Abstract
Background
Nucleoside analog GS‐441524 is effective in treating cats with feline infectious peritonitis (FIP). Investigation into the use of parent nucleotide analog remdesivir (GS‐5734) is needed.
Objectives
To assess efficacy and tolerability of remdesivir with or without transition to GS‐441524 in cats with FIP and document clinical and clinicopathologic progression over 6 months.
Animals
Twenty‐eight client‐owned cats with FIP.
Methods
Cats were prospectively recruited between May 2021 and May 2022. An induction dosage of remdesivir 10 to 15 mg/kg intravenously or subcutaneously q24h was utilized for 4 doses, with a maintenance dosage of remdesivir (6‐15 mg/kg SC) or GS‐441524 (10‐15 mg/kg per os) every 24 hours continued for at least 84 days. Laboratory testing, veterinary, and owner assessments were recorded.
Results
Twenty‐four cats survived to 6 months (86%). Three cats died within 48 hours. Excluding these, survival from 48 hours to 6 months was 96% (24/25). Remission was achieved by day 84 in 56% (14/25). Three cats required secondary treatment for re‐emergent FIP. Remission was achieved in all 3 after higher dosing (15‐20 mg/kg). Adverse reactions were occasional site discomfort and skin irritation with remdesivir injection. Markers of treatment success included resolution of pyrexia, effusions, and presenting signs of FIP in the first half of treatment and normalization of globulin concentration, and continued body weight gains in the latter half of the treatment period.
Conclusions and Clinical Importance
Parenteral administration of remdesivir and oral administration of GS‐441524 are effective and well‐tolerated treatments for FIP. Early emphasis on clinical, and later emphasis on clinicopathologic response, appears prudent when monitoring treatment efficacy.
Keywords: antiviral, clinical trial, feline coronavirus, nucleoside analog
Abbreviations
- AG
albumin to globulin ratio (albumin:globulin)
- ALKP
alkaline phosphatase
- ALT
alanine transaminase
- AST
aspartate transaminase
- BSH
British shorthair
- BW
body weight
- CK
creatine kinase
- DIF
direct immunofluorescence
- DSH
domestic shorthair
- DMH
domestic medium hair
- EDTA
ethylenediamine tetra‐acetic acid
- FeCV
feline enteric coronavirus
- FCoV
feline coronavirus
- FIP
feline infectious peritonitis
- GGT
gamma‐glutamyl transferase
- IHC
immunohistochemistry
- IV
intravenous
- LH
lithium heparin
- PO
per os
- QoL
quality of life
- qRT‐PCR
quantitative reverse transcriptase polymerase chain reaction
- RI
reference interval
- SC
subcutaneous
- TBil
total bilirubin
- total T4
total thyroxine concentration
1. INTRODUCTION
Remission and long‐term survival occur in cats with FIP after administration of the adenosine nucleoside analog GS‐441524. 1 , 2 Additional studies further corroborate the value of GS‐441524 in effecting a clinical cure for effusive, noneffusive, ocular, and neurologic forms of FIP when dosage is sufficiently high. 3 , 4 , 5 Remdesivir (GS‐5734) is a prodrug of GS‐441524. 6 , 7 Knowledge in several species including mice, rhesus monkeys, and humans suggests remdesivir undergoes rapid metabolic conversion in vivo, with GS‐441524 the major circulating metabolite. 7 , 8 , 9 GS‐441524 undergoes intracellular phosphorylation to form a nucleoside monophosphate and subsequently, the active triphosphate (GS‐443902), which disrupts viral replication by mimicking endogenous adenine as it is incorporated into viral RNA. 10 , 11 Absence of veterinary licensing and progression to commercialization of GS‐441524 has seen the emergence of an unlicensed drug market. Purportedly, thousands of cats have undergone treatment globally, with the majority successfully achieving remission and likely cure, however, importation of unregistered compounds for veterinary use remains illegal in many parts of the world. 4 , 12
In November 2020, Australian veterinarians gained legal access to a compounded, injectable formulation of remdesivir (100 mg vials) from a veterinary compounding pharmacy (BOVA Aus, Australia). In late 2021, a 50 mg tablet formulation of GS‐441524 became available from the same compounder. No safety data or pharmacokinetic studies were available for the use of remdesivir in cats, however, considering successful use of GS‐441524, and with no registered alternative, Australian veterinarians began to utilize compounded remdesivir administered parenterally and later GS‐441524 administered orally, for cats with FIP. Extrapolated data from other species was used to estimate a starting dosage 13 , 14 and the administration regimen evolved with clinical experience.
The aim of this prospective case‐series was to document survival and describe the clinical course of cats with FIP treated with compounded remdesivir administered parenterally as monotherapy, with or without transition to compounded GS‐441524 administered orally. Because of the global significance of these treatment options and need for peer‐reviewed studies, this investigation records clinicopathologic changes over 6 months, with monitoring beyond 12 months ongoing.
2. METHODS
2.1. Study cohort, inclusion criteria, and case recruitment
Client‐owned cats with a convincing diagnosis of FIP were recruited for this non‐randomized prospective case series. There was no untreated cohort, as it was deemed unethical to withhold treatment from infected cats given the availability of effective treatment from legal sources. Glucocorticoid treatment was gradually discontinued within 1 to 2 weeks where applicable. Use of other antiviral or immunomodulatory drugs precluded enrolment.
A convincing FIP diagnosis required supportive clinical signs, combined with a positive result from 1 or more of the following 4 tests, with tests 1 to 3 requiring detection of FCoV antigen within macrophages located in tissues, aspirates, or body cavity effusions, as described in Worthing et al (2012).
(a) Immunocytochemistry by direct immunofluorescence (DIF) on effusions with total protein >35 g/L and low cellularity (<5000 cell/μL) of predominantly macrophages and neutrophils. (b) Immunocytochemistry by DIF on aspirates from lesions with pyogranulomatous inflammation on cytology. (c) Immunohistochemistry (IHC) on tissue biopsies with corresponding supportive histopathological changes. (d) Detection of FCoV ribonucleic acid via quantitative reverse transcriptase polymerase chain reaction (qRT‐PCR) on effusions only. 15
FCoV qRT‐PCR on blood, tissue biopsy, or aspirates was not accepted because of the possibility of false positives with avirulent feline enteric coronavirus (FeCV), as transient viremia and detection of FeCV occurs within sentinel organs of healthy cats (with so‐called systemic FeCV infection). 16 , 17 , 18 FIP was classified as effusive or non‐effusive, with or without ocular involvement, and with or without neurologic involvement. Cats were tested on enrolment using Anigen Rapid FIV Ab/FeLV Ag test kits (BioNote Inc, Life Bioscience, Australia), although feline retroviral positivity was only to be noted and was not an exclusion criteria.
Cat owners were required to pay for medications and veterinary services. Monitoring comprising regular, extensive laboratory testing and capture of subjective and objective veterinary and owner assessments throughout the treatment period (minimum 84 days) and continued out to 12 months, was provided at no charge (Figure 1).
FIGURE 1.

Treatment and monitoring protocol for cats with FIP receiving parenteral administered remdesivir with or without transition to GS‐441524 administered orally.
Case recruitment came via an addendum to the laboratory reporting of FIP cases confirmed by DIF and IHC provided by Veterinary Pathology Diagnostic Services (Sydney School of Veterinary Science, The University of Sydney, Australia), currently the only laboratory in Australia offering these tests commercially. The addendum was also applied to FCoV RT‐qPCR results for effusions via an external laboratory (IDEXX Laboratories Pty Ltd, Australia). Recruitment also followed conference presentations, webinars, podcasts, social media platforms, and an article in The Veterinarian. Cats remained in the care of their owners and primary care veterinarians. This study was approved by The University of Sydney Animal Ethics Committee (2021/1874).
2.2. Treatment protocols
Compounded remdesivir and GS‐441524 for parenteral or oral administration, respectively, were obtained by primary care veterinarians via prescription from Bova Aus.
Treatment was continued for a minimum of 84 days and at least 2 weeks beyond achievement of remission. Remission was defined as (a) body condition score of ≥5/9, 19 (b) normothermia, (c) complete resolution of presenting signs of FIP, (d) normalization of the following analytes to within the relevant reference intervals: plasma total bilirubin concentration, plasma globulin concentration (with an albumin:globulin [AG] ratio of ≥0.6), neutrophil, and lymphocyte counts, (d) quality of life (QoL) score equating to a score ≥44/55 (average of 4/5 or higher for questions 1 to 11 in the owner assessment questionnaire [Appendix 2]).
Protocol 1 (Remdesivir‐lo): The original treatment protocol comprised 10 mg/kg remdesivir q24h administered as a slow (10‐15 minutes) intravenous (IV) infusion on days 0, 1, 2, and 3 (induction phase). The maintenance phase used 6 mg/kg administered subcutaneously (SC) q24h for a minimum of 84 days total treatment or extended at least 2 weeks beyond achievement of remission (as defined below). If ocular or neurologic manifestations were apparent, a higher maintenance dosage of 10 mg/kg was utilized. 3
Protocol 2 (Remdesivir‐hi): In response to the suboptimal durability of remission achieved in some of the initial cases, dosages were adjusted upwards in a revised protocol approved by The University of Sydney Animal Ethics Committee (2021/1874) and outlined in Table 1.
TABLE 1.
Treatment protocol “Remdesivir‐hi.”
| FIP presentation | Induction dose of remdesivir | Maintenance dose of remdesivir |
|---|---|---|
| Effusive | 10 mg/kg q24h IV or SC for 4 days | 8‐10 mg/kg SC q24h out to 84 days |
| Non‐effusive | 15 mg/kg q24h IV or SC for 4 days | 10‐12 mg/kg SC q24h out to 84 days |
| Neurologic and/or ocular | 15 mg/kg q24h SC IV or SC for 4 days | 12‐15 mg/kg SC q24h out to 84 days |
Protocol 3 (Remdesivir/GS‐441524): After the release of compounded GS‐441524 50 mg tablets in late 2021 (half‐way through case recruitment), if continued treatment with remdesivir was threatened by adverse responses to daily injections, veterinarians could elect to transition cats to orally administered GS‐441524 at the same dosage but rounded up to the nearest half tablet (25 mg). Tablets were administered in the starved state, followed by 3 to 5 mL of water or a tablespoon of wet food, to ensure passage to the stomach. A meal was offered 30 minutes later. Where oral GS‐441524 dosages were increased to ≥20 mg/kg, total daily dosage was given divided q12h.
For all protocols, it was emphasized to attending veterinarians that dosages be promptly increased to comply with the maintenance dosage (in mg/kg) with BW gain. Veterinarians were also advised not to reduce the dosage administered should cats experience BW reductions. Ancillary medications including appetite stimulants and anti‐emetics were permitted. Use of gabapentin PO and transmucosal buprenorphine were supported in the case of injection site discomfort.
If clinical or clinicopathologic abnormalities persisted, or worsened, during treatment (eg, persistent hyperglobulinemia or development of new or recurrent signs such as uveitis), or cats showed re‐emergent signs consistent with FIP relapse after completion of their primary course of treatment, a dosage increase of 5 mg/kg was recommended for cats receiving either remdesivir or GS‐441524. For cats requiring a second course of treatment, a full 84‐day course was prescribed at a dosage 5 mg/kg higher than their initial course.
2.3. Case management
2.3.1. Clinical assessments
Cats underwent physical examinations weekly for 4 weeks, every 2 weeks to treatment completion, then every 1 or 2 months, out to 12 months (Figure 1). Where available to the treating veterinarian, serial abdominal, and thoracic‐focused assessment with sonography for trauma, triage, and tracking (AFAST and TFAST) was also performed. Online questionnaires via REDCap (Appendices 1 and 2), were utilized to capture owner‐perceived general health and QoL impression (modified Karnofsky score approach), and veterinary‐reported examination findings, adverse events, and drug and sample collection details.
2.3.2. Sample collection and analyses
At each examination (Figure 1), 3.9 mL blood was collected into 1.3 mL plain, lithium heparin (LH) and ethylenediamine tetra‐acetic acid (EDTA) tubes, and fresh blood smears were prepared. Urine (3‐5 mL) was obtained (voided or cystocentesis) and body‐cavity centesis performed, if applicable. Samples were transported on ice to Veterinary Pathology Diagnostic Services (Sydney School of Veterinary Science, The University of Sydney, Australia), for analysis and storage.
A complete blood count was performed using a Sysmex XN‐10 automated hematology analyzer (Sysmex America Inc, Lincolnshire), with RapidDiff‐stained manual blood smear read by a clinical pathology technician. A biochemistry panel consisting of CK, ALP, ALT, AST, GGT, triglycerides, total protein (Biuret method), albumin, globulin, cholesterol (total), glucose, total bilirubin, creatinine, urea, calcium (uncorrected), inorganic phosphate, sodium, potassium, chloride, and total thyroxine was performed using a Konelab 30i (Thermo Fisher Scientific Australia Pty Ltd, Australia) analyzer. Serum was submitted to an external laboratory (IDEXX Laboratories Pty Ltd, Australia) for symmetric dimethylarginine measurement. After centrifugation at 12 000g for 10 minutes, the remaining EDTA cell pellet and plasma, LH plasma, and serum were stored at −80°C. Urine‐specific gravity was measured using a standard human optical refractometer. When present, sequential body cavity effusions were submitted for DIF, including qualitative cytologic evaluation and protein analysis (total protein, albumin, globulin, AG ratio via Biuret method) and qRT‐PCR. On occasion, because of courier issues related to the COVID‐19 pandemic, external laboratories (IDEXX Laboratories Pty Ltd, ASAP Laboratory Ltd, Australia) and in‐house analyzer hematology and biochemical profiles were utilized to ensure timely analysis. Where ultrasonographic or radiographic signs of disease were present, clinicians were encouraged to repeat diagnostic imaging toward the end of the course of treatment.
2.3.3. Statistical analysis
All statistical analyses were conducted in R Studio (v2022.07.1) using R (v 4.2.1) and figures were produced using the package ggplot2, by statistician and author Hall. 20 , 21 The primary outcome was to determine survival rates to 6 months, with the secondary outcome of documenting the clinicopathologic progression in recruited cats. Descriptive statistics were obtained for survival, analytes by week of treatment, days to normalization, and length of treatment. To obtain predicted means for analytes over time, a linear mixed model was used with a fixed effect of Time and random effect of cat. Before analysis, data were assessed for normality using the Shapiro‐Wilk test, and variables not meeting normality requirements were loge transformed to approximate normality.
3. RESULTS
3.1. Study cohort
Twenty‐eight cats with a convincing diagnosis of FIP (Table 2) were recruited between May 2021 and May 2022. Of these, 18 (64%) were pedigree breeds comprising British shorthair (BSH; 5 cats), Burmese (4), Cornish rex (2), Russian blue (2), Ragdoll (1), Exotic shorthair (1), Maine coon (1), Australian mist (1), Tonkinese (1), and 10 (36%) were domestic crossbreds; 8 domestic shorthair (DSH), 1 domestic medium hair (DMH) and 1 DSH cross Scottish shorthair. Of the 28 cats, 22 (78%) were castrated males, 5 (18%) were spayed females, and 1 (4%) entire female. The majority (23/28; 82%) of cats were aged <1 year at diagnosis, median 7.5 months; (IQR 6‐12 months, range 3‐164 months). Fifty‐four percent (15/28) of cases were enrolled in the 2 months of May/June (Fall/Winter) and 36% (10/28) in September/October (Spring). All cats were feline immunodeficiency virus and feline leukemia virus negative.
TABLE 2.
Number of cats with effusive and non‐effusive FIP that tested positive to each confirmatory test.
| Confirmatory test | n | |
|---|---|---|
| Effusive FIP (n = 23) | ||
| DIF positive/PCR positive | 11 | |
| DIF positive/PCR negative | 5 | |
| DIF positive/PCR not performed | 1 | |
| PCR positive (DIF negative) | 6 | |
| Non‐effusive FIP (n = 5) | ||
| IHC positive (tissue) | 3 | |
| DIF positive (aspirates) | 2 | |
Abbreviations: DIF, direct immunofluorescence; FCoV qRT‐PCR, feline coronavirus quantitative reverse transcriptase polymerase chain reaction; IHC, immunohistochemistry.
3.2. Clinicopathologic abnormalities on initiation of treatment
Twenty‐three cats had effusive and 5 had non‐effusive presentations. One cat with effusive disease had concurrent ocular involvement (anterior uveitis). No cats had signs of neurologic disease. Of the 23 effusive FIP cases, 16 (69%) had purely peritoneal effusions, 5 (22%) had pleural effusions, and 2 (9%) had bicavitary effusions. Inappetence (96%) and reduced BW (89%) were the most reported clinical findings, with the remaining presenting clinical abnormalities presented in Table 3 in descending order of prevalence. Five of the 28 cats (18%) were obtunded at time of treatment commencement, 22 were quiet, alert, and responsive (78%) and 1 was bright, alert, and responsive (4%).
TABLE 3.
Clinical signs observed at time of diagnosis in 28 cats with FIP.
| Clinical abnormality | Number of cats | % |
|---|---|---|
| Inappetence | 27 | 96 |
| Low body condition score | 25 | 89 |
| Ascites | 18 | 64 |
| Pyrexia | 12 | 43 |
| Icterus | 10 | 36 |
| Labored breathing | 9 | 32 |
| Palpable abdominal organ enlargement | 7 | 25 |
| Mesenteric lymph node enlargement | 8 | 27 |
| Tachycardia | 4 | 14 |
| Palpably abnormal kidneys (renomegaly) | 3 | 11 |
| Uveitis | 1 | 4 |
| Ileocecocolic mural mass | 1 | 4 |
| Systolic cardiac murmur | 1 | 4 |
| Arrhythmia | 1 | 4 |
Hypoalbuminemia and hyperbilirubinemia were the most common biochemical abnormalities, present in 93% and 79% of cases, respectively. The remaining presenting clinicopathologic abnormalities are presented in Table 4 in descending order of prevalence. All cats had an AG ratio of ≤0.6 on day 0 of treatment, with a median of 0.4 (IQR 0.3‐0.5).
TABLE 4.
Clinicopathologic abnormalities present at time of diagnosis of 28 cats with FIP.
| Clinicopathologic abnormality | Number of cats | % |
|---|---|---|
| Hypoalbuminemia | 26 | 93 |
| Hyperbilirubinemia | 22 | 79 |
| Anemia | 17 | 61 |
| Hyperglobulinemia | 16 | 57 |
| Neutrophilia | 14 | 50 |
| Increases in serum ALT activities | 11 | 39 |
| Lymphopenia | 9 | 32 |
| Leucocytosis | 6 | 21 |
| Hyperproteinemia | 4 | 14 |
| Hypoproteinemia | 3 | 11 |
| Lymphocytosis | 1 | 3 |
3.3. Antiviral treatment regimens
Remdesivir administered parenterally was the sole treatment in 15/28 cats (54%); of these, 11 received Remdesivir‐lo and 4 received Remdesivir‐hi. The remaining 13/28 (46%) cats received Remdesivir/GS‐441524. Median duration of remdesivir administrated parenterally before transition to GS‐441524 administered orally was 20 days (range 5‐160 days). This transition was elected when injection site discomfort became problematic.
3.4. Survival outcomes to 6 months after initiation of treatment
Twenty‐four of 28 cats (86%) survived to 6 months, including 3 that were obtunded at diagnosis. Two cats that presented in moribund states and received Remdesivir‐hi but died within 48 hours of commencing treatment. Another cat receiving Remdesivir‐lo developed a urethral obstruction and owners elected euthanasia on day 2 of treatment (Figure 2). All 3 had effusive FIP. These cats are not included in further discussion of the response to treatment.
FIGURE 2.

Duration of treatment and protocol received in 28 cats with FIP treated with parenteral remdesivir with or without transition onto oral GS‐441524.
Of the 25 cats that survived the initial 48 hours, 24 lived to 6 months, a survival rate of 96% (26/27). A total of 15/25 cats (60%) that survived beyond the first 48 hours met the criteria for clinical remission and completed treatment by day 84 (Figure 2), except for 1 cat that received an additional 6 days of treatment because of an owner communication error. The primary treatment course was extended in 10 of 25 cats (40%), with 5 of these also requiring dosage increases. Three cats were still receiving treatment at the 6‐month time point (Figure 2). Three of the 25 cats (12%) required a secondary course of treatment, when signs referable to FIP (pyrexia, lethargy, and inappetence) recurred between 2 and 13 days after discontinuing treatment (Figure 2); all had received Remdesivir‐lo. All 3 cats commenced a second course of treatment using a dosage 5 mg/kg higher than their primary course, and all achieved remission states just beyond the 6‐month time point (2 received BOVA products for 84 days, 1 received unregistered tablet formulations of GS‐441524 for 75 days).
3.5. Recurrence rates and dosage compliance
The recurrence rate to 6 months for cats that achieved remission whilst receiving Remdesivir‐lo was 30% (3/10), compared with no recurrence for the Remdesivir‐hi (n = 2) and Remdesivir/GS‐441524 (n = 13) groups. Transient inadvertent dosage reductions below 6 mg/kg (5.26‐5.99 mg/kg) occurred in 8/10 cats receiving Remdesivir‐lo because of BW increases between examinations. Of these 8 cats, 1 (13%) experienced indicators of clinical relapse within their primary course of treatment and 3 (37%) relapsed after completion.
These findings prompted treatment protocol revisions and the creation of Remdesivir‐hi and Remdesivir/GS‐441524 protocols. No recurrence of FIP in cats receiving these revised protocols was recorded for 6 months. The stipulation to round up to the nearest half tablet of oral GS‐441524 in Remdesivir/GS‐441524 reduced the occurrence of inadvertent dosage reductions because of BW gains between examinations. This resulted in transiently higher dosages being administered as cats grew into each half‐tablet dosage increase (20.3‐28.1 mg/kg).
3.6. Clinicopathologic findings during the first 12 weeks of treatment
Marked and rapid clinical improvements were observed in all 25 cats that completed 12 weeks of treatment. Pyrexia resolved in a median of 7 days (IQR 3.5‐7; Figure 3). For the 23 effusive cases, effusion resolved within a median of 9.5 days (IQR 6.25‐17.25; Figure 4), commonly coinciding with a transient reduction in BW, reduction in PCV, and increase in plasma globulin concentration (Figure 4). This effect typically peaked by day 14 of treatment. Cats had gained an average of 30% of their day 0 BW at the time of treatment completion and owners reported a rapid improvement in QoL within the first 2 weeks of treatment (Figure 5). Six cats had sufficient residual effusion to allow repeat centesis with paired DIF and FCoV qRT‐PCR. Four cats were negative to both tests at day 7, 1 was negative to both tests at day 21, with the final cat remaining positive for both tests at day 7, with subsequent resolution of effusion by day 14.
FIGURE 3.

Days to normalization for key clinical and clinicopathologic parameters in 25 cats with FIP that survived to complete 84 days of treatment, ordered from the most to least rapid to resolve. Bars indicate average days to normalization for an individual variable. Bar‐lines indicate the SE.
FIGURE 4.

Trend observed in the 20 effusive FIP cases that survived to complete 84 days of treatment using remdesivir administered parenterally with and without a transition onto GS‐441524 PO. The dashed vertical line indicates the average time of effusion resolution. × symbolled line represents the mean globulin concentration by week of treatment expressed in g/L. • symbolled line represents the mean percent of BW change from day 0 by week of treatment. Δ symbolled line represents the mean PCV by week of treatment expressed as L/L. Gray‐shaded areas represent the SE for each variable.
FIGURE 5.
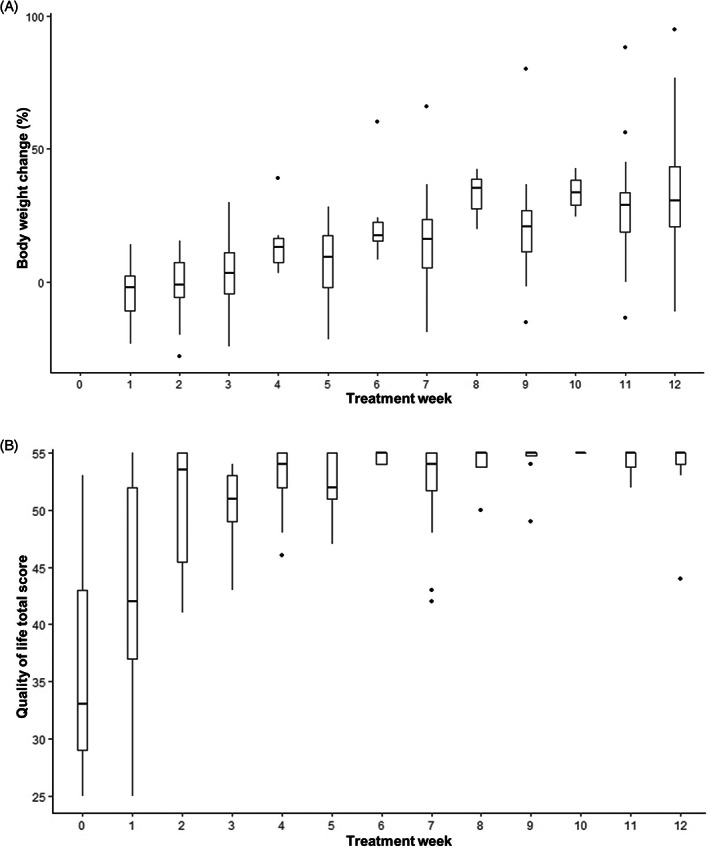
(A) Percentage of BW change relative to day 0, by week of treatment for 25 cats with FIP receiving remdesivir with or without a transition to oral GS‐441524. (B) Quality of life score by week of treatment for 25 cats with FIP receiving remdesivir with or without a transition to oral GS‐441524 (modified Karnofsky score, where the highest possible score is 55). For both (A) and (B) boxes depict the median (horizontal line) and interquartile range, whiskers representing the range, dots representing outliers.
Where present, mesenteric lymphadenomegaly (8 cats), renomegaly (3 cats), and ileocecocolic mass effect (1 cat) resolved palpably and ultrasonographically by 6 weeks, except 2 cats that had residual mild mesenteric lymphadenopathy (width 5.4‐6.3 mm). Anterior uveitis (1 cat) present at diagnosis, resolved by week 4.
Hyperbilirubinemia, hyperproteinemia, and leucocyte abnormalities normalized within 2 to 3 weeks (median 14 days, IQR 7‐24; median 21 days, IQR 11‐28 and median 9 days, IQR 7‐30, respectively; Figure 3). Hyperglobulinemia was slower to normalize, taking a median of 35 days (IQR 23.5‐42.3). Plasma albumin concentration and PCV took longer to improve and in some instances remained slightly below their reference intervals at treatment completion. The AG ratio gradually rose from week 2 of treatment, normalizing above 0.6 between weeks 7 and 9 (median 49 days, IQR 35‐63 days; Figure 3).
For the 9/25 cats (36%) that had serum ALT activity above the reference interval at diagnosis, resolution occurred in 5 cats over the course of treatment. Four cats had persistent mildly high ALT activity after completion of treatment. Of the 16 cats (64%) with serum ALT activity within the reference interval (RI) at the time of diagnosis, 8 cats (50%) subsequently had a serum ALT activity in excess of 128 U/L (>2 × upper extent of RI), median 175 U/L; IQR 128‐266 (RI 4‐64 U/L) at 1 or more time points throughout the treatment, although these resolved during treatment or once remission was achieved.
3.7. Further observations
Eosinophilia was observed in 13/25 cats (52%) that completed 12 weeks of treatment. Of these, 6 received Remdesivir‐lo and 7 received Remdesivir/GS‐441524. For 10 cats this was a single occurrence during treatment and for 1 cat this occurred after treatment ceased. The median time until the first documentation of eosinophilia was 63 days (IQR 37‐89). Eosinophilia was typically mild to moderate (median 2.66 × 109/L; IQR 2.2‐3.0; RI 0.04‐1.90).
During the primary course of treatment, 4 cats developed comorbid conditions (including upper respiratory tract signs and dermatophytosis) requiring specific adjunctive treatment. Two cats underwent surgical procedures (including ovariohysterectomy and enucleation). FIP treatment was continued throughout with no perceived decrease in efficacy or adverse effects.
3.8. Adverse drug reactions
Of the 25 cats that survived 12 weeks of treatment, 2 cats (8%) had extreme adverse reactions because of perceived stinging after subcutaneous administration of remdesivir. This includes aggressive and aversive behavior toward owners and veterinary staff, ultimately prohibiting continued injectable treatment. Mild injection site discomfort was reported in 13/25 (52%) of cats and mild localized injection site reactions observed in 5/25 (20%), characterized by localized thickening of the dermis, mild pruritus with or without focal alopecia. All resolved on completion of injections. No adverse effects were observed in cats receiving GS‐441524 tablets.
4. DISCUSSION
FIP was historically recognized as an invariably fatal disease. This study demonstrated that compounded formulations of injectable remdesivir and GS‐441524 tablets were well tolerated and effective in producing remission states in cats with naturally occurring FIP, with an 86% survival to 6 months, increasing to 96% survival if they are alive after the first 48 hours of treatment.
These high remission rates are consistent with existing reports documenting therapeutic efficacy using GS‐441524 by injection or orally for the treatment of FIP. Subcutaneous administration of GS‐441524 achieves survival rates of 77% beyond 6 months in cats with naturally occurring FIP, excluding those with ocular and severe neurologic forms, in which blood‐brain and blood‐eye drug penetration are uncertain. 1 , 2 , 22 Owner‐reported outcomes for 393 cats receiving treatment for a presumptive diagnosis of FIP demonstrate a 96% survival rate, providing further evidence to support the use of GS‐441524. 4 This study has several limitations: only 54% of cats had reached 6 months post‐treatment at the time of reporting, owners used multiple brands of unregistered formulations of GS‐441524 (parenteral and oral), definitive diagnoses were not documented, and case recruitment is likely biased, with a positive outcome more likely to prompt owner participation. 4 Higher survival rates of 100% are also reported to 6 months in 18 cats using an unregistered oral complex formulation containing GS‐441524, although cats with severe disease were excluded because of perceived risks associated with oral administration and potentially reduced gastrointestinal drug absorption in moribund cats. 5 In contrast, parenteral, and especially intravenous administration negates the limitations of oral therapy.
This current study, therefore, did not exclude advanced disease states, as it was considered important to determine treatment efficacy across the full range of clinical presentations. A total of 5/28 cats (18%) in this study were obtunded or moribund at the time of treatment commencement, yet 3 achieved remission and survived beyond 6 months, suggesting that although cats with advanced disease might be at greater risk for death in the first 48 hours, remission can still be achieved and attempting treatment is worthwhile.
Rapid resolution of clinical signs associated with FIP was achieved in all cats that survived to 6 months, again consistent with existing reports utilizing parenteral and oral administration of GS‐441524. 1 , 2 , 5 Resolution of pyrexia and inappetence was achieved within 1 week, with improved QoL scores, resolution of icterus, effusions, and ophthalmic changes to follow within 2 to 4 weeks of starting treatment. In contrast, clinicopathologic derangements such as hyperglobulinemia, hypoalbuminemia, and anemia tended to resolve later during treatment. This might support a clinical decision to emphasize monitoring physical examination findings in the first half of treatment and hematologic and biochemical measurements in the latter half of treatment. Critically, the presence of hyperglobulinemia beyond the first 6 weeks of treatment should prompt a dosage increase, or consideration of the addition of a second antiviral agent (such as the protease inhibitor GC376 23 or molnupiravir 24 , 25 , 26 ) as treatment data grows and drug availability improves. Treatment extension should be considered if abnormalities referable for FIP remain beyond week 10 of treatment. The trend observed in effusive cases, whereby a BW drop, globulin concentration spike, and PCV drop coincides with effusion resorption at week 2, is another important observation of which clinicians should be aware, with similar trends previously documented. 2 In isolation, these findings could be erroneously interpreted as a negative response to treatment, however, if paired with positive clinical responses (resolution of pyrexia, improved appetite, and demeanor, resolution of effusions), this appears not to be the case. Given the voluminous and highly proteinaceous composition of FIP effusions, hyperglobulinemia and reduction in PCV might represent systemic protein resorption and transient hemodilution because of body cavity fluid shifts.
It is important to note that the protocols outlined in this study evolved over the course of the recruitment period and were not prospectively assigned in a randomized manner. Although differences in recurrence rates were observed (with a 30% likelihood of recurrence using Remdesivir‐lo, compared with zero recurrence with Remdesivir‐hi and Remdesivir/GS‐441524), determination of the statistical significance of these results would require much larger prospective, blind, randomized trials. Of the 8 cats receiving Remdesivir‐lo that experienced inadvertent dosage reductions (dropping below 6 mg/kg) because of BW increases, 4 (50%) went on to develop either re‐emergent signs of FIP during treatment or came out of remission. Although other factors might have influenced these outcomes, it appears prudent to utilize the higher dosages in Remdesivir‐hi and Remdesivir/GS‐441524, weighing cats every 2 weeks and adjusting dosages upwards to accommodate for the anticipated substantial BW increase (average 30%) during the 84‐day treatment period.
The observation that 3 cats (1 receiving Remdesivir‐lo, 2 receiving Remdesivir/GS‐441524) demonstrated signs consistent with clinical relapse of FIP during their initial treatment period might suggest either the emergence of antiviral resistance, variations in drug distribution, initial viral load, or virus strain. Further research is required to better characterize the mechanisms for such variations in clinical response and highlights the need for continued investigation into pharmacokinetics, pharmacodynamics, dosage optimization, and the potential role of combination drug therapy. Parenteral administration of remdesivir and oral administration of GS‐441524 were well tolerated. The transition to GS‐441524 tablets circumvented injection site discomfort (generally mild, though occasionally severe). Thirty‐eight percent of cats had mildly high serum ALT activity (typically less than a 2‐fold increase) at the time of diagnosis. Mechanisms to explain this finding might include FIP‐induced immune‐mediated inflammation, hepatic hypoxia or hypovolemia, and in some instances, hemolysis artifact. 27 Increase in serum ALT activity often resolved during treatment, or in some instances persisted beyond the point where treatment was discontinued (without FIP relapse), and therefore is unlikely to represent an adverse drug effect.
There was no obvious explanation for the eosinophilia documented in 56% of cats in this trial. Fecal examinations were not performed, thus co‐infection with parasitic species cannot be excluded but is considered unlikely, as anthelmintic prophylaxis was current. No dermatologic changes were observed, bar mild alopecia and skin thickening at the injection site in some, but not all, cats with eosinophilia. Krentz 5 also reports mild eosinophilia in 61% of cats receiving an oral formulation containing GS‐441524. It is of great interest that increased eosinophil counts are thought to hold positive prognostic value in human patients with COVID‐19. 28 , 29 , 30 Further investigation as to the implication of eosinophilia in cats receiving treatment with antiviral drugs is required.
At the time of writing, remdesivir and GS‐441524 for veterinary use is only legally available in a limited number of countries. Much of the world, however, remains without easy and timely legal access to both these drugs. As newer antivirals continue to show promise in FIP treatment, 25 legal access to drugs such as molnupiravir, EIDD 1931, and nirmatrelvir/ritonavir (Paxlovid) are likely to become more globally accessible. This study provides a robust framework to guide the expected clinicopathologic response during effective antiviral therapy using nucleoside and nucleotide analog for comparative purposes in future drug trials.
Various methodologies have been used for the detection of FCoV antigen within macrophages from cellular aspirates or effusions (immunocytochemistry) leading to variability in the reported specificity of such testing. 31 , 32 , 33 , 34 , 35 , 36 , 37 The technique used in the current study has 100% specificity and 75% sensitivity and is, therefore, a definitive test when the result is positive. 31 It is important for clinicians to be aware of variations in immunocytochemistry techniques and to be familiar with the specificity of the methodology employed by their local laboratory when interpreting positive results.
Limitations of this study included the lack of a control group (because of ethical reasons), the relatively small number of cases within the 3 treatment groups, and the need to adapt treatment protocols to optimize outcomes in this client‐owned cohort of cats. Full fluid analysis was not performed on every effusive case at the time of diagnosis because of limitations in the study design. This might have prohibited the detection of comorbid disease such as neoplasia or bacterial infection which might have affected outcomes in cats that died in the first 48 hours of treatment.
Compounded remdesivir administered parenterally as monotherapy or with a subsequent transition onto compounded GS‐441524 administered orally can be considered a well‐tolerated and effective treatment for naturally occurring FIP. Access to both parenteral and oral formulations is advantageous, allowing for treatment flexibility across the spectrum of disease states and patient temperaments. Regular monitoring of body weight and dosage adjustments are vital to ensure a sufficient dosage is being maintained. Dosage increases and treatment extensions beyond 84 days might be required, as directed by individual response. Repeat treatment might also be required in a minority of cases, although remission rates after repeat treatment were 100%. Further research is required to refine our understanding of FIP to optimize duration of treatment, accurate determination of remission, and markers for early relapse.
CONFLICT OF INTEREST DECLARATION
All studies were exclusively designed by the researchers with no input from external commercial or non‐commercial organizations. Sally J. Coggins has received paid honorariums to deliver educational webinars and lectures on FIP treatment developments in Australia from: The Australia and New Zealand College of Veterinary Scientists; Bova Au & UK “Bova Scholars Webinar Series”; and Centre for Veterinary Education, The University of Sydney. The content of these presentations has not been influenced by the aforementioned companies and organizations.
OFF‐LABEL ANTIMICROBIAL DECLARATION
This paper discusses the off‐label use of compounded antiviral formulations for the treatment of FIP in cats. Gilead Sciences, Inc. hold the patent for remdesivir and GS‐441524 and have declined registration for veterinary use. In Australia, compounded drugs are not classified as veterinary chemicals under the Agricultural and Veterinary Chemicals Code and are therefore exempt from regulations associated with registered drugs. Australian veterinarians are permitted to write a prescription to a registered pharmacist to compound a drug if there is not a registered veterinary product suitable for use.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by The University of Sydney Animal Ethics Committee Project number: 2021/1874.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENT
The authors acknowledge the funding support of EveryCat Health Foundation through grants W21‐006 and W21‐018, as well as the Cat Protection Society of New South Wales. This manuscript will form part of Sally Coggins doctoral thesis, which is supported by the Lionel Marcellus Lonsdale scholarship, The University of Sydney. Parts of this research have also been presented in oral abstract form at the Australia and New Zealand College of Veterinary Scientists Science Week conference in June 2022 and the International Society of Companion Animal Infectious Diseases Symposium in September 2022. The authors thank Riannon Apicella for data entry, and all the primary care staff involved in the management of enrolled cats. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Coggins SJ, Norris JM, Malik R, et al. Outcomes of treatment of cats with feline infectious peritonitis using parenterally administered remdesivir, with or without transition to orally administered GS‐441524. J Vet Intern Med. 2023;37(5):1772‐1783. doi: 10.1111/jvim.16803
[Correction added after first online publication on 20 July 2023. Placement of sub‐heading in Table 2 has been amended.]
REFERENCES
- 1. Murphy BG, Perron M, Murakami E, et al. The nucleoside analog GS‐441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet Microbiol. 2018;219:226‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pedersen NC, Perron M, Bannasch M, et al. Efficacy and safety of the nucleoside analog GS‐441524 for treatment of cats with naturally occurring feline infectious peritonitis. J Feline Med Surg. 2019;21:271‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dickinson PJ, Bannasch M, Thomasy SM, et al. Antiviral treatment using the adenosine nucleoside analogue GS‐441524 in cats with clinically diagnosed neurological feline infectious peritonitis. J Vet Intern Med. 2020;34:1587‐1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones S, Novicoff W, Nadeau J, Evans S. Unlicensed GS‐441524‐like antiviral therapy can be effective for at‐home treatment of feline infectious peritonitis. Animals. 2021;11:2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krentz D, Zenger K, Alberer M, et al. Curing cats with feline infectious peritonitis with an oral multi‐component drug containing GS‐441524. Viruses. 2021;13:2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amirian ES, Levy JK. Current knowledge about the antivirals remdesivir (GS‐5734) and GS‐441524 as therapeutic options for coronaviruses. One Health. 2020;9:100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilead Sciences Inc . VEKLURY Prescriber Information. Foster City: Gilead Sciences; 2022. [Google Scholar]
- 8. Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS‐5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Cao L, Li G, et al. Remdesivir metabolite GS‐441524 effectively inhibits SARS‐CoV‐2 infection in mouse models. J Med Chem. 2022;65:2785‐2793. [DOI] [PubMed] [Google Scholar]
- 10. Cho A, Saunders OL, Butler T, et al. Synthesis and antiviral activity of a series of 1′‐substituted 4‐aza‐7,9‐dideazaadenosine C‐nucleosides. Bioorg Med Chem Lett. 2012;22:2705‐2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sheahan TP, Sims AC, Graham RL, et al. Broad‐spectrum antiviral GS‐5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CZ/SK FW . Ongoing statistical analysis of GS‐441524 treated cats in The Czech Republic and Slovakia. 2022.
- 13. Hughes D, Howard G, Malik R. Treatment of FIP in cats with remdesivir. Veterinarian. 2021;19‐27. [Google Scholar]
- 14. Malik R. Treatment of FIP in cats with subcutaneous remdesivir followed by oral GS‐441524 tablets. Veterinarian. 2022:19‐27. [Google Scholar]
- 15. Longstaff L, Porter E, Crossley VJ, Hayhow SE, Helps CR, Tasker S. Feline coronavirus quantitative reverse transcriptase polymerase chain reaction on effusion samples in cats with and without feline infectious peritonitis. J Feline Med Surg. 2017;19:240‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gunn‐Moore DA, Gruffydd‐Jones TJ, Harbour DA. Detection of feline coronaviruses by culture and reverse transcriptase‐polymerase chain reaction of blood samples from healthy cats and cats with clinical feline infectious peritonitis. Vet Microbiol. 1998;62:193‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kipar A, Baptiste K, Barth A, Reinacher M. Natural FCoV infection: cats with FIP exhibit significantly higher viral loads than healthy infected cats. J Feline Med Surg. 2006;8:69‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Porter E, Tasker S, Day MJ, et al. Amino acid changes in the spike protein of feline coronavirus correlate with systemic spread of virus from the intestine and not with feline infectious peritonitis. Vet Res. 2014;45:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Association TWSAV . WSAVA Body Condition Score. 2020.
- 20. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer‐Verlag; 2009. [Google Scholar]
- 21. R Core Team . R: A Language and Environment for Statistical computing. Vienna: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 22. Kim Y, Liu H, Galasiti Kankanamalage AC, et al. Reversal of the progression of fatal coronavirus infection in cats by a broad‐spectrum coronavirus protease inhibitor. PLoS Pathog. 2016;12:e1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lv J, Bai Y, Wang Y, Yang L, Jin Y, Dong J. Effect of GS‐441524 in combination with the 3C‐like protease inhibitor GC376 on the treatment of naturally transmitted feline infectious peritonitis. Front Vet Sci. 2022;9:1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malik R. A key role for molnupiravir in the management of cats with FIP in Australia. Veterinarian. 2022;21:17‐21. [Google Scholar]
- 25. Roy M, Jacque N, Novicoff W, Li E, Negash R, Evans SJM. Unlicensed Molnupiravir is an effective rescue treatment following failure of unlicensed GS‐441524‐like therapy for cats with suspected feline infectious peritonitis. Pathogens. 2022;11:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cook S, Wittenburg L, Yan VC, et al. An optimized bioassay for screening combined anticoronaviral compounds for efficacy against feline infectious peritonitis virus with pharmacokinetic analyses of GS‐441524, remdesivir, and molnupiravir in cats. Viruses. 2022;14:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stockham S, Scott M. Chapter 12: Enzymes. In: Scott MA, Stockham SL, eds. Fundamentals of Veterinary Clinical Pathology. 2nd ed. Iowa: Blackwell Publishing; 2008:639‐674. [Google Scholar]
- 28. Mateos González M, Sierra Gonzalo E, Casado Lopez I, et al. The prognostic value of eosinophil recovery in COVID‐19: a multicentre, retrospective cohort study on patients hospitalised in Spanish Hospitals. J Clin Med. 2021;10:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nair AP, Soliman A, Al Masalamani MA, et al. Clinical outcome of eosinophilia in patients with COVID‐19: a controlled study: eosinophilia and COVID‐19. Acta Biomed Ateneo Parm. 2020;91:e2020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fraissé M, Logre E, Mentec H, Cally R, Plantefève G, Contou D. Eosinophilia in critically ill COVID‐19 patients: a French monocenter retrospective study. Crit Care. 2020;24:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Worthing KA, Wigney DI, Dhand NK, et al. Risk factors for feline infectious peritonitis in Australian cats. J Feline Med Surg. 2012;14:405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parodi MC, Cammarata G, Paltrinieri S, Lavazza A, Ape F. Using direct immunofluorescence to detect coronaviruses in peritoneal in peritoneal and pleural effusions. J Small Anim Pract. 1993;34:609‐613. [Google Scholar]
- 33. Hirschberger J, Hartmann K, Wilhelm N, Frost J, Lutz H, Kraft W. Clinical symptoms and diagnosis of feline infectious peritonitis. Tierarztl Prax. 1995;23:92‐99. [PubMed] [Google Scholar]
- 34. Paltrinieri S, Parodi MC, Cammarata G. In vivo diagnosis of feline infectious peritonitis by comparison of protein content, cytology, and direct immunofluorescence test on peritoneal and pleural effusions. J Vet Diagn Invest. 1999;11:358‐361. [DOI] [PubMed] [Google Scholar]
- 35. Hartmann K, Binder C, Hirschberger J, et al. Comparison of different tests to diagnose feline infectious peritonitis. J Vet Intern Med. 2003;17:781‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Felten S, Matiasek K, Gruendl S, Sangl L, Wess G, Hartmann K. Investigation into the utility of an immunocytochemical assay in body cavity effusions for diagnosis of feline infectious peritonitis. J Feline Med Surg. 2017;19:410‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Litster AL, Pogranichniy R, Lin TL. Diagnostic utility of a direct immunofluorescence test to detect feline coronavirus antigen in macrophages in effusive feline infectious peritonitis. Vet J. 2013;198:362‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


