Abstract
Lysozyme (LZ) is a purely natural, nonpolluting and nonspecific immune factor, which has beneficial effects on the healthy development of animals. In this study, the influences of LZ on the growth performance and intestinal barrier of weaned piglets were studied. A total of 48 weaned piglets (Landrace × Yorkshire, 22 d old) were randomly divided into a control group (basal diet) and a LZ group (0.1% LZ diet) for 19 d. The results showed that LZ could significantly improve the average daily gain (ADG, P < 0.05) and average daily feed intake (ADFI, P < 0.05). LZ also improved the intestinal morphology and significantly increased the expression of occludin in the jejunum (P < 0.05). In addition, LZ down-regulated the expression of interleukin-1β (IL-1β, P < 0.05) and tumor necrosis factor-α (TNF-α, P < 0.05), and inhibited the expression of the genes in the nuclear factor-k-gene binding (NF-κB, P < 0.05) signaling pathway. More importantly, the analysis of intestinal flora showed LZ increased the abundance of Firmicutes (P < 0.05) and the ratio of Firmicutes to Bacteroidota (P = 0.09) at the phylum level, and increased the abundance of Clostridium_sensu_stricto_1 (P < 0.05) and reduced the abundance of Olsenella and Prevotella (P < 0.05) at the genus level. In short, this study proved that LZ could effectively improve the growth performance, relieve inflammation and improve the intestinal barrier function of weaned piglets. These findings provided an important theoretical basis for the application of LZ in pig production.
Keywords: Lysozyme, Growth performance, Intestinal barrier, Intestinal flora, Weaned piglet
1. Introduction
Weaning is one of the most stressful processes in piglet feeding, lasting about 3 to 4 weeks. During this period, weaned piglets are faced with physiological, environmental and nutritional challenges and are easily infected with various diseases (Saladrigas-García et al., 2021; Xie et al., 2021). The gut is the largest immune organ in mammals; therefore, a normal gastrointestinal function is particularly important for piglets to adapt to a new environment after birth and weaning (Yu et al., 2020). However, weaning stress causes intestinal damage, gut dysbiosis, and destroys intestinal barrier function (Tang et al., 2021). In the past, farmers tried to use antibiotics to solve the health problems of weaned piglets, but serious problems caused by antibiotics have threatened the safety of animal production and human health (Sarmah et al., 2006). Therefore, it is necessary to find purely natural and nonpolluting feed additives to improve the growth performance of piglets and avoid the adverse effects of pathogenic and nonpathogenic intestinal microorganisms.
Lysozyme (LZ) is a protein peptide that is distributed in animal and plant tissues, which has natural antibacterial activity (Ferraboschi et al., 2021). It mainly destroys the β-1,4-glycosidic bond between N-acetylmuramic acid and N-acetyl glucosamine of peptidoglycan polymers in the cell wall and leads to the division and death of the bacteria (Wu et al., 2019). As a natural immune factor, LZ is expected to develop as the replacement for antibiotics (Huang et al., 2018). It has been confirmed that exogenous LZ can reduce the colonization of Clostridium perfringens and enhance the intestinal barrier function, thereby improving the growth performance of chickens (Liu et al., 2010). LZ can also improve the health status of piglets on sows during lactation (Xu et al., 2018). Further, it can alleviate gastrointestinal disorders and inflammation induced by dextran sodium sulfate in obese mice by modulating the intestinal microbiota (Larsen et al., 2021).
This study aimed to explore the effects of LZ on the growth performance and intestinal barrier function of 22-d-old weaned piglets. Our research will provide the theoretical basis for better development of LZ in feed industries.
2. Materials and methods
2.1. Animal ethics statement
The experimental design and procedures used in this study were approved by the Animal Care and Use Committee of the Institute of Subtropical Agroecology, Chinese Academy of Sciences (IACUC # 201,302).
2.2. Experimental materials
According to the experimental design, 0.1% LZ was added in the basal diet. The LZ product used in the experiment was provided by Zhumadian Huazhong Chia Tai Co. Ltd (Zhumadian, China) in powdered form with an activity of 50 U/mg and the main effective component being LZ dimer. The basal diet (Table 1) met or exceeded the nutritional requirements of weaned piglets as recommended by the NRC (NRC, 1998).
Table 1.
Ingredients and nutrient composition of the piglet diets (as-fed basis, %).
| Item | Content |
|---|---|
| Ingredients | |
| Corn | 38.35 |
| Rice (broken) | 10.00 |
| Wheat flour | 12.80 |
| Soy protein concentrate | 4.00 |
| Soybean meal (CP, 46%) | 13.48 |
| Soy oil | 1.50 |
| Fish meal (CP, 65%) | 4.00 |
| Full-fat soybean | 4.00 |
| Whey power (CP, 3.8%) | 3.76 |
| Glucose | 2.00 |
| CaHPO4 | 0.83 |
| Calcium lactate | 0.85 |
| Salt | 0.14 |
| Sugar | 2.00 |
| Lys (78.8%) | 0.39 |
| Met (99%) | 0.08 |
| L-Thr (98.5%) | 0.12 |
| Lycine | 0.10 |
| Choline (60%) | 0.07 |
| Nucleotides | 1.00 |
| Flavoring agent | 0.13 |
| Vitamin-mineral premix1 | 0.4 |
| Total | 100 |
| Calculated values of nutrient levels | |
| Crude protein | 19.37 |
| Digestible energy, MJ/kg | 15.36 |
| Crude fat | 4.52 |
| Crude fibre | 1.92 |
| Crude ash | 3.89 |
| Salt | 0.24 |
| Ca | 0.72 |
| Available P | 0.45 |
| Lys | 1.60 |
| Met | 0.67 |
| Cys | 0.47 |
| Thr | 1.07 |
| Tyr | 0.30 |
Premix provided the following per kilogram of diet: Fe (FeSO4·H2O), 80 mg; Mn (MnSO4·5H2O), 45 mg; Zn (ZnO), 100 mg; Cu (CuSO4·5H2O), 20 mg; I (KI), 0.70 mg; Se (Na2SeO3·H2O), 0.25 mg; vitamin A, 10,000 IU; vitamin D3, 2,500 IU; vitamin E, 100 IU; vitamin K, 10 IU; vitamin B2, 10 mg; vitamin B6, 1 mg; vitamin B12, 50 μg; biotin, 80 μg; folic acid, 5 mg; nicotinic acid, 15 mg; choline chloride, 1,500 mg.
2.3. Experimental design and treatments
In this experiment, 48 healthy weaned piglets (Landrace × Yorkshire) aged 22 d and of similar size were selected from 6 litters, and were randomly divided into 2 groups with 6 replicates in each group and 4 piglets in each replicate. Dietary treatments were designed as follows: (1) control group (CON, basal diet); (2) LZ group (LZ, basal diet + 0.1% LZ), and the experiment lasted for 19 d. Piglets in each replicate were kept in 1 pen. The ambient temperature was controlled at about 28 °C and the humidity was controlled at 65% to 75%. Piglets were fed 6 times a day (at 06:30, 09:00, 11:30, 14:30, 17:30 and 22:00, respectively) and had free access to water. All piglets were vaccinated according to the regulations of the farm. Piglets were weighed at the beginning and the end of the experiment to calculate average daily gain (ADG). Feed intake was recorded every day.
2.4. Sample collection
Six pigs were randomly selected from each treatment for blood and tissue sampling. Blood samples were collected into 10 mL tubes and then centrifuged at 3,000 × g for 10 min at 4 °C to recover serum samples. Segments of mid-jejunum and ileum were harvested and rinsed several times with ice-cold phosphate-buffered saline and divided into 2 sections. One (approximately 1 to 2 cm) section was fixed with 4% formaldehyde-phosphate buffer and kept at 4 °C for a microscopic assessment of the mucosa morphology. The jejunum and ileum mucosa were scraped off, immediately frozen in liquid nitrogen and stored at −80 °C for RNA extraction and fluorescence-based quantitative RNA determination. Colon content was harvested from all 12 pigs within 30 min after slaughter. Colon samples were collected for 16S rRNA sequencing and volatile fatty acid (VFA) analysis.
2.5. Measurements in serum samples
Serum biochemical parameters, including total protein (TP), albumin (ALB), alkaline phosphatase (ALP), diamine oxidase (DAO), pancreatic amylase (p-AMY), amylase (AMS) and lipase (LIP) were measured using an automated biochemistry analyzer (Synchron CX Pro, Beckman Coulter, Fullerton, CA, United States) and a commercial kit (Aokai (Suzhou) Biotechnology Co., Ltd., Suzhou, China).
2.6. Intestinal morphology
The cross-sections of intestinal samples preserved with paraformaldehyde fixative were prepared by standard paraffin embedding technique. The samples were sectioned at 5 μm thickness, then stained with hematoxylin and eosin. The villus height (VH) and crypt depth (CD) were measured under a microscope with 40× combined magnification using an image processing and analysis system (version 1; Leica Imaging Systems Ltd., Cambridge, UK), and the ratio of villus height to crypt depth (V:C ratio) was calculated. At least 10 well-oriented intact villi and the associated crypt depth of each section were measured for each piglet.
2.7. Quantitative real-time polymerase chain reaction
Total RNA was extracted from the intestine by the TRIZOL method, and the concentration and purity of RNA were determined by Nanodrop-2000. According to the instructions of the Primer-script RT Reagent Kit with gDNA Eraser, gDNA was removed and reverse-transcribed and the reverse-transcribed cDNA was stored in a refrigerator at −20 °C for later use. The RT-qPCR was performed on a Roche LightCyclerfi 480 instrument II (Roche, Basel, Switzerland) with 10 μL total volume reaction, consisting of 5 μL 2× SYBR R Green Pro Taq HS Premix II, 2 μL cDNA template, 0.4 μL each of the forward and reverse primers, and 2.2 μL RNase free water. The relative level of mRNA expression was calculated using the 2−ΔΔCt method after normalization with β-actin as a housekeeping gene. The primers required are shown in Table 2.
Table 2.
Primer sequences of quantitative real-time PCR.
| Gene name | Primer sequences (5′–3′) | Accession no. |
|---|---|---|
| β-Actin | F: TCTGGCACCACACCTTCT R: TGATCTGGGTCATCTTCTCAC |
XM_021086047.1 |
| Occludin | F: AACGTATTTATGACGAGCAGCCC R: CACTTTCCCGTTGGACGAGTA |
XM_005672525.3 |
| ZO-1 | F: AAGGTCTGCCGAGACAACAG R: TCACAGTGTGGTAAGCGCAG |
XM_021098896.1 |
| MUC2 | F: GGACGACACCATCTACCTCAC R: AGGCCAGCTCGGGAATAGA |
XM_021082584.1 |
| TNF-α | F: TCTGCCTACTGCACTTCGAG R: GTTGATGCTCAAGGGGCCA |
NM_214022.1 |
| IL-8 | F: GACTTCCAAACTGGCTGTTGC R: ATTTGGGGTGGAAAGGTGTG |
NM_213867.1 |
| IL-4 | F: CCCGAGTGTCAAGTGGCTTA R: TGATGATGCCGAAATAGCAG |
NM_214340.1 |
| IL-1β | F: CCAAAGAGGGACATGGAGAA R: GGGCTTTTGTTCTGCTTGAG |
NM_001302388.2 |
| IFN-γ | F: CAGCTTTGCGTGACTTTGTG R: GATGAGTTCACTGATGGCTTT |
NM_213948.1 |
| NF-κB | F: GACCTGGTTTCGCTCTTG R: TGCTGTATCCGGGTACTT |
NM_001114281.1 |
| P38 MAPK | F: ATTCTCCGACGGTCTCAAGT R: GCCACATAGCCTGTCATT |
XM_021079285.1 |
| NF-κB p65 | F: TCATCGAGCAGCCCAAGCA R: CAGCCTCATAGAAGCCATCCC |
NM_001114281.1 |
| IκBα | F: CACCCGAGTTAGAAGGGCTC R: GGTATCTGCTGAGGTGTGCTG |
XM_001924394.6 |
β-Actin = β-non-muscle actin; ZO-1 = zonula occludens-1; Muc 2 = mucin 2; TNF-α = tumor necrosis factor-α; IL-8/4/1β = interleukin-8/4/1β; IFN-γ = interferon-γ; NF-κB = nuclear factor-k-gene binding; p38 MAPK = p38 mitogen-activated protein kinase; NF-κB p65 = nuclear factor-κB p65; IκBα = inhibitor of κB alpha.
2.8. DNA extraction and 16S rRNA sequencing
The genomic DNA of the sample was extracted by the CTAB method, and the specific steps were performed according to the instructions. The extracted DNA was used as a template, 16S rDNA was amplified by PCR, and V4 primers (515 F and 806 R) were used to identify bacterial diversity. A TruSeq DNA PCR-Free Sample Preparation Kit was used to construct the library. The constructed library was quantified by Qubit and Q-PCR. After the library was qualified, NovaSeq 6000 was used for computer sequencing. The sequencing service was completed by Shanghai Nuoyuan Zhihe Biotechnology Co., Ltd. The analysis from clustering to Alpha and Beta diversity was performed using QIIME (v1.7.0) and displayed using R software (v2.15.3).
2.9. Short-chain fatty acid (SCFA) composition of colonic contents
A certain mass of colon content samples was accurately weighed, and homogenate was added into an Eppendorf (EP) tube filled with 500 μL ultra-pure water. After vortexing, the supernatant was centrifuged at 6,000 × g at 4 °C for 10 min. Then, the supernatant was collected into another EP tube. Ultra-pure water (400 μL) was added to it. After vortexing, it was centrifuged at 6,000 × g at 4 °C for 10 min. Then, the supernatant was collected into another EP tube. Then, 90 μL metaphosphoric acid was added to 810 μL supernatant. The tube was centrifuged at 6,000 × g at 4 °C for 10 min and allowed to stand for 3 to 4 h. The solution was filtered through a 0.2-μm filter into a sample bottle. The filtrate was more than 500 μL. The contents of acetic acid, propionic acid, isobutyric acid and isovaleric acid were determined by gas chromatography (Agilent 6890, Palo Alto, CA).
2.10. Data analysis
The original data were preliminarily sorted out using Excel 2010, and an independent sample t-test was used to analyze the significance of the data using SPSS 19.0 software (International Business Machines Corporation, Armonk, NY). Data were expressed as least squares means ± standard error of mean (SEM). The values were considered highly significant when P < 0.01, significant when P < 0.05 and a tendency when 0.05 ≤ P < 0.10.
3. Results
3.1. Growth performance
As shown in Table 3, piglets in LZ showed higher (P < 0.05) ADG and average daily feed intake (ADFI) compared with CON, but had no significant effect on feed conversion ratio (FCR, P > 0.05).
Table 3.
Effect of LZ on growth performance of weaned piglets.1
| Item | CON | LZ | P-value |
|---|---|---|---|
| ADFI, g | 327.83 ± 47.02b | 398.93 ± 38.82a | 0.03 |
| ADG, g | 206.10 ± 9.40b | 251.07 ± 17.95a | 0.04 |
| FCR | 1.60 ± 0.09 | 1.59 ± 0.05 | 0.97 |
CON = control group; LZ = lysozyme group; ADFI = average daily feed intake; ADG = average daily gain; FCR = feed conversion ratio.
a, b Within a row, means without a common superscript differ significantly (P < 0.05).
Each value represents the mean ± SEM of 6 replicates (n = 6).
3.2. Serum biochemistry parameters
Piglets in LZ had higher (P < 0.05) serum TP compared with the piglets in CON (Table 4). There was no significant difference in serum ALB, ALP, DAO, p-AMY, AMS or LIP between the 2 groups.
Table 4.
Effect of LZ on serum biochemistry of weaned piglets.1
| Item | CON | LZ | P-value |
|---|---|---|---|
| TP, g/L | 48.95 ± 1.45b | 55.37 ± 1.46a | 0.01 |
| ALB, g/L | 39.83 ± 1.28 | 41.13 ± 1.49 | 0.52 |
| ALP, U/L | 484.67 ± 57.39 | 440.17 ± 21.54 | 0.49 |
| DAO, mmol/L | 2.32 ± 0.34 | 3.28 ± 0.42 | 0.17 |
| p-AMY, U/L | 1518.35 ± 271.94 | 1777.43 ± 75.10 | 0.26 |
| AMS, U/L | 1972.17 ± 333.64 | 1786.00 ± 67.47 | 0.30 |
| LIP, U/L | 123.45 ± 6.67 | 131.53 ± 1.08 | 0.26 |
CON = control group; LZ = lysozyme group; TP = total protein; ALB = albumin; ALP = alkaline phosphatase; DAO = diamine oxidase; p-AMY = pancreatic amylase; AMS = amylase; LIP = lipase.
a, b Within a row, means without a common superscript differ significantly (P < 0.05).
Each value represents the mean ± SEM of 6 replicates (n = 6).
3.3. Intestinal morphology
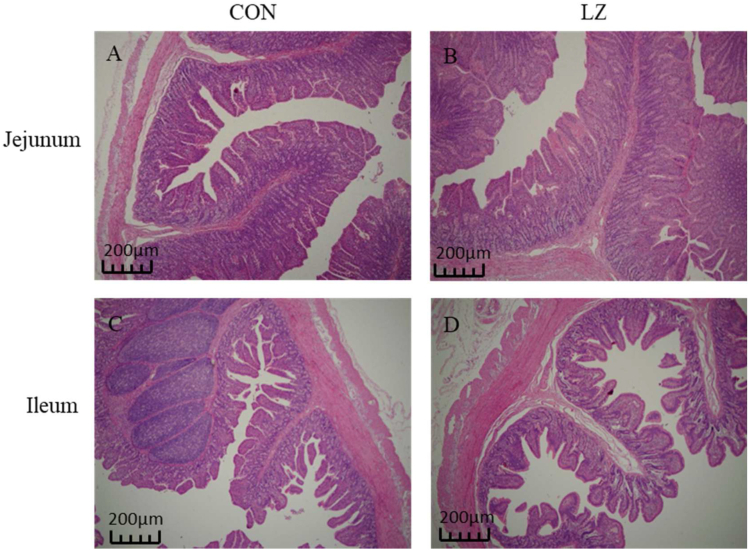
As shown in Table 5 and Fig. 1, dietary LZ had no significant effect on VH, CD and V:C ratio of ileum (P > 0.05) compared with the CON, but had a tendency towards increased VH (P = 0.05) and V:C ratio of the jejunum (P = 0.08).
Table 5.
Effect of LZ on intestinal morphology of weaned piglets.1
| Item | CON | LZ | P-value |
|---|---|---|---|
| Jejunum | |||
| VH, μm | 245.73 ± 22.27 | 321.66 ± 24.88 | 0.05 |
| CD, μm | 212.74 ± 12.82 | 204.68 ± 14.70 | 0.69 |
| V:C ratio | 1.23 ± 0.16 | 1.74 ± 0.19 | 0.08 |
| Ileum | |||
| VH, μm | 292.21 ± 36.50 | 296.20 ± 15.44 | 0.92 |
| CD, μm | 267.57 ± 9.09 | 281.09 ± 8.76 | 0.31 |
| V:C ratio | 1.31 ± 0.12 | 1.11 ± 0.08 | 0.19 |
CON = control group; LZ = lysozyme group; VH = villus height; CD = crypt depth; V:C ratio = villus height to crypt depth ratio.
Each value represents the mean ± SEM of 6 replicates (n = 6).
Fig. 1.
Effect of dietary LZ on intestinal morphology of jejunum and ileum in weaned piglets. (A, B) Jejunal section of piglets in CON and LZ. (C, D) Ileal section of piglets in CON and LZ. CON = control group; LZ = lysozyme group.
3.4. Gene expression of intestinal inflammation and tight junction proteins
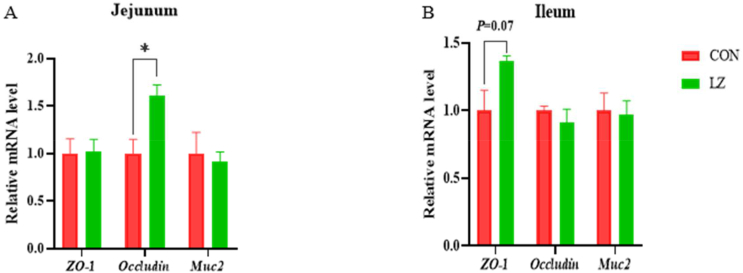
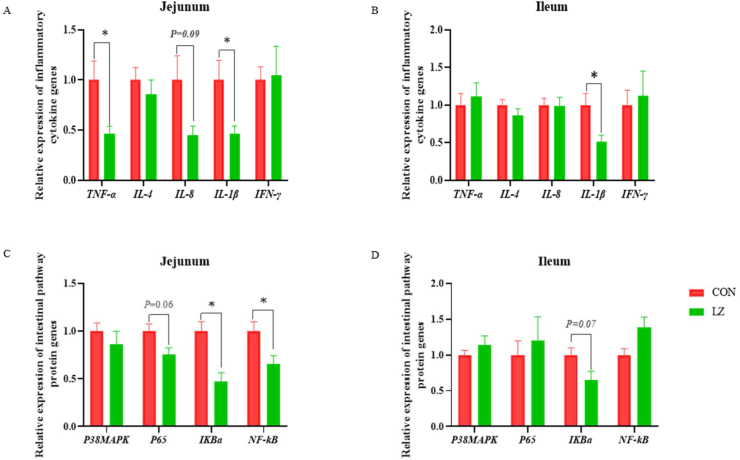
Compared with CON, the expression of occludin mRNA in the jejunum was significantly enhanced in LZ (P < 0.05). There was a trend toward a higher mRNA expression level of zonula occludens-1 (ZO-1) in the ileum of piglets fed LZ diets (P = 0.07) (Fig. 2A and B). As shown in Fig. 3, compared with CON, the mRNA expression level of IL-1β (P < 0.05) in the jejunum and ileum of LZ significantly decreased, and the mRNA expression level of TNF-α in the jejunum of LZ significantly decreased (P < 0.05). The IL-8 mRNA expression of piglets in LZ showed a decreasing trend (P = 0.09) (Fig. 3A and B).
Fig. 2.
Effect of LZ on intestinal tight junction protein mRNA expression in weaned piglets. (A, B) Relative expression of tight junction protein genes in jejunum and ileum (n = 6). CON = control group; LZ = lysozyme group; ZO-1 = zonula occludens-1; Muc 2 = mucin 2. Values are means ± SEM. Statistical differences in mean values of all indices were evaluated by independent t-test. The asterisk (∗) means P < 0.05.
Fig. 3.
Effect of LZ on intestinal immunity of weaned piglets. (A, B) Relative expression of inflammatory cytokine genes in jejunum and ileum (n = 6). TNF-α = tumor necrosis factor-α; IL-8/4/1β = interleukin-8/4/1β; IFN-γ = interferon-γ. (C, D) Relative expression of intestinal pathway protein genes in jejunum and ileum (n = 6). p38 MAPK = p38 mitogen-activated protein kinase; NF-κB p65 = nuclear factor-κB p65; IκBα = inhibitor of κB alpha; NF-κB = nuclear factor-k-gene binding; CON = control group; LZ = lysozyme group. Values are means ± SEM. Statistical differences in mean values of all indices were evaluated by independent t-test. The asterisk (∗) means P < 0.05.
The influence of LZ on the IκBα/NF-κB signaling pathway is shown in Fig. 3C and D. Compared to CON, LZ significantly inhibited (P < 0.05) the expressions of IκBα and NF-κB in the jejunum. A decrease trend in mRNA expression levels of p65 (P = 0.06) in the jejunum and IκBα (P = 0.07) in the ileum in LZ group.
3.5. Colonic microbiota diversity and composition
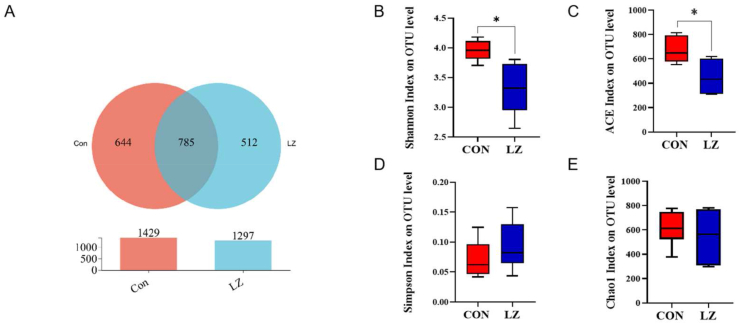
The differences in microbial flora abundance in the colon contents between the 2 groups were detected and the overlap between the 2 groups was illustrated using a Venn diagram (Fig. 4A). Result showed that the CON and LZ had 1429 and 1297 OTU respectively, of which 785 OTU were shared by both. As shown in Fig. 4B and C, the microbial richness indices (ACE) and the diversity indices (Shannon) were significantly decreased (P < 0.05) in the gut of piglets in LZ.
Fig. 4.
Effect of dietary LZ on the composition of weaned piglets gut microbiota. (A) Venn diagrams on OTU level, (B) Shannon index on OUT level, (C) ACE index on OUT level, (D) Simpson index on OUT level, (E) Chao index on OUT level. CON = control group; LZ = lysozyme group; OUT = operational taxonomic unit. Values are means ± SEM. Statistical differences in mean values of all indices were evaluated by independent t-test. The asterisk (∗) means P < 0.05.
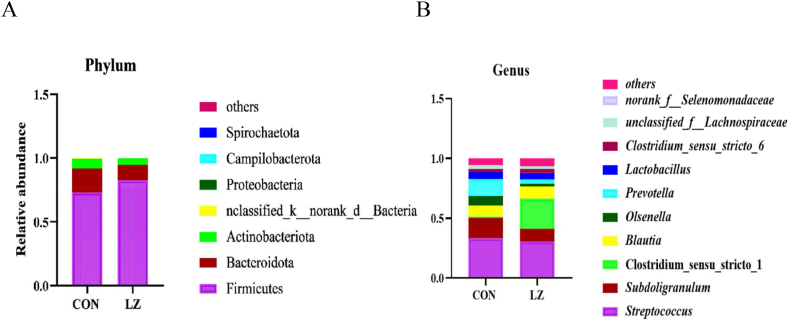
The relative abundance of the colonic microbiota at phylum and genus levels in the CON and LZ groups is shown in Fig. 5A and B. Firmicutes and Bacteroidota were the most dominant bacteria in the colonic microbial communities of CON and LZ, and the predominant genus were Streptococcus and Subdoligranulum.
Fig. 5.
Effect of dietary LZ on the composition of weaned piglets gut microbiota. Relative abundance of the colonic microbiota at phylum (A) and genus (B) levels. CON = control group; LZ = lysozyme group.
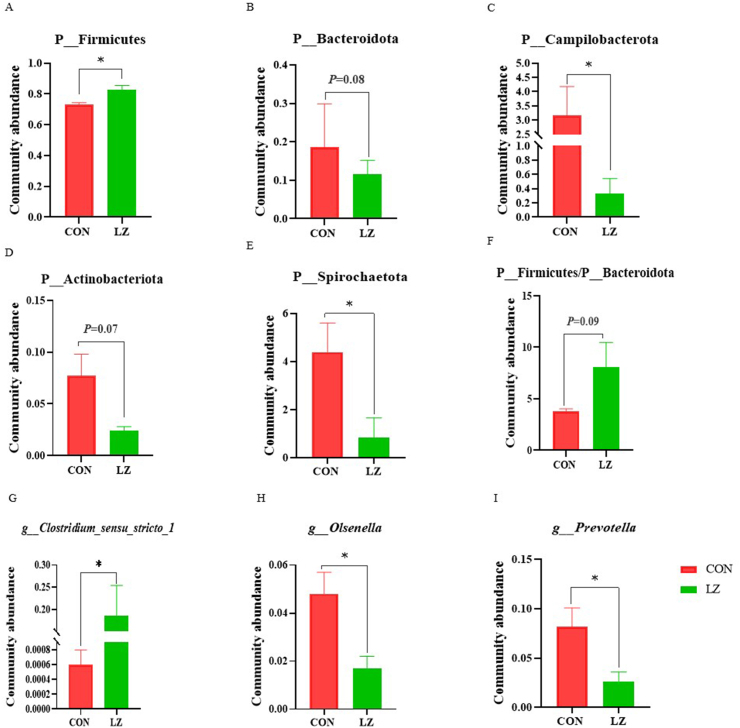
As shown in Fig. 6, LZ increased the abundance of Firmicutes (P < 0.05) and the ratio of Firmicutes to Bacteroidota (P = 0.09), and decreased the abundance of Bacteroidota (P = 0.08), Actinobacteriota (P = 0.07) and Campilobacterota (P < 0.05) at phylum level. At the genus level, LZ significantly increased the abundance of Clostridium_sensu_stricto_1 (P < 0.05), and reduced the abundance of Olsenella and Prevotella (P < 0.05).
Fig. 6.
Effect of LZ on intestinal microorganisms in weaned piglets (n = 6). (A) Community abundance of P_Firmicutes, (B) community abundance of P_Bacteroidota, (C) community abundance of P_Campilobacterota, (D) community abundance of P_Actinobacteriota, (E) community abundance of P_Spirochaetota, (F) ratio of P_Firmicutes to P_Bacteroidota, (G) community abundance of g_Clostridium_sensu_stricto_1, (H) community abundance of g_Olsenella, (I) community abundance of g_Prevotella. CON = control group; LZ = lysozyme group. Values are means ± SEM. Statistical differences in mean values of all indices were evaluated by independent t-test. The asterisk (∗) means P < 0.05.
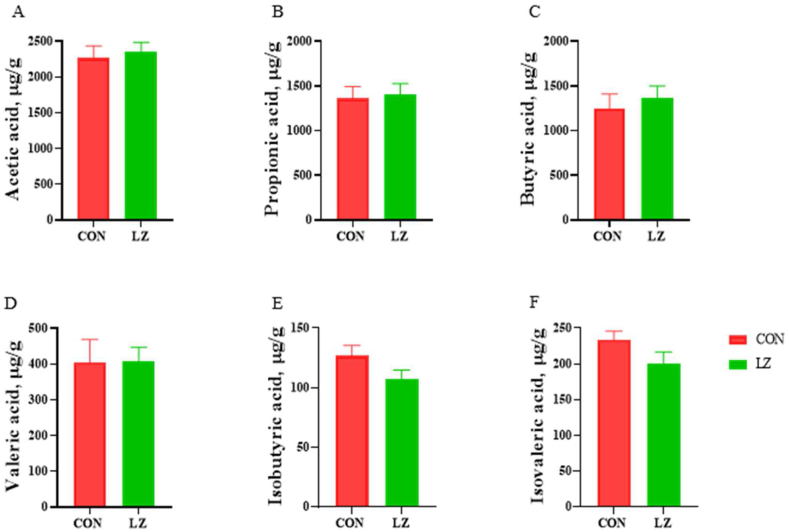
3.6. Concentration of SCFA in the colonic contents
As shown in Fig. 7, there was no significant effect on the concentration of SCFA in the colonic contents of weaned piglets in CON and LZ.
Fig. 7.
Effect of LZ on concentrations of short-chain fatty acids (n = 6). (A) Acetic acid content. (B) Propionic acid content. (C) Butyric acid content. (D) Valeric acid content. (E) Isobutyric acid content. (F) Isovaleric acid content. CON = control group; LZ = lysozyme group. Values are means ± SEM. Statistical differences in mean values of all indices were evaluated by independent t-test.
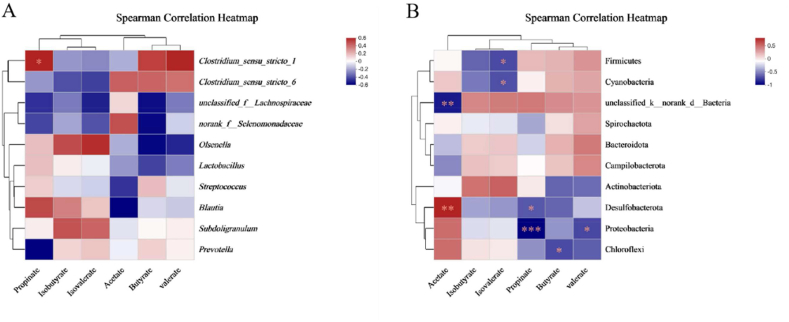
3.7. Correlation between SCFA and intestinal flora
The correlation between SCFA and intestinal flora at the phylum and genus levels was further explored, and the results are shown in Fig. 8. At the phylum level, Firmicutes and Cyanobacteria were negatively correlated with isovalerate, unciassified_k_norank_d_Bacteria was negatively correlated with acetic acid, and Proteobacteria was negatively correlated with propionic acid and isobutyric acid. At the genus level, Clostridium_sensu_stricto_1 was positively correlated with propionic acid (P < 0.05).
Fig. 8.
Correlations heatmap between intestinal flora and short-chain fatty acids (SCFA) in colon. Spearman correlation heatmap of SCFA and intestinal flora at the genus (A) and phylum levels (B). The asterisk (∗) means P < 0.05.
4. Discussion
Immature intestinal development, insufficient passive immunity, and changes in the nutrition and environment of weaned piglets usually lead to severe growth inhibition and diarrhea (Sun et al., 2021). In fact, most breeds of pigs will face poor immune function and gastrointestinal infection during weaning (Xu et al., 2014). At present, it is not feasible to add antibiotics to improve the intestinal health of animals during weaning, so substitutes such as natural extracts and antibacterial proteins are being widely studied. Increasingly, many studies have been focusing on the use of natural antibacterial proteins in animal husbandry, such as LZ and lactoferrin. These proteins exist in the tears, saliva or breast milk of mammals, and provide nonspecific antibacterial defense and activate the immune response (Cooper et al., 2015; Ercan and Demirci, 2016). LZ is one of the main host defense factors of animal body. It directly inhibits or kills pathogenic bacteria by destroying the structure of the cell wall, which is beneficial for enhancing intestinal function and improving growth performance and economic benefits (Wu et al., 2019; Vanderkelen et al., 2012; Wang et al., 2012). Our research mainly studied the effect of LZ as a natural antibacterial protein on the growth performance and intestinal function of weaned piglets, and the results explained the mechanism of how LZ effectively alleviates the effects of weaning stress in piglets.
Firstly, we measured the effects of LZ on the growth performance and blood biochemical indices of weaned piglets. The results showed that dietary LZ in the diet could significantly improve the ADFI and ADG of piglets and improve the growth rate of piglets, yet FCR showed no significant difference. It has been reported that LZ as an antibiotic substitute could significantly improve the growth performance of suckling piglets, and growing-finishing pigs (May et al., 2012; Oliver et al., 2014). In addition, Long et al. found that LZ (45,240 U/mg) could improve the growth performance of 25-d-old weaned ternary pigs (Long et al., 2016). In our study, the 22-d-old (3 d younger than those in Long et al.) dually crossbred pigs, which had weaker immunity than ternary pigs, had poorer resistance to stress. Therefore, we used a higher dose of LZ (50 U/mg, 0.1%) to investigate the effect of LZ on weaned piglets, and our results were consistent with their conclusion.
The blood biochemical indices can reflect the growth performance and liver metabolism of animals. The results of the blood biochemical indices in this study showed that dietary LZ significantly increased the serum TP content of weaned piglets. Previous experiments showed that the addition of LZ also increased the TP content of sows (Zou et al., 2019). Serum TP concentration reflects the absorption, synthesis and decomposition of protein, and to some extent, the ability of the liver to synthesize protein. Hence LZ may be beneficial to liver metabolism and therefore the health of weaned piglets.
Intestinal morphology is an important indicator of intestinal health (Liu et al., 2012). It is reported that weaning stress can cause intestinal damage such as reduced intestinal villi height and deepened recess in piglets, and the morphology of the small intestine changes from dense finger-like villi to smooth tongue-like villi, resulting in reduced active intestinal absorption capacity (Smith et al., 2010). Many researchers have found that adding LZ to piglets’ diet elicits beneficial changes to the height of the ileal, jejunal and duodenal villi (Brundige et al., 2008; Cooper et al., 2011; Xiong et al., 2019). Our results also showed that LZ could increase the height of the jejunal villi. That is why dietary LZ can improve the morphology of the intestinal mucosal epithelium, improve the ability to digest and absorb nutrients, and finally promote the growth and development of weaned piglets.
The intestinal barrier plays an important role in maintaining the balance of the intestinal environment, which can prevent pathogens, toxins and antigens from entering mucosal tissue (McCarty and Lerner, 2021; Turner, 2009). Intestinal mucosal tight junctions are an important part of the intestinal mucosal mechanical barrier, which mainly include proteins such as claudin, occludin, ZO-1 and connecting adhesion molecules (Buckley and Turner, 2018; Runkle and Mu, 2013). Protein ZO-1 is involved in the formation of tight junctions and the regulation of epithelial cell permeability, and also in the regulation of the composition and function of the parietal cytoskeleton (Van Itallie and Anderson, 2014). Occludin is a transmembrane protein, which is closely related to the structural integrity of intestinal epithelial cells and barrier function (Al-Sadi et al., 2011). Our results showed that dietary LZ significantly increased the expression of occludin gene mRNA in the jejunum and ZO-1 gene mRNA in the ileum, indicating that dietary LZ improved the intestinal barrier function of piglets and thus improved animal productivity.
It has been reported that weaning piglets can cause related intestinal inflammation (Tang et al., 2021). The intestinal barrier is also dynamically regulated by cytokines such as proinflammatory factors including IL-1β, IL-8, TNF-α and others, which play a key role in immune stress. The proinflammatory factors change behavior, metabolism, neuroendocrine function and ultimately inhibit the growth of biological organisms by increasing intestinal epithelial permeability, inducing pathological changes in the intestinal tight junction barrier, and mediating systemic effects of inflammation (Al-Sadi et al., 2009; Jacobi et al., 2006; Shin and Kim, 2018; Lee, 2015). It is reported that the addition of LZ decreased the level of TNF-α in intestinal mucosa of weaned piglets after E. coli K88 attack (Nyachoti et al., 2012). In this study, dietary LZ significantly reduced the gene expression level of TNF-α, IL-1β and IL-8 in jejunum and IL-1β in the ileum. Therefore, the improvement in intestinal barrier function by LZ may be related to the down regulation of the gene expression levels of these proinflammatory factors.
We further studied the expression of inflammatory pathway related genes in the jejunal and ileal mucosa of piglets. The results showed that LZ significantly reduced the expression of the NF-κB signaling pathway-related gene mRNA in intestinal mucosa, but there is no significant effect on the p38 MAPK signaling pathway. However, NF-κB is the main transcription factor involved in inflammatory diseases (Napetschnig et al., 2013). IκBα is an inhibitor of NF-κB, which inhibits the activation of this pathway by forming an inactive NF-κB/IκBα complex in the cytoplasm. Under extracellular stimulation, the degradation of IκBα mediated by phosphorylation activates, which in turn activates nuclear translocation and downstream transcriptional genes of NF-κB (Zhang et al., 2022). The current research showed that LZ could significantly inhibit the degradation of intestinal IκBα and the activation of NF-κB, but previous studies have rarely reported the effect of feed with added LZ on the NF-κB signaling pathway. This research found that the regulation of intestinal function by LZ may be related to the inhibition of NF-κB signaling pathway.
Intestinal flora contributes to the maintenance of intestinal physiological structure and function, which is considered to be related to intestinal inflammation, barrier function and host growth performance (Wang et al., 2016). Thus, high-throughput sequencing of 16S rRNA gene analysis of piglet colonic contents revealed that the addition of LZ to the diet decreased the ACE index and Shannon index for α diversity of colonic microorganisms. The α diversity refers to the diversity in a specific region or ecosystem, which is often measured by Chao 1, ACE, Shannon and Simpson indicators. Among them, the Chao1 index and ACE index are related to microbial community richness, whereas the Shannon index and Simpson index are related to the diversity of intestinal flora (Zhang et al., 2020). The results were similar to previous studies in which the addition of LZ in the diet decreased the microbial diversity of the cecum in growing pigs (Zou et al., 2019), and the microbial diversity decreased on the 21st day of lactation when LZ was added from late pregnancy to lactation (Xu et al., 2020). By analyzing the results, it was speculated that the decrease in microbial diversity may be related to the inhibition or direct killing of intestinal pathogens by LZ.
Furthermore, we found that dietary LZ decreased the relative abundance of Bacteroidota, Actinobacteria and Campilobacterota microorganisms, increased the relative abundance of Firmicutes and the ratio of Firmicutes to Bacteroidota microorganisms at the phylum level. Firmicutes and Bacteroidetes are the dominant bacteria in animal intestine. Bacteroidetes are mainly used to degrade carbohydrates, and their enrichment is negatively correlated with the daily gain of piglets (Ban-Tokuda et al., 2017; Spence et al., 2006). The fat deposition of piglets is related to the proportion of Firmicutes and Bacteroidetes, the larger the proportion, the easier it is to promote the host's capacity to absorb or store energy (Magnusson et al., 2015). Lots of studies have confirmed that the ratio of Firmicutes to Bacteroidetes in the intestinal microbiota of obese people is relatively high, allowing them to effectively absorb heat from food and promote the growth of the body (Guida and Venema, 2015). Actinomycetes are associated with inflammatory response. High levels of Actinomycetes were found in the colon of patients with inflammatory bowel disease and obese Ossabaw miniature pigs (Pedersen et al., 2013). Campylobacter, as a symbiotic bacterium, widely exists in the intestines of various wild or domestic animals. It is also a common zoonotic pathogen. Campylobacter can adhere to the surface of intestinal mucosa epithelial cells in the animal gastrointestinal tract and cause a toxin-mediated inflammatory reaction, and the clinical manifestation is gastroenteritis (Dasti et al., 2010). Therefore, the reduction in the relative abundance of Actinomycetes and Campylobacter in the results of this study was beneficial for alleviating the inflammatory response in weaned piglets.
At the genus level, we found that LZ decreased the relative abundance of Olsenella and Prevotella, and increased the relative abundance of Clostridium_sensu_stricto_1. Clostridium plays an important role in the stability of the intestinal environment as the most important symbiotic flora in the intestine. Clostridium can prevent inflammation in vitro and in vivo by blocking the activation of NF-κB (Guo et al., 2020), consistent with our previous results that LZ inhibited the activity of NF-κB. As an anaerobic or microaerobic bacterium, Olsenella is common in the oral cavity or gastrointestinal tract, and further research is needed in view of the minimal research results on it. Prevotella is an important pathogen in the intestine and can participate in human diseases by promoting chronic inflammation. Prevotella can also stimulate epithelial cells to produce cytokines and promote mucosa inflammatory immune response (Larsen, 2017), which was consistent with the finding that adding LZ down-regulated the mRNA gene expression of inflammatory factors. Consequently, it could be explained that LZ may improve the immune response and reduce the inflammatory response by affecting the intestinal flora.
Moreover, intestinal SCFA produced by intestinal flora are involved in regulating intestinal homeostasis (Ríos-Covián et al., 2016). Thus, we further measured the concentration of SCFA in colonic contents to explore the relationship between intestinal flora and SCFA under the action of LZ. Nevertheless, our results showed that the addition of LZ in the diet did not change the concentration of SCFA in the colon of weaned piglets. It indicated that LZ could affect the composition of intestinal flora, but did not further affect the metabolism of SCFA by intestinal flora. Whether LZ can play a role through other metabolites or other forms of microorganisms needs to be further studied.
5. Conclusions
This study found that LZ could significantly improve the growth performance of weaned piglets, and enhanced the mechanical barrier function of the intestinal mucosa of weaned piglets. The mechanism might be that lysozyme could down-regulate the expression of proinflammatory cytokine mRNA and inhibit the NF-κB signaling pathway. Moreover, LZ increased the abundance of beneficial bacteria, and further enhanced the intestinal biological barrier function. This study provided an important theoretical basis for LZ to become a feed additive with broad application prospects in pig production.
Author contributions
Yuying Wu: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Bei Cheng: Conceptualization, Methodology. Longxiang Ji: Resources. Xiangyun Lv: Resources. Yingying Feng: Conceptualization, Methodology. Liu'an Li: Resources, Methodology. Xin Wu: Supervision, Writing - review & editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
The research work was supported by National key R&D program of China (2021YFD1301002), Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-CXRC-031; TSBICIP-CXRC-038).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Yingying Feng, Email: fengyy@tib.cas.cn.
Liu’an Li, Email: anliuli2003@163.com.
References
- Al-Sadi R., Boivin M., Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R., Khatib K., Guo S., Ye D., Youssef M., Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1054–G1064. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban-Tokuda T., Maekawa S., Miwa T., Ohkawara S., Matsui H. Changes in faecal bacteria during fattening in finishing swine. Anaerobe. 2017;47:188–193. doi: 10.1016/j.anaerobe.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Buckley A., Turner J.R. Cell biology of tight junction barrier regulation and mucosa disease. Cold Spring Harbor Perspect Biol. 2018;10:a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundige D.R., Maga E.A., Klasing K.C., Murray J.D. Lysozyme transgenic goats' milk influences gastrointestinal morphology in young pigs. J Nutr. 2008;138:921–926. doi: 10.1093/jn/138.5.921. [DOI] [PubMed] [Google Scholar]
- Cooper C.A., Brundige D.R., Reh W.A., Maga E.A., Murray J.D. Lysozyme transgenic goats' milk positively impacts intestinal cytokine expression and morphology. Transgenic Res. 2011;20:1235–1243. doi: 10.1007/s11248-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C.A., Maga E.A., Murray J.D. Production of human lactoferrin and lysozyme in the milk of transgenic dairy animals: past, present, and future. Transgenic Res. 2015;24:605–614. doi: 10.1007/s11248-015-9885-5. [DOI] [PubMed] [Google Scholar]
- Dasti J.I., Tareen A.M., Lugert R., Zautner A.E., Gross U. Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010;300:205–211. doi: 10.1016/j.ijmm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ercan D., Demirci A. Recent advances for the production and recovery methods of lysozyme. Crit Rev Biotechnol. 2016;36:1078–1088. doi: 10.3109/07388551.2015.1084263. [DOI] [PubMed] [Google Scholar]
- Ferraboschi P., Ciceri S., Grisenti P. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics. 2021;10:1534. doi: 10.3390/antibiotics10121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guida S., Venema K. Gut microbiota and obesity: involvement of the adipose tissue. J Funct Foods. 2015;14:407–423. [Google Scholar]
- Guo P., Zhang K., Ma X., He P. Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol. 2020;11:24. doi: 10.1186/s40104-019-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Li X., Lu D., Liu S., Suo X., Li Q., Li N. Lysozyme improves gut performance and protects against enterotoxigenic escherichia coli infection in neonatal piglets. Vet Res. 2018;49:20. doi: 10.1186/s13567-018-0511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi S.K., Gabler N.K., Ajuwon K.M., Davis J.E., Spurlock M.E. Adipocytes, myofibers, and cytokine biology: new horizons in the regulation of growth and body composition. J Anim Sci. 2006;84(Suppl):E140–E149. doi: 10.2527/2006.8413_supple140x. [DOI] [PubMed] [Google Scholar]
- Larsen I.S., Jensen BaH., Bonazzi E., Choi B.S.Y., Kristensen N.N., Schmidt E.G.W., Süenderhauf A., Morin L., Olsen P.B., Hansen L.B.S., Schröder T., Sina C., Chassaing B., Marette A. Fungal lysozyme leverages the gut microbiota to curb dss-induced colitis. Gut Microb. 2021;13 doi: 10.1080/19490976.2021.1988836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J.M. The immune response to prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Int Res. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Guo Y., Wang Z., Yuan J. Exogenous lysozyme influences clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. 2010;39:17–24. doi: 10.1080/03079450903447404. [DOI] [PubMed] [Google Scholar]
- Liu Y., Chen F., Odle J., Lin X., Jacobi S.K., Zhu H., Wu Z., Hou Y. Fish oil enhances intestinal integrity and inhibits tlr4 and nod 2 signaling pathways in weaned pigs after lps challenge. J Nutr. 2012;142:2017–2024. doi: 10.3945/jn.112.164947. [DOI] [PubMed] [Google Scholar]
- Long Y., Lin S., Zhu J., Pang X., Fang Z., Lin Y., Che L., Xu S., Li J., Huang Y., Su X., Wu D. Effects of dietary lysozyme levels on growth performance, intestinal morphology, non-specific immunity and mrna expression in weanling piglets. Anim Sci J. 2016;87:411–418. doi: 10.1111/asj.12444. [DOI] [PubMed] [Google Scholar]
- Magnusson K.R., Hauck L., Jeffrey B.M., Elias V., Humphrey A., Nath R., Perrone A., Bermudez L.E. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience. 2015;300:128–140. doi: 10.1016/j.neuroscience.2015.05.016. [DOI] [PubMed] [Google Scholar]
- May K.D., Wells J.E., Maxwell C.V., Oliver W.T. Granulated lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology of 10-day-old pigs. J Anim Sci. 2012;90:1118–1125. doi: 10.2527/jas.2011-4297. [DOI] [PubMed] [Google Scholar]
- Mccarty M.F., Lerner A. Perspective: prospects for nutraceutical support of intestinal barrier function. Adv Nutr. 2021;12:316–324. doi: 10.1093/advances/nmaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napetschnig, Johanna, Wu Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Nutrient requirements of swine. 10th revised edition. The National Academies Press; Washington, DC: 1998. [Google Scholar]
- Nyachoti C.M., Kiarie E., Bhandari S.K., Zhang G., Krause D.O. Weaned pig responses to escherichia coli k88 oral challenge when receiving a lysozyme supplement. J Anim Sci. 2012;90:252–260. doi: 10.2527/jas.2010-3596. [DOI] [PubMed] [Google Scholar]
- Oliver W.T., Wells J.E., Maxwell C.V. Lysozyme as an alternative to antibiotics improves performance in nursery pigs during an indirect immune challenge. J Anim Sci. 2014;92:4927–4934. doi: 10.2527/jas.2014-8033. [DOI] [PubMed] [Google Scholar]
- Pedersen R., Ingerslev H.C., Sturek M., Alloosh M., Cirera S., Christoffersen B.O., Moesgaard S.G., Larsen N., Boye M. Characterisation of gut microbiota in ossabaw and gottingen minipigs as models of obesity and metabolic syndrome. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., De Los Reyes-Gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkle E.A., Mu D. Tight junction proteins: from barrier to tumorigenesis. Cancer Lett. 2013;337:41–48. doi: 10.1016/j.canlet.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladrigas-García M., D'angelo M., Ko H.L., Nolis P., Ramayo-Caldas Y., Folch J.M., Llonch P., Solà-Oriol D., Pérez J.F., Martín-Orúe S.M. Understanding host-microbiota interactions in the commercial piglet around weaning. Sci Rep. 2021;11 doi: 10.1038/s41598-021-02754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah A.K., Meyer M.T., Boxall A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (vas) in the environment. Chemosphere. 2006;65:725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Shin W., Kim H.J. Intestinal barrier dysfunction orchestrates the onset of inflammatory host-microbiome cross-talk in a human gut inflammation-on-a-chip. Proc Natl Acad Sci U S A. 2018;115 doi: 10.1073/pnas.1810819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F., Clark J.E., Overman B.L., Tozel C.C., Huang J.H., Rivier J.E., Blikslager A.T., Moeser A.J. Early weaning stress impairs development of mucosa barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C., Wells W.G., Smith C.J. Characterization of the primary starch utilization operon in the obligate anaerobe bacteroides fragilis: regulation by carbon source and oxygen. J Bacteriol. 2006;188:4663–4672. doi: 10.1128/JB.00125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Cui Y., Su Y., Gao Z., Diao X., Li J., Zhu X., Li D., Li Z., Wang C., Shi Y. Dietary fiber ameliorates lipopolysaccharide-induced intestinal barrier function damage in piglets by modulation of intestinal microbiome. mSystems. 2021;6 doi: 10.1128/mSystems.01374-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Liu J., Ma Y., Wei Y., Liu J., Wang H. Impairment of intestinal barrier function induced by early weaning via autophagy and apoptosis associated with gut microbiome and metabolites. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.804870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosa barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Van Itallie C.M., Anderson J.M. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157–165. doi: 10.1016/j.semcdb.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkelen L., Ons E., Van Herreweghe J.M., Callewaert L., Goddeeris B.M., Michiels C.W. Role of lysozyme inhibitors in the virulence of avian pathogenic escherichia coli. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Lo L.F., Forsberg L.S., Maier R.J. Helicobacter pylori peptidoglycan modifications confer lysozyme resistance and contribute to survival in the host. mBio. 2012;3 doi: 10.1128/mBio.00409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li Z., Han Q., Guo Y., Zhang B., D'inca R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by escherichia coli in broilers. Br J Nutr. 2016;116:1878–1888. doi: 10.1017/S0007114516004116. [DOI] [PubMed] [Google Scholar]
- Wu T., Jiang Q., Wu D., Hu Y., Chen S., Ding T., Ye X., Liu D., Chen J. What is new in lysozyme research and its application in food industry? A review. Food Chem. 2019;274:698–709. doi: 10.1016/j.foodchem.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Xie C., Zhang Y., Niu K., Liang X., Wang H., Shan J., Wu X. Enteromorpha polysaccharide-zinc replacing prophylactic antibiotics contributes to improving gut health of weaned piglets. Anim Nutr. 2021;7:641–649. doi: 10.1016/j.aninu.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Zhou J., Liu H., Tang Y., Tan B., Yin Y. Dietary lysozyme supplementation contributes to enhanced intestinal functions and gut microflora of piglets. Food Funct. 2019;10:1696–1706. doi: 10.1039/c8fo02335b. [DOI] [PubMed] [Google Scholar]
- Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y., Wang T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;30:584–589. doi: 10.1016/j.nut.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Xu S., Shi J., Dong Y., Li Z., Shen Y. Fecal bacteria and metabolite responses to dietary lysozyme in a sow model from late gestation until lactation. Sci Rep. 2020;10:3210. doi: 10.1038/s41598-020-60131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Shi J., Shi X., Dong Y., Wu X., Li Z., Fang Z., Lin Y., Che L., Li J., Feng B., Wang J., Wu D., Shen Y. Effects of dietary supplementation with lysozyme during late gestation and lactation stage on the performance of sows and their offspring. J Anim Sci. 2018;96:4768–4779. doi: 10.1093/jas/sky338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Song Y., Yu B., He J., Zheng P., Mao X., Huang Z., Luo Y., Luo J., Yan H., Wang Q., Wang H., Chen D. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J Anim Sci Biotechnol. 2020;11:87. doi: 10.1186/s40104-020-00496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Qi N., Zeng Y., Bao M., Chen Y., Liao J., Wei L., Cao D., Huang S., Luo Q., Jiang Y., Mo Z. The endogenous alterations of the gut microbiota and feces metabolites alleviate oxidative damage in the brain of lancl1 knockout mice. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.557342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Duan X., Wassie T., Wang H.H., Li T., Xie C., Wu X. Enteromorpha prolifera polysaccharide-zinc complex modulates the immune response and alleviates lps-induced intestinal inflammation via inhibiting the tlr4/nf-κb signaling pathway. Food Funct. 2022;13:52–63. doi: 10.1039/d1fo02171k. [DOI] [PubMed] [Google Scholar]
- Zou L., Xiong X., Liu H., Zhou J., Liu Y., Yin Y. Effects of dietary lysozyme levels on growth performance, intestinal morphology, immunity response and microbiota community of growing pigs. J Sci Food Agric. 2019;99:1643–1650. doi: 10.1002/jsfa.9348. [DOI] [PubMed] [Google Scholar]