Abstract
Purpose:
Recombinant human interleukin-1 receptor antagonist (anakinra) is an anti-inflammatory with efficacy in animal models of stroke. We tested the effect of anakinra on perihaematomal oedema in acute intracerebral haemorrhage (ICH) and explored effects on inflammatory markers.
Methods:
We conducted a multicentre, randomised, double-blind, placebo-controlled trial in patients with acute, spontaneous, supratentorial ICH between May 2019 and February 2021. Patients were randomised to 100 mg subcutaneous anakinra within 8 h of onset, followed by five, 12-hourly, 100 mg subcutaneous injections, or matched placebo. Primary outcome was oedema extension distance (OED) on a 72 h CT scan. Secondary outcomes included plasma C-reactive protein (CRP) and interleukin-6 (IL-6).
Findings:
25 patients (target = 80) were recruited, 14 randomised to anakinra, 11 to placebo. Mean age was 67 and 52% were male. The anakinra group had higher median baseline ICH volume (12.6 ml, interquartile range[IQR]:4.8–17.9) versus placebo (5.5 ml, IQR:2.1–10.9). Adjusting for baseline, 72 h OED was not significantly different between groups (mean difference OED anakinra vs placebo -0.05 cm, 95% confidence interval [CI]: −0.17–0.06, p = 0.336). There was no significant difference in area-under-the-curve to Day 4 for IL-6 and CRP, but a post-hoc analysis demonstrated IL-6 was 56% (95% CI: 2%–80%) lower at Day 2 with anakinra. There were 10 and 2 serious adverse events in anakinra and placebo groups, respectively, none attributed to anakinra.
Conclusion:
We describe feasibility for delivering anakinra in acute ICH and provide preliminary safety data. We lacked power to test for effects on oedema thus further trials will be required.
Keywords: Intracerebral haemorrhage, interleukin-1 receptor antagonist, anakinra, clinical trial
Introduction
Spontaneous intracerebral haemorrhage (ICH) accounts for around 10% of incident strokes in Western Europe and has worldwide incidence of over three million per annum. 1 Globally, it is estimated that 62.8 million disability-adjusted life years are lost per year as a result of ICH. 1 Despite this major global burden, ICH has few effective treatments. Current medical treatments for ICH seek to reduce the risk of haematoma expansion with rapid reversal of anticoagulation and intensive lowering of blood pressure. However, haematoma expansion only affects around 20% of patients 2 and thus only a minority can benefit. For all survivors of the hyperacute phase, secondary brain injury over the subsequent hours to days is driven by a cascade of cellular and molecular events including the toxic effects of blood components and inflammation. 3
Within hours of brain injury, a sterile inflammatory response is initiated where activated microglia take on a pro-inflammatory phenotype, releasing cytokines and chemokines that activate astrocytes and endothelial cells and lead to recruitment of neutrophils to the site of injury, exacerbating tissue injury. 4 The prototypical, proinflammatory cytokine interleukin-1 (IL-1) is released from activated microglia via inflammasome-mediated pathways and plays a key detrimental role in the first 3 days after onset, in keeping with peak levels observed in animal models. 5 Inhibiting IL-1 leads to a reduction in damage in diverse experimental acute brain injuries including ischaemic stroke and ICH. 6 The naturally occurring IL-1 blocker, IL-1 receptor antagonist (IL-1Ra), is expressed at almost undetectable levels in healthy brain. 7 Perihaematomal oedema increases rapidly over the first 72 h after ICH 8 and is associated with worse outcomes. 9 Overexpression of IL-1Ra in a rodent model of ICH is associated with a 36% reduction in oedema. 10 Anakinra (recombinant human IL-1Ra) is a licenced treatment (for rheumatoid arthritis, periodic fever syndromes, Still’s disease, and COVID-1911) that has also been given to many other patient groups, without significant safety concerns.11,12 Anakinra is a competitive and highly selective antagonist that blocks all known actions of IL-1α and IL-1β. 12
We sought to assess whether anakinra could reduce subacute perihaematomal oedema after acute spontaneous ICH, test effects on circulating inflammatory markers and clinical outcomes at 3 months, and obtain preliminary safety data.
Methods
BLOC-ICH was a phase II, randomised, double-blind, placebo-controlled, parallel group clinical trial conducted between May 2019 and February 2021 at five UK stroke centres based at five different hospitals in the United Kingdom. The study was approved by National Health Service Research Ethics Committee (IRAS ID:252065) and was registered on ClinicalTrials.gov (NCT03737344) and the EU Clinical Trials Register (2018-000249-38). Substantial amendments to the protocol were made in July 2019 (administrative changes) and February 2020 (including minor alteration to dosing schedule for practicality).
Patients aged 18 or over with acute, spontaneous, non-traumatic, supratentorial ICH were eligible for the trial if there was no suspicion of an underlying macrovascular or neoplastic cause for their index ICH and they could receive treatment with the study drug within 8 h of symptom onset (or time last known to be well if onset unknown). Full eligibility criteria are listed in Supplemental Material, but patients were excluded if they had severe ICH (e.g. Glasgow Coma Scale [GCS] score <6), deemed unlikely to survive to a 72 h CT scan, confirmed or suspected haemorrhagic transformation of infarction, or neurosurgery planned within 72 h of admission. Informed consent was obtained from all participants with capacity. Where capacity to consent was not present, consent was obtained from a personal legal representative and where none was available, from a professional legal representative.
Following consent and prior to randomisation, a baseline assessment was performed, including collection of venous blood for measurement of inflammatory markers, demographics, medical history, GCS score, National Institutes of Health Stroke Scale (NIHSS) score, and concomitant medication check. Participants were randomised 1:1 to active treatment or matched placebo using an independent online randomisation service. Randomisation was stratified by centre and GCS score at time of consent (6–13; 14 or 15). Active treatment and placebo had identical labelling and packaging and treatment allocation was concealed from participants, their representatives, all clinical staff, and all research staff throughout the trial.
The trial intervention was twice daily, subcutaneous (SC) administration of 100 mg of anakinra (marketed as Kineret® in 0.67 mL prefilled syringes for single use) or matched placebo, supplied by Swedish Orphan Biovitrum AB (publ) (Sobi). Study drug was in addition to standard care, which was administered according to current UK stroke guidelines at all centres. 13 Administration of study drug commenced as soon as possible after randomisation and continued twice daily (with a minimum of 8 h and a maximum of 16 h between doses) for up to 3 days (six doses) from onset of symptoms, or until discharge from the treating centre (whichever was sooner). IL-1Ra was commenced as soon as possible after recruitment and no later than 8 h after symptom onset because brain IL-1 protein levels rise 30-fold by 6 h after onset of experiment ICH in rodents, with levels falling up to 7 days, 5 and early signs of microglial activation and initial neutrophil infiltration are observed by 1–4 h from onset.14,15 By blocking IL-1 as early as possible and by treating for 3 days we sought to optimise the detection of any effect of anakinra on perihaematomal oedema.
Participants were assessed on the day after randomisation (Day 1) and daily until Day 4 or discharge from hospital, whichever was sooner. At each daily assessment, blood was collected for inflammatory marker measurement. On Day 3, NIHSS and GCS were recorded, and a CT brain scan was performed (CT performed at 72 h ± 12 h after randomisation, or at discharge, if sooner). Adverse events and concomitant medications were documented at days 1, 4 and 30 (±3 days). The final assessment was performed at Month 3 (±14 days) by telephone by the Chief Investigator who was blinded to treatment allocation.
All trial data were captured in a secure online database. The diagnostic and 72 h CT scans were anonymised and transferred to the Imaging Center at the Brain Injury Outcomes Clinical Trials Coordinating Center at Johns Hopkins School of Medicine, Baltimore USA. Measurement of haematoma volume and perihaematomal oedema volume was performed, blinded to treatment allocation, using previously described methods.16,17 This approach has previously been shown to have excellent inter-rater and intra-rater reliability. 17 Plasma was aliquoted into freezer vials for storage at −70°C. On completion of the trial, samples were transferred to the central laboratory at Salford Royal Hospital for analysis to measure plasma CRP and IL-6. Assay methods are described in the Supplemental Material.
The primary outcome was the oedema extension distance (OED) on the 72 h CT scan, adjusted for baseline OED. OED was chosen as the primary outcome as we have previously shown that it is relatively independent of the haematoma volume and allows testing of a significant treatment effect on oedema with fewer patients in a phase II clinical trial. 18 Secondary outcomes were (1) early neurological decline (END) between baseline and Day 4 (defined as an increase in NIHSS of ⩾4 points and/or a decrease in the GCS by ⩾2 points, lasting at least 8 h or leading to surgery or death) (2) Haematoma expansion (defined as increase in haematoma volume by ⩾ 6ml and/or ⩾33% between baseline and 72 h scans 19 ) (3) area under the curve for CRP and IL-6 to Day 4 (4) Clinical outcomes at 3 months including modified Rankin Scale (mRS), Stroke Impact Scale (SIS), Fatigue Severity Scale (FSS), quality of life (EQ-5D-5L), Hospital Anxiety and Depression Scale (HADS). Target recruitment was 80 patients, aiming for 66 with primary outcome data to provide 90% power at 5% significance level for a 0.1 cm reduction in OED at 72 h, with adjustment for baseline OED. Animals overexpressing IL-1Ra had 36.5% less oedema. 10 If the absolute perihaematomal oedema volume is reduced to a similar extent, this equates to a 30% reduction in OED for a haematoma of around 30 ml. Based on analysis of CT brain scans from day 3 post-onset in conservatively managed (control) patients in the MISTIE II trial 16 (n = 39), mean (SD) OED was 0.57 (0.14) cm. We targetted a conservative reduction of 0.1 cm, approximately half the effect size previously observed in the animal model.
Statistical analyses were conducted according to a pre-specified statistical analysis plan, finalised after completion of recruitment but prior to unblinding. An additional post-hoc analysis of inflammatory markers at Day 2 was undertaken to maximise inclusion of patients in the analysis and to compare markers when all included patients were receiving treatment. All analyses were conducted under the principle of intention-to-treat. OED at 72 h was compared using analysis of covariance to adjust for baseline OED. We decided a priori to include scans conducted between 48–60 and 84–96 h in the primary analysis, to allow inclusion of more patients in the primary analysis. CRP and IL-6 were transformed to their natural logarithm and area-under-the-curve (AUC) to Day 4 was calculated, with adjustment for baseline. If a single time point was missing, interpolation was used to calculate AUC. mRS was dichotomised as 0–3 (good outcome) and 4–6 (poor outcome) for analysis by logistic regression. Given small treatment groups, no adjustment for cofactors was performed. All analyses were performed using Stata software (Version 14).
Findings
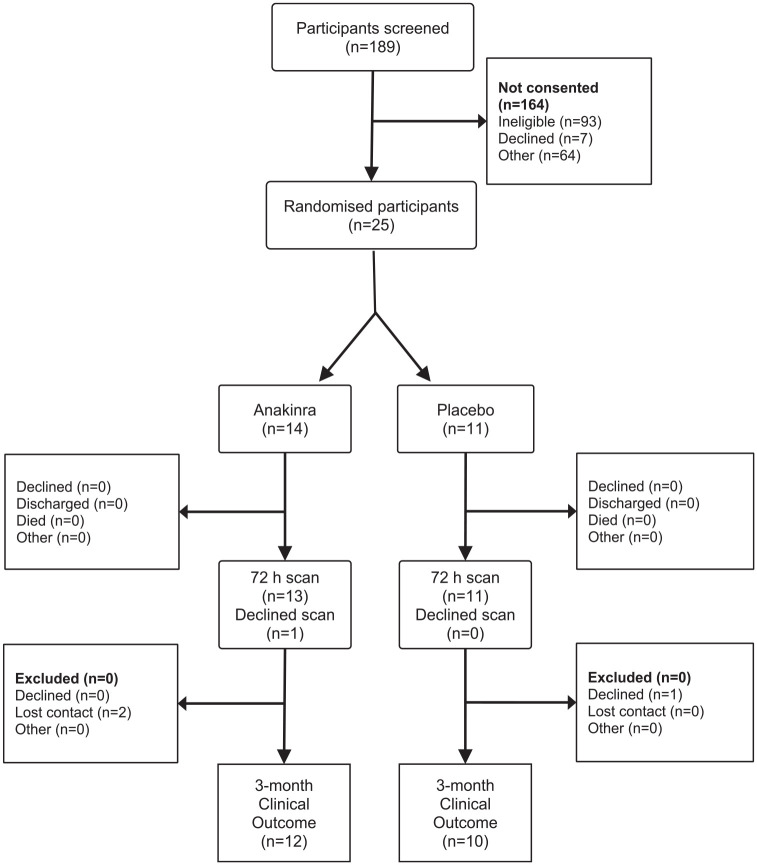
Recruitment to the trial was repeatedly disrupted by the COVID-19 pandemic and we were only able to recruit 25 patients (Figure 1). Fourteen patients were randomised to anakinra and 11 to placebo. Nine patients in each group received all six doses of treatment, one in each group received five (both due to discharge from recruiting hospital). Three in the anakinra group received four doses, one due to a temperature excursion in trial drug storage making the remaining treatment unavailable, one as the patient was palliated then died, and one as the trial treatment was stopped due to an SAE unrelated to the trial treatment. Finally, one in each group had three doses only, one due to early discharge (placebo group) and one due to the participant declining the fourth dose followed by discharge. All the placebo group and 13 of the anakinra group had a 72 h CT for measurement the primary outcome. Twelve of the anakinra group and 10 of the placebo group had a 3-month mRS available. Two in the anakinra group were lost to follow-up and one in the placebo group declined the 3-month assessment.
Figure 1.
CONSORT diagram.
There was a marked baseline imbalance between the groups, with greater baseline severity in the anakinra group: ICH volume and NIHSS were around twice the placebo group, GCS was lower, and intraventricular haemorrhage more frequent (Table 1).
Table 1.
Baseline characteristics.
| Characteristic | Level | Anakinra (N = 14) | Placebo (N = 11) |
|---|---|---|---|
| Age (years) | Mean (SD) Minimum-Maximum |
70 (11.5) 57–88 |
62 (14.6) 38–85 |
| Sex | |||
| Male | N (%) | 8 (57%) | 5 (45%) |
| Female | N (%) | 6 (43%) | 6 (55%) |
| Ethnicity | |||
| White | N (%) | 12 (86%) | 9 (82%) |
| Other | N (%) | 2 (14%) | 2 (18%) |
| Premorbid mRS | |||
| 0 | N (%) | 8 (57%) | 7 (64%) |
| 1 | N (%) | 4 (29%) | 3 (27%) |
| 2 | N (%) | 1 (7%) | 0 |
| 3 | N (%) | 1 (7%) | 1 (9%) |
| 4 | N (%) | 0 | 0 |
| 5 | N (%) | 0 | 0 |
| Time between onset and arrival in hospital (hours) | Median (IQR) Minimum-Maximum |
1.6 (1.0, 4) 0.9–5.6 |
1.8 (1.3, 2.0) 1.1–4.8 |
| Systolic Blood Pressure (mmHg) | Mean (SD) Minimum-Maximum |
160.7 (30.7) 130–237 |
158.7 (22.3) 127–203 |
| Pre-randomisation Glasgow Coma Scale score | Median (IQR) Minimum-Maximum |
14 (11, 15) 8–15 |
15 (14, 15) 8–15 |
| Glasgow Coma Score Classification | |||
| Mild (14–15) | N (%) | 9 (64%) | 9 (82%) |
| Moderate (9–13) | N (%) | 4 (29%) | 1 (9%) |
| Severe (3–8) | N (%) | 1 (7%) | 1 (9%) |
| NIHSS on admission/baseline | Median (IQR) Minimum-Maximum |
12.5 (9, 19) 2–26 |
6 (3, 12) 1–20 |
| NIHSS on admission/baseline | |||
| Minor (1–4) | N (%) | 1 (7%) | 5 (45%) |
| Moderate (5–15) | N (%) | 8 (57%) | 4 (36%) |
| Moderate-severe (16–20) | N (%) | 3 (21%) | 2 (18%) |
| Severe (21–40) | N (%) | 2 (14%) | 0 |
| ICH Volume (mL) | Median (IQR) Minimum-Maximum |
12.6 (4.8, 17.9) 1.4–41.8 |
5.5 (2.1, 10.9) 1.1–45.0 |
| Intraventricular haemorrhage | N (%) | 4 (29%) | 1 (9%) |
| Haematoma location | |||
| Deep | N (%) | 7 (50%) | 8 (73%) |
| Lobar | N (%) | 7 (50%) | 3 (27%) |
| Hypertension | |||
| Yes | N (%) | 7 (50%) | 5 (45%) |
| No | N (%) | 7 (50%) | 5 (45%) |
| Unknown | N (%) | 0 | 1 (9%) |
| Type 2 Diabetes | |||
| Yes | N (%) | 0 | 1 (9%) |
| No | N (%) | 14 (100%) | 10 (91%) |
| Atrial fibrillation | |||
| Yes | N (%) | 3 (21%) | 3 (27%) |
| No | N (%) | 0 | 1 (9%) |
| Missing | N (%) | 11 (79%) | 7 (64%) |
| Prior venous thromboembolism | |||
| Yes | N (%) | 1 (7%) | 1 (9%) |
| No | N (%) | 2 (14%) | 3 (27%) |
| Missing | N (%) | 11 (79%) | 7 (64%) |
| Use of anticoagulants | |||
| Warfarin | N (%) | 3 (21%) | 0 |
| Rivaroxaban | N (%) | 0 | 2 (18%) |
| Apixaban | N (%) | 0 | 2 (18%) |
| Symptom onset to randomisation (h) | Mean (SD) Minimum-Maximu |
4.6 (1.7) 2.7–7.9 |
4.8 (1.7) 3.3–6.2 |
| Randomisation to treatment (min) | Mean (SD) Minimum-Maximum |
33 (26) 3–108 |
28 (18) 12–50 |
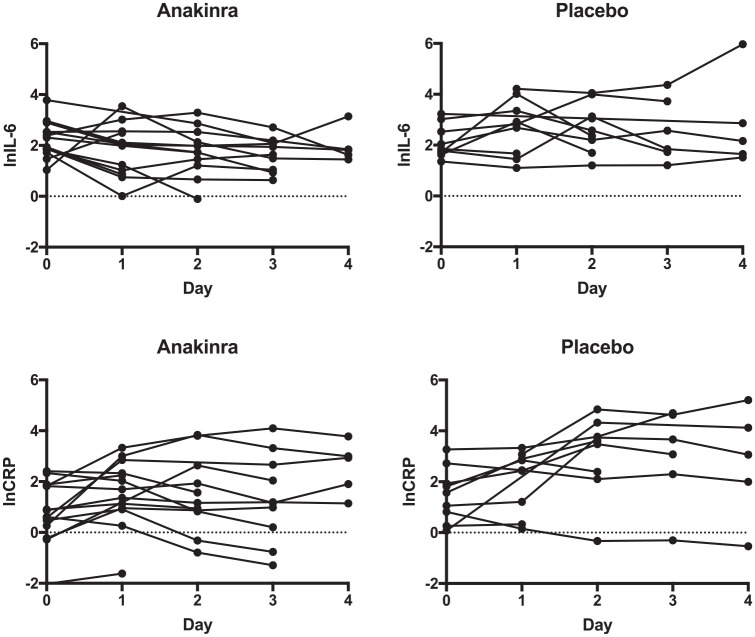
Mean OED was similar at baseline and increased less in the anakinra group (Figure 2, Table 2), but after adjusting for baseline, this did not reach statistical significance (anakinra vs placebo, adjusted OED difference at 72 h: −0.05 [−0.17, 0.06], p = 0.336). OED at 72 h and the difference between OED at baseline and 72 h were normally distributed (Shapiro-Wilk test). Early neurological decline occurred in only one patient in the anakinra group and did not occur in the placebo group (p = 1.0). Two patients in the anakinra group and none in the placebo group had haematoma expansion and this was not statistically significant. At 3 months, more participants had a poor outcome in the anakinra group (7/12; 58%) versus the placebo group (3/10; 30%) but this was not statistically significant on unadjusted logistic regression (OR for poor outcome:3.3, 95% CI: 0.6–19.3). Other clinical outcomes showed no significant difference (Supplemental Table 1). Adjustment for baseline imbalances was not appropriate due to small numbers. The prespecified analysis of plasma inflammatory markers (Table 3, Figure 3) showed that after adjusting for baseline IL-6, the mean difference between the area under the curve for IL-6 to day 4 was −2.90 (n = 15 [n = 9 anakinra, n = 6 placebo], 95% CI: −5.90 to 0.12) when comparing anakinra to placebo. After adjusting for baseline CRP, the mean difference between the area under the curve for CRP to Day 4 was −0.87 (n = 15 [n = 9 anakinra, n = 6 placebo], 95% CI: −7.72 to 5.99) when comparing anakinra to placebo. In a post-hoc analysis, we compared inflammatory markers at the midpoint of sampling (Day 2) when all patients were receiving trial treatment and 19 patients (n = 8 placebo, n = 11 anakinra) had blood samples collected. After adjusting for baseline IL-6 using analysis of covariance, Day 2 IL-6 was 56% (95% CI: 2%–80%) lower in the anakinra group (p = 0.05) versus placebo. CRP at Day 2 was 73% lower (95% CI: 95% lower–31% higher) with anakinra than with placebo (p = 0.10).
Figure 2.
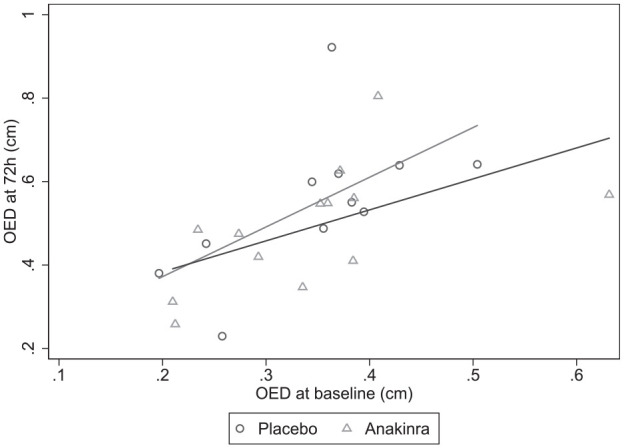
Scatter plot of OED by treatment group, baseline value on X-axis, 72 h value on Y-axis.
Table 2.
Primary outcomes and selected secondary outcomes by treatment group.
| Measurement | Level | Anakinra | Placebo | Mean difference (95% CI) |
|---|---|---|---|---|
| Oedema extension distance | −0.05 (−0.17, 0.06) a | |||
| Baseline |
N
Mean (SD) |
14 0.33 (0.12) |
11 0.35 (0.10) |
|
| 72 h |
N
Mean (SD) |
13 0.49 (0.15) |
11 0.55 (0.18) |
|
| Mean change (72 h – Baseline) |
N
Mean (SD) |
13 0.15 (0.12) |
11 0.20 (0.14) |
|
| Early Neurological Decline | p = 1.0 b | |||
| Participants with data | N | 14 | 11 | |
| Yes | N (%) | 1 (7%) | 0 | |
| No | N (%) | 13 (93%) | 11 (100%) | |
| Haematoma Expansion | p = 0.482 b | |||
| Participants with data | N | 13 | 11 | |
| Yes | N (%) | 2 (15%) | 0 (0%) | |
| Yes (>33% increase) | N (%) | 2 (15%) | 0 (0%) | |
| Yes (> 6mL increase) | N (%) | 1 (8%) | 0 (0%) | |
| No | N (%) | 11 (85%) | 11 (100%) | |
| Modified Rankin Scale (mRS) at 3 months | OR 3.3 (0.6, 19.3) c |
|||
| Participants with data | N | 12 | 10 | |
| 0 | N (%) | 0 | 0 | |
| 1 | N (%) | 2 (17%) | 2 (20%) | |
| 2 | N (%) | 1 (8%) | 3 (30%) | |
| 3 | N (%) | 2 (17%) | 2 (20%) | |
| 4 | N (%) | 3 (25%) | 3 (30%) | |
| 5 | N (%) | 3 (25%) | 0 | |
| 6 | N (%) | 1 (8%) | 0 | |
Difference in 72 h OED adjusted for baseline OED using ANCOVA. Kineret – placebo is shown.
Fisher’s exact test.
mRS was dichotomised as good outcome (0 –3) and poor outcome (4 –6) for comparison by logistic regression, odds ratio of poor outcome, compared to good outcome, and 95% CI displayed.
Table 3.
Inflammatory markers levels by treatment allocation as median and interquartile range.
| Time point | Level | IL-6 (pg/ml) |
CRP (mg/l) |
||
|---|---|---|---|---|---|
| Anakinra | Placebo | Anakinra | Placebo | ||
| Baseline | N | 14 8.5 (6.3, 12.5) |
9 (5.5,12.6) |
14 4.1 (1.3, 6.2) |
9 4.8 (2.3, 6.9) |
| Day 1 | N | 13 7.6 (2.8, 12.1) |
9 16.8 (5.3, 28.5) |
13 5.5 (2.6, 10.3) |
9 11.6 (3.4, 18.1) |
| Day 2 | N | 11 5.7 (3.4, 12.6) |
9 13.2 (9.0, 22.9) |
11 3.2 (2.3, 13.9) |
9 36.7 (10.9, 43.3) |
| Day 3 | N | 10 6.1 (2.8, 8.2) |
6 9.7 (5.7, 41.3) |
10 3.2 (1.2, 14.6) |
6 30.3 (10.0, 102.2) |
| Day 4 | N | 5 6.2 (5.1, 6.3) |
5 8.7 (5.2, 17.5) |
5 18.9 (6.7, 20.0) |
5 21.3 (7.4, 61.5) |
Figure 3.
Plots of the natural logarithms of IL-6 and CRP from baseline to Day 4 by treatment group.
Two serious adverse events (SAEs) were reported in one patient in the placebo group, including aspiration pneumonia (resolved with treatment) and a rapid increase in perihaematomal oedema, and two adverse events (AEs) in two other patients (rash, sciatica). Ten serious adverse events were reported in the anakinra treated group. There were three reports of aspiration pneumonia (all resolved with treatment) and three of worsening neurological deficit. Of the three cases of worsening neurological deficit, the first was reported with the same onset date as aspiration pneumonia in a single participant, suggesting infection was the cause of neurological deterioration. The second had a repeat scan showing worsening mass effect and hydrocephalus. The third had a severe decline in level of consciousness 2 days after symptom onset, was palliated, and died the following day without repeat imaging. The anakinra group also included single SAEs of pulmonary emboli, hypernatraemia (without clinical symptoms, resolved), atrial fibrillation with a slow ventricular response requiring pacing, and an incidental bladder lesion. Two AEs of pyrexia and seizure were reported in the anakinra group. No SAEs or AEs were deemed to be related to the study treatment by the local investigators or the chief investigator. No statistical analyses of the SAEs and AEs was possible, given small numbers.
Discussion
BLOC-ICH is the first clinical trial to test anakinra in spontaneous ICH. We have described the feasibility of delivering anakinra to this patient group as part of a clinical trial and a post-hoc analysis demonstrated a reduction in circulating IL-6 at Day 2 with anakinra compared to placebo, although neither IL-6 nor CRP levels differed significantly between anakinra and placebo over the pre-specified 4 days after trial entry. OED was lower in anakinra-treated patients, but this did not reach statistical significance. No SAEs were attributed to anakinra but assessments of causality by investigators can be unreliable, and a much larger trial will be required to definitively test safety. Whilst more SAEs were seen in the anakinra group, this is likely to be a result of the greater disease severity in the anakinra group at baseline, prior to randomisation. Clinical outcome at 3 months, including the mRS, did not show any statistically significant difference between groups.
Our study has strengths, including the use of concealed, third-party randomisation and a double-blinded, placebo-controlled design. The primary outcome (OED) was measured by expert investigators independent of the trial team, who were also blinded to group allocation. The primary outcome was missing from only one patient (96% complete), suggesting that undertaking a 72 h research CT for subsequent measurement of perihaematomal oedema is feasible in UK Stroke Research Centres and could be employed in future studies. We found that OED at 72 h had a magnitude and variability similar to previous studies. For example, a study in over 1000 patients using patients recruited to ICH clinical trials 9 showed a mean OED at 72 h of 0.54 cm with a SD of 0.26 cm compared to 0.55 cm and 0.18 cm respectively in our placebo group. Our observed 72 h values were also similar to those we found from MISTIE-II data (mean 0.57 cm, SD 0.14 cm), on which we based our power calculation. We observed a difference of 0.05 cm between our groups and had we reached our recruitment target of 80 patients, would have been powered to detect a change of 0.1 cm. These findings, along with the association between change in OED to 72 h with functional outcome, 9 support the use of OED on 72 h CT as a useful biomarker in future studies. Overall, three participants were lost to follow-up at 3 months (88% complete) so later outcomes may be associated with higher levels of attrition in subsequent trials. Additional feasibility challenges were encountered, including seven of 25 patients not completing all six doses of the study drug, with three of these being due to patient discharge prior to completion. Future studies may seek to explore mechanisms to continue study drug post-discharge.
The key weakness of our study was under-recruitment, limiting the power of the study to detect any effect of anakinra on the primary outcome. Prior to the pandemic, recruitment was on schedule to complete in January 2021 and plans were in place to open two additional sites. Unfortunately, recruitment slowed dramatically after Mar 2020, and the trial was forced to cease recruitment in Feb 2021 due to financial constraints. Our mean recruitment rate was 0.6 patients per month per site (range 0–1) prior to March 2020 and was 0.1 thereafter (range 0–0.4). We thus believe recruitment to a similar trial in less adverse conditions would be feasible in the future, with an expected recruitment rate like the pre-pandemic rate in BLOC-ICH. We observed a recruitment rate of 13% of those screened with most patients not recruited due ineligibility (most often as onset >8 h) or because research staff were not available at the time of presentation (Figure 1).
Previous published clinical trials have investigated the effects of anakinra in over 100 patients with ischaemic stroke20,21 and just under 150 with subarachnoid haemorrhage.22,23 Trials in ischaemic stroke include a study of a high dose IV regimen and a low dose SC regimen, both for 72 h, which both demonstrated lower circulating IL-6 and CRP and no safety concerns. Studies in subarachnoid haemorrhage have also tested similar high and low dose regimens with reduction of IL-6 and CRP with anakinra. IL-6 was reduced in the cerebrospinal fluid of one study using high dose anakinra. 22
Another trial of anakinra in ICH (ACTION; NCT04834388) opened to recruitment in Aug 2022 and is randomising patients on a 1:1:1 basis to standard care, high dose anakinra (IV loading with 500 mg, IV infusion at 2 mg/kg/h over 3 days), or low dose anakinra (SC loading with 100 mg, followed by SC 100 mg twice a day for 3 days) with OED at 7 days as the primary outcome. ACTION is expected to complete in Dec 2023 and will provide further data on anakinra in ICH, including preliminary data on the use of higher dose IV anakinra. Further investigation of the optimal treatment window, route of administration and dosing regimen will be required before embarking on a definitive study testing the effect of anakinra on longer-term outcomes after ICH, including death, disability, and quality of life. Demonstrating unequivocal reductions in surrogate markers of inflammation, both in the brain and circulation, will increase confidence in the proposed biological effect of anakinra in ICH.
Conclusion
We describe feasibility for delivering anakinra in ICH patients in a clinical trial and provide preliminary data on safety and outcomes, including perihaematomal oedema, circulating inflammatory markers and clinical outcomes at 3 months. Further trials are needed to further evaluate the therapeutic potential of anakinra in ICH.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231185208 for Phase II randomised, placebo-controlled, clinical trial of interleukin-1 receptor antagonist in intracerebral haemorrhage: BLOcking the Cytokine IL-1 in ICH (BLOC-ICH) by Adrian R Parry-Jones, Katie Stocking, Mary Joan MacLeod, Brian Clarke, David J Werring, Keith W Muir and Andy Vail in European Stroke Journal
Supplemental material, sj-pdf-2-eso-10.1177_23969873231185208 for Phase II randomised, placebo-controlled, clinical trial of interleukin-1 receptor antagonist in intracerebral haemorrhage: BLOcking the Cytokine IL-1 in ICH (BLOC-ICH) by Adrian R Parry-Jones, Katie Stocking, Mary Joan MacLeod, Brian Clarke, David J Werring, Keith W Muir and Andy Vail in European Stroke Journal
Supplemental material, sj-pdf-3-eso-10.1177_23969873231185208 for Phase II randomised, placebo-controlled, clinical trial of interleukin-1 receptor antagonist in intracerebral haemorrhage: BLOcking the Cytokine IL-1 in ICH (BLOC-ICH) by Adrian R Parry-Jones, Katie Stocking, Mary Joan MacLeod, Brian Clarke, David J Werring, Keith W Muir and Andy Vail in European Stroke Journal
Acknowledgments
We are grateful to Sobi for supplying study drug and the NIHR Comprehensive Research Network and clinical teams at the BLOC-ICH sites. We are grateful to the members of the Trial Steering Committee and Independent Data Safety and Monitoring Committee (see Supplemental Materials for committee members).
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This report is independent research arising from a Clinician Scientist Award to APJ (CS-2014-14-005) supported by the National Institute for Health Research. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. Anakinra (Kineret®) and placebo were provided free of charge by Swedish Orphan Biovitrum AB (publ) (Sobi) under an Investigator Initiated Study Agreement. Sobi was not involved in the design or conduct of the study, data collection and analysis, or preparation of the manuscript.
Informed consent: Written informed consent was obtained from all subjects before the study and where this was not possible, informed consent was obtained from legally authorised representatives before the study.
Ethical approval: Ethical approval for this study was obtained from the National Health Service Research Ethics Committee (IRAS ID:252065).
Guarantor: APJ
Contributorship: APJ and AV developed the trial protocol. APJ, MJM, BC, DW & KWM were site PIs. Analyses were completed by KS and AV, APJ drafted, and all authors critically reviewed the manuscript prior to submission.
Trial registration: ClinicalTrials.gov (NCT03737344) and the EU Clinical Trials Register (2018-000249-38).
ORCID iD: Adrian R Parry-Jones  https://orcid.org/0000-0002-4462-3846
https://orcid.org/0000-0002-4462-3846
Supplemental material: Supplemental material for this article is available online.
References
- 1. Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology 2020; 54: 171–179. [DOI] [PubMed] [Google Scholar]
- 2. Al-Shahi Salman R, Frantzias J, Lee RJ, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol 2018; 17: 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Puy L, Parry-Jones AR, Sandset EC, et al. Intracerebral haemorrhage. Nat Rev Dis Primers 2023; 9: 14. [DOI] [PubMed] [Google Scholar]
- 4. Askenase MH, Sansing LH. Stages of the inflammatory response in pathology and tissue repair after intracerebral hemorrhage. Semin Neurol 2016; 36: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res 2007; 1180: 140–154. [DOI] [PubMed] [Google Scholar]
- 6. Sobowale OA, Parry-Jones AR, Smith CJ, et al. Interleukin-1 in stroke: from bench to bedside. Stroke 2016; 47: 2160–2167. [DOI] [PubMed] [Google Scholar]
- 7. Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol 2005; 5: 629–640. [DOI] [PubMed] [Google Scholar]
- 8. Staykov D, Wagner I, Volbers B, et al. Natural course of perihemorrhagic edema after intracerebral hemorrhage. Stroke 2011; 42: 2625–2629. [DOI] [PubMed] [Google Scholar]
- 9. Hurford R, Vail A, Heal C, et al. Oedema extension distance in intracerebral haemorrhage: Association with baseline characteristics and long-term outcome. Eur Stroke J 2019; 4: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masada T, Hua Y, Xi G, et al. Overexpression of interleukin-1 receptor antagonist reduces brain edema induced by intracerebral hemorrhage and thrombin. Acta Neurochir Suppl 2003; 86: 463–467. [DOI] [PubMed] [Google Scholar]
- 11. kineret-epar-product-information_en.pdf, https://www.ema.europa.eu/en/documents/product-information/kineret-epar-product-information_en.pdf (accessed 12 April 2023).
- 12. Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012; 11: 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Intercollegiate Stroke Working Party. National clinical guideline for stroke. 4th ed. London: Royal College of Physicians, 2012. [Google Scholar]
- 14. Wang J, Doré S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain 2007; 130: 1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol 2007; 61: 352–362. [DOI] [PubMed] [Google Scholar]
- 16. Mould WA, Carhuapoma JR, Muschelli J, et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke 2013; 44: 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu TY, Sobowale O, Hurford R, et al. Software output from semi-automated planimetry can underestimate intracerebral haemorrhage and peri-haematomal oedema volumes by up to 41. Neuroradiol 2016; 58: 867–876. [DOI] [PubMed] [Google Scholar]
- 18. Parry-Jones AR, Wang X, Sato S, et al. Edema extension distance: Outcome Measure for Phase II clinical trials targeting edema after intracerebral hemorrhage. Stroke 2015; 46: e137–e140. [DOI] [PubMed] [Google Scholar]
- 19. Dowlatshahi D, Demchuk AM, Flaherty ML, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurol 2011; 76: 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Emsley HC, Smith CJ, Georgiou RF, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry 2005; 76: 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith CJ, Hulme S, Vail A, et al. SCIL-STROKE (Subcutaneous interleukin-1 receptor antagonist in ischemic stroke): A randomized controlled Phase 2 trial. Stroke 2018; 49: 1210–1216. [DOI] [PubMed] [Google Scholar]
- 22. Singh N, Hopkins SJ, Hulme S, et al. The effect of intravenous interleukin-1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: a phase II randomised controlled trial. J Neuroinflammation 2014; 11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galea J, Ogungbenro K, Hulme S, et al. Reduction of inflammation after administration of interleukin-1 receptor antagonist following aneurysmal subarachnoid hemorrhage: results of the subcutaneous Interleukin-1Ra in SAH (SCIL-SAH) study. J Neurosurg 2018; 128: 515–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231185208 for Phase II randomised, placebo-controlled, clinical trial of interleukin-1 receptor antagonist in intracerebral haemorrhage: BLOcking the Cytokine IL-1 in ICH (BLOC-ICH) by Adrian R Parry-Jones, Katie Stocking, Mary Joan MacLeod, Brian Clarke, David J Werring, Keith W Muir and Andy Vail in European Stroke Journal
Supplemental material, sj-pdf-2-eso-10.1177_23969873231185208 for Phase II randomised, placebo-controlled, clinical trial of interleukin-1 receptor antagonist in intracerebral haemorrhage: BLOcking the Cytokine IL-1 in ICH (BLOC-ICH) by Adrian R Parry-Jones, Katie Stocking, Mary Joan MacLeod, Brian Clarke, David J Werring, Keith W Muir and Andy Vail in European Stroke Journal
Supplemental material, sj-pdf-3-eso-10.1177_23969873231185208 for Phase II randomised, placebo-controlled, clinical trial of interleukin-1 receptor antagonist in intracerebral haemorrhage: BLOcking the Cytokine IL-1 in ICH (BLOC-ICH) by Adrian R Parry-Jones, Katie Stocking, Mary Joan MacLeod, Brian Clarke, David J Werring, Keith W Muir and Andy Vail in European Stroke Journal