Abstract
Introduction:
Ischaemic stroke patients with atrial fibrillation (AF) are at high risk of stroke recurrence despite oral anticoagulation therapy. Patients with cardiovascular comorbidities may take both antiplatelet and oral anticoagulation therapy (OAC/AP). Our study aims to evaluate the safety and efficacy of OAC/AP therapy as secondary prevention in people with AF and ischaemic stroke.
Patients and methods:
We performed a post-hoc analysis of pooled individual data from multicenter prospective cohort studies and compared outcomes in the OAC/AP cohort and patients on DOAC/VKA anticoagulation alone (OAC cohort). Primary outcome was a composite of ischaemic stroke, systemic embolism, intracranial bleeding, and major extracranial bleeding, while secondary outcomes were ischaemic and haemorrhagic events considered separately. A multivariable logistic regression analysis was performed to identify independent predictors for outcome events. To compare the risk of outcome events between the two cohorts, the relation between the survival function and the set of explanatory variables were calculated by Cox proportional hazard models and the results were reported as adjusted hazard ratios (HR). Finally another analysis was performed to compare the overall risk of outcome events in both OAC/AP and OAC cohorts after propensity score matching (PSM).
Results:
During a mean follow-up time of 7.5 ± 9.1 months (median follow-up time 3.5 months, interquartile range ±3), 2284 stroke patients were on oral anticoagulants and 215 were on combined therapy. The multivariable model demonstrated that the composite outcome is associated with age (OR: 1.03, 95% CI: 1.01–1.04 for each year increase) and concomitant antiplatelet therapy (OR: 2.2, 95% CI: 1.48–3.27), the ischaemic outcome with congestive heart failure (OR: 1.55, 95% CI: 1.02–2.36) and concomitant antiplatelet therapy (OR: 1.93, 95% CI: 1.19–3.13) and the haemorrhagic outcome with age (OR: 1.03, 95% CI: 1.01–1.06 for each year increase), alcoholism (OR: 2.15, 95% CI: 1.06–4.39) and concomitant antiplatelet therapy (OR: 2.22, 95% CI: 1.23–4.02). Cox regression demonstrated a higher rate of the composite outcome (hazard ratio of 1.93 [95% CI, 1.35–2.76]), ischaemic events (HR: 2.05 [95% CI: 1.45–2.87]) and bleeding outcomes (HR: 1.90 [95% CI, 1.06–3.40]) in OAC/AP cohort. After PSM analysis, the composite outcome remained more frequent in people treated with OAC + AP (RR: 1.70 [95% CI, 1.05–2.74]).
Discussion:
Secondary prevention with combination of oral anticoagulant and antiplatelet therapy after ischaemic stroke was associated with worse outcomes in our cohort.
Conclusion:
Further research is needed to improve secondary prevention by investigating the mechanisms of recurrent ischaemic stroke in patients with atrial fibrillation.
Keywords: Ischaemic stroke, antiplatelet, anticoagulants, secondary prevention, atrial fibrillation
Graphical abstract.
Introduction
Patients with a history of acute ischaemic stroke (AIS) or transient ischaemic attack (TIA) due to cardioembolism are at excessive risk of recurrent stroke despite secondary prevention with direct oral anticoagulants (DOAC) or vitamin K-antagonists (VKA). In fact, the residual stroke risk despite anticoagulant therapy in patients with atrial fibrillation (AF) ranges from 0.7% to 2.3% per year.1–4 The aetiology of recurrent stroke in such patients is not well understood. Possible causes are concomitant competing stroke mechanisms, 5 such as large artery and small vessel disease, 6 insufficient anticoagulation such as lack of compliance, 7 inappropriate dosage of therapy or temporarily withdrawl of medication, cardioembolism despite appropriate anticoagulation and thrombogenic anatomy of the left atrial appendage.8,9 However, most patients have AF-related cardioembolism despite sufficient anticoagulation treatment. 6 Therefore, first of all these patients need to undergo a thorough work-up in order to uncover additional non-cardioembolic ischaemic stroke causes that might require additional secondary prevention strategies and risk factors management. 10 However, if no other causes are identified and anticoagulation is in the therapeutic range, the optimization of secondary prevention therapy can be challenging and no specific clinical strategies are recommended by current guidelines. Recent data suggested that the combination of low dosage rivaroxaban with antiplatelet therapy might be effective for prevention of major adverse cardiovascular events in patients with history of severe atherosclerotic vascular disease of the lower extremities, of the carotid arteries, or coronary arteries.11–14 However, in contrast to this, other data suggested that adding antiplatelet to anticoagulants does not provide any benefit in patients with AF and stroke in terms of secondary prevention at 3 months from the index event. 6 Furthermore, there is no strong evidence that this association can actually change the clinical outcome in this particular subgroup of patients. A better understanding of the long-term risks and benefits associated with combined anticoagulation and antiplatelet treatment in patients with AF is urgently needed. Nevertheless, given the high risk of bleeding associated with the combination of antiplatelet and anticoagulation treatment,15–17 a clinical trial designed to evaluate if this combination actually provides a benefit in terms of secondary prevention in patients with AF and stroke may not be feasible. Yet, some ischaemic stroke patients require dual therapy for various reasons, for example due to the co-existence of AF and coronary artery disease. 18 Therefore, the aim of this study was to evaluate if the therapeutic combination of DOAC/VKA anticoagulation and antiplatelet therapy (OAC/AP cohort) can be both safe and effective as secondary prevention strategy to prevent long-term recurrence of ischaemic events in patients with AF who suffered an acute ischaemic stroke.
Patients and methods
We performed a post-hoc analysis of pooled individual data from an established international collaboration of investigator-initiated prospective cohort studies. The following studies were included: RAF, RAF-NOAC, and RENO-EXTEND. Details about the participating studies can be obtained from the published studies.19–21
The baseline variables provided by the participating studies are reported in the Supplemental Materials.
Inclusion and exclusion criteria
We included patients with: (1) acute ischaemic stroke with a systematic follow-up of at least 3 months or longer after the index event (patients with a fatal event before 3 months were also included in the analysis) (2) diagnosis of nonvalvular AF either known prior to the index event or detected after the event; (3) information on antithrombotic therapy (anticoagulation or antiplatelet) prior to and after index event available; (4) secondary stroke prevention with DOACs or VKA; and (5) information on the presence/absence of recurrent AIS, systemic embolism, intracerebral haemorrhage, major extracranial bleeding and death. We excluded patients with: (1) intracerebral haemorrhage occurred before the index event; (2) mechanical heart valves; (3) rheumatic or severe mitral valve stenosis; (4) long-term secondary stroke prevention with antiplatelet only; or (5) missing information on antithrombotic treatment before and after the index event.
Outcome evaluation
Patients’ follow-up was carried out with neurological examinations performed by physicians participating in this study. The duration of follow-up was at least 3 months. Patient’s follow-up started at the moment of index events.
The primary outcome measure was the composite of ischaemic stroke, systemic embolism, intracranial bleeding, and major extracranial bleeding. Recurrent stroke was defined as the sudden onset of a new focal neurological deficit of vascular origin in a site consistent with the territory of a major cerebral artery and was categorized as ischaemic or hemorrhagic. Intracranial bleeding was defined as a spontaneous intracerebral bleeding, subdural, or subarachnoid haemorrhage. 22 Traumatic intracranial bleeding was not considered as an outcome event. Systemic embolism was defined as an acute vascular occlusion of an extremity or organ confirmed by imaging or either surgery or autopsy. Major extracranial bleeding was defined as a reduction in the haemoglobin level of 2 g per decilitre or more, the requirement of a blood transfusion of at least two units, or symptomatic bleeding in either a critical area or organ. Death data were recorded; functional recovery was assessed by the modified Rankin Scale, dichotomizing between functional independence (modified Rankin Scale score 0–2) and disability (modified Rankin Scale score ⩾3). 23 Secondary outcomes were ischaemic and haemorrhagic outcomes respectively defined as occurrence of ischaemic stroke and systemic embolism, and intracranial bleeding and major extracranial bleeding. Follow-up visits and outcome adjudication were performed by local investigators, not in a blinded fashion.
Statistical analysis
Differences between groups in clinical characteristics and risk factors were calculated using the χ2 test of proportions (with a two-sided α level of 5%). In order to reduce the family wise error rate we used the Bonferroni method to calculate the adjusted p-values for multiple comparisons. Multivariable backward logistic regression analysis was performed to identify independent predictors for outcome events. The variables included in this analysis were the following: age, sex, congestive heart failure, hypertension, diabetes mellitus, history of stroke/TIA, vascular disease, hyperlipidaemia, current alcohol abuse, current smoking habit, paroxysmal AF, antiplatelet therapy in addition to oral anticoagulant therapy after the index stroke. We reported odds ratios (ORs) and their 95% confidence intervals (CIs).
Furthermore, to compare the risk of outcome events between OAC/AP cohort and patients receiving only OAC (OAC cohort), the relation between the survival function and the set of explanatory variables were calculated by Cox proportional hazard models. These models provide an estimate of the treatment effect on survival after an adjustment for other explanatory variables. The results of these analyses were reported as adjusted hazard ratios (HR).
Using propensity score matching (PSM), a further analysis was performed to compare the overall risk of outcome events in both OAC/AP and OAC cohorts. This score was calculated including the variables of interest selected from the univariate analysis, using backward stepwise analysis with a 0.1 level as a screening criterion for the selection of candidate variables. Matching was then done with a 1:1 ratio across the groups, without replacement, and with a forced preservation of bridging cases. In this PSM, survival function and empirical cumulative hazard function were estimated via the Kaplan-Meier estimator for the two groups; any differences between survival functions were tested using the log-rank statistic (or Mantel-Haenszel test), that in the case of large samples has an asymptotic χ2 distribution. 24 Patients were censored at the time of an outcome event, death (even within three months from the index event), or if they had been lost to follow-up. Data were analysed using the SPSS/PC Win package 25.0. Statistical significance was defined as p < 0.05.
Results
Characteristics of the study population
The final cohort for this analysis comprised 2499 of 3396 patients (73.6%) with ischaemic stroke and AF from the pooled data set of individual patient data. We excluded 253 patients due to the fact they were duplicate patients, 449 patients who received only antiplatelets, 113 patients treated with only low molecular weight heparin, and 82 patients who did not receive any antithrombotic therapy. Of the 2499 included patients, 215 patients composed the OAC/AP cohort and 2284 the OAC cohort. All the patients of the OAC/AP cohort received double therapy (OAC + AP) during the entire follow-up time. One hundred seventy-four patients received aspirin, while 36 received clopidogrel and five patients received triple therapy (OAC + aspirin + clopidogrel). The OAC/AP cohort showed a higher prevalence of myocardial infarction, peripheral arterial disease and a lower prevalence of therapy with DOAC as shown in Table 1.
Table 1.
Characteristics of patients with or without antiplatelet therapy.
| OAC/AP cohort (n = 215) | OAC cohort (n = 2284) | p * | |
|---|---|---|---|
| Age | 76.1 ± 9.7 | 76.5 ± 9.5 | 1 |
| Sex (M) | 114 (53.0%) | 1109 (48.6%) | 1 |
| Diabetes | 51 (23.7%) | 520 (22.8%) | 1 |
| Hypertension | 185 (86.0%) | 1792 (78.5%) | 0.1 |
| Hyperlipidaemia | 106 (49.3%) | 931 (40.8%) | 0.28 |
| Alcoholism | 23 (10.7%) | 166 (7.3%) | 1 |
| Current smoker | 25 (11.6%) | 248 (10.9%) | 1 |
| Paroxysmal AF | 96 (44.7%) | 1096 (48.0%) | 1 |
| History of stroke/TIA | 71 (33.0%) | 669 (29.3%) | 1 |
| CHF | 51 (23.7%) | 406 (17.8%) | 0.42 |
| MI | 63 (29.3%) | 357 (15.6%) | 0.001 |
| PAD | 36 (16.7%) | 217 (9.5%) | 0.028 |
| Type of oral anticoagulant | 0.001 | ||
| DOAC | 117 (54.4%) | 1790 (78.4%) | |
| Warfarin | 98 (45.6%) | 494 (21.6%) | |
| CHA2DS2-Vasc ⩾ 3 | 203 (94.4%) | 2080 (91.1%) | 0.98 |
OAC: oral anti-coagulants; AP: anti-platelet; AF: atrial fibrillation; TIA: transient ischaemic attack; CHF: chronic heart failure; MI: myocardial infarction; PAD: peripheral artery disease; DOAC: direct acting oral anti-coagulants; p*: adjusted p-values after Bonferroni correction.
Of the 215 patients in OAC/AP cohort, 78 patients were on antiplatelet therapy before the index ischaemic stroke (63 patients because of myocardial infarction and 15 patients because of vascular disease of coronary arteries) and among them 34 were also on oral anticoagulants. On the other hand, 137 patients were on anticoagulation before the index event and antiplatelet therapy was added after the index event (90 patients with stroke due to large-artery atherosclerosis or small-vessel occlusion, 47 patients with probable cardioembolic aetiology).
Rates and predictive factors of ischaemic and bleeding events
After a mean follow-up time of 7.5 ± 9.1 months (median follow-up time 3.5 months, interquartile range ±3) 231 patients (9.2%) had an outcome event corresponding to an annual rate of 15.4%. The following events were observed: One hundred twenty-three ischaemic strokes, 20 systemic embolisms, 45 intracranial bleedings, and 43 were major extracranial bleedings.
The univariate analysis demonstrates that patients with an occurrence of combined outcome (ischaemic and haemorrhagic events) had a higher prevalence of hypertension, CHA2DS2-Vasc ⩾ 3 and use of concomitant antiplatelet therapy (Table 2).
Table 2.
Characteristics of patients with and without outcome events.
| Combined outcome |
Ischaemic outcome |
Haemorrhagic outcome |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| With (n = 231) | Without (n = 2255) | p * | With (n = 143) | Without (n = 2343) | p * | With (n = 88) | Without (n = 2398) | p * | |
| Age | 78.4 ± 8.3 | 76.3 ± 9.7 | 0.09 | 78.1 ± 8.4 | 76.4 ± 9.6 | 1 | 79.0 ± 7.9 | 76.4 ± 9.6 | 0.45 |
| Sex (M) | 122 (52.8%) | 1096 (48.6%) | 1 | 72 (50.3%) | 1144 (48.8%) | 1 | 51 (57.9%) | 1167 (48.7%) | 1 |
| Diabetes | 65 (28.1%) | 504 (22.4%) | 1 | 42 (29.4%) | 527 (22.5%) | 1 | 24 (27.3%) | 545 (22.7%) | 1 |
| Hypertension | 204 (88.3%) | 1765 (78.3%) | 0.004 | 129 (90.2%) | 1838 (78.4%) | 0.03 | 79 (89.8%) | 1890 (78.8%) | 0.45 |
| Hyperlipidaemia | 109 (47.2%) | 923 (40.9%) | 1 | 71 (49.6%) | 960 (41.0%) | 1 | 40 (45.5%) | 992 (41.4%) | 1 |
| Alcoholism | 19 (8.2%) | 169 (7.5%) | 1 | 9 (6.3%) | 178 (7.6%) | 1 | 10 (11.4%) | 178 (7.4%) | 1 |
| Current smoker | 18 (7.8%) | 251 (11.1%) | 1 | 12 (8.4%) | 263 (11.2%) | 1 | 6 (6.8%) | 263 (11.0%) | 1 |
| Paroxysmal AF | 111 (48.1%) | 1073 (47.6%) | 1 | 73 (51.0%) | 1110 (47.4%) | 1 | 39 (44.3%) | 1145 (47.7%) | 1 |
| History of stroke/TIA | 69 (29.9%) | 673 (29.8%) | 1 | 50 (34.9%) | 692 (29.5%) | 1 | 22 (25.0%) | 720 (30.0%) | 1 |
| CHF | 56 (24.2%) | 398 (17.6%) | 0.9 | 39 (27.3%) | 414 (17.7%) | 0.1 | 17 (19.3%) | 437 (18.2%) | 1 |
| MI | 50 (21.6%) | 370 (16.4%) | 1 | 34 (23.8%) | 386 (16.5%) | 1 | 18 (20.4%) | 402 (16.8%) | 1 |
| PAD | 37 (16.0%) | 211 (9.4%) | 0.09 | 22 (15.4%) | 226 (9.6%) | 1 | 15 (17.0%) | 233 (9.7%) | 0.9 |
| Type of oral anticoagulant | 1 | 1 | 1 | ||||||
| DOAC | 162 (70.1%) | 1731 (76.8%) | 105 (73.4%) | 1790 (76.4%) | 62 (70.4%) | 1831 (76.3%) | |||
| Warfarin | 69 (29.9%) | 524 (23.2%) | 38 (26.6%) | 553 (23.6%) | 26 (29.6%) | 567 (23.7%) | |||
| CHA2DS2-Vasc ⩾ 3 | 225 (97.4%) | 2044 (90.6%) | 0.004 | 142 (99.3%) | 2125 (90.7%) | 0.004 | 85 (96.6%) | 2184 (91.1%) | 1 |
| Concomitant antiplatelet | 40 (17.3%) | 174 (7.7%) | 0.004 | 25 (17.5%) | 189 (8.1%) | 0.004 | 16 (18.2%) | 198 (8.3%) | 0.04 |
AF: atrial fibrillation; TIA: transient ischaemic attack; CHF: chronic heart failure; MI: myocardial infarction; PAD: peripheral artery disease; DOAC: direct acting oral anti-coagulants; p*: adjusted p-values after Bonferroni correction.
Patients with an ischaemic outcome had a higher prevalence of hypertension, CHA2DS2-Vasc ⩾ 3 and use of concomitant antiplatelet therapy (Table 2). Patients with a haemorrhagic outcome had a higher prevalence of concomitant antiplatelet therapy (Table 2).
The multivariable model demonstrated that the composite outcome is associated with age (OR: 1.03, 95% CI: 1.01–1.04 for each year increase) and concomitant antiplatelet therapy (OR: 2.2, 95% CI: 1.48–3.27) (Table 3).
Table 3.
Multivariable logistic regression analysis to identify independent predictors for outcome events. Combined, ischaemic, and haemorrhagic outcomes excluding CHA2DS2-Vasc, including risk factors within the score.
| Combined outcome |
Ischaemic outcome |
Haemorrhagic outcome |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Age (for each year increase) | 1.03 | 1.01–1.04 | 0.007 | 1.02 | 1.00–1.04 | 0.06 | 1.03 | 1.01–1.06 | 0.02 |
| Sex | 1.24 | 0.92–1.68 | 0.16 | 1.06 | 0.74–1.52 | 0.76 | 1.53 | 0.95–2.45 | 0.08 |
| Diabetes | 1.20 | 0.87–1.66 | 0.26 | 1.11 | 0.75–1.64 | 0.61 | 1.17 | 0.70–1.94 | 0.55 |
| Hypertension | 1.69 | 1.09–2.63 | 0.02 | 1.53 | 0.91–2.60 | 0.11 | 1.72 | 0.86–3.45 | 0.13 |
| Hyperlipidaemia | 1.06 | 0.78–1.43 | 0.73 | 1.15 | 0.80–1.65 | 0.46 | 0.97 | 0.61–1.57 | 0.91 |
| Alcoholism | 1.24 | 0.73–2.11 | 0.43 | 0.95 | 0.47–1.66 | 0.89 | 2.15 | 1.06–4.39 | 0.03 |
| Current smoker | 0.79 | 0.46–1.36 | 0.40 | 0.87 | 0.46–1.66 | 0.68 | 0.61 | 0.25–1.50 | 0.28 |
| Paroxysmal AF | 1.13 | 0.84–1.52 | 0.41 | 1.27 | 0.89–1.81 | 0.19 | 0.86 | 0.54–1.36 | 0.52 |
| History of stroke/TIA | 0.88 | 0.64–1.20 | 0.42 | 1.01 | 0.69–1.46 | 0.97 | 0.67 | 0.40–1.12 | 0.12 |
| CHF | 1.28 | 0.9–1.83 | 0.17 | 1.55 | 1.02–2.36 | 0.04 | 0.86 | 0.48–1.55 | 0.61 |
| MI | 1.02 | 0.70–1.48 | 0.91 | 1.04 | 0.67–1.62 | 0.87 | 0.96 | 0.53–1.74 | 0.9 |
| PAD | 1.51 | 1.00–2.28 | 0.05 | 1.31 | 0.79–2.17 | 0.3 | 1.60 | 0.85–3.00 | 0.14 |
| DOAC versus Warfarin | 0.76 | 0.55–1.06 | 0.10 | 0.77 | 0.52–1.14 | 0.19 | 0.75 | 0.46–1.25 | 0.27 |
| Concomitant antiplatelet | 2.20 | 1.48–3.27 | <0.0001 | 1.93 | 1.19–3.13 | 0.007 | 2.22 | 1.23–4.02 | 0.008 |
AF: atrial fibrillation; TIA: transient ischaemic attack; CHF: chronic heart failure; MI: myocardial infarction; PAD: peripheral artery disease; DOAC: direct acting oral anti-coagulants.
The ischaemic outcome is associated with congestive heart failure (OR: 1.55, 95% CI: 1.02–2.36) and concomitant antiplatelet therapy (OR: 1.93, 95% CI: 1.19–3.13) (Table 3). The haemorrhagic outcome is associated with age (OR: 1.03, 95% CI: 1.01–1.06 for each year increase), alcoholism (OR: 2.15, 95% CI: 1.06–4.39) and concomitant antiplatelet therapy (OR: 2.22, 95% CI: 1.23–4.02) (Table 3).
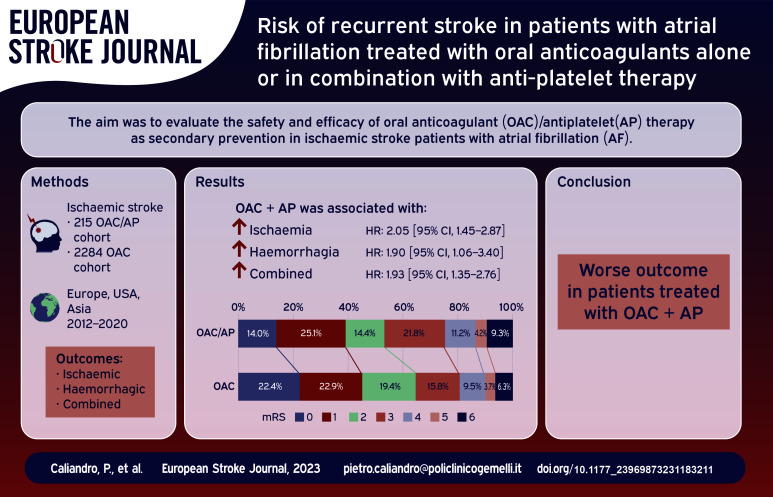
The Cox regression curve analyses comparing the overall outcome events in the OAC/AP cohort and the OAC cohort are reported in Figure 1. The use of antiplatelet therapy in addition to oral anticoagulant therapy was associated with a higher rate of the primary outcome (hazard ratio of 1.93 [95% CI, 1.35–2.76]), ischaemic outcome events (HR: 2.05 [95% CI, 1.35–2.76]) and bleeding outcome events (HR: 1.90 [95% CI, 1.06–3.40]).
Figure 1.
Cox regression results for primary and secondary outcomes (antiplatelet vs no antiplatelet).
After PSM, 212 patients within the OAC cohort were compared with 212 patients of the OAC/AP cohort. In Table 4, we report the characteristics of the patients after PSM.
Table 4.
Characteristics of patients after PSM.
| OAC/AP cohort (n = 212) | OAC cohort (n = 212) | p | |
|---|---|---|---|
| Age | 76.3 ± 9.5 | 76.6 ± 9.2 | 0.8 |
| Sex (M) | 112 (52.8%) | 110 (51.9%) | 0.9 |
| Diabetes | 51 (24.0%) | 58 (27.3%) | 0.5 |
| Hypertension | 182 (85.8%) | 182 (85.8%) | 1.0 |
| Hyperlipidaemia | 105 (49.5%) | 108 (50.9%) | 0.8 |
| Alcoholism | 21 (9.9%) | 19 (9.0%) | 0.9 |
| Current smoker | 24 (11.3%) | 25 (11.8%) | 0.9 |
| Paroxysmal AF | 96 (45.3%) | 84 (40.0%) | 0.3 |
| History of stroke/TIA | 68 (32.1%) | 74 (34.9%) | 0.3 |
| CHF | 50 (23.6%) | 47 (22.2%) | 0.8 |
| MI | 61 (28.8%) | 65 (30.6%) | 0.7 |
| PAD | 35 (16.5%) | 35 (16.5%) | 1.0 |
| Type of oral anticoagulant | 0.9 | ||
| DOAC | 115 (54.2%) | 117 (55.1%) | |
| Warfarin | 97 (45.8%) | 95 (44.9%) | |
| CHA2DS2-Vasc ⩾ 3 | 200 (94.3%) | 198 (93.4%) | 0.7 |
| Outcome events | |||
| Combined outcome | 39 (18.4%) | 23 (10.8%) | 0.01 |
| Ischaemic outcome | 23 (10.8%) | 13 (6.1%) | 0.08 |
| Haemorrhagic outcome | 16 (7.5%) | 10 (4.7%) | 0.2 |
AF: atrial fibrillation; TIA: transient ischaemic attack; CHF: chronic heart failure; MI: myocardial infarction; PAD: peripheral artery disease; DOAC: direct acting oral anti-coagulants.
With regards to outcome events, the composite outcome remained more frequent in people treated with combined therapy (RR: 1.70 [95% CI, 1.05–2.74]) (Figure 2).
Figure 2.
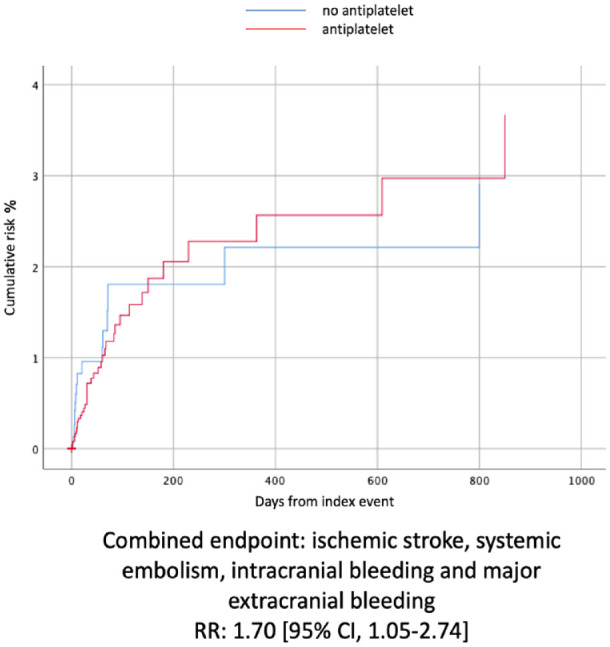
The Kaplan-Meier curves that compared the combined outcome events between the two treatment groups after PSM.
With regards to functional outcome, 905 (36.2%) patients were deceased or disabled (mRS ⩾ 3), and 165 (6.6%) were deceased at the end of follow-up. Specifically, 100 (46.5%) out of the 215 OAC/AP patients were deceased or disabled, compared with 805 (35.2%) patients in the OAC cohort (OR: 1.60 [95% CI, 1.21–2.12]). A total of 20 (9.3%) patients in the OAC/AP cohort were deceased, compared with 144 (6.3%) patients in the OAC cohort (OR: 1.52 [95% CI, 0.93–2.49]).
Supplemental Figure 1 shows the different distribution of mRS score in the two cohorts.
Discussion
In this multicenter, international, prospective cohort study, we found that the combination of antiplatelet and oral anticoagulant therapy after an ischaemic stroke was associated with a greater long term risk of both the composite outcome (ischaemic stroke, systemic embolism, intracranial bleeding, and major extracranial bleeding) and the individual secondary outcomes.
When interpreting these results, it must be taken into account that patients treated with antiplatelet and anticoagulant had a greater prevalence of cardiovascular risk factors in our cohort. Some studies demonstrated that despite anticoagulation, competing mechanisms other than AF-related cardioembolism, may cause stroke recurrence in about one of four cases5,6 and that vascular risk factors are associated with higher stroke recurrence in patients with AF.25–28 Additionally, physicians are more likely to prescribe antiplatelets to patients with the highest burden of vascular risk factors, which had an impact on our non-randomized patient sample reflecting clinical practice. Therefore, in our cohort with antiplatelet-anticoagulant combination, the higher prevalence of outcomes could be interpreted as a consequence of the higher prevalence of vascular risk factors. The association between combination antiplatelet and anticoagulation therapy with higher odds or risk of ischaemic and bleeding outcome events remained significant after adjustment for risk factors on multivariable analyses controlling for potential confounders. Moreover, after having applied PSM for risk factors, the composite outcome remains more frequent in patients with antiplatelet and oral anticoagulant therapy, while secondary outcomes were not different between groups probably because of the low rate of events in the matched samples. Actually, taking a closer look at Figure 2, it looks like double therapy (ASA + OAC), in the first 200 days after the index event, might have a protective role since the risk of combined event is lower than in the OAC group. However, considering the whole follow-up time, the risk of combined outcome remains globally higher in the OAC/AP cohort. Overall, our data provide evidence that adding antiplatelet to oral anticoagulant therapy for secondary prevention is not effective in reducing the long-term risk of ischaemic recurrence over a mean period of 7.5 ± 9.1 months (median follow-up time 3.5 months, interquartile range ±3) in patients with AF and ischaemic stroke. Our findings are in line with a previous study showing no benefit at 3 months after the index event. 6 These findings also broadens the knowledge of previous studies that do not support combining antiplatelet and anticoagulation therapy for primary stroke prevention in patients with AF.29–32 Obviously, this does not mean that in case of specific indications for antiplatelet therapy such as acute ischaemic heart disease, antiplatelet therapy should be avoided. 33 However, current cardiology guidelines do not provide any evidence regarding the timing of double therapy (OAC + AP) specifically in patients with AF, ischaemic cardiovascular disease and a recent acute ischaemic stroke. 33 Therefore, at the time there are no clear indications on how to manage secondary prevention in this kind of patients. In this view, our results could add evidence on this topic, even though our data were obtained before the current ESC guidelines were published.
Besides the occurrence of competing mechanisms, we have to consider that AF-related cardioembolism remains the most common aetiology for stroke recurrence despite sufficient anticoagulation, 6 but our study design does not allow to evaluate if the combination therapy can be effective as a secondary prevention strategy in this subgroup of patients. Moreover, since AF-related cardioembolism still has a pivotal role in stroke recurrence, it could be useful to further explore the concept and the role of AF burden. Recently it has been suggested that cardioembolic risk could be related more to the amount of AF that an individual has (AF burden) than just the presence or absence of AF. In fact, one possibile definition for AF burden is the proportion of time an individual is in AF during a monitoring period, expressed as a percentage. 34 Furthermore, if AF burden plays an actual role in cardioembolism recurrence is still a matter of debate and further studies focused on this topic could improve our knowledge on the aetiopathogenesis of recurrent ischaemic cerebral events especially in patients with AF already on anticoagulation therapy.
Finally, our study has some limitations: it is a post-hoc analysis of observational, not-randomized studies and a selection bias of the studied populations cannot be excluded, although PSM approach reduces this risk. Furthermore another limitation is the follow-up duration (7.5 months in our study). Although it is relatively a long period, it would be useful to have specific information on longer follow-up taking into account that patients with AF have both comulative risk of ischaemic stroke recurrence and haemorrhagic events. Moreover, we don’t have any data on the effectiveness of blood pressure control during the follow-up period. However, given that all the patients included in the study had an ischeamic event, they have been thourougly evaluated during their hospitalization and secondary prevention therapy for all ischaemic risk factors has been maximized, including that of hypertension. However, the study has the advantage to reflect real-life experiences with a multicenter prospective design and large sample size.
Conclusions
In conclusion, in the absence of other definite indications, this study does not provide evidence to support combined antiplatelet and anticoagulant therapy for secondary prevention in patients with AF and stroke.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231183211 for Risk of recurrent stroke in patients with atrial fibrillation treated with oral anticoagulants alone or in combination with anti-platelet therapy in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231183211 for Risk of recurrent stroke in patients with atrial fibrillation treated with oral anticoagulants alone or in combination with anti-platelet therapy in European Stroke Journal
Supplemental material, sj-tif-3-eso-10.1177_23969873231183211 for Risk of recurrent stroke in patients with atrial fibrillation treated with oral anticoagulants alone or in combination with anti-platelet therapy in European Stroke Journal
Footnotes
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all subjects before the study.
Ethical approval: This study was completed in accordance with the Helsinki Declaration as revised in 2013 (include details of relevant legislation where applicable).
Guarantor: Pietro Caliandro
Contributorship: PC researched literature and conceived the study. PC and MP were involved in protocol development. All authors were involved in patient recruitment. PC, VC, MM, GR, AZ, and MP were involved in data analysis. PC, VC, MM, and MP wrote the first draft of the manuscript. All authors critically reviewed and edited the manuscript and approved the final version of the manuscript.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
RAF and RENO-EXTEND Investigators:
Pietro Caliandro, Virginia Cancelloni, Moci Marco, Giuseppe Reale, Aurelia Zauli, Giancarlo Agnelli, Valeria Caso, Cecilia Becattini, Paolo Calabresi, Maria Giulia Mosconi, Michela Giustozzi, Georgios Tsivgoulis, David Julian Seiffge, Stefan T. Engelter, Philippe Lyrer, Alexandros A. Polymeris, Tolga Dittrich, Annaelle Zietz, Gian Marco De Marchis, Jukka Putaala, Daniel Strbian, Liisa Tomppo, Patrik Michel, Davide Strambo, Alexander Salerno, Suzette Remillard, Manuela Buehrer, Odessa Bavaud, Peter Vanacker, Susanna Zuurbier, Laetitia Yperzeele, Caroline M.J. Loos, Manuel Cappellari, Andrea Emiliani, Marialuisa Zedde, Azmil Abdul-Rahim, Jesse Dawson, Robert Cronshaw, Erika Schirinzi, Massimo Del Sette, Christoph Stretz, Narendra Kala, Michael Reznik, Ashley Schomer, Brian Mac Grory, Mahesh Jayaraman, Ryan McTaggart, Shadi Yaghi, Karen L. Furie, Luca Masotti, Elisa Grifoni, Danilo Toni, Angela Risitano, Anne Falcou, Luca Petraglia, Enrico Maria Lotti, Marina Padroni, Lucia Pavolucci, Piergiorgio Lochner, Giorgio Silvestrelli, Alfonso Ciccone, Andrea Alberti, Michele Venti, Ilaria Leone De Magistris, Odysseas Kargiotis, Alessandro Rocco, Marina Diomedi, Simona Marcheselli, Kateryna Antonenko, Eugenia Rota, Tiziana Tassinari, Valentina Saia, Francesco Palmerini, Paolo Aridon, Valentina Arnao, Serena Monaco, Salvatore Cottone, Antonio Baldi, Cataldo D’Amore, Walter Ageno, Samuela Pegoraro, George Ntaios, Dimitrios Sagris, Sotirios Giannopoulos, Maria Kosmidou, Evangelos Ntais, Michele Romoli, Leonardo Pantoni, Silvia Rosa, Pierluigi Bertora, Alberto Chiti, Isabella Canavero, Carlo Emanuele Saggese, Maurizio Plocco, Elisa Giorli, Lina Palaiodimou, Eleni Bakola, Fabio Bandini, Antonio Gasparro, Valeria Terruso, Marina Mannino, Alessandro Pezzini, Raffaele Ornello, Simona Sacco, Nemanja Popovic, Umberto Scoditti, Antonio Genovese, Licia Denti, Yuriy Flomin, Michelangelo Mancuso, Elena Ferrari, Maria Chiara Caselli, Leonardo Ulivi, Nicola Giannini, Kostantinos Vadikolias, Chrysoula Liantinioti, Maria Chondrogianni, Panagiotis Halvatsiotis, Monica Carletti, Efstathia Karagkiozi, George Athanasakis, Kostantinos Makaritsis, Alessia Lanari, Turgut Tatlisumak, Monica Acciarresi, Vieri Vannucchi, Gianni Lorenzini, Rossana Tassi, Francesca Guideri, Maurizio Acampa, Giuseppe Martini, Sung-Il Sohn, Nicola Mumoli, Prasanna Tadi, Federica Letteri, Miriam Maccarrone, Loris Poli, Mauro Magoni, Franco Galati, Cindy Tiseo, Vanessa Gourbali, Giovanni Orlandi, Martina Giuntini, Francesco Corea, Marta Bellesini, Laura Girardi, Mario Maimone Baronello, Theodore Karapanayiotides, Christina Rueckert, Laszló Csiba, Lilla Szabó, Alberto Rigatelli, Davide Imberti, Dorjan Zabzuni, Alessio Pieroni, Kristian Barlinn, Lars-Peder Pallesen, Jessica Barlinn, Boris Doronin, Vera Volodina, Dirk Deleu, Bruno Bonetti, Cesare Porta, Luana Gentile, Ashraf Eskandari, and Maurizio Paciaroni
References
- 1. Diener H-C, Connolly SJ, Ezekowitz MD, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 2010; 9: 1157–1163. [DOI] [PubMed] [Google Scholar]
- 2. Hankey GJ, Patel MR, Stevens SR, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol 2012; 11: 315–322. [DOI] [PubMed] [Google Scholar]
- 3. Easton JD, Lopes RD, Bahit MC, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 2012; 11: 503–511. [DOI] [PubMed] [Google Scholar]
- 4. Rost NS, Giugliano RP, Ruff CT, et al. Outcomes with edoxaban versus warfarin in patients with previous cerebrovascular events: findings from ENGAGE AF-TIMI 48 (Effective anticoagulation with factor Xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48). Stroke 2016; 47: 2075–2082. [DOI] [PubMed] [Google Scholar]
- 5. Purrucker JC, Hölscher K, Kollmer J, et al. Etiology of ischemic strokes of patients with atrial fibrillation and therapy with anticoagulants. J Clin Med 2020; 9: 2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polymeris AA, Meinel TR, Oehler H, et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2022; 93: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salmasi S, Loewen PS, Tandun R, et al. Adherence to oral anticoagulants among patients with atrial fibrillation: a systematic review and meta-analysis of observational studies. BMJ Open 2020; 10: e034778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Senadeera SC, Palmer DG, Keenan R, et al. Left atrial appendage thrombus detected during hyperacute stroke imaging is associated with atrial fibrillation. Stroke 2020; 51: 3760–3764. [DOI] [PubMed] [Google Scholar]
- 9. Stretz C, Wu TY, Wilson D, et al. Ischaemic stroke in anticoagulated patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2021; 92: 1164–1172. [DOI] [PubMed] [Google Scholar]
- 10. Rota E, Testa L, Di Brigida G, et al. The management of patients with acute ischemic stroke while on direct oral anticoagulants (DOACs): data from an Italian cohort and a proposed algorithm. J Thromb Thrombolysis 2020; 50: 732–738. [DOI] [PubMed] [Google Scholar]
- 11. Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018; 391: 219–229. [DOI] [PubMed] [Google Scholar]
- 12. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017; 377: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 13. Perera KS, Ng KKH, Nayar S, et al. Association between low-dose rivaroxaban with or without aspirin and ischemic stroke subtypes: a secondary analysis of the COMPASS Trial. JAMA Neurol 2020; 77: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma M, Hart RG, Connolly SJ, et al. Stroke outcomes in the COMPASS trial. Circulation 2019; 139: 1134–1145. [DOI] [PubMed] [Google Scholar]
- 15. Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am. Heart J 2006; 152: 967–973. [DOI] [PubMed] [Google Scholar]
- 16. Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation 2012; 126: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 17. So CH, Eckman MH. Combined aspirin and anticoagulant therapy in patients with atrial fibrillation. J Thromb Thrombolysis 2017; 43: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lucà F, Giubilato S, Fusco SAD, et al. The combination of oral anticoagulant and antiplatelet therapies: Stay One Step ahead. J Cardiovasc Pharmacol Ther 2020; 25: 391–398. [DOI] [PubMed] [Google Scholar]
- 19. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-K oral anticoagulants (RAF-NOACs) study. JAHA 2017; 6: e007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation. Stroke 2015; 46: 2175–2178. [DOI] [PubMed] [Google Scholar]
- 21. Paciaroni M, Caso V, Agnelli G, et al. Recurrent ischemic stroke and bleeding in patients with atrial fibrillation who suffered an acute stroke while on treatment with nonvitamin K antagonist oral anticoagulants: the RENO-EXTEND study. Stroke 2022; 53: 2620–2627. [DOI] [PubMed] [Google Scholar]
- 22. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 23. Quinn TJ, Dawson J, Walters MR, et al. Functional outcome measures in contemporary stroke trials. Int J Stroke 2009; 4: 200–205. [DOI] [PubMed] [Google Scholar]
- 24. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–170. [PubMed] [Google Scholar]
- 25. Paciaroni M, Agnelli G, Ageno W, et al. Risk factors for cerebral ischemic events in patients with atrial fibrillation on warfarin for stroke prevention. Atherosclerosis 2010; 212: 564–566. [DOI] [PubMed] [Google Scholar]
- 26. Paciaroni M, Agnelli G, Caso V, et al. Causes and risk factors of cerebral ischemic events in patients with atrial fibrillation treated with non-vitamin K antagonist oral anticoagulants for stroke prevention. Stroke 2019; 50: 2168–2174. [DOI] [PubMed] [Google Scholar]
- 27. Lehtola H, Airaksinen KEJ, Hartikainen P, et al. Stroke recurrence in patients with atrial fibrillation: concomitant carotid artery stenosis doubles the risk. Eur J Neurol 2017; 24: 719–725. [DOI] [PubMed] [Google Scholar]
- 28. Du H, Wilson D, Ambler G, et al. Small vessel disease and ischemic stroke risk during anticoagulation for atrial fibrillation after cerebral ischemia. Stroke 2021; 52: 91–99. [DOI] [PubMed] [Google Scholar]
- 29. Fox KAA, Velentgas P, Camm AJ, et al. Outcomes associated with oral anticoagulants plus antiplatelets in patients with newly diagnosed atrial fibrillation. JAMA Netw Open 2020; 3: e200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steinberg BA, Kim S, Piccini JP, et al. Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for better informed treatment of atrial fibrillation (ORBIT-AF) registry. Circulation 2013; 128: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexander JH, Lopes RD, Thomas L, et al. Apixaban vs. Warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2014; 35: 224–232. [DOI] [PubMed] [Google Scholar]
- 32. Shah R, Hellkamp A, Lokhnygina Y, et al. Use of concomitant aspirin in patients with atrial fibrillation: findings from the ROCKET AF trial. Am Heart J 2016; 179: 77–86. [DOI] [PubMed] [Google Scholar]
- 33. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021; 42: 373–498. [DOI] [PubMed] [Google Scholar]
- 34. Chen LY, Chung MK, Allen LA, et al. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation 2018; 137: e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231183211 for Risk of recurrent stroke in patients with atrial fibrillation treated with oral anticoagulants alone or in combination with anti-platelet therapy in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231183211 for Risk of recurrent stroke in patients with atrial fibrillation treated with oral anticoagulants alone or in combination with anti-platelet therapy in European Stroke Journal
Supplemental material, sj-tif-3-eso-10.1177_23969873231183211 for Risk of recurrent stroke in patients with atrial fibrillation treated with oral anticoagulants alone or in combination with anti-platelet therapy in European Stroke Journal