Abstract
Background and objectives:
Acute kidney injury is a common comorbidity in patients with intracerebral hemorrhage. Although there are predictive models to determine risk of AKI in patients in critical care or post-surgical scenarios or in general medical floors, there are no models that specifically determine the risk of AKI in patients with ICH.
Methods:
Clinical features and laboratory tests were selected by previous studies and LASSO (least absolute shrinkage and selection operator) regression. We used multivariable logistic regression with a bidirectional stepwise method to construct ICH-AKIM (intracerebral hemorrhage–associated acute kidney injury model). The accuracy of ICH-AKIM was measured by the area under the receiver operating characteristic curve. The outcome was AKI development during hospitalization, defined as KDIGO (Kidney Disease: Improving Global Outcomes) Guidelines.
Results:
From four independent medical centers, a total of 9649 patients with ICH were available. Overall, five clinical features (sex, systolic blood pressure, diabetes, Glasgow coma scale, mannitol infusion) and four laboratory tests at admission (serum creatinine, albumin, uric acid, neutrophils-to-lymphocyte ratio) were predictive factors and were included in the ICH-AKIM construction. The AUC of ICH-AKIM in the derivation, internal validation, and three external validation cohorts were 0.815, 0.816, 0.776, 0.780, and 0.821, respectively. Compared to the univariate forecast and pre-existing AKI models, ICH-AKIM led to significant improvements in discrimination and reclassification for predicting the incidence of AKI in all cohorts. An online interface of ICH-AKIM is freely available for use.
Conclusion:
ICH-AKIM exhibited good discriminative capabilities for the prediction of AKI after ICH and outperforms existing predictive models.
Keywords: Intracerebral hemorrhage, acute kidney injury, predictive model
Introduction
Acute kidney injury (AKI) is a common comorbidity after spontaneous intracerebral hemorrhage (ICH) and is related to increased mortality, prolonged hospitalization, and frequently being admitted to a critical care unit.1–3 Delayed diagnosis and treatment allow AKI to progress to more severe stages and develop into chronic kidney disease after hospital discharge. 4 Thus, the early prediction of AKI development is critical for doctors to identify high-risk inpatients and optimize the treatment strategy in time.
Several clinical features and laboratory tests to predict AKI in patients with ICH have been identified and evaluated. The 2022 guideline for the management of patients with ICH from the American Heart Association/American Stroke Association suggests that admission hypertension and a high dose of systolic blood pressure reduction are associated with a high risk of AKI.5–8 Several retrospective studies indicated admission renal function tests (e.g. serum creatinine), inflammatory tests (e.g. neutrophils-to-lymphocyte), and medicines (e.g. antihypertensive, mannitol, and contrast6,9–11) could be efficient predictors for identifying ICH inpatients at risk of early AKI.12–14 Based on these clinical features and laboratory tests, some models have been developed for the prediction of AKI in patients undergoing percutaneous coronary intervention, 15 pediatric critical care, 16 cirrhotic, 17 and organ transplantation. 18 However, there is a lack of AKI predictive models for patients with ICH.
In this study, we collected a total of 9649 ICH patients from four medical hospitals, one of which was used for derivation and internal validation and the other three for external validation. The objectives of this study were to develop and validate a precise and convenient AKI predictive model based on clinical features and laboratory tests at admission in a large retrospective cohort of patients with ICH.
Methods
Derivation cohort
We retrospectively included consecutive ICH patients referred to West China Hospital, Sichuan University, between January 2010 and July 2019. This hospital has a comprehensive stroke center which covered both local stroke patients and patients transferred from primary centers. The study was approved by the Institutional Review Boards of West China Hospital, with a waiver of informed consent. The data of this study are available from the corresponding author on reasonable request. Patients were eligible if they were definitively diagnosed with ICH via computed tomography scan. We excluded patients who met the following criteria: (1) age <18 years, (2) prior history of ICH, (3) whose ICH related to a ruptured vascular malformation or aneurysm, head trauma, hemorrhagic infarction, or tumoral hemorrhage, (4) lack of AKI records, (5) patients with pre-existing kidney failure and chronic kidney disease, and (6) excluding variables with more than 20% of missing patients. By random split, 70% of patients were defined as a derivation cohort, and 30% of patients were defined as an internal validation cohort.
External validation cohorts
We reviewed patients with ICH from Longquan Hospital (primary stroke center) between 2016 and 2020, Shanxi Provincial People’s Hospital (comprehensive stroke center) between 2014 and 2021, and Affiliated Hospital of Chengdu University (primary stroke center) between 2012 and 2020 for external validation construction. The study was also approved by the institutional review boards of Longquan hospital, Shanxi provincial people’s hospital, and the affiliated hospital of Chengdu university. The eligibility criteria and excluded criteria were the same for the validation cohort.
Study outcomes and follow-up
The outcome of this study was AKI development during hospitalization in patients with ICH. AKI was defined based on Kidney Disease: Improving Global Outcomes (KDIGO Guidelines) 19 : (1) a rise in serum creatinine of ⩾0.3 mg/dL (26.5 µmol/L) within 48 h; (2) an increase in serum creatinine to ⩾1.5 times during hospitalization.
Variables selection and model development
All variables, including clinical features, laboratory tests, and treatments, were collected from electronic clinical records of each medical center, and were the first values within 24 h of admission. After intersecting variables of the four medical centers and excluding the missing value of laboratory tests beyond 20%, a total of 40 variables were included. Then, based on the prior relevant literature and the knowledge of medical experts, we selected vital risk factors of AKI, and 24 risk factors remained after selection. Next, the least absolute shrinkage and selection operator (LASSO) regression was performed to remove nonsignificant variables for the AKI risk based on the data of the derivation cohort. Finally, 14 risk factors were selected to construct a predictive model called ICH-AKIM by multivariable logistic regression models, with a bidirectional stepwise method to optimize (Supplemental eFigure 1).
The performance of ICH-AKIM was tested in internal and external cohorts. For the convenient use of doctors, the online model was constructed with Shiny. The URL of this model is https://westchina-neurosurgery.shinyapps.io/aki_predictor/. The user interface displays the factors and coefficients of ICH-AKIM, and users can input their own data to generate a prediction by ICH-AKIM.
Previous AKI prediction models
We systemically searched the models for AKI prediction in PubMed, and found some models that have been developed in patients undergoing percutaneous coronary intervention, 15 pediatric critical care, 16 cirrhotic, 17 and organ transplantation. 18 Because there are no existing AKI predictive models for inpatients with ICH, we found two publications of AKI models derived from general wards to compare with our model.20,21 Catalina et al. has developed a risk score to predict the risk of hospital-acquired AKI based on variables present at admission in the electronic clinical records, which included diabetes, hypertension, sepsis, medical ward, pneumonia, skin infection, dehydration, serum albumin, white blood cells, creatinine. Walid et al. developed a regression model for the prediction of AKI in non-ICU patients, and the variables included type of surgery, congestive heart failure, hemiplegia, renal disease, liver disease, malignancy, anemia, diabetes, age, uric acid, urea, calcium, leukocytes, sodium, glucose, potassium. Since multiple studies reported that serum cystatin at admission was a good predictive biomarker for hospital-acquired AKI,22–25 we also compared our model to serum cystatin C.
Calibration and discrimination
The performance of ICH-AKIM was examined by the determination of the discrimination and calibration. Discrimination was evaluated with AUC. The DeLong test was used to compare the area under the curve (AUC). The calibration of ICH-AKIM was assessed using calibration plots and the Hosmer-Lemeshow statistics to check for significant differences between the observed and predicted risks. Based on the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement, we assessed improvement in model performance by calculating the change in AUC, net reclassification improvement (NRI), and relative integrated discrimination improvement (IDI).26,27
Statistical analysis
Statistical analyses were performed using R software version 4.2.1. Categorical variables were reported as absolute numbers (%), and continuous variables were reported as mean (±standard deviation) or median (interquartile ranges). A p-value <0.05 was accepted as statistically significant. We replaced missing values by k-Nearest Neighbor imputation method of each cohort. We also performed a sample size estimation with the formula developed by Riley et al. 28 ; the minimum sample size for a new model was 430 patients.
Results
After filtering by exclusion criteria, a total of 9649 patients were included in this study and 908 patients were excluded. The derivation cohort enrolled 4130 patients, the internal validation cohort enrolled 1771 patients, and three external validation cohorts enrolled 3748 patients (Figure 1). The sample size in the derivation cohort was enough to ensure precise predictions and four validation cohorts to examine the generalizability of this model. Baseline clinical features and laboratory tests were reported in Table 1. Briefly, a vast majority of clinical characteristics were different among these cohorts, which ensured the examination of the transportability of the model.
Figure 1.
Flowchart illustrating participant selection into the derivation and validation cohorts. ICH: intracerebral hemorrhage.
Table 1.
Baseline characteristics of the patients in five cohorts.
| Characteristics # | Derivation cohort (n = 4130) | Internal validation cohort (n = 1771) | External validation cohort 1 (n = 1916) | External validation cohort 2 (n = 1098) | External validation cohort 3 (n = 734) | Missing value count n (%) | p-Value |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, mean (SD), year | 56.81 (14.78) | 56.96 (14.37) | 57.29 (13.53) | 62.22 (14.52) | 64.19 (13.76) | 0 (0) | <0.001 |
| Female, n (%) | 1323 (32.0) | 593 (33.5) | 1264 (66.0) | 380 (34.6) | 275 (37.5) | 0 (0) | <0.001 |
| Smoking, n (%) | 0 (0) | <0.001 | |||||
| Never | 2799 (67.8) | 1222 (69.0) | 1111 (58.0) | 672 (61.2) | 494 (67.3) | ||
| Current | 1062 (25.7) | 463 (26.1) | 670 (35.0) | 362 (33.0) | 204 (27.8) | ||
| Ever | 269 (6.5) | 86 (4.9) | 135 (7.0) | 64 (5.8) | 36 (4.9) | ||
| Alcohol abuse, n (%) | 1274 (30.8) | 551 (31.1) | 609 (31.8) | 431 (39.3) | 211 (28.7) | 0 (0) | <0.001 |
| Medical history | |||||||
| Hypertension, n (%) | 2949 (71.4) | 1268 (71.6) | 1288 (67.2) | 309 (28.1) | 483 (65.8) | 0 (0) | <0.001 |
| Diabetes, n (%) | 419 (10.1) | 196 (11.1) | 158 (8.2) | 84 (7.7) | 73 (9.9) | 0 (0) | 0.005 |
| Hematoma characteristics | |||||||
| Volume of hematoma, mean (SD), ml | 21.34 (26.88) | 21.79 (26.10) | 18.60 (17.68) | 16.58 (21.98) | 19.05 (20.98) | 2791 (29.3) | <0.001 |
| Infratentorial hematoma, n (%) | 888 (21.5) | 342 (19.3) | 278 (14.5) | 173 (15.8) | 53 (7.2) | 0 (0) | <0.001 |
| Intraventricular hematoma, n (%) | 930 (22.5) | 407 (23.0) | 119 (6.2) | 440 (40.1) | 930 (22.5) | 0 (0) | <0.001 |
| Glasgow Coma Scale, mean (SD) | 10.99 (4.12) | 10.84 (4.17) | 9.48 (3.75) | 10.59 (4.19) | 12.19 (3.46) | 157 (1.7) | <0.001 |
| Systolic blood pressure, mean (SD), mmHg | 159.98 (32.31) | 161.38 (33.22) | 150.74 (24.87) | 166.28 (34.36) | 167.03 (32.94) | 97 (1.0) | <0.001 |
| Laboratory tests, mean (SD) | |||||||
| Creatinine | 97.84 (118.97) | 99.18 (121.20) | 99.49 (111.79) | 89.25 (107.99) | 79.23 (90.85) | 69 (0.7) | <0.001 |
| Albumin, g/L | 40.85 (5.66) | 40.64 (5.73) | 38.99 (4.51) | 37.28 (4.68) | 38.52 (4.76) | 160 (1.7) | <0.001 |
| Uric acid | 319.30 (125.75) | 318.87 (124.25) | 321.38 (116.75) | 279.72 (88.46) | 330.50 (127.91) | 853 (9.0) | <0.001 |
| Hemoglobin, g/L | 133.54 (22.63) | 134.50 (23.63) | 134.00 (45.85) | 132.99 (20.62) | 129.76 (20.87) | 258 (2.7) | <0.001 |
| White blood cell count, 1012/L | 10.60 (8.31) | 10.53 (5.88) | 11.92 (12.14) | 9.34 (10.27) | 9.62 (7.29) | 263 (2.8) | <0.001 |
| Platelet count, μmol /L, 109/L | 160.95 (69.36) | 160.06 (74.44) | 191.60 (65.20) | 167.06 (67.64) | 177.78 (88.82) | 260 (2.7) | <0.001 |
| Urea | 6.11 (4.21) | 6.20 (4.68) | 5.59 (3.93) | 5.92 (4.04) | 5.78 (5.68) | 88 (0.9) | <0.001 |
| Sodium | 138.74 (5.32) | 138.76 (5.45) | 138.76 (4.70) | 138.10 (4.36) | 139.47 (6.09) | 54 (0.5) | <0.001 |
| Potassium | 3.56 (0.51) | 3.57 (0.52) | 3.71 (0.52) | 3.68 (0.50) | 3.75 (0.51) | 55 (0.6) | <0.001 |
| Chlorine | 102.05 (6.55) | 102.00 (6.56) | 104.60 (5.49) | 103.33 (4.20) | 104.12 (6.59) | 55 (0.6) | <0.001 |
| Outcome | |||||||
| AKI, n (%) | 589 (14.3) | 257 (14.5) | 203 (10.6) | 226 (20.6) | 50 (6.8) | 0 (0) | <0.001 |
SD: standard deviation; AKI: acute kidney injury.
The definitions of characteristics were shown in Supplemental eTable 2.
A multivariable logistic regression was performed for the prediction of ICH-associated AKI in the derivation cohort and resulted in nine variables after bidirectional stepwise adjustment. The final variables included five clinical features (sex, systolic blood pressure, diabetes, Glasgow coma scale, mannitol infusion) and four laboratory tests at admission (serum creatinine, albumin, uric acid, neutrophils-to-lymphocyte ratio), which were mostly identified as significant independent predictors of AKI (Table 2). The online predictive model was applied by the coefficients from the multivariable logistic regression, as shown in Figure 2.
Table 2.
Multivariable logistic regression model of acute kidney injury in patients with intracerebral hemorrhage in the derivation cohort.
| β-coefficients | SE | Adjusted OR (95% CI) | p | |
|---|---|---|---|---|
| Model intercept | −2.6300 | 0.4190 | ||
| Creatinine, μmol/l | 0.0048 | 0.0005 | 1.005 (1.004–1.006) | <0.001 |
| Neutrophils-to-lymphocyte ratio | 0.0219 | 0.0049 | 1.022 (1.012–1.032) | <0.001 |
| Systolic blood pressure, mmHg | 0.0137 | 0.0016 | 1.014 (1.011–1.017) | <0.001 |
| Glasgow Coma Scale | −0.100 | 0.0121 | 0.905 (0.884–0.927) | <0.001 |
| Albumin, g/l | −0.061 | 0.0086 | 0.941 (0.925–0.957) | <0.001 |
| Uric acid, μmol/l | 0.0021 | 0.0004 | 1.002 (1.001–1.003) | <0.001 |
| Diabetes | 0.6500 | 0.1460 | 1.915 (1.433–2.541) | <0.001 |
| Female | −0.560 | 0.1264 | 0.571 (0.444–0.729) | <0.001 |
| The use of mannitol | 0.6860 | 0.1731 | 1.986 (1.426–2.813) | <0.001 |
SE: standard error; OR: odds ratio.
Figure 2.
The online web-based calculator of the model for predicting AKI in patients with intracerebral hemorrhage (https://westchina-neurosurgery.shinyapps.io/aki_predictor/) AKI: acute kidney injury. SBP: systolic blood pressure; NLR: neutrophils-to-lymphocyte ratio; GCS: Glasgow Coma Scale.
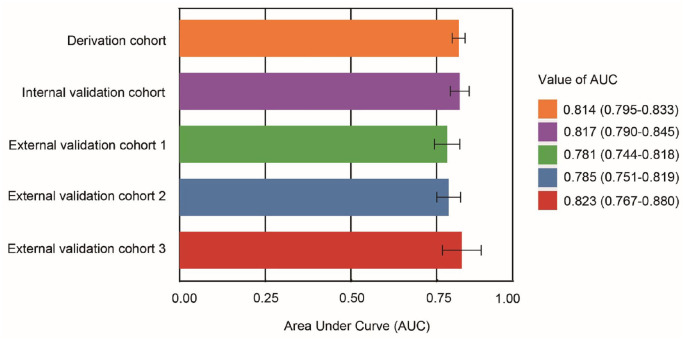
The AUC of ICH-AKIM in the derivation, internal validation, and three external validation cohorts were 0.815 (95% CI: 0.796–0.833), 0.816 (95% CI: 0.788–0.843), 0.776 (95% CI: 0.739–0.814), 0.780 (95% CI: 0.745–0.815), and 0.821 (95% CI: 0.763–0.878), respectively (Figure 3). The model also had good calibration, with a Hosmer-Lemeshow statistic of p = 0.304 (nonsignificant p-values indicate adequate calibration).
Figure 3.
The area under Receiver Operating Characteristic Curve (AUC) in five cohorts. AUC: area under curve.
Compared to univariate forecast and two pre-existing ICH scores, ICH-AKIM improved discrimination (change in AUC = from +0.039 to +0.211) and reclassification (categorical NRI was 0.6%–30.4%) in all five cohorts (Table 3 and Supplemental eFigure 2). Similar improvements in continuous NRI and IDI were found in both cohorts. We also established a sensitivity model without mannitol. The AUC of the sensitivity model without mannitol in the derivation, internal validation, and three external validation cohorts were 0.813 (95% CI: 0.795–0.832), 0.822 (95% CI: 0.796–0.849), 0.777 (95% CI: 0.740–0.814), 0.782 (95% CI: 0.747–0.817), and 0.815 (95% CI: 0.757–0.873), respectively. The detail of the sensitivity model was shown in Supplemental eTable 3. The online version of this sensitivity model was also provided and shown in Supplemental eFigure 3.
Table 3.
AUC of the model in five cohorts and comparison with other models.
| Models | Discrimination |
Reclassification |
||
|---|---|---|---|---|
| ∆AUC (P) | IDI, % (95% CI, P) | Categorical NRI, % (95% CI, P)* | Continuous NRI, % (95% CI, P) | |
| Derivation cohort | ||||
| The ICH-AKIM model | reference | reference | reference | reference |
| vs Cystatin C | 0.097 (<0.001) | 11.0 (9.4–12.6, <0.001) | 8.9 (6.0–11.8, <0.001) | 74.7 (66.6–82.9, <0.001) |
| vs Martin-Cleary et al. 21 | 0.122 (<0.001) | 13.0 (11.3–14.7, <0.001) | 16.0 (12.6–19.4, <0.001) | 72.0 (63.9–80.4, <0.001) |
| vs Elrewihby et al. 20 | 0.160 (<0.001) | 10.7 (7.2–14.1, <0.001) | 12.5 (8.6–16.4, <0.001) | 61.5 (53.3–69.7, <0.001) |
| Internal validation cohort | ||||
| The ICH-AKIM model | reference | reference | reference | reference |
| vs Cystatin C | 0.076 (<0.001) | 9.3 (6.6–11.9, <0.001) | 4.6 (0.2–9.0, <0.001) | 63.4 (50.8–75.9, <0.001) |
| vs Catalina et al. | 0.098 (<0.001) | 12.8 (10.2–15.5, <0.001) | 12.9 (7.8–17.9, <0.001) | 67.9 (55.6–80.2, <0.001) |
| vs Walid et al. | 0.137 (<0.001) | 15.4 (9.8–20.9, <0.001) | 19.3 (12.8–25.7, <0.001) | 72.3 (59.9–84.6, <0.001) |
| External validation cohort 1 | ||||
| The ICH-AKIM model | reference | reference | reference | reference |
| vs Cystatin C | 0.097 (<0.001) | 13.4 (10.0–16.8, <0.001) | 12.9 (7.8–18.0, <0.001) | 76.5 (62.3–90.6, <0.001) |
| vs Catalina et al. | 0.045 (<0.001) | 9.6 (6.6–12.1, <0.001) | 13.2 (8.1–18.3, <0.001) | 64.6 (50.3–78.8, <0.001) |
| vs Walid et al. | 0.079 (<0.001) | 22.5 (16.4–28.6, <0.001) | 23.5 (16.4–30.5, <0.001) | 80.6 (66.5–94.6, <0.001) |
| External validation cohort 2 | ||||
| The ICH-AKIM model | reference | reference | reference | reference |
| vs Cystatin C | 0.132 (<0.001) | 6.2 (4.3–8.1, <0.001) | 3.6 (0.6–6.5, 0.02) | 52.7 (39.3–66.1, <0.001) |
| vs Catalina et al. | 0.119 (<0.001) | 6.0 (3.8–8.3, <0.001) | 5.2 (0.9–9.6, 0.02) | 46.8 (34.1–59.4, <0.001) |
| vs Walid et al. | 0.203 (<0.001) | 4.1 (0.1–8.4, 0.05) | 8.3 (3.7–13.1, <0.001) | 32.9 (22.2–43.6, <0.001) |
| External validation cohort 3 | ||||
| The ICH-AKIM model | reference | reference | reference | reference |
| vs Cystatin C | 0.042 (0.30) | 7.2 (3.1–11.2, <0.001) | 2.2 (−0.5–8.9, 0.53) | 77.4 (49.2–105.5, <0.001) |
| vs Catalina et al. | 0.066 (0.09) | 3.2 (−1.2–7.5, 0.14) | 0.6 (−7–8, 0.88) | 41.9 (15.2–68.7, 0.002) |
| vs Walid et al. | 0.156 (<0.001) | 22.2 (8.9–35.5, 0.001) | 30.4 (16.0–44.7, <0.001) | 66.9 (39.8–94.0, <0.001) |
AUC: area under curve; IDI: integrated discrimination improvement; NRI: net reclassification improvement.
The cut off value of categorical NRI was 0.5.
Discussion
In this large multicenter retrospective study, we developed a novel, robust, and user-friendly online predictive model called ICH-AKIM in patients with ICH. The ICH-AKIM model uses only nine common and readily available clinical variables at admission (sex, 29 systolic blood pressure,5,7 diabetes, 29 Glasgow coma scale, serum creatinine, 1 albumin, 30 uric acid, the neutrophils-to-lymphocyte ratio, 12 and mannitol infusion 11 ), thereby allowing routine application by doctors with electronic medical records. We also evaluated the generalizability of ICH-AKIM via three independent external validation cohorts and found it to be superior to pre-existing “general AKI” models. ICH-AKIM is also more robust than the univariate predictor Cystatin C. Finally, we have made our model freely available online for clinical practice or research.
We anticipate that ICH-AKIM will be of value in the neurosurgical unit, specifically for patients hospitalized for initial onset of intracerebral hemorrhage. Prior to this work, the assessment of AKI risk at admission was usually limited to other clinical contexts, such as patients with chronic kidney disease, critically ill, or receiving nephrotoxic drugs. 31 Even if an ICH patient is identified as critically ill, that is, there is a need to assess the risk of AKI, there was a lack of risk scores or models for that ICH patient. Our ICH-AKIM model predicts the risk of AKI in patients with ICH via only nine common and readily available clinical variables at admission. Previous studies have reported some hospital-acquired AKI were iatrogenic, and the risk factors included the admission of contrast agents and nephrotoxic drugs.32,33 Via the ICH-AKIM model, neurosurgeons could identify the high-risk patients of AKI at admission and implement multiple preventive measures to avoid potential iatrogenic AKI, which included avoiding the use of high-osmolar contrast media, 32 adequate fluid rehydration during the peri-contrast period, 34 and minimizing the use of potentially nephrotoxic drugs such as mannitol and vancomycin.9,11
There are some variables with significant differences between derivation and external validation cohorts such as age and creatinine. To evaluate the external validity, TRIPOD recommends the performance of the predictive model should be evaluated in datasets that were not used to develop the model in different locations consisting of plausibly similar individuals. Although some variables were significantly different among derivation and external validation cohorts, our model retained high performance in three external validation cohorts (the AUC of ICH-AKIM in three external validation cohorts were 0.776, 0.780, and 0.821). To ensure the stability of the model in the general population, we have excluded patients with abnormal clinical features which potentially affected outcomes, such as patients with chronic kidney disease or patients under the age of 18.
There are several limitations of this work. First, all of the patients enrolled in this study were Chinese, thus, the racial differences in kidney disease were not discussed. Future studies may include various racial and ethnic groups through international collaboration. Secondly, because of the lack of several uncommonly measured parameters, we were unable to evaluate the predictive performance of several existing scores (e.g. Trongtrakul et al. 35 ). Third, we note that the performance of pre-existing models applied on our dataset was lower than the original studies, possibly due to the uniqueness of clinical features in patients with ICH. But the ICH-AKIM model was more robust than pre-existing models in the external validation cohorts. Fourth, stepwise selection may increase the risk of type I errors. Previous studies indicated that this risk depend on sample size and number of potential predictors. 36 This risk was small in this study because of the large sample size of the training cohort. We validated the generalizability of our model in three different external cohorts to prove that it does not appear to be overfitting and could apply to new data. Fifth, uric acid was one of the predictor variables in the ICH-AKIM model. But in many other institutions, uric acid is not routinely measured in patients admitted with ICH, which could be a limitation to the generalizability of the ICH-AKIM model during clinical practice. Finally, although the effect of the ICH-AKIM model has been evaluated in three external cohorts, it has not been applied in clinical practice. Thus, further studies may perform a prospective trial to validate the effectiveness of the ICH-AKIM model.
Recently, multivariate models and even more complex models like artificial intelligence algorithms are widely published in the field of AKI prediction. Such studies have illustrated the predictive models of AKI could achieve remarkable performance in multiple clinical contexts, including contrast-associated AKI, post-cardiac surgery, critical care, cirrhotic, and organ transplantation.15–18,37 Our experience indicate that predictive models can be applied to multiple clinical contexts, but may have the best performance when used in the same clinical context. In the future, we anticipate that integration of continuous prediction of future acute kidney injury based on deep learning of massive clinical data4,29 into electronic health systems will have a positive impact on clinician workflows.
Conclusions
This large multicenter retrospective study has developed and validated a robust and convenient model called ICH-AKIM that enables accurate prediction of AKI risk based on clinical features and laboratory tests at admission in patients with ICH. Within patients with ICH in this multi-institutional study, ICH-AKIM exhibited superior performance to univariate Cystatin C model and pre-existing risk models of AKI.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231184667 for Predictive model of acute kidney injury after spontaneous intracerebral hemorrhage: A multicenter retrospective study by Yixin Tian, Yu Zhang, Jialing He, Lvlin Chen, Pengfei Hao, Tiangui Li, Liyuan Peng, Weelic Chong, Yang Hai, Chao You, Lu Jia and Fang Fang in European Stroke Journal
Acknowledgments
Not applicable.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by National Natural Science Foundation of China (82271364) Yu Zhang), the innovation team project of Affiliated Hospital of Clinical Medicine College of Chengdu University (CDFYCX202203) Yu Zhang) and the project of Sichuan Science and Technology Bureau (22ZDYF0798) (Fang Fang).
Ethical approval: The ethics committee of West China Hospital (No. 20211701), the ethics committee of the First of People’s Hospital of Longquan District (No. 2022004), the ethics committee of the Affiliated Hospital of Chengdu University (No. PJ2021-017-03), and the ethics committee of Shanxi Bethune Hospital (No. YXLL-2022-068).
Informed consent: Not applicable.
Guarantor: Fang Fang
Contributorship: Study concept: FF, LJ. Design: All authors. Acquisition, analysis, or interpretation of data: YT, YZ, JH, LC, TL, PH, LP, WC, and YH. Statistical analysis: YT and YZ. Drafting of the manuscript: YT and YZ. Critical revision of the manuscript for important intellectual content: All authors.
Trial registration: Not applicable.
ORCID iDs: Yixin Tian  https://orcid.org/0000-0002-4195-0710
https://orcid.org/0000-0002-4195-0710
Jialing He  https://orcid.org/0000-0001-8871-0012
https://orcid.org/0000-0001-8871-0012
Supplemental material: Supplemental material for this article is available online.
References
- 1. Zhang C, Xia J, Ge H, et al. Long-term mortality related to acute kidney injury following intracerebral hemorrhage: A 10-Year (2010-2019) Retrospective Study. J Stroke Cerebrovasc Dis 2021; 30: 105688. [DOI] [PubMed] [Google Scholar]
- 2. Tsagalis G, Akrivos T, Alevizaki M, et al. Long-term prognosis of acute kidney injury after first acute stroke. Clin J Am Soc Nephrol 2009; 4: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu VC, Wu PC, Wu CH, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc 2014; 3: e000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiofolo C, Chbat N, Ghosh E, et al. Automated continuous acute kidney injury prediction and surveillance: a random forest model. Mayo Clin Proc 2019; 94: 783–792. [DOI] [PubMed] [Google Scholar]
- 5. Qureshi AI, Huang W, Lobanova I, et al. Systolic blood pressure reduction and acute kidney injury in intracerebral hemorrhage. Stroke 2020; 51: 3030–3038. [DOI] [PubMed] [Google Scholar]
- 6. Hewgley H, Turner SC, Vandigo JE, et al. Impact of admission hypertension on rates of acute kidney injury in intracerebral hemorrhage treated with intensive blood pressure control. Neurocrit Care 2018; 28: 344–352. [DOI] [PubMed] [Google Scholar]
- 7. Burgess LG, Goyal N, Jones GM, et al. Evaluation of acute kidney injury and mortality after intensive blood pressure control in patients with intracerebral hemorrhage. J Am Heart Assoc 2018; 7: e008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang Y, Wan C, Wu G. Acute kidney injury after a stroke: a PRISMA-compliant meta-analysis. Brain Behav 2020; 10: e01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sazanami K, Inose R, Yagi T, et al. Incidence of acute kidney injury after teicoplanin- or vancomycin- and piperacillin/tazobactam combination therapy: a comparative study using propensity score matching analysis. J Infect Chemother 2021; 27: 1723–1728. [DOI] [PubMed] [Google Scholar]
- 10. Micozkadioglu H. Higher diastolic blood pressure at admission and antiedema therapy is associated with acute kidney injury in acute ischemic stroke patients. Int J Nephrol Renovasc Dis 2014; 7: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin SY, Tang SC, Tsai LK, et al. Incidence and risk factors for acute kidney injury following mannitol infusion in patients with acute stroke: a retrospective cohort study. Medicine 2015; 94: e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang F, Liu J, Yu X, et al. The Monocyte-to-Lymphocyte ratio predicts acute kidney injury after acute hemorrhagic stroke. Front Neurol 2022; 13: 904249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arnold J, Sims D, Gill P, et al. Acute kidney injury calculated using admission serum creatinine underestimates 30-day and 1-year mortality after acute stroke. Clin Kidney J 2020; 13: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang F, Su L, Xiang H, et al. Incidence, risk factors, and biomarkers predicting ischemic or hemorrhagic stroke associated acute kidney injury and outcome: a retrospective study in a general intensive care unit. Blood Purif 2019; 47: 317–326. [DOI] [PubMed] [Google Scholar]
- 15. Mehran R, Owen R, Chiarito M, et al. A contemporary simple risk score for prediction of contrast-associated acute kidney injury after percutaneous coronary intervention: derivation and validation from an observational registry. J Lancet 2021; 398: 1974–1983. [DOI] [PubMed] [Google Scholar]
- 16. Dong J, Feng T, Thapa-Chhetry B, et al. Machine learning model for early prediction of acute kidney injury (AKI) in pediatric critical care. Crit Care 2021; 25: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gameiro J, Agapito Fonseca J, Monteiro Dias J, et al. Prediction of acute kidney injury in cirrhotic patients: a new score combining renal, liver and inflammatory markers. Int J Nephrol Renovasc Dis 2018; 11: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xin W, Yi W, Liu H, et al. Early prediction of acute kidney injury after liver transplantation by scoring system and decision tree. Ren Fail 2021; 43: 1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830. [DOI] [PubMed] [Google Scholar]
- 20. Elrewihby W, Kasem H, Raghib A, et al. Developing and validating a risk score model for prediction of acute kidney injury in non-ICU hospitalized patients. Clin Nephrol 2021; 95: 182–188. [DOI] [PubMed] [Google Scholar]
- 21. Martin-Cleary C, Molinero-Casares LM, Ortiz A, et al. Development and internal validation of a prediction model for hospital-acquired acute kidney injury. Clin Kidney J 2021; 14: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Z, Lu B, Sheng X, et al. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis 2011; 58: 356–365. [DOI] [PubMed] [Google Scholar]
- 23. Vijay P, Lal BB, Sood V, et al. Cystatin C: best biomarker for acute kidney injury and estimation of glomerular filtration rate in childhood cirrhosis. Eur J Pediatr 2021; 180: 3287–3295. [DOI] [PubMed] [Google Scholar]
- 24. Maiwall R, Kumar A, Bhardwaj A, et al. Cystatin C predicts acute kidney injury and mortality in cirrhotics: A prospective cohort study. Liver Int 2018; 38: 654–664. [DOI] [PubMed] [Google Scholar]
- 25. Abd El, Wahab AM, Awadeen A, Mansour MM, et al. The diagnostic and prognostic utility of serum cystatin C and angiopoietin 2 in patients with liver cirrhosis complicated by acute kidney injury. Ther Apher Dial 2023; 27: 419–427. [DOI] [PubMed] [Google Scholar]
- 26. Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015; 350: g7594. [DOI] [PubMed] [Google Scholar]
- 27. Alba AC, Agoritsas T, Walsh M, et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA 2017; 318: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 28. Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ 2020; 368: m441. [DOI] [PubMed] [Google Scholar]
- 29. Tomašev N, Glorot X, Rae JW, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Our Nat 2019; 572: 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Z, Ho KM, Hong Y. Machine learning for the prediction of volume responsiveness in patients with oliguric acute kidney injury in critical care. Crit Care 2019; 23: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zorrilla-Vaca A, Ziai W, Connolly ES, Jr, et al. Acute kidney injury following acute ischemic stroke and intracerebral hemorrhage: a meta-analysis of prevalence rate and mortality risk. Cerebrovasc Dis 2018; 45: 1–9. [DOI] [PubMed] [Google Scholar]
- 32. Chandiramani R, Cao D, Nicolas J, et al. Contrast-induced acute kidney injury. Cardiovasc Interv Ther 2020; 35: 209–217. [DOI] [PubMed] [Google Scholar]
- 33. McCullough PA, Choi JP, Feghali GA, et al. Contrast-induced acute kidney injury. J Am Coll Cardiol 2016; 68: 1465–1473. [DOI] [PubMed] [Google Scholar]
- 34. Soomro QH, Anand ST, Weisbord SD, et al. The relationship between rate and volume of intravenous fluid administration and kidney outcomes after angiography. Clin J Am Soc Nephrol 2022; 17: 1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trongtrakul K, Patumanond J, Kongsayreepong S, et al. Acute kidney injury risk prediction score for critically-ill surgical patients. BMC Anesthesiol 2020; 20: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whittingham MJ, Stephens PA, Bradbury RB, et al. Why do we still use stepwise modelling in ecology and behaviour? J Anim Ecol 2006; 75: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 37. Ostermann M, Lumlertgul N, Wilson FP. Predictive models for acute kidney injury following cardiac surgery: the importance of accurate and actionable prediction. JAMA 2022; 327: 927–929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231184667 for Predictive model of acute kidney injury after spontaneous intracerebral hemorrhage: A multicenter retrospective study by Yixin Tian, Yu Zhang, Jialing He, Lvlin Chen, Pengfei Hao, Tiangui Li, Liyuan Peng, Weelic Chong, Yang Hai, Chao You, Lu Jia and Fang Fang in European Stroke Journal