Abstract
Excessive alcohol drinking can cause pathological changes including carcinogenesis in the digestive tract from mouth to large intestine, but the underlying mechanisms are not fully understood. In this review, we discuss the effects of alcohol on small and large intestinal functions, such as leaky gut, dysbiosis and alterations of intestinal epithelium and gut immune dysfunctions, commonly referred to as alcohol-associated bowel disease (ABD). To date, detailed mechanistic insights into ABD are lacking. Accumulating evidence suggests a pathogenic role of ethanol metabolism in dysfunctions of the intestinal tract. Ethanol metabolism generates acetaldehyde and acetate, which could potentially promote functional disruptions of microbial and host components of the intestinal barrier along the gastrointestinal tract. The potential involvement of acetaldehyde and acetate in the pathogenesis of the underlying ABD, including cancer, is discussed. We also highlight some gaps in knowledge existing in the field of ABD. Finally, we discuss future directions in exploring the role of acetaldehyde and acetate generated during chronic alcohol intake in various pathologies affecting different sites of the intestinal tract.
INTRODUCTION
Chronic alcohol consumption is one of the leading risk factors for population health worldwide. The harmful consumption of alcohol resulted in approximately 3 million deaths (5.3% of all deaths) globally in 2016. Among the 230 alcohol-associated diseases, gastrointestinal (GI) diseases, unintentional injuries, cardiovascular diseases and diabetes were the major contributors to the estimated 3 million alcohol-attributable deaths in 2016, accounting for 21.3%, 20.9% and 19.0% of these deaths, respectively.1 Digestive diseases are becoming a more frequent cause of mortality and morbidity globally, especially in high-income countries.2 Moreover, cancers of the digestive tract, including cancers of the oral cavity, oesophagus, liver, colorectum and possibly stomach and pancreas, are also associated with chronic alcohol misuse.3 Alcohol-associated colorectal carcinogenesis risk could be linked to accumulation of the toxic product of ethanol metabolism, namely acetaldehyde, oxidative stress as well as genetic and environmental factors.4 Emerging evidence has highlighted that alcohol misuse is related to GI cancers and causes significant damage to the GI track, leading to alcohol-associated GI disease.3 In this review, we mainly discuss the effects of alcohol on small and large intestinal damage, which is termed as alcohol-associated bowel disease (ABD). In contrast to the well-characterised alcohol-associated liver disease (ALD),5 the characterisation of ABD has not been well defined, and underlying mechanisms of ABD pathogenesis remain poorly understood. Therefore, this review aims at outlining the current state of understanding of ABD and highlights potential mechanisms linking ethanol metabolites acetaldehyde and acetate in the pathogenesis of ABD. The effect of alcohol on colon cancer is briefly discussed.
ALCOHOL-ASSOCIATED BOWEL DISEASE
GI diseases are often referred to as disorders of digestive tract from the mouth to the anus. Strictly speaking, GI diseases also include disorders from the accessory digestive glands such as the liver, pancreas, gallbladder and biliary tract. There are two types of GI diseases, functional and structural GI diseases. Functional GI diseases are caused by abnormal GI movement with normal GI structure, such as diarrhoea, nausea, bloating, constipation, irritable bowel syndrome.6 Structural GI diseases are caused by abnormal GI structure with abnormal GI movement, including strictures, stenosis, haemorrhoids, diverticular disease, colon polyps, inflammatory bowel disease, etc.7
Excessive alcohol consumption likely induces significant pathological changes in the small and large intestines leading to ABD, but the clinical diagnosis of this disease has not been defined. Clinical diagnosis could be made based on alcohol drinking history, increased gut permeability (measurement of 51Cr-EDTA urinary excretion, intestinal sugars absorption and albumin in the faeces),8 elevated systemic translocation of microbial products and gut tissue histology. In general, ABD also includes alcohol-associated colon cancer, which is described well in previous publications.3
Structural and functional changes in ABD have not been well defined but can include a variety of pathological changes in the intestine, such as leaky gut (with elevated translocation in the circulation of gut microbial products), changes in the intestinal epithelium (eg, changes in the crypt-villus axis, disruption of tight junctions), gut immune dysfunctions (eg, reduced number of macrophages and T cells) and alterations in the intestinal microbiome (also called dysbiosis).9 10 Even if some elements implicated in the pathophysiology of ABD have been identified, we still do not know how the intestine effectively contributes to the metabolism of ethanol and its principal metabolites acetaldehyde and acetate and, also, in which manner products of ethanol metabolism impacts bowel functions. One current problem in the field of alcohol-induced gut pathogenesis is that studies are largely descriptive. In addition, investigations of the intestinal barrier in humans often take into consideration only advanced stages of ALD (eg, severe alcoholic hepatitis and decompensated cirrhosis), in which additional factors such as portal hypertension or decompensation of liver function are influencing per se the digestive tract.11 Nowadays, we lack a diagnostic definition of ABD (based on histology and/or markers of intestinal dysfunctions) as well as the molecular characterisation of the different stages of ABD, which could precede cancers of the digestive tract.
PRINCIPAL FUNCTIONS OF THE INTESTINE
The intestine is accountable for the digestion and absorption of nutrients and liquids. Smooth muscle peristalsis enables luminal contents to move along the GI tract, while smooth muscle segmentation ensures appropriate contact time and exposure to the absorptive epithelial cells. Nutrient absorption mainly occurs in the small intestine thanks to the presence of long protrusions in the lumen called villi and organised apical structures named microvilli which expands exponentially the total surface area of the intestine.12 Absorption of the massive load of dietary elements, as well as of potential toxic substances, in the GI tract can occur via two routes, paracellular or transcellular. Paracellular permeability is the passage of molecules in between the cells, and the strength of proteins called tight junction proteins controls paracellular trafficking. By contrast, transcellular permeability involves transport inside the cells through endocytosis/exocytosis (transcytosis) or via membrane receptors.13 The general concept though is that receptor-dependent absorption is ‘safe’ as only digested, monomeric substances are absorbed by contrast to paracellular route. However, it remains to be understood whether and how the number of different molecules crossing the gut barrier, as well as the routes used by these molecules to cross this barrier, influence different functions of the intestine in physiologically and pathogenic conditions. Ethanol metabolism in the intestinal tract itself might impair absorption processes as well as defence mechanisms.
ROLE OF THE LIVER AND THE INTESTINE IN ETHANOL METABOLISM
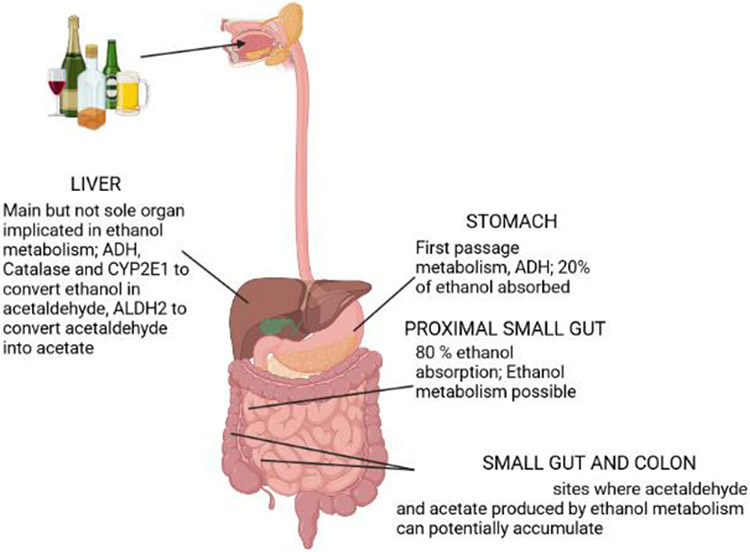
The liver is classically considered the major organ for ethanol metabolism (figure 1). Three oxidative pathways of ethanol metabolism in hepatocytes can convert ethanol to the production of acetaldehyde: (a) alcohol dehydrogenase (ADH), which is localised in the cytosol of hepatocytes is the major pathway of ethanol metabolism. Catalysis of this reaction requires the conversion of nicotinamide adenine dinucleotide NAD+ to NADH; (b) catalase, which is localised in the peroxisomes of hepatocytes, can metabolise ethanol into acetaldehyde through the conversion of H2O2 to 2H2O. This process catalyses a small proportion of remaining ethanol and (c) cytochrome P450-2E1, in the microsomes, also contributes to ethanol conversion to acetaldehyde but is only significant in situations of excessive chronic alcohol consumption. Oxidation of ethanol by this enzyme also leads to the production of reactive oxygen species (ROS).14 Ethanol metabolism in individuals with AUD generates ROS that causes lipid peroxidation, mitochondrial glutathione depletion and S-adenosylmethionine depletion; all these products subsequently prime and sensitise hepatocytes to injury. Excess acetaldehyde can bind covalently to microtubules in the hepatocytes, resulting in the intracellular retention of normally excreted proteins and the subsequent cell swelling. Acetaldehyde is an extremely reactive compound; it is very toxic to hepatocytes because it reacts with a variety of proteins and DNAs to form adducts that promote glutathione depletion, lipid peroxidation and mitochondrial damage.15 Acetaldehyde is metabolised into acetate by the enzyme called acetaldehyde dehydrogenase 2 (ALDH2). Although acetate has no direct hepatotoxicity, it is believed to alter the inflammatory response by upregulating pro-inflammatory cytokines in macrophages.16
Figure 1.
Schematic representation of ethanol metabolism in the gastrointestinal tract. Ethanol is metabolised first in the stomach and absorbed principally in the proximal small gut by simple diffusion. The liver is the major organ involved in ethanol metabolism. Acetaldehyde and acetate can potentially accumulate in the intestine and promote/contribute to ABD pathogenesis. ABD, alcohol-associated bowel disease; ADH, alcohol dehydrogenase; ALDH, acetaldehyde dehydrogenase; CYP2E1, cytochrome P450-2E1.
Ethanol is absorbed by simple diffusion through the mucosa of the GI tract. The amount of ethanol diffusing across the intestine depends on the concentration gradient between the gut lumen and the capillaries in the lamina propria, local blood flow and intestinal permeability. The highest levels of ethanol are reached in the duodenum and jejunum while luminal alcohol concentrations in the ileum, cecum and colon are similar to serum ethanol levels, suggesting that ethanol detected in the distal small intestine and colon is from the systemic circulation. Luminal ethanol can be metabolised by the intestinal epithelial cells because ethanol metabolising enzymes are expressed in the intestinal wall. ADH, the main enzyme involved in the first step of ethanol oxidation, is expressed in all parts of the intestine.17 However, to date no studies have mechanistically dissected how the intestine contributes to ethanol metabolism.
Exposure of the proximal small intestine to ethanol reduces the active transport of numerous nutrients across the epithelium, such as monosaccharides, several L-amino acid residues and lipids (fatty acids, monoglycerides) as well as for some vitamins.17 However, the effects of alcohol on the disturbed digestion and/or absorption of lipids and protein in subjects with alcohol use disorder (AUD) have not been carefully investigated. One study examined such effect in patients with AUD without confounding disorders such as cirrhosis or pancreatic insufficiency by using an intestinal perfusion technique of a solution containing a mixture of proteins, lipids and carbohydrates to evaluate nutrient absorption. The authors clearly showed that the duodenal absorption of all three nutrients was lower in patients with AUD compared with age-matched healthy controls, but absorption rates in jejunum were not reduced,18 which is in agreement with more pronounced alcohol-associated mucosal alterations in the duodenum than in jejunum.18 When duodenal biopsies from patients with AUD were examined, some authors found normal histology while others demonstrated significant histological alterations.17 19 The conflicting results may be explained by the fact that, in most subjects studied, the endoscopy was performed several days after alcohol withdrawal. Considering the high regenerative capacity of the gut epithelium, many changes might have repaired within the interval between starting abstinence and the endoscopy. A main problem in defining ABD is indeed the lack of a histological definition of the disease in patients with AUD in controlled conditions related to active alcohol consumption or abstinence. In this context, we have recently shown that the duodenal villi length of actively drinking patients with AUD is significantly reduced compared with healthy controls in the absence of histological signs of epithelial damage, confirmed by similar levels of circulating intestinal fatty acid binding protein, a marker of intestinal epithelial damage20 in patients with AUD compared with healthy controls. Interestingly, intestinal permeability measured by the gold standard 51Cr-EDTA was elevated in 40% patients with AUD and primarily confined to the proximal small bowel,21 where most of the histological/pathological changes are found and which is the principal site of alcohol absorption. Ethanol and/or its metabolites acetaldehyde and acetate may affect the malabsorption and other dysfunctions of the proximal small intestine as well as of the distal small intestine and colon. Acetate, for example, is a short-chain fatty acid also produced by anaerobic bacteria, especially in the colon and can shape different functions of the gut barrier.22 However, the effects of different concentrations of ethanol, acetaldehyde and acetate on intestinal barrier functions in vivo remain largely unknown.
As mentioned above, acetaldehyde is transformed into acetate by mitochondrial ALDH2. Interestingly, a specific gene polymorphism that drastically reduces ALDH2 activity is the variant ALDH2*2, highly prevalent in East Asians23 and results in high acetaldehyde levels and low acetate. Remarkably, ALDH2 gene polymorphism is associated with the pathogenesis of ALD and cancer.24 To model the contribution of this variant and ALDH2 deficiency in ALD, ALDH2*2 knockin mice and ALDH2 knockout mice have been used to study the pathogenesis of alcohol-associated liver cancer.25 Interestingly, the concept of the liver being the sole organ involved in ethanol metabolism and acetaldehyde detoxification has been recently challenged. It was found that genetic deletion of the Aldh2 gene in the liver caused less than a half elevation of blood acetaldehyde levels compared with those in global Aldh2 knockout mice,26 suggesting that other organs (adipose tissue, heart, intestine, etc) also contribute to the metabolism and clearance of acetaldehyde. In the small and large intestine, ALDH2 activity is relatively lower compared with ADH activity, thus potentially resulting in acetaldehyde accumulation.27 To date, the effects of acetaldehyde and acetate on ABD are largely unknown. We will here discuss current knowledge on potential production and metabolism of ethanol by the gut microbiota. We next highlight how ethanol and/or its metabolites acetaldehyde and acetate could contribute to alcohol-associated bowel pathogenesis.
GUT MICROBIOTA: PRODUCTION AND METABOLISM OF ALCOHOL AND ITS METABOLITES
The intestine is the home for trillions of microbes including bacteria, archaea, fungi and viruses.28 The gut microbiome encodes 100 times more genes than the human genome,29 exerting a remarkable influence on the host, by modulating several host functions, such as supporting the intestinal epithelium, harvesting energy,30 protecting against pathogens31 and coordinating intestinal immunity.32
Several previous studies suggest that the gut microbiota produce ethanol. Some intestinal lactic acid bacteria as well as yeasts can produce ethanol in vitro under different nutritional conditions.33 Another study has confirmed that ethanol production by the gut microbiota can occur also in vivo after consumption of fructooligosaccharides.34 Under anaerobic conditions microbes of the colon can use alcoholic fermentation to produce small amounts of ethanol via the enzymatic action of microbial ADH.35 However, whether gut bacteria produce ethanol in human in vivo is still controversial, further extensive studies are required to clarify this notion.
Gut microbes might convert ethanol into acetaldehyde by microbial ADH.36 37 or in certain bacteria via catalase.38 In which sites of the intestine these microbes metabolise ethanol is largely unknown. A recent investigation in mice chronically fed ethanol showed that ethanol metabolism of the caecal microbiota might be low in vivo, and incorporation of acetate produced by host ethanol metabolism may represent a principal pathway contributing to microbiota changes during alcohol consumption.39 The authors concluded that gut microbiota does not metabolise ethanol directly. However, ethanol metabolism of the microbiota of the proximal sites of the GI track as well as of the colon were not investigated. Acetaldehyde can be converted by microbial ALDH, which was found to be upregulated in the caecal microbiota of ethanol-fed mice.39 However, the ability to metabolise high luminal levels of acetaldehyde in the context of heavy alcohol consumption is low, due to lower microbial and mucosal ALDH activity compared with ADH35 as well as to liver ALDH.26 Consequent acetaldehyde accumulation could result in gut barrier disruption by incompletely known molecular mechanisms and may eventually lead to cancer. Heavy drinking has been associated with a 1.5 times increase in the risk of colorectal cancer (CRC), although the increased risk is modest in magnitude.23 Genetic background, like for example the variant ALDH2*2, may determine increased susceptibility to the carcinogenic effect of acetaldehyde in the gut.40
Large amounts of acetate can be produced by colonic microbes via conversion of fibres into monosaccharides, which are then converted into short-chain fatty acids.41 Acetate produced by host ethanol metabolism can further increase luminal acetate concentrations in individuals with AUD42 and could potentially affect growth of microbial communities.43 To date, we lack experimental evidence of the mechanistic role of the complex interplay between production of alcohol and its metabolites by the gut microbiota and host ethanol metabolism in gut pathogenesis. We now highlight gut barrier dysfunctions and their potential links with alcohol and its products of metabolism.
ETHANOL AND ITS METABOLITES IN ABD
Ethanol as well as acetaldehyde and acetate produced by ethanol metabolism in individuals with AUD may promote and/or contribute to bowel pathogenesis. Alterations in the microbiome.44 45 and increased microbial translocation (translocation of microbes and/or their products in the portal and systemic circulation) are strongly associated with chronic alcohol consumption.46 In addition, alcohol miuse is linked with modifications at multiple levels of the gut barrier, including the epithelium and immune system, which might in turn shape the microbial changes, allow microbes and/or their products to translocate into the portal and systemic circulation. We will here describe important alterations in the intestinal barrier functions highlighting how acetaldehyde and acetate could induce some of these changes in the different sites of the gut.
Changes in the gut microbiota
Many studies have investigated the changes in the microbiota composition specifically bacteria in patients with AUD and ethanol-fed mice. It has been shown that chronic alcohol intake can lead to bacterial overgrowth in the proximal small intestine,44 compositional changes in the microbiota (‘dysbiosis’)47 and elevated translocation of bacterial products to the blood circulation bacteria-derived products, a process called microbial translocation.46 In addition, several other studies have assessed the changes in the intestinal microbiome, mycobiome and virome in ALD.48-50 A comparison between these studies is not always possible since there are significant differences in design, data collection methodology and ALD stage. Usually, studies looked at the composition of the faecal microbiota and only two studies looked at the composition of the microbiota in the proximal small gut21 and in the colon.51
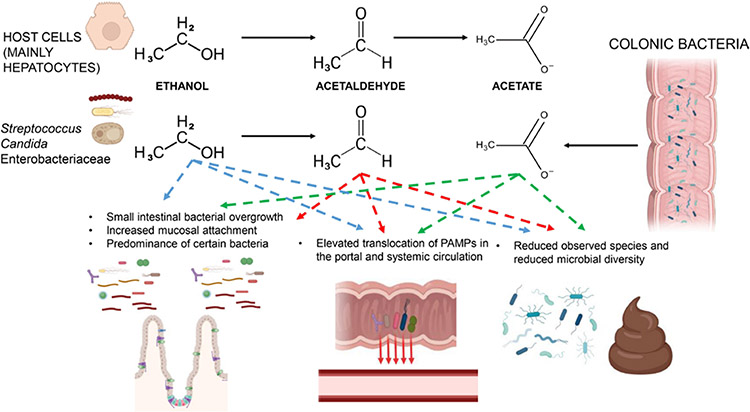
The microbiota is commonly studied at the compositional level. However, we still lack an in-depth comprehension of the functional changes in microbial metabolism in chronic alcohol consumption and how these changes mechanistically affect host intestinal cells. It is known that ethanol can be converted into acetaldehyde by different microbes, such as Candida, Streptococcus, Enterobacteriaceae family.36-38 Furthermore, treatment with ciprofloxacin reduced the acetaldehyde levels in the colonic content from 387 μM to 21 μM,52 supporting a role of microbiota in acetaldehyde accumulation in the colonic lumen. However, more rigorous studies are required to clarify whether gut bacterial produce acetaldehyde in vivo postalcohol drinking. If ethanol metabolism represents a selective advantage for these microbes in vivo is not known and which may be the functional consequences on invasive capacities in the gut. Since the microbiota has a low capacity to metabolise acetaldehyde into acetate, acetaldehyde can accumulate in the intestine potentially predisposing to barrier disruption (figure 2).
Figure 2.
Host and microbial ethanol metabolism and its possible contribution to microbial-related changes during ABD. Acetaldehyde and acetate produced by the host may impact directly or indirectly small gut bacterial overgrowth, microbial translocation and changes in the faecal microbiota. Microbe metabolising ethanol to acetaldehyde may contribute to acetaldehyde accumulation in the gut, but more rigorous studies are required to confirm this notion. Also, colonic bacteria producing acetate could impact gut acetate levels. ABD, alcohol-associated bowel disease; PAMP, pathogen-associated molecular pattern.
Dysbiosis can increase microbial translocation, either directly or in an indirect manner. Over-represented pathogenic microbes can attach and invade the mucosa, but the underlying mechanisms have not been carefully studied. Moreover, some commensal microbes may have some ‘good functions’ such as transformation of bile acids, synthesis of vitamins, short-chain fatty acids and amino acids. Loss of these microbes can negatively affect the integrity of the intestinal epithelium as well as immune responses.53
Changes in the intestinal epithelium
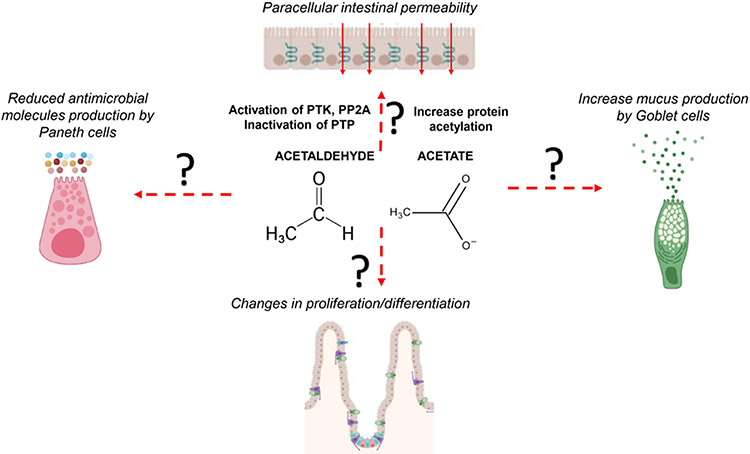
Gut barrier functions are insured by several types of epithelial cells. Absorptive enterocytes play a key role in absorbing nutrients and contribute to mucosal defence by controlling paracellular permeability and directly targeting a variety of pathogen-associated molecular patterns via different pattern recognition receptors, such as toll-like receptors and nucleotide-binding oligomerisation domain-containing proteins.54 Several independent studies have demonstrated that alcohol intake causes the disruption of tight junction proteins (eg, zonula occludens 1, occludin and claudins) and subsequent elevation of paracellular permeability, also called leaky gut, primarily in the proximal small bowel.11 The exact molecular mechanisms underlying increased intestinal permeability remain largely obscure. Studies conducted in animal models and using intestinal cell lines such as CaCo-2 have shown the potential involvement of different pathways in the dysregulation of tight junction proteins.55 56 Acetaldehyde can disrupt tight junctions either directly or indirectly. Using CaCo-2 monolayers, it has been shown that acetaldehyde-induced loss of tight junction integrity is related to activation of protein tyrosine kinase as well as of protein phosphatase 2A and inhibition of protein tyrosine phosphatase, which in turn lead to dysregulation of tight junction expression and protein-protein interactions.57 Acetate could also potentially affect paracellular intestinal permeability by influencing protein acetylation.58 All these studies used colon cancer cell lines to study the effects of ethanol, acetaldehyde and acetate on gut permeability. However, intestinal permeability in patients with AUD without advanced liver disease is primarily confined to the duodenum and jejunum,21 where ethanol is absorbed. To date, no studies have identified pathways and mechanisms related to proximal small gut permeability in patients with AUD at early precirrhotic stages, in which additional factors characterising liver decompensation (eg, portal hypertension) are not involved in the process.11 The pathogenesis of ABD might have different pathogenic mechanisms whether in the proximal intestine (more directly related to local ethanol metabolism) or in the distal intestine/colon (where changes might be secondary to dysbiosis due to acethaldehyde/acetate generated by metabolism of ethanol).
Secretory goblet cells, the frequency of which progressively increases going down the intestine, produce mucus, which is a first physical barrier against luminal microbes. Commensal bacteria degrade glycans in the mucins to extract the energy content which then they share with the intestine in a mutualistic relationship.59 Moreover, goblet cells produce antimicrobial molecules, such as trefoil factor60 and resistin-like molecule beta,61 to protect against microbial invasion in intestines, and they also present bacterial antigens to dendritic cells (DCs) via the so-called ‘goblet cells antigen-associated passages’, thus modulating the intestinal immune system.62 Interestingly, an increased production of mucus in the proximal small intestine is found in both mice and humans postalcohol consumption.63 Deletion of the mucin-2 gene in mice ameliorated alcohol-induced injury by inducing changes in the composition of the microbiota and increased expression of regenerating islet-derived 3 (Reg3) antimicrobial molecules, resulting in reduced bacterial overload and translocation in mice.63 To date, it is not known which factors are contributing to the changes in mucus and antimicrobial Reg3 production and whether acetaldehyde and/or acetate can contribute to this phenomenon in vivo.
Paneth cells reside at the base of the crypts in the small intestine, playing an important role in maintaining intestinal barrier integrity by supporting metabolically adult stem cells and by producing and releasing antimicrobial peptides, such as the Reg3 family of proteins, lysozyme and secretory phospholipase A2.64 Alcohol misuse reduces gene and protein expression of Reg3γ and Reg3β in small intestine in mice and duodenal Reg3γ in patients with AUD, indicating impaired function of specialised small intestinal Paneth cells.47 Genetic deletion of Reg3 lectins in mice enhanced bacterial translocation into the liver, thereby activating immune responses in the liver.47 Furthermore, Reg3 knockout mice had worsened alcoholic liver injury than control wild-type mice, which is due to increased bacterial translocation.44 Interestingly, diminution of the Reg3 is correlated with increased number of bacteria attached to the duodenum.44 A recent report showed that Paneth cell dysfunction is mediated by zinc deficiency and reduced α-defensins production, resulting in bacterial translocation,65 further supporting the notion of impairment of Paneth cell functions during ABD.
Microfold cells, also known as M cells, are present in follicle-associated epithelium and shape immune responses by taking up luminal antigens delivering to organised lymphoid tissues within the mucosa.66 Enteroendocrine cells (EECs) are specialised epithelial cells that represent <1% of the entire gut epithelial population, playing a key role in sensing the intestinal luminal environment. In particular, EECs are able to sense microbial metabolites and can orchestrate immune responses and responses related to the enteric nervous system.67 However, their contributions in ABD remain obscure.
The small intestinal epithelium is organised in villi and invaginations called crypts of Lieberkühn. Adult stem cells are found at the base of the crypts and generate all absorptive and secretory cells that form the epithelial layer.68 Stem cells in the crypt generate new cells that expand and differentiate in the part of the crypts called transit-amplifying zone. The transit-amplifying cells divide there 4–5 times in approximately 2 days before migrating upwards to the villus at which point they are fully differentiated.69 The differentiated cells reach the top of the villus in around 3 days where they undergo spontaneous apoptosis with the remains of the cell being dispersed into the lumen. This constant renewal of the epithelium is of upmost importance for it to be able to correctly perform its functions. Chronic alcohol feeding in mice damaged adult stem cells by dysregulating Wnt/β-catenin signalling and diminishing expression of stem cell markers such as Lgr5 and Bmi1.70 However, chronic plus binge alcohol consumption showed crypt hyperplasia (increased crypt length) with enhanced proliferation.71 Changes in the duodenal epithelium could influence both defective defence mechanisms as well as malabsorption of nutrients, a well-known characteristic of patients with an AUD.72 The villi in patients with AUD even without advanced liver disease is reduced21 and it might influence absorption of nutrients important for gut barrier functions, such as L-amino acids and lipids. In cirrhotic stages, specific ultrastructural changes of the villi have been described, such as atrophy, shorter villosity like in early ALD stages as well as presence of epithelial damage,73 likely due to portal hypertension and pro-inflammatory responses in the gut as such is not found in earlier stages of ALD. The potential contributions of acetaldehyde and acetate in epithelial dysfunctions are represented in figure 3.
Figure 3.
Potential role of acetaldehyde and acetate in alcohol-associated epithelial pathogenesis. The mechanisms described for increased paracellular permeability are relative to studies conducted with Caco-2 cell lines. The in vivo impact of acetaldehyde and acetate on alcohol-induced epithelial dysfunctions in the different sites of the intestinal tract are not known. PP2A, protein phosphatase 2A; PTK, protein tyrosine kinase; PTP, protein tyrosine phosphatase.
Changes in the intestinal immune system
The gut is home for the greatest number of immune cells of any tissue in the human body. These immune cells are strategically localised in different compartments of the intestinal mucosa, which is controlled by the expression of several chemokine receptors, and play an important role in counteracting the invasion of microbes or translocation of microbial products.74 The immune responses against microbes mainly take place in the mucosa, which is formed by the epithelium, the underlying lamina propria and the muscularis mucosa, a thin muscle layer below the lamina propria. The priming adaptive immune cell responses mainly occur in the organised structure of the gut-associated lymphoid tissue (GALT) and the draining lymph nodes in the intestine. The best-characterised gut-associated lymphoid tissues are the macroscopically visible Peyer’s patches located on the antimesenteric side of the small intestine. The size and density of Peyer’s patches rises from the jejunum to the ileum with particularly concentrated from in the distal ileum and rare distribution in the proximal small intestine, although a more heterogeneous distribution exists in humans compared with mice.75
Chronic alcohol consumption is known to reduce intestinal immunity, but it is still not clear how these reductions promote attachment and invasion of microbes in the intestine. Moreover, it has never been explored how ethanol, acetaldehyde and acetate influence intestinal immune cell functionality.
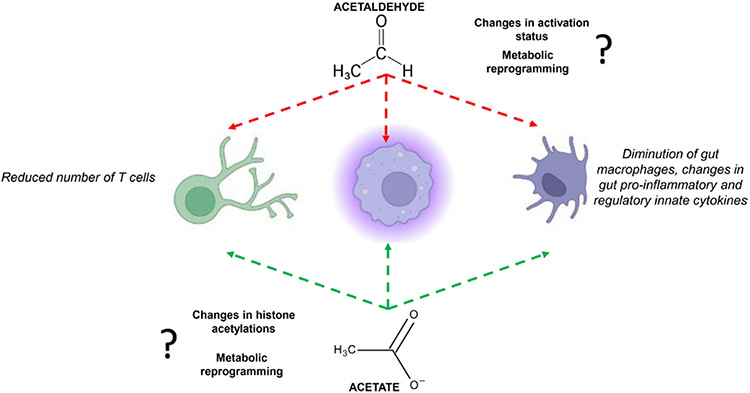
Macrophages in the lamina propria produce tumour necrosis factor-α, and such production is increased in the duodenum of mice chronically fed ethanol as well as in patients with AUD.76 However, the overall number of duodenal CD68+ macrophages in patients with AUD is reduced compared with healthy controls.21 It is known that macrophages have several subsets, but which subset of lamina propria macrophages produce this inflammatory cytokine remain unknown as well as its significance for intestinal barrier integrity. To date, no study has investigated the tolerogenic properties of intestinal macrophages in chronic alcohol consumption and how ethanol metabolism may impact their function. Macrophages in the gut are highly phagocytic and, for example, ethanol and acetaldehyde may influence their phagocytic activity in vivo, as shown in vitro with neutrophils and monocytes.77 Studies conducted with human macrophages cell lines have shown that acetate can influence histone acetylation in promoter regions of pro-inflammatory mediators.16 Acetate produced by ethanol metabolism and/or acetate produced by the gut microbiota could potentially change histone acetylation/deacetylation activity in intestinal macrophages along the intestinal tract. A recent report has shown that acetate can promote antiinflammatory functions, important features of macrophages, in B10 regulatory cells via metabolic changes through the increased production of acetyl-coenzyme A, which fuelled the tricarboxylic acid cycle and promoted post-translational lysine acetylation.78 Alterations in intestinal acetaldehyde/acetate concentrations due to ethanol metabolism in individuals with or without the ALDH2*2 variant may potentially alter gut innate and adaptive immune cells during ABD.
DCs coordinate immune responses in the intestine by presenting processed antigens to adaptive immune cells. The effects of chronic alcohol consumption on gut DCs have not been carefully investigated. It has been reported that acute alcohol intake modifies the function and cytokine production of human monocyte-derived DCs in the systemic circulation.79 Excessive alcohol consumption is associated with alterations of circulating DC distribution, immunophenotype and secretion of inflammatory mediators,80 supporting a negative effect of alcohol on the function of DCs. However, intestinal DCs have not been evaluated during ABD in mice and humans. In addition, DCs can recognise aldehyde-protein adducts, as demonstrated in other intestinal and non-intestinal diseases,81 and potentially promoting impaired gut adaptive immune responses during ABD.
The number of intestinal IgA-secreting plasma cells is reduced in mice chronically fed ethanol.82 In humans, studies on plasma cells during ABD are scarce and contradictory. It was reported that patients with decompensated cirrhosis had reduced intestinal secretion of IgA compared with those with compensated cirrhosis.83 By contrast, two other investigations showed no differences in the levels of IgA secretion84 and IgA-secreting plasma cells.85 To date, no studies have evaluated the changes in GALT related to chronic alcohol consumption and how ethanol and/or its metabolites could impact intestinal lymphoid structures and functions.
Changes in intestinal T cells appear to play an important role in ABD. A reduced number of duodenal T lymphocytes characterised patients with AUD in pre-cirrhotic stages.21 The diminution of T cells was explained by a specific reduction of duodenal CD8+ T resident memory (TRM) lymphocytes. Those TRMs also displayed features of immune dysfunctions and reduced immunosurveillance against microbes. Mechanistically, TRM cells had increased apoptosis related to an altered lipid metabolism and lysosomal membrane permeabilisation. It was found that ethanol had no direct toxicity in vitro, and cell death was rather linked to metabolic changes in TRMs and potentially to increased duodenal sphingolipid production.86 However, the potential role of ethanol metabolites acetaldehyde and acetate in duodenal TRM apoptosis and/or functional changes has not been investigated.
Advanced ALD is related to impaired adaptive immunity due to defective microbial immunosurveillance. For example, increased proportions of CD4+ T helper 17 cells in the ileal and colonic lamina propria have been found in ethanol-fed mice.87 A diminution of mucosa-associated invariant T cells, which play an important role against bacterial infections, have been observed in advanced ALD along with defective antibacterial and cytotoxic responses.88 T lymphocytes in the gut use mitochondrial β-oxidation of exogenous lipids to support their survival and protective function. Treatment with acetaldehyde in vitro inhibits production of cytokine proteins in activated mouse and human T cells. Mechanistically, acetaldehyde treatment inhibits glucose metabolism in T cells by inhibiting aerobic glycolysis-related signalling pathways.89 Alterations in the metabolism of intestinal T lymphocytes due to ethanol metabolism in the intestine as well as direct and indirect effects of acetaldehyde and/or acetate could potentially induce metabolic reprogramming in intestinal T cells potentially causing their dysfunctions, and this hypothesis clearly deserves future investigations.
Emerging data suggest that acetaldehyde and acetate contribute to gut immune defects, which is summarised in figure 4, but the underlying mechanisms are currently elusive and mechanistic approaches are therefore needed to elucidate their potential role on ABD.
Figure 4.
Potential implications of acetaldehyde and acetate in alcohol-related gut immune dysfunctions. Mechanisms potentially involved are listed near the two products of ethanol metabolism.
ALCOHOL-ASSOCIATED COLON CANCER
Substantial epidemiological evidence demonstrates that excessive alcohol consumption is closely associated with an increased risk for developing CRC.90-92 A 10-year retrospective study indicated that long-term alcohol intake is correlated with an increased incidence of KRAS+ and BRAF−/KRAS− but not BRAF+ CRC,93 which strongly suggests the connections between specific genomic alterations and alcohol consumption during carcinogenesis. Although the exact effect of alcohol on CRC and its underlying mechanisms remain incompletely understood, recent studies provided several potential explanations. First, ethanol and acetaldehyde significantly enhance DNA damage response and colonic epithelial proliferation. DNA mismatch repair gene mutation carriers are more susceptible to alcohol-induced CRC.94 A genome-wide study indicated a possible interaction between alcohol intake and genetic variants in the 10q24.2/COX15 region,95 and the A allele of single nucleotide polymorphisms rs2300985 has an increased risk for CRC in light-to-moderate drinkers. For heavy alcohol consumption, however, higher risk and worse prognosis of CRC might be associated with hypomethylation of insulin-like growth factor 2.96 Moreover, ethanol and its metabolites in colon epithelial cells contribute to the formation of ROS, reactive nitrogen species and mucosal inflammatory response,97 98 which may indirectly lead to DNA damage and initiate malignant transformation.99 100 As a very reactive and toxic intermediate metabolite of ethanol, acetaldehyde causes various DNA modifications, including mutagenic DNA lesions and chromosomal instability,100 101 single-strand or double-strand breaks, reacting with deoxyguanosine to form N2-ethylidenede-oxyguanosine, a Schiff base adduct102 and formation of adducts with DNA or proteins,103 which has been shown to correlate with hyperproliferation of colon crypt cells and CRC development.100 104
In addition to the carcinogenic roles, recent evidence suggests that ethanol and its metabolites can directly function on a variety of molecular pathways in CRC. Data from in vitro cell culture studies and experimental models revealed that ethanol elevates epithelial mesenchymal transformation in CRC by activating laminin subunit gamma-2 and integrin beta 1 signals.105 Ethanol and/or acetaldehyde mediates activation of transforming growth factor-beta/Runt-related transcription factor 3/Snail axis and glycogen synthase kinase-3 beta/β-catenin/monocyte chemoattractant protein 1 pathway, subsequently promotes aggressiveness of CRC.106 107 Chronic alcohol intake promotes metastasis of CRC via the chemokine (C-C motif) ligand 5/AMP-activated protein kinase pathway-induced autophagy.108 Acetaldehyde induces Fanconi anaemia DNA repair pathway and inhibits apoptosis signals by triggering nuclear factor kappa B-dependent pathway,100 and accelerates deficient DNA mismatch repair pathway-driven colonic tumourigenesis in a Lynch syndrome model.109 In contrast to ethanol and acetaldehyde, acetate was recently demonstrated to suppress PVR/CD155 expression via the phosphoinositide 3-kinases/protein kinase B pathway in colon cancer cell.110
Moreover, some findings suggest that alcohol metabolic enzymes play a key role in tumour initiation and cancer cell stemness maintenance of CRC. Genetic polymorphisms in ADH1B and ALDH2 correlated with colorectal carcinogenesis.111 It has been identified that ALDH1A confers the stem or progenitor properties to normal and cancer stem cells, while ALDH1B is dramatically upregulated in colonic adenocarcinoma.112 ALDHs are highly expressed in colon cancer cells, which enhance Notch signalling pathway for stemness maintenance113 and drive the transition from colitis to CRC.114 Additionally, one study reported that alcohol exposure upregulated ALDH2 expression in CRC cells, which stabilises the PD-L1 protein expression by inhibiting its proteasome-dependent degradation, and subsequently facilitates alcohol-mediated tumour escape.115
CONCLUSION AND FUTURE DIRECTIONS
Acetaldehyde and acetate have the potential to promote various dysfunctions of the intestine during acute and/or chronic alcohol consumption. Epithelial and non-epithelial cells as well as microbes along the different sites of the intestinal tract could be impacted by ethanol itself and by its metabolites, acetaldehyde and acetate. The exact contribution of the digestive tract in ethanol metabolism remains to be elucidated. Approximately 90% of patients with AUD present with mild forms of liver disease, but the majority likely have certain degrees of bowel dysfunctions (eg, malabsorption, intestinal permeability, immune dysfunctions), suggesting that ABD may precede in certain patients and promote or contribute to ALD. Therefore, future studies aiming at investigating ethanol-related impairment of bowel functions are crucial to identify molecular targets potentially useful for future interventions. Approximately 8% populations in the world present defects in acetaldehyde metabolism and clearance, leading to accumulation of acetaldehyde and low levels of acetate. However, we do not know the consequences of the imbalance between acetaldehyde and acetate in ABD pathogenesis.
To challenge these questions, translational approaches would be necessary to identify key molecular culprits responsible for ABD. Different mouse models (eg, acute, chronic-plus-binge models) could be used to better evaluate mechanistically the impact of ethanol metabolites in acute and chronic alcohol consumption. Combining transgenic mouse models of ABD, ex vivo models of the intestinal barrier (eg, enteroids, immune cell cultures and co-culture experiments) as well as innovative high-throughput technologies for the study of host and microbial functions could help us identify key factors involved in alcohol-induced gut pathogenesis. Nowadays, clinical management of patients with ABD is related to the management of the alcohol use disorder. However, since we do not have clear diagnostic criteria for ABD, the management of this disease is difficult to achieve. To better define the diagnosis of ABD and characterise the impact of acetaldehyde and acetate on human ABD, tissue levels of acetaldehyde and acetate as well as molecular signatures and markers of intestinal barrier disruptions (eg, epithelial barrier disruption, impairment of immune responses, markers of microbial translocation) could be evaluated in actively drinking patients with or without ALDH2*2 variants. Standardisation of clinical settings is fundamental to identify pathological changes implicated in ABD during active alcohol consumption and the recovery of these changes after abstinence. Thanks to highly standardised investigations in humans, it will be possible for investigators worldwide to collaborate and correctly characterise the different stages of ABD and design proper therapeutic strategies for the different populations. These studies will help us elucidate key molecular mechanisms implicated in ABD and identify therapeutic targets to treat and/or ameliorate this disease.
Acknowledgements
The figures were created with Biorender.com.
Funding
The work was supported by the intramural programme of NIAAA, NIH (AA000369, AA000368) (BG, GK) and by grants from Fond National de Recherche Scientifique Belgium (J.0146.17, T.0195.22 and T.0217.18) and Action de Recherche Concertée (ARC), Université Catholique de Louvain, Belgium to PS.
Footnotes
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
REFERENCES
- 1.World Health Organization. Global status report on alcohol and health. 2018. [Google Scholar]
- 2.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–61. [DOI] [PubMed] [Google Scholar]
- 3.Seitz HK, Müller S, Tilg H, et al. Alcohol use and gastrointestinal diseases. Visc Med 2020;36:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grumish EL, Armstrong AR, Voigt RM, et al. Alcohol-induced immune dysregulation in the colon is diurnally variable. Visc Med 2020;36:212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao B, Ahmad MF, Nagy LE, et al. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol 2019;70:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black CJ, Drossman DA, Talley NJ, et al. Functional gastrointestinal disorders: advances in understanding and management. Lancet 2020;396:1664–74. [DOI] [PubMed] [Google Scholar]
- 7.Gao X, Liu J, Li L, et al. A brief review of nutraceutical ingredients in gastrointestinal disorders: evidence and suggestions. Int J Mol Sci 2020;21:1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Llorente C, Hartmann P, et al. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods 2015;421:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maccioni L, Leclercq IA, Schnabl B, et al. Host factors in dysregulation of the gut barrier function during alcohol-associated liver disease. Int J Mol Sci 2021;22:12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barr T, Helms C, Grant K, et al. Opposing effects of alcohol on the immune system. Prog Neuropsychopharmacol Biol Psychiatry 2016;65:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stärkel P, Leclercq S, de Timary P, et al. Intestinal dysbiosis and permeability: the Yin and Yang in alcohol dependence and alcoholic liver disease. Clin Sci 2018;132:199–212. [DOI] [PubMed] [Google Scholar]
- 12.Cheng LK, O’Grady G, Du P, et al. Gastrointestinal system. Wiley Interdiscip Rev Syst Biol Med 2010;2:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ménard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol 2010;3:247–59. [DOI] [PubMed] [Google Scholar]
- 14.Kourkoumpetis T, Sood G. Pathogenesis of alcoholic liver disease: an update. Clin Liver Dis 2019;23:71–80. [DOI] [PubMed] [Google Scholar]
- 15.Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol 2014;20:17756–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendrick SFW, O’Boyle G, Mann J, et al. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology 2010;51:1988–97. [DOI] [PubMed] [Google Scholar]
- 17.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol 2003;17:575–92. [DOI] [PubMed] [Google Scholar]
- 18.Pfeiffer A, Schmidt T, Vidon N, et al. Absorption of a nutrient solution in chronic alcoholics without nutrient deficiencies and liver cirrhosis. Scand J Gastroenterol 1992;27:1023–30. [DOI] [PubMed] [Google Scholar]
- 19.Persson J. Alcohol and the small intestine. Scand J Gastroenterol 1991;26:3–15. [DOI] [PubMed] [Google Scholar]
- 20.Grootjans J, Thuijls G, Verdam F, et al. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg 2010;2:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccioni L, Gao B, Leclercq S, et al. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 2020;12:1782157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández MAG, Canfora EE, Jocken JWE, et al. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019;11:1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JS, Hsiao J-R, Chen C-H. Aldh2 polymorphism and alcohol-related cancers in Asians: a public health perspective TSE-Hua tan. J Biomed Sci 2017;24:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Wang C, Xu H, et al. Aldehyde dehydrogenase, liver disease and cancer. Int J Biol Sci 2020;16:921–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo W, Gao Y, He Y, et al. ALDH2 deficiency promotes alcohol-associated liver cancer by activating oncogenic pathways via oxidized DNA-enriched extracellular Vesicles. J Hepatol 2019;71:1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillot A, Ren T, Jourdan T, et al. Targeting liver aldehyde dehydrogenase-2 prevents heavy but not moderate alcohol drinking. Proc Natl Acad Sci U S A 2019;116:25974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaglenova J, Martínez SE, Porté S, et al. Expression, localization and potential physiological significance of alcohol dehydrogenase in the gastrointestinal tract. Eur J Biochem 2003;270:2652–62. [DOI] [PubMed] [Google Scholar]
- 28.Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell 2012;148:1258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016;164:337–40. [DOI] [PubMed] [Google Scholar]
- 30.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015;11:577–91. [DOI] [PubMed] [Google Scholar]
- 31.Kamada N, Chen GY, Inohara N, et al. Control of pathogens and pathobionts by the gut Microbiota. Nat Immunol 2013;14:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulde M, Hornef MW. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol Rev 2014;260:21–34. [DOI] [PubMed] [Google Scholar]
- 33.Elshaghabee FMF, Bockelmann W, Meske D, et al. Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front Microbiol 2016;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi M, Yang Y, Ando M, et al. Increased intestinal ethanol following consumption of fructooligosaccharides in rats. Biom Rep 2018;9:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salaspuro MP. Acetaldehyde, microbes, and cancer of the digestive tract. Crit Rev Clin Lab Sci 2003;40:183–208. [DOI] [PubMed] [Google Scholar]
- 36.Jokelainen K, Siitonen A, Jousimies-Somer H, et al. In vitro alcohol dehydrogenase-mediated acetaldehyde production by aerobic bacteria representing the normal colonic flora in man. Alcohol Clin Exp Res 1996;20:967–72. [DOI] [PubMed] [Google Scholar]
- 37.Nieminen MT, Salaspuro M. Local acetaldehyde — an essential role in alcohol-related upper gastrointestinal tract carcinogenesis. Cancers (Basel) 2018;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuruya A, Kuwahara A, Saito Y, et al. Ecophysiological consequences of alcoholism on human gut microbiota: implications for ethanol-related pathogenesis of colon cancer. Sci Rep 2016;6:27923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martino C, Zaramela LS, Gao B, et al. Acetate reprograms gut Microbiota during alcohol consumption. Nat Commun 2022;13:4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi M, Jahanzaib Anwar M, Usman A, et al. Colorectal cancer and alcohol consumption—populations to molecules. Cancers (Basel) 2018;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portincasa P, Bonfrate L, Vacca M, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci 2022:23:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong W, Zhou Z. Alterations of the gut microbiome and metabolome in alcoholic liver disease. World J Gastrointest Pathophysiol 2014;5:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr Pharm Des 2003;9:347–58. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Fouts DE, Stärkel P, et al. Intestinal REG3 Lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 2016;19:227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang S, Schnabl B. Microbiota and fatty liver disease-the known, the unknown, and the future. Cell Host Microbe 2020;28:233–f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fouts DE, Torralba M, Nelson KE, et al. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol 2012;56:1283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011;53:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartmann P Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res 2015;39:763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L, Stärkel P, Fan JG, et al. The gut mycobiome: a novel player in chronic liver diseases. J Gastroenterol 2021;56:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu CL, Zhang X, Jiang L, et al. Intestinal virome in patients with alcohol use disorder and after abstinence. Hepatol Commun 2022;6:2058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol 2012;302:G966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Homann N, Tillonen J, Salaspuro M. Microbially produced acetaldehyde from ethanol may increase the risk of colon cancer via folate deficiency. Int J Cancer 2000;86:169–73. [DOI] [PubMed] [Google Scholar]
- 53.Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers 2017;5:e1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells JM, Rossi O, Meijerink M, et al. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci U S A 2011;108 Suppl 1:4607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Sadi R, Ye D, Boivin M, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway. PLoS One 2014;9:e85345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Sadi R, Ye D, Said HM, et al. Cellular and molecular mechanism of Interleukin-1B modulation of Caco-2 intestinal epithelial tight junction barrier. J Cell Mol Med 2011;15:970–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elamin EE, Masclee AA, Jonkers DM. Effects of Acetaldehyde on Intestinal Barrier Function. Molecular aspects of alcohol and nutrition: a volume in the molecular nutrition series. Elsevier Inc, 2016: 171–86. [Google Scholar]
- 58.Ivanov AI, McCall IC, Babbin B, et al. Microtubules regulate disassembly of epithelial apical junctions. BMC Cell Biol 2006;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansson GC. Mucins and the microbiome. Annu Rev Biochem 2020;89:769–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taupin D, Podolsky DK. Trefoil factors: Initiators of mucosal healing. Nat Rev Mol Cell Biol 2003;4:721–32. [DOI] [PubMed] [Google Scholar]
- 61.Herbert DR, Yang J-Q, Hogan SP, et al. Intestinal epithelial cell secretion of RELM-B protects against gastrointestinal worm infection. J Exp Med 2009;206:2947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDole JR, Wheeler LW, McDonald KG, et al. Goblet cells deliver luminal antigen to CD103 + dendritic cells in the small intestine. Nature 2012;483:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartmann P, Chen P, Wang HJ, et al. Deficiency of intestinal Mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology 2013;58:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gassler N. Paneth cells in intestinal physiology and pathophysiology. World J Gastrointest Pathophysiol 2017;8:150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong W, Wei X, Hao L, et al. Paneth cell dysfunction mediates alcohol-related steatohepatitis through promoting bacterial translocation in mice: role of zinc deficiency. Hepatology 2020;71:1575–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kucharzik T, Lügering N, Rautenberg K, et al. Role of M cells in intestinal barrier function. Ann N Y Acad Sci 2000;915:171–83. [DOI] [PubMed] [Google Scholar]
- 67.Worthington JJ, Reimann F, Gribble FM. Enteroendocrine cells-sensory sentinels of the intestinal environment and Orchestrators of Mucosal immunity. Mucosal Immunol 2018;11:3–20. [DOI] [PubMed] [Google Scholar]
- 68.Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol 2019;16:19–34. [DOI] [PubMed] [Google Scholar]
- 69.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 2009;71:241–60. [DOI] [PubMed] [Google Scholar]
- 70.Lu R, Voigt RM, Zhang Y, et al. Alcohol injury damages intestinal stem cells. Alcohol Clin Exp Res 2017;41:727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park J-H, Jung IK, Lee Y, et al. Alcohol stimulates the proliferation of mouse small intestinal epithelial cells via WNT signaling. Biochem Biophys Res Commun 2021;534:639–45. [DOI] [PubMed] [Google Scholar]
- 72.Addolorato G, Capristo E, Greco AV, et al. Three months of abstinence from alcohol normalizes energy expenditure and substrate oxidation in alcoholics: a longitudinal study. Am J Gastroenterol 1998;93:2476–81. [DOI] [PubMed] [Google Scholar]
- 73.Tsiaoussis GI, Assimakopoulos SF, Tsamandas AC, et al. Intestinal barrier dysfunction in cirrhosis: current concepts in pathophysiology and clinical implications. World J Hepatol 2015;7:2058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol 2014;14:667–85. [DOI] [PubMed] [Google Scholar]
- 75.Cornes JS. Number, size, and distribution of Peyer’s patches in the human small intestine: part I the development of Peyer’s patches. Gut 1965;6:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen P, Stärkel P, Turner JR, et al. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 2015;61:883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vrsalovic M, Vrsalovic MM, Presecki AV, et al. Modulating role of alcohol and acetaldehyde on neutrophils and monocytes functions in vitro. J Cardiovasc Pharmacol 2007;50:462–5. [DOI] [PubMed] [Google Scholar]
- 78.Daïen CI, Tan J, Audo R, et al. Gut-derived acetate promotes B10 cells with antiinflammatory effects. JCI Insight 2021;6:e144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szabo G, Catalano D, White B, et al. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin Exp Res 2004;28:824–8. [DOI] [PubMed] [Google Scholar]
- 80.Laso FJ, Vaquero JM, Almeida J, et al. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res 2007;31:846–54. [DOI] [PubMed] [Google Scholar]
- 81.Martín-Sierra C, Laranjeira P, Domingues MR, et al. Lipoxidation and cancer immunity. Redox Biol 2019;23:101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moro-Sibilot L, Blanc P, Taillardet M, et al. Mouse and human liver contain immunoglobulin A-secreting cells originating from Peyer's patches and directed against intestinal antigens. Gastroenterology 2016;151:311–23. [DOI] [PubMed] [Google Scholar]
- 83.Pelletier G, Briantais MJ, Buffet C, et al. Serum and intestinal secretory IgA in alcoholic cirrhosis of the liver. Gut 1982;23:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colombel JF, Vaerman JP, Mesnard B, et al. Jejunal immunoglobulin secretion in alcoholic patients with and without cirrhosis. J Hepatol 1991;12:145–9. [DOI] [PubMed] [Google Scholar]
- 85.Maier A, Bode C, Fritz P, et al. Effects of chronic alcohol abuse on duodenal mononuclear cells in man. Dig Dis Sci 1999;44:691–6. [DOI] [PubMed] [Google Scholar]
- 86.Maccioni L, Loriot A, Dewulf J, et al. Duodenal CD8 + T resident memory cell apoptosis contributes to gut barrier dysfunction and microbial translocation in early associated liver disease in humans. Aliment Pharmacol Ther 2022;56:1055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chu S, Sun R, Gu X, et al. Inhibition of Sphingosine-1-phosphate-induced Th17 cells ameliorates alcohol-associated steatohepatitis in mice. Hepatology 2021;73:952–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Riva A, Patel V, Kurioka A, et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut 2018;67:918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao Y, Zhou Z, Ren T, et al. Alcohol inhibits T-cell glucose metabolism and hepatitis in ALDH2-deficient mice and humans: roles of acetaldehyde and glucocorticoids. Gut 2019;68:1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma R, Abbasi-Kangevari M, Abd-Rabu R, et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterology & Hepatology 2022;7:627–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McNabb S, Harrison TA, Albanes D, et al. Meta-Analysis of 16 studies of the Association of alcohol with colorectal cancer. Int J Cancer 2020;146:861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vieira AR, Abar L, Chan DSM, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR continuous update project. Ann Oncol 2017;28:1788–802. [DOI] [PubMed] [Google Scholar]
- 93.Jayasekara H, MacInnis RJ, Williamson EJ, et al. Lifetime alcohol intake is associated with an increased risk of KRAS+ and BRAF−/KRAS− but not BRAF+ colorectal cancer. Int J Cancer 2017;140:1485–93. [DOI] [PubMed] [Google Scholar]
- 94.Dashti SG, Buchanan DD, Jayasekara H, et al. Alcohol consumption and the risk of colorectal cancer for mismatch repair gene mutation carriers. Cancer Epidemiol Biomarkers Prev 2017;26:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jordahl KM, Shcherbina A, Kim AE, et al. Beyond GWAS of colorectal cancer: evidence of interaction with alcohol consumption and putative causal variant for the 10Q24.2 region. Cancer Epidemiol Biomarkers Prev 2022;31:1077–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishihara R, Wang M, Qian ZR, et al. Alcohol, one-carbon nutrient intake, and risk of colorectal cancer according to tumor methylation level of IGF2 differentially methylated region. Am J Clin Nutr 2014;100:1479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohira H, Tsuruya A, Oikawa D, et al. Alteration of oxidative-stress and related marker levels in mouse colonic tissues and fecal microbiota structures with chronic ethanol administration: implications for the pathogenesis of ethanol-related colorectal cancer. PLoS One 2021;16:e0246580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shukla PK, Chaudhry KK, Mir H, et al. Chronic ethanol feeding promotes Azoxymethane and dextran sulfate sodium-induced Colonic tumorigenesis potentially by enhancing Mucosal inflammation. BMC Cancer 2016;16:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsochantaridis I, Kontopoulos A, Voulgaridou G-P, et al. Aldehyde dehydrogenase 1B1 is implicated in DNA damage response in human colorectal adenocarcinoma. Cells 2022;11:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 2007;7:599–612. [DOI] [PubMed] [Google Scholar]
- 101.Garaycoechea JI, Crossan GP, Langevin F, et al. Alcohol and endogenous aldehydes damage chromosomes and Mutate stem cells. Nature 2018;553:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sonohara Y, Takatsuka R, Masutani C, et al. Acetaldehyde induces NER repairable mutagenic DNA lesions. Carcinogenesis 2022;43:52–9. [DOI] [PubMed] [Google Scholar]
- 103.Yu H-S, Oyama T, Isse T, et al. Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chem Biol Interact 2010;188:367–75. [DOI] [PubMed] [Google Scholar]
- 104.Roy HK, Gulizia JM, Karolski WJ, et al. Ethanol promotes intestinal tumorigenesis in the MIN mouse. Multiple intestinal neoplasia. Cancer Epidemiol Biomarkers Prev 2002;11:1499–502. [PubMed] [Google Scholar]
- 105.Nong FF, Liang YQ, Xing SP, et al. Alcohol promotes epithelial mesenchymal transformation-mediated premetastatic niche formation of colorectal cancer by activating interaction between laminin-Γ2 and integrin-B1. World J Gastroenterol 2022;28:5154–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu M, Wang S, Qi Y, et al. Role of MCP-1 in alcohol-induced aggressiveness of colorectal cancer cells. Mol Carcinog 2016;55:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng K, Yu J, Chen Z, et al. Ethanol promotes alcohol-related colorectal cancer metastasis via the TGF-B/RUNX3/snail axis by inducing TGF-B1 upregulation and RUNX3 cytoplasmic mislocalization. EBioMedicine 2019;50:224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao H, Chen D, Cao R, et al. Alcohol consumption promotes colorectal carcinoma metastasis via a Ccl5-induced and AMPK-pathway-mediated activation of Autophagy. Sci Rep 2018;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cerretelli G, Zhou Y, Müller MF, et al. Ethanol-induced formation of colorectal tumours and precursors in a mouse model of lynch syndrome. J Pathol 2021;255:464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tran NL, Lee IK, Choi J, et al. Acetate decreases PVR/CD155 expression via Pi3K/AKT pathway in cancer cells. BMB Rep 2021;54:431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crous-Bou M, Rennert G, Cuadras D, et al. Polymorphisms in alcohol metabolism genes ADH1B and ALDH2, alcohol consumption and colorectal cancer. PLoS One 2013;8:e80158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y, Orlicky DJ, Matsumoto A, et al. Aldehyde dehydrogenase 1B1 (ALDH1B1) is a potential biomarker for human colon cancer. Biochem Biophys Res Commun 2011;405:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arcaroli JJ, Powell RW, Varella-Garcia M, et al. ALDH+ tumor-initiating cells exhibiting gain in NOTCH1 gene copy number have enhanced regrowth sensitivity to a Γ-Secretase inhibitor and Irinotecan in colorectal cancer. Mol Oncol 2012;6:370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carpentino JE, Hynes MJ, Appelman HD, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res 2009;69:8208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang H, Xia Y, Wang F, et al. Aldehyde dehydrogenase 2 mediates alcohol-induced colorectal cancer immune escape through stabilizing PD-L1 expression. Adv Sci (Weinh) 2021;8:2003404. [DOI] [PMC free article] [PubMed] [Google Scholar]