ABSTRACT
Recently, we have examined the membrane anchoring and subsequent lipidation of six members of the LC3/GABARAP protein family, together with their ability to promote membrane tethering and fusion. GABARAP and GABARAPL1 showed the highest activities. Differences found within LC3/GABARAP proteins suggested the existence of a lipidation threshold as a requisite for tethering and inter-vesicular lipid mixing. The presence of ATG12–ATG5-ATG16L1 (E3 in short) increased and accelerated LC3/GABARAP lipidation and subsequent vesicle tethering. However, E3 hampered LC3/GABARAP capacity to induce inter-vesicular lipid mixing and/or fusion. Our results suggest a model in which, together with the recently described inter-membrane lipid transfer mechanism, LC3/GABARAP could help in the phagophore expansion process through their ability to tether and fuse vesicles. The growing regions would be areas where the LC3/GABARAP proteins could be lipidated in the absence of E3, or else an independent regulatory mechanism would allow lipid/vesicle incorporation and phagophore growth when E3 was present.
Abbreviations: Atg/ATG: autophagy-related protein (in yeast/human); E3: ATG12–ATG5-ATG16L1 complex; GABARAP: gamma-aminobutyric acid receptor associated protein; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3.
KEYWORDS: Autophagy proteins, autophagosome expansion, ATG8, ATG12–ATG5-ATG16L1, LC3/GABARAP, E3 complex
Autophagy in eukaryotes involves the concerted action of two ubiquitin-like conjugation systems, namely ATG12 and LC3/GABARAP (or human ATG8) systems. The product of the ATG12 ubiquitin-like conjugation system, i.e., the ATG12–ATG5-ATG16L1 complex (E3 in short), acts as an E3-like enzyme in the second conjugation system, mediating the LC3/GABARAP covalent binding to the lipid phosphatidyl ethanolamine. Once anchored to the membrane, the LC3/GABARAP proteins are involved in autophagosomal membrane expansion, closure, and fusion with lysosomes, and autophagosomal cargo selection. At least six members of the LC3/GABARAP family are known in humans, suggesting that each of them could play partial different roles in the autophagy process (Figure 1A).
Figure 1.
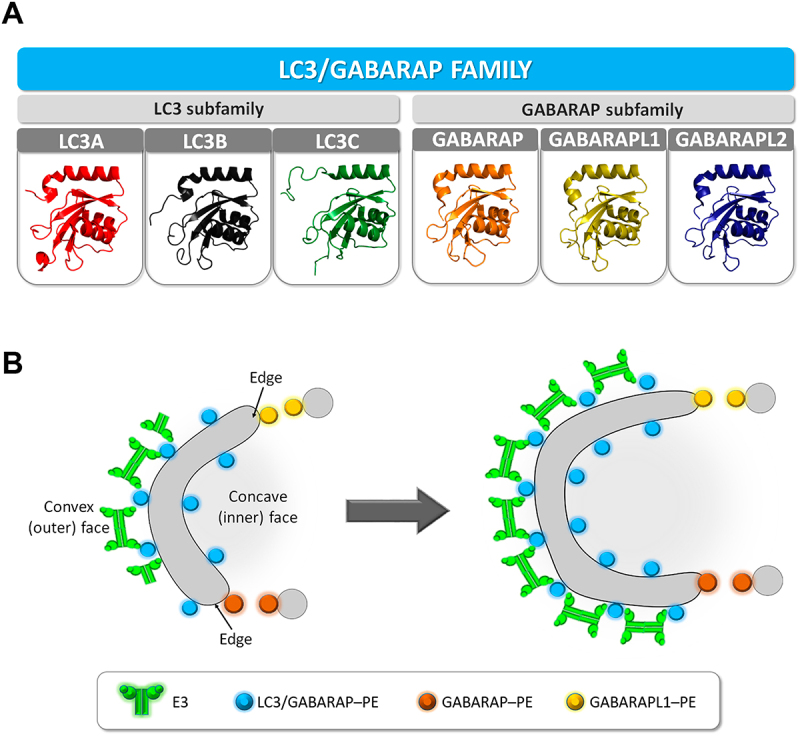
LC3/GABARAP proteins and E3 in the phagophore expansion. (A) Schematic representation of the LC3/GABARAP protein family. 3D structures of each protein in solution, presented using PyMOL. PDB: LC3A (5C×3), LC3B (2ZJD), LC3C (2NCN), GABARAP (1GNU), GABARAPL1 (5 L×I) and GABARAPL2 (4CO7). (B) Hypothetical model based on the results in Iriondo et al. GABARAP and GABARAPL1 are the main candidates to promote phagophore expansion, particularly on the highly curved edges, as these proteins reach faster the necessary lipidation levels to trigger vesicle tethering and inter-vesicular lipid mixing. In this model, E3 is not forming an immobile scaffold with lipidated LC3/GABARAP proteins on the edges or growing zones of the phagophore.
In vitro studies with yeast proteins have uncovered the interplay between these two ubiquitin-like systems and their interaction with membranes showing that the presence of yeast E3 increased Atg3 activity, enhancing the lipid-protein conjugation reaction and specifying the membrane into which Atg8 is anchored. Studies with human proteins are scarce since the entire human E3 was only recently purified upon expression in eukaryotic cells.
We have analyzed the in vitro lipidation of six members of the LC3/GABARAP protein family, namely LC3A, LC3B, LC3C, GABARAP, GABARAPL1 and GABARAPL2, in the presence or absence of E3 [1]. Moreover, we have investigated the molecular mechanisms by which the various family members trigger vesicle tethering/aggregation and fusion, and how the presence of E3 modulates those activities in the phagophore expansion process. In our study, we have used large unilamellar vesicles composed of egg phosphatidyl choline: dioleoyl phosphatidyl ethanolamine: phosphatidyl inositol: dioleoyl glycerol (33:55:10:2 mol ratio) as the model membrane.
When considering the ability of LC3/GABARAP proteins to promote vesicle tethering and fusion, two observations are particularly relevant. One is that under otherwise similar conditions, E3-independent lipidation appeared to differ for each subfamily, GABARAP-subfamily members being most easily lipidated. In turn, LC3A and LC3B reached only low lipidation levels, LC3C being the exception to the rule. The second observation is the existence of a lag phase in tethering and in inter-vesicle lipid mixing, in the absence of E3. This suggests the need to reach a lipidation threshold before proceeding to stronger levels of interaction with the host lipid bilayer. The growing edge of the phagophore should be a narrow area, with a high concentration of intrinsic-negative-curvature lipids, and relatively little space for proteins. Then, a protein that could induce membrane fusion at a low number of protein molecules per area would be needed. The lower lipidation threshold for all the GABARAP proteins, as compared to the LC3 subfamily, suggests that the members of the GABARAP family would be excellent candidates to perform this function.
Under the experimental conditions tested, E3 caused no vesicle tethering on its own. However, the presence of ATG3 elicited membrane tethering, albeit to a low extent. This positive effect could be explained by the well-known interaction between ATG12 and ATG3. Such an interaction possibly increases E3 affinity for the membrane and consequently activates the E3-dependent tethering activity. This could also be a combined effect of both proteins, since the lipid-composition-sensitive tethering activity of ATG3 had already been shown.
Moreover, in our study we observed that, in the presence of E3, lipidation levels of LC3/GABARAP proteins correlated with their tethering ability. The higher lipidation levels obtained with E3 allowed the participation of all LC3/GABARAP family members in tethering events. The absence of a lag phase when E3 was present in the reconstitution system, suggests that the proteins reached their lipidation threshold in a shorter time. E3 interaction with the membranes and the subsequent vesicle tethering, together with the positive effect of ATG3, could explain the acceleration of lipidation and tethering.
However, E3 did not have the same effect on all proteins when the inter-vesicular lipid mixing was measured. E3 clearly lowered the lipid mixing activity of the two most active proteins, GABARAP and GABARAPL1. However, the remaining questions are why E3 decreases their ability to produce inter-vesicular lipid mixing, and why proteins with similar lipidation levels induce similar tethering but different levels of inter-vesicular lipid mixing.
Previous studies in yeast had shown that, once yeast Atg8 is lipidated, it is able to associate with E3 and form a membrane scaffold via an Atg8-interacting motif in Atg12. Although formation of such a scaffold with the different LC3/GABARAP proteins has not been described yet, it is conceivable that in our in vitro system, once the LC3/GABARAP proteins have reached a critical lipidation level, the scaffold describe for the yeast proteins form on the liposomes. Using a liposome flotation assay after lipidation, we have detected co-localization between E3 and GABARAPL1. This result is compatible with the presence of a protein coat on the liposomes that could give rise to the above-mentioned scaffold.
In yeast, E3 had been detected on the external surface of the growing phagophore, together with Atg8, while only Atg8–PE was present on the inner surface. In humans, formation of a scaffold could explain why GABARAP and GABARAPL1 had a lower lipid mixing ability when E3 was present. That is, the scaffold would facilitate vesicle tethering but it would hamper inter-vesicular lipid mixing, for which vesicle hemi-fusion or close apposition is required. The results would be compatible with the hypothesis that E3 could only form an immobile scaffold on the external surface of the growing phagophore. Formation of this scaffold, however, would not happen on the inner surface, nor on the edges of the nascent autophagosome, in order to allow successive steps of phagophore growth (Figure 1B), either through vesicle fusion or lipid transfer.
Altogether, these results show that the GABARAP subfamily is more active than its LC3 homologs in mediating membrane fusion. Since yeast Atg8 promotes vesicle hemi-fusion, LC3s appear to have lost this function during evolution. This is consistent with the notion that GABARAP proteins are more evolutionarily related to Atg8 than LC3 proteins. The LC3 subfamily may have become more specialized in the recognition of autophagic receptors and adapters. This hypothesis is consistent with data about the C. elegans Atg8 orthologs LGG-1 and LGG-2. That is, LGG-1, which is more similar to GABARAP proteins, has the ability to tether and fuse vesicles, while LGG-2, which is more similar to LC3 proteins, has only a limited capacity to induce tethering and none to fuse vesicles.
Future studies should be addressed to clarify why E3 decreases LC3/GABARAP ability to produce inter-vesicular lipid mixing, and why proteins with similar lipidation levels induce similar tethering but different levels of inter-vesicular lipid mixing. Structural studies including atomic force, fluorescence and cryo-electron microscopy techniques should allow a better understanding of these remaining questions.
Funding Statement
This work was supported in part by the Spanish Ministerio de Ciencia e Innovación (MCI), Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER) [grant No. PID2021-124461NB-I00], the Basque Government [grans No. IT1625-22], the Fundación Ramón Areces [CIVP20A6619], the Fundación Biofísica Bizkaia, and the Basque Excellence Research Centre (BERC) program of the Basque Government. MNI was a recipient of a pre-doctoral FPU fellowship from the Spanish Ministry of Science, Innovation and Universities [FPU16/05873].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Iriondo MN, Etxaniz A, Varela YR, et al. Effect of ATG12–ATG5-ATG16L1 autophagy E3-like complex on the ability of LC3/GABARAP proteins to induce vesicle tethering and fusion. Cell Mol Life Sci. 2023;80(2):56. DOI: 10.1007/s00018-023-04704-z [DOI] [PMC free article] [PubMed] [Google Scholar]


