Abstract
Most studies attempting to address the health care needs of the millions of transgender, nonbinary, and/or gender-diverse (TNG) individuals rely on human subjects, overlooking the benefits of translational research in animal models. Researchers have identified many ways in which gonadal steroid hormones regulate neuronal gene expression, connectivity, activity, and function across the brain to control behavior. However, these discoveries primarily benefit cisgender populations. Research into the effects of exogenous hormones such as estradiol, testosterone, and progesterone has a direct translational benefit for TNG individuals on gender-affirming hormone therapies (GAHTs). Despite this potential, endocrinological health care for TNG individuals remains largely unimproved. Here, we outline important areas of translational research that could address the unique health care needs of TNG individuals on GAHT. We highlight key biomedical questions regarding GAHT that can be investigated using animal models. We discuss how contemporary research fails to address the needs of GAHT users and identify equitable practices for cisgender scientists engaging with this work. We conclude that if necessary and important steps are taken to address these issues, translational research on GAHTs will greatly benefit the health care outcomes of TNG people.
Despite blossoming sociopolitical recognition of and conversation about transgender, nonbinary, and/or gender-diverse (TNG) identities in recent years, biomedical literature has dragged woefully behind in its inquiry into gender-affirming care. Some TNG individuals seek gender-affirming medical care to assist in their embodiment goals, often seeking alignment with their internal sense of gender (see the Supplement for relevant definitions and terminology recommendations). This can include gender-affirming hormone therapies (GAHTs) and gender-affirming surgeries. The first animal model of GAHT was only published in 2017 (1,2), and no randomized controlled trials have been completed on GAHT, because with-holding a standard of care medical therapy that improves mental health outcomes (3–8) from individuals is unethical. Due to this lack of research, GAHT is not U.S. Food and Drug Administration–approved, and GAHT prescriptions are all off-label use. Anti-TNG lawmakers and activists exploit this dearth of knowledge to justify discriminatory policies (9), including those that delay and deny GAHT for TNG people (10–12). We believe that animal models of GAHT can help fill this gap by allowing researchers to conduct studies not possible in humans to better understand the specific biological systems affected by GAHT.
Cultural conversations about GAHT almost exclusively center around development of physical characteristics—beards, deep voices, and broad shoulders with T (testosterone)-GAHT versus breasts, hips, and softer skin with E2 (estradiol)- and/or P4 (progesterone)-GAHT. Such portrayals fail to recognize the profound effect of hormonal milieu on neural function and behavior. Here, we review current deficiencies in our understanding of GAHT effects on neurological processes and illustrate how to best model GAHT using current experimental paradigms in common laboratory animals. These deficits are encapsulated by several key research questions:
How does GAHT shape gene expression in the brain at different life stages?
How does GAHT impact neural processes involved in affect, and what is the consequence of this on mental health treatment and outcomes for TNG populations?
How does GAHT regulate the neural networks controlling social behavior, and how does this interact with the unique psychosocial stressors experienced by TNG individuals?
How does GAHT affect cognitive processes such as learning, spatial, and episodic memory?
How does GAHT impact the neurological control of metabolism and physiology?
In this review, we focus on data applicable to adult GAHTs. For more information on translational models for puberty and adolescent GAHTs, see the Supplement.
LIMITATIONS TO CURRENT MODELS
Because animal GAHT models are rarely used, it is worth commenting on the tradeoffs different model species offer. Rodents offer benefits of their low cost, small size, and shorter life span, which collectively enable studies to have a higher study population. In addition, they provide genetic accessibility, allowing precise recording and manipulation of neural and molecular function not possible in other species. Their most relevant limitation is that they lack sex hormone binding globulin (which binds T in the body) postnatally, impacting hormone metabolism (13). Nonhuman primates offer a closer model of human hormone metabolism and facilitate more robust studies of affect. Nevertheless, using nonhuman primates has significant drawbacks pertaining to their high expense and large size (limiting sample size and statistical power), ethical concerns, and specific needs that limit which research facilities are equipped to support nonhuman primate research.
Researchers should consider whether their hypothesis is better tested using classic endocrinological techniques or precise GAHT models (Figure 1A) when designing or reviewing GAHT experiments. For example, gonadectomy and surgical pellet implantation offers the most even, constant hormone levels; however, this is not how human GAHT is conducted (1,3,14–16). Also, animal studies cannot account for the unique chronic stressors that TNG humans endure (17–19), which inevitably impacts their nervous system function and behavior. TNG people experience extraordinary amounts of social rejection (19). Such experiences are known to impact overall stress level, various psychiatric symptoms, and suicidal behaviors in this community (17,18,20–23). Furthermore, health care discrimination predicts future health care avoidance due to increased rejection sensitivity (24). Social rejection and ostracization elicit the same brain activity as physical pain (25), trigger feelings of sadness and anger (26), and are well-established risk factors for developing depression (27–31). Notably, depressed individuals may falsely perceive rejection when it is not in fact expressed, likely compounding its impact (32).
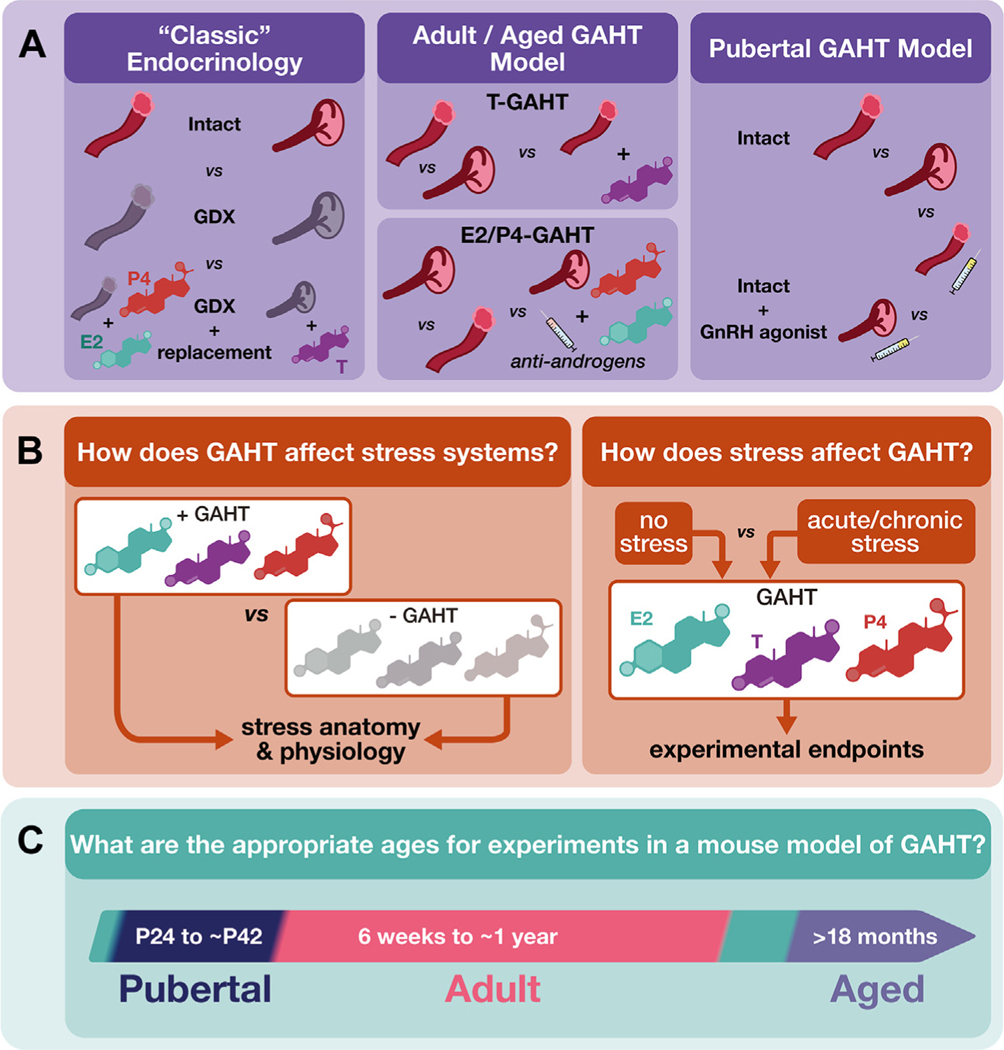
Figure 1.
Recommendations for rodent models of GAHT. (A) Classic endocrinology studies use GDX and hormone replacement to study the role of individual hormones. Researchers should take care to best model the human condition. GAHT in trans men and transmasculine people typically involves exogenous T treatment, GAHT in trans women and transfeminine people typically involves exogenous E2 and antiandrogen treatment, sometimes combined with P4, and transgender people who have their gonads removed do so later in life after the initiation of GAHT (3,15). Therefore, better models of GAHT should use intact animals at pubertal, adult, and aged time points. In adults and aged animals, untreated, intact animals with ovaries and testes should be compared with intact animals with ovaries treated with T and intact animals with testes treated with antiandrogens, E2, and/or P4. Commonly used antiandrogens include spironolactone, cyproterone acetate, and finasteride; all 3 antiandrogens have distinct mechanisms of action and off-target effects (3). Models using all 3 should be tested in translational studies. In peripubertal animals, untreated intact mice should be compared with intact mice treated with a GnRH agonist, such as leuprolide. (B) Owing to the high levels of chronic stress faced by transgender, nonbinary, and/or gender diverse populations, researchers should strive to assess both the effect of GAHT on stress systems in the brain and periphery and the effect of various stressors on the efficacy of GAHT relative to particular experimental end points. Care should be taken to assess the effects of stressors occurring prior to, during, and after the onset of exogenous hormone treatment. (C) Relevant timelines for rodent models of GAHT. E2, estradiol; GAHT, gender-affirming hormone therapy; GDX, gonadectomy; GnRH, gonadotropin hormone-releasing hormone; P4, progesterone; T, testosterone.
Such stressors are often conceptualized by the minority stress model, originally coined by Brooks (33). The minority stress model for TNG people [based on the minority stress model proposed for lesbian, gay, and bisexual people (34)] posits that external stressors related to minority status, internalization of these stressors, and learned anticipation of discrimination impacts general health (35). These external and internal factors result in elevated allostatic load, negatively affecting health. Coping and social support can offer a certain degree of buffering against the negative impact of minority stressors (36,37). It is important for researchers to recognize that animal GAHT models are unable to explore the uniquely human impacts of minority stress on mood and behavior. However, animal models of chronic social stress can provide translational insights into how elevated social allostatic load impacts neural function and the biological mechanisms underlying resiliency and susceptibility (38,39). Thus, as will be discussed below, animal GAHT models should be paired with varied chronic social stress models (Figure 1B). Such studies could potentially translate into personalized GAHT better suited to individual patient needs.
GAHT AND GENE EXPRESSION
Gonadal hormones are typically divided into 3 classes: estrogens, androgens, and progestogens. During mammalian steroidogenesis, P4 acts as a precursor for T, and T is converted to E2. While predominantly made in the gonads, they can be synthesized in the brain to affect neural development and function (40,41). Regardless of their origin, gonadal hormones canonically exert their effects as ligands for their corresponding receptors. Hormone receptors are broadly nuclear or membrane tethered (Table 1). Following ligand binding, nuclear receptors bind to DNA motifs and act as transcription factors, initiating the expression or repression of multiple sets of genes (42–44). Furthermore, individual neurons often coexpress several types of nuclear hormone receptors (45), endowing them with further flexibility to alter their gene expression profile depending on hormone state (46). Membrane-bound receptors can be found on the plasma membrane of both cells and intracellular organelles, and their activation drives rapid alteration of intracellular signaling pathways (47–49). This is in contrast to the timescale of nuclear receptor function, offering parallel mechanisms of action for hormones to influence cell function and behavior (50). Indeed, gonadal hormones and their nuclear receptors regulate gene expression to drive changes in neural circuit wiring, synaptic strength, and neural activity in ways that shape the likelihood of expressing various behaviors (44,50–53).
Table 1.
Nuclear and Membrane Receptors for Sex Steroid Hormones
| Receptor | Estrogen | Androgen | Progestogen |
|---|---|---|---|
| Nuclear | ER-α,ER-β | AR | PR-A, PR-B |
| Membrane-Bound, Steroid Specific | GPER1/GPR30, Gq-mER, ER-X | GPR56 | mPR family PGRMC1 |
| Other | TRPV1 | TRPM8 | TRPV1 |
AR, androgen receptor; ER, estrogen receptor; GPER1/GPR30: G protein–coupled estrogen receptor 1/G protein–coupled receptor 30; Gq-mER, Gq–coupled membrane estrogen receptor; GPR56, G protein–coupled receptor 56; mPR, membrane tethered progesterone receptor; PR, progesterone receptor; PGRMC1, progesterone receptor membrane component 1; TRPM8, transient receptor potential cation channel subfamily M member 8 (menthol receptor 1); TRPV1, transient receptor potential cation channel subfamily V member 1 (capsaicin receptor, vanilloid receptor 1).
Understanding how E2, T, and P4 direct adult neuronal gene expression can provide important insights into the mechanisms that both mediate the effects of and are influenced by GAHT. Changes in gene expression can occur through epigenetic modifications of the accessibility of genomic regions to transcription machinery. Thus, in addition to their role as transcription factors, gonadal hormone receptors can act on the molecular structure of the genome to regulate gene expression (44,54,55). Recent work focusing on the posterior bed nucleus of the stria terminalis, a subcortical structure involved in mammalian social behavior, showed that adult gonadectomy reverts sex differences in gene expression to a less-differentiated state in neurons expressing estrogen receptor (ER)α (44). In adult gonadectomized mice, few sex differences are seen in ERα-mediated genomic organization in response to exogenous E2. This indicates that sex-typical differences in gene expression arise from the acute hormone milieu and that hormones can mount a genomic response even after endogenous hormonal organizational periods of development at birth and puberty (44). This work provides a potential mechanism for neural circuits mediating sex-typical behaviors to retain the capacity for sex-atypical behaviors in adulthood after sexual differentiation (56–59). Importantly, it implicates GAHT in driving flexible changes in human neuronal gene expression. Future investigations should identify whether E2 modulates gene expression similarly in other brain regions that express steroid receptors (Figure 2) and elucidate the specific effects of ERα and ERβ isomers. The expression profiles elicited by T, P4, or combinations thereof through their nuclear receptors should also be characterized (Table 1).
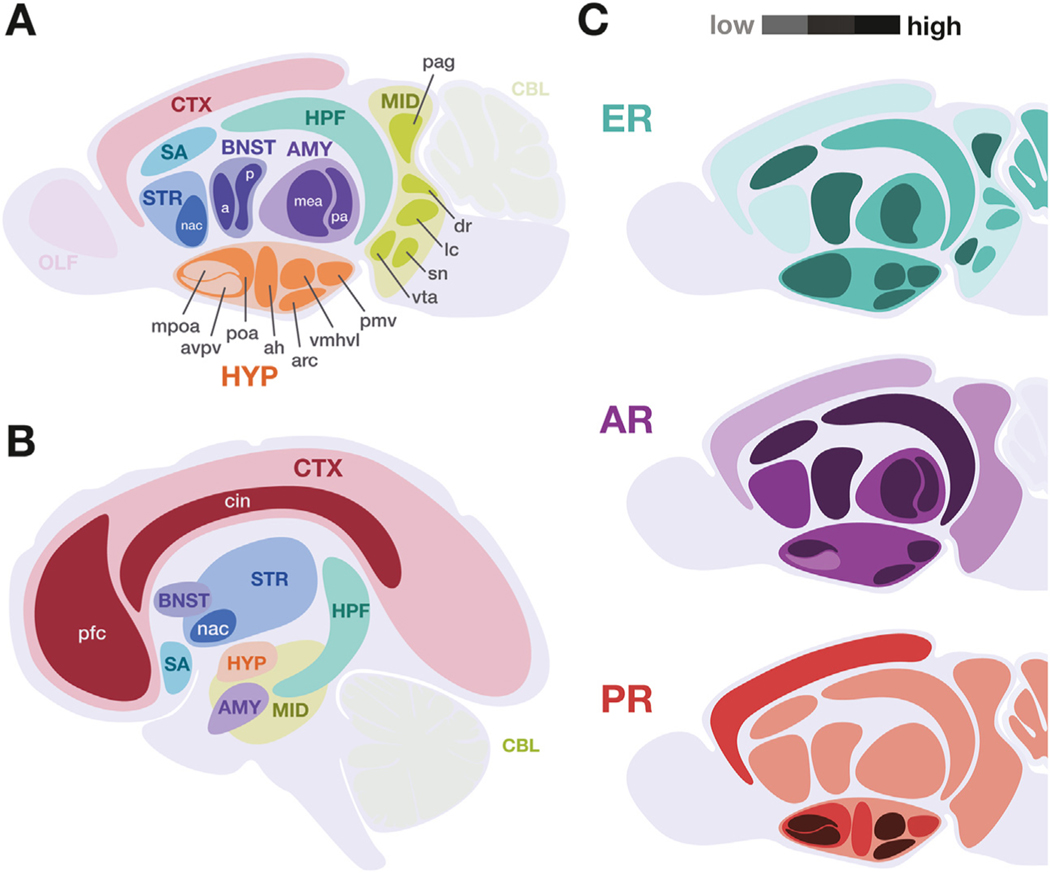
Figure 2.
Key neuroanatomical regions to consider in gender-affirming hormone therapy studies in both rodent animal models and humans. Certain brain regions are especially relevant to the study of gender-affirming hormone therapies, indicated in (A) murine and (B) human central nervous systems. Broad anatomical regions are indicated by uppercase abbreviations, with key subregions indicated in lowercase. (C) Schematic summarizing the relative expression levels of canonical nuclear hormone receptors in the murine brain, with darker shades indicating higher expression. Data represented includes both immunolocalization of receptor protein and receptor messenger RNA in adult rodent models. This summary does not include membrane or other hormone receptors (Table 1) and does not account for known isoform, developmental, and sex variability. [Visualizations based on data presented in (67,71,90–92)]. a, anterior; ah, anterior hypothalamus; AMY, amygdala; AR, androgen receptor; arc, arcuate nucleus; avpv, anteroventral periventricular nucleus; BNST, bed nucleus of the stria terminalis; CBL, cerebellum; cin, cingulate; CTX, cortex; dr, dorsal raphe; ER, estrogen receptor; HPF, hippocampal formation; HYP, hypothalamus; lc, locus coeruleus; mea, medial amygdala; MID, midbrain neuromodulatory centers; mpoa, medial preoptic area; nac, nucleus accumbens; OLF, olfactory bulb; p, posterior; pa, posterior amygdala; pag, periaqueductal gray; pfc, prefrontal cortex; pmv, ventral premammillary nucleus; poa, preoptic area; PR, progesterone receptor; SA, septal area, includes medial and lateral septal nuclei; sn, substantia nigra; STR, striatum; vmhvl, ventromedial hypothalamus ventrolateral division; vta, ventral tegmental area.
Gonadal hormones influence nonneuronal populations and should be considered when studying GAHTs. Microglia, immune cells in the nervous system, are responsive to E2 and are required for male-typical development of the preoptic area of the hypothalamus (60). Notably, microglia are involved in the anti-inflammatory effects of E2 and P4 (61–63) and may contribute to the mental health benefits associated with GAHT. Astrocytes are glial cells that regulate the recycling of neurotransmitters, cell metabolites, and modulate the blood-brain barrier, and these cells can express gonadal hormone receptors and steroidogenesis enzymes (64,65). Linking changes in hormone-regulated neuronal activity and function (53) to changes in hormone-dependent gene expression could provide specific molecular pathways for targeted and personalized GAHT.
GAHT AND MOOD/AFFECT
Understanding how changing hormone levels influence mood and affect could improve evidence-based mental health treatments for TNG individuals. Gonadal hormones can influence many affective disorders by acting on brain regions such as the hypothalamus, prefrontal cortex, nucleus accumbens, hippocampus, and extended amygdala (66–71) (Figure 2). Indeed, depressed cisgender women have reduced E2 levels (72). Low T levels are associated with increased risk of depression in cisgender men, though this is not reliably reversed by T replacement (73,74). In contrast, cisgender women with high T levels and physical manifestations of androgenism are more likely to report depression and eating disorders (75,76)—though this is confounded by societal stigma around facial and body hair for women. In postmenopausal cisgender women, depressive symptoms respond stronger to venlafaxine, a 5-HT (serotonin) and norepinephrine reuptake inhibitor, than to fluoxetine, a 5-HT reuptake inhibitor (77). Yet, supplementing fluoxetine with E2 rescues its antidepressive capacity in this population (78). One potential explanation is that E2 increases prefrontal 5-HT receptor expression in postmenopausal cisgender women (79). Conversely, venlafaxine efficacy in cisgender menopausal women is impaired by E2 (80). Studies on the efficacy of estrogen supplementation on negative affect reported mixed results in postmenopausal cisgender women (81,82). Nevertheless, clinical studies considering mood in the context of endogenous hormonal changes during menopause and pregnancy cannot control for co-occurring psychosocial stressors, sleep deprivation, and body image concerns. Just as hormonal changes influence the mental health needs of cisgender individuals, GAHT likely affects the mental health needs of TNG individuals. However, similar to cisgender individuals, a clear understanding of the relationship between hormone state and mood in TNG individuals is confounded by the reduced distress associated with physical changes from GAHT (8) and by minority stress (17,18). Fortunately, many animal models of affective disorders are clinically relevant, are hormone sensitive (38,39,83), and can be adapted for GAHT models (Figure 1A).
Studies in rodent models have revealed several possible mechanisms to explain variable responses to treatment for mood related to hormone state. One key pathway mediating depressive symptoms in humans and mice involves MAPK (mitogen-activated protein kinase) activity and BDNF (brain-derived neurotrophic factor) expression. BDNF is decreased in depressed patients (84), and translational studies have shown that MAPK activity can increase BDNF expression, leading to downstream changes in 5-HT signaling and improved mood (85). Fluoxetine, E2, and T increase BDNF along with MAPK activity in mice (86–88), while venlafaxine-induced BDNF increases occur independent of MAPK activity (87). Thus, changes in hormone state may alter the effectiveness of antidepressants. This may depend on which pathway is used to control BDNF, which neurotransmitters they influence, or other mechanisms. Indeed, similar to E2 for cisgender women, the T levels of adult male mice impact the effectiveness of fluoxetine but not venlafaxine (89). Tailoring experimental treatments to align with GAHT paradigms would ensure that the diverse hormone states of TNG individuals are accounted for in translational research.
Critically, studies examining mental health outcomes associated with GAHT run the risk of pathologizing gender affirmation rather than addressing patient needs. Therefore, we propose two experimental designs to study stress and affective disorders in models of GAHT (Figure 1B). First, researchers should compare hormone-treated and untreated animals to determine GAHT’s impact on clinically relevant neural systems. Second, researchers should compare between groups that receive hormone treatment but are differentially exposed to stress to understand how GAHT uniquely impacts mental health needs. This isolates the effects of stress on the nervous system within a model of GAHT. For example, researchers may first ask whether GAHT-like manipulation alters the 5-HT system in a mouse model. If a significant effect appears, one should ask how this novel 5-HT system changes after stress and whether such changes influence depressive symptom-sensitive pathways (e.g., MAPK and/or BDNF). These paradigms ensure that the crux of each experimental question is to inform personalized care for the mental health needs of TNG individuals.
GAHT AND SOCIAL BEHAVIOR
Hormone-sensitive receptors are found across the entirety of the social behavior network (45,67,71,90–92), a densely inter-connected network of subcortical brain regions fundamental to the expression of social behavior (Figure 2). These data raise the possibility that GAHT could act on this network to alter how the brain processes relevant social cues to guide ongoing and future social behavior, interacting with social transition and its associated benefits. Here, we review relevant data and emphasize open questions that could improve GAHT-related care.
Despite the importance of social transition—or the changes associated with re-entering the community as one’s lived gender—there is a paucity of research on the relationship between GAHT and social behavior. This is due in part to the outsized influence of culture, family, and other human-specific experiences on social behavior. Nevertheless, GAHT will affect the social behavior network. Consequently, understanding the mechanisms by which GAHT influences neural processing of behaviorally relevant social cues is key to providing TNG individuals informed and personalized transition care.
Because individual species evolved unique, species-specific social behaviors, researchers must be cautious translating work on social behaviors in model organisms to humans (93,94). For instance, mice communicate predominately via ultrasonic vocalizations and pheromones (95,96) whereas humans use complex language systems (97). Similarly, sexual and reproductive behaviors show diverse and varied motivations and behavioral expression across animal species (93,94). Nevertheless, social behaviors can be conceptualized as consisting of initial cue detection and subsequent action phases. By isolating specific cues and behavioral actions for study, this framework allows researchers to compare social behavior within and across species (93) (for examples in rodents, see the Supplement). Thus, despite species-specific differences, animal models can be used to identify the genetic, cell type, circuit, and computational mechanisms by which distinct hormone states influence ongoing behavior.
New advancements in behavioral quantification will broaden our understanding of social behavior (98,99). Animal pose tracking and unsupervised data analysis will allow researchers to holistically identify, quantify, and compare the entire social behavioral space, with fewer a priori biases. By pairing such tools with other techniques, such as multisite, multicolor manipulation, and imaging of neuronal activity (100,101), researchers can test novel hypotheses about how GAHT-like manipulations alters neural computations that translate social cues into behavior. Future development of fluorescent hormone indicators, similar to those developed for other neuromodulators (102), could reveal relationships between specific hormone levels, neural activity, and behavior. With such data, we may be able to develop more personalized GAHT regimens based on an individual’s social transition goals.
In gonadally intact vertebrates, the relationship between hormones and social behavior is bidirectional. Across a variety of species, including humans, social experience is associated with subsequent changes in endogenous gonadal hormone levels, biasing future behavior (50,51,103,104). In particular, it has been proposed that experience-driven changes in hormone state induce neural circuit plasticity, allowing for behavioral flexibility across different social environments (50,51,104). Nevertheless, TNG populations often suppress their endogenous gonadal hormone production via medication or surgery. At present, the effects of a loss or reduction of gonadal feedback on ongoing behavior are unknown. Animal models can help bridge this gap in knowledge by allowing researchers to directly manipulate gonadal hormone production, hormone receptor function, and neural activity. Future research should use both classic endocrinology methods and models of GAHT (Figure 1A) to elucidate how experience-driven hormone release influences neural activity and behavior and how this is affected by GAHT.
Work in humans showed that perceived stress and longitudinal T interact to influence partner desire in cisgender individuals (105). Specifically, in cisgender women, low T is associated with partner desire if individuals also experience low stress, while high stress, high T is associated with partner desire. In cisgender men, partner desire is associated with high stress and low T, as well as low stress and high T. However, the relationship between stress, T, and partner desire is complicated by human cultural expectations related to gender, and the idiosyncrasies of the Western college student sample. Researchers can avoid these confounds by employing models of GAHT in species that form pair bonds and directly manipulating stress and T levels. This will test the specific roles of stress and T on neural circuits during pair-bond formation and behavior displays toward partners or nonpartnered conspecifics. In pair-bonded Mongolian gerbils, acute T supplementation in intact males influences their sociability. Specifically, T following interactions with a pair-bonded partner facilitates prosocial behavior toward novel conspecifics, whereas T following an interaction with unfamiliar conspecifics reduces these behaviors. Finally, acute T paired with partner interactions activates paraventricular hypothalamus oxytocin neurons, implicating potential neuromodulatory circuit mechanisms that support these behaviors (106). Because levels of psychosocial stress in early transition are high (17–19), understanding how stress and changing T levels affect partner desire and sociability could translate into better social support systems for newly transitioning individuals.
As discussed previously, social rejection and ostracization are key components of the minority stress experienced by TNG individuals, which can be modeled by the need-threat temporal model of ostracism (26). Stage 1 of this model describes the immediate period during which the rejection is painful and is perceived as threatening one’s needs. The second stage—coping (or reflective)—refers to when an ostracized person processes the ostracism and may begin planning ways to protect their needs. If experiencing chronic rejection, the individual enters the third long-term (or resignation) stage in which coping reserves are depleted, and the individual may experience alienation, depression, helplessness, and feelings of unworthiness. Research suggests that in some circumstances this negative mental health impact may be buffered by group membership, though this appears to be more or less protective depending on the type of group identity (29,107,108). Together, these data underscore the need to understand how acute and chronic social stress impinge on hormone-sensitive mood and social circuits in models of GAHT (Figure 1B). Future studies should examine how models of GAHT interact with group inclusion/ostracization (38,39). How species-specific social buffering behaviors, such as allogrooming in mice (109), influence neural function in deep-brain regions implicated in stress also needs to be explored.
Work in cisgender and TNG populations shows a complex relationship between psychosocial stress and hormone levels. For example, although T levels are higher in nonheterosexual cisgender women than in heterosexual cisgender women, T levels are also positively associated with increased cortisol, allostatic load, and perceived stress across hetero- and nonheterosexual cisgender individuals (110). In TNG populations, long-term use of GAHT is associated with reductions in cortisol levels (8). However, TNG men who report transition-related minority stress show elevated cortisol and blood pressure (17,18). Although these data indicate that the additional psychosocial stress experienced by TNG populations increases stress-associated biomarkers, the role, if any, of gonadal hormones in physiological responses to social stressors remains unclear. By combining direct manipulations of hormone state and social stress, researchers can leverage translational models of GAHT to test the relative contribution of hormone state and social allostatic load on physiological stress response. In addition, researchers can design targeted experiments assessing the effect of GAHT-like manipulations on neural activity and function of brain regions across the hypothalamic-pituitary-adrenal axis. Together these data could identify specific mechanisms by which GAHT regulates physiological responses to social stressors. Such work is needed to develop novel therapies for TNG and cisgender populations.
GAHT AND COGNITION AND MEMORY
Little is known about GAHT’s influence on cognitive measures in TNG people, either in youth seeking to delay puberty or in aged TNG individuals with years of GAHT. Cognition, memory, and learning (111,112) are influenced by gonadal hormones and may be influenced by GAHT. The brain regions involved in these processes—the hippocampus, prefrontal and perirhinal cortices, medial septum, and neuromodulatory regions such as the locus coeruleus, dorsal raphe, ventral tegmental area, and substantia nigra—all express steroid receptors (Figure 2) (67,71,112,113). In addition, these regions differently express ERα and ERβ, suggesting distinct roles for each in cognitive outcomes (67,113). Furthermore, both nuclear and membrane steroid signaling of estrogens and progestogens are involved in cognitive measures in rodents (112,113). Estrogens and progestogens control particular aspects of memory and learning by modulating neurotransmitters involved in cognition and memory (112,114). Despite these effects, evidence that estrogen replacement therapies can protect against cognitive decline remains controversial (115).
Much of what we know about gonadal hormones on cognition and memory is from studies in animal models; however, studies in cisgender humans partially support the hypothesis that steroids influence cognition and memory in aged adults. In a cohort of postmenopausal cisgender women, hormone replacement did not alter cognitive outcomes (82). However, cognitive decline is found in comparisons of pre/perimenopausal to postmenopausal cisgender women (116). Although evidence is limited, it is suggested that the menstrual cycle, possibly because of P4 fluctuations, modulates emotional recognition, emotional memory consolidation, and fear extinction (117). P4 may play an inhibitory role in the hippocampus during spatial tasks through rapid signaling (118). However, in pre- and postmenopausal cisgender women, there is no consistent effect of P4 on most cognitive measures (119). Indeed, P4 has beneficial and disruptive influences on cognition and memory that depend on dosage, duration and timing of administration, age, type of progestogen, and type of cognitive assay (120). In cisgender men with low and typical T levels, positive associations between cognition and T supplementation are observed that depend on the concentration and administration of T, the age and baseline cognition of the subject, and the type of cognitive test (121). Consistent with the minority stress model, aged adults who identify as sexual and gender minorities are at greater risk for cognitive decline than their cisgender and heterosexual counterparts. Furthermore, sexual and gender minority individuals with cognitive decline were more likely to be depressed, disabled, or a person of color (122,123). In a study of 18 TNG women from Brazil, Full Scale IQ and Rey auditory-verbal learning test measures improved after 60 days of E2-GAHT; however, there was no relationship between E2 concentration and memory (124). More clinical studies are needed to assess the interactions of GAHT and chronic stressors on cognition and memory in adult and aging TNG populations. Future preclinical studies should be designed appropriately to ascertain the circuits and neurotransmitters involved in these processes.
GAHT AND METABOLISM
While much is known about the steroidal control of metabolism and physiology, the field is only starting to examine the long-term consequences of GAHT on metabolism, growth, and bone homeostasis. Circulating levels of gonadal steroid hormones play various roles in a number of metabolic pathways and processes. These include feeding, overall energy homeostasis, and bone health. In feeding, androgens alter leptin sensitivity of cells within the hypothalamic arcuate nucleus. Ovariectomized mice treated with dihydrotestosterone, a nonaromatizable androgen and more potent androgen receptor agonist than T, show an increase in feeding behavior and overall body mass (125). Estrogen receptors of all types are coexpressed with other metabolic receptors including leptin receptors in arcuate nucleus proopiomelanocortin–expressing populations (126). These and other neurons expressing estrogen receptors within the hypothalamus are implicated in feeding and adiposity. Deletion of ERα in proopiomelanocortin neurons causes hyperphagia in mice (127). E2 signaling coordinates hypothalamic MAPK signaling and ceramides to regulate brown adipose tissue thermogenesis (128,129). In addition, ERα neurons in the ventrolateral division of the ventromedial hypothalamus express melanocortin-4 receptors in an E2-dependent manner, allowing them to integrate both estrogen and melanocortin signals to drive physical activity (43). Many studies on metabolism typically have a long-term goal to reduce adiposity in humans. This framework aligns itself with medical systems that associate fat accumulation with a negative health outcome and use weight and body mass index as a metric to deny access to health care (130). Researchers must exercise caution when interpreting data and, in contrast to the traditional framework, design studies to understand the changes in body weight and the localization of fat accumulation from pre- to post-GAHT. Such experiments would align with one of the major goals of medical transition—body fat redistribution—and allow clinicians to tailor individual GAHT regimens to achieve desired transition outcomes.
Finally, hormonal action on skeletal system growth and maintenance must also be taken into account when tailoring GAHT, especially for aged TNG individuals experiencing age-dependent bone loss. Currently, it is known that menopausal individuals exhibit a decrease in overall bone mass (131), and deletion of ERα in arcuate nucleus kisspeptin neurons rapidly increases trabecular bone mass in ovariectomized mice (132). However, the effects of long-term GAHT on bone health remain largely underinvestigated (133). Future studies should address the influence of exogenous hormones on bone health in models of GAHT. These metabolic end points must be taken into account to understand and maintain the long-term health of TNG individuals.
Ultimately, interrogating the dynamics of gonadal steroids in the brain requires careful longitudinal monitoring. We believe that interpreting data on fat deposition, energy homeostasis, and bone health must involve a careful parameterization of sex-related variables (9). We suggest that researchers track changes in gonadal hormone levels in the hypothalamus through microdialysis probes and further parameterize adiposity metrics by including location of fat accumulation (subcutaneous, visceral, gonadal, etc.) in model organisms. Implementation of novel systems neuroscience methods, such as all optical approaches for deep-brain imaging and neuronal manipulation (101,102), will lead to an increased understanding of the relationship between hormone dynamics, neural activity, and metabolism. We also suggest that researchers use rodent models of GAHT at different developmental stages to examine how such treatment regimens affect various metabolic processes across the lifespan (Figure 1C).
CONCLUSIONS
Translational models can lead to improved or novel technologies, but their success is often determined when they leave the lab space and begin to interact with the community of users and providers (134). Both intended and unintended consequences are documented when studies are designed to support or investigate communities facing prejudice within health care and research institutions (135,136). This is notably true for the TNG community; while TNG individuals vary greatly in terms of gender identity and sexual orientation (137), theorists have focused on aspects of TNG experience that are not salient for many. Indeed, past studies have found that nearly 30% of participants do not identify within theoretically provided classifications (138). With increasing sociopolitical recognition of nonbinary and genderqueer identities, traditional binary classification is increasingly unhelpful in research (139). By preemptively using limited and misunderstood aspects of biology and TNG identity, theorists restricted the efficacy of TNG research by continually focusing on and publicizing stigmatized elements not illustrative of all TNG identities (138,140–142). Unfortunately, barriers to STEM (science, technology, engineering, and math) careers exist for TNG individuals (143,144) forcing TNG individuals to work outside of institutional conversations through protests and activism (145). However, social scientists have developed techniques to produce reciprocal relationships between the TNG community and researchers (146). Findings and research methodologies developed through such collaborations are well-received by community members (147) and continually used by researchers (148). Developing animal models of GAHT does not automatically create this reciprocal relationship between practice and evidence production. Thus, biomedical researchers must rely on studies that do. We propose that it is necessary for biomedical scientists to engage with community-based research findings to produce translational GAHT research that materially addresses the mental and physical health needs of the TNG community. The TNG community has identified research priorities for GAHT (Figure 3) (146,149,150) that can be used to develop questions and design experiments. Using these resources will ensure that study outcomes and interpretations align with TNG needs and experiences.
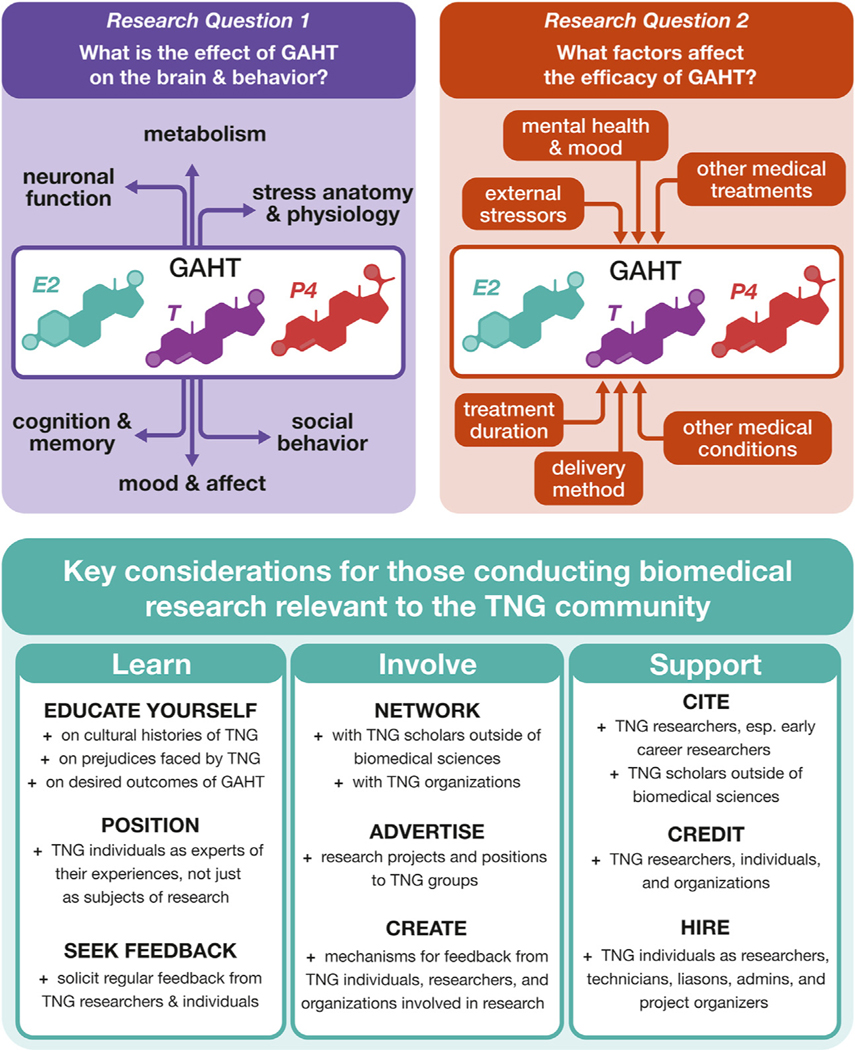
Figure 3.
Questions and considerations for effective biomedical research on GAHTs. Summary for research questions and considerations presented in this review. Understanding GAHT requires understanding how GAHT affects certain brain functions (research question 1) and what factors affect GAHT to achieve beneficial outcomes for TNG individuals (research question 2). For researchers engaging in TNG-relevant research, it is important to properly engage with TNG communities in several ways: learning about TNG people outside of the immediate biological question, involving TNG individuals who are interested in participating, and supporting TNG people who wish to contribute. E2, estradiol; GAHT, gender-affirming hormone therapy; P4, progesterone; T, testosterone; TNG, transgender, nonbinary, and/or gender diverse.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
TGG is supported in part by the National Institutes of Health (NIH) (Grant No. R25MH119043). DRP is supported by Dr. Molly Moravek, Dr. Ariella Shikanov, and Dr. Vasantha Padmanabhan through NIH (Grant No. 5R01HD098233–03). SDS is supported by Dr. Jessica Tollkuhn through NIH (Grant Nos. R01MH113628 and SFARI 736613). TAR is supported by the NIH (Grant No. R01MH123544). EMG is supported by the NIH (Grant No. F32MH126562).
We thank Corin Humphrey, Tyler Earl, the members of the Corin Humphrey Memorial Journal Club, the members of the Falkner lab at Princeton University, and countless members of the TNG community for vibrant discussions on hormones and plasticity.
Footnotes
The authors reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2022.07.002
REFERENCES
- 1.Goetz TG, Mamillapalli R, Devlin MJ, Robbins AE, Majidi-Zolbin M, Taylor HS (2017): Cross-sex testosterone therapy in ovariectomized mice: Addition of low-dose estrogen preserves bone architecture [published correction appears in Am J Physiol Endocrinol Metab. 2020; 319:E658]. Am J Physiol Endocrinol Metab 313:E540–E551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goetz TG, Mamillapalli R, Sahin C, Majidi-Zolbin M, Ge G, Mani A, Taylor HS (2018): Addition of estradiol to cross-sex testosterone therapy reduces atherosclerosis plaque formation in female ApoE–/– mice. Endocrinology 159:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, et al. (2012): Standards of care for the health of trans-sexual, transgender, and gender-nonconforming people, Version 7. Int J Transgend 13:165–232. [Google Scholar]
- 4.Green AE, DeChants JP, Price MN, Davis CK (2022): Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health 70:643–649. [DOI] [PubMed] [Google Scholar]
- 5.White Hughto JM, Reisner SL (2016): A systematic review of the effects of hormone therapy on psychological functioning and quality of life in transgender individuals. Transgend Health 1:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowniak S, Bolt L, Sharifi C (2019): Effect of cross-sex hormones on the quality of life, depression and anxiety of transgender individuals: A quantitative systematic review. JBI Database System Rev Implement Rep 17:1826–1854. [DOI] [PubMed] [Google Scholar]
- 7.Serano J (2007): Whipping Girl: A Transsexual Woman on Sexism and the Scapegoating of Femininity, 2nd ed. Berekeley, CA: Seal Press. [Google Scholar]
- 8.Colizzi M, Costa R, Pace V, Todarello O (2013): Hormonal treatment reduces psychobiological distress in gender identity disorder, independently of the attachment style. J Sex Med 10:3049–3058. [DOI] [PubMed] [Google Scholar]
- 9.Miyagi M, Guthman EM, Sun SED (2021): Transgender rights rely on inclusive language. Science 374:1568–1569. [DOI] [PubMed] [Google Scholar]
- 10.State of Florida Agency for Health Care Administration (2022): Agency for health care administration releases generally accepted professional medical standards determination on the treatment of gender dysphoria. Available at: https://southfloridahospitalnews.com/agency-for-health-care-administration-releases-generally-accepted-professional-medical-standards-determination-on-the-treatment-of-gender-dysphoria. Accessed September 14, 2022.
- 11.Ashley F (2021): The misuse of gender dysphoria: Toward greater conceptual clarity in transgender health. Perspect Psychol Sci 16:1159–1164. [DOI] [PubMed] [Google Scholar]
- 12.Redcay A, Bergquist K, Luquet W (2021): On the basis of gender: A medical-legal review of barriers to healthcare for transgender and gender-expansive patients. Soc Work Public Health 36:615–627. [DOI] [PubMed] [Google Scholar]
- 13.Hammond GL (2011): Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod 85:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingberg E, Theodorsson A, Theodorsson E, Strom JO (2012): Methods for long-term 17β-estradiol administration to mice. Gen Comp Endocrinol 175:188–193. [DOI] [PubMed] [Google Scholar]
- 15.Prior JC (2019): Progesterone is important for transgender women’s therapy-Applying evidence for the benefits of progesterone in ciswomen. J Clin Endocrinol Metab 104:1181–1186. [DOI] [PubMed] [Google Scholar]
- 16.Deutsch M (2016): Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people, 2nd ed. Center of Excellence for Transgender Health, Department of Family and Community Medicine, University of California San Francisco. Available at: https://transcare.ucsf.edu/sites/transcare.ucsf.edu/files/Transgender-PGACG-6-17-16.pdf. Accessed September 27, 2022. [Google Scholar]
- 17.Dubois LZ (2012): Associations between transition-specific stress experience, nocturnal decline in ambulatory blood pressure, and C-reactive protein levels among transgender men. Am J Hum Biol 24:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuBois LZ, Powers S, Everett BG, Juster RP (2017): Stigma and diurnal cortisol among transitioning transgender men. Psychoneuroendocrinology 82:59–66. [DOI] [PubMed] [Google Scholar]
- 19.Bradford J, Reisner SL, Honnold JA, Xavier J (2013): Experiences of transgender-related discrimination and implications for health: Results from the Virginia Transgender Health Initiative Study. Am J Public Health 103:1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements-Nolle K, Marx R, Katz M (2006): Attempted suicide among transgender persons: The influence of gender-based discrimination and victimization. J Homosex 51:53–69. [DOI] [PubMed] [Google Scholar]
- 21.Rood BA, Reisner SL, Surace FI, Puckett JA, Maroney MR, Pantalone DW (2016): Expecting rejection: Understanding the minority stress experiences of transgender and gender-nonconforming individuals. Transgend Health 1:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pariseau EM, Chevalier L, Long KA, Clapham R, Edwards-Leeper L, Tishelman AC (2019): The relationship between family acceptance-rejection and transgender youth psychosocial functioning. Clin Pract Pediatr Psychol 7:267–277. [Google Scholar]
- 23.Zhang J, Lo HH, Au AM (2021): The buffer of resilience in the relations of gender-related discrimination, rejection, and victimization with depression among Chinese transgender and gender non-conforming individuals. J Affect Disord 283:335–343. [DOI] [PubMed] [Google Scholar]
- 24.Hughto JMW, Pachankis JE, Reisner SL (2018): Healthcare mistreatment and avoidance in trans masculine adults: The mediating role of rejection sensitivity. Psychol Sex Orientat Gend Divers 5:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenberger NI, Lieberman MD, Williams KD (2003): Does rejection hurt? An fMRI study of social exclusion. Science 302:290–292. [DOI] [PubMed] [Google Scholar]
- 26.Williams KD (2009): Chapter 6: Ostracism: A temporal need-threat model. In: Advances in Experimental Social Psychology. Academic Press, 41: 275–314. [Google Scholar]
- 27.Williams KD, Nida SA (2011): Ostracism: Consequences and coping. Curr Dir Psychol Sci 20:71–75. [Google Scholar]
- 28.Allen NB, Badcock PBT (2003): The social risk hypothesis of depressed mood: Evolutionary, psychosocial, and neurobiological perspectives. Psychol Bull 129:887–913. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt MT, Branscombe NR, Postmes T, Garcia A (2014): The consequences of perceived discrimination for psychological wellbeing: A meta-analytic review. Psychol Bull 140:921–948. [DOI] [PubMed] [Google Scholar]
- 30.Berger M, Sarnyai Z (2015): “More than skin deep”: Stress neurobiology and mental-health consequences of racial discrimination. Stress 18:1–10. [DOI] [PubMed] [Google Scholar]
- 31.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT (2017): Structural racism and health inequities in the USA: Evidence and interventions. Lancet 389:1453–1463. [DOI] [PubMed] [Google Scholar]
- 32.Coyne JC (1976): Toward an interactional description of depression. Psychiatry 39:28–40. [DOI] [PubMed] [Google Scholar]
- 33.Brooks VR (1981): Minority Stress and Lesbian Women. Lexington, MA: Lexington Books. [Google Scholar]
- 34.Meyer IH (2003): Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: Conceptual issues and research evidence. Psychol Bull 129:674–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendricks ML, Testa RJ (2012): A conceptual framework for clinical work with transgender and gender nonconforming clients: An adaptation of the Minority Stress Model. Prof Psychol Res Pract 43:460–467. [Google Scholar]
- 36.Trujillo MA, Perrin PB, Sutter M, Tabaac A, Benotsch EG (2017): The buffering role of social support on the associations among discrimination, mental health, and suicidality in a transgender sample. Int J Transgend 18:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puckett JA, Matsuno E, Dyar C, Mustanski B, Newcomb ME (2019): Mental health and resilience in transgender individuals: What type of support makes a difference? J Fam Psychol 33:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews GA, Tye KM (2019): Neural mechanisms of social homeostasis. Ann N Y Acad Sci 1457:5–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koolhaas JM, de Boer SF, Buwalda B, Meerlo P (2017): Social stress models in rodents: Towards enhanced validity. Neurobiol Stress 6:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amateau SK, Alt JJ, Stamps CL, McCarthy MM (2004): Brain estradiol content in newborn rats: Sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology 145:2906–2917. [DOI] [PubMed] [Google Scholar]
- 41.London SE (2016): Influences of non-canonical neurosteroid signaling on developing neural circuits. Curr Opin Neurobiol 40:103–110. [DOI] [PubMed] [Google Scholar]
- 42.Kato S, Sato T, Watanabe T, Takemasa S, Masuhiro Y, Ohtake F, Matsumoto T (2005): Function of nuclear sex hormone receptors in gene regulation. Cancer Chemother Pharmacol 56(suppl 1):4–9. [DOI] [PubMed] [Google Scholar]
- 43.Krause WC, Rodriguez R, Gegenhuber B, Matharu N, Rodriguez AN, Padilla-Roger AM, et al. (2021): Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature 599:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gegenhuber B, Wu MV, Bronstein R, Tollkuhn J (2022): Gene regulation by gonadal hormone receptors underlies brain sex differences. Nature 606:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, et al. (2018): Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362:eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfaff DW (1997): Hormones, genes, and behavior. Proc Natl Acad Sci U S A 94:14213–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prossnitz ER, Barton M (2011): The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pohóczky K, Kun J, Szalontai B, Szőke É, Sághy É, Payrits M, et al. (2016): Estrogen-dependent up-regulation of TRPA1 and TRPV1 receptor proteins in the rat endometrium. J Mol Endocrinol 56:135–149. [DOI] [PubMed] [Google Scholar]
- 49.Vail G, Roepke TA (2019): Membrane-initiated estrogen signaling via Gq-coupled GPCR in the central nervous system. Steroids 142:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira RF (2009): Social behavior in context: Hormonal modulation of behavioral plasticity and social competence. Integr Comp Biol 49:423–440. [DOI] [PubMed] [Google Scholar]
- 51.Guthman EM, Falkner AL (2022): Neural mechanisms of persistent aggression. Curr Opin Neurobiol 73:102526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue S, Yang R, Tantry A, Davis CH, Yang T, Knoedler JR, et al. (2019): Periodic remodeling in a neural circuit governs timing of female sexual behavior. Cell 179:1393–1408.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, et al. (2017): Hormonal gain control of a medial preoptic area social reward circuit [published correction appears in Nat Neurosci 2017; 20:1427–1430]. Nat Neurosci 20:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engström O, et al. (1997): Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758. [DOI] [PubMed] [Google Scholar]
- 55.Maggi A (2011): Liganded and unliganded activation of estrogen receptor and hormone replacement therapies. Biochim Biophys Acta 1812:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levine S, Mullins R Jr (1964): Estrogen administered neonatally affects adult sexual behavior in male and female rats. Science 144:185–187. [DOI] [PubMed] [Google Scholar]
- 57.Davis PG, Barfield RJ (1979): Activation of feminine sexual behavior in castrated male rats by intrahypothalamic implants of estradiol benzoate. Neuroendocrinology 28:228–233. [DOI] [PubMed] [Google Scholar]
- 58.Wei YC, Wang SR, Jiao ZL, Zhang W, Lin JK, Li XY, et al. (2018): Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat Commun 9:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, et al. (2018): Functional circuit architecture underlying parental behaviour. Nature 556:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenz KM, McCarthy MM (2015): A starring role for microglia in brain sex differences. Neuroscientist 21:306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gagne C, Piot A, Brake WG (2021): Depression, estrogens, and neuroinflammation: A preclinical review of ketamine treatment for mood disorders in women. Front Psychiatry 12:797577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, et al. (2003): Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A 100:9614–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP (2000): Antiinflammatory effects of estrogen on microglial activation. Endocrinology 141:3646–3656. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Segura LM (2008): Aromatase in the brain: Not just for reproduction anymore. J Neuroendocrinol 20:705–712. [DOI] [PubMed] [Google Scholar]
- 65.Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, et al. (2011): Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A 108:8867–8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krentzel AA, Willett JA, Johnson AG, Meitzen J (2021): Estrogen receptor alpha, G-protein coupled estrogen receptor 1, and aromatase: Developmental, sex, and region-specific differences across the rat caudate–putamen, nucleus accumbens core and shell. J Comp Neurol 529:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, et al. (2003): Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha [published correction appears in Endocrinology 2003; 144:2844]. Endocrinology 144:2055–2067. [DOI] [PubMed] [Google Scholar]
- 68.Williams ES, Manning CE, Eagle AL, Swift-Gallant A, Duque-Wilckens N, Chinnusamy S, et al. (2020): Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol Psychiatry 87:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shors TJ, Chua C, Falduto J (2001): Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci 21:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farrell MR, Gruene TM, Shansky RM (2015): The influence of stress and gonadal hormones on neuronal structure and function. Horm Behav 76:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simerly RB, Chang C, Muramatsu M, Swanson LW (1990): Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol 294:76–95. [DOI] [PubMed] [Google Scholar]
- 72.Young EA, Midgley AR, Carlson NE, Brown MB (2000): Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry 57:1157–1162. [DOI] [PubMed] [Google Scholar]
- 73.McHenry J, Carrier N, Hull E, Kabbaj M (2014): Sex differences in anxiety and depression: Role of testosterone. Front Neuroendocrinol 35:42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seidman SN, Rabkin JG (1998): Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 48:157–161. [DOI] [PubMed] [Google Scholar]
- 75.Pasch L, He SY, Huddleston H, Cedars MI, Beshay A, Zane LT, Shinkai K (2016): Clinician vs self-ratings of hirsutism in patients with polycystic ovarian syndrome: Associations with quality of life and depression. JAMA Dermatol 152:783–788. [DOI] [PubMed] [Google Scholar]
- 76.Morgan J, Scholtz S, Lacey H, Conway G (2008): The prevalence of eating disorders in women with facial hirsutism: An epidemiological cohort study. Int J Eat Disord 41:427–431. [DOI] [PubMed] [Google Scholar]
- 77.Zhou J, Wang X, Feng L, Xiao L, Yang R, Zhu X, et al. (2021): Venlafaxine vs. fluoxetine in postmenopausal women with major depressive disorder: An 8-week, randomized, single-blind, active-controlled study. BMC Psychiatry 21:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu P, He FF, Bai WP, Yu Q, Shi W, Wu YY, et al. (2004): Menopausal depression: Comparison of hormone replacement therapy and hormone replacement therapy plus fluoxetine. Chin Med J (Engl) 117:189–194. [PubMed] [Google Scholar]
- 79.Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, et al. (2003): Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry 160:1522–1524. [DOI] [PubMed] [Google Scholar]
- 80.Thase ME, Entsuah R, Cantillon M, Kornstein SG (2005): Relative antidepressant efficacy of venlafaxine and SSRIs: Sex-age interactions. J Womens Health (Larchmt) 14:609–616. [DOI] [PubMed] [Google Scholar]
- 81.Sherwin BB (1988): Affective changes with estrogen and androgen replacement therapy in surgically menopausal women. J Affect Disord 14:177–187. [DOI] [PubMed] [Google Scholar]
- 82.Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, et al. (2015): Effects of hormone therapy on cognition and mood in recently postmenopausal women: Findings from the randomized, controlled KEEPS–cognitive and affective study. PLoS Med 12:e1001833; discussion e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Czéh B, Fuchs E, Wiborg O, Simon M (2016): Animal models of major depression and their clinical implications. Prog Neuro-psychopharmacol Biol Psychiatry 64:293–310. [DOI] [PubMed] [Google Scholar]
- 84.Lee BH, Kim H, Park SH, Kim YK (2007): Decreased plasma BDNF level in depressive patients. J Affect Disord 101:239–244. [DOI] [PubMed] [Google Scholar]
- 85.Popova NK, Naumenko VS (2019): Neuronal and behavioral plasticity: The role of serotonin and BDNF systems tandem. Expert Opin Ther Targets 23:227–239. [DOI] [PubMed] [Google Scholar]
- 86.Boonyaratanakornkit V, Edwards DP (2007): Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med 25:139–153. [DOI] [PubMed] [Google Scholar]
- 87.Carlini VP, Poretti MB, Rask-Andersen M, Chavan RA, Ponzio MF, Sawant RS, et al. (2012): Differential effects of fluoxetine and venlafaxine on memory recognition: Possible mechanisms of action. Prog Neuropsychopharmacol Biol Psychiatry 38:159–167. [DOI] [PubMed] [Google Scholar]
- 88.Hung YY, Huang YL, Chang C, Kang HY (2019): Deficiency in androgen receptor aggravates the depressive-like behaviors in chronic mild stress model of depression. Cells 8:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shao S, Cui Y, Chen ZB, Zhang B, Huang SM, Liu XW (2020): Androgen deficit changes the response to antidepressant drugs in tail suspension test in mice. Aging Male 23:1259–1265. [DOI] [PubMed] [Google Scholar]
- 90.Cara AL, Henson EL, Beekly BG, Elias CF (2021): Distribution of androgen receptor mRNA in the prepubertal male and female mouse brain. J Neuroendocrinol 33:e13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kato J, Hirata S, Nozawa A, Yamada-Mouri N (1994): Gene expression of progesterone receptor isoforms in the rat brain. Horm Behav 28:454–463. [DOI] [PubMed] [Google Scholar]
- 92.Shughrue PJ, Lane MV, Merchenthaler I (1997): Comparative distribution of estrogen receptor-alpha and - beta mRNA in the rat central nervous system. J Comp Neurol 388:507–525. [DOI] [PubMed] [Google Scholar]
- 93.Tinbergen N (1951): The Study of Instinct. Oxford: Clarendon Press/Oxford University Press. [Google Scholar]
- 94.Roughgarden J (2004): Evolution’s Rainbow: Diversity, Gender, and Sexuality in Nature and People. Berkeley, CA: University of California Press. [Google Scholar]
- 95.Brennan PA, Zufall F (2006): Pheromonal communication in vertebrates. Nature 444:308–315. [DOI] [PubMed] [Google Scholar]
- 96.Egnor SR, Seagraves KM (2016): The contribution of ultrasonic vocalizations to mouse courtship. Curr Opin Neurobiol 38:1–5. [DOI] [PubMed] [Google Scholar]
- 97.Hauser MD, Chomsky N, Fitch WT (2002): The faculty of language: What is it, who has it, and how did it evolve? Science 298:1569–1579. [DOI] [PubMed] [Google Scholar]
- 98.Pereira TD, Tabris N, Matsliah A, Turner DM, Li J, Ravindranath S, et al. (2022): SLEAP: A deep learning system for multi-animal pose tracking [published correction appears in Nat Methods 2022; 19:628]. Nat Methods 19:486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Willmore L, Cameron C, Yang J, Witten I, Falkner A (2022): Behavioral and dopaminergic signatures of resilience. bioRxiv. 10.1101/2022.03.18.484885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sych Y, Chernysheva M, Sumanovski LT, Helmchen F (2019): High-density multi-fiber photometry for studying large-scale brain circuit dynamics. Nat Methods 16:553–560. [DOI] [PubMed] [Google Scholar]
- 101.Siciliano CA, Tye KM (2019): Leveraging calcium imaging to illuminate circuit dysfunction in addiction. Alcohol 74:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, et al. (2020): Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat Methods 17:1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Anders SM, Goldey KL, Kuo PX (2011): The Steroid/Peptide Theory of Social Bonds: Integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology 36:1265–1275. [DOI] [PubMed] [Google Scholar]
- 104.Wingfield JC, Hegner RE, Dufty AM Jr, Ball GF (1990): The “challenge hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846. [Google Scholar]
- 105.Raisanen JC, Chadwick SB, Michalak N, van Anders SM (2018): Average associations between sexual desire, testosterone, and stress in women and men over time. Arch Sex Behav 47:1613–1631. [DOI] [PubMed] [Google Scholar]
- 106.Kelly AM, Gonzalez Abreu JA, Thompson RR (2022): Beyond sex and aggression: Testosterone rapidly matches behavioural responses to social context and tries to predict the future. ProcBiolSci 289:20220453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wirth JH, Williams KD (2009): ‘They don’t like our kind’: Consequences of being ostracized while possessing a group membership. Group Processes Intergroup Relat 12:111–127. [Google Scholar]
- 108.Steffens NK, Haslam SA, Schuh SC, Jetten J, van Dick R (2017): A meta-analytic review of social identification and health in organizational contexts. Pers Soc Psychol Rev 21:303–335. [DOI] [PubMed] [Google Scholar]
- 109.Wu YE, Dang J, Kingsbury L, Zhang M, Sun F, Hu RK, Hong W (2021): Neural control of affiliative touch in prosocial interaction. Nature 599:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Juster RP, Almeida D, Cardoso C, Raymond C, Johnson PJ, Pfaus JG, et al. (2016): Gonads and strife: Sex hormones vary according to sexual orientation for women and stress indices for both sexes. Psychoneuroendocrinology 72:119–130. [DOI] [PubMed] [Google Scholar]
- 111.Hampson E (2018): Estrogens, aging, and working memory. Curr Psychiatry Rep 20:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pompili A, Iorio C, Gasbarri A (2020): Effects of sex steroid hormones on memory. Acta Neurobiol Exp (Wars) 80:117–128. [PubMed] [Google Scholar]
- 113.Frick KM, Kim J (2018): Mechanisms underlying the rapid effects of estradiol and progesterone on hippocampal memory consolidation in female rodents. Horm Behav 104:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamson DK, Roes MM, Galea LAM (2016): Sex hormones and cognition: Neuroendocrine influences on memory and learning. Compr Physiol 13:1295–1337. [DOI] [PubMed] [Google Scholar]
- 115.Galea LAM, Frick KM, Hampson E, Sohrabji F, Choleris E (2017): Why estrogens matter for behavior and brain health. Neurosci Biobehav Rev 76:363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rentz DM, Weiss BK, Jacobs EG, Cherkerzian S, Klibanski A, Remington A, et al. (2017): Sex differences in episodic memory in early midlife: Impact of reproductive aging. Menopause 24:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sundström Poromaa I, Gingnell M (2014): Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci 8:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lacasse JM, Patel S, Bailey A, Peronace V, Brake WG (2022): Progesterone rapidly alters the use of place and response memory during spatial navigation in female rats. Horm Behav 140:105137. [DOI] [PubMed] [Google Scholar]
- 119.Henderson VW (2018): Progesterone and human cognition. Climacteric 21:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barros LA, Tufik S, Andersen ML (2015): The role of progesterone in memory: An overview of three decades. Neurosci Biobehav Rev 49:193–204. [DOI] [PubMed] [Google Scholar]
- 121.Hua JT, Hildreth KL, Pelak VS (2016): Effects of testosterone therapy on cognitive function in aging: A systematic review. Cogn Behav Neurol 29:122–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Flatt JD, Cicero EC, Lambrou NH, Wharton W, Anderson JG, Bouldin ED, et al. (2021): Subjective cognitive decline higher among sexual and gender minorities in the United States, 2015–2018. Alzheimers Dement (N Y) 7:e12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Flatt JD, Johnson JK, Karpiak SE, Seidel L, Larson B, Brennan-Ing M (2018): Correlates of subjective cognitive decline in lesbian, gay, bisexual, and transgender older adults. J Alzheimers Dis 64:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schneider MA, Spritzer PM, Suh JS, Minuzzi L, Frey BN, Schwarz K, et al. (2020): The link between estradiol and neuroplasticity in transgender women after gender-affirming surgery: A bimodal hypothesis. Neuroendocrinology 110:489–500. [DOI] [PubMed] [Google Scholar]
- 125.Kanaya N, Vonderfecht S, Chen S (2013): Androgen (dihydrotestosterone)-mediated regulation of food intake and obesity in female mice. J Steroid Biochem Mol Biol 138:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stincic TL, Rønnekleiv OK, Kelly MJ (2018): Diverse actions of estradiol on anorexigenic and orexigenic hypothalamic arcuate neurons. Horm Behav 104:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. (2011): Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction [published correction appears in Cell Metab 2019; 29:1232]. Cell Metab 14:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martínez de Morentin PB, González-García I, Martins L, Lage R, Fernández-Mallo D, Martínez-Sánchez N, et al. (2014): Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 20:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.González-García I, Contreras C, Estévez-Salguero Á, Ruíz-Pino F, Colsh B, Pensado I, et al. (2018): Estradiol regulates energy balance by ameliorating hypothalamic ceramide-induced ER stress. Cell Rep 25:413–423.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mold F, Forbes A (2013): Patients’ and professionals’ experiences and perspectives of obesity in health-care settings: A synthesis of current research. Health Expect 16:119–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Flores VA, Pal L, Manson JE (2021): Hormone therapy in menopause: Concepts, controversies, and approach to treatment. Endocr Rev 42:720–752. [DOI] [PubMed] [Google Scholar]
- 132.Herber CB, Krause WC, Wang L, Bayrer JR, Li A, Schmitz M, et al. (2019): Estrogen signaling in arcuate Kiss1 neurons suppresses a sex-dependent female circuit promoting dense strong bones. Nat Commun 10:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davidge-Pitts C, Clarke BL (2019): Transgender bone health. Maturitas 127:35–42. [DOI] [PubMed] [Google Scholar]
- 134.Minkler M, Wallerstein N, editors. (2011). Community-Based Participatory Research for Health: From Process to Outcomes. San Francisco, CA: Jossey-Bass. [Google Scholar]
- 135.Murthy VH, Krumholz HM, Gross CP (2004): Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 291:2720–2726. [DOI] [PubMed] [Google Scholar]
- 136.Lett E, Adekunle D, McMurray P, Asabor EN, Irie W, Simon MA, et al. (2022): Health equity tourism: Ravaging the justice landscape. J Med Syst 46:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuper LE, Nussbaum R, Mustanski B (2012): Exploring the diversity of gender and sexual orientation identities in an online sample of transgender individuals. J Sex Res 49:244–254. [DOI] [PubMed] [Google Scholar]
- 138.Rosser BRS, Oakes JM, Bockting WO, Miner M (2007): Capturing the social demographics of hidden sexual minorities: An internet study of the transgender population in the United States. Sex Res Soc Policy 4:50–64. [Google Scholar]
- 139.Richards C, Bouman WP, Seal L, Barker MJ, Nieder TO, T’Sjoen G (2016): Non-binary or genderqueer genders. Int Rev Psychiatry 28:95–102. [DOI] [PubMed] [Google Scholar]
- 140.Serano JM (2010): The case against autogynephilia. Int J Transgend 12:176–187. [Google Scholar]
- 141.Levitt HM, Ippolito MR (2014): Being transgender: The experience of transgender identity development. J Homosex 61:1727–1758. [DOI] [PubMed] [Google Scholar]
- 142.Restar AJ (2020): Methodological critique of Littman’s (2018) parental-respondents accounts of “rapid-onset gender Dysphoria. Arch Sex Behav 49:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cech EA, Waidzunas TJ (2021): Systemic inequalities for LGBTQ professionals in STEM. Sci Adv 7:eabe0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Armada-Moreira A, Cizauskas C, Fleury G, Forslund SK, Guthman EM, Hanafiah A, et al. (2021): STEM Pride: Perspectives from transgender, nonbinary, and genderqueer scientists. Cell 184:3352–3355. [DOI] [PubMed] [Google Scholar]
- 145.Carey B (2007): Criticism of gender theory, and a scientist under siege. New York Times F1:F6. [Google Scholar]
- 146.Puckett JA, Barr SM, Wadsworth LP, Thai J (2018): Considerations for clinical work and research with transgender and gender diverse individuals. Behav Therapist 41:253–262. [Google Scholar]
- 147.Clements-Nolle K, Bachrach A (2008): CBPR with a hidden population: The transgender community health project a decade later. In: Minkler M, Wallerstein N, editors. Community-Based Research for Health: From Process to Outcomes. San Francisco, CA: Jossey-Bass Publishing. [Google Scholar]
- 148.Rhodes SD, Malow RM, Jolly C (2010): Community-based participatory research: A new and not-so-new approach to HIV/AIDS prevention, care, and treatment. AIDS Educ Prev 22:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bauer GR, Hammond R, Travers R, Kaay M, Hohenadel KM, Boyce M (2009): “I don’t think this is theoretical; this is our lives”: How erasure impacts health care for transgender people. J Assoc Nurses AIDS Care 20:348–361. [DOI] [PubMed] [Google Scholar]
- 150.DuBois LZ, Gibb JK, Juster RP, Powers SI (2021): Biocultural approaches to transgender and gender diverse experience and health: Integrating biomarkers and advancing gender/sex research. Am J Hum Biol 33:e23555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.