Abstract
Hydrogen sulphide (H2S) is a gaseous neurotransmitter that can be self‐synthesized by living organisms. With the deepening of research, the pathophysiological mechanisms of endogenous H2S in cancer have been increasingly elucidated: (1) promote angiogenesis, (2) stimulate cell bioenergetics, (3) promote migration and proliferation thereby invasion, (4) inhibit apoptosis and (5) activate abnormal cell cycle. However, the increasing H2S levels via exogenous sources show the opposite trend. This phenomenon can be explained by the bell‐shaped pharmacological model of H2S, that is, the production of endogenous (low concentration) H2S promotes tumour growth while the exogenous (high concentration) H2S inhibits tumour growth. Here, we review the impact of endogenous H2S synthesis and metabolism on tumour progression, summarize the mechanism of action of H2S in tumour growth, and discuss the possibility of H2S as a potential target for tumour treatment.
Like Yin and Yang, hydrogen sulphide plays a dual role in tumours. Increased production at low concentrations (endogenous) promotes tumorigenesis and development, whereas supplementation at high concentrations (exogenous) suppresses tumours.
1. INTRODUCTION
Hydrogen sulphide (H2S) is one of the three known gaseous signalling molecules in biological systems. Together with carbon monoxide (CO) and nitric oxide (NO), it forms a family of endogenous gases. These gases are involved in regulating a variety of physiological and pathological processes 1 , 2 , 3 and show pleiotropy and dose dependence on a variety of diseases, including cancer. 4 , 5 , 6 , 7 At present, some compounds that can inhibit or induce the synthesis of these gases have been tested in preclinical research, including NO‐releasing drugs for cancer prevention and treatment and CO‐releasing drugs for immune inflammation or autoimmune diseases. 8 , 9 , 10 , 11 , 12 , 13
H2S mainly comes from different substrates catalysed by cystathionine (CTH) β‐synthase (CBS), CTH γ‐lyase (CSE), and 3‐mercaptopyruvate sulfurtransferase (3‐MST). 14 , 15 , 16 In cancer cells, H2S shows cytoprotective or cytotoxic effects, depending on the concentration: that is, a low concentration (endogenous) of H2S can induce tumorigenesis, while a high level (exogenous) of H2S can inhibit tumorigenesis. 17 , 18 , 19 This provides two different ideas for the treatment of cancer, namely inhibiting the production of endogenous H2S or adding exogenous H2S. This review summarizes the effect of endogenous H2S on cancer, focuses on the impact of the change of endogenous H2S concentration on cancer cells, and expounds its implication on cancer treatments, hoping to provide insight for follow‐up research and drug development.
2. ENZYMES THAT SYNTHESIZE H2S
2.1. The distribution of CBS and its catalysed reaction
In mammals, CBS is mainly found in the liver, brain, kidney, and pancreas. 20 , 21 In the liver, the content of CBS is most abundant in hepatocytes and least in the hepatic stellate cells (HSCs) and Kupffer cells. 22 , 23 CBS is expressed in all brain regions except the hippocampus, with the highest content present in the cerebellum and cerebral cortex. 24 CBS is also expressed in neural stem cells and regulates their differentiation. 25 In the kidney, CBS is mainly distributed in the glomeruli, the epithelium of the proximal tubules, collecting ducts, and the inter‐lobular arteries of the kidney. 26 , 27 Moreover, CBS is abundantly expressed in acinar cells of the pancreas, and can also be detected in pancreatic islet cells and exocrine cells. 28 , 29 CBS content in other tissues is relatively low. In the digestive system, CBS exists in the gastric mucosa, colonic epithelium, small intestine, jejunum, and ileum. 30 , 31 , 32 , 33 CBS is also significantly expressed in the spleen. 34 CBS has been suggested to play an important role in the female reproductive system since it is well expressed in the ovary and uterus but is relatively low in the prostate and testis. 35 It is also expressed in the prostate epithelium, bladder, and urethra. 36 , 37 , 38 In the heart, CBS is expressed in cardiomyocytes, coronary arteries, and perivascular adipose tissue. 39 , 40 Meanwhile, in the lung, it is expressed in the epithelial cells of the alveoli, bronchiole, and trachea, as well as the endothelial cells (ECs) and smooth muscle cells of the pulmonary artery. 41 , 42 , 43 , 44 In addition, the content of CBS in the thyroid is low and it is significantly increased in thyroid cancer. 45 Likewise, CBS is not contained in breast tissue but is overexpressed in breast cancer (BC). 46
CBS can generate H2S through several condensation reactions including those of two molecules of L‐cysteine into L‐lanthionine, two L‐homocysteine molecules into L‐homolanthionine, and L‐cysteine and L‐homocysteine into L‐cystathionine. 3 , 47 Although a large amount of cystathionine (CTH) can theoretically inhibit or even reverse the overall response of CBS, the level of CTH in most tissues is very low, so it is difficult to achieve this reverse reaction in vivo. 48
2.2. The distribution of CSE and its catalysed reaction
As the main H2S synthase, CSE is mainly expressed in the cardiovascular and respiratory systems, 49 , 50 including in the liver, kidney, pancreas, uterus, and prostate. 50 , 51 , 52 In addition, a small amount of CSE mRNA has also been detected in the brain, but because the inhibitor of CSE could not impede the production of H2S in the brain, it is thought that CSE is not the main H2S producing enzyme in the brain. 24
CSE can decompose cysteine into pyruvate, ammonia, and thiocysteine, and further catalyse thiocysteine to produce H2S. CSE can also use homocysteine as a substrate to generate H2S. CSE deficiency can lead to cystathioniuria and hyperhomocysteinemia. 53
2.3. The distribution of 3‐MST and its catalytic reaction
3‐MST is found in almost all tissues of mammals; however, its expression is tissue‐specific. In the central nervous system, 3‐MST is mainly located in hippocampal vertebral neurons, cerebellar Purkinje cells, and olfactory bulb mitral valve cells. 54 In addition, 3‐MST is also relatively high in the kidney, liver, testis, large intestine, and endocrine organs. 55
3‐MST catalyses the production of H2S and requires the assistance of cysteine aminotransferase (CAT). 56 CAT converts cysteine to 3‐mercaptopyruvate, and 3‐MST transfers sulphur from 3‐mercaptopyruvate to sulphite, sulphur acceptor, or sulphur. However, this method can only generate sulphane sulphur or combined sulphur, but for producing H2S, the action of reducing agents (e.g., thioredoxin, dihydrolipoic acid) or various enzymes in the cell is required. 57 , 58
3. THE TUMOUR‐PROMOTING MECHANISM OF H2S
3.1. H2S promotes angiogenesis
Angiogenesis is a multi‐step process involving ECs that is characterized by endothelial extracellular matrix remodelling, including initiation, migration, catheter formation, and differentiation. 59 When gene mutations accumulate and cause cancer, the solid tumour will form a highly vascularized state. These vessels provide oxygen and nutrition for the development or local spread of the tumour. 60
H2S promotes EC angiogenesis by regulating cyclic nucleotides, kinases, and ion channels. 61 , 62 H2S donors increase the phosphorylation levels of Akt, p38, and ERK1/2, while the pharmacological inhibition of PI3K/Akt and MAPK inhibits the proliferation and migration of EC. H2S also promotes angiogenesis through the KATP channel. In addition, in human EC, the KATP channel plays a role upstream of p38. 63 , 64 , 65 , 66 , 67 The inhibition of endothelial nitric oxide synthase, soluble guanylyl cyclase, or cyclic guanosine monophosphate (cGMP) dependent protein kinase weakens H2S‐stimulated angiogenesis, indicating H2S can interact with multiple molecules of the NO/cGMP pathway to promote angiogenesis. 68 , 69
Vascular endothelial growth factor (VEGF) can promote vascular permeability, extracellular matrix degeneration, vascular EC migration, proliferation, and angioplasty. 70 , 71 Many studies have shown that there is extensive interaction between H2S and VEGF. In particular, the incubation of human EC with VEGF increases the concentration of H2S, and the silencing or pharmacological inhibition of CSE weakens the angiogenesis of VEGF‐stimulated EC. 66 Although the mechanism of this phenomenon has not been clarified, some experiments show that it may be caused by CSE‐mediated Ca2+/calmodulin‐dependent activation. 69 In addition, the inhibition of CSE can markedly block the activation of p38 and ERK1/2 stimulated by VEGF. 66 CBS silencing also reduced the expression of VEGFR2 and neuropilin‐1, thereby reducing the signal intensity of VEGF. The S‐sulfhydration of specificity protein 1 (Sp1) at Cys68 and Cys755 by H2S enhances the stability of Sp1, and subsequently, promotes the transcription of VEGFR2. 72 H2S can also enhance binding of VEGF to VEGFR2 (thereby increasing activity of the latter). 73
3.2. H2S inhibits apoptosis
Apoptosis refers to the autonomous and orderly death of cells controlled by genes to maintain the stability of the internal environment. It involves the activation, expression, and regulation of a series of genes. 74 Evasion of apoptosis is an important mechanism in the development of cancer, allowing cancer cells to survive under physiological stress. 75 H2S has been found to play an anti‐apoptotic effect in the cardiovascular system, ischaemia–reperfusion injury, and various cancers. 76 , 77 , 78 , 79 , 80 One of the potential anti‐apoptotic mechanisms of H2S is its anti‐oxidant effect achieved by scavenging reactive oxygen species (ROS) and reactive nitrogen species (RNS). Although H2S is usually at a low concentration under baseline conditions, its small molecular structure and ability to penetrate freely on the cell membrane make it a more effective antioxidant than glutathione (GSH). However, it is reasonable to believe that H2S‐mediated antioxidant protection is caused by a wide range of intermediate signals it regulates rather than direct ROS/RNS clearance. 81 Another potential mechanism is the activation of anti‐apoptotic pathways via S‐sulfhydrating NF‐κB, Kelch‐like ECH‐associated protein 1, and mitogen‐activated protein kinase kinase 1 (MEK1). 82 , 83 , 84
3.3. H2S boosts cellular bioenergetics
Cellular bioenergetics plays an important role in the occurrence and development of different types of cancer. 85 , 86 Initially, H2S was reported to exhibit cytotoxic effects on mitochondria by inhibiting the cytochrome c oxidase system, but recent studies demonstrate a more complex, concentration‐dependent regulation of mitochondrial and cellular bioenergetics by H2S. In normal intestinal epithelial cells, H2S acts as a substrate for bioenergy production. 87 , 88 Further research shows that in colon and ovarian cancer, H2S can serve both as a regulator and a substrate of bioenergetics. 89 , 90 CBS silencing reduces oxygen consumption and adenosine triphosphate (ATP) production. Silencing of 3‐MST in hepatoma cells also shows similar effects. Likewise, the pharmacological inhibition of CBS and 3‐MST blocks electron transport and mitochondrial energy production in various cancer cells, whereas replenishment of substrates for these enzymes reversed this process. 91 , 92 , 93 , 94 , 95 It is worth adding that, H2S by itself cannot initiate or maintain the mitochondrial electron transport system, but can affect glycolysis‐derived electron donors.
The H2S‐mediated mitochondrial electron transport requires the participation of sulphide quinone oxidoreductase (SQR), 87 , 96 , 97 and the expression of SQR in tumour cells is up‐regulated under hypoxic conditions that may be a potential mechanism for tumour cells to use H2S to generate energy. 98 On the other hand, electrons from SQR can also be transported in reverse when cells are exposed to higher concentrations of H2S. 87 In cancer cells, this mechanism does not aid in electron transport, proton pump, or ATP generation, but instead it stimulates mitochondrial ROS production. 99 In addition, H2S can directly S‐sulfhydrate glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) to enhance its activity in ATP generation. 52
3.4. H2S promotes DNA repair and tumour growth
Recent studies have shown that cell cycle checkpoints, DNA damage and repair, and the expression of proteins involved in maintaining gene stability are regulated by both exogenous and endogenous H2S. 100 , 101 , 102 The effect is suggested to be due to be associated with activities of MEK1 and poly [ADP‐ribose] polymerase 1 (PARP‐1). Specifically, PARP1 can sense DNA single‐strand or double‐strand breaks and initiate DNA damage repair pathways. PARP inhibitors have been developed to block DNA repair in BRCA‐mutated cancers, thereby initiating signalling pathways that trigger apoptosis and ultimately inhibit tumour growth. 103 H2S is abnormally elevated in a variety of cancers, and inhibition of CBS or CSE activity suppresses tumour growth in colon, lung, prostate, and BCs. 89 , 93 , 104 , 105 MEK1 belongs to the classical MAPK kinase pathway, and the activation of MEK1 is closely related to cell proliferation and tumorigenesis. 106 S‐sulfhydration of MEK1 by H2S at Cys341 promotes phosphorylation and nuclear translocation of MEK1, thereby activating PARP‐1‐mediated DNA damage repair, which is most likely a key driver of tumour growth due to CBS or CSE overexpression. 84 In addition, H2S can S‐sulfhydrate Exo/endonuclease G at Cys76 in mitochondria to mediate DNA damage repair in mitochondria. 107
4. ENDOGENOUS H2S, CELL SIGNAL TRANSDUCTION, AND CANCER
4.1. Endogenous H2S and BC
BC is the most common malignancy in women and is divided into different subtypes with widely varying prognoses and treatment modalities. 108 , 109 Endocrine therapy is suitable for hormone receptor (HR)‐positive patients, and targeted therapy is suitable for human epidermal growth factor receptor 2 (HER2)‐positive patients. 110 Triple‐negative breast cancer (TNBC) is a highly aggressive subtype lacking oestrogen receptor, progesterone receptor, and HER2, and it is easy to metastasize to the nervous system and lungs. 111 , 112 , 113
CBS and CSE have been shown to be highly expressed in various BC types and are closely related to their development. In HR+/HER2+ BC cell line MCF7, knockdown of CBS and CSE inhibited cell growth by inhibiting the Akt signalling pathway, while in TNBC cell line MDA‐MB‐231, knockdown of CBS inhibited cell growth by inhibiting signal transducer and activator of transcription 3 (STAT3). 114 NO regulates the growth of various tumours and has a positive feedback loop with H2S. 115 Knockdown of CBS and CSE mitigates the production of NO, while the addition of NO donors attenuates the antitumor effect of CBS and CSE knockdown. 114 CSE overexpression also promotes the metastasis of BC, especially in TNBC. In vitro experiments have shown that CSE promotes the growth, migration, and invasion of BC. Nude mice experiments show that CSE promotes the migration of BC cells to the lungs, which may be related to the elevated expression of matrix metallopeptidase (MMP)‐2 and MMP‐9. In addition, knockdown of CBS and CSE inhibits the PI3K‐Akt (PI3K, Akt, and pAkt), focal adhesion kinase‐paxillin, and Ras‐MAPK (Ras, Raf, ERK1/2, and pERK1/2) pathways, 116 thus confirming the promoting effect of CBS and CSE on BC. CBS is also highly expressed in basal‐like breast cancer (BLBC). Protein‐Cysteine persulfidation by CBS causes an increase in GSH synthesis. Silencing CBS increases the expression of angiogenesis inhibitor SERPINF1 and inhibits the expression of Ki67, CD31, CD34, and hypoxia‐inducible factor (HIF‐1α), and reduces GSH synthesis enhanced oxidative stress, thereby inhibiting tumour cell growth. CSE abolishes the tumour suppressor effect due to CBS knockdown to a certain extent, indicating that simultaneous targeting of CBS and CSE can produce a more obvious inhibitory effect on BLBC. 117 STAT3 is a transcription factor that is highly activated in BC and promotes the growth of cancer cells. 118 STAT3 can directly regulate the expression of CSE. At the same time, STAT3 is also regulated by CSE and is positively correlated with the expression of CSE. Knocking down CSE significantly reduces proliferation and migration activities in BC cells. 105 In addition, CBS and CSE also regulate the immunogenicity of BC cells. After silencing CBS and CSE, the expression of Natural Killer Group 2D ligands (ULBP2 and MICA) increases, which improves the targeting of NK cells to BC cells. At the same time, the expression of co‐stimulatory ligands CD86 and 41BBL on BC cells increased, and these ligands bind to homologous receptors CD28 and 41BB on T cells to activate T cells and enhance their function. Tumour necrosis factor α (TNF‐α) promotes immune cell apoptosis in the tumour microenvironment, and TNF‐α expression is also reduced after CBS and CSE knockdown. 114 In addition, the level of reactive aldehyde (such as 4‐hydroxynonenal and malondialdehyde) adducts in BC cells co‐cultured with macrophages increases after CBS silencing, resulting in cytotoxicity. 119 The recently discovered novel CSE inhibitor I157172 up‐regulates sirtuin 1 (SIRT1) and inhibits the phosphorylation and deacetylation of STAT3, the expression of MMP2/9, p‐Akt, and Bcl‐2, which in turn inhibits the migration and invasion of BC cell line MCF7. 120
In addition, CTH, an intermediate metabolite of the CBS‐catalysed synthesis of H2S, has recently been found to exert anti‐apoptotic effects in cells. 121 Due to the elevated levels of CBS in BC, the production of CTH intensifies, but its downstream metabolite CGL remains insignificantly affected, resulting in the accumulation of CTH in BC cells. Exogenously added CTH attenuates H2O2‐induced and doxorubicin‐induced apoptosis and maintains mitochondrial stability by increasing intra‐cytoplasmic calcium concentration, restoring the number of mitochondrial cristae, and increasing mitochondrial reserve and exerting anti‐apoptotic effects in BC cells 46 (Figure 1).
FIGURE 1.
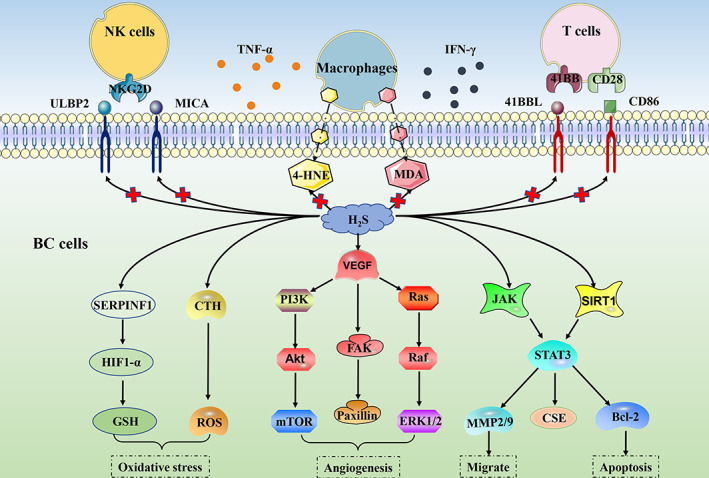
Hydrogen sulphide (H2S) can regulate the occurrence and development of breast cancer cells by regulating oxidative stress, angiogenesis, migration, apoptosis, and immunogenicity. 4‐HNE, 4‐hydroxynonenal; CD80, CD86, 41BBL, T cells co‐stimulate the ligand; CSE, cystathionine γ‐lyase; CTH, cystathionine; FAK, focal adhesion kinase; GSH, glutathione; HIF1‐α, hypoxia‐inducible factor; JAK, Janus kinase; MDA, malondialdehyde; MMP, matrix metallopeptidase; NKG2D, Natural Killer Group 2D; ROS, reactive oxygen species; SERPINF1, angiogenesis inhibitor; SIRT1, sirtuin 1; STAT3, signal transducer and activator of transcription 3; ULBP2 and MICA, NKG2D ligands; VEGF, vascular endothelial growth factor;
4.2. Endogenous H2S and hepatoma
The role of endogenous H2S in hepatoma was shown to be twofold, which appears to be related to the cell type and the reaction mechanism of H2S synthase. In addition, a variety of factors also affect hepatoma by regulating endogenous H2S. CSE is highly expressed in hepatoma cell lines HepG2 and PLC/PRF/5, but low in Hep3B, and its silencing shows an inhibitory effect in HepG2 and PLC/PRF/5, while the effect on Hep3B was not obvious. Subsequent experiments showed that the tumour suppressor effect caused by the knockdown of CSE was achieved by regulating apoptotic proteins (p53, Bax, Bcl‐2, p21, and caspase‐3), key proteins of EGFR and MAPK signalling pathways, and increasing the production of ROS to induce RNA damage. 122 High expression and over‐activation of indoleamine 2,3‐dioxygenase 1 (IDO1) are important reasons for the immune evasion of cancer cells. 123 , 124 In hepatocellular carcinoma (HCC) patients, the expression of IDO1 is negatively correlated with the expression of CSE. The deletion of H2S in CSE−/− mice leads to the increased expression and activity of IDO1. Exogenously added H2S down‐regulates the expression of IDO1 through the NF‐κB and STAT3 pathways and inhibits the activity of IDO1 through the nitric oxide synthase/NO pathway. In addition, exogenously added H2S also inhibited tumour growth in H22 hepatoma mice by inducing effector T cells and suppressing myeloid‐derived suppressor cells. 125 The effect of other factors on cancer may also play a role through the CSE/H2S axis. Irradiation increased the long‐term migration and invasion ability of HepG2 cells. This effect was due to the increased expression of CBS and CSE caused by radiation, and the activation of epithelial–mesenchymal transition (EMT) and P38/MAPK signalling pathways. After knocking down CBS and CSE, the p38/EMT signalling pathway was inhibited, and the effect was more obvious after knocking down CSE. 126 The PI3K/Akt signalling pathway plays a role in promoting invasive phenotype, malignancy, angiogenesis, and so forth in a variety of cancers. 127 , 128 The activated PI3K /Akt pathway promotes the occurrence and development of HCC through Sp1‐mediated regulation of CSE promoter and protein expression, indicating a positive feedback loop between CSE and PI3K/Akt. 129 CBS exhibits different roles in different hepatoma cell lines. In HCC cell lines Hep3B and MHCC97H, CBS inhibits tumour growth by regulating apoptosis‐related proteins (cleaved Caspase‐3 and Bcl‐3) and inhibiting the transcription of paired related homeobox 2 by interleukin‐ 6 (IL‐6), which negatively regulates the expression of STAT3. Regulatory T cells are the main inhibitory component of the immune system and are controlled by forkhead box P3 (FOXP3). In regulatory T cells, the absence of CBS leads to the activation of IL‐6/STAT3 and promotes the expression of FOXP3, which activates regulatory T cells and suppresses T cells to help HCC cells evade immune attack. 130 But in the human hepatoma cell line HepG2, inhibiting CBS results in cancer suppression. The combined application of curcumenol and laminarin inhibited the proliferation and metastasis of HepG2 cells. Subsequent experiments found that this effect was caused by attenuating the expression of CBS as well as STAT3 (pSTAT3), Bcl‐2, MMP2, MMP9, VEGF, and their downstream signalling pathways (ERK1/2, pERK1/2, Akt, pAkt). 131 3‐MST inhibited the Akt/FOXO3a/p27 and cyclin D1/CDK4/Rb/E2F1 signalling pathways by increasing the production of H2S and inhibited the proliferation, migration, and invasion of HCC cell lines HepG2 and MHCC‐LM3 by increasing the cleaved caspase‐3 and PARP levels. 132
Other cells in the liver can also inhibit the occurrence and development of HCC by secreting H2S. HSCs can play a role in suppressing tumours in the development of HCC. Activated HSCs release H2S and H2S increases the pro‐apoptotic factor TNFSF14 through the JNK/JunB signalling pathway 133 (Figure 2).
FIGURE 2.
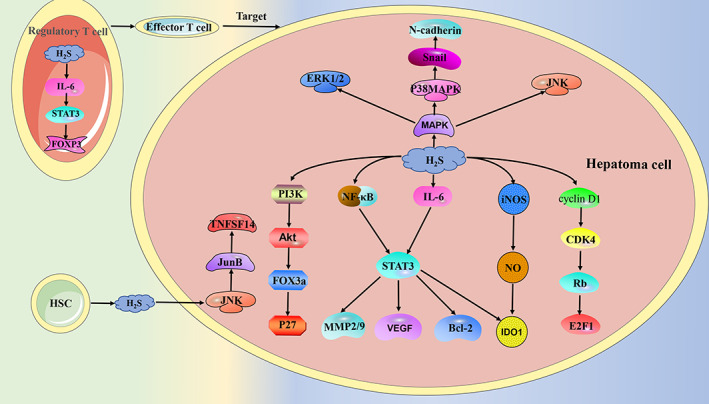
In addition to the hydrogen sulphide (H2S) synthesized by the hepatoma cells themselves, the H2S secreted by the hepatic stellate cells also has an effect on the hepatoma cells. CDK4, recombinant cyclin dependent Kinase 4; E2F1, E2F transcription factor 1; FOX3a, forkhead box O3; FOXP3, forkhead box P3; HSC, hepatic stellate cell; IDO1, indoleamine 2, 3 ‐dioxygenase 1; IL‐6, interleukin‐ 6; iNOS, nitric oxide synthase; JNK, c‐Jun N‐terminal kinase; JunB, JunB Proto‐Oncogene; MMP, matrix metallopeptidase; NF‐κB, nuclear factor kappa‐B; NO, nitric oxide; P27, CDK inhibitor P27; Rb, retinoblastoma; STAT3, signal transducer and activator of transcription 3; TNFSF14, pro‐apoptotic factor; VEGF, vascular endothelial growth factor
4.3. Endogenous H2S and colorectal cancer
CBS shows different effects in different colorectal cancer cell lines. In HT‐29 cells, high expression of CBS inhibits cell proliferation, clone formation, spheroid formation, migration, cell growth, and liver metastasis in vitro and in vivo by inhibiting transcription factor Sp1 and down‐regulating CD44 (a transmembrane glycoprotein and an important biomarker of cancer stem cells) expression. 134 In SW480 and DLD1 cells, there is a positive feedback regulation between CBS and VEGF. Knockdown of CBS diminishes the expression of VEGF by regulating activating protein 1, while bevacizumab (anti‐VEGF monoclonal antibody) reduces the binding of NF‐κB to the CBS promoter and inhibits CBS gene activation. Moreover, CBS knockdown reduces colon cancer migration, invasion, and angiogenesis by inhibiting VEGF. 135 In HCT116 and HT29 cells, aminooxyacetic acid (AOAA) hinders cell survival and intracellular H2S synthesis in a concentration‐dependent manner. Furthermore, the combined application of AOAA and oxaliplatin (OXA)‐enhanced OXA‐induced apoptosis by modulating the level of apoptotic markers (up‐regulate cleaved caspase‐9, cleaved PARP, Bax, and p53 and down‐regulate Bcl‐2, total caspase‐9, total caspase‐3, and total PARP), inducing the production of ROS, and reducing the generation of intracellular GSH in vitro, while in vivo AOAA and OXA decreased the expression of Ki67 and proliferating cell nuclear antigen (PCNA) thus enhancing the chemotherapeutic effect of OXA. 136 AOAA also enhances the sensitivity of colon cancer cells to 5‐Fluorouracil (5‐FU) Combination treatment of AOAA and 5‐FU‐induced apoptosis, cell cycle arrest, disturbance of bioenergetic production, and increased oxidative stress. MiR‐215‐5p is a key tumour suppressor in colon cancer, AOAA and sh‐CBS can both increase the expression of miR‐215‐5p and decrease the expression of epiregulin and thymidylate synthetase, and enhance the sensitivity of acquired 5‐FU‐resistant cell lines to 5‐FU. 137 In HCT116 and NCM356 of colon cancer cells, sh‐CBS and AOAA mitigate basal respiration, ATP synthesis, and glycolysis by inhibiting GAPDH and L‐cysteine. 89 N1, N12‐Diacetylspermine can up‐regulate the expression of CBS and promote the proliferation of colorectal cancer cell lines SW480 and Caco2, while miR‐559 can target and inhibit CBS and inhibit the proliferation of the cells. 138 These opposite results of CBS may be caused by the difference in cell lines, and AOAA, as a pyridoxal phosphate‐dependent enzymes inhibitor, showed an inhibitory effect on both CBS and CSE. 139 , 140 3‐MST is highly expressed in murine colon cancer cell line CT26. HMPSNE (a 3‐MST inhibitor) inhibited the growth, migration, and viability of CT26 cells by inhibiting the cell metabolic capacity and glycolysis parameters but had no obvious effect on cell apoptosis and necrosis. 93 Furthermore, in HCT116 cells, AOAA and HMPSNE induced mesenchymal‐epithelial transition of cells via transcription factor Sp3‐ATP citrate lyase‐Wnt‐β‐Catenin. N‐acetylcysteine (NAC) is a widely used antioxidant that acts as a substrate for 3‐MST with higher efficiency than cysteine, but its role in cancer is bidirectional. 141 , 142 In SW480 cells, exogenous supplementation of NAC increased the expression and activity of 3‐MST and SQR. Since 3‐MST exists as a cancer promoter in colorectal cancer, it seems that NAC can lead to the development of colorectal cancer, but whether it can lead to drug resistance in colorectal cancer cells by regulating H2S and oxidative stress needs further evaluation. 143 When converted to cysteine, xCT (also known as SLC7A11) acts as a precursor for GSH biosynthesis, and increased xCT expression is associated with chemoresistance and nutrient dependence in a variety of cancers. 144 , 145 In human colon cancer cells HCT116 and HT29, xCT is highly expressed, and CES‐derived H2S S‐sulfhydrates OTU domain‐containing ubiquitin aldehyde‐binding protein 1 (OTUB1) at Cys91 to regulate its binding to xCT. Inhibition of CSE attenuates the S‐sulfhydration of OTUB1 and reduces xCT production. In addition, the production of GSH and H2S was reduced after the inhibition of xCT and CSE, which may lead to cell oxidative stress‐induced apoptosis. In vivo experiments also showed that after the knockdown of CSE and xCT, the expression of PCNA was decreased, and the expression of prostaglandin‐endoperoxide synthase was increased, which significantly inhibited tumour growth. 146
Microorganisms in the gut environment can degrade cysteine to generate H2S, resulting in high levels of H2S in the gut environment. 147 There may be some crosstalk between these H2S and intracellular H2S so that the three H2S‐producing enzymes exhibit such a complex and biphasic role in colorectal cancer (Figure 3).
FIGURE 3.
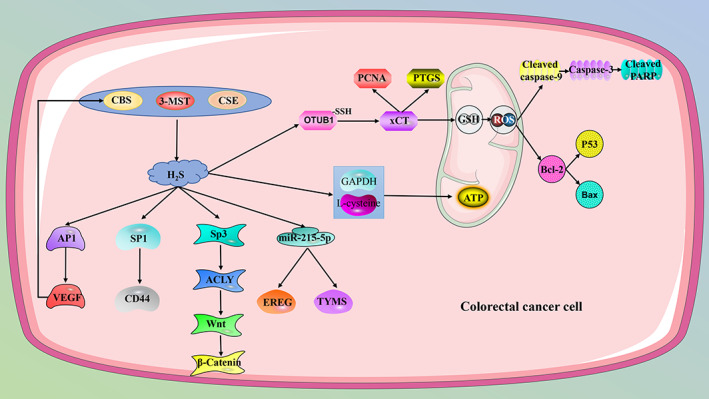
Hydrogen sulphide (H2S) synthase has different functions in colorectal cancer, which may be due to the crosstalk between different molecules and the high concentration of H2S in the intestinal environment. However, in general, inhibition of endogenous H2S can inhibit tumour growth. 3‐MST, 3‐mercaptopyruvate sulfurtransferase; ACLY, ATP citrate lyase; AP1, activating protein 1; ATP, adenosine triphosphate; CBS, cystathionine β‐synthase; CD44, a transmembrane glycoprotein and an important biomarker of cancer stem cells; CSE, cystathionine γ‐lyase; EREG, epiregulin; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; GSH, glutathione; OTUB1, OTU domain‐containing ubiquitin aldehyde‐binding protein 1; PCNA, proliferating cell nuclear antigen; PTGS, prostaglandin‐endoperoxide synthase; ROS, reactive oxygen species; Sp1, specificity protein 1; Sp3, transcription factor Sp3; ‐SSH, S‐sulfhydration; TYMS, thymidylate synthetase; VEGF, vascular endothelial growth factor; xCT, SLC7A11.
4.4. Endogenous H2S and gastric cancer
Gastric cancer (GC) is one of the leading causes of cancer death worldwide, and the CpG Island methylator phenotype (CIMP) is an epigenetic molecular subtype that suppresses the expression of tumour suppressors in a variety of malignancies. 148 Novel associations between CBS epimutations and CIMP subtypes in GC, marked reduction of CBS staining in malignant gastric epithelium, and in vitro models of CBS deficiency can lead to aberrant DNA methylation, down‐regulation of subsets of genes involved in tumour suppressor activity, including annexin A6, VANGL planar cell polarity protein 2, bridging integrator 1, and cAMP responsive element binding protein 3 like 1. Deletion of CBS also leads to regulation of inflammation and H2S production, particularly, through the elevation of TNF‐α and NF‐κB activity, which is accompanied by the reduction of H2S. In addition, epimutation of CBS has also been associated with CIMP in bladder urothelial carcinoma, oesophageal adenocarcinoma, head and neck squamous cell carcinoma, HCC, and uterine corpus endometrial carcinoma. 15 CSE is highly expressed in GC cell line AGS and its inhibition by DL‐propargylglycine (PAG) and β‐cyano‐L‐alanine (BCA) markedly lowers the proliferation ability of AGS and promotes apoptosis. 149 AOAA or PAG also enhances the sensitivity of GC cells to 3,3′‐diindolylmethane (DIM). In BGC‐823 and SGC‐7901 cells, AOAA or PAG was shown to augment the sensitivity of GC cells to DIM by activating the p38‐p53 signalling pathway and regulating downstream signalling molecules. AOAA and PAG combined with DIM suppresses cell proliferation by regulating Cyclin D1, PCNA, and p21, cell migration by impeding VEGF, and facilitates cell apoptosis by regulating cleaved PARP, cleaved caspase‐3, Bax, and Bcl‐2. 150 In addition, fatty‐acid receptor CD36‐dependent lipid metabolism is an important component of metabolic reprogramming in cancer cells. 151 In GC cells, overexpression of CD36 induces lipid metabolism reprogramming and promotes GC metastasis, and endogenous H2S mediates CD36‐induced resistance to antiangiogenic drugs and up‐regulated CD36 expression by inducing nuclear translocation of antioxidant transcriptional factor Nrf2. 152 Helicobacter pylori infection induces peptic ulcer and GC, and H2S production is increased in H. pylori‐infected AGS cells, suggesting that H2S may be involved in H. pylori‐induced gastric mucosal disease 153 (Figure 4).
FIGURE 4.
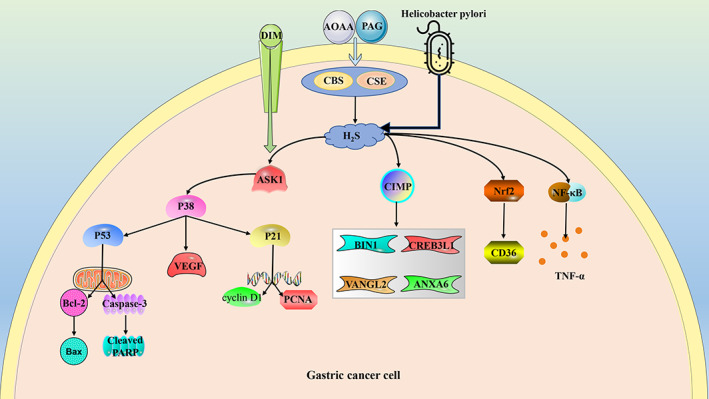
The role of hydrogen sulphide (H2S) in gastric cancer is a multifactorial process. ANXA6, annexin A6; AOAA, aminooxyacetic acid; ASK1, apoptosis signal‐regulating kinase 1; BIN1, bridging integrator 1; CBS, cystathionine β‐synthase; CD36, fatty‐acid receptor CD36; CIMP, CpG island methylator phenotype; CREB3L1, cAMP responsive element binding protein 3 like 1; CSE, cystathionine γ‐lyase; DIM, 3,3′‐Diindolylmethane; NF‐κB, nuclear factor kappa‐B; Nrf2, nuclear factor erythroid 2‐related factor 2; PAG, DL‐Propargylglycine; PCNA, proliferating cell nuclear antigen; TNF‐α, tumour necrosis factor α; VANGL2, VANGL planar cell polarity protein 2; VEGF, vascular endothelial growth factor.
4.5. Endogenous H2S and ovarian cancer
The synthesis of H2S in ovarian cancer is mainly regulated by CBS and CBS is highly expressed in both primary epithelial ovarian cancer and ovarian cancer cell lines, and down‐regulation of CBS in vitro induces oxidative stress to trigger apoptosis cascade by regulating GSH, ROS, p53, and NF‐κB in ovarian cancer cells, and reduces NAD/NADH ratio and ATP production by inhibiting mitochondrial respiration. In vivo CBS silencing inhibits tumour angiogenesis by reducing Ki67 and CD31. Meanwhile, down‐regulation of CBS enhances ovarian cancer sensitivity to cisplatin both in vivo and in vitro. 90 Mitomycin 2 (MFN2) plays an important role in cell proliferation and death. 154 High expression of CBS and MFN2 has a poor prognosis for ovarian cancer. Metabolites GSH and H2S of CBS can increase the expression of MFN2. 155 Also, Nrf2 enhances the expression of CBS through antioxidant response element (ARE) and high expression of CBS mitigates ferroptosis induced by erastin (xCT‐specific inhibitors) in ovarian cancer by regulating S‐adenosyl homocysteine, homocysteine, and CTH. 156 In addition, inhibition of CBS‐induced and CSE‐induced cell death in ES2 cell line. 157 Selenium‐containing chrysin can inhibit cancer by inhibiting CBS 158 (Figure 5).
FIGURE 5.
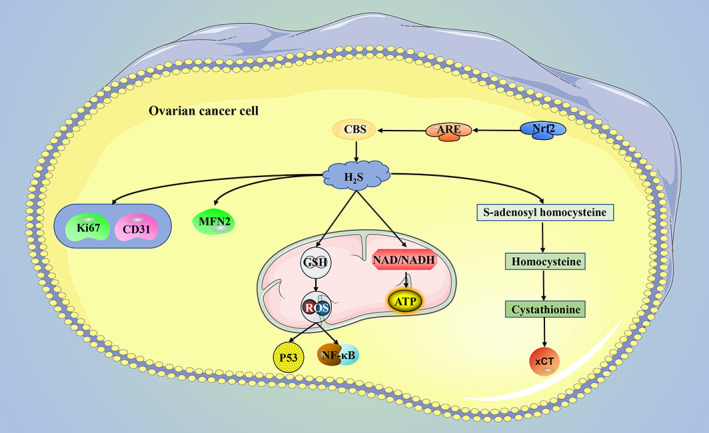
The main hydrogen sulphide (H2S) producing enzyme in ovarian cancer is CBS. In ovarian cancer, inhibition of CBS increases cancer cell apoptosis and ferroptosis and decreases ATP production. ARE, antioxidant response element; ATP, adenosine triphosphate; CBS, cystathionine β‐synthase; CD31, Platelet endothelial cell adhesion molecule‐1; GSH, glutathione; MFN2, mitomycin 2; Nrf2, nuclear factor erythroid 2‐related factor 2; ROS, reactive oxygen species; xCT, SLC7A11.
4.6. Endogenous H2S and prostate cancer
The presence of 3‐MST was not detected in prostate cancer tissues. 159 However, the expression of the other two H2S synthases is also influenced by a variety of factors. Studies have shown that CBS is not detected in benign prostatic epithelium, while low and high levels of CBS are detected in benign hyperplasia prostate cell lines and androgen‐dependent prostate cancer cell lines (LNCaP and DU145), respectively, which seems to indicate that high expression of CBS promotes the progression of prostate cancer, while low expression of CBS is found in bone metastatic cell lines of prostate cancer PC‐3. This may also be due to the fact that the expression of CBS is regulated by androgens, but the results of this regulation are contradictory: in LNCaP cells, dihydrotestosterone up‐regulates the expression of CBS while testosterone down‐regulates the expression of CBS. 36 , 160 CSE is highly expressed in both PC tissues and cells, but its amount varies in different types of PC cells. The expression of CSE in bone metastatic PC‐3 cell lines was higher than that in tumour‐derived PC‐3 cells, which may mean that the high expression of CSE accelerated the metastasis of PC. 104 Experiments show that in the bone metastatic prostate cancer cell line PC‐3, CSE‐derived H2S leads to increased IL‐1β production through S‐sulfhydration of NF‐κB at Cys38S and increases the expression of downstream MMP‐13 and VEGF to increase cell migration and invasion. In the mouse in‐situ transplantation model, CSE knockdown inhibits tumour growth by inhibiting CD31 and vessel endothelial hyaluronan receptor 1 expression and reduces the incidence of para‐aortic lymph nodes and bone metastases. 104 In the androgen‐resistant prostate cancer cell line LNCap, CSE‐derived H2S inhibits the growth of prostate cancer cells by inhibiting the trans‐activation of androgen receptor through S‐sulfhydrate at Cys611/614. 161 However, it has been reported that H2S can increase LNCap mitosis by activating T‐type calcium channels, 162 and dihydrotestosterone can down‐regulate CBS and CSE expression. 36 In general, H2S plays a different role in prostate cancer by regulating different molecules.
4.7. Endogenous H2S and other cancers
Endogenous H2S also has different effects on other types of cancer. In non‐small cell lung cancer (NSCLC) cell lines A549 and 95D, the expressions of CBS and CSE were significantly up‐regulated. AOAA and PAG inhibited the growth of NSCLC by regulating the expressions of cleaved caspase‐3, Bax, and Bcl‐2, and inhibited the growth of NSCLC by regulating the expressions of E‐cadherin, vimentin, MMP2, and MMP9. Silencing of CBS or CSE shows the same tumour‐suppressing effect. HIF‐1α is critical for H2S‐mediated EMT and angiogenesis, and up‐regulation of HIF‐1α resulted in up‐regulation of CBS and CSE expression followed by increased production of VEGF, PI3K, and p‐PI3K, which was reversed by AOAA and PAG. 163 Ribosomal proteins (rp) play a key role in the therapeutic effects of 5‐FU on tumours, and rpL3 is a key sensing molecule for 5‐FU and oxaliplatin‐induced ribosomal stress in colon and lung cancers. In lung cancer tissues, the expression of rpL3 was down‐regulated and the expression of CBS was up‐regulated. In the lung cancer cell line Calu‐6 after 5‐FU treatment, rpL3 decreased its stability at transcriptional and post‐translational levels by targeting CBS to enhance the effect of 5‐FU on cancer cells' lethality. 164 In lung cancer cell lines A549 and H1944, high levels of H2S enhance the activity of mitochondrial DNA repair enzymes and improves ATP production in cancer cells, and AOAA reverses this effect and increases the sensitivity of tumour‐bearing mice to chemotherapeutics. 107 CBS expression is also elevated in chronic myeloid leukaemia, and knockdown of CBS or AOAA treatment promotes apoptosis and triggers S‐phase arrest by regulating cleaved caspase‐9, Bax, cyt C, and NF‐κB. The high expression of CBS boosts the proliferation, migration, and invasion of oesophageal squamous cell carcinoma in vitro and induces angiogenesis and lymphatic metastasis in vivo by up‐regulating VEGF and activating the SIRT1 signalling pathway. These effects could be reversed by the knockdown of CBS. In clear cell renal cell carcinoma, either hydroxylamine (HA; a dual inhibitor of CBS and CSE) or PAG exerts tumour‐suppressive effects both in vivo and in vitro. 165 In addition, in various types of thyroid cancer, the expression of CBS is up‐regulated to varying degrees, and the highly expressed CSE can also activate the hedgehog signalling pathway to promote the occurrence and development of thyroid papillary carcinoma. 45 , 166
In the adoptive cell transfer mouse model, T cells that overexpressed CSE showed better tumour inhibitory effect than normal T cells, due to the fact that in T cells, the overexpression of CSE does not promote its proliferation and change its phenotype, but rather enhances the inhibition of tumour growth by regulating the concentration of serine, proline, and glycine in the metabolic environment. Inhibiting tumour growth by changing its microenvironment represents a novel approach for tumour immunotherapy. 167
5. METHODS FOR INHIBITING ENDOGENOUS H2S
5.1. Pharmacological inhibitor
The commonly used inhibitors of endogenous H2S are usually inhibitors of H2S‐generating enzymes, mainly PAG, BCA, AOAA, HA, and HMPSNE. AOAA was first thought to be a specific inhibitor of CBS. Recent studies have found that AOAA can act as a bidirectional inhibitor of CBS and CSE, possibly due to its inhibitory effect on pyridoxal‐5′‐phosphate, which is a catalytically active cofactor for various enzymes, including CBS and CSE. 168 PAG is an irreversible and specific inhibitor of CSE, but it also inhibits several transamination reactions in muscle and exhibits some nephrotoxicity, and it cannot cross the blood–brain barrier. 168 BCA is a reversible CSE inhibitor with broad bioavailability, but it has inevitable neurotoxicity. 169 HA is a cellular metabolite that can release NO and has antioxidant properties. It can be used as an inhibitor of heme‐containing enzymes including CBS, 170 but it has an inhibitory effect on CSE at low concentrations. 171 I157172 is a novel CSE inhibitor that exerts a tumour suppressor effect in BC. 120 HMPSNE is a newly discovered specific inhibitor of 3‐MST, which acts on the activated cysteine residues in the active site of 3‐MST. 172 In addition, trifluoroalanine can also inhibit CBS and CSE, and aminoethoxyvinylglycine can inhibit CSE, but these two inhibitors have not been used in cancer research. 171 In general, AOAA and HA, which are widely used in basic research, have poor targeting, and the side effects of PGA and BCA are relatively large. However, HMPSNE and I157172 are well targeted and have no related side effects reported, which requires further verification of their biological safety. In addition, compounds with aromatic ring‐carbonyl‐S‐pyrimidone structures may be used as new 3‐MST inhibitors. 172
5.2. Target gene sequence
Existing targeted gene silencing approaches including siRNA, shRNA, and CRISPR/Cas9 effectively inhibit endogenous H2S production, 126 , 135 and all have significant effects. Although 3‐MST‐targeted knockdown has been shown to attenuate cellular biology energetics, 95 this approach has not been directly applied to cancer research. When compared with pharmacological inhibitors, targeted gene sequences have better specificity, but most researchers still prefer pharmacological inhibition due to the need for expertise and related equipment. 171
5.3. MicroRNA and transcription factors
MicroRNA (miR) and transcription factors can also act as direct regulators of H2S synthases. MiR‐24‐3p, miR‐203, and miR‐376a can target CBS to induce apoptosis, and miR‐559 can also target CBS to inhibit cell proliferation. 130 , 138 , 173 , 174 MiR‐30 family can induce oxidative stress by inhibiting CSE, 175 , 176 while miR‐216a also directly targeted CSE to inhibit its expression. 177 And miR‐4137 can inhibit both CBS and CSE in BC to yield a tumour‐suppressing effect. 114
In addition, transcription factor Sp1 can increase the expression of CSE, and Nrf2 can activate the ARE upstream of CBS to up‐regulate its expression. 129 , 156 Moreover, many molecules can regulate the expression of H2S synthases, such as miR‐106a and rpL3, but they cannot directly target these three enzymes and may require other molecules as intermediate bridges to function 164 , 178 (Table 1).
TABLE 1.
Targeted ways to inhibit H2S synthases.
| Type | Appellation | Target(s) | Refs. |
|---|---|---|---|
| Pharmacological inhibitors | PAG | CSE | 168 |
| BCA | CSE | 169 | |
| AOAA | CSE, CBS | 168 | |
| HA | CSE, CBS | 170 | |
| HMPSNE | 3‐MST | 172 | |
| I157172 | CSE | 120 | |
| Trifluoroalanine | CSE, CBS | 171 | |
| Aminoethoxyvinylglycine | CSE | 171 | |
| Target gene sequence | siRNA | CSE, CBS | 126 |
| shRNA | CSE, CBS | 117 , 146 | |
| CRISPR/Cas9 | CSE, CBS | 125 , 135 | |
| MicroRNA | MiR‐24‐3p | CBS | 130 |
| MiR‐203 | CBS | 173 | |
| MiR‐376a | CBS | 174 | |
| MiR‐559 | CBS | 138 | |
| MiR‐30 | CSE | 175 , 176 | |
| MiR‐216a | CSE | 177 | |
| MiR‐4137 | CSE, CBS | 114 | |
| Transcription factor | SP1 | CSE | 129 |
Abbreviations: 3‐MST, 3‐mercaptopyruvate sulfurtransferase; AOAA, aminooxyacetic acid; BCA, β‐cyano‐L‐alanine; CBS, cystathionine β‐synthase; CSE, cystathionine γ‐lyase; H2S, hydrogen sulphide; HA, hydroxylamine; HMPSNE, 2‐[(4‐hydroxy‐6‐methylpyrimidin‐2‐yl)sulfanyl]‐1‐ (naphthalen‐1‐yl)ethan‐1‐one; PAG, DL‐Propargylglycine; SP1, specificity protein 1.
6. DISCUSSION
The role of H2S in cancer has been increasingly elaborated, and the different roles of endogenous and exogenous H2S in cancer provide two approaches for cancer treatment: inhibition of endogenous H2S or supplementation of exogenous H2S. Although some papers have shown that high expression of H2S synthases can promote tumours, this may be due to the high level of H2S in the tumour environment (digestive system). In general, inhibiting the synthesis of endogenous H2S can inhibit the occurrence and development of cancer. The existing methods of inhibiting endogenous H2S include (1), pharmacological inhibitors, (2), siRNA, shRNA, CRISPR/Cas9, (3), miR, and transcription factors. However, pharmacological inhibitors have poor targeting, and their way of action may not only inhibit the production of endogenous H2S, and some inhibitors have relatively large side effects. siRNA, shRNA, and CRISPR/Cas9 cannot be applied to human experiments, only to verify the effect of the H2S synthases through cell or animal experiments. The same problem exists with miRs and transcription factors. Therefore, in addition to elucidating the deep mechanism of endogenous H2S in cancer, the next goal is to develop better‐targeted endogenous H2S inhibitors with fewer side effects or endogenous H2S inhibition methods that can be applied to humans. Furthermore, in addition to the endogenous H2S in tumour cells themselves, H2S in T cells and HSCs, for example, can also exert tumour‐suppressive effects by regulating the microenvironment.
In conclusion, although there are various difficulties and challenges, the inhibition of endogenous H2S production is a potential cancer treatment.
AUTHOR CONTRIBUTIONS
Dong‐Dong Wu, Chang‐Yong Yang, and Xin‐Ying Ji conceived the study and drafted the article. Hao‐Jie Chen, Ke Li, Yang‐Zhe Qin, Jing‐Jing Zhou, Tao Li, and Lei Qian, prepared the figures. All authors read and approved the final article.
FUNDING INFORMATION
This work was supported by grants from the National Natural Science Foundation of China (No. 81802718), the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038), the Natural Science Foundation of Education Department of Henan Province, China (No. 21A310003), and the Foundation of Science & Technology Department of Henan Province, China (Nos. 222102310490, 222102310495).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Chen H‐J, Li K, Qin Y‐Z, et al. Recent advances in the role of endogenous hydrogen sulphide in cancer cells. Cell Prolif. 2023;56(9):e13449. doi: 10.1111/cpr.13449
Contributor Information
Chang‐Yong Yang, Email: ycy0378@163.com.
Xin‐Ying Ji, Email: 10190096@vip.henu.edu.cn.
Dong‐Dong Wu, Email: ddwubiomed2010@163.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Mani S, Untereiner A, Wu L, Wang R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid Redox Signal. 2014;20(5):805‐817. [DOI] [PubMed] [Google Scholar]
- 2. Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6(11):917‐935. [DOI] [PubMed] [Google Scholar]
- 3. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92(2):791‐896. [DOI] [PubMed] [Google Scholar]
- 4. Fagone P, Mazzon E, Bramanti P, Bendtzen K, Nicoletti F. Gasotransmitters and the immune system: mode of action and novel therapeutic targets. Eur J Pharmacol. 2018;834:92‐102. [DOI] [PubMed] [Google Scholar]
- 5. Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9(9):728‐743. [DOI] [PubMed] [Google Scholar]
- 6. Wallace JL. Hydrogen sulfide‐releasing anti‐inflammatory drugs. Trends Pharmacol Sci. 2007;28(10):501‐505. [DOI] [PubMed] [Google Scholar]
- 7. Ianaro A, Cirino G, Wallace JL. Hydrogen sulfide‐releasing anti‐inflammatory drugs for chemoprevention and treatment of cancer. Pharmacol Res. 2016;111:652‐658. [DOI] [PubMed] [Google Scholar]
- 8. Rothweiler F, Michaelis M, Brauer P, et al. Anticancer effects of the nitric oxide‐modified saquinavir derivative saquinavir‐NO against multidrug‐resistant cancer cells. Neoplasia. 2010;12(12):1023‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMurtry V, Saavedra JE, Nieves‐Alicea R, Simeone AM, Keefer LK, Tari AM. JS‐K, a nitric oxide‐releasing prodrug, induces breast cancer cell death while sparing normal mammary epithelial cells. Int J Oncol. 2011;38(4):963‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao L, Williams JL. Nitric oxide‐donating aspirin induces G2/M phase cell cycle arrest in human cancer cells by regulating phase transition proteins. Int J Oncol. 2012;41(1):325‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nikolic I, Saksida T, Mangano K, et al. Pharmacological application of carbon monoxide ameliorates islet‐directed autoimmunity in mice via anti‐inflammatory and anti‐apoptotic effects. Diabetologia. 2014;57(5):980‐990. [DOI] [PubMed] [Google Scholar]
- 12. Fagone P, Mangano K, Quattrocchi C, et al. Prevention of clinical and histological signs of proteolipid protein (PLP)‐induced experimental allergic encephalomyelitis (EAE) in mice by the water‐soluble carbon monoxide‐releasing molecule (CORM)‐A1. Clin Exp Immunol. 2011;163(3):368‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fagone P, Mangano K, Coco M, et al. Therapeutic potential of carbon monoxide in multiple sclerosis. Clin Exp Immunol. 2012;167(2):179‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu J, Shao X, Qin W, et al. Quantitative chemoproteomics reveals O‐GlcNAcylation of cystathionine γ‐lyase (CSE) represses trophoblast syncytialization. Cell Chem Biol. 2021;28(6):788‐801.e5. [DOI] [PubMed] [Google Scholar]
- 15. Padmanabhan N, Kyon HK, Boot A, et al. Highly recurrent CBS epimutations in gastric cancer CpG Island methylator phenotypes and inflammation. Genome Biol. 2021;22(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Augsburger F, Szabo C. Potential role of the 3‐mercaptopyruvate sulfurtransferase (3‐MST)‐hydrogen sulfide (H(2)S) pathway in cancer cells. Pharmacol Res. 2020;154:104083. [DOI] [PubMed] [Google Scholar]
- 17. Wu D, Si W, Wang M, Lv S, Ji A, Li Y. Hydrogen sulfide in cancer: friend or foe? Nitric Oxide. 2015;50:38‐45. [DOI] [PubMed] [Google Scholar]
- 18. Lee ZW, Zhou J, Chen CS, et al. The slow‐releasing hydrogen sulfide donor, GYY4137, exhibits novel anti‐cancer effects in vitro and in vivo. PLoS One. 2011;6(6):e21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi B, Yan Q, Tang J, et al. Hydrogen sulfide‐Activatable second near‐infrared fluorescent nanoassemblies for targeted photothermal cancer therapy. Nano Lett. 2018;18(10):6411‐6416. [DOI] [PubMed] [Google Scholar]
- 20. Bao L, Vlček Č́, Pačes V, Kraus JP. Identification and tissue distribution of human cystathionine beta‐synthase mRNA isoforms. Arch Biochem Biophys. 1998;350(1):95‐103. [DOI] [PubMed] [Google Scholar]
- 21. Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal. 2011;15(2):363‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dicker‐Brown A, Fonseca VA, Fink LM, Kern PA. The effect of glucose and insulin on the activity of methylene tetrahydrofolate reductase and cystathionine‐beta‐synthase: studies in hepatocytes. Atherosclerosis. 2001;158(2):297‐301. [DOI] [PubMed] [Google Scholar]
- 23. Damba T, Zhang M, Buist‐Homan M, van Goor H, Faber KN, Moshage H. Hydrogen sulfide stimulates activation of hepatic stellate cells through increased cellular bio‐energetics. Nitric Oxide. 2019;92:26‐33. [DOI] [PubMed] [Google Scholar]
- 24. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16(3):1066‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Z, Liu DX, Wang FW, et al. L‐cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H₂S pathway. Neuroscience. 2013;237:106‐117. [DOI] [PubMed] [Google Scholar]
- 26. Huang P, Chen S, Wang Y, et al. Down‐regulated CBS/H2S pathway is involved in high‐salt‐induced hypertension in dahl rats. Nitric Oxide. 2015;46:192‐203. [DOI] [PubMed] [Google Scholar]
- 27. Yuan X, Zhang J, Xie F, et al. Loss of the protein cystathionine β‐synthase during kidney injury promotes renal Tubulointerstitial fibrosis. Kidney Blood Press Res. 2017;42(3):428‐443. [DOI] [PubMed] [Google Scholar]
- 28. Kaneko Y, Kimura T, Taniguchi S, et al. Glucose‐induced production of hydrogen sulfide may protect the pancreatic beta‐cells from apoptotic cell death by high glucose. FEBS Lett. 2009;583(2):377‐382. [DOI] [PubMed] [Google Scholar]
- 29. Tamizhselvi R, Moore PK, Bhatia M. Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: in vitro studies using isolated mouse pancreatic acinar cells. J Cell Mol Med. 2007;11(2):315‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magierowski M, Magierowska K, Surmiak M, et al. The effect of hydrogen sulfide‐releasing naproxen (ATB‐346) versus naproxen on formation of stress‐induced gastric lesions, the regulation of systemic inflammation, hypoxia and alterations in gastric microcirculation. J Physiol Pharmacol. 2017;68(5):749‐756. [PubMed] [Google Scholar]
- 31. Tomuschat C, O'Donnell AM, Coyle D, Puri P. Reduction of hydrogen sulfide synthesis enzymes cystathionine‐β‐synthase and cystathionine‐γ‐lyase in the colon of patients with Hirschsprungs disease. J Pediatr Surg. 2018;53(3):525‐530. [DOI] [PubMed] [Google Scholar]
- 32. Wu C, Xu Z, Huang K. Effects of dietary selenium on inflammation and hydrogen sulfide in the gastrointestinal tract in chickens. Biol Trace Elem Res. 2016;174(2):428‐435. [DOI] [PubMed] [Google Scholar]
- 33. Saghazadeh‐Dezfuli M, Fanaei H, Gharib‐Naseri MK, Nasri S, Mard SA. Antidiarrheal effect of sodium hydrosulfide in diabetic rats: In vitro and in vivo studies. Neurogastroenterol Motil. 2018;30(10):e13273. [DOI] [PubMed] [Google Scholar]
- 34. Ahmad A, Gerö D, Olah G, Szabo C. Effect of endotoxemia in mice genetically deficient in cystathionine‐γ‐lyase, cystathionine‐β‐synthase or 3‐mercaptopyruvate sulfurtransferase. Int J Mol Med. 2016;38(6):1683‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue‐based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 36. Guo H, Gai JW, Wang Y, Jin HF, du JB, Jin J. Characterization of hydrogen sulfide and its synthases, cystathionine β‐synthase and cystathionine γ‐lyase, in human prostatic tissue and cells. Urology. 2012;79(2):483.e1‐483.e5. [DOI] [PubMed] [Google Scholar]
- 37. Gai JW, Wahafu W, Guo H, et al. Further evidence of endogenous hydrogen sulphide as a mediator of relaxation in human and rat bladder. Asian J Androl. 2013;15(5):692‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li G, Xie ZZ, Chua JMW, Wong PC, Bian J. Hydrogen sulfide protects testicular germ cells against heat‐induced injury. Nitric Oxide. 2015;46:165‐171. [DOI] [PubMed] [Google Scholar]
- 39. Donovan J, Wong PS, Garle MJ, Alexander SPH, Dunn WR, Ralevic V. Coronary artery hypoxic vasorelaxation is augmented by perivascular adipose tissue through a mechanism involving hydrogen sulphide and cystathionine‐β‐synthase. Acta Physiol (Oxf). 2018;224(4):e13126. [DOI] [PubMed] [Google Scholar]
- 40. Li N, Wang MJ, Jin S, et al. The H2S donor NaHS changes the expression pattern of H2S‐producing enzymes after myocardial infarction. Oxid Med Cell Longev. 2016;2016:6492469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han W, Dong Z, Dimitropoulou C, Su Y. Hydrogen sulfide ameliorates tobacco smoke‐induced oxidative stress and emphysema in mice. Antioxid Redox Signal. 2011;15(8):2121‐2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Talaei F, Bouma HR, Hylkema MN, et al. The role of endogenous H2S formation in reversible remodeling of lung tissue during hibernation in the Syrian hamster. J Exp Biol. 2012;215(Pt 16):2912‐2919. [DOI] [PubMed] [Google Scholar]
- 43. Rashid S, Heer JK, Garle MJ, Alexander SPH, Roberts RE. Hydrogen sulphide‐induced relaxation of porcine peripheral bronchioles. Br J Pharmacol. 2013;168(8):1902‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luo L, Liu D, Tang C, et al. Sulfur dioxide upregulates the inhibited endogenous hydrogen sulfide pathway in rats with pulmonary hypertension induced by high pulmonary blood flow. Biochem Biophys Res Commun. 2013;433(4):519‐525. [DOI] [PubMed] [Google Scholar]
- 45. Turbat‐Herrera EA, Kilpatrick MJ, Chen J, et al. Cystathione β‐synthase is increased in thyroid malignancies. Anticancer Res. 2018;38(11):6085‐6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sen S, Kawahara B, Mahata SK, et al. Cystathionine: a novel oncometabolite in human breast cancer. Arch Biochem Biophys. 2016;604:95‐102. [DOI] [PubMed] [Google Scholar]
- 47. Jhee KH, Kruger WD. The role of cystathionine beta‐synthase in homocysteine metabolism. Antioxid Redox Signal. 2005;7(5–6):813‐822. [DOI] [PubMed] [Google Scholar]
- 48. Jhee KH, Niks D, McPhie P, Dunn MF, Miles EW. The reaction of yeast cystathionine beta‐synthase is rate‐limited by the conversion of aminoacrylate to cystathionine. Biochemistry. 2001;40(36):10873‐10880. [DOI] [PubMed] [Google Scholar]
- 49. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237(3):527‐531. [DOI] [PubMed] [Google Scholar]
- 50. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20(21):6008‐6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma‐lyase. Science. 2008;322(5901):587‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mustafa AK, Gadalla MM, Sen N, et al. H2S signals through protein S‐sulfhydration. Sci Signal. 2009;2(96):ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sen U, Sathnur PB, Kundu S, et al. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am J Physiol Cell Physiol. 2012;303(1):C41‐C51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shibuya N, Tanaka M, Yoshida M, et al. 3‐Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11(4):703‐714. [DOI] [PubMed] [Google Scholar]
- 55. Nagahara N. Multiple role of 3‐mercaptopyruvate sulfurtransferase: antioxidative function, H(2) S and polysulfide production and possible SO(x) production. Br J Pharmacol. 2018;175(4):577‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuo SM, Lea TC, Stipanuk MH. Developmental pattern, tissue distribution, and subcellular distribution of cysteine: alpha‐ketoglutarate aminotransferase and 3‐mercaptopyruvate sulfurtransferase activities in the rat. Biol Neonate. 1983;43(1–2):23‐32. [DOI] [PubMed] [Google Scholar]
- 57. Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, Kimura H. Thioredoxin and dihydrolipoic acid are required for 3‐mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J. 2011;439(3):479‐485. [DOI] [PubMed] [Google Scholar]
- 58. Kimura H. Physiological roles of hydrogen sulfide and polysulfides. Handb Exp Pharmacol. 2015;230:61‐81. [DOI] [PubMed] [Google Scholar]
- 59. Baru O, Nutu A, Braicu C, et al. Angiogenesis in regenerative dentistry: are we far enough for therapy? Int J Mol Sci. 2021;22(2):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jośko J, Gwóźdź B, Jedrzejowska‐Szypułka H, Hendryk S. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000;6(5):1047‐1052. [PubMed] [Google Scholar]
- 61. Katsouda A, Bibli SI, Pyriochou A, Szabo C, Papapetropoulos A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol Res. 2016;113(Pt A):175‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Szabó C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol. 2011;164(3):853‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med. 2013;17(7):879‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76(1):29‐40. [DOI] [PubMed] [Google Scholar]
- 65. Jang H, Oh MY, Kim YJ, et al. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res. 2014;92(11):1520‐1528. [DOI] [PubMed] [Google Scholar]
- 66. Papapetropoulos A, Pyriochou A, Altaany Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106(51):21972‐21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Umaru B, Pyriochou A, Kotsikoris V, Papapetropoulos A, Topouzis S. ATP‐sensitive potassium channel activation induces angiogenesis in vitro and in vivo. J Pharmacol Exp Ther. 2015;354(1):79‐87. [DOI] [PubMed] [Google Scholar]
- 68. Bibli SI, Yang G, Zhou Z, Wang R, Topouzis S, Papapetropoulos A. Role of cGMP in hydrogen sulfide signaling. Nitric Oxide. 2015;46:7‐13. [DOI] [PubMed] [Google Scholar]
- 69. Coletta C, Papapetropoulos A, Erdelyi K, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium‐dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109(23):9161‐9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hoeben A, Landuyt B, Highley MS, Wildiers H, van Oosterom AT, de Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549‐580. [DOI] [PubMed] [Google Scholar]
- 71. Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29(6):789‐791. [DOI] [PubMed] [Google Scholar]
- 72. Saha S, Chakraborty PK, Xiong X, et al. Cystathionine β‐synthase regulates endothelial function via protein S‐sulfhydration. FASEB J. 2016;30(1):441‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tao BB, Liu SY, Zhang CC, et al. VEGFR2 functions as an H2S‐targeting receptor protein kinase with its novel Cys1045‐Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal. 2013;19(5):448‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 76. Kar S, Kambis TN, Mishra PK. Hydrogen sulfide‐mediated regulation of cell death signaling ameliorates adverse cardiac remodeling and diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2019;316(6):H1237‐h1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia‐reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295(2):H801‐H806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rose P, Moore PK, Ming SH, Nam OC, Armstrong JS, Whiteman M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta‐phenylethyl isothiocyanate induced apoptosis. World J Gastroenterol. 2005;11(26):3990‐3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zheng D, Chen Z, Chen J, Zhuang X, Feng J, Li J. Exogenous hydrogen sulfide exerts proliferation, anti‐apoptosis, migration effects and accelerates cell cycle progression in multiple myeloma cells via activating the Akt pathway. Oncol Rep. 2016;36(4):1909‐1916. [DOI] [PubMed] [Google Scholar]
- 80. Zhen Y, Pan W, Hu F, et al. Exogenous hydrogen sulfide exerts proliferation/anti‐apoptosis/angiogenesis/migration effects via amplifying the activation of NF‐κB pathway in PLC/PRF/5 hepatoma cells. Int J Oncol. 2015;46(5):2194‐2204. [DOI] [PubMed] [Google Scholar]
- 81. Murphy B, Bhattacharya R, Mukherjee P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019;33(12):13098‐13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sen N, Paul BD, Gadalla MM, et al. Hydrogen sulfide‐linked sulfhydration of NF‐κB mediates its antiapoptotic actions. Mol Cell. 2012;45(1):13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang G, Zhao K, Ju Y, et al. Hydrogen sulfide protects against cellular senescence via S‐sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal. 2013;18(15):1906‐1919. [DOI] [PubMed] [Google Scholar]
- 84. Zhao K, Ju YJ, Li S, Altaany Z, Wang R, Yang G. S‐sulfhydration of MEK1 leads to PARP‐1 activation and DNA damage repair. EMBO Rep. 2014;15(7):792‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nagano H, Hashimoto N, Nakayama A, et al. p53‐inducible DPYSL4 associates with mitochondrial supercomplexes and regulates energy metabolism in adipocytes and cancer cells. Proc Natl Acad Sci U S A. 2018;115(33):8370‐8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhu J, Thompson CB. Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol. 2019;20(7):436‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797(8):1500‐1511. [DOI] [PubMed] [Google Scholar]
- 88. Mimoun S, Andriamihaja M, Chaumontet C, et al. Detoxification of H(2)S by differentiated colonic epithelial cells: implication of the sulfide oxidizing unit and of the cell respiratory capacity. Antioxid Redox Signal. 2012;17(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 89. Szabo C, Coletta C, Chao C, et al. Tumor‐derived hydrogen sulfide, produced by cystathionine‐β‐synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A. 2013;110(30):12474‐12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bhattacharyya S, Saha S, Giri K, et al. Cystathionine beta‐synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One. 2013;8(11):e79167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Druzhyna N, Szczesny B, Olah G, et al. Screening of a composite library of clinically used drugs and well‐characterized pharmacological compounds for cystathionine β‐synthase inhibition identifies benserazide as a drug potentially suitable for repurposing for the experimental therapy of colon cancer. Pharmacol Res. 2016;113(Pt A):18‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chao C, Zatarain JR, Ding Y, et al. Cystathionine‐beta‐synthase inhibition for colon cancer: enhancement of the efficacy of aminooxyacetic acid via the prodrug approach. Mol Med. 2016;22:361‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Augsburger F, Randi EB, Jendly M, Ascencao K, Dilek N, Szabo C. Role of 3‐mercaptopyruvate sulfurtransferase in the regulation of proliferation, migration, and bioenergetics in murine colon cancer cells. Biomolecules. 2020;10(3):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zuhra K, Panagaki T, Randi EB, et al. Mechanism of cystathionine‐β‐synthase inhibition by disulfiram: The role of bis(N,N‐diethyldithiocarbamate)‐copper(II). Biochem Pharmacol. 2020;182:114267. [DOI] [PubMed] [Google Scholar]
- 95. Módis K, Coletta C, Erdélyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3‐mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27(2):601‐611. [DOI] [PubMed] [Google Scholar]
- 96. Ackermann M, Kubitza M, Maier K, Brawanski A, Hauska G, Piña AL. The vertebrate homolog of sulfide‐quinone reductase is expressed in mitochondria of neuronal tissues. Neuroscience. 2011;199:1‐12. [DOI] [PubMed] [Google Scholar]
- 97. Mishanina TV, Yadav PK, Ballou DP, Banerjee R. Transient kinetic analysis of hydrogen sulfide oxidation catalyzed by human sulfide quinone oxidoreductase. J Biol Chem. 2015;290(41):25072‐25080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Malagrinò F, Zuhra K, Mascolo L, et al. Hydrogen sulfide oxidation: adaptive changes in mitochondria of SW480 colorectal cancer cells upon exposure to hypoxia. Oxid Med Cell Longev. 2019;2019:8102936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jia J, Wang Z, Zhang M, et al. SQR mediates therapeutic effects of H(2)S by targeting mitochondrial electron transport to induce mitochondrial uncoupling. Sci Adv. 2020;6(35):eaaz5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Attene‐Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical‐associated DNA damage. Mol Cancer Res. 2007;5(5):455‐459. [DOI] [PubMed] [Google Scholar]
- 101. Baskar R, Li L, Moore PK. Hydrogen sulfide‐induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J. 2007;21(1):247‐255. [DOI] [PubMed] [Google Scholar]
- 102. Takeuchi H, Setoguchi T, Machigashira M, Kanbara K, Izumi Y. Hydrogen sulfide inhibits cell proliferation and induces cell cycle arrest via an elevated p21 Cip1 level in Ca9‐22 cells. J Periodontal Res. 2008;43(1):90‐95. [DOI] [PubMed] [Google Scholar]
- 103. Brown JS, O'Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang YH, Huang JT, Chen WL, et al. Dysregulation of cystathionine γ‐lyase promotes prostate cancer progression and metastasis. EMBO Rep. 2019;20(10):e45986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. You J, Shi X, Liang H, et al. Cystathionine‐ γ‐lyase promotes process of breast cancer in association with STAT3 signaling pathway. Oncotarget. 2017;8(39):65677‐65686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58(11):621‐631. [DOI] [PubMed] [Google Scholar]
- 107. Szczesny B, Marcatti M, Zatarain JR, et al. Inhibition of hydrogen sulfide biosynthesis sensitizes lung adenocarcinoma to chemotherapeutic drugs by inhibiting mitochondrial DNA repair and suppressing cellular bioenergetics. Sci Rep. 2016;6:36125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288‐300. [DOI] [PubMed] [Google Scholar]
- 109. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 110. Nielsen DL, Kümler I, Palshof JAE, Andersson M. Efficacy of HER2‐targeted therapy in metastatic breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Breast. 2013;22(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 111. Kumar P, Aggarwal R. An overview of triple‐negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247‐269. [DOI] [PubMed] [Google Scholar]
- 112. Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple‐negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638‐2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sihto H, Lundin J, Lundin M, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res. 2011;13(5):R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Youness RA, Gad AZ, Sanber K, et al. Targeting hydrogen sulphide signaling in breast cancer. J Adv Res. 2021;27:177‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Youness RA, Assal RA, Abdel Motaal A, Gad MZ. A novel role of sONE/NOS3/NO signaling cascade in mediating hydrogen sulphide bilateral effects on triple negative breast cancer progression. Nitric Oxide. 2018;80:12‐23. [DOI] [PubMed] [Google Scholar]
- 116. Wang L, Shi H, Liu Y, et al. Cystathionine‐γ‐lyase promotes the metastasis of breast cancer via the VEGF signaling pathway. Int J Oncol. 2019;55(2):473‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Erdélyi K, Ditrói T, Johansson HJ, et al. Reprogrammed transsulfuration promotes basal‐like breast tumor progression via realigning cellular cysteine persulfidation. Proc Natl Acad Sci U S A. 2021;118(45):e2100050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lin L, Hutzen B, Zuo M, et al. Novel STAT3 phosphorylation inhibitors exhibit potent growth‐suppressive activity in pancreatic and breast cancer cells. Cancer Res. 2010;70(6):2445‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sen S, Kawahara B, Gupta D, et al. Role of cystathionine β‐synthase in human breast cancer. Free Radic Biol Med. 2015;86:228‐238. [DOI] [PubMed] [Google Scholar]
- 120. Wang L, Shi H, Zhang X, et al. I157172, a novel inhibitor of cystathionine γ‐lyase, inhibits growth and migration of breast cancer cells via SIRT1‐mediated deacetylation of STAT3. Oncol Rep. 2019;41(1):427‐436. [DOI] [PubMed] [Google Scholar]
- 121. Maclean KN, Greiner LS, Evans JR, et al. Cystathionine protects against endoplasmic reticulum stress‐induced lipid accumulation, tissue injury, and apoptotic cell death. J Biol Chem. 2012;287(38):31994‐32005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pan Y, Ye S, Yuan D, Zhang J, Bai Y, Shao C. Hydrogen sulfide (H2S)/cystathionine γ‐lyase (CSE) pathway contributes to the proliferation of hepatoma cells. Mutat Res. 2014;763–764:10‐18. [DOI] [PubMed] [Google Scholar]
- 123. Prendergast GC, Smith C, Thomas S, et al. Indoleamine 2,3‐dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63(7):721‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, Wolchok JD. Targeting myeloid‐derived suppressor cells with colony stimulating factor‐1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3‐dioxygenase‐expressing tumors. EBioMedicine. 2016;6:50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yang D, Li T, Li Y, et al. H(2)S suppresses indoleamine 2, 3‐dioxygenase 1 and exhibits immunotherapeutic efficacy in murine hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhang H, Song Y, Zhou C, et al. Blocking endogenous H(2)S signaling attenuated radiation‐induced long‐term metastasis of residual HepG2 cells through inhibition of EMT. Radiat Res. 2018;190(4):374‐384. [DOI] [PubMed] [Google Scholar]
- 127. Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal. 2009;21(4):470‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3‐kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yin P, Zhao C, Li Z, et al. Sp1 is involved in regulation of cystathionine γ‐lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell Signal. 2012;24(6):1229‐1240. [DOI] [PubMed] [Google Scholar]
- 130. Zhou YF, Song SS, Tian MX, et al. Cystathionine β‐synthase mediated PRRX2/IL‐6/STAT3 inactivation suppresses Tregs infiltration and induces apoptosis to inhibit HCC carcinogenesis. J Immunother Cancer. 2021;9(8):e003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Han H, Wang L, Liu Y, et al. Combination of curcuma zedoary and kelp inhibits growth and metastasis of liver cancer in vivo and in vitro via reducing endogenous H(2)S levels. Food Funct. 2019;10(1):224‐234. [DOI] [PubMed] [Google Scholar]
- 132. Li M, Song X, Jin Q, et al. 3‐Mercaptopyruvate sulfurtransferase represses tumour progression and predicts prognosis in hepatocellular carcinoma. Liver Int. 2022;42(5):1173‐1184. [DOI] [PubMed] [Google Scholar]
- 133. Ma Y, Wang S, Wu Y, et al. Hepatic stellate cell mediates transcription of TNFSF14 in hepatocellular carcinoma cells via H(2)S/CSE‐JNK/JunB signaling pathway. Cell Death Dis. 2022;13(3):238. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 134. Zhang Y, Chen S, Zhu J, et al. Overexpression of CBS/H(2)S inhibits proliferation and metastasis of colon cancer cells through downregulation of CD44. Cancer Cell Int. 2022;22(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Guo S, Li J, Huang Z, et al. The CBS‐H(2)S axis promotes liver metastasis of colon cancer by upregulating VEGF through AP‐1 activation. Br J Cancer. 2022;126(7):1055‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yue T, Zuo S, Bu D, et al. Aminooxyacetic acid (AOAA) sensitizes colon cancer cells to oxaliplatin via exaggerating apoptosis induced by ROS. J Cancer. 2020;11(7):1828‐1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chen S, Yue T, Huang Z, et al. Inhibition of hydrogen sulfide synthesis reverses acquired resistance to 5‐FU through miR‐215‐5p‐EREG/TYMS axis in colon cancer cells. Cancer Lett. 2019;466:49‐60. [DOI] [PubMed] [Google Scholar]
- 138. Mu T, Chu T, Li W, Dong Q, Liu Y. N1, N12‐Diacetylspermine is elevated in colorectal cancer and promotes proliferation through the miR‐559/CBS Axis in cancer cell lines. J Oncol. 2021;2021:6665704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Du K, Hyun J, Premont RT, et al. Hedgehog‐YAP signaling pathway regulates Glutaminolysis to control activation of hepatic stellate cells. Gastroenterology. 2018;154(5):1465‐1479.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hellmich MR, Coletta C, Chao C, Szabo C. The therapeutic potential of cystathionine β‐synthetase/hydrogen sulfide inhibition in cancer. Antioxid Redox Signal. 2015;22(5):424‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Park EJ, Min KJ, Lee TJ, Yoo YH, Kim YS, Kwon TK. β‐Lapachone induces programmed necrosis through the RIP1‐PARP‐AIF‐dependent pathway in human hepatocellular carcinoma SK‐Hep1 cells. Cell Death Dis. 2014;5(5):e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fu Y, Yang G, Zhu F, et al. Antioxidants decrease the apoptotic effect of 5‐Fu in colon cancer by regulating Src‐dependent caspase‐7 phosphorylation. Cell Death Dis. 2014;5(1):e983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zuhra K, Tomé CS, Masi L, et al. N‐acetylcysteine serves as substrate of 3‐mercaptopyruvate sulfurtransferase and stimulates sulfide metabolism in colon cancer cells. Cells. 2019;8(8):828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Daher B, Parks SK, Durivault J, et al. Genetic ablation of the Cystine transporter xCT in PDAC cells inhibits mTORC1, growth, survival, and tumor formation via nutrient and oxidative stresses. Cancer Res. 2019;79(15):3877‐3890. [DOI] [PubMed] [Google Scholar]
- 145. Shin CS, Mishra P, Watrous JD, et al. The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat Commun. 2017;8:15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chen S, Bu D, Zhu J, et al. Endogenous hydrogen sulfide regulates xCT stability through persulfidation of OTUB1 at cysteine 91 in colon cancer cells. Neoplasia. 2021;23(5):461‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Braccia DJ, Jiang X, Pop M, Hall AB. The capacity to produce hydrogen sulfide (H(2)S) via cysteine degradation is ubiquitous in the human gut microbiome. Front Microbiol. 2021;12:705583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Miller BF, Sánchez‐Vega F, Elnitski L. The emergence of pan‐cancer CIMP and its elusive interpretation. Biomolecules. 2016;6(4):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sekiguchi F, Sekimoto T, Ogura A, Kawabata A. Endogenous hydrogen sulfide enhances cell proliferation of human gastric cancer AGS cells. Biol Pharm Bull. 2016;39(5):887‐890. [DOI] [PubMed] [Google Scholar]
- 150. Ye F, Li X, Sun K, et al. Inhibition of endogenous hydrogen sulfide biosynthesis enhances the anti‐cancer effect of 3,3'‐diindolylmethane in human gastric cancer cells. Life Sci. 2020;261:118348. [DOI] [PubMed] [Google Scholar]
- 151. Pascual G, Avgustinova A, Mejetta S, et al. Targeting metastasis‐initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41‐45. [DOI] [PubMed] [Google Scholar]
- 152. Wang R, Tao B, Fan Q, et al. Fatty‐acid receptor CD36 functions as a hydrogen sulfide‐targeted receptor with its Cys333‐Cys272 disulfide bond serving as a specific molecular switch to accelerate gastric cancer metastasis. EBioMedicine. 2019;45:108‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kawahara Y, Hirashita Y, Tamura C, et al. Helicobacter pylori infection modulates endogenous hydrogen sulfide production in gastric cancer AGS cells. Helicobacter. 2020;25(5):e12732. [DOI] [PubMed] [Google Scholar]
- 154. Xin Y, Li J, Wu W, Liu X. Mitofusin‐2: a new mediator of pathological cell proliferation. Front Cell Dev Biol. 2021;9:647631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chakraborty PK, Murphy B, Mustafi SB, et al. Cystathionine β‐synthase regulates mitochondrial morphogenesis in ovarian cancer. FASEB J. 2018;32(8):4145‐4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Liu N, Lin X, Huang C. Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin‐induced ferroptosis resistance. Br J Cancer. 2020;122(2):279‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nunes SC, Ramos C, Santos I, et al. Cysteine boosts fitness under hypoxia‐mimicked conditions in ovarian cancer by metabolic reprogramming. Front Cell Dev Biol. 2021;9:722412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Santos I, Ramos C, Mendes C, et al. Targeting glutathione and cystathionine β‐synthase in ovarian cancer treatment by selenium‐Chrysin Polyurea dendrimer Nanoformulation. Nutrients. 2019;11(10):2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Khattak S, Rauf MA, Khan NH, et al. Hydrogen sulfide biology and its role in cancer. Molecules. 2022;27(11):3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Prudova A, Albin M, Bauman Z, Lin A, Vitvitsky V, Banerjee R. Testosterone regulation of homocysteine metabolism modulates redox status in human prostate cancer cells. Antioxid Redox Signal. 2007;9(11):1875‐1881. [DOI] [PubMed] [Google Scholar]
- 161. Zhao K, Li S, Wu L, Lai C, Yang G. Hydrogen sulfide represses androgen receptor transactivation by targeting at the second zinc finger module. J Biol Chem. 2014;289(30):20824‐20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Fukami K, Sekiguchi F, Yasukawa M, et al. Functional upregulation of the H2S/Cav3.2 channel pathway accelerates secretory function in neuroendocrine‐differentiated human prostate cancer cells. Biochem Pharmacol. 2015;97(3):300‐309. [DOI] [PubMed] [Google Scholar]
- 163. Wang M, Yan J, Cao X, Hua P, Li Z. Hydrogen sulfide modulates epithelial‐mesenchymal transition and angiogenesis in non‐small cell lung cancer via HIF‐1α activation. Biochem Pharmacol. 2020;172:113775. [DOI] [PubMed] [Google Scholar]
- 164. Russo A, Saide A, Cagliani R, Cantile M, Botti G, Russo G. rpL3 promotes the apoptosis of p53 mutated lung cancer cells by down‐regulating CBS and NFκB upon 5‐FU treatment. Sci Rep. 2016;6:38369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sonke E, Verrydt M, Postenka CO, et al. Inhibition of endogenous hydrogen sulfide production in clear‐cell renal cell carcinoma cell lines and xenografts restricts their growth, survival and angiogenic potential. Nitric Oxide. 2015;49:26‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Xu Y, Ma N, Wei P, Zeng Z, Meng J. Expression of hydrogen sulfide synthases and Hh signaling pathway components correlate with the clinicopathological characteristics of papillary thyroid cancer patients. Int J Clin Exp Pathol. 2018;11(3):1818‐1824. [PMC free article] [PubMed] [Google Scholar]
- 167. Lancien M, Gueno L, Salle S, et al. Cystathionine‐gamma‐lyase overexpression in T cells enhances antitumor effect independently of cysteine autonomy. Cancer Sci. 2021;112(5):1723‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Whiteman M, le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond). 2011;121(11):459‐488. [DOI] [PubMed] [Google Scholar]
- 169. Farran MT, Darwish AH, Uwayjan MG, Sleiman FT, Ashkarian VM. Vicine and convicine in common vetch (Vicia sativa) seeds enhance beta‐cyanoalanine toxicity in male broiler chicks. Int J Toxicol. 2002;21(3):201‐209. [DOI] [PubMed] [Google Scholar]
- 170. Spooren AA, Evelo CT. Hydroxylamine treatment increases glutathione‐protein and protein‐protein binding in human erythrocytes. Blood Cells Mol Dis. 1997;23(3):323‐336. [DOI] [PubMed] [Google Scholar]
- 171. Asimakopoulou A, Panopoulos P, Chasapis CT, et al. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol. 2013;169(4):922‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hanaoka K, Sasakura K, Suwanai Y, et al. Discovery and mechanistic characterization of selective inhibitors of H(2)S‐producing enzyme: 3‐mercaptopyruvate sulfurtransferase (3MST) targeting active‐site cysteine Persulfide. Sci Rep. 2017;7:40227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zhang Q, Shen Z, Shen Y, et al. The regulatory role of MiR‐203 in oxidative stress induced cell injury through the CBS/H(2)S pathway. Nitric Oxide. 2022;118:31‐38. [DOI] [PubMed] [Google Scholar]
- 174. Lv L, Xi HP, Huang JC, Zhou XY. LncRNA SNHG1 alleviated apoptosis and inflammation during ischemic stroke by targeting miR‐376a and modulating CBS/H(2)S pathway. Int J Neurosci. 2021;131(12):1162‐1172. [DOI] [PubMed] [Google Scholar]
- 175. Shen Y, Shen Z, Miao L, et al. miRNA‐30 family inhibition protects against cardiac ischemic injury by regulating cystathionine‐γ‐lyase expression. Antioxid Redox Signal. 2015;22(3):224‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hu X, Liu B, Wu P, Lang Y, Li T. LncRNA Oprm1 overexpression attenuates myocardial ischemia/reperfusion injury by increasing endogenous hydrogen sulfide via Oprm1/miR‐30b‐5p/CSE axis. Life Sci. 2020;254:117699. [DOI] [PubMed] [Google Scholar]
- 177. Gong D, Cheng HP, Xie W, et al. Cystathionine γ‐lyase(CSE)/hydrogen sulfide system is regulated by miR‐216a and influences cholesterol efflux in macrophages via the PI3K/AKT/ABCA1 pathway. Biochem Biophys Res Commun. 2016;470(1):107‐116. [DOI] [PubMed] [Google Scholar]
- 178. Behera J, Kumar A, voor MJ, Tyagi N. Exosomal lncRNA‐H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2‐NO signaling in CBS‐heterozygous mice. Theranostics. 2021;11(16):7715‐7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


