases are limited, 16 , 17 which may contribute to their lower vaccination rates. Our results suggested that the presence of D/EE was a barrier to vaccination, possibly due to the unique characteristics of this disorder, such as neurodevelopmental disruption, developmental regression, and unresponsiveness to multiple ASMs. 18 , 19 , 20 Furthermore, in China, there is a recommendation to delay vaccination for individuals who have had active epilepsy within the last 6 months, 21 and thus seizure freedom can have a direct effect on vaccine uptake, with caregivers declined to vaccinate children with D/EE, particularly those with unfavorable ASM responses.
In contrast to previous research, 22 the educational attainment of caregivers was not associated with their willingness or decision to vaccinate. This could be ascribed to the vaccination strategy of the Chinese government, which emphasizes building population immunity 23 and enhancing primary healthcare in rural areas. 24
In the study cohort, we observed no significant differences in the incidence of seizures between the two groups after COVID‐19 infection. Although these results suggest that vaccination may not reduce the risk of seizure recurrence, three unvaccinated DS cases presented with status epilepticus during the acute phase of COVID‐19 infection. Given that COVID‐19 vaccination is well tolerated by individuals with DS 7 and that those with underlying neurological disease, such as epilepsy, are at a higher risk of developing severe neurological conditions when infected by COVID‐19, 25 vaccination should still be recommended, even in younger children with D/EE. In addition, further large‐scale prospective trials are warranted to elucidate the effectiveness of COVID‐19 vaccination in reducing the risk of seizure recurrence.
Consistent with previous reports, 4 , 7 the primary reason for caregiver vaccine hesitancy was concern over vaccine‐associated increases in seizure activity. Additionally, post‐traumatic stress disorder in parents with children with severe diseases, such as epilepsy, may affect the decision to vaccinate against COVID‐19. 26 However, mounting evidence suggests that COVID‐19 vaccines are generally safe and well tolerated in patients with epilepsy, even for DS with high sensitivity to fever. 7 , 27 Concerns over interactions between ASMs and vaccines was also identified as a barrier to vaccination. Indeed, some CYP enzyme subtypes can be affected by anti‐influenza vaccinations, resulting in increased carbamazepine and phenytoin toxicity. 28 Similar interactions between COVID‐19 vaccines and ASMs are assumed to be possible and need to be further investigated. Nevertheless, current data strongly suggest that the benefits of COVID‐19 vaccination outweigh any potential risks. 29 Considering the high percentage of caregivers who expressed an intention to vaccinate, concerns regarding potential interactions between COVID‐19 vaccines and ASMs should not be a perceived barrier to vaccination, nor should there be a view that COVID‐19 vaccination would be less effective. To address concerns that extend beyond vaccination, robust and improved public health service measures should be implemented and advocated, thereby increasing the likelihood of vaccination. The importance of physicians should be emphasized and valued, given the pivotal role they play in propagating the benefits of vaccination. 30
This study has several limitations. As a retrospective study, our research may be influenced by memory bias, resulting in data loss due to incomplete information recall in the questionnaires. In addition, the small size of some specific epilepsy syndromes makes it difficult to clarify the impact of vaccination on certain epilepsy syndromes. Lastly, the side‐effects following vaccination may have been underestimated, particularly for younger children who are unable to accurately describe their symptoms.
In summary, this study provides valuable information for clinical practice, particularly from the viewpoint of epilepsy syndrome, as well as for caregivers and health providers regarding COVID‐19 vaccination decisions for the most frequent epilepsy syndromes, particularly for younger children with D/EE. Although the protective effects of vaccination against seizure aggravation following COVID‐19 infection were not significant, COVID‐19 vaccination is considered safe and well tolerated in children and is still recommended considering the potential for severe illness and death caused by COVID‐19 infection in individuals with pre‐existing epilepsy.
AUTHOR CONTRIBUTIONS
These authors contributed equally to this work. Tingsong Li designed the study and revised the final draft of the article. Congjie Chen analyzed the data and drafted the article. Ningning Chen and Li Xie designed the questionnaire and collected the completed questionnaires. Jiannan Ma and Yuanyuan Luo collected clinical data.
CONFLICT OF INTEREST STATEMENT
None of the authors have any relevant conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Appendix S1.
ACKNOWLEDGMENTs
This study was supported by CQMU Program for Youth Innovation in Future Medicine (W0031).
Chen C, Chen N, Xie L, Luo Y, Ma J, Li T. Vaccination against COVID‐19 and potential protective effects on seizure recurrence in children with epilepsy: A cross‐sectional survey. Epilepsia Open. 2023;8:1133–1141. 10.1002/epi4.12794
DATA AVAILABILITY STATEMENT
All the data of this study can be reached upon the qualified request.
REFERENCES
- 1. World Health Organization . COVID‐19 advice for the public: getting vaccinated. Accessed February 21, 2023. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/covid‐19‐vaccines/advice
- 2. Lu L, Zhang Q, Xiao J, Zhang Y, Peng W, Han X, et al. COVID‐19 vaccine take‐up rate and safety in adults with epilepsy: data from a multicenter study in China. Epilepsia. 2022;63(1):244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valencia I, Berg AT, Hirsch LJ, Lopez MR, Melmed K, Rosengard JL, et al. Epilepsy and COVID 2021. Epilepsy Curr. 2022;22(6):398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li N, Chu C, Lin W. A survey of hesitancy and response to the COVID‐19 vaccine among patients with epilepsy in Northeast China. Front Neurol. 2021;12:778618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang S, Lv J, He C, Yang Y, Zheng Y, Ye L, et al. COVID‐19 vaccination hesitancy and safety among adult people with epilepsy in eastern China. Epilepsy Behav. 2023;138:108984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romozzi M, Rollo E, Quintieri P, Dono F, Evangelista G, Consoli S, et al. Impact of COVID‐19 vaccine on epilepsy in adult subjects: an Italian multicentric experience. Neurol Sci. 2022;43(8):4627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hood V, Berg AT, Knupp KG, Koh S, Laux L, Meskis MA, et al. COVID‐19 vaccine in patients with Dravet syndrome: observations and real‐world experiences. Epilepsia. 2022;63(7):1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X, Wu L, Zheng D, Yang B, Wu D. COVID‐19 vaccination for patients with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2022;134:108744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy children and adolescents: a double‐blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(12):1645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang Y, Zou N, Luo Y, Cheng M, Liao S, Hong S, et al. Cohort study of infantile epileptic spasms syndrome: etiological analysis and treatment of corticosteroids. Seizure. 2022;101:120–6. [DOI] [PubMed] [Google Scholar]
- 11. Zhao B, Liao S, Zhong X, Luo Y, Hong S, Cheng M, et al. Effectiveness and safety of oxcarbazepine vs. levetiracetam as monotherapy for infantile focal epilepsy: a longitudinal cohort study. Front Neurol. 2022;13:909191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jara A, Undurraga EA, Zubizarreta JR, González C, Acevedo J, Pizarro A, et al. Effectiveness of CoronaVac in children 3‐5 years of age during the SARS‐CoV‐2 omicron outbreak in Chile. Nat Med. 2022;28(7):1377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandes EG, López‐Lopes GIS, Silva VO, Yamashiro R, Madureira KCR, Gallo JF, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in inadvertently vaccinated healthy children. Rev Inst Med Trop Sao Paulo. 2021;63:e83 Published December 6, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muñoz FM, Sher LD, Sabharwal C, Gurtman A, Xu X, Kitchin N, et al. Evaluation of BNT162b2 COVID‐19 vaccine in children younger than 5 years of age. N Engl J Med. 2023;388(7):621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Craiu D, Rener Primec Z, Lagae L, Vigevano F, Trinka E, Specchio N, et al. Vaccination and childhood epilepsies. Eur J Paediatr Neurol. 2022;36:57–68. [DOI] [PubMed] [Google Scholar]
- 16. Wang Z, Fang X, Han T, Lv S, Li C, Ma A, et al. Safety and tolerability of COVID‐19 vaccine in children with epilepsy: a prospective, multicenter study. Pediatr Neurol. 2023;140:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Núñez I, García‐Grimshaw M, Castillo Valencia CY, Aguilera Callejas DE, Moya Alfaro ML, Saniger‐Alba MDM, et al. Seizures following COVID‐19 vaccination in Mexico: a nationwide observational study. Epilepsia. 2022;63(10):e144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zuberi SM, Wirrell E, Yozawitz E, Wilmshurst JM, Specchio N, Riney K, et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: position statement by the ILAE task force on nosology and definitions. Epilepsia. 2022;63(6):1349–97. [DOI] [PubMed] [Google Scholar]
- 19. Specchio N, Wirrell EC, Scheffer IE, Nabbout R, Riney K, Samia P, et al. International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: position paper by the ILAE task force on nosology and definitions. Epilepsia. 2022;63(6):1398–442. [DOI] [PubMed] [Google Scholar]
- 20. Riney K, Bogacz A, Somerville E, Hirsch E, Nabbout R, Scheffer IE, et al. International league against epilepsy classification and definition of epilepsy syndromes with onset at a variable age: position statement by the ILAE task force on nosology and definitions. Epilepsia. 2022;63(6):1443–74. [DOI] [PubMed] [Google Scholar]
- 21. Sheng Y. Expert consensus on vaccination of children with special health status—epilepsy and vaccination. Chin J Pract Pediatr. 2019;34(2):82–4. [Google Scholar]
- 22. Goldman RD, Staubli G, Cotanda CP, Brown JC, Hoeffe J, Seiler M, et al. Factors associated with parents' willingness to enroll their children in trials for COVID‐19 vaccination. Hum Vaccin Immunother. 2021;17(6):1607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. An Z, Wang F, Pan A, Yin Z, Rodewald L, Feng Z. Vaccination strategy and challenges for consolidating successful containment of COVID‐19 with population immunity in China. BMJ. 2021;1(375):e066125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu D, Zhang B, Huang M, Liu M, Xia X, Zuo Y, et al. Evaluation of a medical education policy with compulsory rural service in China. Front Public Health. 2023;11:1042898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID‐19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78(5):536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corsi M, Orsini A, Pedrinelli V, Santangelo A, Bertelloni CA, Carli N, et al. PTSD in parents of children with severe diseases: a systematic review to face COVID‐19 impact. Ital J Pediatr. 2021;47(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clayton LM, Balestrini S, Cross JH, Wilson G, Eldred C, Evans H, et al. The impact of SARS‐CoV‐2 vaccination in Dravet syndrome: a UK survey. Epilepsy Behav. 2021;124:108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kow CS, Hasan SS. Potential interactions between COVID‐19 vaccines and antiepileptic drugs. Seizure. 2021;86:80–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers For Disease Control And Prevention . Selected Adverse Events Reported after COVID‐19 Vaccination. Accessed February 21, 2023. https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/safety/adverse‐events.html
- 30. Miraglia Del Giudice G, Folcarelli L, Della Polla G, Napoli A, Angelillo IF. Investigating the reasons for receiving the second booster dose of the COVID‐19 vaccine in adults and in people with chronic medical conditions in southern Italy. Vaccines (Basel). 2023;11(4):737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
All the data of this study can be reached upon the qualified request.


