Abstract
Purpose of Review:
In the gastro-intestinal tract, the complex network of multiple innate cell populations play critical roles not only as a first line of defense against invading pathogens and in driving adaptive immune responses, but also in maintaining intestinal homeostasis. Here, we describe the roles of various innate immune cell populations in gut immunity and detail studies investigating the impact of acute and chronic HIV infection on these cell populations.
Recent Findings:
Alterations in frequencies, phenotype and/or function of innate lymphoid cells, dendritic cells, macrophages, neutrophils and innate-like T cells have been reported in people with HIV (PWH), with many of these features persisting despite anti-retroviral therapy and virological suppression.
Summary:
Dysregulated gut innate immunity in PWH is a feature of gut pathogenesis. A greater understanding of the mechanisms driving impairment in the multiple different gut innate immune cell populations and the downstream consequences of an altered innate immune response on host defense and gut homeostasis in PWH is needed to develop more effective HIV treatments and cure strategies.
Keywords: HIV, innate immunity, gut
Introduction.
Innate immunity occurs rapidly and is considered the first line of defense against invading pathogens. Innate immune responses are typically mediated through cell-dependent mechanisms (e.g. phagocytosis, cytotoxicity) and/or via secreted factors (e.g cytokines, Type I Interferons [Type I IFNs]) [1]. In the gastro-intestinal (GI) tract, innate immune cells such as innate lymphoid cells (ILCs), myeloid and plasmacytoid dendritic cells (mDCs; pDCs) and macrophages reside in the intra-epithelial layer as well as deeper into the lamina propria where they play roles not only in providing protection against invading pathogens and in driving adaptive immunity, but in maintaining immunological tolerance [1,2]. Furthermore, the human gut contains unconventional T cells with innate like properties, including mucosal-associated invariant T (MAIT) cells, γδ T cells and invariant Natural Killer T (iNKT) cells. These unusual T cells typically recognize conserved microbial molecules (e.g. lipid antigens, bacterial metabolites) presented via MHC-like molecules, but in some situations they can also be activated independently of MHC molecules [3,4]. Fundamental to the role of innate immune cells in maintaining gut homeostasis is their close interaction with the gut microbiome, a collection of commensal microbiota representing bacteria, viruses, bacteriophages and fungi. The most well studied of these, the bacterial microbiome is essential for the development and function of innate and adaptive immunity [5]. These critical innate immune cell-microbiome interactions are mediated both directly (e.g. bacteria-specific ligands binding to their specific receptors expressed by multiple innate immune cells) and indirectly via production of proteins and metabolites such as short chain fatty acids that regulate immune cell function and metabolism. Alterations in the microbial community structure, as occurs in a number of gut-associated inflammatory diseases including HIV, may therefore have a profound effect on gut innate immunity.
Frequencies of gut innate and innate-like immune cells are typically low in healthy tissue making investigations into their contributions to HIV pathogenesis difficult. However, a number of groups have met this challenge, and their investigations into the impact of HIV infection on these innate immune cells are reviewed below.
Innate Lymphoid Cells.
ILCs populate mucosal barriers including the GI tract where they play critical roles in maintaining homeostasis and in defense against pathogens [6–10]. Group 1 ILCs, which include Natural Killer (NK) cells and the more recently discovered ILC1 subset, express the transcription factors Tbet and Eomes and are primarily anti-viral with cytolytic functions and production of IFNγ. Group 2 ILCs (ILC2s) express GATA3, produce the Type 2 cytokines IL-4, IL-5 and IL-13, and are involved in immunity against helminths. Group 3 ILCs (ILC3s) express RORγt, produce IL-22, GM-CSF, IL-17 and the lymphotoxin LT-α1β2, and in addition to limiting extracellular bacterial and fungal infections, ILC3s play a role in epithelial barrier maintenance and tissue repair. A number of studies have highlighted the plasticity of ILCs whereby different subsets modify their phenotype and cytokine profiles to reflect those of their counterparts (e.g. ILC3 to ILC1 profile) in response to tissue-dependent cues including cytokines and microbiota [11].
Group 1 ILCs.
Mela and colleagues were first to show depletion of NK cells (identified as CD3−CD56+) in colonic lamina propria of untreated, viremic PWH versus uninfected controls [12]. Colonic intraepithelial and lamina propria NK cells, identified by NKp46 expression, were also reduced in frequency during untreated chronic HIV infection [13]. Interestingly, rectal NK cells (CD56+CD16+) from chronically infected, viremic PWH were lower in frequency, but more activated (based on CD69 expression) versus anti-retroviral therapy (ART)-naïve PWH with plasma viral loads <2000 HIV RNA copies/ml (HIV controllers) [14]. Providing further support for a role of viral replication in driving the depletion of gut NK cells , similar frequencies of rectal NK cells relative to uninfected controls were observed in HIV controllers (rectal NK cells identified as CD56+CD16+) [15], and in the colon (identified as CD3−CD56+) [12] as well as throughout the GI tract (identified as CD56+CD94+NKp44−) [16], in ART-treated PWH with viral suppression. Of note, CD94 expression was recently shown to identify a population of blood memory-like CD56hi NK cells with greater cytolytic activity versus CD94− NK cells and frequencies of which were increased in ART-naïve PWH, but not impacted in either HIV controllers or in ART-treated PWH [17]. In a recent study, Utay and colleagues highlighted that the timing of ART initiation differentially impacted frequencies of rectal NK cell subsets, identified based on CD56 and CD16 expression [18]. For example, lower frequencies of CD56brightCD16− NK cells were observed in PWH who initiated ART during either acute or chronic infection versus untreated, chronically infected PWH; however, ART initiation during the chronic phase was associated with higher frequencies of CD56brightCD16dim NK cells versus frequencies observed when ART was started during the acute stage. Few studies have directly investigated the impact of HIV infection on gut ILC1s. In chronically-infected and ART-treated PWH, ileum and colonic ILC1s were depleted [16,19], and ILC1s were not restored with ART, a finding linked to impaired IL-7 responses [16].
Group 2 and 3 ILCs.
ILC2s constitute only a minor fraction of total ILCs throughout the human GI tract [16] and have not been studied extensively in PWH. In one study, similar frequencies of ILC2s were observed in ART-treated PWH versus uninfected controls in the small intestine (duodenum, ileum) and in the colon [16].
In 2009, Cella and colleagues described a population of human tonsil CD3− cells that co-expressed NKp44 and CD56 and were capable of producing IL-22 (termed NK22 cells) [20], a population of cells now considered to be ILC3s. Using this early definition of ILC3s, frequencies of colonic CD3− IL-22-producing cells were increased during acute HIV infection [21], reduced in frequency in chronic infection [22] and restored to levels similar to controls during long term ART [23]. We evaluated frequencies of NKp44±CD56± colonic cells producing IFNγ or IL-22 and observed higher frequencies of colonic NKp44+CD56− producing IFNγ in untreated, PWH versus controls [24]. In uninfected persons, this population of ILCs typically produced IL-22, suggesting a switch to an inflammatory phenotype in PWH. Frequencies of NKp44+CD56− IFNγ+ ILC associated with colonic mDC and T cell activation and with the relative abundance of mucosa-associated Prevotella, a commensal bacteria found at a significantly higher relative abundance in our cohort of PWH [25]. Our observations suggested that a critical interplay between gut ILCs, other local immune cells, and dysbiotic enteric bacteria may exist in chronic, untreated HIV infection.
In a recent study, utilizing an updated scheme to identify ILC3s based on co-expression of CD117 and CD127 and exclusion of other cells that also expressed these makers (“lineage-“) [26,27], Kloverpris et al. observed no difference in frequencies of lineage−CD127+CD117+ ILC3s in the colon of PWH versus uninfected controls [28]. However, ART status of the PWH was not detailed in that study. In other studies, ILC3s identified as Lin−CD127+Rorγt+ using flow cytometry or evaluated by histology as CD3−CD117+ were decreased in ART-treated PWH [17]●. Investigations of Lin−CD127+CD117+ ILC3s across the GI tract of ART-treated PWH also noted a decrease in colonic ILC3s, but intriguingly, frequencies were increased in the duodenum [16]. These compartment-specific differences were extended into functional differences, such that IL-22 production by colonic ILC3s was significantly higher in ART-treated PWH. Conversely, no HIV-associated differences were observed in frequencies of IL-22+ duodenum or ileum ILC3s. In a recent study, Wang and colleagues postulated that the loss of protective gut-resident ILC3s and the associated inflammatory state in the setting of HIV infection may drive the expansion of circulating memory NK cells [17]. Given that memory NK cells may have a greater ability to control HIV, studies directly investigating the role of gut ILC3s in concert with gut memory NK cells are warranted.
Although as a whole, these studies suggest that HIV infection impacts gut ILC3 frequencies, future studies will need to more consistently adopt the current standard nomenclature for ILC3 identification [27] and investigate not only how HIV infection impacts frequencies, but also ILC3 function particularly in light of the their critical role in gut epithelial barrier maintenance and gut homeostasis.
Myeloid cells: mDCs and macrophages.
The mononuclear phagocyte system has historically been categorized into mDCs, monocytes, and macrophages based on phenotypical and functional characteristics; however, advancing technologies such as single cell RNAseq (scRNAseq) have highlighted that many of these phenotypic features are often overlapping [29,30]. Macrophages and mDCs are found throughout the intestinal tract where they serve as gatekeepers between the external (i.e. lumen) and internal (i.e. intra-epithelial and lamina propria) environments and mediate the delicate balance between immunogenic and tolerogenic intestinal immune responses [31–34]. In their role as potent antigen presenting cells, mDCs are instrumental in the induction of adaptive immunity against enteric microbes, including orchestrating high-affinity IgA antibodies against enteric pathogens, induction of regulatory T cells, and driving cytotoxic and T helper (Th) cell responses [33]. Our recent in vitro mechanistic studies demonstrated that human gut mDCs were required for commensal and pathogenic bacteria-induced IL-22 production by ILC3s [35], for IFNγ production by Group I ILCs [36] and in the commensal bacteria-induced expansion and enhanced HIV infection of lamina propria CD4 T cells [37]. These findings provide further evidence of the importance of mDC in human gut immunity via cross talk with other local innate and adaptive immune cells. Tissue macrophages consist of populations of both long-lived resident cells, primarily found in the deeper layers of the intestine, and populations of cells located close to the epithelial layer and in the lamina propria that are continuously replaced by circulating monocytes [31,32,34]. Of note, although classically known for their ability to phagocytose pathogens and dead cells, macrophages can also serve many similar functions to mDCs including to mediate interactions with resident enteric microbiota leading to production of cytokines, to promote regulatory T cells, or to promote IL-22 production by ILC3s.
Myeloid Dendritic Cells.
To date, few studies have investigated gut mDCs in the setting of HIV infection. In a recent study extensively interrogating the impact of untreated HIV infection on colonic mDC frequency and phenotype, we observed that, despite no significant differences in frequency, CD1c+ mDCs in PWH expressed an altered activation phenotype characterized by increased levels of CD40, but lower expression of CD83 [38]. Increased CD40 expression by mDC was associated with increased colonic and peripheral blood CD4 and CD8 T cell activation, constitutive production of colonic mucosa-associated inflammatory cytokines including IL-23, IL-6, TNFα and IFNγ, and with the relative abundances of dysbiotic enteric bacteria. In other studies, increased levels of indoleamine 2,3-dioxygenase 1 (IDO1) in rectosigmoid DEC205+ mDCs was linked to the inversion of Th17/T regulatory cell ratio in chronically infected PWH [39]. Taken together, these studies provided the initial evidence that gut mDCs may play a central role in driving gut inflammation in the setting of untreated HIV-1 infection. However, we believe it will be necessary to cultivate a greater appreciation of human gut mDC biology throughout the course of HIV disease. Given the recent observation that ART did not fully restore gene transcriptional changes in peripheral blood mDC that had occurred during untreated HIV infection [40], these future studies should include an evaluation of the function of gut mDC during viral suppression with ART to determine if a failure to restore gut mDC phenotype and function is a contributing factor to the persistence of gut dysfunction despite effective viral suppression with ART. These types of studies will be necessary to permit the design of novel treatments that target gut mDCs with the intent on reducing mDC-mediated inflammation (e.g. via anti-cytokine therapy, induction of tolerogenic mDC subsets).
Macrophages.
Frequencies of CD68+ or CD169+ macrophages were increased in duodenum of untreated PWH, normalized with ART, and related in vitro studies suggested that the accumulated macrophages in untreated PWH displayed impaired phagocytic ability [41]. In histological staining of colonic tissue of untreated PWH and uninfected controls, we observed higher levels of both lipoteichoic acid (LTA), a Gram-positive cell wall component, and lipopolysaccharide (LPS), a Gram-negative cell wall component in PWH [38]. Although more LTA+ HAM56+ macrophages were observed in PWH than in uninfected controls, no similar increase in frequencies of LPS+ macrophages was observed in that cohort, an observation in keeping with a potential defect in macrophage function and uptake of LPS and/or Gram-negative bacteria. Accumulation of colon CD14+ inflammatory macrophages were also noted in African AIDS patients presenting with diarrhea and/or weight loss, but without overt opportunistic infections, with frequencies positively associated with plasma LPS [42]. Thus, despite accumulation of gut macrophages during chronic HIV infection, their potential dysfunction may be a contributing factor to an apparent inability to limit microbial translocation.
Role of gut mDC and macrophages in cell-to-cell transmission of HIV-1.
HIV infection of target cells can occur with both cell-free viral particles and via cell-to-cell transfer of viral material [43]. In addition to membrane protrusions, cell fusion and cell engulfment processes, cell-associated HIV-1 transmission occurs through the establishment of ‘infectious’ or ‘virological’ synapses. Although a number of cellular factors have been implicated in HIV trans-infection of CD4 T cells [44], the role of CD169, also known as Siglec-1, has recently garnered attention. CD169 is induced by Type I IFNs, which consist of several cytokines that include the 12 IFNα subtypes and IFNβ [45] (discussed in more detail below). Early in vitro studies implicated CD169 expression by both blood-derived and tonsil mDCs and by blood-derived macrophages in mediating HIV-1 transfer to CD4 T cells [46–52]. Recent in vitro studies highlighted that upon virus capture, HIV-1 particles co-localize with CD169 within virus containing compartments (VCC), thus providing potential mechanisms of viral evasion [53,54] and suggesting that inhibiting CD169 uptake of HIV-1 may provide additional strategies to reduce HIV transmission and/or eradiate HIV reservoirs. In vivo, CD169 was required for dissemination of a murine retrovirus [55], while HIV p24 was detected within CD169+ cervical cells of a viremic PWH [56] and CD169+ p27gag+ cells detected in lymph nodes of SHIV-infected macaques [46].
We recently reported that Type I IFN expression is compartmentalized during untreated, chronic HIV infection, with higher IFNβ, but lower IFNα gene expression observed in the colonic mucosa of untreated, viremic PWH versus uninfected controls [57]. Therefore, to probe potential contributions of IFNβ expression on CD169-mediated HIV trans-infection of gut CD4 T cells, we utilized an in vitro human intestinal cell model [58] to evaluate CD169 expression on gut mDC and macrophages in response to varying doses of exogenously added recombinant IFNβ (Fig. 1). Our initial studies suggest that IFNβ primarily drives CD169 expression on gut macrophages with minimal impact on gut mDC. Studies are underway to further investigate the underlying mechanisms governing how Type I IFNs impact CD169 expression and HIV trans-infection of gut CD4 T cells. Given that bacterial Toll-like receptor ligands such as LPS enhance CD169 expression on monocyte-derived DC [53], it will be important to determine if the threshold for IFNβ-induced CD169 by gut myeloid cells is altered in the presence of bacteria or bacterial ligands and to potentially link microbial translocation to HIV transmission and to shifting the role of Type I IFNs from protective to pathogenic [59].
Figure 1. In vitro exposure of gut macrophages to IFNβ increases CD169 expression.
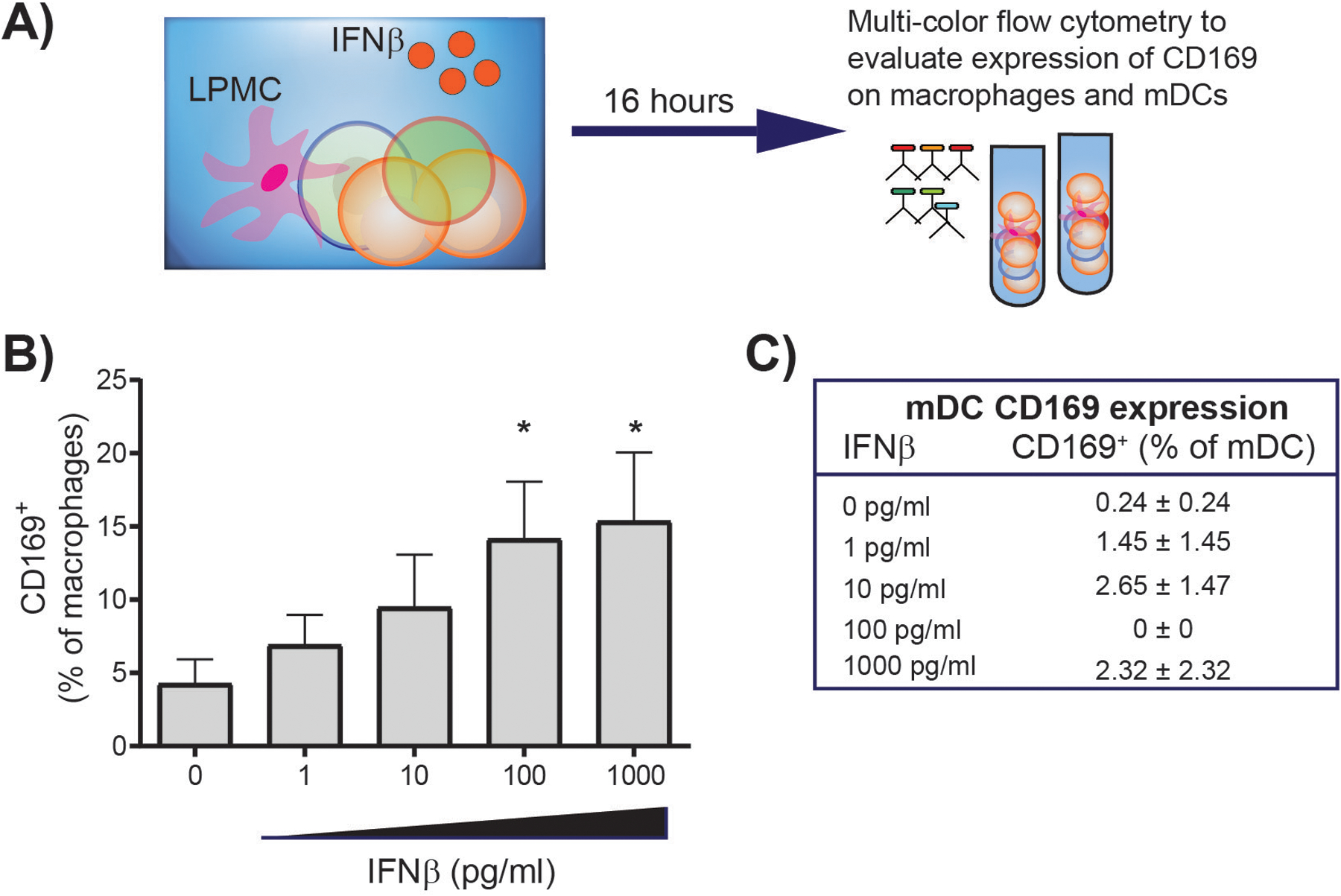
Jejunum lamina propria mononuclear cells (LPMC) were cultured with increasing doses of IFNβ for 16–18hrs (N=5). Cells were collected and multi-color flow cytometry used to evaluate expression of CD169 in macrophages and mDC. Macrophages were identified as HLA-DR+CD11c±CD64+ and mDC as HLA-DR+CD11c+CD64− within viable, CD45+ CD3− CD19− cells [105,106]. A minimum of 25 events were required for analysis of CD169 expression therefore CD169 expression on mDC is in shown for 3 LPMC samples. Statistical analysis: Paired t test comparing IFNβ condition to no IFNβ; *P<0.05. LPMC were isolated from patients undergoing elective abdominal surgery and were designated discarded tissue from macroscopically normal sites. Samples from patients with a history of Inflammatory Bowel Disease, HIV-1 infection, treatment with immunosuppressive drugs, or recent chemotherapy (within 8 weeks) were excluded from the study. LPMC were isolated from tissue samples in a two step-procedure to remove epithelial cells followed by collagenase-digestion to release LPMC as previously described [37,58,107]. All patients undergoing surgery signed a release form to allow unrestricted use of discarded tissue for research purposes. Protected patient information was de-identified to the laboratory investigators. Research associated with the use of LPMC was reviewed by the Colorado Multiple Institutional Review Board (COMIRB) at UC-AMC and deemed Not Human Subject Research.
Plasmacytoid Dendritic Cells.
Gut pDCs play a critical role both in anti-viral immunity and in immune regulation primarily due to being the primary producers of Type I IFNs [60,61]. Increased numbers of activated (CD40+) colonic CD303+ pDCs were identified in untreated PWH, and activated gut pDCs were associated with levels of colonic mDC activation, suggesting that drivers of gut DC activation may be similar for both subsets and/or that a form of cross talk occurs between these two DC subsets [38]. In duodenum of persons with HIV/AIDS, accumulating pDCs expressed Ki67 [62]. The authors postulated that, given that mature pDCs do not typically proliferate, the presence of Ki67+ CD303+ pDCs in colons of untreated PWH is due to the accumulation of naïve pDCs, likely of bone marrow origin. Of note, Boichuk and colleagues also noted that accumulated pDCs expressed the cytolytic molecule granzyme B and proposed that expression of this granzyme by pDC was an additional contributor to gut HIV pathogenesis [62]. In another study, higher frequencies of ileum CD303+/CD123+ pDCs were found in untreated PWH, and pDC frequencies were associated with plasma levels of IFNα and with mucosal CD8 T cell activation. Further, pDC frequencies had not normalized after 6 months of ART [63]. In HIV-infected humanized mouse models, pDC and Type I IFNs were linked to ILC apoptosis and death and postulated as one mechanism driving depletion of gut ILC3s and ILC1s in chronic HIV infection [19,22].
Other gut immune cells with innate or innate-like properties.
Neutrophils.
Neutrophils are polymorphonuclear leukocytes whose primary function is to kill microbes via phagocytosis, degranulation and establishment of neutrophil extracellular traps (NETosis) [64,65]. As ‘first responders’, neutrophils are also important in the establishment of the ensuing immune response and both recruit and interact with multiple other immune cells including macrophages and DCs. Few studies have directly investigated gut neutrophils in the setting of HIV infection. Neutrophil infiltration, quantified by histological staining of the enzyme myeloperoxidase (MPO), was shown to be increased in the colons of both untreated and ART-treated acute and chronically infected PWH [17,66,67]. Hensley-McBain and colleagues expanded on this work and utilized multi-color flow cytometry to confirm that colonic neutrophils accumulated in ART-treated individuals [68]●. Further, they linked increased neutrophil frequencies to prolonged neutrophil survival, a finding potentially related to features of dysbiosis (i.e. reduced Lactobacillus:Prevotella ratio).
Mucosal-associated invariant T cells.
MAIT cells are recently identified unconventional innate-like T cells that express CD161 and a semi-invariant T cell receptor (TCR; Vα7.2) and recognize microbial vitamin B metabolites presented by the highly conserved MHC Class I-like molecule MR1 [69]. The majority of MAIT cells express CD8 [70]. Activated MAIT cells are capable of killing infected cells, of inhibiting microbial growth and of secreting cytokines such as IFNγ, TNFα, IL-17 and IL-22. MAIT cells can also be activated in an MHC/TCR-independent manner via IL-12 and IL-18 and may be important in anti-viral immunity. MAIT cells, measured as the proportion of CD161+ cells within the CD8 T cell population, were reduced in colonic biopsies from untreated, chronically infected PWH versus uninfected controls, with restoration of MAIT cells noted in ART-treated PWH [71]. Of note, histological enumeration of colonic MAIT cells (identified using MDR1 as a substitute for CD161) also demonstrated a reduction in the proportion of MAIT cells within total CD8 T cells of chronically infected PWH; however, evaluation of frequencies per area of tissue eliminated this difference, suggesting that reduced proportions were likely a result of an overall increase in CD8 T cell frequencies [72]. Similarly, no significant loss of rectal CD161+Vα7.2+ MAIT cells within CD8 T cells was observed in rectal tissue of chronically-infected PWH [70] or within CD3+ CD4− T cells [73]. Of note, the low frequencies of CD4-expressing MAIT cells noted in controls was further reduced in PWH [70].
γδ T cells.
γδ T cells, defined by their expression of a unique TCR composed of one γ-chain and one δ-chain, are capable of mounting rapid cytolytic and inflammatory cytokine-mediated immune responses typically following activation via MHC Class II-independent presentation of antigens (e.g. phosphoantigens) and/or signaling through activation receptors (e.g. NKG2d) [74]. Gut γδ T cells, located in the intra-epithelial layer and underlying lamina propria, have essential roles in monitoring epithelial barrier function, scanning for indicators of cellular stress and mediating host protection against bacteria and viral infections [75]. The impact of HIV infection on gut γδ T cell frequencies may vary by location within the gut, both throughout the GI tract and between the intra-epithelial and lamina propria layers. Early studies utilizing histological analysis to specifically identify duodenal γδ T cells located in the intra-epithelial layer demonstrated an expansion of this T cell subset in individuals with late-stage HIV-1 infection and found that frequencies remained elevated despite ART [76,77]. Subsequent studies utilized multi-color flow cytometry analyses of pinch biopsies and therefore quantified γδ T cells without the ability to differentiate between those located in the intra-epithelial or lamina propria layers. In one study, percentages of rectal γδ T cells, as a fraction of total CD3+ T cells, were decreased in untreated, chronically infected PWH versus controls and in PWH who initiated ART treatment either early in HIV infection or later during the chronic phase [73]. In contrast, Poles and colleagues observed an overall increase of rectal γδ T cells (similarly enumerated as a percentage of CD3+ T cells) in untreated, chronically-infected PWH, and they noted an expansion of γδ T cells expressing the Vδ1 chain, but a decreased fraction of the Vδ2 γδ T cell subset [78]. In ART-treated PWH with effective viral suppression, frequencies of rectal γδ T cells and associated Vδ1 and Vδ2 subsets appeared to partially normalize [78]. Frequencies of duodenal Vδ1 γδ T cells amongst CD3+ T cells were significantly lower in both acutely-infected PWH (1 of 15 were receiving ART) and chronically-infected PWH (3 of 14 were receiving ART) versus healthy controls with no differences in Vδ2 frequencies between the cohorts [79]. Interestingly, the ability of Vδ2 γδ T cells to constitutively produce IFNγ was lost during chronic infection suggesting that these cells were functionally exhausted [79]. Similar frequencies of Vδ1 γδ T cells in colon, duodenum and ileum were found between HIV controllers and uninfected controls, further suggesting a role for HIV-1 replication in alterations in γδ T cell frequencies [80].
Invariant Natural Killer T cells.
Human iNKT cells are a rare population of T cells characterized by expression of semi-invariant TCR (Vα24-Jα18 preferentially paired with Vβ11) that recognizes glycolipids presented by MHC Class I-related glycoprotein CD1d [81]. iNKT cells play important roles in immunity against a wide range of microbes including bacteria, fungi, parasites and viruses. Human iNKT cell repertoire consists of CD4+, CD8+, and CD4−CD8− cells, among which CD4+ iNKT cells account for approximately 50% of total iNKT cells and predominantly produce IL-4 [82]. CD4− cells mainly produce Th1 cytokines and have cytolytic activity [82,83]. In one study, rectal iNKT cells represented <0.5% of total CD3+ T cells, and lower levels were observed in chronically infected PWH due to a preferential depletion of CD4+ iNKT cells. Frequencies of CD4+ iNKT cells were inversely associated with plasma viremia [84]. In contrast, preservation of rectal iNKT cells were observed in ART-treated PWH compared to healthy controls, with no differences in the proportion of iNKT cells expressing CD4 [85]; production of IL-4 and IL-10 by gut iNKT cells inversely associated with systemic indicators of microbial translocation [85]. Furthermore, abundance of Bacteroides positively associated with iNKT frequencies and production of IL-4. Thus, iNKT cells may play an important role in local immune activation and microbial translocation and their function is potentially influenced by the enteric microbiota [85].
Type I Interferons.
Type I IFNs are a diverse family of innate cytokines that, in humans, include 12 distinct IFNα subtypes and IFNβ, all of which signal through the Type I IFN receptor (IFNR) to induce hundreds of interferon-stimulated genes (ISGs) that drive a wide range of biological activities [86]. During acute HIV-1 infection, Type I IFNs are generally considered to play a protective anti-viral role; however, during chronic infection, Type I IFNs have been associated with features of HIV-1 pathogenesis including inflammation and immune activation [59]. We recently showed compartmentalization of the Type I IFN response during untreated, chronic HIV infection [57]. IFNα subtypes were upregulated in peripheral blood mononuclear cells (PBMC), but were downregulated in colonic tissue versus controls; IFNβ gene transcripts were undetectable in PBMC, but significantly higher in colonic tissue of PWH. Furthermore, expression of canonical Type I IFN-induced viral restriction factors was elevated in colonic tissue of PWH and was associated with colon and systemic features of HIV pathogenesis. In follow up studies, in vitro exposure of uninfected gut CD4 T cells to dominant IFNα subtypes or IFNβ identified sets of ISGs upregulated by all Type I IFNs tested (core ISGs) as well as genes specifically induced by IFNβ (βISGs) [87]. In untreated, chronically infected PWH, core ISG expression positively correlated with IFNβ rather than IFNα transcripts, suggesting that IFNβ drove these responses. Core ISG expression positively correlated with plasma LPS, and βISGs positively correlated with plasma IL-6 levels. These findings further strengthen the link between gut Type I IFN expression and indicators of HIV pathogenesis.[88]
Several studies support a potential role of the gut microbiome in regulating Type I IFN responses. Many ISGs were upregulated in gut CD4 T cells following in vitro exposure with Prevotella stercorea [89], and Type I IFN response genes (IFNB, IFNAR1 and Mx2) in gut tissue inversely correlated with the abundance of stool Prevotella in ART-treated PWH [90]. In ART-treated PWH, a multi-strain probiotic supplement (Visbiome) taken twice daily over a 6 month period increased gut transcript levels of certain IFNα subtypes [91], including subtypes previously shown to exhibit the greatest anti-viral activity in human gut immune cells in vitro [92].
Contribution of gut innate immunity to HIV cure strategies.
ART is highly effective at suppressing HIV viral loads in the periphery; however, it is unable to completely eradicate the virus due to the persistence of latent infection and HIV-1 viral reservoirs found throughout the body, including the GI tract with gut CD4 T cells reportedly representing up to 95% of all HIV-infected cells in ART-treated PWH [93–97].To date, therapeutic cure strategies involve either eliminating HIV (sterilizing cure) or providing control of HIV in the absence of ART (functional cure). The “shock and kill” approach targets latently-infected CD4 T cells by utilizing latency reversal treatments to reactivate HIV gene expression, making them susceptible to killing by cytotoxic CD8 T cell responses, a process that may be dependent on effective innate immunity [95]. Surprisingly, NK cells, pDCs and Type I IFNs, but not HIV-specific CD8 T cells were implicated in the decline in CD4 T cell-associated HIV-1 DNA following treatment with the histone deacetylase inhibitor panobinostat [98]. Blood pDC and multiple Type I IFNs inhibited establishment of in vitro latent infection in blood CD4 T cells; however once latency was established, IFNα demonstrated an ability to initiate HIV-1 transcription [99]. Consequently, the dysfunction that remains in gut innate immunity in ART-treated PWH may be a significant barrier to the elimination of gut HIV-1 reservoirs and development of fully-effective HIV-1 cure strategies.
In addition to harnessing the innate immune system to reduce the gut HIV-1 reservoir, HIV-1 cure strategies may also need to target innate cellular sources of the reservoir. Although debate remains, gut macrophages have been proposed as one of these potential sources [100]. Early in vivo studies demonstrated expression of intracellular p24 in duodenal CD68+ and CD64+ macrophages [101] and HIV DNA was detected in rectal CD13+ myeloid cells [102]. However, Gag HIV-DNA was rarely detected in highly purified colonic myeloid cells [103]. With the recent identification of long-lived, self-renewing tissue resident gut macrophages, further studies are needed to dissect the role of this population of cells, versus myeloid cells potentially replenished by infected blood monocytes, as a potential HIV-1 reservoir. Furthermore, given that viral SIV DNA detected within small and large intestinal myeloid cells of untreated, SIV-infected macaques was associated with phagocytosis of SIV-infected T cells [104], it will be important to utilize technologies (e.g. RNAScope and single cell assays) that not only accurately define and isolate to high purity the various gut-associated myeloid subsets, but also identify bone-fide latently-infected cells, a task made more challenging in light of the low frequency of these cell populations [100].
Conclusions.
HIV-1 infection dramatically alters the gut innate immune system landscape impacting many, if not all, of the multiple innate cell populations that play vital roles in gut immunity and homeostasis. While some of these defects appear to normalize with ART and viral suppression, suggesting a role for viral replication in driving their dysfunction, many defects persist and likely contribute to the ongoing gut inflammation and epithelial barrier disruption associated with chronic immune activation in PWH. However, despite the dedication of numerous groups in overcoming the inherent difficulties in studying these typically rare immune cells in human gut tissue and understanding their contribution to HIV-associated gut pathogenesis, knowledge gaps remain. The nature of impairment needs to be expanded beyond evaluation of frequencies alone, and the impact of HIV-1 infection on innate immune function should be further explored. Moreover, although correlative analyses typically undertaken in clinical studies are informative, these should be followed up with mechanistic studies to provide possible avenues for the development of novel treatments that target specific innate immune cells with the ultimate goal of reducing chronic inflammation. Finally, a better understanding of the contribution of gut innate immune cells as a source of the HIV-1 reservoir as well as how their functional properties can be harnessed to provide more effective functional control of HIV are needed for the development of optimal HIV cure strategies.
Acknowledgements.
We would like to acknowledge and give our sincere thanks to all the study participants who generously contributed their time and biological samples to the many clinical studies detailed in this review. We would also like to acknowledge and thank Steven Lada for his assistance with the in vitro studies detailed in Figure 1.
Financial support and sponsorship.
S.M.D. and C.C.W. are currently supported by funding from NIH (R01AI118983; R01AI134220; R21AG062932). Previously unpublished studies included in this review were supported by R01AI134220. Published studies conducted by Drs. Dillon and Wilson mentioned in this review were supported by R01DK088663, R01AI118983 and R01AI108404.
Footnotes
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Human and Animal Rights and Informed Consent. All reported studies/experiments with human or animal subjects performed by the authors that were previously published complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Human and Animal rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Stephanie M. Dillon, University of Colorado Anschutz Medical Campus, Department of Medicine, Division of Infectious Diseases, Aurora, CO, USA..
Cara C. Wilson, University of Colorado Anschutz Medical Campus, Department of Medicine, Division of Infectious Diseases, Aurora, CO, USA.
References.
- 1.Gasteiger G, D’Osualdo A, Schubert DA, Weber A, Bruscia EM, Hartl D: Cellular Innate Immunity: An Old Game with New Players. J Innate Immun 2017, 9:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R: Control of adaptive immunity by the innate immune system. Nat Immunol 2015, 16:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett MS, Round JL, Leung DT: Innate-like lymphocytes in intestinal infections. Curr Opin Infect Dis 2015, 28:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou C, Li MO: Tissue-Resident Lymphocytes Across Innate and Adaptive Lineages. Front Immunol 2018, 9:2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng D, Liwinski T, Elinav E: Interaction between microbiota and immunity in health and disease. Cell Res 2020, 30:492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellanos JG, Longman RS: The balance of power: innate lymphoid cells in tissue inflammation and repair. J Clin Invest 2019, 129:2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbert DR, Douglas B, Zullo K: Group 2 Innate Lymphoid Cells (ILC2): Type 2 Immunity and Helminth Immunity. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pantazi E, Powell N: Group 3 ILCs: Peacekeepers or Troublemakers? What’s Your Gut Telling You?! Front Immunol 2019, 10:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poggi A, Benelli R, Vene R, Costa D, Ferrari N, Tosetti F, Zocchi MR: Human Gut-Associated Natural Killer Cells in Health and Disease. Front Immunol 2019, 10:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo GY, Giles DA, Kronenberg M: The role of innate lymphoid cells in response to microbes at mucosal surfaces. Mucosal Immunol 2020, 13:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colonna M: Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity. Immunity 2018, 48:1104–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mela CM, Steel A, Lindsay J, Gazzard BG, Gotch FM, Goodier MR: Depletion of natural killer cells in the colonic lamina propria of viraemic HIV-1-infected individuals. AIDS 2007, 21:2177–2182. [DOI] [PubMed] [Google Scholar]
- 13.Sips M, Sciaranghella G, Diefenbach T, Dugast AS, Berger CT, Liu Q, Kwon D, Ghebremichael M, Estes JD, Carrington M, et al. : Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol 2012, 5:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taborda NA, Gonzalez SM, Alvarez CM, Correa LA, Montoya CJ, Rugeles MT: Higher Frequency of NK and CD4+ T-Cells in Mucosa and Potent Cytotoxic Response in HIV Controllers. PLoS One 2015, 10:e0136292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taborda NA, Gonzalez SM, Correa LA, Montoya CJ, Rugeles MT: Spontaneous HIV Controllers Exhibit Preserved Immune Parameters in Peripheral Blood and Gastrointestinal Mucosa. J Acquir Immune Defic Syndr 2015, 70:115–121. [DOI] [PubMed] [Google Scholar]
- 16.Kramer B, Goeser F, Lutz P, Glassner A, Boesecke C, Schwarze-Zander C, Kaczmarek D, Nischalke HD, Branchi V, Manekeller S, et al. : Compartment-specific distribution of human intestinal innate lymphoid cells is altered in HIV patients under effective therapy. PLoS Pathog 2017, 13:e1006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.● Wang Y, Lifshitz L, Gellatly K, Vinton CL, Busman-Sahay K, McCauley S, Vangala P, Kim K, Derr A, Jaiswal S, et al. : HIV-1-induced cytokines deplete homeostatic innate lymphoid cells and expand TCF7-dependent memory NK cells. Nat Immunol 2020, 21:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study elegantly demonstrates that depletion of gut ILC3s persists in People with HIV (PWH) despite effective virologic suppression by anti-retroviral therapy and lower frequencies of gut ILC3s are associated with neutrophil accumulation and with Type I Interferons.
- 18.Utay NS, Vigil KJ, Somasunderam A, Aulicino PC, Smulevitz B, Chiadika S, Wolf DS, Kimata JT, Arduino RC: Timing of Antiretroviral Therapy Initiation Determines Rectal Natural Killer Cell Populations. AIDS Res Hum Retroviruses 2020, 36:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, Cheng L, Wang H, Yu H, Tu B, Fu Q, Li G, Wang Q, Sun Y, Zhang X, et al. : Infection and depletion of CD4+ group-1 innate lymphoid cells by HIV-1 via type-I interferon pathway. PLoS Pathog 2018, 14:e1006819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M: A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009, 457:722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LY, et al. : A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol 2012, 5:670–680. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Cheng L, Zhao J, Li G, Zhang L, Chen W, Nie W, Reszka-Blanco NJ, Wang FS, Su L: Plasmacytoid dendritic cells promote HIV-1-induced group 3 innate lymphoid cell depletion. J Clin Invest 2015, 125:3692–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes SM, Pires AR, Ferreira C, Foxall RB, Rino J, Santos C, Correia L, Pocas J, Veiga-Fernandes H, Sousa AE: Enteric mucosa integrity in the presence of a preserved innate interleukin 22 compartment in HIV type 1-treated individuals. J Infect Dis 2014, 210:630–640. [DOI] [PubMed] [Google Scholar]
- 24.Dillon SM, Castleman MJ, Frank DN, Austin GL, Gianella S, Cogswell AC, Landay AL, Barker E, Wilson CC: Brief Report: Inflammatory Colonic Innate Lymphoid Cells Are Increased During Untreated HIV-1 Infection and Associated With Markers of Gut Dysbiosis and Mucosal Immune Activation. J Acquir Immune Defic Syndr 2017, 76:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, et al. : An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 2014, 7:983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. : Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 2013, 13:145–149. [DOI] [PubMed] [Google Scholar]
- 27.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. : Innate Lymphoid Cells: 10 Years On. Cell 2018, 174:1054–1066. [DOI] [PubMed] [Google Scholar]
- 28.Kloverpris HN, Kazer SW, Mjosberg J, Mabuka JM, Wellmann A, Ndhlovu Z, Yadon MC, Nhamoyebonde S, Muenchhoff M, Simoni Y, et al. : Innate Lymphoid Cells Are Depleted Irreversibly during Acute HIV-1 Infection in the Absence of Viral Suppression. Immunity 2016, 44:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S: Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014, 14:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, et al. : Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bain CC, Schridde A: Origin, Differentiation, and Function of Intestinal Macrophages. Front Immunol 2018, 9:2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller PA, Matheis F, Mucida D: Gut macrophages: key players in intestinal immunity and tissue physiology. Curr Opin Immunol 2020, 62:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun T, Nguyen A, Gommerman JL: Dendritic Cell Subsets in Intestinal Immunity and Inflammation. J Immunol 2020, 204:1075–1083. [DOI] [PubMed] [Google Scholar]
- 34.Viola MF, Boeckxstaens G: Intestinal resident macrophages: Multitaskers of the gut. Neurogastroenterol Motil 2020, 32:e13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castleman MJ, Dillon SM, Purba CM, Cogswell AC, Kibbie JJ, McCarter MD, Santiago ML, Barker E, Wilson CC: Commensal and Pathogenic Bacteria Indirectly Induce IL-22 but Not IFNgamma Production From Human Colonic ILC3s via Multiple Mechanisms. Front Immunol 2019, 10:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castleman MJ, Dillon SM, Purba C, Cogswell AC, McCarter M, Barker E, Wilson C: Enteric bacteria induce IFNgamma and Granzyme B from human colonic Group 1 Innate Lymphoid Cells. Gut Microbes 2019:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillon SM, Manuzak JA, Leone AK, Lee EJ, Rogers LM, McCarter MD, Wilson CC: HIV-1 infection of human intestinal lamina propria CD4+ T cells in vitro is enhanced by exposure to commensal Escherichia coli. J Immunol 2012, 189:885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dillon SM, Lee EJ, Kotter CV, Austin GL, Gianella S, Siewe B, Smith DM, Landay AL, McManus MC, Robertson CE, et al. : Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol 2016, 9:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, et al. : Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2010, 2:32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray SM, Zhang Y, Douek DC, Sekaly RP: Myeloid Cells Enriched for a Dendritic Cell Population From People Living With HIV Have Altered Gene Expression Not Restored by Antiretroviral Therapy. Front Immunol 2020, 11:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allers K, Fehr M, Conrad K, Epple HJ, Schurmann D, Geelhaar-Karsch A, Schinnerling K, Moos V, Schneider T: Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J Infect Dis 2014, 209:739–748. [DOI] [PubMed] [Google Scholar]
- 42.Cassol E, Rossouw T, Malfeld S, Mahasha P, Slavik T, Seebregts C, Bond R, du Plessis J, Janssen C, Roskams T, et al. : CD14(+) macrophages that accumulate in the colon of African AIDS patients express pro-inflammatory cytokines and are responsive to lipopolysaccharide. BMC Infect Dis 2015, 15:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bracq L, Xie M, Benichou S, Bouchet J: Mechanisms for Cell-to-Cell Transmission of HIV-1. Front Immunol 2018, 9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coleman CM, Gelais CS, Wu L: Cellular and viral mechanisms of HIV-1 transmission mediated by dendritic cells. Adv Exp Med Biol 2013, 762:109–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivashkiv LB, Donlin LT: Regulation of type I interferon responses. Nat Rev Immunol 2014, 14:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akiyama H, Ramirez NP, Gibson G, Kline C, Watkins S, Ambrose Z, Gummuluru S: Interferon-Inducible CD169/Siglec1 Attenuates Anti-HIV-1 Effects of Alpha Interferon. J Virol 2017, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, et al. : Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol 2012, 10:e1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jobe O, Trinh HV, Kim J, Alsalmi W, Tovanabutra S, Ehrenberg PK, Peachman KK, Gao G, Thomas R, Kim JH, et al. : Effect of cytokines on Siglec-1 and HIV-1 entry in monocyte-derived macrophages: the importance of HIV-1 envelope V1V2 region. J Leukoc Biol 2016, 99:1089–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pino M, Erkizia I, Benet S, Erikson E, Fernandez-Figueras MT, Guerrero D, Dalmau J, Ouchi D, Rausell A, Ciuffi A, et al. : HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology 2015, 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, Gummuluru S: Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog 2013, 9:e1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puryear WB, Yu X, Ramirez NP, Reinhard BM, Gummuluru S: HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc Natl Acad Sci U S A 2012, 109:7475–7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rempel H, Calosing C, Sun B, Pulliam L: Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS One 2008, 3:e1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akiyama H, Ramirez NG, Gudheti MV, Gummuluru S: CD169-mediated trafficking of HIV to plasma membrane invaginations in dendritic cells attenuates efficacy of anti-gp120 broadly neutralizing antibodies. PLoS Pathog 2015, 11:e1004751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammonds JE, Beeman N, Ding L, Takushi S, Francis AC, Wang JJ, Melikyan GB, Spearman P: Siglec-1 initiates formation of the virus-containing compartment and enhances macrophage-to-T cell transmission of HIV-1. PLoS Pathog 2017, 13:e1006181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sewald X, Ladinsky MS, Uchil PD, Beloor J, Pi R, Herrmann C, Motamedi N, Murooka TT, Brehm MA, Greiner DL, et al. : Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science 2015, 350:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez-Zsolt D, Cantero-Perez J, Erkizia I, Benet S, Pino M, Serra-Peinado C, Hernandez-Gallego A, Castellvi J, Tapia G, Arnau-Saz V, et al. : Dendritic Cells From the Cervical Mucosa Capture and Transfer HIV-1 via Siglec-1. Front Immunol 2019, 10:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dillon SM, Guo K, Austin GL, Gianella S, Engen PA, Mutlu EA, Losurdo J, Swanson G, Chakradeo P, Keshavarzian A, et al. : A compartmentalized type I interferon response in the gut during chronic HIV-1 infection is associated with immunopathogenesis. AIDS 2018, 32:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dillon SM, Guo K, Castleman MJ, Santiago ML, Wilson CC: Quantifying HIV-1-Mediated Gut CD4+ T Cell Death in the Lamina Propria Aggregate Culture (LPAC) Model. Bio-protocol 2020, 10:: e3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scagnolari C, Antonelli G: Type I interferon and HIV: Subtle balance between antiviral activity, immunopathogenesis and the microbiome. Cytokine Growth Factor Rev 2018, 40:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lombardi VC, Khaiboullina SF: Plasmacytoid dendritic cells of the gut: relevance to immunity and pathology. Clin Immunol 2014, 153:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Won HY, Lee JY, Ryu D, Kim HT, Chang SY: The Role of Plasmacytoid Dendritic Cells in Gut Health. Immune Netw 2019, 19:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boichuk SV, Khaiboullina SF, Ramazanov BR, Khasanova GR, Ivanovskaya KA, Nizamutdinov EZ, Sharafutdinov MR, Martynova EV, DeMeirleir KL, Hulstaert J, et al. : Gut-Associated Plasmacytoid Dendritic Cells Display an Immature Phenotype and Upregulated Granzyme B in Subjects with HIV/AIDS. Front Immunol 2015, 6:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehmann C, Jung N, Forster K, Koch N, Leifeld L, Fischer J, Mauss S, Drebber U, Steffen HM, Romerio F, et al. : Longitudinal analysis of distribution and function of plasmacytoid dendritic cells in peripheral blood and gut mucosa of HIV infected patients. J Infect Dis 2014, 209:940–949. [DOI] [PubMed] [Google Scholar]
- 64.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A: Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012, 30:459–489. [DOI] [PubMed] [Google Scholar]
- 65.Fournier BM, Parkos CA: The role of neutrophils during intestinal inflammation. Mucosal Immunol 2012, 5:354–366. [DOI] [PubMed] [Google Scholar]
- 66.Deleage C, Schuetz A, Alvord WG, Johnston L, Hao XP, Morcock DR, Rerknimitr R, Fletcher JL, Puttamaswin S, Phanuphak N, et al. : Impact of early cART in the gut during acute HIV infection. JCI Insight 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, Martin JN, Deeks SG, McCune JM, Hunt PW: Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS 2015, 29:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.● Hensley-McBain T, Wu MC, Manuzak JA, Cheu RK, Gustin A, Driscoll CB, Zevin AS, Miller CJ, Coronado E, Smith E, et al. : Increased mucosal neutrophil survival is associated with altered microbiota in HIV infection. PLoS Pathog 2019, 15:e1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights that the accumulation of neutrophils in the gut of chronically-infected, anti-retroviral therapy-treated people with HIV (PWH) is linked to alterations in the enteric microbiome.
- 69.Ioannidis M, Cerundolo V, Salio M: The Immune Modulating Properties of Mucosal-Associated Invariant T Cells. Front Immunol 2020, 11:1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leeansyah E, Ganesh A, Quigley MF, Sonnerborg A, Andersson J, Hunt PW, Somsouk M, Deeks SG, Martin JN, Moll M, et al. : Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 2013, 121:1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greathead L, Metcalf R, Gazzard B, Gotch F, Steel A, Kelleher P: CD8+/CD161++ mucosal-associated invariant T-cell levels in the colon are restored on long-term antiretroviral therapy and correlate with CD8+ T-cell immune activation. AIDS 2014, 28:1690–1692. [DOI] [PubMed] [Google Scholar]
- 72.Cosgrove C, Ussher JE, Rauch A, Gartner K, Kurioka A, Huhn MH, Adelmann K, Kang YH, Fergusson JR, Simmonds P, et al. : Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood 2013, 121:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kok A, Hocqueloux L, Hocini H, Carriere M, Lefrou L, Guguin A, Tisserand P, Bonnabau H, Avettand-Fenoel V, Prazuck T, et al. : Early initiation of combined antiretroviral therapy preserves immune function in the gut of HIV-infected patients. Mucosal Immunol 2015, 8:127–140. [DOI] [PubMed] [Google Scholar]
- 74.Lawand M, Dechanet-Merville J, Dieu-Nosjean MC: Key Features of Gamma-Delta T-Cell Subsets in Human Diseases and Their Immunotherapeutic Implications. Front Immunol 2017, 8:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCarthy NE, Eberl M: Human gammadelta T-Cell Control of Mucosal Immunity and Inflammation. Front Immunol 2018, 9:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nilssen DE, Brandtzaeg P: Intraepithelial gammadelta T cells remain increased in the duodenum of AIDS patients despite antiretroviral treatment. PLoS One 2012, 7:e29066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nilssen DE, Muller F, Oktedalen O, Froland SS, Fausa O, Halstensen TS, Brandtzaeg P: Intraepithelial gamma/delta T cells in duodenal mucosa are related to the immune state and survival time in AIDS. J Virol 1996, 70:3545–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poles MA, Barsoum S, Yu W, Yu J, Sun P, Daly J, He T, Mehandru S, Talal A, Markowitz M, et al. : Human immunodeficiency virus type 1 induces persistent changes in mucosal and blood gammadelta T cells despite suppressive therapy. J Virol 2003, 77:10456–10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cimini E, Agrati C, D’Offizi G, Vlassi C, Casetti R, Sacchi A, Lionetti R, Bordoni V, Tumino N, Scognamiglio P, et al. : Primary and Chronic HIV Infection Differently Modulates Mucosal Vdelta1 and Vdelta2 T-Cells Differentiation Profile and Effector Functions. PLoS One 2015, 10:e0129771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olson GS, Moore SW, Richter JM, Garber JJ, Bowman BA, Rawlings CA, Flagg M, Corleis B, Kwon DS: Increased frequency of systemic pro-inflammatory Vdelta1(+) gammadelta T cells in HIV elite controllers correlates with gut viral load. Sci Rep 2018, 8:16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kinjo Y, Kitano N, Kronenberg M: The role of invariant natural killer T cells in microbial immunity. J Infect Chemother 2013, 19:560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, Rugeles MT, Atkinson MA, Landay AL, Wilson SB: Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology 2007, 122:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou L, Adrianto I, Wang J, Wu X, Datta I, Mi QS: Single-Cell RNA-Seq Analysis Uncovers Distinct Functional Human NKT Cell Sub-Populations in Peripheral Blood. Front Cell Dev Biol 2020, 8:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ibarrondo FJ, Wilson SB, Hultin LE, Shih R, Hausner MA, Hultin PM, Anton PA, Jamieson BD, Yang OO: Preferential depletion of gut CD4-expressing iNKT cells contributes to systemic immune activation in HIV-1 infection. Mucosal Immunol 2013, 6:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paquin-Proulx D, Ching C, Vujkovic-Cvijin I, Fadrosh D, Loh L, Huang Y, Somsouk M, Lynch SV, Hunt PW, Nixon DF, et al. : Bacteroides are associated with GALT iNKT cell function and reduction of microbial translocation in HIV-1 infection. Mucosal Immunol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schneider WM, Chevillotte MD, Rice CM: Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol 2014, 32:513–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo K, Shen G, Kibbie J, Gonzalez T, Dillon SM, Smith HA, Cooper EH, Lavender K, Hasenkrug KJ, Sutter K, et al. : Qualitative Differences Between the IFNalpha subtypes and IFNbeta Influence Chronic Mucosal HIV-1 Pathogenesis. PLoS Pathog 2020, 16:e1008986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hughes SM, Levy CN, Calienes FL, Stekler JD, Pandey U, Vojtech L, Berard AR, Birse K, Noel-Romas L, Richardson B, et al. : Treatment with Commonly Used Antiretroviral Drugs Induces a Type I/III Interferon Signature in the Gut in the Absence of HIV Infection. Cell Rep Med 2020, 1:100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoder AC, Guo K, Dillon SM, Phang T, Lee EJ, Harper MS, Helm K, Kappes JC, Ochsenbauer C, McCarter MD, et al. : The transcriptome of HIV-1 infected intestinal CD4+ T cells exposed to enteric bacteria. PLoS Pathog 2017, 13:e1006226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pinacchio C, Scagnolari C, Iebba V, Santinelli L, Innocenti GP, Frasca F, Bitossi C, Scordio M, Oliveto G, Ceccarelli G, et al. : High abundance of genus Prevotella is associated with dysregulation of IFN-I and T cell response in HIV-1-infected patients. AIDS 2020, 34:1467–1473. [DOI] [PubMed] [Google Scholar]
- 91.Pinacchio C, Scheri GC, Statzu M, Santinelli L, Ceccarelli G, Innocenti GP, Vullo V, Antonelli G, Brenchley JM, d’Ettorre G, et al. : Type I/II Interferon in HIV-1-Infected Patients: Expression in Gut Mucosa and in Peripheral Blood Mononuclear Cells and Its Modification upon Probiotic Supplementation. J Immunol Res 2018, 2018:1738676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harper MS, Guo K, Gibbert K, Lee EJ, Dillon SM, Barrett BS, McCarter MD, Hasenkrug KJ, Dittmer U, Wilson CC, et al. : Interferon-alpha Subtypes in an Ex Vivo Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms. PLoS Pathog 2015, 11:e1005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chaillon A, Gianella S, Dellicour S, Rawlings SA, Schlub TE, De Oliveira MF, Ignacio C, Porrachia M, Vrancken B, Smith DM: HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J Clin Invest 2020, 130:1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, Kottilil S, Moir S, Mican JM, Mullins JI, et al. : Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis 2008, 197:714–720. [DOI] [PubMed] [Google Scholar]
- 95.Ward AR, Mota TM, Jones RB: Immunological approaches to HIV cure. Semin Immunol 2020:101412. [DOI] [PubMed] [Google Scholar]
- 96.Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, Choi AL, Girling V, Ho T, Li P, et al. : Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis 2010, 202:1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, Deeks SG, Luciw PA, Chipman JG, Beilman GJ, et al. : Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med 2017, 23:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olesen R, Vigano S, Rasmussen TA, Sogaard OS, Ouyang Z, Buzon M, Bashirova A, Carrington M, Palmer S, Brinkmann CR, et al. : Innate Immune Activity Correlates with CD4 T Cell-Associated HIV-1 DNA Decline during Latency-Reversing Treatment with Panobinostat. J Virol 2015, 89:10176–10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van der Sluis RM, Zerbato JM, Rhodes JW, Pascoe RD, Solomon A, Kumar NA, Dantanarayana AI, Tennakoon S, Dufloo J, McMahon J, et al. : Diverse effects of interferon alpha on the establishment and reversal of HIV latency. PLoS Pathog 2020, 16:e1008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong ME, Jaworowski A, Hearps AC: The HIV Reservoir in Monocytes and Macrophages. Front Immunol 2019, 10:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zalar A, Figueroa MI, Ruibal-Ares B, Bare P, Cahn P, de Bracco MM, Belmonte L: Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antiviral Res 2010, 87:269–271. [DOI] [PubMed] [Google Scholar]
- 102.Yukl SA, Sinclair E, Somsouk M, Hunt PW, Epling L, Killian M, Girling V, Li P, Havlir DV, Deeks SG, et al. : A comparison of methods for measuring rectal HIV levels suggests that HIV DNA resides in cells other than CD4+ T cells, including myeloid cells. AIDS 2014, 28:439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cattin A, Wiche Salinas TR, Gosselin A, Planas D, Shacklett B, Cohen EA, Ghali MP, Routy JP, Ancuta P: HIV-1 is rarely detected in blood and colon myeloid cells during viral-suppressive antiretroviral therapy. AIDS 2019, 33:1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calantone N, Wu F, Klase Z, Deleage C, Perkins M, Matsuda K, Thompson EA, Ortiz AM, Vinton CL, Ourmanov I, et al. : Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity 2014, 41:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bujko A, Atlasy N, Landsverk OJB, Richter L, Yaqub S, Horneland R, Oyen O, Aandahl EM, Aabakken L, Stunnenberg HG, et al. : Transcriptional and functional profiling defines human small intestinal macrophage subsets. J Exp Med 2018, 215:441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mann ER, Bernardo D, English NR, Landy J, Al-Hassi HO, Peake ST, Man R, Elliott TR, Spranger H, Lee GH, et al. : Compartment-specific immunity in the human gut: properties and functions of dendritic cells in the colon versus the ileum. Gut 2016, 65:256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dillon SM, Lee EJ, Donovan AM, Guo K, Harper MS, Frank DN, McCarter MD, Santiago ML, Wilson CC: Enhancement of HIV-1 infection and intestinal CD4+ T cell depletion ex vivo by gut microbes altered during chronic HIV-1 infection. Retrovirology 2016, 13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


