Abstract
Introduction:
High systolic blood pressure (SBP) is associated with poor functional outcome. We analysed whether the association of SBP with outcomes after endovascular treatment (EVT) is modified by prior intravenous thrombolysis (IVT).
Patients and methods:
This was a post-hoc analysis of MR CLEAN-NO IV, a randomised trial of IVT with alteplase followed by EVT versus EVT alone, within 4.5 h from stroke onset. SBP was recorded on hospital admission. The primary outcome was 90-day modified Rankin Scale (mRS) score and secondary outcomes included symptomatic intracranial haemorrhage (sICH) and successful reperfusion (eTICI 2b-3), analysed with (ordinal) logistic regression. Estimates were calculated per 10 mmHg change in SBP. We assessed whether IVT modified the associations of SBP with these outcomes using multiplicative interaction terms.
Results:
Of 539 randomised patients, 266 received IVT. The association of SBP with mRS score was J-shaped, with an inflection point at 150 mmHg. Using 150 mmHg as a reference point, SBPs higher than 150 mmHg were associated with poor functional outcome (acOR: 1.23, 95% CI: 1.09–1.38), but lower SBPs were not (acOR: 1.14, 95% CI: 0.99–1.30). Higher SBP was not associated with the risk of sICH (aOR: 1.09, 95% CI: 0.93–1.27) nor with the probability of successful reperfusion (aOR: 1.00, 95% CI: 0.91–1.10). Our main result was that we found no effect modification by IVT (p-values for interaction, mRS = 0.94; sICH = 0.26; successful reperfusion = 0.58).
Discussion and conclusion:
There was no effect modification of IVT with SBP for any of the clinical outcomes. Therefore, the level of SBP (if ⩽185/110 mmHg) should not guide IVT decisions in patients otherwise eligible for both IVT and EVT within the 4.5-h time window.
Trial registration:
ISRCTN80619088, https://www.isrctn.com/ISRCTN80619088.
Keywords: Blood pressure, hypertension, endovascular treatment, intravenous thrombolysis, acute ischaemic stroke, thrombectomy, alteplase, randomised controlled trial
Introduction
Many studies have found that a high systolic blood pressure (SBP) on admission is associated with a lower likelihood of functional independence in patients with acute ischaemic stroke treated with intravenous thrombolysis (IVT) with alteplase. 1 Several studies have shown a U- or J-shaped curve for the relation of SBP with death or dependency, with lower and higher SBPs associated with worse outcomes.2–4 More recently, a similar association was reported for patients with ischaemic stroke due to a large vessel occlusion who underwent endovascular treatment (EVT).5–7 Still, the beneficial effects of both IVT and EVT on functional outcome are independent of admission SBP level.7–9 The pathophysiological mechanisms of worse functional outcome with higher SBP are poorly understood, but this is probably partly explained by an increased occurrence of symptomatic intracranial haemorrhage (sICH).1,2 While evidence suggests that elevated SBP levels are associated with a higher risk of sICH, it is unknown whether this association is modified by IVT before EVT.
We analysed whether the association of blood pressure (BP) with functional outcome after EVT differs between patients treated with or without IVT prior to EVT. Second, we determined if the risk of sICH with higher BPs is modified by IVT.
Methods
We analysed data from MR CLEAN-NO IV (Multicenter Randomized CLinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands – IVT followed by EVT vs EVT alone for acute ischaemic stroke caused by a proximal intracranial occlusion). 10 Trial design has been reported previously. 11 MR CLEAN-NO IV was a multicenter randomised trial of patients with a large vessel occlusion of the anterior circulation who were randomised to receive either IVT with alteplase (0.9 mg/kg) followed by EVT, or EVT without preceding alteplase, upon direct presentation at an EVT-capable hospital, within 4.5 h after symptom onset or time of last seen well. Rescue IVT was permitted if successful reperfusion (extended thrombolysis in cerebral infarction (eTICI) score 2b-3) was not achieved with EVT alone.
Noninvasive SBP and diastolic BP (DBP) were measured with an automatic sphygmomanometer at hospital admission, before start of IVT, if given, and before EVT. Only the first BP reading at the emergency department was documented in the trial database. BP exceeding 185/110 mmHg was an exclusion criterion for entry into the study. Therefore, in patients with BP > 185/110 mmHg at hospital admission, their BP was lowered to below this threshold before randomisation with antihypertensive treatment or fell spontaneously, but this was not recorded. BP lowering treatment to achieve IVT eligibility was allowed, but the use of BP lowering drugs during hospital admission was not documented in the trial database. Imaging was analysed by a core laboratory and safety endpoints were evaluated by an adverse event committee; both committees were blinded to treatment allocation. The trial is registered as ISRCTN80619088. Data can be made available upon reasonable request.
Outcome measures
The primary outcome was the score on the modified Rankin Scale (mRS) at 90 days. The mRS is a scale for functional outcome, ranging from 0 (no symptoms) to 6 (death). 12 Secondary outcome measures were functional independence (mRS score 0–2) at 90 days, stroke severity at 5–7 days or discharge, if earlier, assessed with the National Institutes of Health Stroke Scale (NIHSS), successful reperfusion (eTICI score 2b-3), and final lesion volume on MRI at 24 h or non-contrast CT at 5–7 days. Safety outcomes were all-cause death at 90 days, any intracranial haemorrhage on follow-up imaging, sICH and ischaemic stroke progression. The Heidelberg Bleeding Classification was used for scoring of any intracranial haemorrhage and sICH. 13 Ischaemic stroke progression was defined as neurological deterioration of ⩾4 points on total NIHSS, or ⩾2 points on one category, not explainable by intracranial haemorrhage on imaging.
Statistical analysis
First, we evaluated whether SBP or DBP had a better correlation with mRS score, by comparing the Akaike Information Criterion of the univariable ordinal logistic regression models, and the optimal BP parameter was used for further analyses.
Second, we examined whether the association of the BP parameter with the outcome measure was linear or nonlinear, by comparing the likelihood ratios of a regression model with a linear BP term to a model with a restricted cubic spline transformation allowing 3 knot for BP. When a nonlinear relation was found, regression analyses were performed for two subgroups using the nadir value of the curve (the point with the lowest y-value) as a reference point, to estimate the differential effects of lower and higher ranges of BPs on outcome. Otherwise, analyses were done using the full range of BP.
Third, to determine the effect of IVT on the associations of BP with outcomes, we used multiplicative interaction terms (BP*prior IVT). This was the analysis of our main interest. We performed analyses on the basis of an as-treated approach (not intention-to-treat), in order to approximate the clinical practice situation. Prior IVT was defined as start of IVT prior to EVT, irrespective of whether full-dose alteplase was given.
We assessed binary outcomes with logistic regression, mRS score with ordinal logistic regression and continuous outcomes with linear regression. Outcome estimates are expressed as odds ratios (OR), common ORs (cOR), or β coefficients with accompanying 95% confidence interval (CI), and are reported per 10 mmHg change in BP. All interaction and regression analyses were adjusted for variables pre-specified in the MR CLEAN-NO IV statistical analysis plan 11 (age, pre-stroke mRS score, onset-to-randomisation time, baseline NIHSS and collateral score) with additional adjustment for history of hypertension, which is considered a confounder since it potentially influences both admission SBP level and functional outcome.
Fourth, sensitivity analyses were performed for the primary outcome for (1) patients in the formal as-treated population as defined in the main publication, in which patients were removed from the analyses after crossover or when they did not receive full-dose IVT prior to EVT, 10 and (2) patients with admission BP that did not exceed the guideline-recommended BP threshold for IVT and EVT of 185/110 mmHg.1,14 The E-value, a measure related to the evidence for causality, was calculated for all models. 15
Missing values, including missing BPs, were replaced with multiple imputation methods, for the use in regression analyses only. Analyses were performed with R studio (Version 4.0.5 R Foundation).
Results
Of 539 included patients included in the trial, 266 received IVT prior to EVT. For two patients in the trial no admission blood pressure was documented in the database. SBP was normally distributed (eFigure 1 in the Supplemental Material). Mean SBP was 151 (SD ±25) and mean DBP was 84 (SD ±16) mmHg. Admission BP exceeded 185/110 mmHg in 46 (9%) of 539 patients. SBP alone exceeded this threshold in 25 patients, DBP alone in 12 patients and both SBP and DBP in 9 patients.
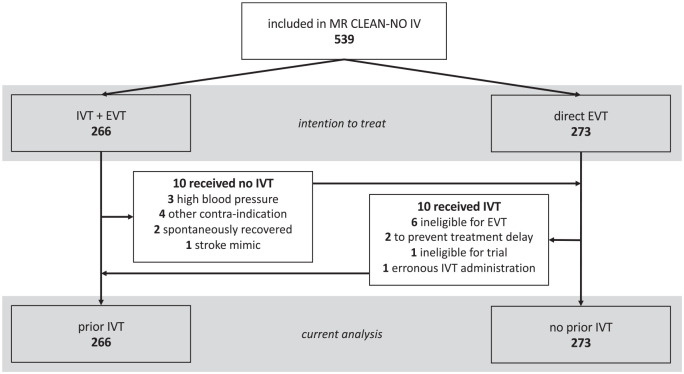
Ten patients who were randomised to the IVT group did not receive IVT, of whom three because of high blood pressure; 10 patients randomised to EVT alone received IVT prior to EVT (Figure 1). Nineteen patients from the EVT-alone group received rescue alteplase after EVT. There was no loss to follow-up.
Figure 1.
Flowchart of patient inclusion.
INR: international normalised ratio; IVT: intravenous thrombolysis; EVT: endovascular treatment.
Baseline characteristics are presented according to SBP tertiles (Supplemental eTable 1). Patients with higher SBP were on average older, were more likely to have a history of hypertension or diabetes mellitus, a tandem lesion or distal occlusion, and less often a thrombectomy was performed.
Admission BP and outcomes
Model fit for the univariable association of BP with mRS score was slightly better when using SBP compared to DBP (Akaike Information Criterion: 1926 for SBP, 1932 for DBP), so SBP was used for further analyses.
The association of SBP with 90-day mRS score (shift analysis towards poor outcome) was nonlinear, which was derived from the comparison of a linear SBP term to a model allowing 3 knot for SBP (p-value for likelihood ratio test: 0.001). This resulted in a J-shaped curve with an inflection point at around an SBP of 150 mmHg (Figure 2). Using 150 mmHg as a reference point, SBPs higher than 150 mmHg were associated with worse functional outcome (adjusted common OR (acOR) per 10 mmHg increment: 1.23, 95% CI: 1.09–1.38), but lower SBPs were not (acOR per 10 mmHg decrement: 1.14, 95% CI: 0.99–1.30; Table 1).
Figure 2.
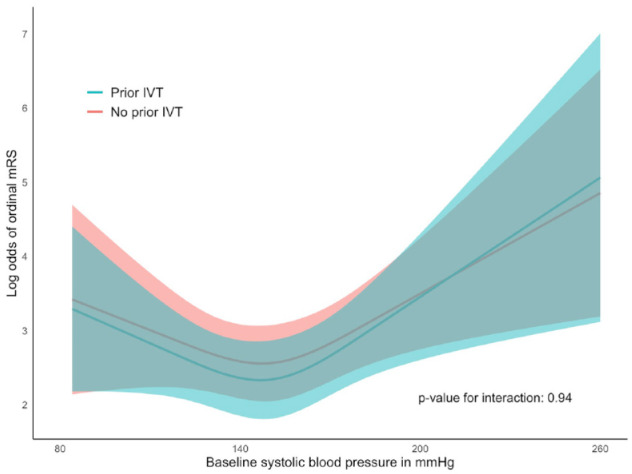
Admission systolic blood pressure and shift towards poorer functional outcome after EVT, with and without prior IVT.
EVT: endovascular treatment; IVT: intravenous thrombolysis; mRS: modified Rankin Scale.
The graph depicts the log odds for a shift towards poorer modified Rankin Scale (mRS) score with corresponding 95% CI, for admission systolic blood pressure (SBP) level, with practically parallel lines for prior intravenous thrombolysis (IVT) and no prior IVT (p-value for interaction: 0.94). Admission SBP ranged from 84 to 260 mmHg. In patients with BP exceeding 185/110 mmHg, SBP lowered to below this threshold before enrolment.
Table 1.
Modified Rankin scale according to admission systolic blood pressure.
| SBP < 150 (n = 260) | SBP ⩾ 150 (n = 277) | SBP < 150 a(c)OR (95% CI)* | SBP ⩾ 150 a(c)OR (95% CI)* | p-Value for interaction † | |
|---|---|---|---|---|---|
| mRS score at 90 days, shift analysis towards poor outcome – median (IQR) | 2 (2–4) | 2 (2–5) | 1.14 (0.99–1.30) | 1.23 (1.09–1.38) | 0.94 |
| mRS score 0–2 at 90 d – n (%) | 139 (53%) | 130 (47%) | 0.89 (0.75–1.06) | 0.82 (0.70–0.96) | 0.91 |
mRS = modified Rankin Scale; SBP = systolic blood pressure.
For nonlinear models (ordinal mRS and mRS 0–2; p-values for likelihood ratio test comparing linear SBP term with restricted cubic spline transformation allowing 3 knot for SBP were respectively 0.001 and 0.016), regression analyses were performed using the nadir value of the model as a reference point, to estimate the effects of lower and higher ranges of BPs on outcomes. For SBP < 150 mmHg, adjusted (common) ORs and 95% CIs are given per 10 mmHg decrement in SBP and for SBP > 150 mmHg per 10 mmHg increment in SBP.
SBP*prior IVT.
We found no association of SBP with successful reperfusion (eTICI 2b-3; aOR: 1.00, 95% CI: 0.91–1.10) nor with the risk of sICH (aOR: 1.09, 95% CI: 0.93–1.27), using SBP as a linear term (Table 2). Exploratory analysis showed no effect modification by successful reperfusion (eTICI 2b-3) on the associations of SBP with mRS score or with sICH (p-values for interaction, mRS: 0.16, sICH: 0.75).
Table 2.
Outcomes according to admission systolic blood pressure*.
| All patients, n = 539 | aOR/β (95% CI) † | p-Value for interaction ‡ | |
|---|---|---|---|
| Death at 90 days – n (%) | 98 (18%) | 1.08 (0.97–1.20) | 0.19 |
| NIHSS at 5–7 days – median (IQR) | 4 (1–11) | 0.09 (−0.26–0.45) | 0.12 |
| Successful reperfusion (eTICI 2b-3) – n (%) | 388 (81%) | 1.00 (0.91–1.10) | 0.58 |
| sICH – n (%) | 30 (6%) | 1.09 (0.93–1.27) | 0.29 |
| Any intracranial haemorrhage – n (%) | 174 (36%) | 1.02 (0.94–1.11) | 0.04 § |
| Final lesion volume on follow-up imaging – median (IQR) | 20 (6–74) | 1.17 (−3.03–5.36) | 0.36 |
| Stroke progression – n (%) | 15 (3%) | 1.14 (0.92–1.41) | 0.78 |
eTICI: extended Thrombolysis in Cerebral Infarction; NIHSS: National Institutes of Health Stroke Scale; sICH: symptomatic intracranial haemorrhage.
Number of missing values: SBP: 2; NIHSS 5–7d: 56; successful reperfusion: 59; any intracranial haemorrhage: 52; final lesion volume: 63.
For all outcomes restricted cubic spline transformation allowing 3 knot for SBP did not improve model fit. Adjusted ORs and 95% CIs are estimated per 10 mmHg change in SBP.
SBP*prior IVT.
Because of a significant interaction test (p < 0.05), IVT subgroup analysis was performed for the outcome any intracranial haemorrhage. Prior IVT – aOR: 1.10 (95% CI: 0.98–1.24); no prior IVT – aOR:0.95 (95% CI: 0.84–1.08).
Prior IVT effect on admission BP and outcomes
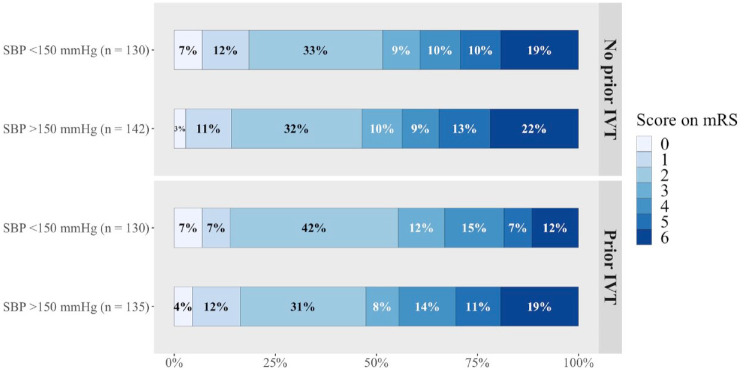
Treatment with IVT prior to EVT did not modify the relation of SBP with mRS score (p-value for interaction: 0.90; Table 1, Figure 2). The odds of a shift towards poorer functional outcome were similar for the two treatment groups (data not given; Figures 2 and 3).
Figure 3.
Functional outcome at 90 days after EVT according to received IVT and SBP groups.
EVT: endovascular treatment; IVT: intravenous thrombolysis; mRS: modified Rankin Scale; SBP: systolic blood pressure.
For intracranial haemorrhage, including asymptomatic haemorrhage, we found effect modification by IVT (p-value: 0.04) with higher SBP. For patients who received IVT, there was a non-statistically significant increased risk of any intracranial haemorrhage with higher SBPs (aOR: 1.10, 95% CI: 0.98–1.24). Conversely, SBP was not associated with risk of any intracranial haemorrhage in those without IVT (aOR: 0.95, 95% CI: 0.84–1.08; Table 2). We found no effect modification of IVT on the association of SBP with symptomatic intracranial haemorrhage after EVT (p-value for interaction: 0.29), nor for other secondary outcomes (Table 2).
Sensitivity analyses
In patients with admission BP below 185/110 mmHg (n = 491), the association of SBP with mRS score was linear (p-value for comparison with the restricted cubic spline model, likelihood ratio test: 0.11) and showed no association of SBP with functional outcome (acOR: 0.98, 95% CI: 0.91–1.07 per 10 mmHg increment) nor effect modification by IVT (p-value for interaction: 0.60; eTable 2 in the Supplemental Material). Exploratory analysis of BP dichotomised in groups below and above BP 185/110 mmHg demonstrated that the BP > 185/110 mmHg group was strongly associated with worse functional outcome (acOR: 2.69, 95% CI: 1.52–4.76 per 10 mmHg increment).
Results from sensitivity analyses for the formal as-treated population (n = 507), were largely similar to results from our main analyses (Supplemental eTable 2). E-values were reported in Supplemental eTable 3.
Discussion
In this post hoc analysis of a randomised trial of IVT prior to EVT versus EVT alone, we found that IVT did not modify the association of SBP with clinical outcomes. In line with previous studies,2–7 we identified a nonlinear, J-shaped, association of SBP with mRS score, with worse functional outcome for SBPs higher than the inflection point of 150 mmHg. We found no clear increased risk of sICH with higher SBPs, and prior IVT did not modify this association.
It has previously been demonstrated that high admission BP is strongly associated with worse functional outcome in acute ischaemic stroke and after IVT and EVT,5,6 which was confirmed in the present study. Increased haemorrhage risk after IVT has been suggested to at least partly explain worse functional outcome in patients with high BP.2–4 Still, not all studies that observed an increased risk of poor outcome with higher BPs concomitantly report higher sICH proportions,3,4 but this could be due to underpowered sample sizes, lack of follow-up imaging, the way symptomatic neurological deterioration is defined or alternative causes such as cerebral oedema. On the other hand, the association of high BP with poor outcome may not be causal, but high BP may be indicative of larger cerebral dysregulation – a theory supported by the failure of many randomised trials that attempted to reduce sICH or improve outcomes by BP lowering, despite the large amount of epidemiological evidence that exists. 1
Several randomised trials have recently compared the effects of EVT alone versus IVT followed by EVT.10,16–20 Overall, while outcomes between treatment groups were largely similar, non-inferiority could not be established. Treatment with IVT prior to EVT may carry an additional risk of haemorrhagic transformation compared with EVT alone, although most were asymptomatic.19,21 It is likely that there will be a paradigm shift towards individualised treatment decisions for patients with ischaemic stroke caused by large vessel occlusion. EVT alone could be favoured in situations upon direct presentation in an EVT-capable hospital in which EVT can be initiated quickly, or in patients who may be at increased risk of IVT-related complications.
In this study, we found that patients who received IVT, higher SBP tended to increase the risk of any intracranial haemorrhage, but not the risk of symptomatic haemorrhage, compared to patients without IVT. In MR CLEAN-NO IV, the proportion of sICH did not differ for the allocated treatment groups, 10 which could have affected our findings regarding SBP and risk of sICH. In our study, the association of SBP with functional outcome was also not different with or without IVT. In other words, IVT did not change the associations of high SBP with worse functional outcome or the risk of sICH in patients undergoing EVT. It has been demonstrated previously that the benefit of both IVT and EVT were independent of SBP level, despite the strong relation of BP with functional outcome.7–9 Therefore, the exact level of BP (up to 185/110 mmHg) should not influence IVT decisions in patients who are otherwise eligible for both IVT and EVT within the 4.5 h time window.
Some studies have reported an inverse relation of higher admission BP with lower odds of achieving successful reperfusion with EVT,5,22,23 whereas we observed no such association. This dissimilarity may be explained by the different treatment modalities, imaging techniques, or grading of flow restoration applied in these studies. Reperfusion grade was missing for 59 patients (11%) in our analyses which could have affected these results and our finding that there was no reperfusion effect modification of SBP on mRS score, even though missing scores were imputed. Also, other factors may be at play, such as patient characteristics or chance.
Current guidelines advocate a BP threshold of ⩽185/110 mmHg before EVT (with or without IVT) and a limit of ⩽180/105 mmHg during and for the first day after treatment, in order to avoid reperfusion haemorrhages with elevated BPs.1,14 We did not find effect modification by reperfusion status on the risk of sICH imposed by admission SBP, analogous to reported previously for post-EVT BP. 24 Interestingly, sensitivity analyses of patients with admission BP below 185/110 mmHg showed no clear association of SBP with functional outcome. This suggests that the association of worse outcome with higher BPs is mainly driven by BP extremes, as was confirmed by our post hoc analysis demonstrating that admission BPs above the 185/110 mmHg threshold were strongly associated with worse functional outcome. However, these patients had their BPs lowered medically or spontaneously to below 185/110 mmHg before treatment, and poorer outcome in this group could also be an adverse effect of BP lowering or from longer time to reperfusion therapy. Nonetheless, this BP threshold was established during a IVT pilot study, after three cases of sICH,25,26 and was afterwards adopted in acute ischaemic stroke guidelines.1,14 In the 3035 patients included in the Third International Stroke Trial (IST-3), the associations of BP (inclusion if BP < 220/130 mmHg) with functional outcome and with occurrence of sICH were independent of IVT (vs control).8,9 Also, the effectiveness and safety of EVT were found to be similar for the SBP range of 105–200 mmHg in the MR CLEAN trial (n = 500). 7 Although the risk of worse outcomes with higher BPs has been established, the treatment effects of IVT or EVT are not modified BP by level, and thereby provide no clear evidence for a strict BP threshold.
Our study has limitations. First, since BP > 185/110 mmHg was an exclusion criterion for entering the trial, we must assume that the proportion of 9% of patients who had admission BPs exceeding this level had their BPs lowered medically or spontaneously before randomisation. Second, this was a post-hoc analysis of the MR CLEAN-NO IV trial and not a pre-specified analysis, therefore results should be interpreted with caution. Documentation of serial BP measurements (blood pressure variability) and antihypertensive treatment would have been of great value to study the effects of BP lowering or natural BP course and their relation with the occurrence of sICH and outcomes. Also, documentation of a single, retrospectively collected BP value increases the risk of measurement error. Several crossovers occurred in the trial, which was reason to use an as-treated approach, in order to approximate the clinical practice situation. Finally, our findings only apply to patients with anterior-circulation stroke presenting at an EVT-capable hospital within 4.5 h of stroke onset who are eligible for both IVT and EVT according to international guidelines. Our results cannot be extrapolated to transferred patients, those presenting outside the 4.5 h time window or with a BP above 185/110 mmHg at initiation or during IVT or EVT.
In conclusion, in this trial of randomised IVT in patients undergoing EVT, higher admission SBPs were associated with poorer outcome, independent of IVT treatment. Therefore, the exact level of SBP (if ⩽185/110 mmHg) should not influence IVT decisions in patients otherwise eligible for both IVT and EVT within the 4.5 h time window.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231173274 for Admission blood pressure and clinical outcomes in patients with acute ischaemic stroke treated with intravenous alteplase and endovascular treatment versus endovascular treatment alone: A MR CLEAN-NO IV substudy by Sophie A van den Berg, Simone M Uniken Venema, Natalie E LeCouffe, Alida A Postma, Geert J Lycklama à Nijeholt, Leon A Rinkel, Kilian M Treurniet, Manon Kappelhof, Agnetha E Bruggeman, Katinka R van Kranendonk, Charles BLM Majoie, Diederik WJ Dippel, H Bart van der Worp, Jonathan M Coutinho, Paul J Nederkoorn and Yvo BWEM Roos in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231173274 for Admission blood pressure and clinical outcomes in patients with acute ischaemic stroke treated with intravenous alteplase and endovascular treatment versus endovascular treatment alone: A MR CLEAN-NO IV substudy by Sophie A van den Berg, Simone M Uniken Venema, Natalie E LeCouffe, Alida A Postma, Geert J Lycklama à Nijeholt, Leon A Rinkel, Kilian M Treurniet, Manon Kappelhof, Agnetha E Bruggeman, Katinka R van Kranendonk, Charles BLM Majoie, Diederik WJ Dippel, H Bart van der Worp, Jonathan M Coutinho, Paul J Nederkoorn and Yvo BWEM Roos in European Stroke Journal
Acknowledgments
We would like to thank the MR CLEAN-NO IV investigators; a complete list of the MR CLEAN-NO IV investigators is provided in the Appendix.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AP reports an institutional grant by Siemens Healthineers. CM reports funding from CVON/Dutch Heart Foundation and Stryker (related to this project) and funding from European Commission, Healthcare Evaluation Netherlands TWIN Foundation (unrelated to this project), all paid to institution, and is shareholder of Nicolab. DD reports funding from the Dutch Heart Foundation, Brain Foundation Netherlands, The Netherlands Organisation for Health Research and Development, Health Holland Top Sector Life Sciences & Health, and unrestricted grants from Penumbra Inc., Stryker, Medtronic, Thrombolytic Science, LLC and Cerenovus for research, all paid to institution. HW reports funding from Stryker, paid to the CONTRAST consortium, and consultation fees from Bayer and LivaNova, paid to his institution. JC reports funding from Boehringer Ingelheim, Bayer and Portola, all to fund medical research and paid to his institution. PN reports funding from Stryker, paid to the CONTRAST consortium. YR is minor shareholder of Nicolab. All other authors report no conflicting interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The trial was funded through the Collaboration for New TReatments of Acute Stroke (CONTRAST) Consortium. The CONTRAST consortium acknowledges the support from the Netherlands Cardiovascular Research Initiative, an initiative of the Dutch Heart Foundation (CVON2015-01: CONTRAST), and from the Brain Foundation Netherlands (HA2015.01.06). The collaboration project is additionally financed by the Ministry of Economic Affairs by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships (LSHM17016). This work was funded in part through unrestricted funding by Stryker, Medtronic and Cerenovus. The funding sources were not involved in study design, monitoring, data collection, statistical analyses, interpretation of results or manuscript writing.
Informed consent: Written deferred consent was provided by all included patients or their representatives.
Ethical approval: The trial protocol for the MR CLEAN-NO IV trial was approved by Dutch (Erasmus MC University Medical Center; MEC-2017-368), Belgian (ID-RCB: 2018-A00764-51) and French (B322201939935, 19/20/987) ethical committees and the research board of each participating centre.
Guarantor: YR.
Contributorship: SB: study concept, statistical analysis, interpretation of the results, drafting of the manuscript; SUV and YR: interpretation of the results, critical revision of the manuscript; NL, AP, GL, LR, KT, MK, AB, KK, CM, DD, HW, JC and PN: critical revision of the manuscript.
ORCID iDs: Sophie A van den Berg  https://orcid.org/0000-0001-6437-6486
https://orcid.org/0000-0001-6437-6486
Simone M Uniken Venema  https://orcid.org/0000-0002-5374-3858
https://orcid.org/0000-0002-5374-3858
Natalie E LeCouffe  https://orcid.org/0000-0002-6668-8755
https://orcid.org/0000-0002-6668-8755
Leon A Rinkel  https://orcid.org/0000-0002-0291-8515
https://orcid.org/0000-0002-0291-8515
H Bart van der Worp  https://orcid.org/0000-0001-9891-2136
https://orcid.org/0000-0001-9891-2136
Yvo BWEM Roos  https://orcid.org/0000-0001-9205-5882
https://orcid.org/0000-0001-9205-5882
Supplemental material: Supplemental material for this article is available online.
References
- 1. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J 2021; 6: II. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Bangalore S, Schwamm L, Smith EE, et al. Blood pressure and in-hospital outcomes in patients presenting with ischaemic stroke. Eur Heart J 2017; 38: 2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vemmos KN, Tsivgoulis G, Spengos K, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med 2004; 255: 257–265. [DOI] [PubMed] [Google Scholar]
- 4. Leonardi-Bee J, Bath PM, Phillips SJ, et al. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002; 33: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 5. van den Berg SA, Uniken Venema SM, Mulder MJHL, et al. Admission blood pressure in relation to clinical outcomes and successful reperfusion after endovascular stroke treatment. Stroke 2020; 51: 3205–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maïer B, Gory B, Taylor G, et al. Mortality and disability according to baseline blood pressure in acute ischemic stroke patients treated by thrombectomy: a collaborative pooled analysis. J Am Heart Assoc 2017; 6: e006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mulder MJHL, Ergezen S, Lingsma HF, et al. Baseline blood pressure effect on the benefit and safety of intra-arterial treatment in MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands). Stroke 2017; 48: 1869–1876. [DOI] [PubMed] [Google Scholar]
- 8. Berge E, Cohen G, Lindley RI, et al. Effects of blood pressure and blood pressure-lowering treatment during the first 24 hours among patients in the third international stroke trial of thrombolytic treatment for acute ischemic stroke. Stroke 2015; 46: 3362–3369. [DOI] [PubMed] [Google Scholar]
- 9. Lindley RI, Wardlaw JM, Whiteley WN, et al. Alteplase for acute ischemic stroke: outcomes by clinically important subgroups in the Third International Stroke Trial. Stroke 2015; 46: 746–756. [DOI] [PubMed] [Google Scholar]
- 10. LeCouffe NE, Kappelhof M, Treurniet KM, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. New Engl J Med 2021; 385: 1833–1844. [DOI] [PubMed] [Google Scholar]
- 11. Treurniet KM, LeCouffe NE, Kappelhof M, et al. MR CLEAN-NO IV: intravenous treatment followed by endovascular treatment versus direct endovascular treatment for acute ischemic stroke caused by a proximal intracranial occlusion-study protocol for a randomized clinical trial. Trials 2021; 22: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 13. von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke. A guideline for healthcare professionals from the American Heart Association/American Stroke. Stroke 2019; 50: E344–E418. [DOI] [PubMed] [Google Scholar]
- 15. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167: 268–274. [DOI] [PubMed] [Google Scholar]
- 16. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. New Engl J Med 2020; 382: 1981–1993. [DOI] [PubMed] [Google Scholar]
- 17. Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT Randomized Clinical Trial. JAMA 2021; 325: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP Randomized Clinical Trial. JAMA 2021; 325: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fischer U, Kaesmacher J, Strbian D, et al. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. Lancet 2022; 400: 104–115. [DOI] [PubMed] [Google Scholar]
- 20. Mitchell PJ, Yan B, Churilov L, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4.5 h of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. Lancet 2022; 400: 116–125. [DOI] [PubMed] [Google Scholar]
- 21. Katsanos AH, Turc G, Psychogios M, et al. Utility of intravenous alteplase prior to endovascular stroke treatment: a systematic review and meta-analysis of RCTs. Neurology 2021; 97: e777–e784. [DOI] [PubMed] [Google Scholar]
- 22. Tsivgoulis G, Saqqur M, Sharma VK, et al. Association of pretreatment blood pressure with tissue plasminogen activator-induced arterial recanalization in acute ischemic stroke. Stroke 2007; 38: 961–966. [DOI] [PubMed] [Google Scholar]
- 23. Nogueira RG, Liebeskind DS, Sung G, et al. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the mechanical embolus removal in cerebral ischemia (MERCI) and multi MERCI trials. Stroke 2009; 40: 3777–3783. [DOI] [PubMed] [Google Scholar]
- 24. Samuels N, van de Graaf RA, van den Berg CAL, et al. Blood pressure in the first 6 hours following endovascular treatment for ischemic stroke is associated with outcome. Stroke 2021; 52: 3514–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brott TG, Haley EC, Jr, Levy DE, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke 1992; 23: 632–640. [DOI] [PubMed] [Google Scholar]
- 26. NINDS t-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. New Engl J Med 1999; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231173274 for Admission blood pressure and clinical outcomes in patients with acute ischaemic stroke treated with intravenous alteplase and endovascular treatment versus endovascular treatment alone: A MR CLEAN-NO IV substudy by Sophie A van den Berg, Simone M Uniken Venema, Natalie E LeCouffe, Alida A Postma, Geert J Lycklama à Nijeholt, Leon A Rinkel, Kilian M Treurniet, Manon Kappelhof, Agnetha E Bruggeman, Katinka R van Kranendonk, Charles BLM Majoie, Diederik WJ Dippel, H Bart van der Worp, Jonathan M Coutinho, Paul J Nederkoorn and Yvo BWEM Roos in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231173274 for Admission blood pressure and clinical outcomes in patients with acute ischaemic stroke treated with intravenous alteplase and endovascular treatment versus endovascular treatment alone: A MR CLEAN-NO IV substudy by Sophie A van den Berg, Simone M Uniken Venema, Natalie E LeCouffe, Alida A Postma, Geert J Lycklama à Nijeholt, Leon A Rinkel, Kilian M Treurniet, Manon Kappelhof, Agnetha E Bruggeman, Katinka R van Kranendonk, Charles BLM Majoie, Diederik WJ Dippel, H Bart van der Worp, Jonathan M Coutinho, Paul J Nederkoorn and Yvo BWEM Roos in European Stroke Journal