Abstract
Background
Intestinal health plays a pivotal role in broiler chicken growth. Oregano aqueous extract (OAE) effectively exerts anti-inflammatory and antibacterial effects. However, the protective effects of OAE on intestinal health in broilers and the underlying mechanism remain unclear. This study aimed to investigate the potential effects of OAE on growth performance, the gut microbiota and intestinal health. A total of 840 1-d-old male and female broilers (Arbor Acres) were randomly allocated into 6 groups as follows: basal diet (Con), Con + antibiotics (Anti, colistin sulfate 7 g/kg, roxarsone 35 g/kg), Con + 400, 500, 600 and 700 mg/kg OAE (OAE400, OAE500, OAE600 and OAE700). Subsequently, fermentation in vitro together with oral administration trials were carried out to further assess the function of OAE on intestinal health of broilers.
Results
Dietary 700 mg/kg OAE supplementation resulted in an increase (P < 0.05) in body weight and a decrease (P < 0.05) in feed conversion ratio when compared with the control during d 22 to 42 of the trial. OAE addition resulted in lower (P < 0.05) jejunal crypt depth and mRNA expression of IL-4 and IL-10 at d 42. In addition, dietary OAE addition increased the abundance of Firmicutes (P = 0.087) and Lactobacillus (P < 0.05) in the cecum, and increased (P < 0.05) the content of acetic acid and butyric acid. In the in vitro fermentation test, OAE significantly increased (P < 0.05) the abundance of Lactobacillus, decreased (P < 0.05) the abundance of unspecified_Enterobacteriaceae, and increased the content of acetic acid (P < 0.05). In the oral administration trial, higher (P < 0.05) IL-4 expression was found in broilers when oral inoculation with oregano fermentation microorganisms at d 42. And SIgA content in the ileum was significantly increased (P = 0.073) when giving OAE fermentation supernatant.
Conclusions
Dietary OAE addition could maintain intestinal health and improve growth performance through enhancing intestinal mucosal immunity and barrier function mediated by gut microbiota changes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-023-00857-w.
Keywords: Broilers, Gut microbiota, Intestinal health, Oregano aqueous extract
Introduction
Intestinal health is closely related to growth performance of animals which depends on light and strong digestion and absorption, complete physical barrier, specific chemical barrier, moderate mucosal immunity and stable microorganism [1]. The gut microbiota also develops most functions including boosting the immune system, improving digestion, and affecting the nervous system [2–4]. Hence, gut microbiota plays a pivotal role in maintaining normal intestinal physiology and health. Necrotizing enteritis caused by excessive proliferation of Clostridium perfringens is common in the poultry industry, which leads to huge economic losses. Dietary additives supplementation could strengthen the gut microbiota, intestinal barrier and colonization resistance to pathogens for improving intestinal health.
Natural plants could produce active secondary metabolites and have emerged as safe, easily accessible, and inexpensive sources of feed additives. Oregano has anti-inflammatory, antibacterial and antioxidant effects because it contains terpenoids, phenols and other active substances. A large number of studies have shown that the phenolic hydroxyl in phenolic compounds, as a cationic trans-membrane carrier, caused proton influx and potassium ion outflow, which leads to the loss of proton kinetic force and the obstruction of ATP synthesis, and ultimately damaged bacterial cells [5, 6]. Oregano’s main components is carvacrol and thymol. The phenolic hydroxyl groups contained in carvacrol and thymol can act as hydrogen donors to bind to peroxyradicals in the first step of the oxidation reaction, thus preventing and delaying lipid oxidation [7]. Carvacrol and thymol can enhance the cellular and humoral immunity of broilers [8]. In addition, oregano could greatly improve production performance [9], gut microbiota and microbiota-driven SCFAs [10], and could activate immune responses [11]. However, the underlying mechanism by which oregano improves performance still remain unclear, and the effects of OAE on the intestinal health of broilers await further studies.
Therefore, based on the protective effects of oregano, the present study was performed to evaluate the modulation effect of OAE on growth performance and intestinal health, and reveal the regulation mechanism via fermentation in vitro together with oral administration trials in vivo.
Materials and methods
Experimental design, animals, and diets
All animal protocols for this study were approved by the Institutional Animal Care and Use Committee of Northwest A&F University. All broilers used in the study were obtained from the Xianyang Dacheng Poultry Industry Co., Ltd. (Xianyang, China), and housed in three-tier battery cages (cage size: 45 cm × 45 cm × 45 cm). The broiler house was initially set at 35 ℃, but the temperature was gradually decreased to 27 ℃ at week 3 and maintained thereafter. The light cycle was one-hour of darkness per day.
Exp. 1
A total of 840 1-d-old male and female broilers (Arbor Acres) were individually weighed and randomly divided into 6 groups with 7 replicates of 20 birds each. Detailed groups are as follows: basal diet without (Con) or with antibiotics (Anti, colistin sulfate 7 g/kg, roxarsone 35 g/kg) or 400, 500, 600 and 700 mg/kg OAE (OAE400, OAE500, OAE600 and OAE700). The study lasted for 42 d. The addition range of OAE was determined as 400–700 mg/kg in our pre-experiment. OAE (carvacrol > 100 g/kg, powder form) was provided by Baoding Jizhong Pharmaceutical Co., Ltd. The powder ingredients were first added to the premixed feed using a step-by-step dilution method, and then mixed with other feed ingredients. The basal diets did not include antibiotics or anticoccidials but included nonstarch polysaccharide degrading enzyme and phytase enzyme. All treatment diets were pelleted after mixing with a conditioning temperature range from 78 to 80 ℃. Each fed a starter diet from 1 to 21 d and a finisher diet from 22 to 42 d (Table1). At d 21 and 42, samples were collected after slaughter.
Table 1.
Ingredients and nutrients composition of Exp. 1 diets
| Ingredients, % | Starter | Finisher |
| Corn | 54.99 | 67.64 |
| Soybean meal | 28.16 | 17.40 |
| Corn DDGS | 8.00 | - |
| Cottonseed meal | 4.00 | 5.00 |
| Corn gluten meal | - | 5.00 |
| Soybean oil | 0.50 | 1.17 |
| Mountain flour | 1.57 | 1.24 |
| Calcium hydrophosphate | 1.27 | 1.26 |
| L-lysine hydrochloride | 0.36 | 0.45 |
| DL-methionine | 0.27 | 0.16 |
| NaCl | 0.54 | 0.26 |
| Mineral premixa | 0.15 | 0.15 |
| L-threonine | 0.09 | 0.07 |
| Choline chloride | 0.08 | 0.08 |
| Vitamin premixb | 0.02 | 0.02 |
| L-tryptophan | - | 0.11 |
| Phytase | 0.02 | 0.02 |
| Total | 100.00 | 100.00 |
| Nutritional levelc, % | ||
| Apparent metabolizable energy, kcal/kg | 2,673.00 | 2,922.00 |
| Dry matter | 86.41 | 86.27 |
| Crude protein | 21.00 | 18.50 |
| Crude fat | 3.24 | 3.84 |
| Crude ash | 6.26 | 5.10 |
| Calcium | 1.00 | 0.85 |
| Total phosphorus | 0.65 | 0.58 |
| Available phosphorus | 0.34 | 0.29 |
| Lysine | 1.20 | 1.04 |
aMineral premix provided the following per kg of the diet: Mn, 95.4 mg; I, 0.38 mg; Fe, 66 mg; Cu, 15 mg; Zn, 96.6 mg; Se, 0.41 mg
bVitamin premix provided the following per kg of the diet: vitamin A, 9,200 IU; vitamin D, 3,000 IU; vitamin E, 38 mg; vitamin K3, 3 mg; vitamin B1, 3 mg; vitamin B2, 10 mg; vitamin B6, 5 mg; vitamin B12, 0.04 mg; niacin, 40 mg; D-calcium pantothenate, 16 mg; folic acid, 2 mg; biotin, 0.3 mg
cThe nutrient levels were calculated values
Exp. 2
Fresh cecal contents were obtained from 42-d-old Arbor Acres broilers fed a basal diet and immediately transferred into an anaerobic chamber. Three volumes of basic culture medium were added to the samples and vortexed until dispersed, then the supernatant was collected. The basic culture medium was prepared according to the method of Chen et al. [12]. Fecal inocula (1%) and without (C group) or with (T group) 1% (w/v) of OAE were mixed with the culture medium, the mixtures were incubated at 37 °C, and culture samples were collected and centrifuged at 12, 24, and 48 h. The microorganism (sediment) of T48 samples (fermented for 48 h in T group) was stored in glycerol, and the supernatant was stored at −80 ℃ for subsequent oral administration trials.
In the oral administration trial, a total of 90 1-d-old male and female broilers (Arbor Acres) were randomized to three groups (six replicates with 5 birds per replicate) as follows: C group (water), S group (supernatant) and M group (microorganism). During d 17–20, each chicken in S and M groups was given orally 1 mL of supernatant, and microorganism (sediment) obtained from the fermentation experiment mentioned, and each chicken in C group was given orally 1 mL of drinking water. The basal diets consisted of monensin, mannanase and phytase enzymes. All birds were fed with the same corn-soybean meal basal diet (crumble form), each fed a starter diet from 1 to 21 d and a finisher diet from 22 to 42 d (Table 2). At d 21 and 42, samples were collected after slaughter.
Table 2.
Ingredients and nutrients composition of Exp. 2 diets
| Ingredients, % | Starter | Finisher |
| Corn | 57.27 | 60.99 |
| Soybean meal | 35.20 | 30.00 |
| Cottonseed meal | 2.00 | 4.00 |
| Soybean oil | 2.00 | 2.00 |
| NaCl | 0.36 | 0.35 |
| Limestone | 2.00 | 1.70 |
| Calcium hydrogen phosphate | 0.30 | 0.30 |
| Choline chloride | 0.05 | 0.05 |
| L-lysine hydrochloride | 0.14 | 0.06 |
| Mineral premixa | 0.30 | 0.30 |
| Phytase | 0.10 | 0.10 |
| Vitamin premixb | 0.03 | 0.03 |
| DL-methionine | 0.25 | 0.12 |
| Total | 100.00 | 100.00 |
| Nutritional levelc, % | ||
| Apparent metabolizable energy, kcal/kg | 2,949.26 | 3,044.86 |
| Crude protein | 21.91 | 19.89 |
| Calcium | 0.96 | 0.91 |
| Total phosphorus | 0.61 | 0.60 |
| Available phosphorus | 0.40 | 0.40 |
| Methionine | 0.57 | 0.45 |
| Methionine + Cysteine | 0.90 | 0.75 |
| Lysine | 1.21 | 1.05 |
aMineral premix provided the following per kg of the diet: Mn, 80 mg; I, 0.40 mg; Fe, 80 mg; Cu, 10 mg; Zn, 70 mg; Se, 0.30 mg
bVitamin premix provided the following per kg of the diet: vitamin A, 250,000 IU; vitamin D, 50,000 IU; vitamin K3, 53 mg; vitamin B1, 40 mg; vitamin B2, 120 mg; vitamin B12, 0.50 mg; vitamin E, 600 IU; biotin, 0.65 mg; folic acid, 25 mg; pantothenic acid, 240 mg; niacin, 1,000 mg
cThe nutrient levels were calculated values
Growth performance
Feed intake and body weight (BW) per pen were measured at d 21 and 42 and used to calculate the average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR, FCR = ADFI/ADG).
Sample collection
Birds were randomly selected from each replicate and slaughtered, then the middle portion of jejunum (defined as the section between duodenum and ileum) and ileum (defined as the section between Meckel’s diverticulum and ileocecal junction) were isolated and approximately 1 cm segments of the midpoints of jejunum and ileum were fixed in 10% neutral-buffered formalin for histological analysis. The jejunum and ileum mucosa were stored at −80 ℃ for mRNA analysis. Cecum digesta of d 42 were stored at −80 ℃ for analysis of microbial composition.
UPLC-Q/TOF–MS analysis
Preparation of test solution: 20 mg of oregano aqueous extract was accurately weighed and dissolved in 1 mL 60% methanol solution. The mixture was centrifuged at 10,000 r/min for 20 min, and the supernatant was collected.
The samples were separated at 50 ℃ on a Waters ACQUITYTM UPLC system (Waters Corporation, Milford, MA, USA) equipped with an ACQUITY UPLC HSS T3 column (150 mm × 2.1 mm, 1.8 µm). The mobile phase consisted of solvent A (H2O containing 0.1% formic acid, v/v) and solvent B (acetonitrile containing 0.1% formic acid, v/v). The gradient program for biosamples included three segments: 5%–40% B from 0 to 32 min, followed by 40%–95% B from 32 to 37 min. The flow rate was 0.3 mL/min, and the temperature was at 50 ℃ throughout the analysis.
Electrospray ion source (ESI), detected in positive and negative ion modes, scanned in primary and multistage modes, with a scanning range of m/z 50 to 1000 and a resolution of 30,000. Ion source voltage is 3.5 kV; The capillary heating temperature is 350 ℃. The flow rate of sheath gas is 40 arb. The flow rate of auxiliary gas is 10 arb. The voltage of tube lens is 120 V; the collision energy of collision-induced dissociation is adjusted to 35% of the maximum value.
The test conditions for UPLC-Q/TOF–MS were set according to the procedure described by Zhou et al. [13]. The corresponding compounds were identified according to the ion mass charge ratio of primary and secondary fragments, the cracking laws of these compounds reported in the literature, and the search and screening of the UNIFI online software.
Intestinal morphological analysis (Exp. 1)
Jejunal and ileal tissues fixed in formalin were embedded in paraffin, and paraffin sections were sliced using a microtome (Leica Microsystems K. K., Tokyo, Japan) and mounted on glass slides. The sections were dewaxed with xylene, hydrated, and then stained with hematoxylin and eosin (H and E). For each sample, five intact villi-crypt units were selected for morphology observation using a light microscope (Olympus Corporation, Tokyo, Japan) coupled with image processing software (Image J 1.53). Villus height (VH, the height from the tip of the villus to the villus-crypt junction) and crypt depth (CD, the depth of invagination between adjacent villi) were measured. VH to CD ratio (VH/CD) was calculated.
The concentration of SIgA (Exp. 1 and 2)
The secreted immunoglobulin A (SIgA) content in jejunum and ileum was measured by immunohistochemical staining as described by Wang et al. [14]. The ileum tissue was dewaxed, rehydrated, microwave irradiated, and treated with 3% H2O2 at room temperature for 25 min, blocked with normal rabbit serum, incubated with the primary antibody overnight at 4℃ (dilution ratio 1:200), incubated with the secondary antibody at room temperature for 50 min (dilution ratio 1:200), and stained by 3,3-diaminoben-zidine (DAB). Finally, the slides were observed with a light microscope (Leica Microsystems K. K., Tokyo, Japan) and Quantitative analysis with Image-Pro Plus software, Version 6.0.
RNA isolation and real-time quantitative PCR (Exp. 1 and 2)
Total RNA was extracted from the jejunum and ileum mucosa following Trizol Reagent protocol (AG21102, Accurate Biotechnology (Hunan) Co., Ltd., Changsha, China). The purity and concentration of the total RNA were measured and cDNA was synthesized with an Evo M-MLV RT Kit for qPCR (AG11707, Accurate Biotechnology (Hunan) Co., Ltd.). The mRNA expression was analyzed with a SYBR® Green Premix Pro Taq HS qPCR Kit (AG11701, Accurate Biotechnology (Hunan) Co., Ltd.) on the iCycler IQ5 (Bio-Rad, Hercules, CA, USA). Primer sequences used in this study are shown in Table 3. The reaction conditions were as follows: 95 ℃ for 30 s; 40 cycles of 95 ℃ for 5 s, 60 ℃ for 30 s. Each sample was measured in duplicate and the relative mRNA expression levels were analyzed using β-actin as an internal control by the 2−ΔΔCt method.
Table 3.
Sequences of real-time PCR primers
| Genes | Primer sequence (5’ → 3’) | Accession No. |
|---|---|---|
| IL-4 | F: AGCACTGCCACAAGAACC | NM_001398460.1 |
| R: GCTAGTTGGTGGAAGAAGGTA | ||
| IL-10 | F: CGCTGTCACCGCTTCTTCA | NM_001004414.3 |
| R: CGTCTCCTTGATCTGCTTGATG | ||
| TNF-α | F: TATGTGCAGCAACCCGTAGT | NM_204267.2 |
| R: AACAACCAGCTATGCACCCCA | ||
| MUC2 | F: AGCGAGATGTTGGCGATGAT | NM_001318434.1 |
| R: AAGTTGCCACACAGACCACA | ||
| β-actin | F: ATTGTCCACGCAAATGCTTC | L08165 |
| R: AAATAAAGCCATGCCAACTCGTC |
F Forward primer, R Reverse primer, IL Interleukin, TNF-α Tumor necrosis factor-α, MUC2 Mucin 2
Microbiota analysis (Exp. 1 and 2)
The microbiota analysis was commissioned by Microeco Tech Co., Ltd. (Shenzhen, China), and the methods were performed according to the procedure described by Peng et al. [15]. The V3-V4 hypervariable regions of the bacteria 16S rRNA gene were amplified with primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) by thermocycler PCR system (GeneAmp 9700, ABI, USA). Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, USA) according to the standard protocols. The analysis was conducted by following the “Atacama soil microbiome tutorial” of QIIME2docs along with customized program scripts (https://docs.qiime2.org/2019.1/). Briefly, raw data FASTQ files were imported into the format which could be operated by QIIME2 system using qiime tools import program. Demultiplexed sequences from each sample were quality filtered and trimmed, de-noised, merged, and then the chimeric sequences were identified and removed using the QIIME2 DADA2 plugin to obtain the feature table of amplicon sequence variant (ASV). The QIIME2 feature-classifier plugin was then used to align ASV sequences to a pre-trained GREENGENES 13_8 99% database (trimmed to the V3V4 region bound by the 338F/806R primer pair) to generate the taxonomy table. Any contaminating mitochondrial and chloroplast sequences were filtered using the QIIME2 feature-table plugin. Feature level alpha diversity indices, such as observed OTUs, Chao1 richness estimator, Shannon diversity index, and Faith’s phylogenetics diversity index were calculated to estimate the microbial diversity within an individual sample. Beta diversity distance measurements, including Bray-Curtis, unweighted UniFrac and weighted UniFrac were performed to investigate the structural variation of microbial communities across samples and then visualized via principal coordinate analysis (PCoA) and nonmetric multidimensional scaling (NMDS) [16].
Measurement of short-chain fatty acids (Exp. 1 and 2)
Approximately 0.3 g of fecal samples were thawed and diluted with 1 mL of ultrapure water, and 0.5 mL supernatant was obtained by centrifuging at 13,500 r/min for 10 min. Then the supernatant was mixed with 0.1 mL of 25% metaphosphoric acid solution and the mixed solution was placed at 4 ℃ for 4 h before centrifuging at 13,500 r/min for 15 min, afterwards the 0.4 mL of supernatant was mixed with 0.1 mL of 25% crotonic acid and the mixed solution was placed at 4 ℃ for 1 h, filtered by 0.45 μm filter (Millipore Co., Bedford, MA, USA). SCFAs concentrations were determined via gas chromatography (Agilent 7890A, Agilent Technologies, Santa Clara, CA, USA) according to the procedures described by Shen et al. [17], the chromatography was performed on Shodex RSpak KC-811 column (6 μm, 8.0 mm × 300 mm). The formula for calculating the concentration of short-chain fatty acids is as follows:
C is the concentration of SCFAs, S is the area of the corresponding peak, and k is the concentration of the corresponding SCFA standard substances/the concentration of crotonic acid.
Statistical analysis
Data were expressed as means with standard error of mean (SEM). The data were analyzed for the homogeneity of variances and normality using Levene’s and Shapiro–Wilk’s tests, respectively. C group and T group in Exp. 2, Student’s t-test was applied to the normal data, the heterogeneous or non-normally-distributed data were analyzed using Mann–Whitney U-test, and pairwise differences in rank sums were evaluated using selected comparisons tests. For all other variables in Exp. 1 and C, M and T group in Exp. 2, the normal data were assessed for statistical significance using a one-way analysis of variance (ANOVA) and Duncan’s multiple range test for pairwise comparisons. The heterogeneous or non-normally-distributed data were analyzed using a non-parametric Kruskal–Wallis test, and pairwise differences in rank sums were evaluated using selected comparisons tests. IBM SPSS 26.0 (Chicago, IL, USA) was used to perform statistical analysis. Statistical significance was considered at P < 0.05 and trends at P < 0.1.
Results
Composition analysis of OAE
Fifteen flavonoids, including seven flavonoid glycosides and eight flavonoid aglycones, were identified from oregano aqueous extract by UPLC-Q-TOF–MS. There were 5 phenylpropanoids and their esters or glycosides. The top ingredients were flavonoids, such as vicenin-2, taxifolin and eriodictyol (Fig. S1).
OAE improved the performance of broilers (Exp. 1)
As shown in Table 4, dietary 700 mg/kg OAE addition significantly improved (P < 0.01) BW at d 42 in comparison with the control. At the same time, the supplementation of 700 mg/kg OAE enhanced (P < 0.01) the ADG from 22 to 42 d and from 1 to 42 d. In addition, 700 mg/kg OAE significantly reduced (P < 0.05) the FCR from 22 to 42 d, but OAE had no effect on ADFI.
Table 4.
Effects of dietary supplementation with oregano aqueous extract on growth performance of broilers1 (Exp. 1)
| Items | Treatments2 | SEM3 | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Con | Anti | OAE400 | OAE500 | OAE600 | OAE700 | |||
| d 1–21 | ||||||||
| BW, g | 1,017.52ab | 1,066.22a | 997.60b | 997.26b | 1,035.96ab | 1,043.10ab | 7.321 | 0.030 |
| ADG, g | 46.55ab | 48.87a | 45.60b | 45.58b | 47.43ab | 47.77ab | 0.349 | 0.030 |
| ADFI, g | 67.61 | 70.36 | 67.39 | 66.80 | 71.55 | 70.61 | 0.578 | 0.069 |
| FCR | 1.48 | 1.45 | 1.51 | 1.48 | 1.48 | 1.51 | 0.011 | 0.630 |
| d 22–42 | ||||||||
| BW, g | 2,847.05c | 3,102.39b | 3,010.53bc | 2,940.42bc | 3,016.13bc | 3,278.39a | 31.194 | < 0.001 |
| ADG, g | 87.12c | 96.96b | 85.85bc | 92.53bc | 94.29bc | 106.44a | 1.417 | 0.001 |
| ADFI, g | 197.28 | 183.17 | 194.31 | 175.48 | 186.67 | 202.28 | 3.583 | 0.300 |
| FCR | 2.28a | 1.90b | 2.03ab | 1.91b | 1.91b | 1.91b | 0.040 | 0.019 |
| d 1–42 | ||||||||
| ADG, g | 66.83c | 72.91b | 70.73bc | 69.06bc | 70.86bc | 77.10a | 0.743 | < 0.001 |
| ADFI, g | 64.63 | 70.36 | 66.76 | 65.50 | 68.06 | 67.06 | 1.020 | 0.685 |
| FCR | 1.86 | 1.72 | 1.81 | 1.75 | 1.78 | 1.72 | 0.028 | 0.650 |
1 n = 7 replicates per treatment
2Con, OAE400, OAE500, OAE600 and OAE700, broilers received a basal diet supplemented with 0, 400, 500, 600 or 700 mg/kg oregano aqueous extract, respectively; Anti, broilers received a basal diet supplemented with 7 g/kg mycolistin sulfate and 35 g/kg locke sand arsine
3SEM Standard error of the mean
a,b,cValues within a row with no common superscripts differ significantly (P < 0.05)
OAE affected intestinal health (Exp. 1)
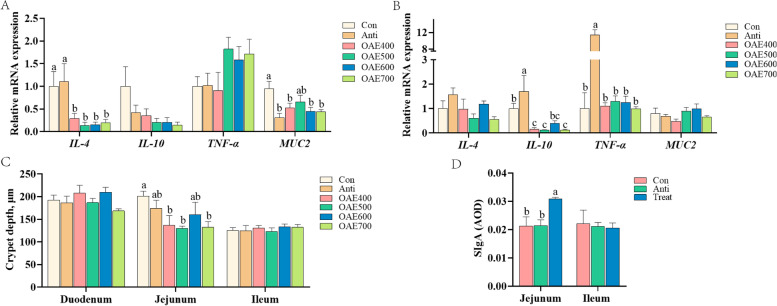
In order to investigate the influence of OAE on intestinal mucosal immunity, the relative mRNA expression of cytokines and the secretion of SIgA in intestinal mucosa were measured. With regard to d 21, no significant effect (P > 0.05) was found on interleukin-4 (IL-4), interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α) expression (Fig. S2A and B). At d 42, the mRNA levels of IL-4 and IL-10 in the jejunum decreased significantly (P < 0.05) with the OAE in a dose-dependent manner (Fig. 1A), and dietary supplementation with OAE down-regulated (P < 0.05) the relative mRNA expression of IL-10 in the ileum (Fig. 1B). At d 21, 400 mg/kg OAE significantly increased the secretion of mucin 2 (MUC2) in the jejunum (Fig. S2A). Nevertheless, jejunal mucin 2 expression was significantly decreased (P < 0.05) with OAE addition, except in OAE500 group at d 42 (Fig. 1A). In the jejunum, intestinal crypt depth was significantly decreased (P < 0.05) in OAE supplementation group, and OAE supplementation at 500 mg/kg and 700 mg/kg showed the best improvement (Fig. 1C). Based on the above results, OAE700 group was used to carry out subsequent studies, and changed its name to Treat. Results showed that the secretion of SIgA increased significantly (P < 0.05) in jejunum at d 42 with OAE addition, but there was no change in the ileum (Fig. 1D).
Fig. 1.
Oregano aqueous extract (OAE) affected intestinal health (Exp. 1). The relative mRNA expression of IL-4, IL-10, TNF-α and MUC2 in jejunum (A) and ileum (B) at d 42. Intestinal crypt depth at d 42 (C). At d 42, the secretion of SIgA in jejunum and ileum (D). Data are expressed as means ± standard deviation. a−cTreatments with no common superscripts differ significantly (P < 0.05). IL, interleukin; TNF-α, tumor necrosis factor-α; MUC2, mucin 2; SIgA, Secreted immunoglobulin A
OAE modulated gut microbiota and SCFAs (Exp. 1)
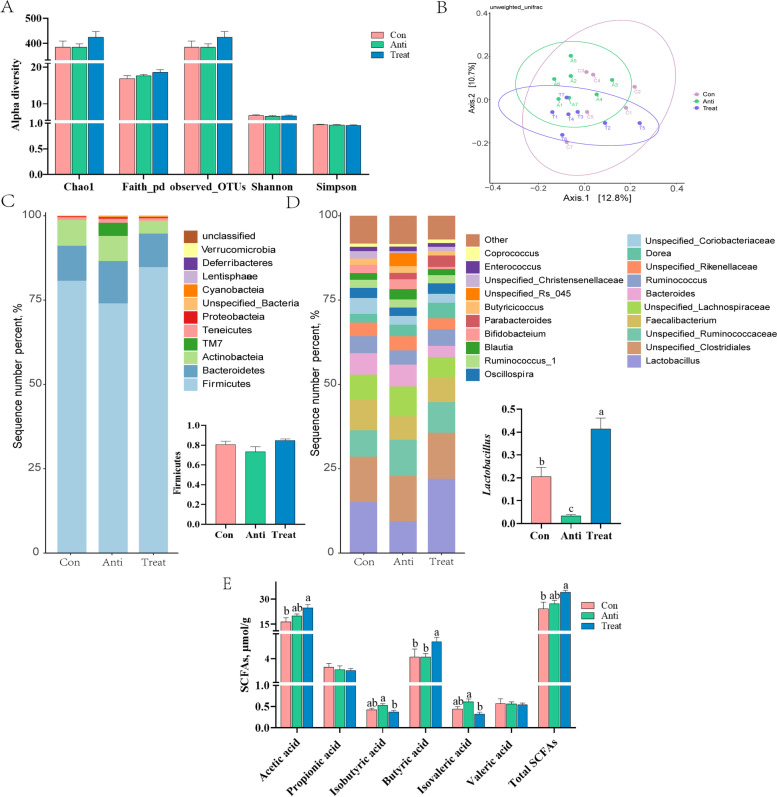
We further explored the regulation of OAE on gut microbiota. In alpha diversity indexes, there was no influence (P > 0.05) on Chao1, Faith_pd, observed_OTUs, Shannon and Simpson indices among all groups (Fig. 2A). Beta diversity analysis was illustrated by principal coordinate analysis (PCoA), the results based on the unweighted UniFrac distance showed separation of microbial communities between Anti and OAE-supplemented groups (P < 0.05; Fig. 2B). To further understand the specific changes in the microbial community, we analyzed the microbiota taxonomic composition. At the phylum level, Firmicutes abundance was somewhat reduced in the Anti group, and increased (P = 0.087) with the OAE supplementation and returned to normal levels. The ratio of Firmicutes/Bacteroidetes was higher (P = 0.067) in OAE than those in Con and Anti group (Fig. 2C). At the genus level, Lactobacillus was decreased significantly (P < 0.05) in the Anti group and reversed in the OAE group (Fig. 2D). The SCFAs results showed that OAE could increase the contents of acetic acid, butyric acid and total SCFAs (P < 0.05), while the contents of isobutyric acid and isovaleric acid was decreased (P < 0.05; Fig. 2E).
Fig. 2.
Oregano aqueous extract (OAE) modulated gut microbiota and SCFAs (Exp. 1). Alpha (A) and Beta (B) diversity analysis of cecum microbiota from broilers. Beta diversity analysis with principal coordinates analysis (PCoA) was based on the unweighted UniFrac distance. Relative abundance of microbiota at the phylum (C) and genus (D) level. Short-chain fatty acid content of cecal digesta (E). Data are expressed as means ± standard deviation. a−cTreatments with no common superscripts differ significantly (P < 0.05). PCoA, Principal coordinate analysis; SCFA, Short-chain fatty acid
Effects of OAE on microorganisms and SCFAs in vitro (Exp. 2)
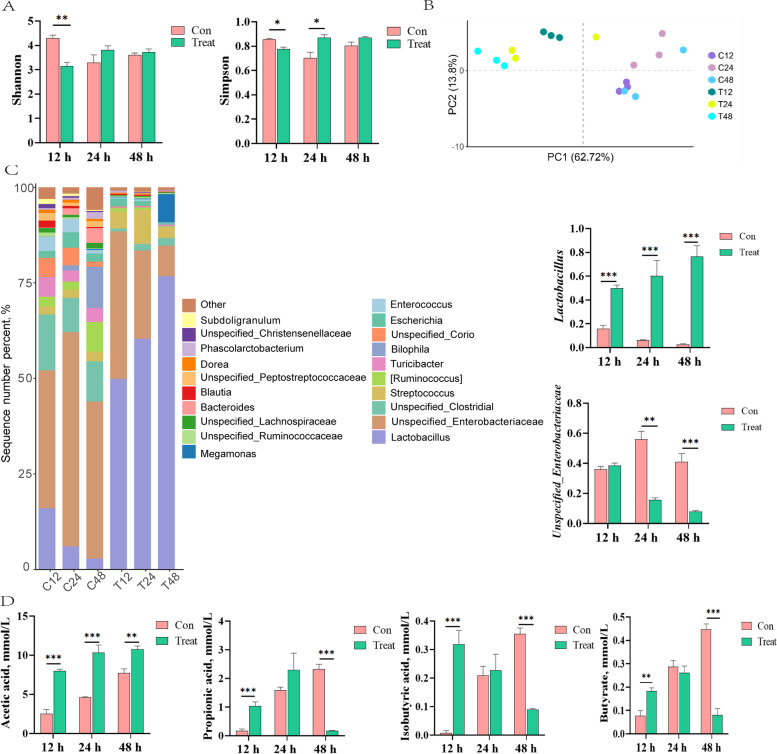
Then, in order to explore the direct effect of OAE on gut microbiota, OAE was fermented together with cecum microbiota in vitro. The results showed that both Shannon and Simpson indices were decreased in the OAE group at 12 h, while Simpson indices were increased in the presence of OAE at 24 h (P < 0.05; Fig. 3A). Additionally, principal components analysis (PCA) result displayed that the microbial community structure in Treat group was significantly different from Con group at each time period, the composition of microorganisms within the groups was relatively similar (Fig. 3B). It is noteworthy that the abundance of Lactobacillus in the Treat group was significantly higher (P < 0.05) than that in the Con group at each time period. Similar observations can be obtained in previous experiments. However, the abundance of unspecified_Enterobacteriaceae in the Treat group decreased significantly (P < 0.05) compared with the Con group at 24 h and 48 h (Fig. 3C). In addition, the concentration of acetic acid in the Treat group was significantly higher (P < 0.05) than that in the Con group at each time period, while the content of propionic acid and butyric acid in the Treat group was significantly reduced (P < 0.05) after 24 h. The content of isobutyric acid was increased in Con group and decreased with time in Treat group (P < 0.05; Fig. 3D).
Fig. 3.
Effects of oregano aqueous extract (OAE) on microorganisms and SCFAs in vitro (Exp. 2). Alpha (A) and Beta (B) diversity analysis of microorganisms. Beta diversity analysis with principal coordinates analysis (PCoA) was based on the unweighted UniFrac distance. Relative abundance of microbiota at the genus (C) level. Content of short-chain fatty acids in fermentation supernatant (D). Data are expressed as means ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001
OAE regulated intestinal health mediated by gut microbiota (Exp. 2)
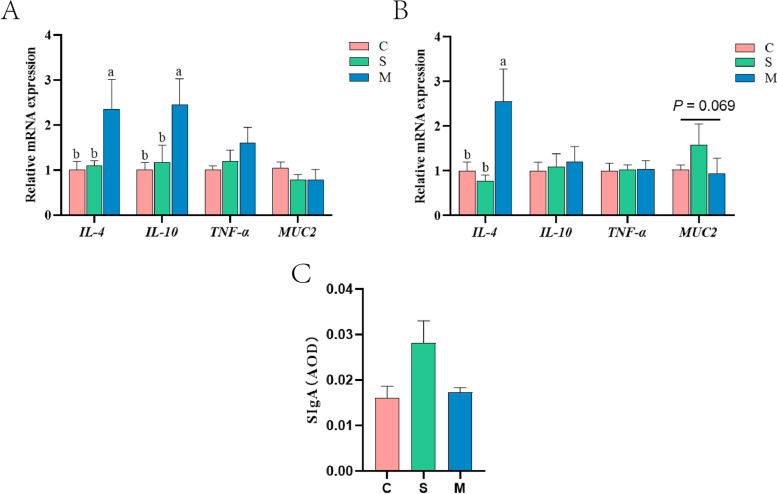
To investigate whether OAE regulated intestinal health through gut microbiota, we gavaged oregano fermentation supernatant or microbe to verify the role of microbe in regulating intestinal health. The results showed that the gavage of microbe significantly increased (P < 0.05) the relative mRNA expression of IL-4 in jejunum at d 21 (Fig. S3A and B). And the relative mRNA expression of IL-4 was significantly increased in M group in the jejunum and ileum at d 42, and the mRNA expression of IL-10 was significantly increased through microbial regulation in jejunum at d 42 (P < 0.05; Fig. 4A and B). At d 42, the mRNA expression of mucin 2 in the ileum showed an upward trend in S group (P = 0.069; Fig. 4B). In addition, gavage of supernatant increased the secretion of SIgA in the ileum (P = 0.073; Fig. 4C).
Fig. 4.
Oregano aqueous extract (OAE) mediated intestinal health by gut microbiota (Exp. 2). The relative mRNA expression of IL-4, IL-10, TNF-α and MUC2 in jejunum (A) and ileum (B) at d 42. At d 42, the secretion levels of SIgA in ileum (C). Data are expressed as means ± standard deviation. a−cTreatments with no common superscripts differ significantly (P < 0.05). IL, interleukin; TNF-α, tumor necrosis factor-α; MUC2, mucin 2; SIgA, Secreted immunoglobulin A
Discussion
In the present study, dietary OAE supplementation increased body weight, average daily gain and decreased feed conversion ratio in broilers and in a dose-dependent manner. Consistent with our findings, several recent studies have indicated that oregano extract improved the growth performance [18–20]. Aromatic substances of phytogenic feed additives were also reported to stimulate the intestinal secretion of digestive enzymes in broilers, increase absorption area and contribute to the stabilization of the gut microbiota, and growth performance improvement [21]. In addition, in this study, the reduction of intestinal crypt depth, improved immune homeostasis and altered microorganisms structure all suggested the role of OAE in enhancement of digestion absorption function and maintaining healthy intestinal condition as well as benefiting feed utilization in broiler.
Inflammatory reactions result in the production of numerous cytokine and inflammatory mediators, causing tissue and intestinal epithelial cell damage, which decreases intestinal barrier functions [22, 23]. In this study, the mRNA expression levels of IL-4 and IL-10 were decreased in the OAE group at d 42. This result might be associated with flavonoids in OAE (including apigenin and toxifolin), which were reported to inhibit Th2-type cytokine production [24–26]. Berry polyphenol components such as flavonoids, proanthocyanidins, and anthocyanins have developed functions in suppressing the secretion of cytokines such as IL-4 [27]. In addition, the decreased expression of IL-4 and IL-10 might be due to the inhibition of intestinal pathogens by OAE and the reduction of the intestinal inflammatory response, so there is no need to secrete excessive anti-inflammatory factors. Furthermore, it has been demonstrated that SIgA acts as the first-line defense barrier in protecting the intestinal epithelium. In the present study, dietary OAE addition significantly increased SIgA secretion [18]. Studies have found that SIgA could enhance the immune function of the intestinal mucosa [28]. SIgA was dysregulated and led to the change of microbial communities [29]. And the relationship between beneficial bacteria and IgA was bilateral, which contribute to improving the intestinal barrier. Thus, the reduced inflammatory response and improved intestinal barrier in response to OAE treatment would be beneficial for the maintenance of intestinal health and growth performance.
To better understand the positive effects of OAE, further analysis was conducted on gut microbiota. There are hundreds of millions of microorganisms in the intestine. The gut microbiota interactions play an important role in preventing pathogen colonization, maintaining immune homeostasis and nutrient metabolism. The active ingredient of oregano (such as thymol and carvacrol) has strong lipid solubility, which can quickly penetrate the cell membrane of pathogenic microorganisms, change their permeability and cause the loss of contents. Moreover, it could effectively prevent the oxidative energy supply process of mitochondria, damaging pathogenic microorganisms due to lack of energy [6, 30]. Apigenin and toxifolin have been reported to significantly increased the abundance of Lactobacillus and inhibited the reproduction of E. coli [31–33]. In this study, OAE treatment increased the abundance of some beneficial bacteria such as Lactobacillus, which were helpful for the maintenance of the overall microbial structure. Lactobacillus is recognized as beneficial bacteria which can promote the growth of animals, regulate the normal flora of the gastrointestinal tract and improve the body’s immunity. Intestinal colonization resistance means the inhibition of resident bacteria overgrowth within the intestinal tract. Lactobacillus can maintain intestinal colonization resistance and resist invasion [34, 35]. Thus, these results indicated that OAE effectively inhibits the colonization of pathogenic bacteria, which may be related to apigenin and toxifolin. Invasion of pathogens can cause secretion of pro-inflammatory factors such as IL-1β and TNF-α [36], while beneficial bacteria could resist the invasion of pathogens and inhibit the increase of pro-inflammatory factors. As reported, Firmicutes is the dominant species in poultry and most of them are beneficial bacteria. The ratio of Firmicutes/Bacteroidetes was usually used to represent the distribution of beneficial and harmful bacteria. These beneficial bacteria, such as all members of the Lactobacillus family, maintained intestinal health by modulating cytokine and chemokine gene expression [37, 38]. In this study, gut microbiota structure alteration might conduce to the enhanced intestinal barrier function and alleviate inflammation, thereby improving intestinal health.
Beneficial bacteria in the gut could ferment carbohydrates to produce short-chain fatty acids, which also inhibit the growth of harmful bacteria [39, 40]. Short-chain fatty acids provide energy for epithelial cell to meet the requirements of epithelial barrier function and cell division. SCFAs are regarded as mediators in the communication between the gut microbiota and the immune system [41–43]. Especially, acetate could regulate intestinal inflammation by stimulation of GPR43 [44], helping to maintain intestinal epithelial barrier function [45]. It was reported that the main components of short-chain fatty acids were acetic acid, propionic acid and butyric acid, accounting for more than 95%, among which acetic acid content was the highest [46], which was consistent with our experimental results. It has been reported that dietary acetic acid could increase appetite and regulate metabolism [47, 48]. Therefore, the increase in growth performance might be positively correlated with acetic acid content. Butyric acid as the main substitute for Firmicutes could also develop function in anti-inflammatory and regulating gut microbiota [49, 50]. In the current study, OAE increased the abundance of Lactobacillus and Firmicutes, then increased the contents of acetic acid and butyric acid, thereby maintaining intestinal health.
OAE contains not only alcohol-soluble substances but also water-soluble substances, which contain the complete complex of the plant [51]. Moreover, the contents of total phenols and flavonoids in aqueous extract are high [52]. Quercetin, apigenin and other flavonoids have low bioavailability and need to be metabolized by hindgut microbiota [53, 54]. This suggests that OAE had the potential to improve the growth performance in birds through modulating gut microbiota. Subsequently, cecal microorganisms were added to the basal medium with OAE, and the enriched microorganisms and their metabolites were orally administered to broilers to study the direct effect of microorganisms on intestinal health. The results showed that Lactobacillus was increased and Unclassified_Enterobacter was decreased in the OAE group. Enterobacter is the most common cause of Gram-negative bacterial infection. It mainly includes Yersinella, Escherichia coli, Klebsiella and so on. E. coli produced toxins that disrupted the intestinal barrier, causing disorders of the gut microbiota and metabolic diseases [55]. Gut homeostasis is mediated by the preponderance of obligate anaerobic members of Firmicutes and Bifidobacteriaceae, whereas the increase in facultative anaerobic Enterobacteriaceae is a common marker of gut dysbiosis [56]. However, the gut microbiota imbalance appeared in the absence of OAE. These results indicated that the OAE can directly affect the microorganisms, and has good antibacterial activity in vitro, which was consistent with the results in vivo in the study. In addition, OAE increased SCFAs contents, then propionic and butyric acids might be converted to acetic acid in the absence of carbon sources, resulting in a decrease in their concentrations after 24 h.
Subsequently, we evaluated the direct regulation of the gut microbiota and the role of SCFAs driven by the microbiota on intestinal health. The results showed that the mRNA expression levels of IL-4 and IL-10 increased in microorganism group to a certain extent, while the mRNA expression levels of MUC2 and secretion of SIgA were increased in supernatant group. Lactobacillus plantarum increased the mRNA expression of IFN-γ and IL-4 in the jejunum of broilers [57, 58]. The results implied that enriched Lactobacillus could regulate the expression of cytokines to improve intestinal health. In addition, it was reported that acetic acid could maintain the integrity of intestinal epithelium [59]. Burger-van Paassen et al. [60] found that acetic acid, propionic acid and butyric acid could all improve the expression of MUC2 gene and protein in LS174T cells. SCFAs were involved in the activation of B cells, thereby promoting the secretion of SIgA. The excellent effects in supernatant group may be mainly caused by acetic acid. Therefore, OAE could promote the growth of Lactobacillus, and inhibit the growth of harmful bacteria, consequently improving mucosal immunity. While the special microbiota could drive the production of acetic acid, which could enhance the protection of mucin and SIgA in the intestine.
Conclusions
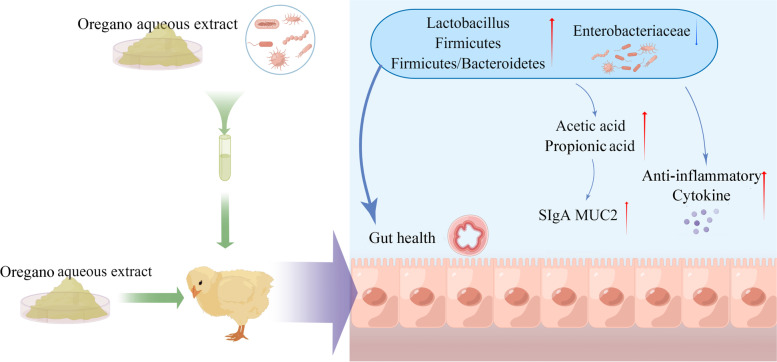
In conclusion, our results demonstrated a potential beneficial role of OAE in improving the growth performance and intestinal health in broilers, which may be related to the improvement of intestinal barrier function and mucosal immunity mediated by microbial changes (Fig. 5). These findings support the potential application of OAE as a safe and effective nutritional intervention strategy to maintain intestinal health and enhance growth performance in broilers.
Fig. 5.
Proposed functions of oregano aqueous extract (OAE) in broilers. Items with a red up-arrow indicated the increased bacteria, SCFAs, SIgA or mucosal gene expression in the OAE-supplemented group compared to the control, whereas those with a blue down-arrow indicated the decreased ones in the OAE-supplemented group
Supplementary Information
Additional file 1: Fig. S1. Identification of OAE. Fig. S2. OAE affected intestinal morphology and mucosal immunity. The relative mRNA expression of IL-4, IL-10, TNF-α and MUC2 in jejunum (A) and ileum (B) at d 21. Intestinal villus height (C) and villus height/crypt depth (D) at d 42. Data are expressed as means ± standard deviation. a−cTreatments with no common superscripts differ significantly (P < 0.05). IL, interleukin; TNF-α, tumor necrosis factor-α; MUC2, mucin 2. Fig. S3. Microorganism and supernatant for intestinal health. The relative mRNA expression of IL-4, IL-10, TNF-α and MUC2 in jejunum (A) and ileum (B) at d 21. At d 42, the secretion levels of SIgA in jejunum (C). Data are expressed as means ± standard deviation. a−cTreatments with no common superscripts differ significantly (P < 0.05). IL, interleukin; TNF-α, tumor necrosis factor-α; MUC2, mucin 2; SIgA, Secreted immunoglobulin A.
Acknowledgements
We would like to thank the staff at our laboratory for their ongoing assistance. We would like to thank Baoding Jizhong Pharmaceutical Co., Ltd. for providing experimental materials and assistance.
Abbreviations
- ADFI
Average daily feed intake
- ADG
Average daily gain
- BW
Body weight
- FCR
Feed conversion ratio
- IL
Interleukin
- MUC2
Mucin 2
- OAE
Oregano aqueous extract
- PCA
Principal components analysis
- PCoA
Principal coordinate analysis
- SCFA
Short-chain fatty acid
- SIgA
Secreted immunoglobulin A
- TNF-α
Tumor necrosis factor-α
Authors’ contributions
FZ and JTY conceived and designed the experiments; FZ, JTY, and YHL mainly performed the experiments; FZ analyzed the data and wrote the manuscript; FZ, JTY, QYZ, HS, YHL, DGL and YGL participated in the revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the National Science Foundation of China (31972529, 32272916), Shaanxi Livestock and Poultry Breeding Double-chain Fusion Key Project ( 2022GD-TSLD-46-0302), the Shaanxi Feed Engineering Technology Research Center (2019HBGC-16), the Program for Shaanxi Science & Technology (NYKJ-2018-YL15, 2019ZDXM3-02 and 2021TD-30) as well as Special Funding for Animal Husbandry from Department of Agriculture and Rural Affairs (XN06). The authors acknowledge members of the Innovative Research Team of Animal Nutrition & Healthy Feeding (Northwest A&F University, Shaanxi, China) and the Yongjiang Innovative Research Team for their help in animal care and sample harvesting.
Availability of data and materials
The data produced or analyzed during the current study are available from the corresponding author by reasonable request.
Declarations
Ethics approval and consent to participate
The use of animals and all experimental protocols were authorized by the Institutional Animal Care and Use Committee of Northwest A&F University (Yangling, Shaanxi, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Fan Zhang and Jiantao Yang contributed equally to this work.
Contributor Information
Fan Zhang, Email: Zhangfan0501@Nwafu.edu.cn.
Jiantao Yang, Email: 380724539@qq.com.
Qinyi Zhan, Email: zhan13@Nwafu.edu.cn.
Hao Shi, Email: dongkeshihao@nwafu.edu.cn.
Yanhe Li, Email: dklyh2020011223@nwafu.edu.cn.
Dinggang Li, Email: lukeldg@163.com.
Yingge Li, Email: 516597870@qq.com.
Xiaojun Yang, Email: yangxj@nwsuaf.edu.cn.
References
- 1.Bindari YR, Gerber PF. Centennial Review: Factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poult Sci. 2022;101(1):101612. doi: 10.1016/j.psj.2021.101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills S, Stanton C, Lane JA, Smith GJ, Ross RP. Precision nutrition and the microbiome, part I: current state of the science. Nutrients. 2019;11(4):923. doi: 10.3390/nu11040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 4.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ultee A, Bennik MH, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68(4):1561–8. 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed]
- 6.Langeveld WT, Veldhuizen EJ, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol. 2014;40(1):76–94. doi: 10.3109/1040841X.2013.763219. [DOI] [PubMed] [Google Scholar]
- 7.Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85(4):633–640. doi: 10.1016/j.foodchem.2003.07.024. [DOI] [Google Scholar]
- 8.Hashemipour H, Kermanshahi H, Golian A, Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult Sci. 2013;92(8):2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- 9.Ding X, Wu X, Zhang K, Bai S, Wang J, Peng H, et al. Dietary supplement of essential oil from oregano affects growth performance, nutrient utilization, intestinal morphology and antioxidant ability in Pekin ducks. J Anim Physiol Anim Nutr (Berl) 2020;104(4):1067–1074. doi: 10.1111/jpn.13311. [DOI] [PubMed] [Google Scholar]
- 10.Bauer BW, Gangadoo S, Bajagai YS, Van TTH, Moore RJ, Stanley D. Oregano powder reduces streptococcus and increases SCFA concentration in a mixed bacterial culture assay. PLoS One. 2019;14(12):e0216853. doi: 10.1371/journal.pone.0216853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashidian G, Boldaji JT, Rainis S, Prokić MD, Faggio C. Oregano (Origanum vulgare) extract enhances zebrafish (Danio rerio) growth performance, serum and mucus innate immune responses and resistance against aeromonas hydrophila challenge. Animals (Basel). 2021;11(2):299. 10.3390/ani11020299. [DOI] [PMC free article] [PubMed]
- 12.Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. Fiber-utilizing capacity varies in Prevotella-versus Bacteroides-dominated gut microbiota. Sci Rep. 2017;7(1):2594. doi: 10.1038/s41598-017-02995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M, Huo J, Wang C, Wang W. UPLC/Q-TOF MS screening and identification of antibacterial compounds in forsythia suspensa (Thunb.) Vahl Leaves. Front Pharmacol. 2022;12:704260. doi: 10.3389/fphar.2021.704260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Zeng L, Tan B, Li G, Huang B, Xiong X, et al. Developmental changes in intercellular junctions and Kv channels in the intestine of piglets during the suckling and post-weaning periods. J Anim Sci Biotechnol. 2016;7:4. doi: 10.1186/s40104-016-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng J, Lu X, Xie K, Xu Y, He R, Guo L, et al. Dynamic alterations in the gut microbiota of collagen-induced arthritis rats following the prolonged administration of total glucosides of paeony. Front Cell Infect Microbiol. 2019;9:204. doi: 10.3389/fcimb.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vázquez-Baeza Y, Pirrung M, Gonzalez A, Knight R. EMPeror: a tool for visualizing high-throughput microbial community data. Gigascience. 2013;2(1):16. doi: 10.1186/2047-217X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen H, Lu Z, Xu Z, Shen Z. Antibiotic pretreatment minimizes dietary effects on reconstructure of rumen fluid and mucosal microbiota in goats. Microbiologyopen. 2018;7(1):e00537. doi: 10.1002/mbo3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang LY, Peng QY, Liu YR, Ma QG, Zhang JY, Guo YP, et al. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poult Sci. 2021;100(7):101163. doi: 10.1016/j.psj.2021.101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irawan A, Hidayat C, Jayanegara A, Ratriyanto A. Essential oils as growth-promoting additives on performance, nutrient digestibility, cecal microbes, and serum metabolites of broiler chickens: a meta-analysis. Anim Biosci. 2021;34(9):1499–1513. doi: 10.5713/ab.20.0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amer SA, Tolba SA, AlSadek DMM, Abdel Fattah DM, Hassan AM, Metwally AE. Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Vet Res. 2021;17(1):312. doi: 10.1186/s12917-021-03022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng C, Xia M, Zhang X, Wang C, Jiang S, Peng J. Supplementing oregano essential oil in a reduced-protein diet improves growth performance and nutrient digestibility by modulating intestinal bacteria, intestinal morphology, and antioxidative capacity of growing-finishing pigs. Animals (Basel) 2018;8(9):159. doi: 10.3390/ani8090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014;20(5):1192–1210. doi: 10.3748/wjg.v20.i5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stecher B. The roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Microbiol Spectr. 2015;3(3):3.3.12. 10.1128/microbiolspec.MBP-0008-2014. [DOI] [PubMed]
- 24.Hirano T, Higa S, Arimitsu J, Naka T, Shima Y, Ohshima S, et al. Flavonoids such as luteolin, fisetin and apigenin are inhibitors of interleukin-4 and interleukin-13 production by activated human basophils. Int Arch Allergy Immunol. 2004;134(2):135–140. doi: 10.1159/000078498. [DOI] [PubMed] [Google Scholar]
- 25.Park CH, Min SY, Yu HW, Kim K, Kim S, Lee HJ, et al. Effects of apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: anti-allergic, anti-inflammatory, and skin-protective activities. Int J Mol Sci. 2020;21(13):4620. doi: 10.3390/ijms21134620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sunil C, Xu B. An insight into the health-promoting effects of taxifolin (dihydroquercetin) Phytochemistry. 2019;166:112066. doi: 10.1016/j.phytochem.2019.112066. [DOI] [PubMed] [Google Scholar]
- 27.González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr. 2010;104(Suppl 3):S15–27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Wang J, Wang H, Huang Y, Qi M, Liao S, et al. Effects of GABA supplementation on intestinal SIgA secretion and gut microbiota in the healthy and ETEC-infected weanling piglets. Mediators Inflamm. 2020;2020:7368483. doi: 10.1155/2020/7368483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336(6080):485–9. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 30.Lesjak M, Simin N, Orcic D, Franciskovic M, Knezevic P, Beara I, et al. Binary and tertiary mixtures of Satureja hortensis and Origanum vulgare essential oils as potent antimicrobial agents against helicobacter pylori. Phytother Res. 2016;30(3):476–84. 10.1002/ptr.5552. [DOI] [PubMed]
- 31.Kim BK, Choi IS, Kim J, Han SH, Suh HJ, Hwang JK. Effects of fermented milk with mixed strains as a probiotic on the inhibition of loperamide-induced constipation. Korean J Food Sci Anim Resour. 2017;37(6):906–916. doi: 10.5851/kosfa.2017.37.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Zhang L, Xu Q, Yang W, Zhao J, Ren Y, et al. Taxifolin alleviates DSS-induced ulcerative colitis by acting on gut microbiome to produce butyric acid. Nutrients. 2022;14(5):1069. doi: 10.3390/nu14051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei W, Jiang W, Tian Z, Wu H, Ning H, Yan G, et al. Fecal g. Streptococcus and g. Eubacterium_coprostanoligenes_group combined with sphingosine to modulate the serum dyslipidemia in high-fat diet mice. Clin Nutr. 2021;40(6):4234–45. 10.1016/j.clnu.2021.01.031. [DOI] [PubMed]
- 34.Heeney DD, Gareau MG, Marco ML. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr Opin Biotechnol. 2018;49:140–147. doi: 10.1016/j.copbio.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stecher B, Hardt WD. The role of microbiota in infectious disease. Trends Microbiol. 2008;16(3):107–114. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Boehme JD, Stegemann-Koniszewski S, Autengruber A, Peters N, Wissing J, Jänsch L, et al. Chronic lung inflammation primes humoral immunity and augments antipneumococcal resistance. Sci Rep. 2017;7(1):4972. doi: 10.1038/s41598-017-05212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haghighi HR, Abdul-Careem MF, Dara RA, Chambers JR, Sharif S. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet Microbiol. 2008;126(1–3):225–233. doi: 10.1016/j.vetmic.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Brisbin JT, Gong J, Parvizi P, Sharif S. Effects of Lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin Vaccine Immunol. 2010;17(9):1337–1343. doi: 10.1128/CVI.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 40.Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One. 2014;9(3):e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217(2):133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 42.Pajak B, Orzechowski A, Gajkowska B. Molecular basis of sodium butyrate-dependent proapoptotic activity in cancer cells. Adv Med Sci. 2007;52:83–88. doi: 10.1007/978-3-540-45456-4_43. [DOI] [PubMed] [Google Scholar]
- 43.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 44.Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121(3):1163–1173. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu J, Kyrou I, Tan BK, Dimitriadis GK, Ramanjaneya M, Tripathi G, et al. Short-chain fatty acid acetate stimulates adipogenesis and mitochondrial biogenesis via GPR43 in brown adipocytes. Endocrinology. 2016;157(5):1881–1894. doi: 10.1210/en.2015-1944. [DOI] [PubMed] [Google Scholar]
- 48.Kaji I, Karaki S, Kuwahara A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion. 2014;89(1):31–36. doi: 10.1159/000356211. [DOI] [PubMed] [Google Scholar]
- 49.Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18(1):2. doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu W, Xiao Z, An W, Dong Y, Zhang B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS One. 2018;13(5):e0197762. doi: 10.1371/journal.pone.0197762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scocco P, Forte C, Franciosini MP, Mercati F, Casagrande-Proietti P, Dall’Aglio C, et al. Gut complex carbohydrates and intestinal microflora in broiler chickens fed with oregano (Origanum vulgare L.) aqueous extract and vitamin E. J Anim Physiol Anim Nutr (Berl). 2017;101(4):676–84. 10.1111/jpn.12588. [DOI] [PubMed]
- 52.Duleti-Lauevi S, Alimpic AZ, Kolarevi S, Vukovi-Gai B, Oale M, Živković J, et al. Antineurodegenerative, antioxidant and antibacterial activities and phenolic components of Origanum majorana L. (Lamiaceae) extracts of different origin. J Appl Bot Food Qual. 2018;91:126–34. 10.5073/JABFQ.2018.091.018.
- 53.Tang D, Chen K, Huang L, Li J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin Drug Metab Toxicol. 2017;13(3):323–330. doi: 10.1080/17425255.2017.1251903. [DOI] [PubMed] [Google Scholar]
- 54.Freitas PL, Miranda JPN, França LM, Paes AMA. Plant-derived (poly) phenols and their metabolic outcomes: the pursuit of a role for the gut mmicrobiota. Nutrients. 2022;14(17):3510. doi: 10.3390/nu14173510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313(5788):848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 56.Martin-Gallausiaux C, Béguet-Crespel F, Marinelli L, Jamet A, Ledue F, Blottière HM, et al. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci Rep. 2018;8(1):9742. doi: 10.1038/s41598-018-28048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z, Wang W, Liu D, Guo Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J Anim Sci Biotechnol. 2018;9:25. doi: 10.1186/s40104-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Liu C, Chen M, Ya T, Huang W, Gao P, et al. A novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int Immunopharmacol. 2015;29(2):901–907. doi: 10.1016/j.intimp.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 60.Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;420(2):211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Identification of OAE. Fig. S2. OAE affected intestinal morphology and mucosal immunity. The relative mRNA expression of IL-4, IL-10, TNF-α and MUC2 in jejunum (A) and ileum (B) at d 21. Intestinal villus height (C) and villus height/crypt depth (D) at d 42. Data are expressed as means ± standard deviation. a−cTreatments with no common superscripts differ significantly (P < 0.05). IL, interleukin; TNF-α, tumor necrosis factor-α; MUC2, mucin 2. Fig. S3. Microorganism and supernatant for intestinal health. The relative mRNA expression of IL-4, IL-10, TNF-α and MUC2 in jejunum (A) and ileum (B) at d 21. At d 42, the secretion levels of SIgA in jejunum (C). Data are expressed as means ± standard deviation. a−cTreatments with no common superscripts differ significantly (P < 0.05). IL, interleukin; TNF-α, tumor necrosis factor-α; MUC2, mucin 2; SIgA, Secreted immunoglobulin A.
Data Availability Statement
The data produced or analyzed during the current study are available from the corresponding author by reasonable request.