Abstract
Although potassium channelopathies have been linked to a wide range of neurological conditions, the underlying pathogenic mechanism is not always clear, and a systematic summary of clinical manifestation is absent. Several neurological disorders have been associated with alterations of calcium-activated potassium channels (KCa channels), such as loss- or gain-of-function mutations, post-transcriptional modification, etc. Here, we outlined the current understanding of the molecular and cellular properties of three subtypes of KCa channels, including big conductance KCa channels (BK), small conductance KCa channels (SK), and the intermediate conductance KCa channels (IK). Next, we comprehensively reviewed the loss- or gain-of-function mutations of each KCa channel and described the corresponding mutation sites in specific diseases to broaden the phenotypic-genotypic spectrum of KCa-related neurological disorders. Moreover, we reviewed the current pharmaceutical strategies targeting KCa channels in KCa-related neurological disorders to provide new directions for drug discovery in anti-seizure medication.
Keywords: Potassium channels, channelopathies, modulators, pharmacology, epilepsy, action potential
1. INTRODUCTION
Ion channels play a critical role in membrane transport activities that are essential for the optimal physiological function of the body. It has been suggested that pathogenic changes in ion channel activity and transporter function might cause diseases, such as migraines with hemiplegic symptoms, epilepsy, and cardiac arrhythmias, which are commonly referred to as ‘ion channelopathies’ [1]. Among ion channels, potassium channels (K+ channels) may control neuronal excitability during development due to their variable gating capabilities and vast temporal and spatial expression patterns [2]. For example, they may modulate the resting membrane potential, control the repolarization rate of action potentials (AP), and spike frequency adaptation patterns [3]. Consequently, K+ channel dysfunction is associated with a variety of neurological disorders, including epilepsy (Ohthara syndrome, Temple-Baraitser syndrome, and malignant migrating partial seizures of infancy), arrhythmia, myokymia, and developmental abnormalities of neural crest-derived tissues (Andersen syndrome) [4]. However, the underlying molecular mechanisms and their correlation with corresponding clinical manifestations are unclear.
Recent studies have found that even very little alterations in the activity of calcium-activated potassium channels (KCa) may have significant effects on neuronal growth and cognitive ability [5]. The KCa channel is a large family of potassium channels found in almost every human cell type, including nerve cells, muscles, secretory cells, etc. KCa channels are formed with three different isoforms, which are classified by their activation and conductance features, namely big conductance KCa channels (BK channels), small conductance KCa channels (SK channels), and intermediate conductance KCa channels (IK channels). However, more studies are focusing on the BK and SK channels with little knowledge about IK channels so far [6]. Increases in cytosolic Ca2+ via voltage-gated Ca2+ channels and intracellular sources (endoplasmic reticulum, mitochondria) can gate all types of KCa channels and subsequently terminate the AP. They typically function by connecting membrane potential to intracellular Ca2+ concentration, which is crucial in a variety of physiological processes, such as regulating the interval of APs, presynaptic neurotransmitter release, and postsynaptic cell firing in certain neurons [7]. KCa channels can also be triggered by other messengers like kinases, protein phosphatases, G proteins, etc. [8]. In addition to the direct electrophysiological role, K+ channels have been reported to participate in diverse physiological processes via regulating cellular signaling pathways, including gene expression, and proliferation
In this review, we focused on the molecular and cellular properties of KCa channels. We comprehensively reviewed the loss- or gain-of-function of each KCa channel and attempted to bridge the phenotypic-genotypic spectrum in KCa-related neurological disorders. Here, we systematically summarized the association between all the subtypes of KCa channels and KCa-related neurological channelopathies. Moreover, we reviewed the current pharmaceutical strategies targeting KCa channels in KCa-related neurological disorders to provide new directions for drug discovery in anti-seizure medication.
2. BK CHANNELS IN KCa-RELATED NEUROLOGICAL DISORDERS
2.1. The Physiological Properties of the BK Channel
The BK channel (also known as Slo1 or KCa1.1) was first discovered in the chromaffin cell membrane [9]. To date, documentation of BK channel expression shows that it is highly expressed in a variety of mammalian cells and tissues, including neurons, excretory cells, cardiac and smooth muscles, as well as skeletal and internal sensory hair cells of the ear [10, 11] (Table 1). In the brain, BK channels are widely expressed in numerous regions, including the cerebral cortex and hippocampus [10].
Table 1.
A summary of the structural, physiological, and pharmacological properties of KCa channels.
| Properties | BK Channel | SK Channel | IK Channel (SK4) | |
|---|---|---|---|---|
| SK1 and SK2 Channels | SK3 Channel | |||
| Structure characters |
β and γ subunits, seven transmembrane domains, 2 RCK domains in intracellular C terminal. |
Six transmembrane domains with amino acid sequence (S-Y-A) between the S3-S4 domain. |
Six transmembrane domains with amino acid sequence (S-Y-T) between the S3-S4 domain. |
Six transmembrane domains with shorter N-terminus. |
| Channel gating |
Voltage, Ca2+, Mg2+, H+, etc | Ca2+ via CaM | Ca2+ via CaM. | |
| CNS expression |
Olfactory bulb, cortex, basal ganglia, caudate putamen, CA1 region, cerebellum. |
CA1 region, amygdala, thalamus, cerebellum. | Midbrain, brain cell nuclei. | |
| Electrophy-siological function |
Fast AHP in neurons, dendritic excitability, synaptic plasticity. |
Medium AHP in neurons, dendritic excitability, synaptic plasticity. |
Volume regulation in erythrocytes. |
|
| Biological function |
Intercellular Ca2+↑ | EDHF response. | Ca2+ signal in T-cell, Proliferation; EDHF response. |
|
| Pharmacology | Blockers: Dihydropyridines, Iberiotoxin, Paxillin, Penitrem A Openers: NS004, NS1619, BMS-204352 |
Blockers: Apamin, Leiurotoxin I, UCL1684, Openers: Dichloro-EBIO, NS309, riluzole. |
Blockers: Charybdotoxin, Maurotoxin, SKA-31, Margatoxin, TRAM-34. Openers: EBIO, NS309, riluzole, methylxanthine. |
|
Abbreviations: AHP, After hyperpolarization; CaM, calmodulin; EDHF, Endothelium-Derived Hyperpolarizing Factor; Dichloro-EBIO, Dichloro-Ethylbenzimidazolone.
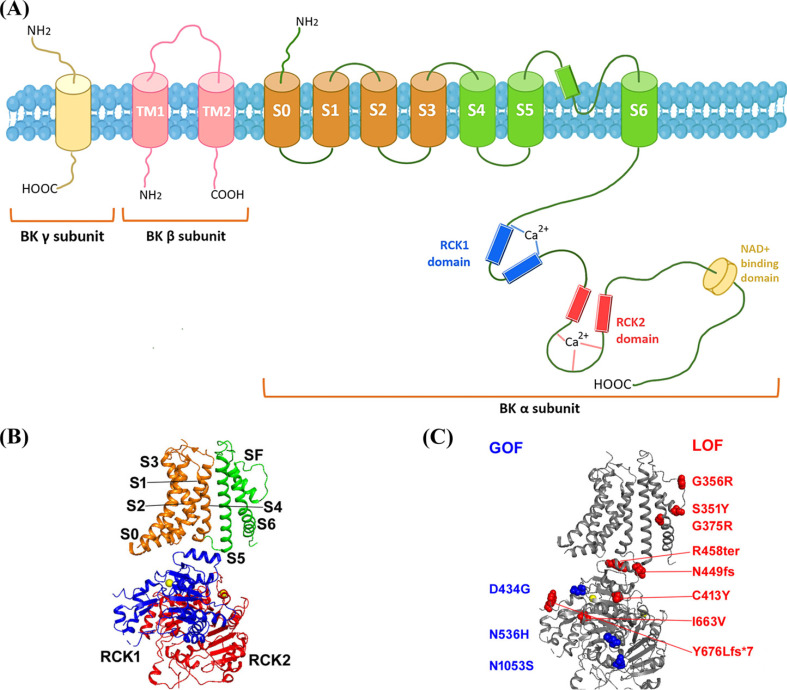
BK channel is a tetrameric structure composed of a pore-forming domain (BK-α) encoded by KCNMA1 and two accessory domains (BK-β and BK-γ). The BK-α subunit of the BK channel in mammals shares 98 percent of its amino acid sequence with that of other members of the voltage-activated and ligand-gated K+ channel superfamily, which participates in voltage-dependent electrophysiological property of the BK channel like voltage-dependent (Kv) channels and other types of potassium channels. There are seven transmembrane domains in BK-α (S0~S6) and a substantial cytoplasmic C-terminus (Table 1, Fig. 1). The extracellular N-terminus and presence of the S0 transmembrane region distinguish BK from other voltage-gated potassium channels and allow for the interaction with additional proteins β and γ subunits [12]. There are two K+ conductance regulator (RCK) domains in the intracellular C-terminus of the BK channel, the allosteric gating mediated by RCK1 and RCK2, containing 2 unique high-affinity Ca2+ binding sites. BK channel activation is influenced by electrostatic interaction between Mg2+ and RCK1. Furthermore, RCK1 controls the sensitivity of channels to Zn2+ and Cd2+ [13-15].
Fig. (1).
A topography of the BK channel with reported human mutation sites. (A) Each α-subunit of the BK channel has seven transmembrane domains (S0~S6), with the pore area between S5 and S6. A functional channel is made up of four of these subunits, with the C-terminus being one of the longest of all potassium channels. The C-terminus comprises two regulators of potassium conductance (RCK) domains, RCK1, and RCK2, which stack on top of one another to create a gating ring beneath the channel opening pore. (B) A single BK channel cry-EM subunit (PDB entry: 6V38). S0-S6 denote the transmembrane segments; Selectivity filter (SF) denotes the selectivity property; the two Ca2+ molecules that are coupled to the channel are represented in yellow. (C) GOF mutation sites are shown in blue, LOF mutation sites are in red mapped onto the BK channel structure and Ca2+ ions bound to the channel are shown in yellow circles. (B and C Copyright from 2022 [Jianmin Cui]. All Rights Reserved) [15].
BK channels are widely believed to prevent neural high-frequency and repeated firing. Reduced Cereblon (Crbn), a well-known target of the immunomodulatory drug thalidomide, was originally identified to cause human intellectual disability. It has previously been shown that BK channel activity is modulated by crbn in primary cultured neurons [16]. To better understand the biological underpinnings of intellectual impairment, research has shown that synaptic abnormalities are the most common factor in cognitive dysfunction. It was discovered that behavioral defects in Crbn KO mice could be restored by treatment with the BK blocker paxilline [17]. This strongly suggests that Crbn KO mice exhibit abnormally increased BK channel activity-induced decreased excitatory presynaptic release, which is closely related to cognitive dysfunction. However, BK channel activity paradoxically increases the firing of early high-frequency spikes of rat hippocampal pyramidal cells [18]. Other studies reported that increasing BK channel activity tends to promote synchronization of neuronal activity in animal models, such as Angelman syndrome. Sun et al. found that enhanced BK channel activity led to increased intrinsic excitability in hippocampal neurons and contributed to subsequent synchronization in the network [19]. BK channels are now receiving more consideration as potential therapeutic targets because of their wide distribution and involvement in an extensive range of physiological processes [20].
2.2. Physiological Modulation of the BK Channels
When the BK channel is activated by increasing intracellular Ca2+ concentration (Ca2+) as a result of membrane excitability and depolarization, it terminates the action potential with K+ outflow, resulting in hyperpolarization. This characteristic is advantageous in excitable cells because it enables negative feedback control of Ca2+ influx [21, 22]. In addition to Ca2+, the cytosolic Mg2+ and H+ can also modulate BK channels. The H+ activates the BK channel via electrostatic interactions between histidine residues and a nearby negatively charged residue. A homologous motif in the RCK1 domain regulates both the stimulatory and inhibitory activities of H+ and Ca2+, allowing bidirectional linkage of cell metabolism and membrane electrical excitability. Moreover, it is possible that the affinity of BK channels towards H+, which is associated with cytoplasmic neural acidification, is an important element in the cessation of epilepsy events [23]. In addition to being regulated by several ions, enzyme-mediated modifications, such as ubiquitination, palmitoylation, and myristoylation, also influence BK channel expression and activity. Furthermore, endogenous modulators, including arachidonic acid, nitric oxide, zinc, GMP, cGMP, and cAMP-mediated phosphorylation of the channel, may also control BK channel function (Table 1).
Studies also revealed that cysteine string protein α (CSPα) regulates BK channel expression [24]. CSPα is a synaptic vesicle-associated protein that is broadly expressed in the nervous system and displays unique anti-neurodegenerative properties [25]. The CSPα is identified as a major regulator of BK channel density in the neuronal plasma membrane. CSP null mice expressed 2.5-fold more BK channels than wild-type mice. Additionally, BK channel levels were significantly enhanced in neuroblastoma cells generated from the murine central nervous system (CNS) after the expression of a dominant negative variant of CSPα containing a mutant HPD (residues 43-45) sequence. The HPD motif is required for the heterotrimeric CSPα chaperone
complex to function properly. Mutations of CSPα in the N terminal J domain or central cysteine string region led to an increase in total and cell surface BK channel expression, resulting in greater BK channel current density [26]. Loss of CSPα function alters BK channel expression in the intact (CNS), which may lead to altered neuronal membrane excitability and contribute to the pathogenesis of neurodegeneration associated with either genetic loss or dysfunction of CSPα [27].
2.3. BK Channel-Linked Neurological Disorders
Both GOF and LOF mutations in KCNMA1 have been associated with neurological diseases, resulting in a spectrum of moderate to severe abnormalities. A summary of GOF, LOF of KCNMA1, as well as overlap symptoms, particularly in the areas of neurodegenerative disorders, is shown in Table 2. Previous studies have found that LOF mutations in KCNMA1 might result in widespread hyperexcitability, causing seizure activity and other related neurological conditions [28]. In addition, GOF mutations in KCNMA1 are linked to numerous neurological conditions, suggesting that alterations in the activity of BK channels may affect the brain's equilibrium. BK channels are found in both inhibitory and excitatory neuronal networks, and their influence on firing varies depending on their expression [10, 29-36].
Table 2.
Mutations in KCNMA1 associated with human neurological diseases.
| Clinical Phenotypes |
Homo
Variants |
Location | Mutant Types | Function Test; Model | Results |
|---|---|---|---|---|---|
| GOF | |||||
| E, PNKD [30] | D434G | RCK domain | Missense mutation | Yes; transfected oocytes or subcloned CHO cell | BK channel opening increased due to a three- to five-fold increase in Ca2+ sensitivity. |
| E, DD [31] | N995S | NAD domain | Missense mutation | Yes; pcDNA3.1 cloned by human KCNMA1 cDNA | BK channel opening increased with higher sensitivity to the voltage change. |
| LOF | |||||
| Ataxia, tremor, apraxia, hypertelorism [32] |
S351Y | Pore domain | Missense mutation | Yes; NA | The BK channel is eliminated, and the K+ current is blocked. |
| Cognitive delay, axial hypotonia, ataxia dysarthria [32] |
G356R | Pore domain | Missense mutation | Yes; NA | BK channel is eliminated, and the K+ current ranging from -160~60 mV is blocked. |
| DD, visceral and cardiac malformation dysplasia, dysmorphic features [32, 33] |
G375R | S6 domain | Missense mutation | Yes; NA | The BK channel is eliminated, and the K+ current is blocked. |
| Congenital abnormalities, DD, ID, axial hypotonia, ataxia [33] | C413Y | AC region of RCK | Missense mutation | Yes; NA | The amplitude of BK current is reduced, and its activation curves shift toward positive potentials. |
| DD, ID, axial hypotonia, ataxia [32] |
I663V | RCK domain | Missense mutation | Yes; NA | The BK channel is eliminated, and the K+ current is blocked. |
| DD, E, cerebellar and corticospinal tract atrophy [34] |
Y676Lfs*7 | RCK domain | Frameshift mutation |
No; NA | The activity of the BK channel decreased. |
| Speech delay, DD, ID, apraxia [33] |
P805L | RCK domain | Missense mutation | Yes; NA | The amplitude of BK current is reduced, and its activation curves shift toward positive potentials. |
| E, PNKD [32] | D984N | RCK domain | Missense mutation | Yes; oocytes injected with cRNA | The amplitude of BK current is reduced, and its activation curves shift toward positive potentials. |
| Overlap GOF/LOF | |||||
| PNKD, DD, visual impairment [33] |
E884K | RCK domain | Missense mutation | No; NA | Not determined. |
| PNDK, DD, ID [35] | G354S | Pore domain | Missense mutation | Yes; transfected Xenopus oocytes | Reduced BK channel activity and delayed activation. |
| DD, ID, myoclonic seizures [36] | R458Ter* | AC domain | Nonsense mutation | No; NA | Putative truncations (premature truncation mutations). |
Abbreviations: AC, Cytosolic domain; RCK, Regulators of K+ conductance GOF, gain of functional mutation; LOF, loss of functional mutation; E, epilepsy; PNKD, Paroxysmal Non-Kinesigenic Dyskinesia; DD, developmental delay; ID, intellectual disability, NA; Not available.
As mentioned before, the BK channel is a tetramer composed of four pore-forming subunits. It seems that 2 GOF and 10 LOF variants of the BK are mainly located on the pore gate domain (PGD; S5, pore and S6 segments) and cytosolic domain, which regulate the function of BK via changing the gating characteristics and activating by intracellular Ca2+. Although there are fewer GOF variants of KCNMA1, 20/37 patients carried GOF variants while 13/37 carried LOF variants. However, reviewing 37 patients with KCNMA1-linked channelopathy, paroxysmal non-kinesigenic dyskinesia (PNKD) was reported in 17 of 20 GOF patients and only 2 of 13 LOF patients. In addition, although seizure is a predominant symptom and equally associated with both GOF and LOF KCNMA1 mutations, all nine GOF KCNMA1 mutations patients with seizures experienced absence seizures. Notably, patients with both GOF and LOF KCNMA1 showed developmental delay and intellectual disability; a subset of neurodevelopmental symptoms has only been described in patients with LOF KCNMA1. Due to the lack of detailed clinical description, it is difficult to determine if particular symptoms are specific to GOF versus LOF channel mutations [37].
BK channel accessory subunit-β family, which is composed of four different isoforms and encoded by KCNMB1-4, is linked to a variety of nervous system diseases. For instance, alcohol escalation was accelerated, and the chronic tolerance to ethanol-induced sedation and hypothermia was reduced in β1 knockout mice [38, 39]. As for the β2 subunit, it is encoded by KCNMB2 and also linked to hippocampal sclerosis, a concomitant neuropathological characteristic of Alzheimer's disease [40]. On the other hand, KCNMB3 deletion increases the risk of idiopathic generalized epilepsy in children. However, the precise mechanism of how such mutation affects neuronal due to the low levels of the β3 subunit is still unclear [40,41]. The β3 encoding gene KCNMB3 is duplicated in some patients with the dup (3q) syndrome [43]. Furthermore, the β4 subunit, which is highly expressed in specific brain regions like the lateral hypothalamus, the Purkinje layer, and the striatum, has been found to control ethanol tolerance at the molecular, cellular, and behavioral levels [44]. In another study, after hyperpolarization (AHP), latency decreased and the firing rate elevated in dentate gyrus granule cells in β4 knockout mice. Moreover, nonconvulsive strokes occurred spontaneously in β4 knockout mice [45].
Single nucleotide polymorphisms (SNPs) are an important class of genetic mutations. When an SNP disrupts secondary structural elements (for example, replacement of proline in the alpha helix region), the resulting mutation often has an impact on the entire protein structure and function. Missense SNPs in KCNMA1 have been related to human neurological diseases. An SNP in KCNMB4 is also associated with an increased risk of developing mesial temporal lobe epilepsy [46]. Many studies revealed a possible link between Alzheimer’s disorder (AD) pathophysiology (onset age or duration of symptoms) and an SNP in the gene KCNMA1, rs16934131 [40, 47]. In addition, numerous cases of mental disability have been associated with a potential LOF SNP mutation in KCNMA1 [48, 49]. Studies on the Fragile X Mental Retardation Protein (FMRP), which regulates dendrite-specific protein synthesis and is essential for brain development, have demonstrated the relationship between the BK channel and cognitive disorders. FMRP deficiency results in excessive AP broadening during repetitive activity, and increased presynaptic Ca2+ influx, in cortical pyramidal neurons [50]. However, these presynaptic actions are mediated selectively by BK channels via the interaction of FMRP with the BK channel’s regulatory β4 subunits [51].
2.4. Binding Site of BK Channel Compounds
There are two putative high affinity Ca2+ binding sites in the BK channel domains: one at Asp362/Asp367 in the RCK1 domain (these residue numbers are derived from the mbr5 sequence of the mouse subunit) [52] and the other in a region known as the Ca2+ bowl, which has a number of Asp residues in it [53], situated inside the RCK2 domain [54]. By binding with certain residues in the cytosolic domain, additional signaling chemicals, like carbon monoxide and heme [55, 56], may also change the gating characteristics of the channel. The activation of ion channels by intracellular ligands is often described as a conformational change in the cytosolic domain caused by ligand binding, which then pulls to open the activation gate at the peptide link between the membrane-spanning and cytosolic domains, called the tugging model [57, 58]. In contrast, Mg2+ activates BK channels by pushing the voltage sensor through electrostatic interaction and emphasizes the connection among side chains in distinct structural domains, also known as a nudging model. Since the Ca2+ sensitivity of BK channels is dependent on the length of the C-linker [59], it has been hypothesized that the activation of the channel by Ca2+ takes place through a tugging model. However, new evidence suggests that Ca2+ binding to the two separate high-affinity sites activates the channel with different mechanisms, and that the processes underlying Ca2+ dependent activation through the two sites may be more complicated.
The distinction between the Ca2+ binding sites was first described in a study where the Ca2+ bowl was mutated [60]. The results showed that the channel retained a partial sensitivity to Ca2+ while sustaining its sensitivity to Cd2+. It has been hypothesized that BK channels have a second Ca2+ binding site that may also bind to Cd2+ to activate the channel. Later research confirmed that the Cd2+ sensitivity of RCK1 is due to its putative second Ca2+ binding site (Asp362/Asp367, where Asp362 has a small influence on Ca2+ sensitivity) [61]. A recent study discovered that mutating ten residues in the N-terminal area of the RCK1 domain from the AC region may specifically modify the Ca2+-dependent activation originating from the location in RCK1 [62].
Furthermore, the change of voltage and Ca2+-dependent activation by the disease-associating mutations also provides unique insights for further understanding BK molecular mechanisms. Since G375R [32] is located in the diglycine hinge for the BK channel activation gate [63], it is possible that this mutation interacts with the opening of the activation gate and that G356R [32] eliminates the selectivity filter [64]. The effects of cytosolic domain mutations on voltage-dependent activation or intrinsic gate opening in BK channels are unknown. Some examples of such mutations are N1053S [48], N536H [65], C413Y, and I663V. Genetic data from human patients, biophysical characterizations, and animal models of GOF or LOF BK channel mutations suggest that BK channels might be a suitable therapeutic target for treating neurological diseases.
2.5. The BK Channel-pharmacology
For the majority of genetic channelopathies, the only option for pharmacological therapy is to modulate the particular activity of mutant ion channels. Precision medicine for KCNMA1 and other channelopathies begins with classifying patient mutations into functional classifications. In addition, GOF, LOF, and possibly benign mutations in the BK channel activity are linked with overlapping symptoms. It is unclear whether specific BK channel agonists or antagonists would truly result in the intended consequence on neuronal activity. It is difficult to determine which BK channel components target which neuron or muscle loci in KCNMA1-linked channelopathy without more precise information. Research on BK channel pharmacotherapy has been in progress for over two decades; these efforts aim primarily to address neurological dysfunction caused by LOF mutations in BK channel activity [66].
BK agonists have been found in a variety of substances, including those that are endogenous, naturally occurring, and synthetic [67]. In the endogenous class are heme and heme-breakdown products [68], long-chain free polyunsaturated acids, cytochrome P450 metabolites, epoxygenase, and lipoxygenase [69], as well as 17-estradiol [70]. The naturally existing class includes several compounds found in herbs, roots, and leaves that have been utilized in traditional medicine to treat asthma and other conditions caused by myocyte dysfunction, such as dehydrosoyasaponin I (DHS-I) [70, 71]. Ca2+ channel inhibitors, like dihydropyridines (DHPs), are another possible FDA-approved medication for normalizing BK channel activity associated with GOF mutations. Some cases of dyskinesia can be treated with DHPs, nifedipine and nimodipine, as well as the non-DHP verapamil that is now being utilized to treat refractory epilepsy and hyperkinetic movement disorders [73]. Furthermore, the study on BK channels has been accelerated significantly by the development of specific inhibitors, such as iberiotoxin, paxilline, lolitrem B, and penitremA. Mehranfard et al. indicated that paxillin and iberiotoxin reversed firing characteristics of dentate gyrus granule cells in the chronic phase of pilocarpine-induced status epilepticus, suggesting that BK channels may have the potential to treat epilepsy [74].
The synthetic class includes GoSlo-SR, which is a newer family of BK channel activators that include NS004 and NS1619, as well as the more powerful and selective NS11021 [75]. When injected two hours after the onset of occlusion in rat models of persistent large-vessel stroke, Bristol-Myers Squibb (BMS-204352), a fluoro-oxindole potassium channel opener, demonstrated significant cortical neuroprotection [76]. Similarly, NS11021 activated the BK channel in a concentration-dependent manner at concentrations greater than 0.3 M by parallel-shifting the channel activation curves to more negative potentials. The single-channel analysis demonstrated that NS11021 improved channel open probability without influencing single-channel conductance by modifying gating kinetics [77]. Furthermore, NS11021 was shown to be a highly selective and efficient BK channel activator, making it an ideal candidate for investigating the physiological and pathological aspects of BK channels.
3. SK CHANNELS IN KCa-RELATED NEUROLOGICAL DISORDERS
3.1. The Physiological Properties of the SK Channel
Three distinct SK channels encoded by KCNN1-3 exist in the mammalian brain: SK1 (KCa2.1, KCNN1), SK2 (KCa2.2, KCNN2), and SK3 (KCa2.3, KCNN3). These channels are structurally similar throughout their transmembrane cores (80-90%) but different in sequence at their N and C termini (Fig. 2). Two SK2 isoforms have been identified: SK2-long (SK2-L), having a 207 amino acid N-terminal extension and SK2-short (SK2-S) [78]. SK channels are abundant throughout the nervous system and significantly expressed in certain brain areas, including the hippocampus, amygdala [79], and thalamus [80]. In addition, brain areas, such as the cortex, hippocampus, and limbic system, all have low levels of SK3 channels [81]. In many cases, both SK1 and SK2 channels are co-located in the same neurons, which may explain why heteromeric SK1/SK2 channels are preferentially assembled [82]. SK channels have vital functions outside the nervous system, such as regulating blood pressure and metabolic processes, as shown in Table 1.
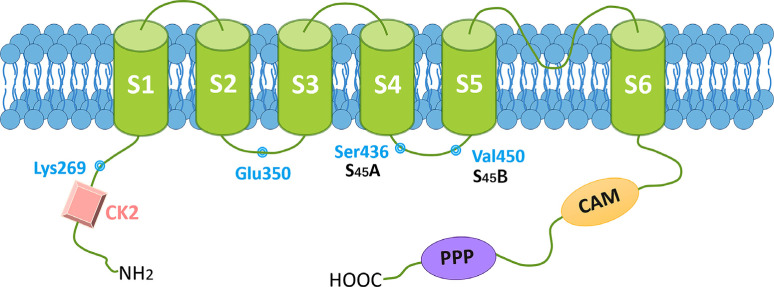
Fig. (2).
A topography of the SK channel with reported human mutation sites. SK channel has six transmembrane domains (S1~S6), with the pore region between S5 and S6. Both the N-terminus and the C-terminus point toward the cytoplasm. The CaM binding site, protein kinase CK2 and protein phosphatase (PPP) binding sites are indicated by yellow, pink, and purple, respectively. GOF mutation sites are shown in blue.
SK channels are required for the proper function of all excitable cells. SK currents are activated when calcium enters neurons via voltage-gated calcium channels, which are activated during the AHP following an AP. As soon as SK channels open, K+ is expelled from the cell, resulting in higher negative membrane potential, which regulates neuronal excitability and spike firing rates [83]. They may also be functionally coupled to post-synaptic calcium sources, such as N-methyl-d-aspartate (NMDA) and nicotinic acetylcholine receptors, calcium released from intracellular ryanodine or inositol 1,4,5-trisphosphate receptors [82].
SK channels modulate membrane excitability in CA1 neurons and regulate hippocampal neural plasticity [84, 85], as well as play a significant role in learning and memory due to their expression in the post-synaptic membrane of glutamatergic terminals, where they regulate neurotransmission and induce neural plasticity [66]. Many studies revealed that learning is impaired by increased activity in SK channels [86, 87]; in contrast, animal studies showed that SK channel antagonists improve learning and memory [88].
3.2. Modulation of the SK Channels
SK channels have positive and negative gating modulators that change the Ca2+ response curve of these Ca2+/calmodulin-gated channels to the left or right, respectively, increasing or decreasing the channel's sensitivity to Ca2+ [25]. Negatively modulated molecules are more likely to cross biological barriers if they are uncharged at physiological pH. In contrast, the more recently disclosed (-)CM-TMPF and the structurally similar (-)B-TMPF operate as SK1-selective positive and negative gating modulators having EC50 or IC50 values of 24 and 31 nM, respectively [89]. Dichloro-EBIO and the more potent benzimidazolone 1-EBIO are the most often utilized positive gating modulators [90]. They serve as important ex vivo chemicals used in brain slices, endothelia and epithelia, and smooth muscle preparations.
Additionally, in one study, melatonin induced downregulation of KCNN1 and 2 expressions restored cognitive impairment caused by cerebral hypoperfusion [91]. Ethanol-induced synaptic excitability in the ventral but not the dorsal hippocampus has recently been linked to changes in the expression of the SK2 and GluA2 subunits in the synaptosomal membrane of the hippocampal neurons [92]. Morphine sensitization may potentially be influenced by increased SK2 channel-mediated negative feedback of NMDA receptor [93]. Withdrawal of cocaine eliminates the neuroplasticity mediated by SK2 in the nucleus accumbens neurons [94]. These findings suggest that drug-induced plasticity alters the activity of SK channels, which may play a crucial role in rewarding behavior.
3.3. SK Channel-Linked Neurological Disorders
It is not surprising that aberrant levels and/or activities of SK channels have been associated with a variety of CNS disorders as these channels play key roles in synaptic transmission, rhythmic activity, and other CNS processes. Lee et al. indicated that the three amino acid replacements in SK3 channels were found in patients with Zimmermann-Laband syndrome (ZLS), a rare genetic disorder characterized by abnormalities of the head and facial (craniofacial) area and the hands and feet [95]. The KCNN3 mutation has recently been also implicated in bipolar disorder [96]. Overexpressing SK3 channels in mice showed impairments in long-term potentiation and memory deficits as well as decreased high cognitive function in the hippocampus [96-98].
Decreased SK3-mediated current and increased neuronal excitability in the nucleus accumbens core are essential processes that enhance the desire for alcohol during abstinence [99]. Similarly, the expression and function of SK channels were dramatically decreased in the pilocarpine model of epilepsy [100]. Decreased SK activity was also shown to be related to increased seizure activity in Dravet syndrome [101]. Schizophrenia patients also have a spontaneous mutation in the KCNN3 gene [102]. Based on these findings, balanced SK channel activity is required for proper neurodevelopment and cognitive functions (Fig. 2). Unfortunately, due to the lack of detailed clinical description and reported variants, it is limited to determine if particular symptoms are specific to GOF versus LOF channel mutations and find ‘hot spots in the channel.
3.4. The SK Channels-Pharmacology
SK channel is inhibited by bee venom toxin apamin by interacting with an outer pore histidine residue which is expressed in all SK subtypes via an allosteric mechanism [103, 104]. The SK channel blockers like Syllatoxin (isolated from the scorpion Leiurus quinquestriatus) or leiurotoxin I, a stronger scorpion toxin, have similar efficacy to apamin [105]. An entirely new benzimidazole derivative, NS8593, has just been described as an inhibitor of the KCa channel with a completely different chemical structure. A gating modulator, NS8593, shifts the Ca2+ activation curve of KCa channels approximately 10-fold to the right, decreasing their Ca2+ sensitivity. NS8593 subsequently inhibits cloned SK1, SK2, and SK3 with IC50 values of 420, 600, and 730 nM at a concentration of 500 nM [106]. UCL1684 and UCL1848, which are less utilized blockers, are equally strong as apamin in inhibiting the SK channel. Previous research has shown that bicuculine methiodide has an apamin-like effect on dopamine neurons that enhances the effects of NMDA receptor activation.
The development of specific SK channel activators resulted in the discovery of NS309, one of the most effective pan-SK2 activators and a critical mechanical tool compound [107]. NS309 and SKA-31 equally stimulate all three SK channels [108]. CyPPA and its recently reported derivative NS13001 are examples of subtype-specific SK activators [109]. The notion of utilizing SK activators is based on the discovery that SK channel silencing in deep cerebellar neurons causes ataxia in mice [110]. In contrast, the treatment of mice with spinocerebellar ataxia type-2 with NS13001 alleviated movement disorders and delayed neurodegeneration in Purkinje cells [111]. Riluzole, a more effective neuroprotectant, causes SK channels to shift to the left and increases AHP in cultured hippocampal neurons by increasing their Ca2+ sensitivity [112-118] (Table 3).
Table 3.
GOF Mutations in KCNN1-4 associated with human neurological diseases.
| Clinical Phenotypes | Gene Type |
Homo
Variants |
Location | Mutant Types | Function Test; Model | Results |
|---|---|---|---|---|---|---|
| ZLS, DD, ID, hypotonia [113] | KCNN1 | G350D | S2-S3 domain | Missense mutation | Yes; pcDNA3.1 cloned by human KCNN3 cDNA | Increased Ca2+ sensitivity and faster channel activation in mutant SK channel. |
| DD, ID, ZLS [113] | KCNN1 | S436K | S45A domain | Missense mutation | Yes; subcloned CHO cell | |
| ZLS, ID, DD, PDA [113] | KCNN2 | K269E | N-terminus | Missense mutation | Yes; HEK293T cells | |
| DD, Hypo-tonia [114] | KCNN3 | V555F | NA | Missense mutation | Yes; NA | |
| ID, DD [114] | KCNN3 | V539del | NA | Nonsense mutation | Yes; NA | |
| DD, Mild seizures [114] | KCNN3 | A287S | NA | Missense mutation | Yes; NA | |
| INCPH [115] | KCNN3 | V450L | S45B domain | Missense mutation | Yes; NA | |
| HX [116] | KCNN4 | R352H | CaMB domain | Missense mutation | Yes; HEK293T cells | Increased 10 folds Ca2+ sensitivity and current density in encoded mutant channel. |
| DHSt [117] | KCNN4 | V282M | S6 domain | Missense mutation | Yes; CD34+ cells and K562 cells | Increased Ca2+ sensitivity in mutant channel. |
| DHSt [118] | KCNN4 | V282E | S6 domain | Missense mutation | Yes; CD34+ cells and K562 cells |
Abbreviations: DD, developmental delay; GOF, gain of functional mutation; ID, intellectual disability; INCPH, idiopathic non-cirrhotic portal hypertension; NA; not available. PDA, patent ductus arteriosus; ZLS, Zimmermann–Laband syndrome. CaMB, Calmodulin domain; HX, Hereditary xerocytosis; DHSt, Dehydrated hereditary stomatocytosis.
4. IK CHANNELS IN KCa-RELATED NEUROLOGICAL DISORDERS
4.1. The Physiological Properties of the IK Channel
The intermediate-conductance channel (IK) encoded by KCNN4 was initially identified in erythrocytes by Gardos in 1958 [119] and is known as SK4 because it is ∼40% identical to the three SK channels [25]. It was finally cloned from pancreatic, placental, or T-lymphocytes in 1997 [26-28]. Additionally, IK was also discovered in the lung, salivary glands, distal colon, and prostate; and was absent in the heart, brain, liver, kidney, and skeletal muscle [120-123]. The expression of the IK channel in some enteric neurons has been shown [124]. IK and BK channels are structurally nearly identical. Each IK channel has six transmembrane domains (numbered S1-S6) and has an active pore, which is located between S5 and S6 [125]. Acidic residues in the IK channel -subunit S4 transmembrane region render it insensitive to voltage changes compared to the BK channels. Since the S0 transmembrane domain is absent in IK channels, the enhanced Ca2+ sensitivity of these channels is due to the calmodulin-binding domain on the α-subunit C-terminal region of the protein that binds Ca2+.
The IK channel is generally activated by Ca2+ release from intracellular stores or Ca2+ inflow through store-operated channels (SOCs) in the endoplasmic reticulum (ER). In addition, activation of the IK channel in excitable cells causes hyperpolarization, which, in turn, enhances the Ca2+ driving force for SOC entrance. This enormous influx specifically activates voltage-dependent ion channels, and the subsequent K+ and Ca2+ outflow of ions hyperpolarizes the membrane and enhances the driving force for Ca2+ efflux [126]. Recent studies suggest that IK may be expressed in neurons as well and have a role in the lowering of AHP [127]. However, the significance of the IK channel in neurons remains unclear.
4.2. IK Channel-linked Neurological Disorders and Pharmacology
In studying how Aß oligomers trigger reactive astrogliosis, researchers have discovered that IK channels can increase the driving force for Ca2+ influx, making them a potential therapeutic target for AD. Additionally, APP/PSEN1 AD mice with the deletion of the KCNN4 mutation in the hippocampus were shown to improve memory impairments and neuronal loss [128]. Despite the lack of clear evidence that IK channels are involved in the pathogenesis of AD, multiple investigations have shown that oxidative stress impairs IK function in older animals [129].
Peptide blockers of the IK channels include charybdotoxin, (ChTx), a 37-amino acid scorpion toxin, which binds to the outer vestibule through two salt bridges while inserting its core lysine residue into the specific segment [130]. ChTx also inhibits BK channels, making it an extremely promiscuous blocker of channels [131]. Additionally, maurotoxin, a 34-residue scorpion toxin cross-linked with four disulfide bridges, is the most powerful peptide blocker of IK [132]. More surprisingly, maurotoxin tends to block Kv voltage-gated channels with higher IC50 (100 pM). ShK (IC50 30 nM), BgK (IC50 172 nM), and margatoxin (IC50 450 nM) are several other toxins that inhibit IK at high concentration [133]. The triaryl-methane IK channel blockers clotrimazole, TRAM-34, and senicapoc, interact with threonine 250 in the pore loop and valine 275 in S6, as demonstrated by the fact that mutations of these residues completely abolish the sensitivity of IK channel to triaryl-methanes [134]. Recently, it was shown that negative-gating modulation is also responsible for the actions of some Di-benzoates, such as RA2 [1,3-phenylenebis(methylene)bis(3-fluoro-4-hydroxybenzoate], which blocks both SK and IK channels with comparable efficacy [135]. Even though its binding site has to be fully discovered, the inner vestibule of both channels (IK and SK) is large enough to accommodate it. Sankaranarayanan et al. developed a series of benzothiazoles, including SKA-31 and SKA-121, that have increased selectivity for the IK channel and demonstrated that selective IK activation could decrease blood pressure in mice [108].
EBIO (ethylbenzimidazolone) was the first compound to be shown to activate IK currents in T84 cells when applied at high micromolar concentrations. Evidence suggests that EBIO enhanced chloride secretion by activating IK channels located on the cell surface of T84 monolayers and secretory endothelia [136]. EBIO activates human cloned IK channels (hIK) by raising the single-channel open probability in a calcium-dependent manner, leading to an apparent leftward shift of the Ca2+ activation curve by order of magnitude. Activation of the IK channel by 8-methoxypsoralen, the methylxanthines, caffeine and theophylline, and a clotrimazole-sensitive K+current in mouse jejunum preparations (100 mM to 1 mM) are further examples of chemical classes that have been found to activate the IK channel. The most potent therapeutic drugs for BK, SK, and IK channels are shown in Fig. (3).
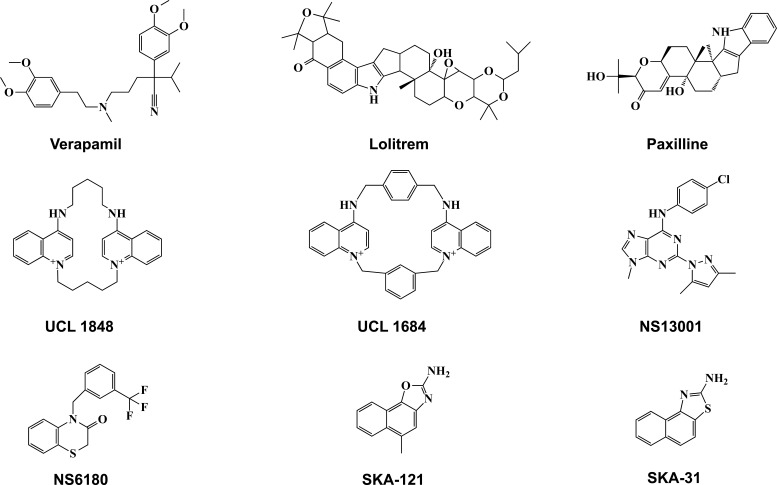
Fig. (3).
Names and structures of BK, SK, and IK channel modulators.
CONCLUSION
KCa channels modulated by intracellular Ca2+ concentration play a critical role in neuronal excitation. Growing studies have reported that mutations in KCa channels are associated with neurological disorders, such as developmental, epileptic encephalopathies, and severe psychomotor and intellectual disabilities. In this review, we first summarized the molecular and cellular properties of three KCa channels, BK, SK and IK channels. Next, we comprehensively reviewed the LOF and GOF mutations of each subtype of the KCa channel and discussed the corresponding disease to broaden the phenotypic-genotypic spectrum of KCa-related neurological disorders. Moreover, we explored the various underlying modulatory mechanisms of KCa channels and the current pharmaceutical strategies to provide new directions for developing more potent and selective treatment strategies for KCa-related neurological disorders.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AC
Cytosolic Domain
- AHP
After Hyperpolarization
- AP
Action Potential
- BK Channels
Big Conductance KCa Channels
- CaM
Calmodulin
- CNS
Central Nervous System
- CSPα
Cysteine String Protein α
- DD
Developmental Delay
- DHPs
Dihydropyridines
- E
Epilepsy
- EBIO
Ethyl-benzimidazolone
- EDHF
Endothelium-derived Hyperpolarizing Factor
- GABAA
Gamma-aminobutyric Acid A Receptors
- GOF
Gain of Function
- ID
Intellectual Disability
- IK Channels
Intermediate Conductance KCa Channels
- KCa
Calcium-activated Potassium Channel
- LOF
Loss of Function
- NMDAR
N-methyl-D-aspartate Receptor
- PNKD
Paroxysmal Dyskinesia
- RCK
Regulators of K+ Conductance
- SK Channels
Small Conductance KCa Channels
AUTHORS’ CONTRIBUTIONS
A.Z. and R.L conducted the literature review and wrote the initial draft of the manuscript. W.H., H.M., Q.W. W.Y., and SC made preliminary revisions to the manuscript. J.W. made critical revisions and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by grants from the National Natural Science Foundation of China (No. 81870935) and the Scientific Research Found of Wuhan University of Technology (No. 40122070) to WJ.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Nappi P., Miceli F., Soldovieri M.V., Ambrosino P., Barrese V., Taglialatela M. Epileptic channelopathies caused by neuronal Kv7 (KCNQ) channel dysfunction. Pflugers Arch. 2020;472(7):881–898. doi: 10.1007/s00424-020-02404-2. [DOI] [PubMed] [Google Scholar]
- 2.Niday Z., Tzingounis A.V. Potassium channel gain of function in epilepsy: An unresolved paradox. Neuroscientist. 2018;24(4):368–380. doi: 10.1177/1073858418763752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kole M.H.P., Stuart G.J. Signal processing in the axon initial segment. Neuron. 2012;73(2):235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Gutman G.A., Chandy K.G., Grissmer S., Lazdunski M., Mckinnon D., Pardo L.A., Robertson G.A., Rudy B., Sanguinetti M.C., Stühmer W., Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005;57(4):473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 5.Barcia G., Fleming M.R., Deligniere A., Gazula V.R., Brown M.R., Langouet M., Chen H., Kronengold J., Abhyankar A., Cilio R., Nitschke P., Kaminska A., Boddaert N., Casanova J.L., Desguerre I., Munnich A., Dulac O., Kaczmarek L.K., Colleaux L., Nabbout R. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat. Genet. 2012;44(11):1255–1259. doi: 10.1038/ng.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sah P., Louise Faber E.S. Channels underlying neuronal calcium-activated potassium currents. Prog. Neurobiol. 2002;66(5):345–353. doi: 10.1016/S0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 7.D’Adamo M.C., Catacuzzeno L., Di Giovanni G., Franciolini F., Pessia M. K+ channelepsy: Progress in the neurobiology of potassium channels and epilepsy. Front. Cell. Neurosci. 2013;7:134. doi: 10.3389/fncel.2013.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zang K., Zhang Y., Hu J., Wang Y. The large conductance calcium-and voltage-activated potassium channel (BK) and epilepsy. CNS Neurol Disord Targets (Formerly. Curr. Drug Targets CNS Neurol. Disord. 2018;17:248–254. doi: 10.2174/1871527317666180404104055. [DOI] [PubMed] [Google Scholar]
- 9.Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J. Physiol. 1985;367(1):117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contet C., Goulding S.P., Kuljis D.A., Barth A.L. BK channels in the central nervous system. Int. Rev. Neurobiol. 2016;128:281–342. doi: 10.1016/bs.irn.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surguchev A., Bai J.P., Joshi P., Navaratnam D. Hair cell BK channels interact with RACK1, and PKC increases its expression on the cell surface by indirect phosphorylation. Am. J. Physiol. Cell Physiol. 2012;303(2):C143–C150. doi: 10.1152/ajpcell.00062.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan J., Aldrich R.W. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 2010;466(7305):513–516. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- 13.Yuan P., Leonetti M.D., Hsiung Y., MacKinnon R. Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel. Nature. 2012;481(7379):94–97. doi: 10.1038/nature10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belyaeva E.A., Sokolova T.V. Mechanism(s) of modulation of Cd2+-induced cytotoxcity by paxilline and NS1619/NS004: An involvement of Ca2+-activated big-conductance potassium channel and/or respiratory chain of mitochondria? Zh. Evol. Biokhim. Fiziol. 2020;56(7):737. doi: 10.31857/S0044452920071523. [DOI] [Google Scholar]
- 15.Cui J. BK channel gating mechanisms: Progresses toward a better understanding of 533 variants linked neurological diseases. Front Physiol. 2021. p. 1867. [DOI] [PMC free article] [PubMed]
- 16.Liu J., Ye J., Zou X., Xu Z., Feng Y., Zou X., Chen Z., Li Y., Cang Y. CRL4ACRBN E3 ubiquitin ligase restricts BK channel activity and prevents epileptogenesis. Nat. Commun. 2014;5(1):3924. doi: 10.1038/ncomms4924. [DOI] [PubMed] [Google Scholar]
- 17.Choi T.Y., Lee S.H., Kim Y.J., Bae J.R., Lee K.M., Jo Y., Kim S.J., Lee A.R., Choi S., Choi L.M., Bang S., Song M.R., Chung J., Lee K.J., Kim S.H., Park C.S., Choi S.Y. Cereblon maintains synaptic and cognitive function by regulating BK channel. J. Neurosci. 2018;38(14):3571–3583. doi: 10.1523/JNEUROSCI.2081-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu H., Shao L.R., Chavoshy S., Gu N., Trieb M., Behrens R., Laake P., Pongs O., Knaus H.G., Ottersen O.P., Storm J.F. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J. Neurosci. 2001;21(24):9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun AX, Yuan Q, Fukuda M, Yu W, Yan H, Lim GGY. Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science (80- ) 2019;366:1486–1492. doi: 10.1126/science.aav5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikitin E.S., Vinogradova L.V. Potassium channels as prominent targets and tools for the treatment of epilepsy. Expert Opin. Ther. Targets. 2021;25(3):223–235. doi: 10.1080/14728222.2021.1908263. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Yan J. Regulation of BK channels by auxiliary Î3 subunits. Front. Physiol. 2014;5:401. doi: 10.3389/fphys.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan C., Sukomon N., Flood E., Rheinberger J., Allen T.W., Nimigean C.M. Ball-and-chain inactivation in a calcium-gated potassium channel. Nature. 2020;580(7802):288–293. doi: 10.1038/s41586-020-2116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toro L., Li M., Zhang Z., Singh H., Wu Y., Stefani E. MaxiK channel and cell signalling. Pflugers Arch. 2014;466(5):875–886. doi: 10.1007/s00424-013-1359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyle B.D., Ahrendt E., Braun A.P., Braun J.E.A. The large conductance, calcium-activated K+ (BK) channel is regulated by cysteine string protein. Sci. Rep. 2013;3(1):2447. doi: 10.1038/srep02447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Junco-Clemente P., Cantero G., Gómez-Sánchez L., Linares-Clemente P., Martínez-López J.A., Luján R., Fernández-Chacón R. Cysteine string protein-α prevents activity-dependent degeneration in GABAergic synapses. J. Neurosci. 2010;30(21):7377–7391. doi: 10.1523/JNEUROSCI.0924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamberlain L.H., Burgoyne R.D. Cysteine-string protein. J. Neurochem. 2000;74(5):1781–1789. doi: 10.1046/j.1471-4159.2000.0741781.x. [DOI] [PubMed] [Google Scholar]
- 27.Ahrendt E., Kyle B., Braun A.P., Braun J.E.A. Cysteine string protein limits expression of the large conductance, calcium-activated K+ (BK) channel. PLoS One. 2014;9(1):e86586. doi: 10.1371/journal.pone.0086586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benton M.D., Lewis A.H., Bant J.S., Raman I.M. Iberiotoxin-sensitive and -insensitive BK currents in Purkinje neuron somata. J. Neurophysiol. 2013;109(10):2528–2541. doi: 10.1152/jn.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graber D., Imagawa E., Miyake N., Matsumoto N., Miyatake S., Graber M. Polymicrogyria in a child with KCNMA1-related channelopathy. Brain Dev. 2021 doi: 10.1016/j.braindev.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Du W., Bautista J.F., Yang H., Diez-Sampedro A., You S.A., Wang L., Kotagal P., Lüders H.O., Shi J., Cui J., Richerson G.B., Wang Q.K. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 2005;37(7):733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Poschmann S., Chen Q., Fazeli W., Oundjian N.J., Snoeijen-Schouwenaars F.M., Fricke O., Kamsteeg E.J., Willemsen M., Wang Q.K. De novo BK channel variant causes epilepsy by affecting voltage gating but not Ca2+ sensitivity. Eur. J. Hum. Genet. 2018;26(2):220–229. doi: 10.1038/s41431-017-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang L., Li X., Moutton S., Schrier Vergano S.A., Cogné B., Saint-Martin A., Hurst A.C.E., Hu Y., Bodamer O., Thevenon J., Hung C.Y., Isidor B., Gerard B., Rega A., Nambot S., Lehalle D., Duffourd Y., Thauvin-Robinet C., Faivre L., Bézieau S., Dure L.S., Helbling D.C., Bick D., Xu C., Chen Q., Mancini G.M.S., Vitobello A., Wang Q.K. De novo loss-of-function KCNMA1 variants are associated with a new multiple malformation syndrome and a broad spectrum of developmental and neurological phenotypes. Hum. Mol. Genet. 2019;28(17):2937–2951. doi: 10.1093/hmg/ddz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mameli C., Cazzola R., Spaccini L., Calcaterra V., Macedoni M., La Verde P.A., D’Auria E., Verduci E., Lista G., Zuccotti G.V. Neonatal diabetes in patients affected by liang-wang syndrome carrying KCnma1 variant p.(Gly375Arg) suggest a potential role of Ca2+ and voltage-activated K+ channel activity in human insulin secretion. Curr. Issues Mol. Biol. 2021;43(2):1036–1042. doi: 10.3390/cimb43020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabarki B., AlMajhad N., AlHashem A., Shaheen R., Alkuraya F.S. Homozygous KCNMA1 mutation as a cause of cerebellar atrophy, developmental delay and seizures. Hum. Genet. 2016;135(11):1295–1298. doi: 10.1007/s00439-016-1726-y. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho-de-Souza J.L., Kubota T., Du X., Latorre R., Gomez C.M., Bezanilla F. A missense mutation in the selectivity filter of BK affects the channel’s potassium conductance. Biophys. J. 2016;110(3):449a. doi: 10.1016/j.bpj.2015.11.2412. [DOI] [Google Scholar]
- 36.Yeşil G., Aralaşmak A., Akyüz E., İçağasıoğlu D., Uygur Şahin T., Bayram Y. Expanding the phenotype of homozygous KCNMA1 mutations; dyskinesia, epilepsy, intellectual disability, cerebellar and corticospinal tract atrophy. Balkan Med. J. 2018;35(4):336–339. doi: 10.4274/balkanmedj.2017.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey C.S., Moldenhauer H.J., Park S.M., Keros S., Meredith A.L. KCNMA1-linked channelopathy. J. Gen. Physiol. 2019;151(10):1173–1189. doi: 10.1085/jgp.201912457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendler K.S., Kalsi G., Holmans P.A., Sanders A.R., Aggen S.H., Dick D.M., Aliev F., Shi J., Levinson D.F., Gejman P.V. Genomewide association analysis of symptoms of alcohol dependence in the molecular genetics of schizophrenia (MGS2) control sample. Alcohol. Clin. Exp. Res. 2011;35(5):963–975. doi: 10.1111/j.1530-0277.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreifeldt M., Cates-Gatto C., Roberts A.J., Contet C. BK channel β1 subunit contributes to behavioral adaptations elicited by chronic intermittent ethanol exposure. Alcohol. Clin. Exp. Res. 2015;39(12):2394–2402. doi: 10.1111/acer.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beecham G.W., Hamilton K., Naj A.C., Martin E.R., Huentelman M., Myers A.J., Corneveaux J.J., Hardy J., Vonsattel J.P., Younkin S.G., Bennett D.A., De Jager P.L., Larson E.B., Crane P.K., Kamboh M.I., Kofler J.K., Mash D.C., Duque L., Gilbert J.R., Gwirtsman H., Buxbaum J.D., Kramer P., Dickson D.W., Farrer L.A., Frosch M.P., Ghetti B., Haines J.L., Hyman B.T., Kukull W.A., Mayeux R.P., Pericak-Vance M.A., Schneider J.A., Trojanowski J.Q., Reiman E.M., Schellenberg G.D., Montine T.J. Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s disease and related dementias. PLoS Genet. 2014;10(9):e1004606. doi: 10.1371/journal.pgen.1004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenz S., Heils A., Kasper J.M., Sander T. Allelic association of a truncation mutation of theKCNMB3 gene with idiopathic generalized epilepsy. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144B(1):10–13. doi: 10.1002/ajmg.b.30369. [DOI] [PubMed] [Google Scholar]
- 42.Poulsen A.N., Wulf H., Hay-Schmidt A., Jansen-Olesen I., Olesen J., Klaerke D.A. Differential expression of BK channel isoforms and β-subunits in rat neuro-vascular tissues. Biochim. Biophys. Acta Biomembr. 2009;1788(2):380–389. doi: 10.1016/j.bbamem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Riazi M.A., Brinkman-Mills P., Johnson A., Naylor S.L., Minoshima S., Shimizu N., Baldini A., McDermid H.E. Identification of a putative regulatory subunit of a calcium-activated potassium channel in the dup(3q) syndrome region and a related sequence on 22q11.2. Genomics. 1999;62(1):90–94. doi: 10.1006/geno.1999.5975. [DOI] [PubMed] [Google Scholar]
- 44.Martin G.E., Hendrickson L.M., Penta K.L., Friesen R.M., Pietrzykowski A.Z., Tapper A.R., Treistman S.N. Identification of a BK channel auxiliary protein controlling molecular and behavioral tolerance to alcohol. Proc. Natl. Acad. Sci. USA. 2008;105(45):17543–17548. doi: 10.1073/pnas.0801068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenner R., Chen Q.H., Vilaythong A., Toney G.M., Noebels J.L., Aldrich R.W. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 2005;8(12):1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 46.Cavalleri G.L., Weale M.E., Shianna K.V., Singh R., Lynch J.M., Grinton B., Szoeke C., Murphy K., Kinirons P., O’Rourke D., Ge D., Depondt C., Claeys K.G., Pandolfo M., Gumbs C., Walley N., McNamara J., Mulley J.C., Linney K.N., Sheffield L.J., Radtke R.A., Tate S.K., Chissoe S.L., Gibson R.A., Hosford D., Stanton A., Graves T.D., Hanna M.G., Eriksson K., Kantanen A.M., Kalviainen R., O’Brien T.J., Sander J.W., Duncan J.S., Scheffer I.E., Berkovic S.F., Wood N.W., Doherty C.P., Delanty N., Sisodiya S.M., Goldstein D.B. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: A case-control study. Lancet Neurol. 2007;6(11):970–980. doi: 10.1016/S1474-4422(07)70247-8. [DOI] [PubMed] [Google Scholar]
- 47.Jafari A., Noursadeghi E., Khodagholi F., Saghiri R., Sauve R., Aliaghaei A., Eliassi A. Brain mitochondrial ATP-insensitive large conductance Ca+2-activated K+ channel properties are altered in a rat model of amyloid-β neurotoxicity. Exp. Neurol. 2015;269:8–16. doi: 10.1016/j.expneurol.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z.B., Tian M.Q., Gao K., Jiang Y.W., Wu Y. De novo KCNMA1 mutations in children with early-onset paroxysmal dyskinesia and developmental delay. Mov. Disord. 2015;30(9):1290–1292. doi: 10.1002/mds.26216. [DOI] [PubMed] [Google Scholar]
- 49.N'Gouemo P. Targeting BK (big potassium) channels in epilepsy. Expert Opin Ther Targets. 2011;15(11):1283–1295. doi: 10.1517/14728222.2011.620607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salkoff L., Butler A., Ferreira G., Santi C., Wei A. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 2006;7(12):921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 51.Deng P.Y., Rotman Z., Blundon J.A., Cho Y., Cui J., Cavalli V., Zakharenko S.S., Klyachko V.A. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77(4):696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia X.M., Zeng X., Lingle C.J. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418(6900):880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 53.Moczydlowski E.G. BK channel news: Full coverage on the calcium bowl. J. Gen. Physiol. 2004;123(5):471–473. doi: 10.1085/jgp.200409069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan P, Leonetti MD, Pico AR, Hsiung Y, MacKinnon R. Structure of the human BK 636 channel Ca2+-activation apparatus at 3.0 Å resolution. Science (80- ) 2010;329:182–186. doi: 10.1126/science.1190414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu R., Alioua A., Kumar Y., Eghbali M., Stefani E., Toro L. MaxiK channel partners: Hysiological impact. J. Physiol. 2006;570(1):65–72. doi: 10.1113/jphysiol.2005.098913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou S., Xu R., Heinemann S.H., Hoshi T. The RCK1 high-affinity Ca2+ sensor confers carbon monoxide sensitivity to Slo1 BK channels. Proc. Natl. Acad. Sci. USA. 2008;105(10):4039–4043. doi: 10.1073/pnas.0800304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang Y., Lee A., Chen J., Cadene M., Chait B.T., MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417(6888):515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 58.Nishida M., Cadene M., Chait B.T., MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 2007;26(17):4005–4015. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niu X., Qian X., Magleby K.L. Linker-gating ring complex as passive spring and Ca2+-dependent machine for a voltage- and Ca2+-activated potassium channel. Neuron. 2004;42(5):745–756. doi: 10.1016/j.neuron.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Schreiber M., Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys. J. 1997;73(3):1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng X.H., Xia X.M., Lingle C.J. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J. Gen. Physiol. 2005;125(3):273–286. doi: 10.1085/jgp.200409239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J., Krishnamoorthy G., Saxena A., Zhang G., Shi J., Yang H., Delaloye K., Sept D., Cui J. An epilepsy/dyskinesia-associated mutation enhances BK channel activation by potentiating Ca2+ sensing. Neuron. 2010;66(6):871–883. doi: 10.1016/j.neuron.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magidovich E., Yifrach O. Conserved gating hinge in ligand- and voltage-dependent K+ channels. Biochemistry. 2004;43(42):13242–13247. doi: 10.1021/bi048377v. [DOI] [PubMed] [Google Scholar]
- 64.Tao X., MacKinnon R. Molecular structures of the human Slo1 K+ channel in complex with β4. eLife. 2019;8:e51409. doi: 10.7554/eLife.51409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y., Xia X.M., Lingle C.J. The functionally relevant site for paxilline inhibition of BK channels. Proc. Natl. Acad. Sci. USA. 2020;117(2):1021–1026. doi: 10.1073/pnas.1912623117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu R., Zhang Z., Liu H., Hou P., Lang J., Wang S., Yan H., Li P., Huang Z., Wu H., Rong M., Huang J., Wang H., Lv L., Qiu M., Ding J., Lai R. Human β-defensin 2 is a novel opener of Ca2+-activated potassium channels and induces vasodilation and hypotension in monkeys. Hypertension. 2013;62(2):415–425. doi: 10.1161/HYPERTENSIONAHA.111.01076. [DOI] [PubMed] [Google Scholar]
- 67.Hoshi T., Heinemann S.H. Modulation of BK channels by small endogenous molecules and pharmaceutical channel openers. Int. Rev. Neurobiol. 2016;128:193–237. doi: 10.1016/bs.irn.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Horrigan F.T., Heinemann S.H., Hoshi T. Heme regulates allosteric activation of the Slo1 BK channel. J. Gen. Physiol. 2005;126(1):7–21. doi: 10.1085/jgp.200509262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou S., Heinemann S.H., Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda) 2009;24(1):26–35. doi: 10.1152/physiol.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science (80- ) 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 71.McManus O.B., Harris G.H., Giangiacomo K.M., Feigenbaum P., Reuben J.P., Addy M.E., Burka J.F., Kaczorowski G.J., Garcia M.L. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993;32(24):6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- 72.Nardi A., Calderone V., Chericoni S., Morelli I. Natural modulators of large-conductance calcium-activated potassium channels. Planta Med. 2003;69(10):885–892. doi: 10.1055/s-2003-45095. [DOI] [PubMed] [Google Scholar]
- 73.Lakshmikanthcharan S., Chaitanya J.S.K., Nandakumar S.M., Nandakumar S.M. Verapamil as an adjuvant treatment for drug-resistant epilepsy. Indian J. Crit. Care Med. 2018;22(9):680–682. doi: 10.4103/ijccm.IJCCM_250_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehranfard N., Gholamipour-Badie H., Motamedi F., Janahmadi M., Naderi N. Long-term increases in BK potassium channel underlie increased action potential firing in dentate granule neurons following pilocarpine-induced status epilepticus in rats. Neurosci. Lett. 2015;585:88–91. doi: 10.1016/j.neulet.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 75.Roy S., Morayo Akande A., Large R.J., Webb T.I., Camarasu C., Sergeant G.P., McHale N.G., Thornbury K.D., Hollywood M.A. Structure-activity relationships of a novel group of large-conductance Ca2+-activated K(+) (BK) channel modulators: The GoSlo-SR family. ChemMedChem. 2012;7(10):1763–1769. doi: 10.1002/cmdc.201200321. [DOI] [PubMed] [Google Scholar]
- 76.Cheney J.A., Weisser J.D., Bareyre F.M., Laurer H.L., Saatman K.E., Raghupathi R., Gribkoff V., Starrett J.E., Jr, McIntosh T.K. The maxi-K channel opener BMS-204352 attenuates regional cerebral edema and neurologic motor impairment after experimental brain injury. J. Cereb. Blood Flow Metab. 2001;21(4):396–403. doi: 10.1097/00004647-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Bentzen B.H., Nardi A., Calloe K., Madsen L.S., Olesen S.P., Grunnet M. The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol. Pharmacol. 2007;72(4):1033–1044. doi: 10.1124/mol.107.038331. [DOI] [PubMed] [Google Scholar]
- 78.Allen D., Bond C.T., Luján R., Ballesteros-Merino C., Lin M.T., Wang K., Klett N., Watanabe M., Shigemoto R., Stackman R.W., Jr, Maylie J., Adelman J.P. The SK2-long isoform directs synaptic localization and function of SK2-containing channels. Nat. Neurosci. 2011;14(6):744–749. doi: 10.1038/nn.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pedarzani P., Stocker M. Molecular and cellular basis of small and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell. Mol. Life Sci. 2008;65(20):3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarpal D., Koenig J.I., Adelman J.P., Brady D., Prendeville L.C., Shepard P.D. Regional distribution of SK3 mRNA-containing neurons in the adult and adolescent rat ventral midbrain and their relationship to dopamine-containing cells. Synapse. 2004;53(2):104–113. doi: 10.1002/syn.20042. [DOI] [PubMed] [Google Scholar]
- 81.Stocker M., Pedarzani P. Differential distribution of three Ca2+-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol. Cell. Neurosci. 2000;15(5):476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- 82.Adelman J.P., Maylie J., Sah P. Small-conductance Ca2+-activated K+ channels: Form and function. Annu. Rev. Physiol. 2012;74(1):245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 83.Bond C.T., Maylie J., Adelman J.P. Small-conductance calcium-activated potassium channels. Ann. N.Y. Acad. Sci. 1999;868(1):370–378. doi: 10.1111/j.1749-6632.1999.tb11298.x. [DOI] [PubMed] [Google Scholar]
- 84.Stackman R.W., Hammond R.S., Linardatos E., Gerlach A., Maylie J., Adelman J.P., Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 2002;22(23):10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Z., Shi G., Liu Y., Xing H., Kabakov A.Y., Zhao A.S., Agbortoko V., Kim J., Singh A.K., Koren G., Harrington E.O., Sellke F.W., Feng J. Coronary endothelial dysfunction prevented by small-conductance calcium-activated potassium channel activator in mice and patients with diabetes. J. Thorac. Cardiovasc. Surg. 2020;160(6):e263–e280. doi: 10.1016/j.jtcvs.2020.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vick K.A., IV, Guidi M., Stackman R.W., Jr. in vivo pharmacological manipulation of small conductance Ca2+-activated K+ channels influences motor behavior, object memory and fear conditioning. Neuropharmacology. 2010;58(3):650–659. doi: 10.1016/j.neuropharm.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kushwah N., Jain V., Kadam M., Kumar R., Dheer A., Prasad D., Kumar B., Khan N. Ginkgo biloba L. Prevents hypobaric hypoxia-induced spatial memory deficit through small conductance calcium-activated potassium channel inhibition: The role of ERK/] CaMKII/CREB signaling. Front. Pharmacol. 2021;12:669701. doi: 10.3389/fphar.2021.669701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hammond R.S., Bond C.T., Strassmaier T., Jennifer Ngo-Anh T., Adelman J.P., Maylie J., Stackman R.W. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J. Neurosci. 2006;26(6):1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jenkins D.P., Strøbæk D., Hougaard C., Jensen M.L., Hummel R., Sørensen U.S., Christophersen P., Wulff H. Negative gating modulation by (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphthylamine (NS8593) depends on residues in the inner pore vestibule: Pharmacological evidence of deep-pore gating of K(Ca)2 channels. Mol. Pharmacol. 2011;79(6):899–909. doi: 10.1124/mol.110.069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh S., Syme C.A., Singh A.K., Devor D.C., Bridges R.J. Benzimidazolone activators of chloride secretion: Potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J. Pharmacol. Exp. Ther. 2001;296(2):600–611. [PubMed] [Google Scholar]
- 91.Al Dera H., Alassiri M., Eleawa S.M., AlKhateeb M.A., Hussein A.M., Dallak M., Sakr H.F., Alqahtani S., Khalil M.A. Melatonin improves memory deficits in rats with cerebral hypoperfusion, possibly, through decreasing the expression of small-conductance Ca2+-activated K+ channels. Neurochem. Res. 2019;44(8):1851–1868. doi: 10.1007/s11064-019-02820-6. [DOI] [PubMed] [Google Scholar]
- 92.Ewin S.E., Morgan J.W., Niere F., McMullen N.P., Barth S.H., Almonte A.G., Raab-Graham K.F., Weiner J.L. Chronic intermittent ethanol exposure selectively increases synaptic excitability in the ventral domain of the rat hippocampus. Neuroscience. 2019;398:144–157. doi: 10.1016/j.neuroscience.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fakira A.K., Portugal G.S., Carusillo B., Melyan Z., Morón J.A. Increased small conductance calcium-activated potassium type 2 channel-mediated negative feedback on N-methyl-D-aspartate receptors impairs synaptic plasticity following context-dependent sensitization to morphine. Biol. Psychiatry. 2014;75(2):105–114. doi: 10.1016/j.biopsych.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ishikawa M., Mu P., Moyer J.T., Wolf J.A., Quock R.M., Davies N.M., Hu X., Schlüter O.M., Dong Y. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J. Neurosci. 2009;29(18):5820–5831. doi: 10.1523/JNEUROSCI.5703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee C-H, MacKinnon R. Activation mechanism of a human SKcalmodulin channel complex elucidated by cryo-EM structures. Science (80) 2018;360:508–513. doi: 10.1126/science.aas9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chandy K.G., Fantino E., Kalman K., Gutman G.A., Gargus J.J. Gene encoding neuronal calcium-activated potassium channel has polymorphic CAG repeats, a candidate role in excitotoxic neurodegeneration and maps to 22q11-q13, critical region for bipolar disease and Schizophrenia disorder 4. Am. J. Hum. Genet. 1997;61:A305–A305. [Google Scholar]
- 97.Blank T., Nijholt I., Kye M.J., Radulovic J., Spiess J. Small-conductance, Ca2+-activated K+ channel SK3 generates age-related memory and LTP deficits. Nat. Neurosci. 2003;6(9):911–912. doi: 10.1038/nn1101. [DOI] [PubMed] [Google Scholar]
- 98.Grube S., Gerchen M.F., Adamcio B., Pardo L.A., Martin S., Malzahn D., Papiol S., Begemann M., Ribbe K., Friedrichs H., Radyushkin K.A., Müller M., Benseler F., Riggert J., Falkai P., Bickeböller H., Nave K.A., Brose N., Stühmer W., Ehrenreich H. A CAG repeat polymorphism of KCNN3 predicts SK3 channel function and cognitive performance in schizophrenia. EMBO Mol. Med. 2011;3(6):309–319. doi: 10.1002/emmm.201100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hopf F.W., Bowers M.S., Chang S.J., Chen B.T., Martin M., Seif T., Cho S.L., Tye K., Bonci A. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron. 2010;65(5):682–694. doi: 10.1016/j.neuron.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oliveira M.S., Skinner F., Arshadmansab M.F., Garcia I., Mello C.F., Knaus H.G., Ermolinsky B.S., Otalora L.F.P., Garrido-Sanabria E.R. Altered expression and function of small-conductance (SK) Ca2+-activated K+ channels in pilocarpine-treated epileptic rats. Brain Res. 2010;1348:187–199. doi: 10.1016/j.brainres.2010.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ritter-Makinson S., Clemente-Perez A., Higashikubo B., Cho F.S., Holden S.S., Bennett E., Chkhaidze A., Eelkman Rooda O.H.J., Cornet M.C., Hoebeek F.E., Yamakawa K., Cilio M.R., Delord B., Paz J.T. Augmented reticular thalamic bursting and seizures in Scn1a-Dravet syndrome. Cell Rep. 2019;26(1):54–64.e6. doi: 10.1016/j.celrep.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller M.J., Rauer H., Tomita H., Rauer H., Gargus J.J., Gutman G.A., Cahalan M.D., Chandy K.G. Nuclear localization and dominant-negative suppression by a mutant SKCa3 N-terminal channel fragment identified in a patient with schizophrenia. J. Biol. Chem. 2001;276(30):27753–27756. doi: 10.1074/jbc.C100221200. [DOI] [PubMed] [Google Scholar]
- 103.Hugues M., Romey G., Duval D., Vincent J.P., Lazdunski M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: Voltage-clamp and biochemical characterization of the toxin receptor. Proc. Natl. Acad. Sci. USA. 1982;79(4):1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mourre C., Fournier C., Soumireu-Mourat B. Apamin, a blocker of the calcium-activated potassium channel, induces neurodegeneration of Purkinje cells exclusively. Brain Res. 1997;778(2):405–408. doi: 10.1016/S0006-8993(97)01165-7. [DOI] [PubMed] [Google Scholar]
- 105.Pedarzani P., D’hoedt D., Doorty K.B., Wadsworth J.D.F., Joseph J.S., Jeyaseelan K., Kini R.M., Gadre S.V., Sapatnekar S.M., Stocker M., Strong P.N. Tamapin, a venom peptide from the Indian red scorpion (Mesobuthus tamulus) that targets small conductance Ca2+-activated K+ channels and afterhyperpolarization currents in central neurons. J. Biol. Chem. 2002;277(48):46101–46109. doi: 10.1074/jbc.M206465200. [DOI] [PubMed] [Google Scholar]
- 106.Strøbæk D., Hougaard C., Johansen T.H., Sørensen U.S., Nielsen E.Ø., Nielsen K.S., Taylor R.D.T., Pedarzani P., Christophersen P. Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons. Mol. Pharmacol. 2006;70(5):1771–1782. doi: 10.1124/mol.106.027110. [DOI] [PubMed] [Google Scholar]
- 107.Strøbæk D., Teuber L., Jørgensen T.D., Ahring P.K., Kjær K., Hansen R.S., Olesen S.P., Christophersen P., Skaaning-Jensen B. Activation of human IK and SK Ca2+-activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime). Biochim. Biophys. Acta Biomembr. 2004;1665(1-2):1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 108.Sankaranarayanan A., Raman G., Busch C., Schultz T., Zimin P.I., Hoyer J., Köhler R., Wulff H. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol. Pharmacol. 2009;75(2):281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hougaard C., Eriksen B.L., Jørgensen S., Johansen T.H., Dyhring T., Madsen L.S., Strøbaek D., Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+ -activated K+ channels. Br. J. Pharmacol. 2007;151(5):655–665. doi: 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shakkottai V.G., Chou C., Oddo S., Sailer C.A., Knaus H.G., Gutman G.A., Barish M.E., LaFerla F.M., Chandy K.G. Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia. J. Clin. Invest. 2004;113(4):582–590. doi: 10.1172/JCI200420216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kasumu A.W., Hougaard C., Rode F., Jacobsen T.A., Sabatier J.M., Eriksen B.L., Strøbæk D., Liang X., Egorova P., Vorontsova D., Christophersen P., Rønn L.C.B., Bezprozvanny I. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem. Biol. 2012;19(10):1340–1353. doi: 10.1016/j.chembiol.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cao Y.J., Dreixler J.C., Couey J.J., Houamed K.M. Modulation of recombinant and native neuronal SK channels by the neuroprotective drug riluzole. Eur. J. Pharmacol. 2002;449(1-2):47–54. doi: 10.1016/S0014-2999(02)01987-8. [DOI] [PubMed] [Google Scholar]
- 113.Bauer C.K., Schneeberger P.E., Kortüm F., Altmüller J., Santos-Simarro F., Baker L., Keller-Ramey J., White S.M., Campeau P.M., Gripp K.W., Kutsche K. Gain-of-function mutations in KCNN3 encoding the small-conductance Ca2+-activated K+ channel SK3 cause Zimmermann-Laband syndrome. Am. J. Hum. Genet. 2019;104(6):1139–1157. doi: 10.1016/j.ajhg.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gripp K.W., Smithson S.F., Scurr I.J., Baptista J., Majumdar A., Pierre G., Williams M., Henderson L.B., Wentzensen I.M., McLaughlin H., Leeuwen L., Simon M.E.H., van Binsbergen E., Dinulos M.B.P., Kaplan J.D., McRae A., Superti-Furga A., Good J.M., Kutsche K. Syndromic disorders caused by gain-of-function variants in KCNH1, KCNK4, and KCNN3—a subgroup of K+ channelopathies. Eur. J. Hum. Genet. 2021;29(9):1384–1395. doi: 10.1038/s41431-021-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koot B.G.P., Alders M., Verheij J., Beuers U., Cobben J.M. A de novo mutation in KCNN3 associated with autosomal dominant idiopathic non-cirrhotic portal hypertension. J. Hepatol. 2016;64(4):974–977. doi: 10.1016/j.jhep.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 116.Rapetti-Mauss R., Lacoste C., Picard V., Guitton C., Lombard E., Loosveld M., Nivaggioni V., Dasilva N., Salgado D., Desvignes J.P., Béroud C., Viout P., Bernard M., Soriani O., Vinti H., Lacroze V., Feneant-Thibault M., Thuret I., Guizouarn H., Badens C. A mutation in the Gardos channel is associated with hereditary xerocytosis. Blood. 2015;126(11):1273–1280. doi: 10.1182/blood-2015-04-642496. [DOI] [PubMed] [Google Scholar]
- 117.Glogowska E., Lezon-Geyda K., Maksimova Y., Schulz V.P., Gallagher P.G. Mutations in the Gardos channel (KCNN4) are associated with hereditary xerocytosis. Blood. 2015;126(11):1281–1284. doi: 10.1182/blood-2015-07-657957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andolfo I., Russo R., Manna F., Shmukler B.E., Gambale A., Vitiello G., De Rosa G., Brugnara C., Alper S.L., Snyder L.M., Iolascon A. Novel Gardos channel mutations linked to dehydrated hereditary stomatocytosis (xerocytosis). Am. J. Hematol. 2015;90(10):921–926. doi: 10.1002/ajh.24117. [DOI] [PubMed] [Google Scholar]
- 119.Gárdos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim. Biophys. Acta. 1958;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- 120.Ishii T.M., Silvia C., Hirschberg B., Bond C.T., Adelman J.P., Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. USA. 1997;94(21):11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Logsdon N.J., Kang J., Togo J.A., Christian E.P., Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J. Biol. Chem. 1997;272(52):32723–32726. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- 122.Ghanshani S., Wulff H., Miller M.J., Rohm H., Neben A., Gutman G.A., Cahalan M.D., Chandy K.G. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000;275(47):37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 123.Chen M.X., Gorman S.A., Benson B., Singh K., Hieble J.P., Michel M.C., Tate S.N., Trezise D.J. Small and intermediate conductance Ca2+ -activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369(6):602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- 124.Nguyen T.V., Matsuyama H., Baell J., Hunne B., Fowler C.J., Smith J.E., Nurgali K., Furness J.B. Effects of compounds that influence IK (KCNN4) channels on afterhyperpolarizing potentials, and determination of IK channel sequence, in guinea pig enteric neurons. J. Neurophysiol. 2007;97(3):2024–2031. doi: 10.1152/jn.00935.2006. [DOI] [PubMed] [Google Scholar]
- 125.Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion N V, Maylie J. Small-conductance, calcium-activated potassium channels from mammalian brain. Science (80- ) 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 126.Nilius B., Vriens J., Prenen J., Droogmans G., Voets T. TRPV4 calcium entry channel: A paradigm for gating diversity. Am J Physiol Physiol. 2004;286(2):C195–205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 127.Sahu G., Asmara H., Zhang F.X., Zamponi G.W., Turner R.W. Activity-dependent facilitation of CaV1. 3 calcium channels promotes KCa3. 1 activation in hippocampal neurons. J. Neurosci. 2017;37(46):11255–11270. doi: 10.1523/JNEUROSCI.0967-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu Z., Dou F., Wang Y., Hou L., Chen H. Ca2+-dependent endoplasmic reticulum stress correlation with astrogliosis involves upregulation of KCa3.1 and inhibition of AKT/mTOR signaling. J. Neuroinflammation. 2018;15(1):316. doi: 10.1186/s12974-018-1351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choi S., Kim J.A., Li H., Shin K.O., Oh G.T., Lee Y.M., Oh S., Pewzner-Jung Y., Futerman A.H., Suh S.H. KCa 3.1 upregulation preserves endothelium‐dependent vasorelaxation during aging and oxidative stress. Aging Cell. 2016;15(5):801–810. doi: 10.1111/acel.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rauer H., Lanigan M.D., Pennington M.W., Aiyar J., Ghanshani S., Cahalan M.D., Norton R.S., Chandy K.G. Structure-guided transformation of charybdotoxin yields an analog that selectively targets Ca2+-activated over voltage-gated K(+) channels. J. Biol. Chem. 2000;275(2):1201–1208. doi: 10.1074/jbc.275.2.1201. [DOI] [PubMed] [Google Scholar]
- 131.Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- 132.Visan V., Fajloun Z., Sabatier J.M., Grissmer S. Mapping of Maurotoxin Binding Sites on hKv1.2, hKv1.3, and hIKCa1 Channels. Mol. Pharmacol. 2004;66(5):1103–1112. doi: 10.1124/mol.104.002774. [DOI] [PubMed] [Google Scholar]
- 133.Jensen B.S., Strøbæk D., Christophersen P., Jørgensen T.D., Hansen C., Silahtaroglu A., Olesen S.P., Ahring P.K. Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am. J. Physiol. Cell Physiol. 1998;275(3):C848–C856. doi: 10.1152/ajpcell.1998.275.3.C848. [DOI] [PubMed] [Google Scholar]
- 134.Wulff H., Gutman G.A., Cahalan M.D., Chandy K.G. Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J. Biol. Chem. 2001;276(34):32040–32045. doi: 10.1074/jbc.M105231200. [DOI] [PubMed] [Google Scholar]
- 135.Oliván-Viguera A., Valero M.S., Coleman N., Brown B.M., Laría C., Divina M.M., Gálvez J.A., Díaz-de-Villegas M.D., Wulff H., Badorrey R., Köhler R. A novel pan-negative-gating modulator of KCa2/3 channels, fluoro-di-benzoate, RA-2, inhibits endothelium-derived hyperpolarization-type relaxation in coronary artery and produces bradycardia in vivo. Mol. Pharmacol. 2015;87(2):338–348. doi: 10.1124/mol.114.095745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Devor D.C., Singh A.K., Frizzell R.A., Bridges R.J. Modulation of Cl- secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am. J. Physiol. 1996;271(5 Pt 1):L775–L784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]