Abstract
Background:
Data on the impact of competing stroke etiologies in stroke patients with atrial fibrillation (AF) are scarce.
Methods:
We used prospectively obtained data from an observational registry (Novel-Oral-Anticoagulants-in-Ischemic-Stroke-Patients-(NOACISP)-LONGTERM) of consecutive AF-stroke patients treated with oral anticoagulants. We compared the frequency of (i) the composite outcome of recurrent ischemic stroke (IS), intracerebral hemorrhage (ICH) or all-cause death as well as (ii) recurrent IS alone among AF-stroke patients with versus without competing stroke etiologies according to the TOAST classification. We performed cox proportional hazards regression modeling adjusted for potential confounders. Furthermore, the etiology of recurrent IS was assessed.
Results:
Among 907 patients (median age 81, 45.6% female), 184 patients (20.3%) had competing etiologies, while 723 (79.7%) had cardioembolism as the only plausible etiology. During 1587 patient-years of follow-up, patients with additional large-artery atherosclerosis had higher rates of the composite outcome (adjusted HR [95% CI] 1.64 [1.11, 2.40], p = 0.017) and recurrent IS (aHR 2.96 [1.65, 5.35 ], p < 0.001), compared to patients with cardioembolism as the only plausible etiology. Overall 71 patients had recurrent IS (7.8%) of whom 26.7% had a different etiology than the index IS with large-artery-atherosclerosis (19.7%) being the most common non-cardioembolic cause.
Conclusion:
In stroke patients with AF, causes other than cardioembolism as competing etiologies were common in index or recurrent IS. Concomitant presence of large-artery-atherosclerosis seems to indicate an increased risk for recurrences suggesting that stroke preventive means might be more effective if they also address competing stroke etiologies in AF-stroke patients.
Clinical Trial Registration:
Keywords: Stroke, stroke etiology, atrial fibrillation, large artery atherosclerosis, oral anticoagulation
Graphical abstract.
Introduction
Cardioembolism (CE) related to atrial fibrillation (AF) is a major cause for ischemic stroke (IS). Oral anticoagulation (OAC) is the treatment of choice for primary and secondary stroke prevention in AF patients.1,2 Nevertheless, acute IS despite OAC in AF patients does occur in clinical practice3,4 challenging physicians whether and how stroke prevention strategy can be improved.
In a multicenter case control study (RENo Study), 5 inadequate low dose of direct oral anticoagulants (DOAC), atrial enlargement, hyperlipidemia and a high CHA2DS2-VASc score were found to be associated with the recurrence of ischemic events. Here, the proportion of cardioembolic stroke was 63.9% using the ASCOD (A for atherosclerosis; S for small-vessel disease, C for cardiac pathology, and O for other causes D for dissection) classification system. 6 Given the co-occurrence of AF, large artery atherosclerosis (LAA) and small vessel disease (SVD) in the elderly population, AF may not be the only mechanism leading to IS. Data on the prognostic impact of competing etiologies in AF patients is scarce. In addition, knowledge on the etiology of the recurrent IS in this patient cohort is limited. Further investigations on the prognostic role of distinct etiologies in IS patients with AF may be helpful to improve strategies for stroke prevention.
With these considerations in mind, we aimed to comprehensively compare AF-stroke patients with CE as the only plausible index stroke etiology to those with competing etiologies regarding the frequency of (i) a composite outcome including recurrent IS, intracranial hemorrhage (ICH) or death and (ii) recurrent IS alone during follow-up. Furthermore, we systematically assessed the etiology of recurrent IS and the difference of etiology between index and recurrent IS.
Patients and methods
Study design and patient cohort
This analysis is based on prospectively collected data from the registry entitled “Novel oral anticoagulants in Ischemic Stroke Patient (NOACISP)-LONGTERM” enrolling adult AF patients with acute recent (<3 months) IS (i.e. acute focal neurological deficits with a corresponding lesion and/or persistent deficit >24 h), transient ischemic attack (TIA) (i.e. acute focal neurological deficits of presumed ischemic origin lasting <24 h) or intracerebral hemorrhage (ICH) between April 2013 and December 2020 as described previously.7,8 All patients were treated with OAC, while the prescribed type (i.e. vitamin K antagonist (VKA) or DOAC) was chosen by consensus of patients and the treating physicians. Follow-up data were obtained by scheduled visits 3, 6, 12, and at least 24 months after inclusion. The visits were conducted by trained study personnel using standardized forms through telephone calls, out-patient visits, and hospital or general practitioner’s records until October 30th 2021.
For the current study we included patients from the registry with (i) non-valvular AF (ii) presenting with IS or TIA, (iii) presence of data about the etiology of the index and, if applicable, a recurrent IS, based on the criteria of the TOAST classification 9 and a follow-up period of at least 3 months after the index event. We excluded patients with ICH as index event.
In line with previous studies7,8 we used the following variables from NOACISP-LONGTERM: age, sex, body mass index, hypertension, diabetes, hyperlipidemia, peripheral artery disease, regular alcohol consumption, active smoking, history of IS and/or ICH, and the CHA2DS2-VASc score, 1 the initial clinical presentation using the National Institutes of Health Stroke Scale (NIHSS) 10 as well as the antithrombotic therapy before and after the index event and concomitant medications. Follow-up data up included (i) functional outcome measured by the modified Rankin scale (mRS) 11 (ii) occurrence of recurrent IS with the etiology based on the TOAST classification, (iii) ICH, or (iv) all-cause death during follow up.
The study followed the STROBE guidelines. 12
Study outcomes
Our primary outcome was the time between index event to the composite of recurrent IS, ICH, and all-cause death. Our secondary outcome was recurrent IS alone.
Competing stroke etiologies
The index event was classified using the TOAST classification by the study physician that included the patient in the registry. We operationalized the presence of competing stroke etiologies by applying the TOAST classification as follows. For patients with two or more potential causes of stroke the study physician named the potential competing stroke etiologies (e.g. cardioembolism (CE) and large artery atherosclerosis (LAA) or small vessel disease (SVD) and CE). Taking into consideration that every patient had atrial fibrillation and thus CE generally as a potential etiology, we regrouped the patients as follows (i) CE only were patients with cardioembolism as the sole plausible etiology. (ii) LAA+: included patients with LAA (defined as stenosis greater than >50% of an appropriate intracranial or extracranial artery) as most likely etiology or as potential competing stroke etiology identified in two or more potential causes of stroke (iii) SVD+: included patients with SVD as either most likely etiology or as competing stroke etiology identified in two or more potential causes of stroke. (iv) Other determined etiology: including patients with other determined etiology as suspected main etiology or as potential competing stroke etiology identified in two or more potential causes of stroke.
Statistical analysis
We compared patients using descriptive statistics stratified by the occurrence of the primary or secondary outcome during follow-up. We used the Chi 2 test to compare the categorical variables, we presented the data accordingly with numbers and proportions. For continuous variables we used the t-test and reported the mean values and standard deviations. In case of non-normally distributed data, the Whitney U test or the Kruskal-Wallis rank sum test was used.
We performed time-to-event-analyses for the primary and secondary outcomes in relation to the index stroke etiology using cox proportional hazard models with Firth penalization for rare events if necessary. The follow-up time was censored at the last visit or death for patients who had no outcome event. We adjusted the models for the following known confounding factors age, sex, hyperlipidemia, diabetes mellitus, hypertension, smoking, concomitant statin use at admission as well as the CHA2DS2-VASc score. Only patients with complete overall data were included in the time to event analysis.
For our Kaplan-Meier curves presenting the primary and secondary outcome, curves were stratified by the index stroke etiology with CE as the reference level.
We performed a post-hoc sensitivity analysis excluding patients with revascularization therapy (i.e. carotid endarterectomy (CEA) or carotid stenting (CAS)) with LAA+ as competing stroke etiology at the index event.
Statistical analyses were performed using R version 4.1.2.
Results
Among 1060 patients of the NOACISP registry, 907 patients (85.6%) were eligible for our analyses (Supplemental Figure 1). The remaining patients were excluded for the following reasons: 31 patients with missing follow-up visits, 35 patients without definite diagnosis of AF, 3 patients with incomplete follow-up data as well as 35 patients with ICH as index event. Finally, 48 patients with stroke mimics or missing information on the index event were excluded and 1 patient with missing data on the TOAST classification of the index event.
The median age was 81 years (Interquartile range (IQR) [74, 86]), 54.4% were male and patients had a median NIHSS of 4 [2, 9] (Supplemental Table 1). One hundred and eighty-four of 907 patients (20.3%) had competing etiologies, while 723 (79.7%) had CE as sole plausible etiology of the index event.
Primary outcome
The composite outcome occurred in 230/907 (25.3%) patients of whom 71 (31%) had an IS, 14 (6%) suffered from an ICH and 145 patients died (63%) during a median follow-up time of 2.01 years (1587 patient-years).
Patients with the occurrence of the primary composite outcome during follow-up compared to those without were older and had more often risk factors for a recurrent IS and ICH with a higher burden of concomitant vascular diseases including arterial hypertension, coronary heart disease, and peripheral artery disease. Patients with IS, ICH, or death during follow up also had higher CHA2DS2-VASc and HAS-BLED scores at baseline (see Table 1).
Table 1.
Comparison of baseline demographics and clinical characteristics, concomitant medication and clinical information’s at baseline (index stroke) between patient who suffered from at least one primary event (i.e. recurrent acute IS, ICH, or death) and those who did not.
| No primary outcome | ⩾1 primary outcome | p-Value | |
|---|---|---|---|
| Demographics | |||
| N | 677 | 230 | |
| Age, years (median, [IQR]) | 80 [74, 85] | 84 [77,88] | <0.001 |
| Male sex, n (%) | 360 (53.2) | 133 (57.8) | 0.25 |
| Medication before index event, n (%) | |||
| DOAC | 163 (24.1) | 62 (27) | 0.43 |
| VKA | 117 (17.3) | 63 (27.4) | 0.001 |
| Antiplatelet | 183 (27) | 73 (31.7) | 0.20 |
| DOAC/antiplatelet | 14 (2.1) | 7 (3.0) | 0.55 |
| VKA/antiplatelet | 11 (1.6) | 7 (3.0) | 0.29 |
| Dual antiplatelet | 3 (0.4) | 0 (0) | 0.73 |
| Vascular risk factors, n (%) | |||
| Hypertension | 540 (79.8) | 202 (87.8) | 0.008 |
| Diabetes | 153 (22.6) | 66 (28.7) | 0.07 |
| Hyperlipidemia | 348 (51.4) | 111 (48.3) | 0.45 |
| Non- Smoking | 496 (73.3) | 179 (77.8) | 0.32 |
| No regular alcohol consumption | 517 (76.4) | 175 (76.1) | 0.98 |
| Concomitant diseases, n (%) | |||
| Coronary heart disease | 169 (25) | 82 (35.7) | 0.002 |
| Heart failure | 107 (15.8) | 41 (17.8) | 0.54 |
| Peripheral artery disease | 53 (7.8) | 35 (15.2) | 0.002 |
| Renal insufficiency | 31 (4.6) | 18 (7.8) | 0.09 |
| CHA2DS2-VASc-score | 6 [5,6] | 6 [5,7] | <0.001 |
| HAS-BLED score | 2 [2,3] | 3 [2,3] | <0.001 |
| NIHSS at index stroke | 4 [2,8] | 4 [2,9] | 0.015 |
| Creatinin (µmol/l), median [IQR] | 83 [70, 103] | 90 [72.7, 115.2] | 0.002 |
SD: standard deviation; IQR: interquartile range; DOAC: direct oral anticoagulants; VKA: vitamin K antagonist.
LAA+ as competing stroke etiology was nearly twice as frequent (13.9% vs 7.5%) in patients with the occurrence of the primary outcome during follow-up compared to those without and slightly less patients had an index stroke due to CE alone 76.5% versus 80.8% (see Table 2).
Table 2.
Comparison of the most likely cause of index event according to the criteria of the TOAST classification between patients who endured at least one primary outcome event (recurrent IS, ICH, or death). Categories are presented with numbers and percentages.
| No primary outcome (n = 677) (%) | ⩾1 primary outcome (n = 230) (%) | |
|---|---|---|
| Cardiac embolism (CE) only | 547 (80.8) | 176 (76.5) |
| Large artery atherosclerosis (LAA+) | 51 (7.5) | 32 (13.9) |
| Small vessel disease (SVD+) | 67 (9.9) | 20 (8.7) |
| Other determined etiology | 12 (1.8%) | 2 (0.9) |
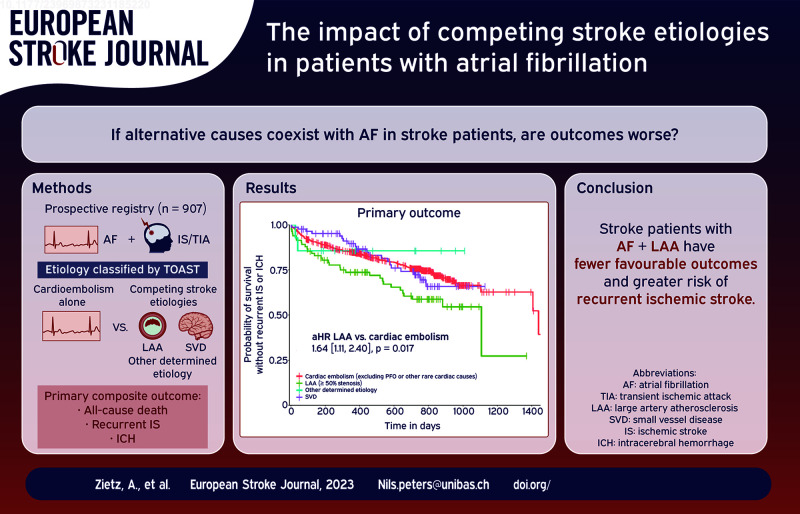
An index event with LAA+ as competing stroke etiology was associated with the primary composite outcome after adjusting for age, sex, hypertension, diabetes, hyperlipidemia, the CHA2DS2-VASc score, smoking, and concomitant statin use at admission (aHR 1.64, 95% CI [1.11, 2.40], p = 0.017, Figure 1) such an association was absent in SVD+ (aHR 0.85, 95% [0.53, 1.36], p = 0.49, Figure 1).
Figure 1.
Kaplan-Meier curve representing the probability of being alive or free of the composite outcome compromising recurrent ischemic stroke (IS), intracerebral hemorrhage (ICH) and death in relation to the potential index stroke etiology based on the TOAST classification. The reference level of the interpretation of hazard ratios is cardiac embolism. Vertical dashes represent censored data. Analysis were adjusted for age, sex, hyperlipidemia, diabetes mellitus, hypertension, the CHA2DS2-VASc score, smoking and statin at admission. One patient was excluded due to missing data.
Secondary outcome
The baseline characteristics of patients with recurrent IS compared to patients without were balanced; however, the subgroups differed with respect to the presence of peripheral artery disease (PAD), hyperlipidemia, and the intake of direct oral anticoagulants before the index event (see Supplemental Table 2).
In Table 3 the stroke etiologies according to the occurrence of the secondary outcome are demonstrated. In patients with the occurrence of a recurrent IS during follow-up compared to those without recurrent IS, the proportion of an index event due to CE alone was lower (47/71 patients (66.2%) vs 676/836 patients (80.8%)) and LAA+ was more frequent as competing index stroke etiology (16/71 patients (22.5%) vs 67/836 patients (8%)).
Table 3.
Comparison of the potential cause of index event according to the TOAST classification between patients with and without at least one recurrent IS during follow up. In three patients the secondary outcome was missing. Categories are presented with numbers and percentages.
| No recurrent acute IS (n = 836) (%) | ⩾1 recurrent acute IS (n = 71) (%) | |
|---|---|---|
| Cardiac embolism (CE) only | 676 (80.8) | 47 (66.2) |
| Large artery atherosclerosis (LAA+) | 67 (8) | 16 (22.5) |
| Small vessel disease (SVD+) | 80 (9.6) | 7 (9.9) |
| Other determined etiology | 13 (1.6) | 1 (1.4) |
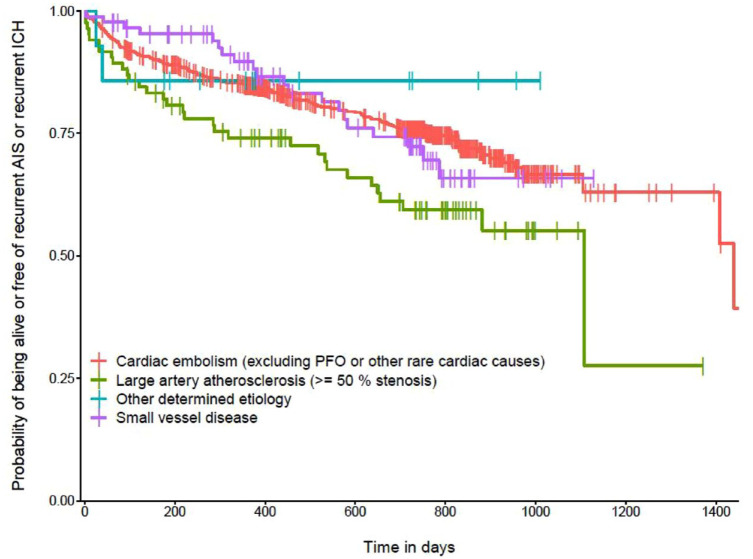
In our adjusted time to event analysis, LAA+ (aHR 2.96, 95% CI [1.65, 5.35], p < 0.001) was associated with a higher risk for a recurrent IS compared to patients with an index event due to CE alone as illustrated in Figure 2. No association was found for SVD+ (aHR 1.36, 95% CI [0.60–3.04], p = 0.465).
Figure 2.
Kaplan-Meier curve representing the probability of being free a recurrent ischemic stroke (IS) in relation to the potential index stroke etiology based on the TOAST classification. The reference level of the interpretation of hazard ratios is cardiac embolism. Vertical dashes represent censored data. Analysis were adjusted for age, sex, hyperlipidemia, diabetes mellitus, hypertension, the CHA2DS2-VASc score smoking and statin at admission. Thirty-two patients were excluded due to missing data.
Post-hoc sensitivity analysis
Additionally, we performed a post-hoc sensitivity analysis excluding 17 patients (CEA: n = 13, CAS: n = 4) with revascularization therapy out of 83 patients with LAA+ as competing index etiology.
Regarding our primary outcome, the hazard ratio was elevated in subjects with LAA+ as competing etiology compared to CE alone although missing statistical significance (aHR 1.46 [0.95, 2.26], p = 0.095, also see Table 4 in the Supplemental Material). LAA+ remained significantly associated with recurrent ischemic stroke (aHR 2.57 [1.31, 5.04], p = 0.011, also see Table 5 in the Supplemental Material).
Etiology of the recurrent IS
In 70 of 71 patients (98,6%) with recurrent IS, the TOAST classification was available. In 46/71 (64.8%) patients with recurrent IS the etiology was CE alone, LAA+ in 14/71 patients (19.7%), SVD+ in 8/71 (11.3%) and other determined etiology in 2/71 (2.8%), respectively (also see table 4).
Table 4.
Etiology of the recurrent IS according to the TOAST classification.
| Etiology of the recurrent IS (n = 71) | Cases (%) |
|---|---|
| Cardiac embolism (CE) only | 46 (64.8) |
| Large artery atherosclerosis (LAA+) | 14 (19.7) |
| Small vessel disease (SVD+) | 8 (11.3) |
| Other determined etiology+ | 2 (2.8) |
| Missing data | 1 (1.4) |
Categories are presented with number and percentages.
As illustrated in Figure 3, in patients with recurrent IS the etiologies differed between the index and recurrent event in 19/71 (26.7%) of the cases.
Figure 3.
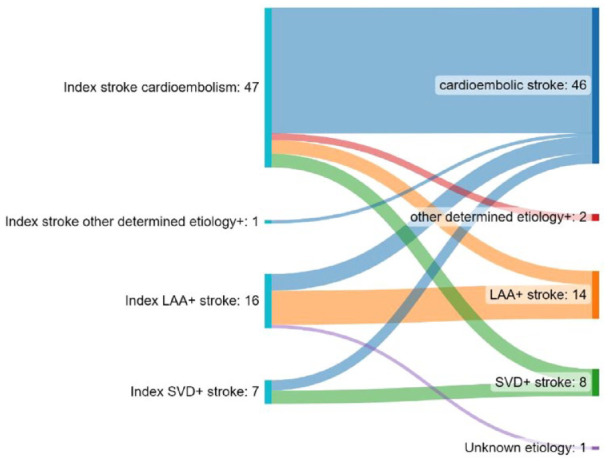
Detailed Illustration of patients with recurrent ischemic stroke. On the left side, the index stroke etiology is noted, on the right side the etiology of the recurrent stroke based on the TOAST classification. The flows represent the changes between the index and recurrent stroke etiology. TOAST classification is missing for one recurrent event (n = 71).
Discussion
This registry-based study on the prognostic impact of competing stroke etiologies among AF-stroke patients had the following key finding: (i) competing stroke etiologies were present in about every fifth AF-stroke patient. (ii) LAA+ indicated a higher risk for complications including recurrent IS in comparison to patients with CE as the only plausible etiology of the index event, including patients that underwent revascularization treatment for carotid artery stenosis. (iii) Index stroke and recurrent stroke differed regarding their etiology in more than a quarter of the patients and LAA+ was the most important non-cardioembolic etiology.
The frequency of non-cardioembolic stroke at the index event in AF patients was described in other studies – where LAA was reported in 11.3% and SVD in 13.2% 13 – recording similar rates compared to our study (20.3%).
Recent studies on AF patients focused on the impact of competing etiologies in acute IS despite OAC, a patient population with a high risk of recurrent events. 14 Competing stroke etiologies were reported in 24% by Polymeris et al., 3 while other studies described slightly higher rates (32.7%) in stroke patients under OAC 5 based on the ASCOD classification. 6
We demonstrated that LAA+ as competing stroke etiology was significantly associated with the primary composite endpoint in a stroke population with AF. Interestingly, in a prior study investigating competing stroke etiologies, patients with CE and LAA as potential stroke etiology had the highest rate of all-cause mortality following the index event compared to other stroke etiologies, pointing toward an important high risk patient population, 15 although data on IS and ICH as clinically important outcome events were lacking.
Looking at recurrent IS alone, LAA+ as competing stroke etiology at the index event was associated with an elevated risk for recurrent IS compared to CE as only plausible index etiology. Taking into consideration that LAA is known to be associated with a high risk for recurrent IS in a general stroke population 16 our findings focused on patients with AF-related stroke underlines the poor prognostic meaning of concomitant LAA in AF patients. This is clinically relevant because concomitant LAA in AF patients is common, in elderly patients percentages up to 12% were reported. 17
In our study, an index event due to SVD was not associated with our primary or secondary outcome. However, we did not incorporate imaging markers of SVD that has been shown to be associated with a higher risk for recurrent IS in stroke patients with AF. 18 Even though neuroimaging marker of SVD were associated with recurrent events in the study of Du et al, the etiology of the recurrent IS was only attributed to SVD in 5.7%, whereas in our analysis 11.3% of the recurrent IS had SVD as potential etiology.
A recently published study demonstrated that competing stroke etiologies were more common in AF stroke patients despite OAC compared to those without prior OAC. In particular, small vessel occlusion and arterial atheroma were associated with IS despite OAC. 19 In contrast to this cross sectional study, we were able to investigate the occurrence of recurrent events (including the etiology of recurrent IS) in detail, demonstrating that especially LAA+ at the index event was associated with recurrent IS. Targeting competing stroke etiologies in AF patients at the index event may thus be relevant to reduce the risk for a recurrent IS under OAC.
Of note, 17/83 patients with LAA+ as competing stroke etiology received a revascularization therapy. A large part of the LAA+ patients (79.5%) received no carotid endarterectomy (CEA) or a carotid stenting (CAS). Treatment decision was based on interdisciplinary neurovascular board recommendation. Potentially the rather low rate of performed revascularization treatment in these patients with both, LAA and AF, may point toward a less stringent indication for revascularization in stroke patients with AF and competing etiologies. Overall, our findings of an increased risk for both the primary and secondary outcome in the presence of LAA were observed for the overall cohort, including subjects that underwent revascularization. Especially the risk of recurrent IS was consistent, irrespective of the inclusion or exclusion of patients that received an interventional treatment of the carotid stenosis, which may also reflect the general high vascular burden in this at risk population.
The etiologies of the index and recurrent IS varied in approximately one quarter of the cases in our study. These findings underline that an effective secondary stroke prevention strategy based on the etiology of the index event does not necessarily prevent a recurrent event due to a different etiology. Thus, the treatment of shared cardiovascular risk factors and potential causes of concomitant diseases such as SVD and LAA should not be overlooked in stroke patients with AF.
Up to date, no clear benefit of add-on antiplatelet therapy in stroke patients with AF under OAC could by demonstrated in observational studies.3,20 Interestingly, a recent study demonstrated that in patients with ischemic stroke despite OAC concomitant antiplatelet use was associated with an increased risk of all-cause death, ICH and IS as well as recurrent IS alone. 3 Data derived from cardiac studies on newly diagnosed AF patients also found a higher risk of bleeding events and IS during follow-up in patients treated with concomitant antiplatelet agents compared to OAC alone. 21 Of note, the duration of the concomitant antiplatelet use was unknown in these observational studies and the elevated risk of recurrent ischemic events may only reflect a high risk population group with an increased vascular load than a causative effect of the antiplatelet agents. In fact, especially stroke patients with large artery arteriosclerosis are at high risk of early recurrence 16 and short term antiplatelet use may be beneficial in this time period. However – to the best of our knowledge – no randomized controlled studies investigated the short term use of an additional antiplatelet therapy in AF-stroke patients with concomitant LAA. Our findings underline that AF associated stroke patients witch competing stroke etiologies – majorly concomitant LAA – have a substantial residual risk of recurrence and further research is needed to improve secondary stroke prevention strategies.
Our study has the following limitations: (i) we used observational data and thus unknown confounders may have introduced bias even though we adjusted our analysis for variables known to affect the risk of ischemic or hemorrhagic complications. (ii) We did not perform an interrater-reliability assessment of the determination of stroke etiology and the operationalization of our dichotomization in presence versus absence of competing etiologies. However, even though the TOAST classification was not determined centrally, the stroke assessment was conducted by an experienced neurovascular study physician. (iii) The management to optimize the vascular risk factor profile including diabetes mellitus, hypertension, lipids was not standardized and details were not systematically available. (iv) The study is based on a single-center registry which reduces the generalizability of the results even though we included a rather large cohort in this analysis, treated at a specialized comprehensive stroke center, thus reflecting the current standard of care for stroke patients.
Our study has several strengths: (i) it is based on a well curated registry including AF patients on OAC following recent IS treated at a specialized stroke center, (ii) we were able to describe not only the etiology of the index event but also the recurrent IS and thus to extend and refine the findings of previous studies,3,22 and (iii) our results were robust also after adjustment for known vascular risk factors.
In conclusion, in stroke patients with AF stroke etiologies other than CE are common in index or recurrent IS. In particular, the concomitant presence of LAA seems to indicate an increased risk for recurrences. These observations suggest that stroke preventive means might be more effective if they also address stroke etiologies other than CE in AF-stroke patients. In particular, stroke patients with AF and concomitant LAA may be in need of a more individualized approach in secondary stroke prevention strategies.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231185220 for The impact of competing stroke etiologies in patients with atrial fibrillation by Annaelle Zietz, Alexandros A Polymeris, Fabrice Helfenstein, Sabine Schaedelin, Lisa Hert, Benjamin Wagner, David J Seiffge, Christopher Traenka, Valerian L Altersberger, Tolga Dittrich, Josefin Kaufmann, Flavia Ravanelli, Joachim Fladt, Urs Fisch, Sebastian Thilemann, Gian Marco De Marchis, Henrik Gensicke, Leo H Bonati, Mira Katan, Urs Fischer, Philippe Lyrer, Stefan T Engelter and Nils Peters in European Stroke Journal
Acknowledgments
We would like to thank the patients for their participations.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AZ, AP, DJS, FH, SS, LH, BW, FR, TS, GMDM, JK, VLA, JF, BL, MK, UF, PL, HG, STE, NP reports no conflict of interest related to this work. BW: No conflict of interest related to this work. BW receives funding from the Doc.Mobility grant of the University of Basel and the Prof. Dr. med. Karl und Rena Theiler-Haag foundation. CT No conflict of interest related to this work. CT received travel grants from Bayer. CT received personal scholarships or research support; from the Swiss Heart Foundation, Freiwillige Akademische Gesellschaft Basel, Bangerter-Rhyner Foundation, University of Basel, Novartis Foundation for Medical and Biological Research.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a grant of the Swiss Heart foundation and by the Basel Stroke Funds, the Science Funds Rehabilitation Felix-Platter-Hospital Basel, and the Neurology Research Pool of the University Hospital Basel. The NOACISP registry was supported by grants from the Science Funds, Bayer AG (Switzerland) and the Stroke Fund of the University Hospital Basel.
Informed consent: Written informed consent was obtained from the patients for their anonymized information to be published.
Ethical approval: The NOACISP-LONGTERM registry has been approved by the local ethics committee (BASEC PB_2016-00662, former EKBB 52/13).
Guarantor: NP
Contributorship: NP, STE, and AZ contributed to the conception and design of study the acquisition of data and wrote the first draft of the manuscript. FH, SS. NP, STE, AZ were involded in the data analysis. LH, BW, DS, CT, VLA, TD; JK, FR, JF, UF, ST, GMDM, HG, LHB, MK, UF, PL contributed to the acquisition of data and/or critical revision of the manuscript. All authors reviewed and edited the manuscript and approved of the final version of the manuscript.
ORCID iDs: Annaelle Zietz  https://orcid.org/0000-0002-4362-2497
https://orcid.org/0000-0002-4362-2497
Alexandros A Polymeris  https://orcid.org/0000-0002-9475-2208
https://orcid.org/0000-0002-9475-2208
Benjamin Wagner  https://orcid.org/0000-0001-9330-1790
https://orcid.org/0000-0001-9330-1790
David Seiffge  https://orcid.org/0000-0003-3890-3849
https://orcid.org/0000-0003-3890-3849
Tolga Dittrich  https://orcid.org/0000-0002-9987-3631
https://orcid.org/0000-0002-9987-3631
Gian Marco De Marchis  https://orcid.org/0000-0002-0342-9780
https://orcid.org/0000-0002-0342-9780
Leo H Bonati  https://orcid.org/0000-0003-1163-8133
https://orcid.org/0000-0003-1163-8133
Nils Peters  https://orcid.org/0000-0001-8451-7389
https://orcid.org/0000-0001-8451-7389
Supplemental material: Supplemental material for this article is available online.
References
- 1. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 2. Mant J, Hobbs FR, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham atrial fibrillation treatment of the aged study, BAFTA): a randomised controlled trial. J Lancet 2007; 370: 493–503. [DOI] [PubMed] [Google Scholar]
- 3. Polymeris AA, Meinel TR, Oehler H, et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2022; 93: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seiffge DJ, De Marchis GM, Koga M, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol 2020; 87: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paciaroni M, Agnelli G, Caso V, et al. Causes and risk factors of cerebral ischemic events in patients with atrial fibrillation treated with non-vitamin K antagonist oral anticoagulants for stroke prevention: the RENo study. Stroke 2019; 50: 2168–2174. [DOI] [PubMed] [Google Scholar]
- 6. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (Updated ASCO phenotyping). Cerebrovasc Dis 2013; 36: 1–5. [DOI] [PubMed] [Google Scholar]
- 7. Meya L, Polymeris AA, Schaedelin S, et al. Oral anticoagulants in atrial fibrillation patients with recent stroke who are dependent on the daily help of others. Stroke 2021; 52: 3472–3481. [DOI] [PubMed] [Google Scholar]
- 8. Hert L, Polymeris AA, Schaedelin S, et al. Small vessel disease is associated with an unfavourable outcome in stroke patients on oral anticoagulation. Eur Stroke J 2020; 5: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 10. Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 11. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 13. Bogousslavsky J, Van Melle G, Regli F, et al. Pathogenesis of anterior circulation stroke in patients with nonvalvular atrial fibrillation: the Lausanne Stroke Registry. Neurol 1990; 40: 1046–1050. [DOI] [PubMed] [Google Scholar]
- 14. Yaghi S, Henninger N, Giles JA, et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: the IAC study. J Neurol Neurosurg Psychiatry 2021; 92: 1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim YD, Cha M-J, Kim J, et al. Long-term mortality in patients with coexisting potential causes of ischemic stroke. Int J Stroke 2015; 10: 541–546. [DOI] [PubMed] [Google Scholar]
- 16. Petty GW, Brown RD, Jr, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke 2000; 31: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 17. Kanter MC, Tegeler CH, Pearce LA, et al. Carotid stenosis in patients with atrial fibrillation. Prevalence, risk factors, and relationship to stroke in the Stroke Prevention in Atrial Fibrillation Study. Arch Intern Med 1994; 154: 1372–1377. [PubMed] [Google Scholar]
- 18. Du H, Wilson D, Ambler G, et al. Small vessel disease and ischemic stroke risk during anticoagulation for atrial fibrillation after cerebral ischemia. Stroke 2021; 52: 91–99. [DOI] [PubMed] [Google Scholar]
- 19. Herlekar R, Sur Roy A, Hajiev S, et al. The contribution of competing mechanisms in stroke despite anticoagulation in patients with atrial fibrillation. Eur Stroke J 2023; 8: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soo Y, Zietz A, Yiu B, et al. Impact of cerebral microbleeds in stroke patients with atrial fibrillation. Ann Neurol. Epub ahead of print 17 March 2023. DOI: 10.1002/ana.26642. [DOI] [PubMed] [Google Scholar]
- 21. Fox KAA, Velentgas P, Camm AJ, et al. Outcomes associated with oral anticoagulants plus antiplatelets in patients with newly diagnosed atrial fibrillation. JAMA Netw Open 2020; 3: e200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paciaroni M, Caso V, Agnelli G, et al. Recurrent ischemic stroke and bleeding in patients with atrial fibrillation who suffered an acute stroke while on treatment with nonvitamin K antagonist oral anticoagulants: the RENO-EXTEND study. Stroke 2022; 53: 2620–2627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231185220 for The impact of competing stroke etiologies in patients with atrial fibrillation by Annaelle Zietz, Alexandros A Polymeris, Fabrice Helfenstein, Sabine Schaedelin, Lisa Hert, Benjamin Wagner, David J Seiffge, Christopher Traenka, Valerian L Altersberger, Tolga Dittrich, Josefin Kaufmann, Flavia Ravanelli, Joachim Fladt, Urs Fisch, Sebastian Thilemann, Gian Marco De Marchis, Henrik Gensicke, Leo H Bonati, Mira Katan, Urs Fischer, Philippe Lyrer, Stefan T Engelter and Nils Peters in European Stroke Journal