Abstract
Background:
Ischaemic stroke may occur despite antiplatelet therapy (APT). We aimed to investigate frequency, potential causes and outcomes in patients with ischaemic stroke despite APT.
Methods:
In this cohort study, we enrolled patients with imaging-confirmed ischaemic stroke from the Swiss Stroke Registry (01/2014-07/2022). We determined the frequency of prior APT, assessed stroke aetiology (modified TOAST classification) and determined the association of prior APT with unfavourable functional outcome (modified Rankin Scale score 3–6) and recurrent ischaemic stroke at 3 months using regression models.
Results:
Among 53,352 patients, 27,484 (51.5%) had no prior antithrombotic treatment, 17,760 (33.3%) were on APT, 7039 (13.2%) on anticoagulation and 1069 (2.0%) were on APT + anticoagulation. In patients with a history of ischaemic stroke/TIA (n = 11,948; 22.4%), 2401 (20.1%) had no prior antithrombotic therapy, 6594 (55.2%) were on APT, 2489 (20.8%) on anticoagulation and 464 (3.9%) on APT + anticoagulation. Amongst patients with ischaemic stroke despite APT, aetiology was large artery atherosclerosis in 19.8% (n = 3416), cardiac embolism in 23.6% (n = 4059), small vessel disease in 11.7% (n = 2011), other causes in 7.4% (n = 1267), more than one cause in 6.3% (n = 1078) and unknown cause in 31.3% (n = 5388). Prior APT was not independently associated with unfavourable outcome (aOR = 1.06; 95% CI: 0.98–1.14; p = 0.135) or death (aOR = 1.10; 95% CI: 0.99–1.21; p = 0.059) at 3-months but with increased odds of recurrent stroke (6.0% vs 4.3%; aOR 1.26; 95% CI: 1.11–1.44; p < 0.001).
Conclusions:
One-third of ischaemic strokes occurred despite APT and 20% of patients with a history of ischaemic stroke had no antithrombotic therapy when having stroke recurrence. Aetiology of breakthrough strokes despite APT is heterogeneous and these patients are at increased risk of recurrent stroke.
Keywords: Acute ischaemic stroke, antiplatelet therapy, anticoagulation, incidence, aetiology, stroke severity, functional outcome, recurrent stroke
Introduction
Antiplatelet therapy (APT) is the cornerstone of primary and secondary prevention of a variety of cardiovascular conditions including secondary prevention of non-cardioembolic stroke and transient ischaemic attack. 1 Aspirin, in particular, is a well-established antiplatelet agent that reduces the overall cardiovascular risk in secondary prevention of cardiovascular disease and cerebrovascular events by about a fifth to a quarter per year.2,3 However, strokes do (re-) occur despite APT with a yearly risk of around 3%–4% after the index event. In the US, approximately 185,000 (23%) of the 795,000 incident ischaemic strokes each year are recurrent strokes. 4 It is estimated that about one-third to one-half of these strokes occur while on APT. 4 Furthermore, about one-third of ischaemic strokes in patients with atrial fibrillation occurs while on oral anticoagulation (AC)5,6 and there has been a number of recent studies on ischaemic stroke despite anticoagulant therapy elucidating aetiology, risk of recurrent stroke and secondary prevention strategies.7,8
Data from a large, national stroke registry in a health care setting with universal coverage and without major barriers for acute stroke treatment and secondary prevention therapies may help to characterise this problem and provide the groundwork for future studies assessing specific diagnostic and therapeutic interventions for this vulnerable population.
The aim of this study was to explore and characterise ‘breakthrough’ ischaemic strokes occurring despite APT both in the overall population and in patients with a history of ischaemic stroke or transient ischaemic attack. We thought to assess frequency, aetiology, clinical characteristics and outcomes, including functional outcome, recurrent strokes, intracranial haemorrhage and death in patients who had ischaemic stroke despite APT using data from a large, prospective national stroke registry.
Methods
Study cohort
This study is a retrospective analysis of prospectively collected clinical data from consecutive stroke patients enrolled in the Swiss Stroke Registry (SSR) between January 2014 and July 2022. The SSR 9 is a national registry enrolling all patients with acute ischaemic or haemorrhagic stroke and transient ischaemic attack treated at one of the Swiss Stroke Units or Centres (certified according to national criteria that are in line with those of the European Stroke Organisation 10 ).
We included patients ⩾18 years of age who had an imaging-confirmed ischaemic stroke and were enrolled in the SSR. We excluded patients with missing data on prior APT. We did not include patients with transient ischaemic attacks or stroke mimics.
Ethics
Enrolment in the SSR is mandatory by Swiss law for the purpose of quality control. Patients are informed about the use of their data for research purposes. In accordance with Swiss law, patients refusing the use of their data for research, were excluded from the study. The project was approved by both the local ethics (KEK Bern 2022-01465) and the SSR steering committees and complies with the Declaration of Helsinki.
Baseline and outcome variables from the SSR
Local investigators at each SSR site collect patient data on admission and during hospitalisation using electronic case report forms, which are centrally stored in a secured database at the clinical trials unit in Basel (CH).
For the analyses, we used the following prospectively collected variables: Demographics (age, sex), event characteristics (time of symptom onset/admission), medical history (hypertension, dyslipidaemia, diabetes mellitus, atrial fibrillation, active smoking, previous ischaemic stroke, previous transient ischaemic attack, previous intracranial haemorrhage, peripheral arterial disease, coronary heart disease), baseline medical data (premorbid modified Rankin scale (mRS), National Institutes of Health Stroke Scale (NIHISS) on admission, blood pressure, blood glucose), prior antithrombotic treatment (APT and/or AC), acute treatment (intravenous thrombolysis, endovascular treatment) and aetiology (modified Trial of Org 10172 in Acute Stroke Treatment – TOAST 11 (we consolidated the TOAST subgroups ‘negative evaluation’ and ‘incomplete evaluation’ to ‘unknown causes’)).
Clinical follow-up
As part of the SSR, local investigators conduct a standardised follow-up after 3 months for all patients treated at the respective centre. During these structured telephone or in person visits information on recurrent events after discharge (recurrent ischaemic stroke, intracerebral haemorrhage, death), as well as the mRS is collected.
Primary and secondary outcomes
The primary outcome of our study was the frequency of prior antiplatelet therapy. Secondary outcomes were stroke severity at baseline (measured with the admission NIHSS), stroke aetiology, unfavourable functional outcome at 3 months (defined as mRS 3–6), death at 3 months, intracerebral haemorrhage at 3 months and recurrent ischaemic stroke at 3 months.
Statistical analysis
Patients meeting the inclusion and exclusion criteria were grouped based on their prior antithrombotic treatment (no prior antithrombotic treatment, prior APT, prior AC, prior APT + AC). Applying the appropriate statistical methods, we compared the four groups concerning baseline medical data and stroke aetiologies (modified TOAST). We reported median values with interquartile range (IQR) or mean values with standard deviation (SD) as appropriate.
To investigate the association between prior antithrombotic treatment and the outcome variables NIHSS on admission and 3-month follow-up outcomes (unfavourable functional outcome, recurrent ischaemic stroke, intracerebral haemorrhage and death), we used quantile regression and binomial logistic regression models. We performed the analysis both in the overall population and in patients with a history of ischaemic stroke or transient ischaemic attack.
In the analysis of the effect of prior antithrombotic treatment on stroke severity (NIHSS), we included age (continuous), sex (categorical), premorbid mRS (ordinal), serum glucose on admission (continuous), systolic blood pressure on admission (continuous), time between stroke onset and admission (continuous), history of ischaemic stroke, intracranial haemorrhage, hypertension, diabetes, atrial fibrillation (categorical variables) as covariates (known epidemiologic, clinical and therapeutic confounders).
For the analysis of 3 months outcomes, we included intravenous thrombolysis (categorical), endovascular treatment (categorical) and NIHSS on admission (continuous). For recurrent stroke and ICH at follow-up premorbid mRS was omitted as they are clinically unlikely to influence the outcome. However, TOAST aetiology (categorical) was included for the analysis of recurrent stroke at follow-up because stroke aetiology influences recurrence risk. We calculated adjusted odds ratios (aOR) or adjusted β-coefficients with corresponding 95% confidence intervals (95% CI). The significance level was set at 0.05 and p-values were two-tailed. Results were not corrected for multiple testing. As to our pre-defined statistical analysis plan, we excluded patients with missing data items from the multivariable analysis. As a post-hoc sensitivity analysis, we repeated the analyses after performing 85 repetitions of multiple imputation of missing variables included in the regression analysis using the STATA ‘mi impute chained’ and ‘mi estimate’ commands. The number of imputations needed for reliable results was calculated as described by von Hippel 12 to be at least 81.
Furthermore, in another post-hoc analysis, we performed the regression analysis of stroke severity and 3-months outcomes in the subgroup of patients on dual antiplatelet therapy. Therefore, we subdivided the original APT group into patients on single and dual antiplatelet therapy and compared each of the groups with the group without prior antithrombotic treatment.
All statistical analyses were performed using STATA (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.).
Results
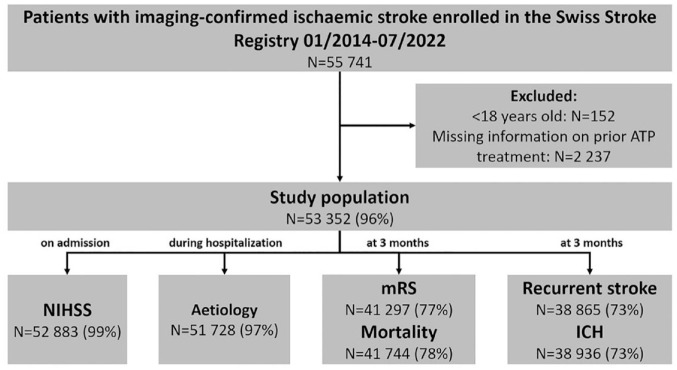
Of 55,741 patients with imaging-proven ischaemic stroke enrolled in the SSR, 152 patients were excluded because of age <18 and 2237 patients because of missing information on prior APT. Thus, the final study population consisted of 53,352 patients (Figure 1). Mean age was 72.4 years (SD ± 13.7), 42.5% were female, 18.8% had a history of ischaemic stroke, 6.2% had a history of transient ischaemic attack, median NIHSS was 3 (IQR 1–8), 32.4% received acute revascularization treatment (intravenous thrombolysis (16.5%), endovascular treatment (8.3%) or both (7.5%), see Table 1).
Figure 1.
Flow diagram of the study population.
ICH: intracerebral haemorrhage; mRS: modified Rankin scale; N: number of patients; NIHSS: National Institutes of Health Stroke Scale.
Table 1.
Baseline characteristics according to pre-stroke antithrombotic treatment.
| Characteristic | All |
No ATT |
APT |
AC |
APT + AC |
p |
|---|---|---|---|---|---|---|
| N = 53,352 | N = 27,484 (51.5%) | N = 17,760 (33.3%) | N = 7039 (13.2%) | N = 1069 (2.0%) | ||
| Age (years; mean ± SD) | 72.4 ± 13.7 | 68.6 ± 14.8 | 75.7 ± 11.1 | 78.1 ± 10.7 | 75.2 ± 10.5 | <0.001 |
| Sex (female) | 22,650 (42.5%) | 12,152 (44.3%) | 6980 (39.3%) | 3196 (45.5%) | 322 (30.1%) | <0.001 |
| BMI (kg/m2; median (p25, p75)) | 25.4 (23.0, 28.4) | 25.4 (22.9, 28.3) | 25.5 (23.1, 28.4) | 25.3 (22.9, 28.4) | 25.8 (23.3, 29.0) | 0.009 |
| History of stroke (yes) | 9794 (18.8%) | 1862 (7.0%) | 5372 (31.0%) | 2154 (31.3%) | 406 (39.0%) | <0.001 |
| History of TIA (yes) | 3211 (6.2%) | 648 (2.4%) | 1835 (10.6%) | 588 (8.6%) | 140 (13.5%) | <0.001 |
| History of ICH (yes) | 965 (1.9%) | 474 (1.8%) | 347 (2.0%) | 130 (1.9%) | 14 (1.3%) | 0.19 |
| History of hypertension (yes) | 38,644 (74.2%) | 17,254 (64.4%) | 14,774 (85.2%) | 5695 (82.7%) | 921 (88.3%) | <0.001 |
| History of diabetes (yes) | 11,348 (21.8%) | 4166 (15.5%) | 5156 (29.8%) | 1671 (24.3%) | 355 (34.0%) | <0.001 |
| History of smoking (yes) | 10,750 (20.7%) | 6083 (22.8%) | 3568 (20.7%) | 871 (12.7%) | 228 (22.0%) | <0.001 |
| History of AF (yes) | 12,635 (24.3%) | 4032 (15.0%) | 3165 (18.3%) | 4831 (70.1%) | 607 (58.2%) | <0.001 |
| History of CHD (yes) | 9498 (18.3%) | 1435 (5.4%) | 5853 (33.8%) | 1664 (24.2%) | 546 (52.5%) | <0.001 |
| History of PAD (yes) | 2901 (5.6%) | 411 (1.5%) | 1774 (10.3%) | 492 (7.2%) | 224 (21.6%) | <0.001 |
| Time onset to admission (min; mean ± SD) | 635.9 ± 831.9 | 647.5 ± 834.2 | 638.7 ± 845.7 | 594.5 ± 792.2 | 564.1 ± 788.0 | <0.001 |
| Glucose on admission (mmol/l; mean ± SD) | 7.2 ± 2.7 | 7.0 ± 2.6 | 7.4 ± 2.8 | 7.2 ± 2.4 | 7.3 ± 2.6 | <0.001 |
| Syst. BP on admission (mmHg; mean ± SD) | 156.7 ± 28.0 | 157.5 ± 28.4 | 156.9 ± 27.3 | 154.0 ± 27.4 | 149.7 ± 28.1 | <0.001 |
| Diast. BP on admission (mmHg; mean ± SD) | 85.1 ± 17.5 | 86.7 ± 17.5 | 83.0 ± 16.9 | 84.4 ± 18.3 | 81.7 ± 18.2 | <0.001 |
| NIHSS on admission (points; median (p25, p75)) | 3.0 (1.0, 8.0) | 3.0 (1.0, 8.0) | 3.0 (1.0, 8.0) | 4.0 (2.0, 11.0) | 4.0 (1.0, 9.0) | <0.001 |
| Recanalisation treatment | <0.001 | |||||
| Only IVT | 8781 (16.5%) | 5104 (18.6%) | 3255 (18.4%) | 375 (5.3%) | 47 (4.4%) | |
| Only EVT | 4435 (8.3%) | 1943 (7.1%) | 1141 (6.4%) | 1198 (17.1%) | 153 (14.4%) | |
| IVT and EVT | 4013 (7.5%) | 2486 (9.1%) | 1282 (7.2%) | 221 (3.1%) | 24 (2.3%) |
AC: anticoagulation; AF: atrial fibrillation; APT: antiplatelet therapy; ATT: antithrombotic therapy; BMI: body-mass-index; BP: blood pressure; CHD: coronary heart disease; diast.: diastolic; EVT: endovascular treatment; ICH: intracerebral haemorrhage; IVT: intravenous thrombolysis; N: number of patients; NIHSS: National Institutes of Health Stroke Scale; p25: 25th percentile (first quartile); p75: 75th percentile (third quartile); PAD: peripheral artery disease; SD: standard deviation; syst.: systolic; TIA: transient ischaemic attack.
Of the study population, 27,484 patients (51.5%) had no previous antithrombotic treatment, 17,760 patients (33.3%) were on APT (of those 78.6% were on aspirin, 12.5% on clopidogrel, 8.6% (n = 1534) on dual antiplatelet therapy (mostly aspirin + clopidogrel or ticagrelor); <0.3% were on prasugrel, ticagrelor or dipyridamole), 7039 patients (13.2%) had prior AC (of those 65.2% were on direct oral anticoagulation and 34.8% on vitamin K antagonists) and 1069 patients (2.0%) were on both, APT and AC (67 patients (0.1%) were treated according to the COMPASS regimen 13 (aspirin and rivaroxaban; no atrial fibrillation, but coronary heart disease or peripheral artery disease), 7380 patients (13.8%) potentially eligible for this regimen).
In the subgroup of patients with a history of ischaemic stroke or transient ischaemic attack (n = 11,948), 2401 patients (20.1%) had no prior antithrombotic therapy, 6594 patients (55.2%) were on APT, 2489 patients (20.8%) on AC and 464 patients (3.9%) on both, APT and AC. The baseline characteristics of this subgroup are summarised in Supplemental Table 1.
The distribution of prior antithrombotic treatment per year during the study period is displayed in Figure 2 (a – upper panel: all patients with ischaemic stroke, b – lower panel: patients with a history of ischaemic stroke or transient ischaemic attack).
Figure 2.
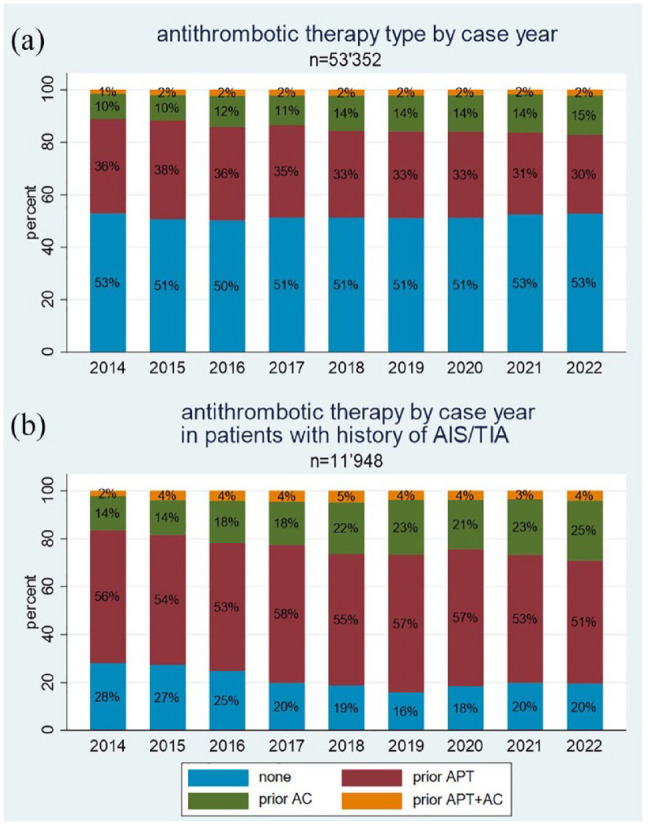
Antithrombotic therapy by case year in the overall population (a) and in patients with history of acute ischaemic stroke (AIS) or transient ischaemic attack (TIA) (b).
AC: anticoagulation; APT: antiplatelet therapy.
Over the study period, the proportion of patients on anticoagulation increases (5%) and APT decreases by approximately the same proportion (5%–6%), while the other categories remain relatively constant (p < 0.001 for trend). In the subgroup of patients with a history of ischaemic stroke and transient ischaemic attack, this effect is even more accentuated (p < 0.001 for trend).
Aetiology
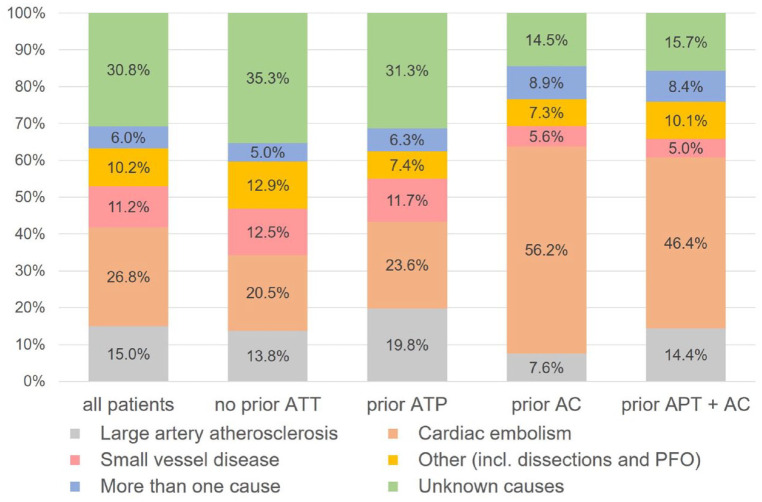
Information on aetiology was available for 51,728 patients (97%). Among patients with ischaemic stroke despite prior APT, aetiology was due to large artery atherosclerosis in 3416 (19.8%), cardiac embolism in 4059 (23.6%), small vessel disease in 2011 (11.7%), other causes in 1267 (7.4%), more than one cause in 1078 (6.3%) and unknown causes in 5388 (31.3%). The aetiologies (modified TOAST classification) according to different antithrombotic pre-treatments are shown in Figure 3.
Figure 3.
Stroke aetiologies (modified TOAST) according to pre-stroke antithrombotic treatment.
AC: anticoagulation; APT: antiplatelet therapy; ATT: antithrombotic treatment; PFO: patent foramen ovale.
Stroke severity on admission
Information on admission NIHSS was available for 52,883 patients (99%). Prior antithrombotic treatment was independently associated with lower admission severity. For prior APT median NIHSS was 0.31 points lower (β = −0.31; 95% CI −0.47 to −0.15; p < 0.001), for prior AC 0.81 points lower (β = −0.81; 95% CI −1.05 to −0.58; p < 0.001) and for prior APT + AC 1.10 points lower (β = −1.10; 95% CI −1.61 to −0.59; p < 0.001) compared to patients without any prior antithrombotic treatment (Table 2).
Table 2.
Regression analysis of stroke severity and follow-up outcomes according to pre-stroke antithrombotic therapy in overall study cohort and in the subpopulation with a history of ischaemic stroke or transient ischaemic attack.
| Characteristic | No ATT | APT | p | AC | p | APT + AC | p | N |
|---|---|---|---|---|---|---|---|---|
| Stroke severity at admission | ref. | β (95% CI) | β (95% CI) | β (95% CI) | ||||
| NIHSS at admission a | 1.00 | −0.31 (−0.47 to −0.15) | <0.001 | −0.81 (−1.05 to −0.58) | <0.001 | −1.10 (−1.61 to −0.59) | <0.001 | 34,483 |
| n = 18,096 | n = 11,187 | n = 4574 | n = 626 | |||||
| History of AIS/TIA | 1.00 | −0.74 (−1.11 to −0.37) | <0.001 | −1.12 (−1.59 to −0.66) | <0.001 | −1.20 (−1.99 to −0.41) | 0.003 | 7892 |
| n = 1568 | n = 4334 | n = 1693 | n = 297 | |||||
| 3 month follow-up outcomes | ref. | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | ||||
| Functional outcome (mRS > 3) b | 1.00 | 1.06 (0.98–1.14) | 0.135 | 1.28 (1.15–1.42) | <0.001 | 1.40 (1.11–1.75) | 0.003 | 28,464 |
| n = 14,912 | n = 9226 | n = 3808 | n = 518 | |||||
| History of AIS/TIA | 1.00 | 0.90 (0.75–1.07) | 0.239 | 1.01 (0.81–1.26) | 0.939 | 1.15 (0.81–1.63) | 0.447 | 6424 |
| n = 1217 | n = 3562 | n = 1399 | n = 246 | |||||
| Recurrent ischaemic stroke c | 1.00 | 1.26 (1.11–1.44) | <0.001 | 1.54 (1.29–1.83) | <0.001 | 1.09 (0.75–1.59) | 0.650 | 29,503 |
| n = 15,616 | n = 9554 | n = 3790 | n = 543 | |||||
| History of AIS/TIA | 1.00 | 1.01 (0.79–1.30) | 0.923 | 1.23 (0.91–1.66) | 0.186 | 0.89 (0.53–1.51) | 0.673 | 6671 |
| n = 1250 | n = 3749 | n = 1404 | n = 268 | |||||
| ICH d | 1.00 | 1.27 (1.05–1.53) | 0.013 | 0.98 (0.74–1.29) | 0.867 | 0.79 (0.40–1.58) | 0.512 | 29,640 |
| n = 15,689 | n = 9629 | n = 3784 | n = 538 | |||||
| History of AIS/TIA | 1.00 | 1.09 (0.67–1.77) | 0.718 | 1.14 (0.63–2.03) | 0.670 | 0.59 (0.17–2.03) | 0.402 | 6698 |
| n = 1247 | n = 3777 | n = 1410 | n = 264 | |||||
| Deathe | 1.00 | 1.10 (0.99–1.21) | 0.059 | 1.60 (1.41–1.80) | <0.001 | 1.69 (1.29–2.21) | <0.001 | 28,524 |
| n = 14,939 | n = 9252 | n = 3814 | n = 519 | |||||
| History of AIS/TIA | 1.00 | 0.71 (0.57–0.88) | 0.002 | 0.99 (0.77–1.27) | 0.940 | 0.89 (0.57–1.39) | 0.620 | 6442 |
| n = 1218 | n = 3577 | n = 1401 | n = 246 |
AC: anticoagulation; AIS: acute ischaemic stroke; aOR: adjusted odds ratio; APT: antiplatelet therapy; ATT: antithrombotic therapy; ICH: intracerebral haemorrhage; mRS: modified Rankin scale; N: number of patients; NIHSS: National Institutes of Health Stroke Scale; ref.: reference; TIA: transient ischaemic attack; 95% CI: 95% confidence interval.
Adjusted beta-coefficients and odds ratios of outcome events in the respective antithrombotic therapy group as compared to no antithrombotic therapy (reference beta coefficient/odds ratio of 1.00). Comparisons of stroke severities were calculated using a quantile regression model, the comparisons of the 3-months follow-up outcomes using binomial logistic regression models. The analyses were adjusted for the following covariates.
Covariates included in all analyses: age (continuous), sex (categorical), serum glucose on admission (continuous), systolic blood pressure on admission (continuous), time between stroke onset and admission (continuous), history of ischaemic stroke, intracranial haemorrhage, hypertension, diabetes, atrial fibrillation (categorical variables).
Additional covariates for each analysis:
Premorbid mRS (ordinal).
Premorbid mRS (ordinal), intravenous thrombolysis (categorical), endovascular treatment (categorical), NIHSS on admission (continuous).
Intravenous thrombolysis (categorical), endovascular treatment (categorical), NIHSS on admission (continuous), modified TOAST aetiology (categorical).
Intravenous thrombolysis (categorical), endovascular treatment (categorical), NIHSS on admission (continuous).
In the subgroup of patients with a history of ischaemic stroke or transient ischaemic attack, we observed the same associations (APT: β = −0.74; 95% CI −1.11 to −0.37; p < 0.001; AC: β = −1.12; 95% CI −1.59 to −0.66; p < 0.001; APT + AC: β = −1.20; 95% CI −1.99 to −0.41; p = 0.003; Table 2).
Functional outcome at 3-months
Information on functional outcome at 3 months was available for 41,297 patients (77%). The median mRS was 2 (IQR 0–3) for the entire cohort; the median mRS was 1 (IQR 0–3) for patients without prior antithrombotic therapy, 2 (IQR 1–4) for patients on prior APT, 3 (IQR 1–5) for patients on prior AC and 2 (IQR 1–4) for patients on prior APT + AC. Overall, 14,682 patients (35.6%) had unfavourable outcome (mRS 3–6); 6101 patients (28.8%) among those had no prior antithrombotic therapy, 5380 patients (39.3%) were on prior APT, 2810 patients (50.6%) were on prior AC and 391 patients (47.1%) were on prior APT + AC.
There was no statistically significant association between unfavourable functional outcome at 3-months and prior APT compared to no antithrombotic therapy (aOR 1.06; 95% CI 0.98–1.14; p = 0.135). However, prior AC and APT + AC was associated with increased odds for unfavourable outcome (AC: aOR 1.28; 95% CI 1.15–1.42; p < 0.001; APT + AC: aOR 1.40; 95% CI 1.11–1.75; p = 0.003; Table 2).
Among patients with a history of ischaemic stroke or transient ischaemic attack, overall 3912 patients (42.2%) had an unfavourable outcome. Six hundred eighty-eight patients (38.7%) had no prior antithrombotic pre-treatment, 2060 patients (40.0%) were on prior APT, 1001 patients (50.7%) were on prior AC and 163 patients (44.4%) were on prior APT + AC. In multivariate analysis in this subgroup, there was no association between prior antithrombotic therapy and unfavourable outcome (Table 2).
Recurrent ischaemic stroke, intracerebral haemorrhage and death at 3-months
Information on recurrent stroke was available in 38,865 patients (73%), for intracerebral haemorrhage in 38,936 (73%) and on mortality in 41,744 patients (78%).
Overall, 2045 patients (5.3%) had recurrent ischaemic stroke at 3 months; 863 patients (4.3%) among those had no prior antithrombotic pre-treatment, 766 patients (6.0%) were on prior APT, 361 patients (7.3%) were on prior AC and 55 patients (7.2%) were on prior APT + AC. In multivariate analysis, prior APT (aOR 1.26; 95% CI 1.11–1.44; p < 0.001) and prior AC (aOR 1.54; 95% CI 1.26–1.83; p < 0.001) were associated with increased odds for recurrent stroke (Table 2). Among patients with a history of ischaemic stroke or transient ischaemic attack, overall 704 patients (8.1%) suffered recurrent stroke. Among those 130 patients (7.9%) had no prior antithrombotic treatment, 382 patients (7.8%) were on prior APT, 160 patients (9.0%) were on prior AC and 32 patients (9.1%) were on prior APT + AC. In multivariate analysis, we did not observe any significant association between prior antithrombotic treatment and risk of recurrent stroke in this subgroup (Table 2).
Overall, 839 patients (2.2%) had intracerebral haemorrhage at 3 months; 400 patients (2.0%) among those had no prior antithrombotic pre-treatment, 316 patients (2.4%) were on prior APT, 110 patients (2.2%) were on prior AC and 13 patients (1.7%) were on prior APT + AC. In multivariate analysis, prior APT (aOR 1.27; 95% CI 1.05–1.53; p = 0.013), but not prior AC (aOR 0.98; 95% CI 0.74–1.29; p = 0.867) was associated with increased odds for intracerebral haemorrhage. Among patients with a history of ischaemic stroke or transient ischaemic attack, overall 179 patients (2.1%) had intracerebral haemorrhage. Among those, 32 patients (2.0%) had no prior antithrombotic treatment, 104 patients (2.1%) were on prior APT, 39 patients (2.2%) were on prior AC and 4 patients (1.2%) were on prior APT + AC. After adjusting for confounders, we did not find any association between prior antithrombotic therapy and intracerebral haemorrhage at follow-up (Table 2).
Overall, at 3 months 5865 patients (14.0%) died; 2316 patients (10.8%) among those without prior antithrombotic therapy, 2032 patients (14.6%) among those on prior APT, 1333 patients (23.8%) among those on prior AC and 184 patients (21.8%) among those on prior APT + AC. In multivariate analysis, there was a statistically significant association between death and prior AC (aOR 1.60; 95% CI 1.41–1.80; p < 0.001) and prior APT + AC (aOR 1.69; 95% CI 1.29–2.21; p < 0.001) but not with prior APT (aOR 1.10; 95% CI 0.99–1.21; p = 0.059). Among patients with a history of ischaemic stroke or transient ischaemic attack, 1486 patients (16.0%) died; 309 patients (17.4%) among those had no prior antithrombotic treatment, 708 patients (13.7%) were on prior APT, 412 patients (20.8%) were on prior AC and 57 patients (15.5%) were on prior APT + AC. In multivariate analysis, we found a significant association between prior APT and decreased odds for death (aOR 0.71; 95% CI 0.57–0.88; p = 0.002; Table 2).
Sensitivity analysis via multiple imputation models
The results of the sensitivity analysis using multiple imputation of missing variables are consistent with the main analysis (Supplemental Table 2). Only in the analysis of functional outcome, the association between prior APT and unfavourable functional outcome now reached the level of significance (aOR 1.06; 95% CI 1.00–1.11; p = 0.041; Supplemental Table 2).
Post-hoc analysis of stroke severity and 3-months outcomes in patients on dual antiplatelet therapy
The results of the regression analyses are summarised in Supplemental Table 3. While the recurrence rate in the overall population is significantly elevated in both single and dual APT, in the subgroup of patients with a history of ischaemic stroke or transient ischaemic attack the odds are only elevated in patients treated with dual APT (aOR 1.76; 95% CI 1.18–2.61; p < 0.001).
Discussion
This large national stroke-registry based study yielded the following key findings: (1) A third of all incident ischaemic strokes are breakthrough strokes despite prior APT. (2) Among patients with a history of ischaemic stroke or transient ischaemic attack, one out of five patients did not have any antithrombotic therapy at the time of stroke recurrence. (3) Aetiology of ischaemic stroke despite prior APT was heterogeneous covering the entire spectrum of stroke aetiologies according to (modified) TOAST criteria. (4) At 3 months, ischaemic stroke despite prior APT was associated with increased odds for recurrent ischaemic stroke and intracerebral haemorrhage, but there was no association with unfavourable functional outcome or death.
Taken together, our study refines and expands our knowledge on breakthrough ischaemic strokes despite APT. The percentage of patients with ischaemic stroke despite APT in our national stroke registry-based study aligns with previous studies worldwide ranging between 16% and 50% from small single centre or multi-centre studies including a few 100 up to over 90,000 patients.5,14–21 However, the vast majority of these studies had been conducted in the early 2000 up to 2015 and only one study reported patients from 2018 but included only 200 patients. Therefore, our study provides the most up-to date information on the prevalence of prior APT in patients with incident ischaemic stroke in the setting of modern antithrombotic therapy including the availability of novel agents (i.e. ticagrelor, prasugrel) and secondary prevention strategies (e.g. dual antiplatelet therapy). Therefore, the observed differences are probably related to the studies being performed in different health care settings (US, Europe, Asia) and time-periods.
The finding that every fifth patient with a history of ischaemic stroke or transient ischaemic attack did not take any antithrombotic therapy at the time of recurrent stroke is alarming as these strokes might have been prevented. The percentage of patients with a history of ischaemic stroke or transient ischaemic attack without any antithrombotic treatment in our study is comparable to other studies, that is, a US Get-with-the-Guidelines multi-centre study (14.2% only previous stroke) 22 or a smaller study from China (34%). 23
Moreover, this is the largest study to report detailed information on aetiology of breakthrough strokes despite APT. While a meta-analysis including nine studies presented a pooled number of 5739 patients, 24 our study enrolled more than 17,000 patients on prior APT. Our study revealed that the aetiology of breakthrough strokes despite APT is heterogeneous and includes the entire spectrum of stroke aetiologies. Most importantly, about one-fourth of these strokes were found to be due to cardiac-embolism. Although APT has some effect in patients with atrial fibrillation 25 to prevent stroke, the optimal preventive treatment remains oral anticoagulation. 26 Furthermore, our study found that only one-third of ischaemic strokes despite APT are due to atherosclerotic disease (either large artery or small vessel disease) indicating potential treatment failure of APT while a large proportion of strokes was due to other determined or unknown causes. In theory, reasons for breakthrough stroke despite APT may include a variety of reasons beyond cardio-embolic and arteriosclerotic disease including conditions unlikely to respond to APT (e.g. endocarditis, vasculitis). Other possible factors are compliance and medication errors (drug interactions, non-label dosage). Especially adherence, 27 antiplatelet resistance28–30 and high on-treatment platelet reactivity 31 have been reported to be of great importance in breakthrough strokes despite APT.
Our data provide evidence that patients on prior APT show decreased NIHSS on admission compared to no prior antithrombotic therapy, which is in line with some smaller studies (174 of 260 patients on APT 32 and 401 of 1862 patients on aspirin 33 ) and a multicentre stroke register analysis amongst 10,433 patients (of these 1914 patients were on aspirin). 14
In contrast to our study, in other analyses (260 patients, 32 1862 patients, 33 5700 patients, 15 10,433 patients 14 ) APT was associated with better functional outcome (mRS) at discharge14,32 or at 3 months.15,33 Whereas some studies showed an unfavourable outcome at 3 months of patients on APT (234 patients 18 ) or no difference regardless of antithrombotic therapy (433 patients, 34 2048 patients 35 ). Besides the significantly larger cohort, the percentage of patients on prior APT was significantly higher in our study (except for one small study 32 ) and different studies defined outcomes differently (favourable mRS 0–1,32,33 favourable mRS 0–2,15,18,34 mRS absolute values 14 ). Finally, one US Get-with-the-Guidelines multi-centre registry with more than half a million patients found a favourable functional outcome (mRS 0–1) in patients on prior antithrombotic therapies (APT and AC) compared to no antithrombotic pre-treatment, but this was assessed at hospital discharge and not at 3 months as in our study. 22
Furthermore, one study compared the incidents of recurrent stroke and/or death at 3 or 12 months respectively, in a post hoc analysis of the NINDS rt-PA and TOAST study cohorts (1275 and 624 patients respectively) and found no difference between patients with and without prior APT. 36 Another two studies found no difference in the mortality rates at 3 months (234 patients) 18 or 12 months (4275 patients) in patients with APT compared to no antithrombotic therapy as well. While one older study found a lower 4-week mortality with prior aspirin use. 20
In our study, the odds for stroke recurrence at 3-months were higher in patients on prior APT (and AC). However, this effect was not significant in the subgroup of patients with a history of ischaemic stroke or transient ischaemic attack.
It is conceivable that the higher recurrence risk in the overall APT population might reflect a higher overall vascular risk profile compared to patients specifically with a history of ischaemic stroke or transient ischaemic attack. In addition, while in the overall population the odds for death were higher (except for APT), in those with a history of ischaemic stroke or transient ischaemic attack they were not. In fact, in patients on APT, the odds were slightly lower, in favour of a ‘protective’ effect.
These results should be taken with caution because firstly, the correction for co-variables should have controlled a portion of the differences. Secondly, the sample size of the subgroup of patients with a history of ischaemic stroke or transient ischaemic attack is relatively small compared to the effect size we would expect (and that have seen in the overall cohort). Furthermore, the proportions of patients without prior antithrombotic treatment and prior APT differ in the overall study cohort and in the subgroup (see Table 2), therefore, there is a possibility that the analysis for this subgroup might be underpowered.
The optimal therapy for breakthrough stroke despite APT is unknown. As demonstrated by our study, about one-fourth of these patients have cardio-embolic strokes and these patients should receive oral anticoagulation therapy for secondary prevention. Moreover, there was a trend towards an increased proportion of patients on anticoagulation between 2014 and 2022 that may be attributed to an increasingly extensive search for and detection of atrial fibrillation. 37
For the remaining three-fourths, there is persistent uncertainty about optimal treatment, despite available data from several studies and one meta-analysis indicating that the addition of or a switch to another APT might lower the probability of future ischaemic vascular events.38–40 Most importantly, this data originates from a period before pivotal trials on dual antiplatelet therapy and the combination of aspirin and low-dose rivaroxaban. 13 In our study only a fraction of patients were treated according to the COMPASS regimen, however, the percentage of possibly eligible patients was significantly higher (over 10%) and there might be potential in reducing cardiovascular risk in our cohort. In particular, ischaemic strokes despite APT had the highest proportion of large artery atherosclerosis aetiology (and thus probably the highest atherosclerotic burden). Therefore, these patients might be particularly interesting for therapy according to the COMPASS regimen at discharge. Finally, it is to be seen whether other add-on antithrombotic (e.g. factor XI inhibitors)41,42 and anti-inflammatory therapies (e.g. colchicine) 43 can convey additional benefits.
Our study has several limitations. First, despite the prospectively collected data, this is a retrospective analysis that introduces a certain degree of bias. Second, this is a hospital based cohort including only patients treated at certified stroke units and stroke centres. Therefore, patients treated outside this setting are not included. However, the vast majority of stroke patients in Switzerland is treated at one of these certified centres. Third, there are limitations introduced through the available variables in the SSR. Therefore, there is unfortunately no granular data on discharge medication, on change of stroke aetiology at recurrent stoke, on medication adherence and the follow-up period is fixed (3 months). Finally, the analyses in the subgroup of patients with a history of ischaemic stroke or transient ischaemic attack might be underpowered considering the expected odds ratios.
The strengths of our study are the large study sample size, prospective data collection, the availability of TOAST classification and long-term follow-up data beyond hospital discharge. Furthermore, we provide sensitivity analyses through multiple imputation models that underline the robustness of our data. Finally, our study included regional stroke units, large non-academic stroke centres and university hospitals advocating generalisability of our findings.
In conclusion, this large multi-centre study using data from a prospective national stroke registry demonstrated the important frequency of breakthrough ischaemic strokes despite APT and their clinical relevance. Most importantly, one out of five patients with a history of ischaemic stroke or transient ischaemic attack was not taking any antithrombotic therapy at the time of stroke recurrence. Furthermore, those patients with stroke despite APT were at increased risk of recurrent stroke at 3 months advocating for novel prevention strategies, which may combine patient education and novel antithrombotic treatments.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231174942 for Ischaemic stroke despite antiplatelet therapy: Causes and outcomes by Norbert Silimon, Boudewijn Drop, Leander Clénin, Krassen Nedeltchev, Timo Kahles, Alexander A Tarnutzer, Mira Katan, Leo Bonati, Stephan Salmen, Sylvan Albert, Alexander Salerno, Emmanuel Carrera, Christian Berger, Nils Peters, Friedrich Medlin, Carlo Cereda, Manuel Bolognese, Georg Kägi, Susanne Renaud, Julien Niederhauser, Christophe Bonvin, Michael Schärer, Marie-Luise Mono, Andreas Luft, Biljana Rodic-Tatic, Urs Fischer, Simon Jung, Marcel Arnold, Thomas Meinel and David Seiffge in European Stroke Journal
Supplemental material, sj-pdf-2-eso-10.1177_23969873231174942 for Ischaemic stroke despite antiplatelet therapy: Causes and outcomes by Norbert Silimon, Boudewijn Drop, Leander Clénin, Krassen Nedeltchev, Timo Kahles, Alexander A Tarnutzer, Mira Katan, Leo Bonati, Stephan Salmen, Sylvan Albert, Alexander Salerno, Emmanuel Carrera, Christian Berger, Nils Peters, Friedrich Medlin, Carlo Cereda, Manuel Bolognese, Georg Kägi, Susanne Renaud, Julien Niederhauser, Christophe Bonvin, Michael Schärer, Marie-Luise Mono, Andreas Luft, Biljana Rodic-Tatic, Urs Fischer, Simon Jung, Marcel Arnold, Thomas Meinel and David Seiffge in European Stroke Journal
Acknowledgments
The authors thank the patients who participated in the study.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AAT reported consultant and/or speaker fees from Mitsubishi Tanabe Pharma, Medtronic, AstraZeneca and Schwabe Pharma outside the submitted work. MK received research grants from the Swiss National Science Foundation (NR 182267; NR 204977; NR 198783), the Swiss Heart Foundation; the USZ-Foundation and has received nonfinancial support from B.R.A.H.M.S. Thermofisher Scientific as well as ROCHE Diagnostics, none related to this study. MK has served on the advisory board of Medtronic, Astra Zeneca, BMS/Pfizer and Bayer. MB reported consultant fees from Astra Zeneca and Sandoz outside the submitted work. TRM reported grants from Bangerter Rhyner Foundation, Swiss Heart Foundation and Swiss National Science Foundation outside the submitted work. DJS reported grants from Bangerter Rhyner Foundation and Swiss Heart Foundation outside the study; personal fees from Bayer, Alexion and VarmX outside the submitted work. No other disclosures/conflicts of interest were reported.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The project was approved by both the local ethics (KEK Bern 2022-01465) and the SSR steering committees and complies with the Declaration of Helsinki.
Informed consent: Enrolment in the SSR is mandatory by Swiss law for the purpose of quality control. Patients are informed about the use of their data for research purposes. In accordance with Swiss law, patients refusing the use of their data for research, were excluded from the study.
Guarantor: DJS.
Contributorship: NS, TRM, DJS were involved in conceiving the study, protocol development, gaining ethical approval. NS and DJS conducted the literature research. All authors were involved in data acquisition. NS and DJS conducted analysis and interpretation of data. NS and DJS wrote the first draft of the manuscript. All authors revised the manuscript for important intellectual content and approved the final version of the manuscript.
ORCID iDs: Norbert Silimon  https://orcid.org/0009-0001-5431-422X
https://orcid.org/0009-0001-5431-422X
Boudewijn Drop  https://orcid.org/0000-0002-3415-6834
https://orcid.org/0000-0002-3415-6834
Timo Kahles  https://orcid.org/0000-0002-1569-6376
https://orcid.org/0000-0002-1569-6376
Thomas Meinel  https://orcid.org/0000-0002-0647-9273
https://orcid.org/0000-0002-0647-9273
David Seiffge  https://orcid.org/0000-0003-3890-3849
https://orcid.org/0000-0003-3890-3849
Supplemental material: Supplemental material for this article is available online.
References
- 1. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 2. Antiplatelet Trialists’ Collaboration. Secondary prevention of vascular disease by prolonged antiplatelet treatment. Antiplatelet Trialists’ Collaboration. Br Med J 1988; 296: 320–331. [PMC free article] [PubMed] [Google Scholar]
- 3. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373: 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xian Y, O’Brien EC, Liang L, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes among patients with atrial fibrillation. JAMA 2017; 317: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 6. Meinel TR, Branca M, De Marchis GM, et al. Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol 2021; 89: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polymeris AA, Meinel TR, Oehler H, et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2022; 93: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stretz C, Wu TY, Wilson D, et al. Ischaemic stroke in anticoagulated patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2021; 92: 1164–1172. [DOI] [PubMed] [Google Scholar]
- 9. Bonati L, Baumgartner RW, Bonvin C, et al. Ein Werkzeug für die Qualitätssicherung und Forschung. Swiss Medical Forum – Schweizerisches Medizin-Forum 2016; 16: 168–169. [Google Scholar]
- 10. Waje-Andreassen U, Nabavi DG, Engelter ST, et al. European Stroke Organisation certification of stroke units and stroke centres. Eur Stroke J 2018; 3: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 12. von Hippel PT. How many imputations do you need? A two-stage calculation using a quadratic rule. Sociol Methods Res 2020; 49: 699–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017; 377: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 14. Park JM, Kang K, Cho YJ, et al. Comparative effectiveness of prestroke aspirin on stroke severity and outcome. Ann Neurol 2016; 79: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ryu WS, Schellingerhout D, Hong KS, et al. Relation of pre-stroke aspirin use with cerebral infarct volume and functional outcomes. Ann Neurol 2021; 90: 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim W-J, Ko Y, Yang MH, et al. Differential effect of previous antiplatelet use on stroke severity according to stroke mechanism. Stroke 2010; 41: 1200–1204. [DOI] [PubMed] [Google Scholar]
- 17. Béjot Y, Aboa-Eboulé C, de Maistre E, et al. Prestroke antiplatelet therapy and early prognosis in stroke patients: the Dijon Stroke Registry. Eur J Neurol 2013; 20: 879–890. [DOI] [PubMed] [Google Scholar]
- 18. Krieger P, Melmed KR, Torres J, et al. Pre-admission antithrombotic use is associated with 3-month mRS score after thrombectomy for acute ischemic stroke. J Thromb Thrombolysis 2022; 54: 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Telman G, Kouperberg E, Sprecher E, et al. Pretreatment with aspirin and etiology of first-ever ischemic stroke in young and middle-aged patients. J Neurol Sci 2009; 281: 2–5. [DOI] [PubMed] [Google Scholar]
- 20. Kalra L, Perez I, Smithard DG, et al. Does prior use of aspirin affect outcome in ischemic stroke? Am J Med 2000; 108: 205–209. [DOI] [PubMed] [Google Scholar]
- 21. Gallo A, Galliazzo S, Grazioli S, et al. Epidemiology and secondary prevention of ischemic stroke in patients on antiplatelet drug: a retrospective cohort study. J Thromb Thrombolysis 2019; 48: 336–344. [DOI] [PubMed] [Google Scholar]
- 22. Myint PK, Hellkamp AS, Fonarow GC, et al. Prior antithrombotic use is associated with favorable mortality and functional outcomes in acute ischemic stroke. Stroke 2016; 47: 2066–2074. [DOI] [PubMed] [Google Scholar]
- 23. Qin H, Wang P, Zhang R, et al. Stroke history is an independent risk factor for poor prognosis in ischemic stroke patients: results from a large nationwide stroke registry. Curr Neurovasc Res 2020; 17: 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rajkumar CA, Floyd CN, Ferro A. Antiplatelet therapy as a modulator of stroke aetiology: a meta-analysis. Br J Clin Pharmacol 2015; 80: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benz AP, Johansson I, Dewilde WJM, et al. Antiplatelet therapy in patients with atrial fibrillation: a systematic review and meta-analysis of randomized trials. Eur Heart J Cardiovasc Pharmacother 2022; 8: 648–659. [DOI] [PubMed] [Google Scholar]
- 26. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 27. Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE study): a prospective epidemiological survey. Lancet 2011; 378: 1231–1243. [DOI] [PubMed] [Google Scholar]
- 28. Bath PM, May J, Flaherty K, et al. Remote assessment of platelet function in patients with acute stroke or transient ischaemic attack. Stroke Res Treat 2017; 2017: 7365684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sibbing D, Aradi D, Alexopoulos D, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y(12) receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019; 12: 1521–1537. [DOI] [PubMed] [Google Scholar]
- 30. Zeb I, Krim N, Bella J. Role of CYP2C19 genotype testing in clinical use of clopidogrel: is it really useful? Expert Rev Cardiovasc Ther 2018; 16: 369–377. [DOI] [PubMed] [Google Scholar]
- 31. Fiolaki A, Katsanos AH, Kyritsis AP, et al. High on treatment platelet reactivity to aspirin and clopidogrel in ischemic stroke: a systematic review and meta-analysis. J Neurol Sci 2017; 376: 112–116. [DOI] [PubMed] [Google Scholar]
- 32. Sanossian N, Saver JL, Rajajee V, et al. Premorbid antiplatelet use and ischemic stroke outcomes. Neurology 2006; 66: 319–323. [DOI] [PubMed] [Google Scholar]
- 33. Lin J, Han Z, Yi X, et al. Prestroke aspirin use is associated with clinical outcomes in ischemic stroke patients with atherothrombosis, small artery disease, and cardioembolic stroke. J Atheroscler Thromb 2019; 26: 528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vibo R, Kõrv J, Roose M. One-year outcome after first-ever stroke according to stroke subtype, severity, risk factors and pre-stroke treatment. A population-based study from Tartu, Estonia. Eur J Neurol 2007; 14: 435–439. [DOI] [PubMed] [Google Scholar]
- 35. Sylaja PN, Nair SS, Pandian J, et al. Impact of pre-stroke antiplatelet use on 3-month outcome after ischemic stroke. Neurol India 2021; 69: 1645–1649. [DOI] [PubMed] [Google Scholar]
- 36. Georgiadis AL, Cordina SM, Vazquez G, et al. Aspirin treatment failure and the risk of recurrent stroke and death among patients with ischemic stroke. J Stroke Cerebrovasc Dis 2013; 22: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015; 14: 377–387. [DOI] [PubMed] [Google Scholar]
- 38. Lee M, Saver JL, Hong KS, et al. Antiplatelet regimen for patients with breakthrough strokes while on aspirin: a systematic review and meta-analysis. Stroke 2017; 48: 2610–2613. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013; 369: 11–19. [DOI] [PubMed] [Google Scholar]
- 40. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018; 379: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharma M, Molina CA, Toyoda K, et al. Rationale and design of the AXIOMATIC-SSP phase II trial: antithrombotic treatment with factor XIa inhibition to optimize management of acute thromboembolic events for secondary stroke prevention. J Stroke Cerebrovasc Dis 2022; 31: 106742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shoamanesh A, Mundl H, Smith EE, et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet 2022; 400: 997–1007. [DOI] [PubMed] [Google Scholar]
- 43. Kelly PJ, Murphy S, Coveney S, et al. Anti-inflammatory approaches to ischaemic stroke prevention. J Neurol Neurosurg Psychiatry 2018; 89: 211–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231174942 for Ischaemic stroke despite antiplatelet therapy: Causes and outcomes by Norbert Silimon, Boudewijn Drop, Leander Clénin, Krassen Nedeltchev, Timo Kahles, Alexander A Tarnutzer, Mira Katan, Leo Bonati, Stephan Salmen, Sylvan Albert, Alexander Salerno, Emmanuel Carrera, Christian Berger, Nils Peters, Friedrich Medlin, Carlo Cereda, Manuel Bolognese, Georg Kägi, Susanne Renaud, Julien Niederhauser, Christophe Bonvin, Michael Schärer, Marie-Luise Mono, Andreas Luft, Biljana Rodic-Tatic, Urs Fischer, Simon Jung, Marcel Arnold, Thomas Meinel and David Seiffge in European Stroke Journal
Supplemental material, sj-pdf-2-eso-10.1177_23969873231174942 for Ischaemic stroke despite antiplatelet therapy: Causes and outcomes by Norbert Silimon, Boudewijn Drop, Leander Clénin, Krassen Nedeltchev, Timo Kahles, Alexander A Tarnutzer, Mira Katan, Leo Bonati, Stephan Salmen, Sylvan Albert, Alexander Salerno, Emmanuel Carrera, Christian Berger, Nils Peters, Friedrich Medlin, Carlo Cereda, Manuel Bolognese, Georg Kägi, Susanne Renaud, Julien Niederhauser, Christophe Bonvin, Michael Schärer, Marie-Luise Mono, Andreas Luft, Biljana Rodic-Tatic, Urs Fischer, Simon Jung, Marcel Arnold, Thomas Meinel and David Seiffge in European Stroke Journal