Abstract
Background
The weight-adjusted waist index (WWI) is a new measure of obesity, and this study aimed to determine the association between the WWI and stroke.
Methods
Using the National Health and Nutrition Examination Survey (NHANES) 2011–2020 dataset, cross-sectional data from 23,389 participants were analysed. The correlation between the WWI and stroke was investigated through multivariate logistic regression and smoothing curve fitting. Subgroup analysis and interaction tests were also carried out.
Results
The research involved 23,389 participants, of whom 893 (3.82%) had a stroke. The fully adjusted model revealed a positive correlation between the WWI and stroke [1.25 (1.05, 1.48)]. Individuals who were in the highest quartile of WWI exhibited a 62% higher likelihood of experiencing a stroke than those in the lowest quartile [1.62 (1.06, 2.48)]. Subgroup analysis and interaction tests revealed that this positive correlation was similar in different population settings (all P for interaction > 0.05).
Conclusion
A higher WWI was associated with a higher prevalence of stroke. The results of this study underscore the value of the WWI in stroke prevention and management.
Keywords: Weight-adjusted waist index, Stroke, Obesity, NHANES, Cross-sectional study
Introduction
Stroke is a cerebrovascular illness characterized by blood vessel obstruction [1]. Stroke was reported to be ranked second globally among the most common causes of mortality and third among the leading causes of death and disability combined [2, 3]. With the ageing population, the frequency of stroke incidence continues to escalate [4]. Specifically, the occurrence of stroke surged by 70%, and its global prevalence rose by 85% between 1990 and 2019 [2]. The high rates of morbidity, mortality, and disability linked to stroke implicate it as a serious threat to public health [5]. Furthermore, the estimated annual global expenditure related to stroke exceeds $89.1 billion, exerting an enormous socioeconomic impact and placing a significant strain on health care systems [6, 7]. As a result, it is necessary to identify preventable and controllable factors to lower the prevalence of stroke.
The prevalence of obesity has reached epidemic levels globally, affecting an increasingly large number of individuals [8–10]. Obesity has been associated with the onset of various diseases, including diabetes, nonalcoholic fatty liver disease, cardiovascular disease, and several malignancies [11–14]. Body mass index (BMI) and waist circumference (WC) serve as common obesity evaluation metrics. However, those indicators cannot be used to differentiate between fat and muscle mass [15–17]. Recent studies have proposed that body composition and fat distribution can be used to more accurately assess poor metabolic characteristics [18, 19]. The weight-adjusted waist index (WWI), a new type of obesity index, standardizes WC with weight, incorporating the strengths of WC while dampening the connection with BMI [20]. The WWI not only differentiates between fat and muscle mass but also accounts for central obesity issues unrelated to weight [21, 22]. Previous research has demonstrated that an elevated WWI is closely linked to several diseases, such as abdominal aortic calcification, osteoporosis, and heart failure [23–25].
The etiopathogenesis of stroke is complex, and obesity is considered to be one of the major risk factors [26]. While the WWI serves as an effective predictor of risk for numerous cardiovascular diseases, no studies to date have explored a potential relationship between the WWI and stroke. Therefore, we aimed to examine this relationship through a cross-sectional analysis using data from the 2011–2020 National Health and Nutrition Examination Survey (NHANES).
Methods
Survey description
NHANES is a continuous US health investigation run by the Centers for Disease Control and Prevention (CDC) [27]. To accurately evaluate the health and nutritional status of the US population, the survey employed stratified multistage probability sampling to generate a representative sample [28]. An in-home interview, physical examination, and a battery of laboratory tests were performed on all participants.
The study procedure was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. Before the commencement of research, every participant furnished written permission [29]. The comprehensive depiction of the NHANES research and its corresponding statistics can be found at https://www.cdc.gov/nchs/nhanes/.
Study population
This investigation employed the NHANES dataset from 2011–2020. Participants with complete stroke and WWI data were included in our study. Initially, 45,462 participants were recruited. After eliminating participants with absent or incomplete data concerning weight (n = 2,976), WC (n = 4,767), and stroke (n = 14,330), our final analysis included 23,389 participants (Fig. 1).
Fig. 1.
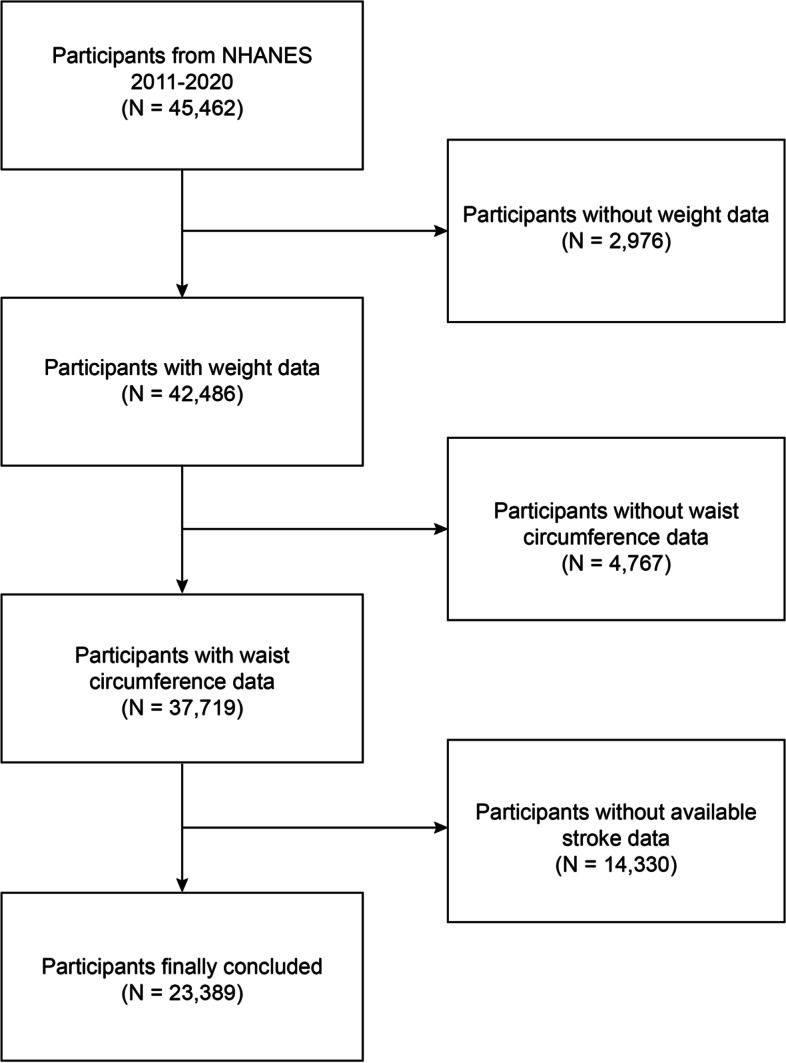
Flow chart of participant selection. NHANES, National Health and Nutrition Examination Survey
Weight-adjusted waist index
The WWI is a new obesity assessment indicator. A greater WWI score indicates a higher level of obesity. The WWI (cm/√kg) is calculated by dividing WC (cm) by the square root of weight (kg) [20]. Anthropometric measurements were taken at a mobile examination centre by skilled medical personnel and recorded by professional recorders to ensure the accuracy of the data. Digital scales were used for weight measurement. Participants wore examination clothing prior to weighing and subsequently stood barefoot in the middle of a digital scale with their arms next to their bodies and their eyes gazing forwards [23]. Tape measures were used for WC measurement. A tape measure was positioned at the junction of the mid-axillary line and a horizontal line above the uppermost lateral edge of the right patella [24]. Our research considered the WWI to be an exposure variable.
Stroke
A medical conditions questionnaire was used to determine the occurrence of stroke. Participants were judged to have experienced a stroke if they replied "yes" to this question: "Has a doctor or other health professional ever told you that you had a stroke?" Previous research has validated the use of self-reported stroke [30, 31]. In our study, stroke was regarded as an outcome variable.
Covariates
In the present study, we included covariates that could obfuscate the relationship between the WWI and stroke, including age, sex, race, education level, smoking, diabetes, high blood pressure, coronary heart disease, cancer, ratio of family income to poverty (PIR), average alcohol consumption in the past 12 months, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and total cholesterol (TC).
Statistical analysis
Statistical analyses conducted for this study were executed in compliance with guidelines provided by the CDC, with proper NHANES sample weights, and taking into account complex multistage clustering surveys. The research assessed the characteristics of participants based on quartiles of the WWI utilizing chi-square tests or t-tests. Multivariate logistic regression was used to examine the linear association between the WWI and stroke in three different models. Model 1 did not involve any covariate adjustments. Model 2 involved the adjustment of age, sex, and race. Model 3 adjusted for age, sex, race, education level, alcohol consumption, smoking, diabetes, high blood pressure, coronary heart disease, cancer, PIR, triglycerides, TC, HDL-C, and LDL-C. After converting the WWI score from continuous to classified variables (quartiles), trend tests were applied to explore trends in the linear correlation between the WWI and stroke. Subgroup analyses of the relationship between the WWI and stroke were performed using stratifying factors, comprising age, sex, race, education level, diabetes, high blood pressure, coronary heart disease, and cancer, and interaction tests were used to test the consistency of this association within distinct groupings. Moreover, smoothing curve fitting was conducted to investigate a nonlinear correlation between the WWI and stroke [32]. We carried out all statistical analyses using R (version 4.2) and Empower software (version 5.0). A two-sided p < 0.05 was considered to indicate a statistically significant difference.
Results
Baseline characteristics
The study involved 23,389 participants with an average age of 49.32 ± 17.42 years, of whom 11,409 (48.78%) were male and 11,980 (51.22%) were female. Among all participants, the prevalence of stroke was 3.82%, and it increased with the higher WWI quartiles. The mean WWI for all participants was 11.09 ± 0.86 cm/√kg, with the values for the different quartiles as follows: quartile 1: < 10.51; quartile 2: 10.51–11.09; quartile 3: 11.10–11.67; and quartile 4: > 11.67 cm/√kg. Compared to those with the lowest quartile of WWI, individuals with the highest quartile of WWI were more likely to be older, female, non-Hispanic white, less educated, and smokers, and they were more likely to have diabetes, high blood pressure, coronary heart disease, cancer, and stroke. Meanwhile, a higher WWI tended to be accompanied by a higher BMI, WC, body weight, triglyceride, LDL-C, and TC levels, while PIR and HDL-C levels were lower (Table 1).
Table 1.
Basic characteristics of participants by weight-adjusted waist index quartile
| Characteristics | Weight-adjusted waist index | P value | |||
|---|---|---|---|---|---|
|
Q1 (< 10.51) N = 5,847 |
Q2 (10.51–11.09) N = 5,847 |
Q3 (11.10–11.67) N = 5,847 |
Q4 (> 11.67) N = 5,848 |
||
| Age (years) | 37.06 ± 13.45 | 46.33 ± 15.05 | 51.74 ± 15.90 | 57.47 ± 16.14 | < 0.001 |
| Sex, (%) | < 0.001 | ||||
| Male | 59.02 | 52.33 | 46.07 | 32.29 | |
| Female | 40.98 | 47.67 | 53.93 | 67.71 | |
| Race/ethnicity, (%) | < 0.001 | ||||
| Non-Hispanic White | 64.39 | 64.73 | 64.07 | 67.08 | |
| Non-Hispanic Black | 14.97 | 10.25 | 10.01 | 9.27 | |
| Mexican American | 5.58 | 8.57 | 10.60 | 9.91 | |
| Other race/multiracial | 15.06 | 16.45 | 15.32 | 13.74 | |
| Education level, (%) | < 0.001 | ||||
| Less than high school | 9.01 | 12.32 | 16.07 | 19.36 | |
| High school | 18.20 | 21.31 | 24.15 | 26.44 | |
| More than high school | 72.79 | 66.33 | 59.76 | 54.17 | |
| Smoking, (%) | < 0.001 | ||||
| Ever | 36.91 | 43.59 | 46.87 | 47.93 | |
| Never | 63.09 | 56.41 | 53.13 | 52.07 | |
| Diabetes, (%) | < 0.001 | ||||
| Yes | 3.01 | 8.60 | 14.85 | 26.76 | |
| No | 96.99 | 91.40 | 85.15 | 73.24 | |
| High blood pressure, (%) | < 0.001 | ||||
| Yes | 13.89 | 29.05 | 38.80 | 52.73 | |
| No | 86.11 | 70.95 | 61.20 | 47.27 | |
| Coronary heart disease, (%) | < 0.001 | ||||
| Yes | 0.83 | 2.23 | 4.28 | 7.20 | |
| No | 99.17 | 97.77 | 95.72 | 92.80 | |
| Cancer, (%) | < 0.001 | ||||
| Yes | 5.09 | 9.75 | 11.87 | 16.89 | |
| No | 94.91 | 90.25 | 88.13 | 83.11 | |
| Stroke, (%) | < 0.001 | ||||
| Yes | 0.90 | 2.00 | 3.08 | 5.49 | |
| No | 99.10 | 98.00 | 96.92 | 94.51 | |
| BMI (kg/m2) | 24.96 ± 4.70 | 28.32 ± 5.32 | 30.67 ± 6.37 | 34.22 ± 7.82 | < 0.001 |
| Waist circumference (cm) | 86.09 ± 10.56 | 97.24 ± 11.61 | 104.79 ± 13.19 | 115.34 ± 16.13 | < 0.001 |
| PIR | 3.08 ± 1.62 | 3.14 ± 1.59 | 2.96 ± 1.60 | 2.61 ± 1.52 | < 0.001 |
| Weight (kg) | 74.70 ± 16.85 | 82.06 ± 19.41 | 86.29 ± 21.81 | 91.65 ± 25.21 | < 0.001 |
| Average alcohol consumption past 12 months | 3.24 ± 18.67 | 4.29 ± 39.96 | 2.99 ± 20.49 | 3.33 ± 29.64 | 0.187 |
| Triglycerides (mg/dL) | 107.49 ± 63.03 | 117.45 ± 67.70 | 124.53 ± 80.28 | 126.97 ± 61.62 | < 0.001 |
| HDL-C (mg/dL) | 57.27 ± 16.23 | 54.00 ± 16.65 | 52.22 ± 16.84 | 51.20 ± 14.41 | < 0.001 |
| LDL-C (mg/dL) | 109.30 ± 22.37 | 113.27 ± 23.74 | 113.47 ± 24.32 | 111.61 ± 24.87 | < 0.001 |
| Total cholesterol (mg/dL) | 183.55 ± 37.46 | 194.32 ± 39.38 | 196.08 ± 42.12 | 192.35 ± 42.86 | < 0.001 |
Mean ± SD for continuous variables: the P value was calculated by the weighted linear regression model
(%) for categorical variables: the P value was calculated by the weighted chi-square test
Abbreviations: Q Quartile, BMI Body mass index, PIR Ratio of family income to poverty, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol
Association between the WWI and stroke
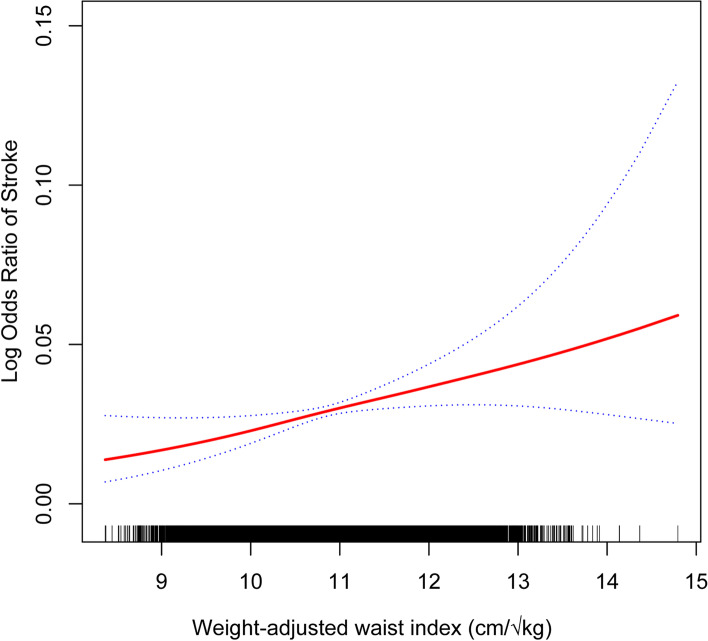
Table 2 shows the correlation between the WWI and stroke. In the crude [1.94 (1.79, 2.10)] and partially adjusted [1.36 (1.23, 1.50)] models, the WWI and stroke showed a significant positive association. Upon complete adjustment, the aforementioned positive association remained statistically significant [1.25 (1.05, 1.48)], with a 25% increase in stroke prevalence for every unit increase in the WWI. This positive association remained stable after transforming the WWI into quartiles (all P for trend < 0.05). Participants in the highest WWI quartile had a 62% increased prevalence of stroke compared to those in the lowest quartile [1.62 (1.06, 2.48)]. Furthermore, the findings of smoothed curve fitting analysis corroborated the nonlinear positive correlation between the WWI and stroke (Fig. 2).
Table 2.
The association between weight-adjusted waist index and stroke
| Exposure | Model 1 [OR (95% CI)] | Model 2 [OR (95% CI)] | Model 3 [OR (95% CI)] |
|---|---|---|---|
| Weight-adjusted waist index (continuous) | 1.94 (1.79, 2.10) | 1.36 (1.23, 1.50) | 1.25 (1.05, 1.48) |
| Weight-adjusted waist index (quartile) | |||
| Quartile 1 | reference | reference | reference |
| Quartile 2 | 2.12 (1.61, 2.79) | 1.34 (1.01, 1.78) | 1.36 (0.93, 1.99) |
| Quartile 3 | 3.56 (2.75, 4.60) | 1.70 (1.30, 2.23) | 1.35 (0.91, 1.99) |
| Quartile 4 | 5.35 (4.18, 6.86) | 2.05 (1.56, 2.69) | 1.62 (1.06, 2.48) |
| P for trend | < 0.001 | < 0.001 | 0.038 |
Model 1: no covariates were adjusted. Model 2: adjusted for age, sex, and race. Model 3: adjusted for age, sex, race, education level, alcohol consumption, smoking, diabetes, high blood pressure, coronary heart disease, cancer, PIR, triglycerides, total cholesterol, HDL-C, and LDL-C
Abbreviations: PIR Ratio of family income to poverty, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol
Fig. 2.
The nonlinear association between the WWI and stroke. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. WWI, weight-adjusted waist index
Subgroup analyses
We performed subgroup analyses and interaction tests stratified by sex, age, race, education level, diabetes, high blood pressure, coronary heart disease, and cancer to evaluate the robustness of the relationship between the WWI and stroke and to discover possible population differences (Table 3). Our outcomes showed that the relationship between the WWI and stroke was not dependent on the above factors (all P for interaction > 0.05). Moreover, the positive correlation of the WWI with stroke was similar across populations with different sex, age, race, education level, diabetes, high blood pressure, coronary heart disease, and cancer status, and it may be appropriate for different populations.
Table 3.
Subgroup analysis of the association between weight-adjusted waist index and stroke
| Subgroup | Stroke [OR (95% CI)] | P for interaction |
|---|---|---|
| Sex | 0.103 | |
| Male | 1.39 (1.11, 1.74) | |
| Female | 1.11 (0.90, 1.38) | |
| Age | 0.131 | |
| < 60 years | 1.72 (1.39, 2.13) | |
| ≥ 60 years | 1.41 (1.15, 1.73) | |
| Race/ethnicity | 0.698 | |
| Non-Hispanic White | 1.39 (1.08, 1.80) | |
| Non-Hispanic Black | 1.13 (0.84, 1.52) | |
| Mexican American | 1.09 (0.54, 2.22) | |
| Other race | 1.34 (0.85, 2.12) | |
| Education level | 0.442 | |
| Less than high school | 1.55 (1.07, 2.23) | |
| High school | 1.07 (0.78, 1.48) | |
| More than high school | 1.23 (0.96, 1.57) | |
| Diabetes | 0.309 | |
| Yes | 1.42 (1.06, 1.91) | |
| No | 1.19 (0.97, 1.45) | |
| High blood pressure | 0.163 | |
| Yes | 1.16 (0.96, 1.41) | |
| No | 1.44 (1.10, 1.88) | |
| Coronary heart disease | 0.173 | |
| Yes | 1.75 (1.06, 2.90) | |
| No | 1.21 (1.01, 1.46) | |
| Cancer | 0.110 | |
| Yes | 1.66 (1.13, 2.43) | |
| No | 1.18 (0.97, 1.43) |
Age, sex, race, education level, alcohol consumption, smoking, diabetes, high blood pressure, coronary heart disease, cancer, PIR, triglycerides, total cholesterol, HDL-C and LDL-C were adjusted
Abbreviations: PIR Ratio of family income to poverty, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol
Discussion
In this cross-sectional study involving 23,389 representative adults, we discovered a positive association between the WWI and stroke prevalence, signifying that individuals with a higher WWI had an elevated probability of experiencing a stroke. The subgroup analyses and interaction tests confirmed the robustness of this positive association across a diverse array of demographic contexts. These observations suggested that an elevated WWI could be an independent risk factor for stroke, thus underscoring the significance of the WWI in the prevention and management of stroke.
To the best of our knowledge, this is the first study to examine the link between the WWI and stroke. Prior research has investigated the correlation between the WWI and cardiovascular diseases. Ding et al. conducted a prospective analysis with 12,447 Chinese individuals and discovered a nonlinear positive correlation between the WWI and all-cause and cardiovascular mortality, with a WWI > 11.2 cm/√kg substantially increasing the risk of death [33]. Zhang et al. revealed that a greater WWI may be an independent predictor of heart failure in cross-sectional research of 25,509 people [25]. Cai et al. also reported that the WWI was related to left ventricular hypertrophy, indicating that the WWI may serve as a significant predictor of cardiometabolic risk [34]. Furthermore, Li et al. discovered that WWI was related to a higher incidence of hypertension in rural China, which could potentially serve as an indicator of the risk of hypertension among a rural population in China [35]. We identified a positive correlation between WWI and stroke, which was in line with the detrimental consequences of WWI on cardiovascular health described in earlier research.
Accumulating evidence confirms that obesity is a significant stroke risk factor. BMI and WC are traditional indicators of obesity. Kurth et al. analysed data from 39,553 female participants and found that BMI was a significant risk indicator for overall stroke and for ischaemic varieties of stroke [26]. Each unit rises in BMI was related to an increase of 6% in the odds of total, ischaemic, and haemorrhaging stroke, as revealed in prospective research including 21,000 males in the US [36]. A cross-sectional study of 21,749,261 Korean participants by Cho et al. showed a linear positive correlation between WC and the prevalence of ischaemic stroke [37]. Despite growing evidence that these traditional indicators of obesity are associated with stroke, the obesity paradox persists [38]. The underlying cause of the controversy may be attributed in part to the limitations of traditional indicators, which do not differentiate between fat mass and muscle mass [15–17]. WWI, a new obesity indicator, combines the benefits of WC while weakening the association with BMI, accurately indicating central obesity regardless of body weight. Studies have shown that central obesity is strongly associated with the risk of cardiovascular disease [39]. WWI as an indicator of central obesity, may more accurately reflect risk factors associated with stroke, such as metabolic syndrome, diabetes, and hypertension [40–42]. WWI can also be used to evaluate fat and muscle mass regardless of BMI category [21]. As a result, WWI might serve as a more comprehensive and accurate measure of obesity, and it may more accurately show the correlation that exists between obesity and stroke. WWI was found to be the strongest predictor of a wide range of diseases in recent studies, outperforming BMI and WC [23, 43]. Similar to our findings, we suggest that the WWI may be an independent risk factor for stroke. Different from BMI, which focuses only on weight in relation to height, WWI also considers WC, which helps to capture the risk of central obesity. Second, WWI provides a more direct, simple, and specific way to assess central obesity than other metrics associated with fat distribution, such as the waist-to-hip ratio (WHR). And WWI is applicable to different races and populations and may be more stable and reliable, especially in cross-racial or multicenter studies [44]. In summary, WWI is simple to calculate, economical, and practical, with superior performance in predicting disease risk, and deserves the attention of health care professionals.
Several potential mechanisms could account for this positive correlation between the WWI and stroke. First, the increased WWI might be a reflection of malfunction in adipose tissue, thereby promoting the production and release of various proinflammatory cytokines [45]. These cytokines are involved in all stages of the formation, progression, erosion, and rupture of atherosclerotic plaques, which leads to thrombo-embolic events [46–48]. Second, central obesity can increase oxidative stress in individuals, which is closely associated with the development of atherosclerosis [49, 50]. Adipose tissue releases more reactive oxygen species (ROS) in individuals with obesity [51]. Excess ROS reduces the bioavailability of nitric oxide (NO), and superoxide readily reacts with NO to produce harmful hydrogen peroxide, which ultimately results in malfunction of endothelial cells [52]. Moreover, the increase in ROS promotes the oxidation of low-density lipoprotein in atherosclerotic lesions, which promotes immune responses in endothelial cells, including elevated levels of adhesion molecule expression, macrophage migration, and the formation of lipid-containing macrophages [53, 54]. These processes exacerbate damage to the vascular endothelium. Third, other disease states that coexist with obesity, such as impaired glucose tolerance, hypertriglyceridaemia, and high blood pressure, also increase the risk of stroke [55].
The strength of this study is that it was based on NHANES data, which were collected using a stratified multistage probability sampling strategy, thus making the study more reliable and representative. Second, we conducted a subgroup analysis to further clarify the association of WWI with stroke in different population settings. Finally, we adjusted for confounding factors including age, sex, race, education level, alcohol consumption, smoking, diabetes, high blood pressure, coronary heart disease, cancer, PIR, triglycerides, TC, HDL-C, and LDL-C to lessen the impact of confounding and obtain more reliable findings. Nevertheless, this research also presents certain constraints. The causal association between WWI and stroke could not be determined due to limitations inherent in the cross-sectional study design. Furthermore, the stroke inclusion criteria relied on self-reported stroke history, and subtypes of stroke were not clear. We were unable to further assess the association between different stroke subtypes and WWI. And the average age of the participants was 49.32 ± 17.42 years, at this age the risk and prevalence of stroke are low. Moreover, even if some potential confounders had been adjusted for, the effect of other potential confounders could not be completely excluded. However, the existing link between WWI and stroke was robust enough that it was unlikely to be greatly altered by confounders that had not been considered.
Conclusion
The results of this investigation indicated that a greater WWI was associated with an increased risk of stroke. This finding may provide new insights for the future of stroke prevention and treatment. However, higher-quality prospective studies are needed to corroborate our results.
Acknowledgements
We would like to thank all participants in this study.
Abbreviations
- WWI
Weight-adjusted waist index
- BMI
Body mass index
- WC
Waist circumference
- NHANES
National health and nutrition examination survey
- NCHS
National center for health statistics
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- TC
Total cholesterol
- PIR
Ratio of family income to poverty
- CDC
Centers for disease control and prevention
- WHR
Waist-to-hip ratio
- ROS
Reactive oxygen species
- NO
Nitric oxide
Authors’ contributions
Jiayi Ye, Xinrong Chen, and Zhe Yin designed the research. Jiayi Ye, Yanjie Hu, Xinrong Chen, Xingzhu Yuan, and Liping Huang collected, analyzed the data, and drafted the manuscript. Jiayi Ye, Yanjie Hu, and Ka Li revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Natural Science Foundation of China Regional Joint Project (No. U22A20334), Chengdu Science and Technology Bureau (No. 2022-YF05-01440-SN), and Chengdu Municipal Health Commission (No. 2022040).
Availability of data and materials
Publicly available datasets were analyzed in this study. These data can be found here: https://www.cdc.gov/nchs/nhanes/.
Declarations
Ethics approval and consent to participate
The portions of this study involving human participants, human materials, or human data were conducted in accordance with the Declaration of Helsinki and were approved by the NCHS Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiayi Ye and Yanjie Hu these authors have contributed equally to this article.
References
- 1.Kuriakose D, Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int J Mol Sci. 2020;21(20):7609. doi: 10.3390/ijms21207609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. [DOI] [PMC free article] [PubMed]
- 3.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott M, Brown DL, Chervin RD. Sleep disorders and the risk of stroke. Expert Rev Neurother. 2018;18(7):523–531. doi: 10.1080/14737175.2018.1489239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, Fisher M, Pandian J, Lindsay P. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29. doi: 10.1177/17474930211065917. [DOI] [PubMed] [Google Scholar]
- 6.Owolabi MO, Thrift AG, Mahal A, Ishida M, Martins S, Johnson WD, Pandian J, Abd-Allah F, Yaria J, Phan HT, et al. Primary stroke prevention worldwide: translating evidence into action. Lancet Public Health. 2022;7(1):e74–e85. doi: 10.1016/S2468-2667(21)00230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren Z, Fu X. Stroke risk factors in United States: an analysis of the 2013–2018 national health and nutrition examination survey. Int J Gen Med. 2021;14:6135–6147. doi: 10.2147/IJGM.S327075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanovski JA. Obesity: trends in underweight and obesity - scale of the problem. Nat Rev Endocrinol. 2018;14(1):5–6. doi: 10.1038/nrendo.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worldwide trends in body-mass index underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–96. [DOI] [PMC free article] [PubMed]
- 11.Liu W, Liu J, Shao S, Lin Q, Wang C, Zhang X, Tu J, Wang J, Ning X. Obesity at a young age is associated with development of diabetes mellitus: a prospective cohort study in rural China. Postgrad Med. 2020;132(8):709–713. doi: 10.1080/00325481.2020.1778383. [DOI] [PubMed] [Google Scholar]
- 12.Vusirikala A, Thomas T, Bhala N, Tahrani AA, Thomas GN, Nirantharakumar K. Impact of obesity and metabolic health status in the development of non-alcoholic fatty liver disease (NAFLD): a United Kingdom population-based cohort study using the health improvement network (THIN) BMC Endocr Disord. 2020;20(1):96. doi: 10.1186/s12902-020-00582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 14.Freisling H, Arnold M, Soerjomataram I, O'Doherty MG, Ordóñez-Mena JM, Bamia C, Kampman E, Leitzmann M, Romieu I, Kee F, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer. 2017;116(11):1486–1497. doi: 10.1038/bjc.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonopoulos AS, Oikonomou EK, Antoniades C, Tousoulis D. From the BMI paradox to the obesity paradox: the obesity-mortality association in coronary heart disease. Obes Rev. 2016;17(10):989–1000. doi: 10.1111/obr.12440. [DOI] [PubMed] [Google Scholar]
- 16.Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36 Suppl 2(Suppl 2):S276–281. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17(5):374–380. doi: 10.1016/j.cardfail.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Shieh A, Karlamangla AS, Karvonen-Guttierez CA, Greendale GA. Menopause-related changes in body composition are associated with subsequent bone mineral density and fractures: study of women’s health across the nation. J Bone Miner Res. 2023;38(3):395–402. doi: 10.1002/jbmr.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma M, Liu X, Jia G, Geng B, Xia Y. The association between body fat distribution and bone mineral density: evidence from the US population. BMC Endocr Disord. 2022;22(1):170. doi: 10.1186/s12902-022-01087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. 2018;8(1):16753. doi: 10.1038/s41598-018-35073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim NH, Park Y, Kim NH, Kim SG. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 2021;50(3):780–786. doi: 10.1093/ageing/afaa208. [DOI] [PubMed] [Google Scholar]
- 22.Qin Z, Chang K, Yang Q, Yu Q, Liao R, Su B. The association between weight-adjusted-waist index and increased urinary albumin excretion in adults: a population-based study. Front Nutr. 2022;9:941926. doi: 10.3389/fnut.2022.941926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie F, Xiao Y, Li X, Wu Y. Association between the weight-adjusted-waist index and abdominal aortic calcification in United States adults: results from the national health and nutrition examination survey 2013–2014. Front Cardiovasc Med. 2022;9:948194. doi: 10.3389/fcvm.2022.948194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao J, Zhang Y, Tan C, Tan W. Associations between weight-adjusted waist index and fractures: a population-based study. J Orthop Surg Res. 2023;18(1):290. doi: 10.1186/s13018-023-03776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Shi W, Ding Z, Park J, Wu S, Zhang J. Association between weight-adjusted-waist index and heart failure: results from National Health and Nutrition Examination Survey 1999–2018. Front Cardiovasc Med. 2022;9:1069146. doi: 10.3389/fcvm.2022.1069146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurth T, Gaziano JM, Rexrode KM, Kase CS, Cook NR, Manson JE, Buring JE. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation. 2005;111(15):1992–1998. doi: 10.1161/01.CIR.0000161822.83163.B6. [DOI] [PubMed] [Google Scholar]
- 27.Borrud L, Chiappa MM, Burt VL, Gahche J, Zipf G, Johnson CL, Dohrmann SM. National health and nutrition examination survey: national youth fitness survey plan, operations, and analysis, 2012. Vital Health Stat 2. 2014;163:1–24. [PubMed] [Google Scholar]
- 28.Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National health and nutrition examination survey: sample design, 2007–2010. Vital Health Stat 2. 2013;160:1–23. [PubMed] [Google Scholar]
- 29.Xie R, Zhang Y. Association between 19 dietary fatty acids intake and rheumatoid arthritis: results of a nationwide survey. Prostaglandins Leukot Essent Fatty Acids. 2023;188:102530. doi: 10.1016/j.plefa.2022.102530. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Yang L, Zhang Y, Liu J. Relationship between circadian syndrome and stroke: a cross-sectional study of the national health and nutrition examination survey. Front Neurol. 2022;13:946172. doi: 10.3389/fneur.2022.946172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Chen X, Cheng H, Zhang L. Dietary copper intake and risk of stroke in adults: a case-control study based on national health and nutrition examination survey 2013–2018. Nutrients. 2022;14(3):409. doi: 10.3390/nu14030409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie R, Zhang Y. Associations between dietary flavonoid intake with hepatic steatosis and fibrosis quantified by VCTE: evidence from NHANES and FNDDS. Nutr Metab Cardiovasc Dis. 2023;33(6):1179–1189. doi: 10.1016/j.numecd.2023.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Ding C, Shi Y, Li J, Li M, Hu L, Rao J, Liu L, Zhao P, Xie C, Zhan B, et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in China: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2022;32(5):1210–1217. doi: 10.1016/j.numecd.2022.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Cai S, Zhu T, Ding Y, Cheng B, Zhang A, Bao Q, Sun J, Li M, Liu X, Wang S. The relationship between the weight-adjusted-waist index and left ventricular hypertrophy in Chinese hypertension adults. Hypertens Res. 2023;46(1):253–260. doi: 10.1038/s41440-022-01075-z. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Qie R, Qin P, Zhang D, Guo C, Zhou Q, Tian G, Liu D, Chen X, Liu L, et al. Association of weight-adjusted-waist index with incident hypertension: the Rural Chinese cohort study. Nutr Metab Cardiovasc Dis. 2020;30(10):1732–1741. doi: 10.1016/j.numecd.2020.05.033. [DOI] [PubMed] [Google Scholar]
- 36.Kurth T, Gaziano JM, Berger K, Kase CS, Rexrode KM, Cook NR, Buring JE, Manson JE. Body mass index and the risk of stroke in men. Arch Intern Med. 2002;162(22):2557–2562. doi: 10.1001/archinte.162.22.2557. [DOI] [PubMed] [Google Scholar]
- 37.Cho JH, Rhee EJ, Park SE, Kwon H, Jung JH, Han KD, Park YG, Park HS, Kim YH, Yoo SJ, et al. The risk of myocardial infarction and ischemic stroke according to waist circumference in 21,749,261 Korean adults: a nationwide population-based study. Diabetes Metab J. 2019;43(2):206–221. doi: 10.4093/dmj.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forlivesi S, Cappellari M, Bonetti B. Obesity paradox and stroke: a narrative review. Eat Weight Disord. 2021;26(2):417–423. doi: 10.1007/s40519-020-00876-w. [DOI] [PubMed] [Google Scholar]
- 39.Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fahed G, Aoun L, BouZerdan M, Allam S, BouZerdan M, Bouferraa Y, Assi HI. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23(2):786. doi: 10.3390/ijms23020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorimachi H, Omote K, Omar M, Popovic D, Verbrugge FH, Reddy YNV, Lin G, Obokata M, Miles JM, Jensen MD, et al. Sex and central obesity in heart failure with preserved ejection fraction. Eur J Heart Fail. 2022;24(8):1359–1370. doi: 10.1002/ejhf.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nurdiantami Y, Watanabe K, Tanaka E, Pradono J, Anme T. Association of general and central obesity with hypertension. Clin Nutr. 2018;37(4):1259–1263. doi: 10.1016/j.clnu.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Cao S, Hu X, Shao Y, Wang Y, Tang Y, Ren S, Li X. Relationship between weight-adjusted-waist index and erectile dysfunction in the United State: results from NHANES 2001–2004. Front Endocrinol (Lausanne) 2023;14:1128076. doi: 10.3389/fendo.2023.1128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JY, Choi J, Vella CA, Criqui MH, Allison MA, Kim NH. Associations between weight-adjusted waist index and abdominal fat and muscle mass: multi-ethnic study of atherosclerosis. Diabetes Metab J. 2022;46(5):747–755. doi: 10.4093/dmj.2021.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117(25):3238–3249. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 47.Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. 2016;109(12):708–715. doi: 10.1016/j.acvd.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 49.Čolak E, Pap D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J Med Biochem. 2021;40(1):1–9. doi: 10.5937/jomb0-24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Na IJ, Park JS, Park SB. Association between abdominal obesity and oxidative stress in Korean adults. Korean J Fam Med. 2019;40(6):395–398. doi: 10.4082/kjfm.18.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 53.Azumi H, Inoue N, Ohashi Y, Terashima M, Mori T, Fujita H, Awano K, Kobayashi K, Maeda K, Hata K, et al. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: important role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2002;22(11):1838–1844. doi: 10.1161/01.atv.0000037101.40667.62. [DOI] [PubMed] [Google Scholar]
- 54.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84(4):1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 55.Wiklund P, Toss F, Weinehall L, Hallmans G, Franks PW, Nordström A, Nordström P. Abdominal and gynoid fat mass are associated with cardiovascular risk factors in men and women. J Clin Endocrinol Metab. 2008;93(11):4360–4366. doi: 10.1210/jc.2008-0804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://www.cdc.gov/nchs/nhanes/.