Abstract
Background
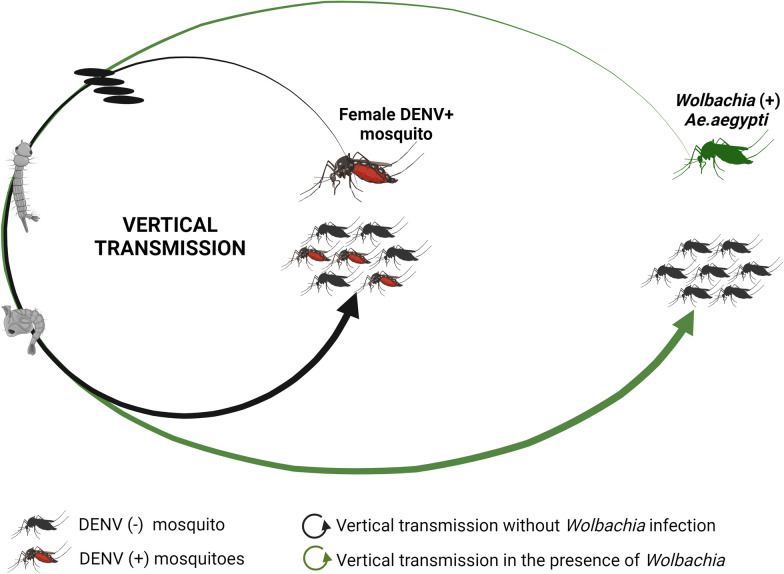
Dengue virus serotypes (DENV-1 to -4) can be transmitted vertically in Aedes aegpti mosquitoes. Whether infection with the wMel strain of the endosymbiont Wolbachia can reduce the incidence of vertical transmission of DENV from infected females to their offspring is not well understood.
Methods
A laboratory colony of Vietnamese Ae. aegypti, both with and without wMel infection, were infected with DENV-1 by intrathoracic injection (IT) to estimate the rate of vertical transmission (VT) of the virus. VT in the DENV-infected mosquitoes was calculated via the infection rate estimation from mosquito pool data using maximum likelihood estimation (MLE).
Results
In 6047 F1 Vietnamese wild-type Ae. aegypti, the MLE of DENV-1 infection was 1.49 per 1000 mosquitoes (95% confidence interval [CI] 0.73–2.74). In 5500 wMel-infected Ae. aegypti, the MLE infection rate was 0 (95% CI 0–0.69). The VT rates between mosquito lines showed a statistically significant difference.
Conclusions
The results reinforce the view that VT is a rare event in wild-type mosquitoes and that infection with wMel is effective in reducing VT.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-023-05921-y.
Keywords: Vertical transmission, Dengue virus, Mosquitoes, Aedes aegypti, Wolbachia, wMel
Background
Dengue is a mosquito-borne viral infection caused by one of four dengue virus serotypes (DENV-1 to -4) that is endemic in many tropical and sub-tropical countries [1]. The global incidence of dengue has increased dramatically in the last 50 years, with approximately 50–100 million symptomatic infections and 20,000 deaths reported annually in over 125 countries [2, 3]. DENV is transmitted between humans through the bite of an infected female Aedes sp. mosquito (Ae. aegypti or Ae. albopictus). However, DENV can also be transmitted vertically, from the DENV-infected female mosquito to her offspring during follicle development or oviposition [4–6]. This latter transmission mode is hypothesized to contribute to DENV persistence in the mosquito population [7]. A sign of vertical transmission (VT) of DENV in a mosquito populations is the presence of infected male mosquitoes (which do not blood feed) and the presence of viruses in immature forms of mosquitoes of any sex. VT is a rare event in nature [8–11] but it has been observed in laboratory studies in both Ae. aegypti and Ae. albopictus [12, 13]. Several studies have also shown that VT is influenced by a number of factors, including mosquito-rearing temperature, mosquito strain and virus strain [14–18].
Although there are two licensed dengue vaccines, vector control has been the mainstay of dengue control efforts for decades. However, it is obvious that existing vector control methods have not removed the public health burden of dengue in any endemic country. A new approach that involves the insect endosymbiont Wolbachia (wMel or wAlbB strains) is being applied to render Ae. aegypti populations much less competent at transmitting DENV between humans [19–21]. Wolbachia inhibits virus replication in mosquito cells via multiple mechanisms, including altering the intracellular environment, activating the innate immune system and interacting with the cell machinery involved in RNA virus infection [22]. Multiple epidemiological studies, including a cluster randomized trial, have shown a large decrease in dengue cases in communities with wMel-Wolbachia-treated mosquitoes, demonstrating that wMel introgression is an effective disease control measure [23, 24].
We previously found a very low DENV VT rate (0.23%) among wild-type (Wt) Ae. aegypti orally infected with DENV after feeding on viremic blood from dengue patients [9]. To assess whether wMel would eliminate VT of DENV, we established an experimental model system to measure VT in the presence and absence of wMel infection.
Methods
Mosquito lines and rearing
The wMel-infected Ae. aegypti population (wMel-Ae. aegypti; Vietnamese genetic background) was generated by backcrossing, as previously described [25]. Generations G59 to G61 of colonized wMel-Ae. aegypti and generations F54 and F55 of colonized Wt Ae. aegypti were used in this study, as previously described [9, 26]. The presence of wMel in each wMel-Ae. agypti generation was confirmed by testing [27]. The mosquitoes were reared and maintained under laboratory conditions, at 26–28 °C, 65–85% relative humidity, and a 12:12-h light/dark cycle, with access to 10% sucrose solution ad libitum.
Generating DENV-infected F0 mosquitoes
Both Wt and wMet-infected adult female mosquitoes, aged 2–3 days, were injected intrathoracically with 1 µl of solution containing DENV-1 grown in cell culture (105 pfu/ml; Genbank Accession Number: FJ432735). DENV-1 was used to establish infection based on the findings of our previous study which indicated that this serotype was less inhibited by wMel than the other three DENV serotypes [28]. Ten injected females were then kept in cups containing 10 male mosquitoes (female:male ratio = 1:1) where they were maintained on sucrose for 10 days.
A human blood meal (non-infectious blood provided via a membrane feeder) was provided to surviving F0 females on day 10 post-injection [28]. After 30 min of blood feeding, fully engorged females were isolated and placed into separate cups (isofemales) containing wet cotton balls for oviposition. Sugar (a piece of cotton soaked in 10% sucrose) was provided for 14 days. On day 14 post non-infectious blood meal, individual F0 females were harvested for testing of their DENV infection status.
Hatching and harvesting F1 mosquitoes
F1 eggs were collected at 5–7 days after the F0 females had taken their non-infectious blood meal and placed in trays filled with fresh water; the trays were kept in incubators at 28 °C under a 12/12-h light/dark cycle. Each tray was provided with one 100 mg tablet of fish food. The larval density was maintained at approximately 3300–3500 larvae per 1.5 l of water. F1 mosquitoes were individually stored and sorted by sex within cohorts originating from the same mother.
F0 and F1 mosquitoes homogenized in a TissueLyser II instrument (Qiagen, Hilden, Germany) at 30 Hz for 2–5 min. Each F0 mosquito homogenate was stored individually, while F1 mosquito homogenates (50 µl from each sample) were pooled before conducting the reverse transcriptase PCR (RT-PCR) assays to detect the presence of Wolbachia [27] and DENV [29]. The positive pooled samples were then un-pooled to determine the number of infected individuals using the remaining volume of mosquito homogenate.
Estimating VT of DENV-1 in colonized Ae. aegypti with and without wMel infection
To estimate the VT of DENV-1 in a large number of F1 mosquitoes, we utilized the maximum likelihood estimate (MLE) method. This method estimated the proportion of DENV-infected individuals in pooled samples, defined as the infection rate most likely observed given the test results and an assumed probabilistic model (binomial distribution of infected individuals in a positive pool) [30–35]. To account for biases, we utilized bias-corrected likelihood methods and calculated a skew-corrected score confidence interval (95% CI) [36]. The infection rate was reported as the number of infected mosquitoes per 1000 individuals. In addition, to perform comparisons of CIs, we utilized the Wilson score-based interval of the Newcombe method, which relies on exact calculations of coverage probabilities [37]. This approach allowed us to assess the statistical significance of the differences in infection rates between populations.
To accurately estimate the proportion of infected individuals in a population using MLE, determining the appropriate pool size is crucial to minimize the likelihood of false-negative results. In this study, the pool size was examined using a sample-media pooling approach, adapted from the Clinical and Laboratory Standards Institute (CLSI) document EP12 (CLSI EP12 [38]). The positive percent agreement (PPA) and negative percent agreement (NPA) values were calculated for different pool sizes. The highest PPA value (96%) was obtained with a pool size of four mosquitoes, whereas a pool size of eight mosquitoes showed a PPA of 89% (95% CI 0.8–0.9) and a pool size of sixteen showed only 68% agreement [39]. All three sizes showed 100% agreement fir NPA values. Therefore, a pool size of eight was selected [39].
Results and discussion
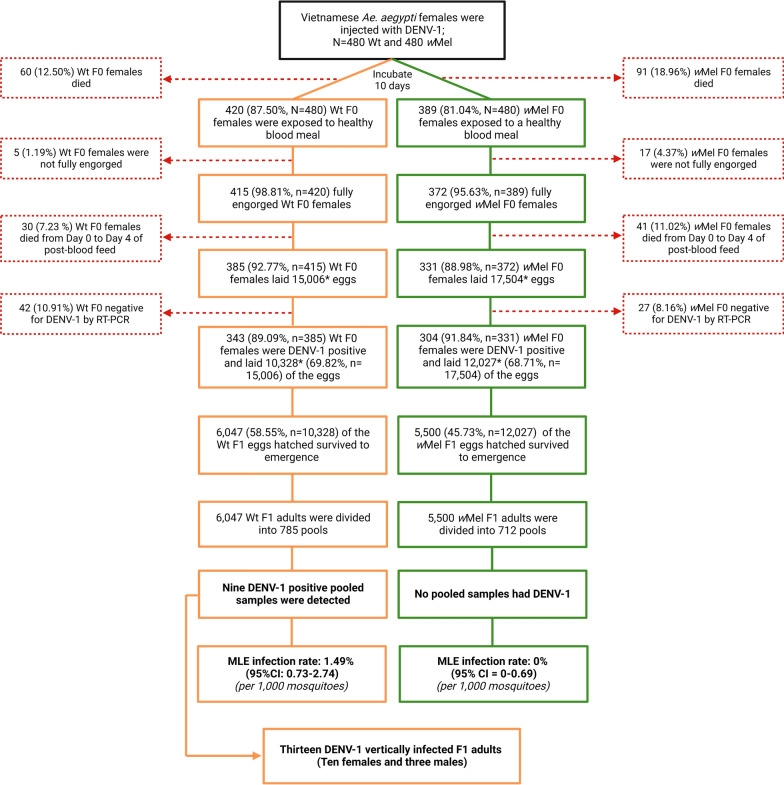
Our study aimed to measure whether VT was less likely to occur in wMel-infected Ae. aegypti mosquitoes (wMel-Ae. aegypti) versus their Wt counterparts. The estimation of VT was conducted in 480 wMel-Ae. aegypti females and in 480 Wt females (Fig. 1). Ten days after intrathoracic (IT) inoculation of DENV-1, 420 (87.50%, N = 480) Wt and 389 (81.04%, N = 480) wMel-Ae. aegypti mosquitoes survived and were given a non-infectious blood meal. Between 5 and 7 days later, approximately 10,328 eggs were collected from 343 (89.09%, n = 385) virus-infected Wt F0 females (out of 385 Wt F0 females that survived and laid eggs), and approximately 12,027 F1 eggs were collected from 304 (91.84%, n = 331) virus-infected wMel-Ae. aegypti F0 females (out of 331 wMel-Ae. aegypti F0 females that survived and laid eggs). In both mosquito lines, high parental infection rates of DENV-1 were observed, with 89.09% (343/385, surviving mosquitoes only) of Wt mosquitoes infected and 91.84% (304/331, surviving mosquitoes only) of wMel-Ae. aegypti mosquitoes infected, respectively (Fig. 1). Consistent with previous findings, IT injection resulted in a higher prevalence of DENV-1 compared to oral feeding [9, 40]. DENV-1 RNA concentrations in whole bodies of F0 Wt and wMel-Ae. aegypti were comparable and high: 7.7 (95% CI 7.63–7.72) and 7.6 (95% CI 7.57–7.67) log10 copies/ml, respectively (Fig. 2). The DENV copy numbers detected in the whole bodies of each mosquito line were not significantly different (Mann–Whitney test: U = 48216, P = 0.09, 95% CI = -0.01-0.13), possibly due to the IT inoculation with a large inoculum of the virus.
Fig. 1.
Flowchart of vertical transmission estimation of DENV-1 in Wt and wMel-infected Ae. aegypti with a Vietnamese background. Shown is the fate of mosquitoes as they were processed in order to determine the frequency of vertical transmission of F0 females after being infected by microinjection of DENV-1 (strain FJ432735). The orange boxes represent Wt Ae. aegypti, the green boxes represent wMel-Ae. aegypti and the red boxes indicate excluded samples. The asterisk (*) indicates the numbers are estimated. DENV-1, Dengue virus serotype 1; MLE, maximum likelihood estimate of infection rate; RT-PCR, reverse transcriptase-PCR; wMel, Wolbachia strain wMel-infected Ae. aegypti; Wt, wild type
Fig. 2.
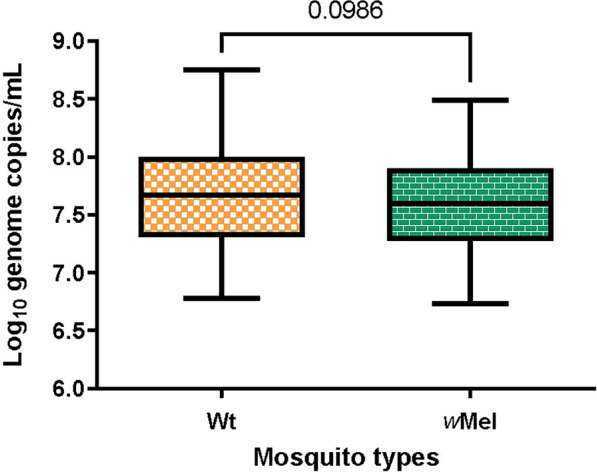
DENV-1 copy numbers in F0 female Ae. aegypti with and without wMel infection. The infectivity of DENV-1 by intrathoracic injection in F0 females represents log10 viral genome copy numbers. Wt mosquitoes are shown in orange and wMel-mosquitoes are shown in green. wMel, Wolbachia strain wMel-infected Ae. aegypti; Wt, wild type
A total of 6047 F1 adults emerged from the approximately 10,328 eggs collected from 343 virus-infected Wt F0 females. Similarly, the approximately 12,027 F1 eggs from 304 virus-infected wMel-Ae. aegypti F0 females completed their development, providing 5500 wMel-Ae. aegypti F1 adults (Fig. 1). All Wt F1 adult mosquitoes were grouped into 785 pools, and 5500 wMel-Ae. aegypti F1 adult mosquitoes were grouped into 712 pools (for details, see Additional file 1: Table S1). These pools were tested for DENV-1, and nine positive pools were detected from the Wt group, while no positive pools were found in the wMel-Ae. aegypti group. The MLE for the VT rate of DENV-1 in Wt mosquitoes was estimated to be 1.49 (95% CI 0.73–2.74) per 1000 adults. However, in wMel-Ae. aegypti mosquitoes, the transmission rate was estimated to be zero (95% CI 0–0.69). The observed difference in the infection rates between the Wt group and the wMel-Ae. aegypti group is 1.49 (95% CI 0.67–5.05). As the 95% CI is entirely above 0, the null hypothesis of no difference (H0: Wt − wMel = 0) can be rejected at a significance level of P < 0.05. Consequently, it can be concluded that the VT rate of the Wt group is significantly higher than that of the wMel group.
Thirteen DENV-1 infected Wt F1 individuals were identified from the nine positive pools, of which 10 were females. When assessing the frequency of mothers transmitting the virus to their progeny and determining the proportion of progeny born to DENV-infected mothers [9] based on positive pools, we observed a slight increase in rates compared to those found in field-collected mosquitoes [9]. Our estimates indicated that for every 343 DENV-infected Wt Ae. aegypti female mosquitoes that survived and laid eggs, 11 individuals (3.21%) transmitted the virus to their offspring. However, experimentally we found that only 0.13% of progeny born to DENV-infected mothers would be infected with DENV-1(13 out of 10,328 eggs acquiring the infection). This proportion increased to 0.21% when calculated based on 6047 F1 adults. The frequency of mothers transmitting the virus to any of their progeny in Wt Ae. aegypti (3.21%) compared to the frequency identified in field-collected Ae. aegypti (2.43%) using patient-derived blood meals [9] might be attributed to the direct introduction of virus into systemic tissues via IT inoculation.
The MLE of DENV-1 infection rate observed in wMel-infected Ae. aegypti was comparable to that found in wMel-infected Ae. aegypti from Brazil [40]. In our study, we optimized the conditions to increase the probability of a VT event in Wt Ae. aegypti, by employing IT inoculation of virus and a long extrinsic incubation period. Furthermore, our study was conducted with a large sample size, approximately 11,547 total F1 Ae. aegypti (6047 Wt and 5500 wMel-Ae. aegypti). Despite these advantages, the VT event was not recorded in wMel-infected Ae. aegypti. The absence of DENV infection in wMel-infected F1 mosquitoes and the observed difference in VT rates between mosquito lines provide evidence that wMel is effective in reducing VT. The ability of wMel to decrease DENV replication in wMel-carrying mosquitoes has been used to reduce the incidence of dengue through the deployment of Wolbachia mosquitoes in endemic areas [23, 24]. The ability of wMel to reduce the VT of DENV can be attributed to various mechanisms, including resource competition, immune system activation and interference with viral replication in the reproductive tissues of mosquitoes [41–44]. These mechanisms are recognized for their ability to reduce the replication of DENV and could therefore limit its transmission from one generation to the next generation. The capacity of wMel to diminish dengue transmission in both horizontal and vertical modes of transmission is critical in mitigating the incidence of dengue, ultimately decreasing the overall disease burden.
Conclusions
The results of the present study support the belief that VT is a rare phenomenon. wMel infection reduces VT in wMel-carrying Ae. aegypti population.
Supplementary Information
Additional file 1: Table S1. Number of F1 adults distributed in each pooled sample.
Acknowledgements
We thank the insectary staff in OUCRU for rearing and maintaining the large numbers of mosquitoes in the project.
Abbreviations
- DENV
Dengue virus
- IT
Intrathoracic
- MLE
Maximum likelihood estimate (of infection rate)
- NPA
Negative percent agreement
- RT-PCR
Reverse transcriptase PCR
- PPA
Positive percent agreement
- VT
Vertical transmission
- wMel
Wolbachia pipientis, wMel strain
- Wt
Wild type
Author contributions
KDTH, DSG, SY and CPS participated in the study design. KDTH, VTT, LTV, DLT, VTTN, GNT, THTX and NVT performed the experiments. KDTH and DSG coordinated data management. KDTH analyzed the data. CPS contributed with funding and reagents. KDTH and CPS wrote the report. All authors read and approved the final report.
Funding
This study was supported by the World Mosquito Program (WMP).
Availability of data and materials
All relevant data generated or analyzed during this study are included in this published article and its additional files.
Declarations
Ethics approval and consent to participate
All work was conducted at the Hospital for Tropical Diseases (HTD), Ho Chi Minh City (HCMC), Vietnam. The enrolment of healthy volunteers to provide non-infectious blood meals to Wt Ae. aegypti and wMel-Ae. aegypti mosquitoes was approved by the Ethics Committee HTD EC and OxTREC (approval nos. HTD EC CS/NÐ/14/12 and OxTREC 45–14). Informed consent was obtained from all participants by trained study staff.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–23. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messina JP, Brady OJ, Pigott DM, Brownstein JS, Hoen AG, Hay SI. A global compendium of human dengue virus occurrence. Sci Data. 2014;1:140004. doi: 10.1038/sdata.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams B, Boots M. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics. 2010;2:1–10. doi: 10.1016/j.epidem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Haddow AD, Guzman H, Popov VL, Wood TG, Widen SG, Haddow AD, et al. First isolation of Aedes flavivirus in the Western hemisphere and evidence of vertical transmission in the mosquito Aedes (Stegomyia) albopictus (Diptera: Culicidae) Virology. 2013;440:134–9. doi: 10.1016/j.virol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Arunachalam N, Tewari SC, Thenmozhi V, Rajendran R, Paramasivan R, Manavalan R, et al. Natural vertical transmission of dengue viruses by Aedes aegypti in Chennai, Tamil Nadu India. Indian J Med Res. 2008;127:395–7. [PubMed] [Google Scholar]
- 7.Khin MM, Than KA. Transovarial transmission of dengue 2 virus by Aedes aegypti in nature. Am J Trop Med Hyg. 1983;32:590–4. doi: 10.4269/ajtmh.1983.32.590. [DOI] [PubMed] [Google Scholar]
- 8.Grunnill M, Boots M. How important is vertical transmission of dengue viruses by mosquitoes (Diptera: Culicidae)? J Med Entomol. 2016;53:1–19. doi: 10.1093/jme/tjv168. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves DDS, Hue KDT, Thuy VT, Tuyet NV, Thi GN, Thi Thuy VH, et al. Assessing the vertical transmission potential of dengue virus in field-reared Aedes aegypti using patient-derived blood meals in Ho Chi Minh City, Vietnam. Parasit Vectors. 2020;13:468. doi: 10.1186/s13071-020-04334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thangamani S, Huang J, Hart CE, Guzman H, Tesh RB. Vertical transmission of Zika virus in Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2016;95:1169–73. doi: 10.4269/ajtmh.16-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Costa CF, da Silva AV, do Nascimento VA, de Souza VC, Monteiro D, Terrazas WCM, et al. Evidence of vertical transmission of Zika virus in field-collected eggs of Aedes aegypti in the Brazilian Amazon. PLoS Negl Trop Dis. 2018;12:e0006594. doi: 10.1371/journal.pntd.0006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi V, Mourya DT, Sharma RC. Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2002;67:158–61. doi: 10.4269/ajtmh.2002.67.158. [DOI] [PubMed] [Google Scholar]
- 13.Gunther J, Martinez-Munoz JP, Perez-Ishiwara DG, Salas-Benito J. Evidence of vertical transmission of dengue virus in two endemic localities in the state of Oaxaca, Mexico. Intervirology. 2007;50:347–52. doi: 10.1159/000107272. [DOI] [PubMed] [Google Scholar]
- 14.Farnesi LC, Martins AJ, Valle D, Rezende GL. Embryonic development of Aedes aegypti (Diptera: Culicidae): influence of different constant temperatures. Mem Inst Oswaldo Cruz. 2009;104:124–6. doi: 10.1590/S0074-02762009000100020. [DOI] [PubMed] [Google Scholar]
- 15.Hardy JL, Rosen L, Kramer LD, Presser SB, Shroyer DA, Turell MJ. Effect of rearing temperature on transovarial transmission of St. Louis encephalitis virus in mosquitoes. Am J Trop Med Hyg. 1980;29:963–8. doi: 10.4269/ajtmh.1980.29.963. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell CJ, Miller BR. Vertical transmission of dengue viruses by strains of Aedes albopictus recently introduced into Brazil. J Am Mosq Control Assoc. 1990;6:251–3. [PubMed] [Google Scholar]
- 17.Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Adv Virus Res. 2003;60:187–232. doi: 10.1016/S0065-3527(03)60006-0. [DOI] [PubMed] [Google Scholar]
- 18.Buckner EA, Alto BW, Lounibos LP. Larval temperature-food effects on adult mosquito infection and vertical transmission of dengue-1 virus. J Med Entomol. 2016;53:91–8. doi: 10.1093/jme/tjv145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugo LE, Rasic G, Maynard AJ, Ambrose L, Liddington C, Thomas CJE, et al. Wolbachia wAlbB inhibit dengue and Zika infection in the mosquito Aedes aegypti with an Australian background. PLoS Negl Trop Dis. 2022;16:e0010786. doi: 10.1371/journal.pntd.0010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad NA, Mancini MV, Ant TH, Martinez J, Kamarul GMR, Nazni WA, et al. Wolbachia strain wAlbB maintains high density and dengue inhibition following introduction into a field population of Aedes aegypti. Philos Trans R Soc Lond B Biol Sci. 1818;376:20190809. doi: 10.1098/rstb.2019.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, Brown-Kenyon J, et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2019;3:1547. doi: 10.12688/gatesopenres.13061.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsey ARI, Bhattacharya T, Newton ILG, Hardy RW. Conflict in the intracellular lives of endosymbionts and viruses: a mechanistic look at Wolbachia-mediated pathogen-blocking. Viruses. 2018;10:141. doi: 10.3390/v10040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med. 2021;384:2177–86. doi: 10.1056/NEJMoa2030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dos Santos GR, Durovni B, Saraceni V, Souza Riback TI, Pinto SB, Anders KL, et al. Estimating the effect of the wMe release programme on the incidence of dengue and chikungunya in Rio de Janeiro, Brazil: a spatiotemporal modelling study. Lancet Infect Dis. 2022;22:1587–95. doi: 10.1016/S1473-3099(22)00436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrington LB, Tran BCN, Le NTH, Luong TTH, Nguyen TT, Nguyen PT, et al. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2018;115:361–366. doi: 10.1073/pnas.1715788115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyet MN, Duong TH, Trung VT, Nguyen TH, Tran CN, Long VT, et al. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2013;110:9072–9077. doi: 10.1073/pnas.1303395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser JE, De Bruyne JT, Iturbe-Ormaetxe I, Stepnell J, Burns RL, Flores HA, et al. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 2017;13:e1006751. doi: 10.1371/journal.ppat.1006751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson NM, Kien DT, Clapham H, Aguas R, Trung VT, Chau TN, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7:27937ra. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hepworth G. Estimation of proportions by group testing. PhD dissertation. Melbourne: University of Melbourne; 1999.
- 31.Walter SD, Hildreth SW, Beaty BJ. Estimation of infection rates in populations of organisms using pools of variable size. Am J Epidemiol. 1980;112:124–128. doi: 10.1093/oxfordjournals.aje.a112961. [DOI] [PubMed] [Google Scholar]
- 32.Biggerstaff B. Mosquito surveillance software. Atlanta: Division Vector-Borne Diseases, US Centers for Disease Control and Prevention; 2007. https://www.cdc.gov/mosquitoes/mosquito-control/professionals/MosqSurvSoft.h. Accessed May 2022.
- 33.Gu W, Lampman R, Novak RJ. Assessment of arbovirus vector infection rates using variable size pooling. Med Vet Entomol. 2004;18:200–4. doi: 10.1111/j.0269-283X.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 34.Hepworth G, Watson R. Debiased estimation of proportions in group testing. J R Stat Soc Ser C Appl Stat. 2009;58:105.
- 35.Gu W, Lampman R, Novak RJ. Problems in estimating mosquito infection rates using minimum infection rate. J Med Entomol. 2003;40:595–6. doi: 10.1603/0022-2585-40.5.595. [DOI] [PubMed] [Google Scholar]
- 36.Hepworth G, Biggerstaff BJ. Bias correction in estimating proportions by imperfect pooled testing. J Agric Biol Environ Stat. 2021;26:90–104. doi: 10.1007/s13253-020-00411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biggerstaff BJ. Confidence intervals for the difference of two proportions estimated from pooled samples. J Agric Biol Environ Stat. 2008;13:478–96. 10.1198/108571108X379055.
- 38.Garrett PE. EP12-user protocol for evaluation of qualitative test performance. 2. Wayne: Clinical and laboratory Standards Institute; 2021. [Google Scholar]
- 39.US Food and Drug Administration. Pooled sample testing and screening testing for COVID-19. 2020. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/pooled-sample-testing-and-screening-testing-covid-19. Accessed Oct 2021.
- 40.Pacidonio EC, Caragata EP, Alves DM, Marques JT, Moreira LA. The impact of Wolbachia infection on the rate of vertical transmission of dengue virus in Brazilian Aedes aegypti. Parasit Vectors. 2017;10:296. doi: 10.1186/s13071-017-2236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Y, Guo J, Li Y. Wolbachia wPip blocks Zika virus transovarial transmission in Aedes albopictus. Microbiol Spectr. 2022;10:e0263321. doi: 10.1128/spectrum.02633-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussain M, Zhang G, Leitner M, Hedges LM, Asgari S. Wolbachia RNase HI contributes to virus blocking in the mosquito Aedes aegypti. iScience. 2023;26:105836. doi: 10.1016/j.isci.2022.105836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stouthamer R, Breeuwer JA, Hurst GD. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- 44.Mejia AJ, Dutra HLC, Jones MJ, Perera R, McGraw EA. Cross-tissue and generation predictability of relative Wolbachia densities in the mosquito Aedes aegypti. Parasit Vectors. 2022;15:128. doi: 10.1186/s13071-022-05231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Number of F1 adults distributed in each pooled sample.
Data Availability Statement
All relevant data generated or analyzed during this study are included in this published article and its additional files.