Abstract
Alzheimer’s disease (AD) is the most common cause of dementia that affects millions of predominantly elderly individuals worldwide. Despite intensive research over several decades, controversies still surround the etiology of AD and the disease remains incurable. Meanwhile, new molecular players of the central amyloid cascade hypothesis have emerged and among these is a protease known as β-site APP cleavage enzyme 2 (BACE2). Unlike BACE1, BACE2 cleaves the amyloid-β protein precursor within the Aβ domain that accordingly prevents the generation of Aβ42 peptides, the aggregation of which is commonly regarded as the toxic entity that drives neurodegeneration in AD. Given this non-amyloidogenic role of BACE2, it is attractive to position BACE2 as a therapeutic target for AD. Indeed, several groups including ours have demonstrated a neuroprotective role for BACE2 in AD. In this review, we discuss emerging evidence supporting the ability of BACE2 in mitigating AD-associated pathology in various experimental systems including human pluripotent stem cell-derived cerebral organoid disease models. Alongside this, we also provide an update on the identification of single nucleotide polymorphisms occurring in the BACE2 gene that are linked to increased risk and earlier disease onset in the general population. In particular, we highlight a recently identified point mutation on BACE2 that apparently leads to sporadic early-onset AD. We believe that a better understanding of the role of BACE2 in AD would provide new insights for the development of viable therapeutic strategies for individuals with dementia.
Keywords: Alzheimer’s disease, amyloid-β protein precursor secretase, beta-secretase, neuroprotection
INTRODUCTION
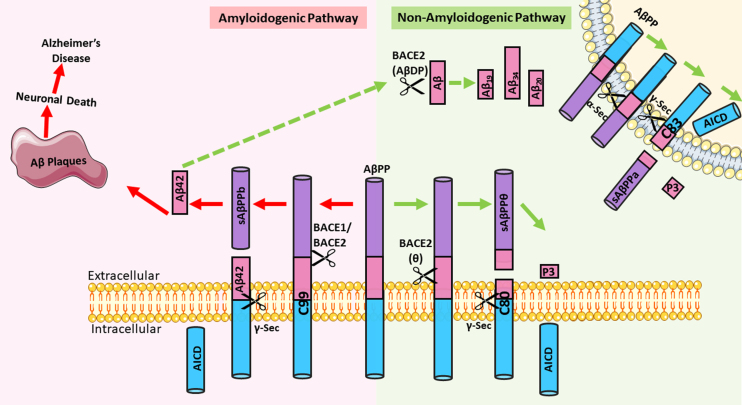
With the passing of every three seconds, someone in the world develops dementia [1]. This is a worrying trend of global concern as dementia remains incurable and is permanently incapacitating that consequently exacts high disease and socio-economic burden throughout the patient’s remaining lifetime. There are currently about 55 million people in the world afflicted with dementia [2], of which Alzheimer’s disease (AD) is the most common form, accounting for about 60% to 80% of dementia cases worldwide [3]. Like other dementia subtypes, AD is a progressively debilitating neurological disorder that is characterized by cognitive decline, memory deficits, and behavioral alterations. The pathological hallmarks of AD include abnormal accumulation of extracellular amyloid-β (Aβ) peptide aggregations (plaques), intracellular neurofibrillary tangles (NFTs) of hyperphosphorylated tau protein, and extensive neuronal loss in the brain parenchyma [4]. The link between these hallmarks to the etiopathogenesis of AD may be explained by the amyloid cascade hypothesis, which remains the most prominent hypothesis to date. The hypothesis states that Aβ aggregation triggers the cascade of events that ultimately lead to AD pathology and symptoms. It posits that the Aβ42 peptide produced via the successive cleavage of the amyloid-β protein precursor (AβPP) by β- and γ-secretases is more aggregate-prone as compared to other Aβ isoforms (Fig. 1). Consequently, these insoluble aggregates form amyloid plaques that trigger a cascade of events promoting the formation of NFTs as well as inflammation and oxidative stress that culminates to neuronal dysfunction and death [5–7]. Supporting this, mutations enhancing β-cleavage of AβPP or altering γ-cleavage of AβPP-C99 (C-terminal fragment generated by β-cleavage of AβPP) are known to be causative of early-onset AD. Furthermore, a study has revealed that a missense mutation in the AβPP suppresses Aβ production and reduces the risk of AD [8]. Notwithstanding the persuasiveness of the amyloid cascade hypothesis, it is tau pathology, rather than Aβ, that seems to correlate with progressive gray matter loss and cognitive impairment [9], which fuels the alternative hypothesis that tau pathology is the primary cause of neurodegeneration in AD. Regardless of the ensuing debate between the amyloid and tau hypotheses, it is generally accepted that both Aβ42 and hyperphosphorylated tau are the two key pathogenic players contributing to the pathogenesis of AD. Accordingly, a targeting strategy focusing on the clearance of both pathogenic entities would be useful, especially given the limited success of anti-amyloid treatments in sporadic AD.
Fig. 1.
Processing of amyloid-β protein precursor (AβPP) via the amyloidogenic or non-amyloidogenic pathway. In the amyloidogenic pathway, AβPP undergoes sequential cleavage first by BACE1 or BACE2 to generate a C99 fragment, which is then cleaved by γ-secretase to release Aβ42. This Aβ42 is aggregate prone and can form neurotoxic plaques that are implicated in the amyloid cascade hypothesis to cause Alzheimer’s disease. In the non-amyloidogenic pathway, Aβ42 can undergo further degradation by BACE2, which can also function as an Aβ-degrading protease (AβDP). The degradation products include non-toxic Aβ species Aβ19, Aβ20, and Aβ34. In addition, AβPP can be either cleaved by BACE2 at the θ-site, or by α-secretase to generate a C80 or C83 fragment, respectively. These C-terminal fragments undergo further cleavage by γ-secretase to release the AβPP intracellular domain (AICD) and a short fragment P3. In this case, no Aβ42 is generated. [Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)].
INSIGHTS FROM DOWN SYNDROME AND THE ENTRY OF BACE2 AS A POTENTIAL NEUROPROTECTIVE MODIFIER OF AD
The APP gene is found on human chromosome 21, and individuals born with Down syndrome (DS) attributed to Trisomy 21 are born with an extra copy of this gene, which enhances their propensity for developing AD. Consistent with this, non-DS individuals with a rare triplication of the APP gene due to the duplication of a single APP locus (DupAPP) develop early-onset AD with a 100% penetrance by the age of 60 [10, 11]. Technically, the same should be observed for DS individuals with APP gene triplication. However, the intriguing paradox is that only about 70% of individuals with DS develop AD by age 60, i.e., 30% of individuals with DS are spared of clinical signs of dementia. This suggests the attractive possibility that chromosome 21 might harbor potentially neuroprotective genetic modifiers of dementia [12]. One such modifier is BACE2, which like the APP gene, is also located on chromosome 21, i.e., an extra copy of the BACE2 gene is similarly found in individuals with DS [13].
The BACE2 gene is located within the DS Critical Region in 21q22.3 and encodes a type 1 transmembrane aspartyl protease spanning 518 amino acids long [14, 15]. BACE2 is a close homolog of BACE1 which shares about 64% amino acid sequence similarity [16]. While BACE1 is recognized to be the main β-secretase in the brain that participates in the amyloidogenic pathway by partnering with γ-secretase to release the Aβ peptide from AβPP [17], BACE2 can process AβPP via the amyloidogenic or non-amyloidogenic route (Fig. 1). In the latter route, BACE2 cleaves AβPP within the Aβ domain particularly at the θ-site (Phe20) to prevent Aβ42 generation [18, 19] (Fig. 1). Given this amyloidosis-protective role of BACE2, it is not surprising to note that several studies have reported a neuroprotective role for BACE2 in dementia [13, 18–22].
EXPRESSION OF BACE2 IN THE BRAIN
Although BACE2 is intimately involved in AβPP processing, its expression is not confined to the brain. Rather, BACE2 is ubiquitously expressed and found in many peripheral tissues such as the kidneys, pancreas, colon, placenta, prostate, trachea, and stomach. Intriguingly, reports regarding its expression in the brain have been conflicting. BACE2 expression was initially deemed to be low in the brain [14, 23, 24]. This being the case, the lower expression of BACE2 in the brain might render neurons more susceptible to Aβ accumulation as compared to other organs with higher BACE2 activity [25]. This would be consistent with its proposed neuroprotective role of BACE2. However, another study demonstrated considerable levels of BACE2 in the human brain [26], especially in the cortical layer and areas near blood vessels. Notably, BACE2 expression in subregions of the brain such as the ventromedial hypothalamus and mammillary body has also been documented [23]. At the cellular level, BACE2 is found to be expressed in both neurons and astrocytes [27], with higher BACE2 levels residing in the latter [25]. Other studies have shown that BACE2 is also expressed in spinal cord neurons [23], hippocampal neurons, and temporal cortical pyramidal neurons [28]. Thus, BACE2 expression is found in many regions of the central nervous system.
Interestingly, individuals diagnosed with mild cognitive impairment, preclinical AD, and AD exhibit an increase in the expression and enzymatic activities of BACE2 [27]. Importantly, in the AD brain, BACE2 protein levels inversely correlate with higher Braak stages [29], suggesting that low BACE2 levels portends increasing severity of AD. Although why BACE2 expression describes such a trend in the AD brain remains unclear, it is noteworthy that a recent report has identified RCAN1 (regulator of calcineurin 1) to be a regulator of BACE2 expression [30]. In their report, Qiu and colleagues demonstrated that RCAN1 promotes BACE2 expression by inhibiting its turnover by the proteasome [30]. Notably, RCAN1 is highly expressed in the human brain and its expression is further increased in aged and AD brains, which BACE2 expression mirrors. RCAN1 levels are reportedly upregulated by stress (cortisol), APOE4, and inflammation (NF-κB), all of which are associated with AD. Thus, the initial increased levels of BACE2 observed in AD brains may be at least in part explained by the positive effects of RCAN1 on its expression. However, whether there is a corresponding decrease in RCAN1 levels with higher Braak stages of AD remains to be characterized. Moreover, RCAN1 also promotes BACE1 expression [31], which makes it a challenging candidate to target to enhance BACE2 expression as a strategy to afford neuroprotection in AD.
AMYLOIDOGENIC AND NON-AMYLOIDOGENIC ACTIVITIES OF BACE2
Although this review is focused on the non-amyloidogenic role of BACE2, it is important for us to also highlight some studies that implicate an amyloidogenic role for BACE2. For example, Wang and colleagues have recently reported that artificial and AD-associated mutations that perturb the juxtamembrane helix of AβPP may—at a slight extent—trigger BACE2 to function as a conditional β-secretase to cleave at the same β-site as BACE1 [32]. BACE2 can also cleave at the β-site when clusterin, also known as the third most predominant genetic risk factor for late-onset AD [33, 34], binds to the juxtamembrane helix of AβPP [32]. Other studies have also hinted at the prospect of BACE2’s involvement in Aβ generation and AD by being a β-secretase. In one of these studies, cells co-transfected with AβPP bearing the Flemish mutation (which typically causes early-onset AD with cerebral amyloid angiopathy and large senile plaques [35]) and BACE2 resulted in an increase in C99 fragments that can possibly be cleaved by γ-secretase to yield the eponymous Aβ peptide [36]. Another study suggested that the triplication of BACE2 as a result of Trisomy 21 could contribute to AD in individuals with DS, as immunoreactivity of BACE2 was found to co-localize with NFTs in the brains of individuals with DS-AD but not in their counterparts without AD pathology [37]. Furthermore, the cleavage activity of BACE2 at the β-site was confirmed in mass spectrometric analyses that revealed identical cleavage fragments when BACE1 or BACE2 was incubated with a shorter AβPP peptide containing the β-site [36]. In addition, BACE2 overexpression increased the presence of soluble peptide AβPPb (sAβPPb) which is a BACE1 cleavage product [28]. It was assumed that an increase in sAβPPb would lead to a concomitant increase in Aβ levels. Unexpectedly, this increase in sAβPPb concentration is associated with diminished (rather than increased) Aβ levels [23] which is of a significant seven-fold decrease in magnitude [28]. This is probably due to the alternative non-amyloidogenic pathways by which BACE2 processes AβPP and Aβ, which will be elaborated below.
Two in vivo studies utilizing transgenic mice revealed that BACE2 might not play a role in the amyloidogenic pathway. Firstly, mice that overexpressed BACE2 revealed no alterations in the concentration of Aβ40 and Aβ42 levels in the hippocampus and cerebral cortex—areas that are implicated in AD. Moreover, there were no observable differences in cognitive dysfunction and cholinergic degeneration, which are typical hallmarks synonymous with people with AD [38]. Subsequently, the same group observed similar findings in double transgenic mice that overexpressed BACE2 and APP. This suggests that BACE2 overexpression is not involved in the amyloidogenic pathway and does not trigger cognitive dysfunction or cholinergic degeneration associated with AD [39]. Interestingly, the authors also hinted that BACE2 may have some protective roles in the double mutant mice, as its expression was increased in response to the transgenic co-expression of AβPP [39]. Besides the transgenic approach, BACE2 knockout mice have also been generated. Like its transgenic counterparts, BACE2 exon 6 knockout mice lacked any observable physical and behavioral changes, even though exon 6 comprises one of two active sites of BACE2. However, fibroblasts derived from these mice secreted more Aβ although this was not the case for BACE2 knockout-derived primary neurons [40]. Notwithstanding these findings, the potential protective role of BACE2 was further corroborated in several overexpression-based experiments involving transfected cell lines. Firstly, conditioned medium collected from SK-N-SH cells overexpressing BACE2, and not cells overexpressing BACE1, demonstrated a sevenfold decrease in Aβ40 levels [28]. Next, in HEK293T cells transfected with BACE2-expressing plasmids, BACE2 was more efficient at cleaving the product of β-secretase (AβPP β-CTF) than cleaving at the β-site. The cleavage of AβPP β-CTF occurs within the Aβ region after Phe-19 and Phe-20 to generate a non-neurotoxic Aβ19 fragment [36]. Subsequent studies reported similar observations regarding the strong affinity of BACE2 for the Phe-19 and Phe-20 θ-cleavage site [19, 20, 41, 42]. For this reason, BACE2 is widely regarded as a θ-secretase. In alignment with these studies, antisense oligomer-mediated knockdown of BACE1, not BACE2, decreased Aβ levels whereas the overexpression of BACE2 reduced Aβ peptide levels [41]. Consistent with this, HEK293 cells expressing BACE2 alone or double transfected with BACE2 and BACE1 generated less Aβ40/42 as compared to cells expressing BACE1 alone [20]. Furthermore, targeted RNAi-mediated inactivation of BACE2 in HEK293 cells transfected with wildtype APP695 or APP695sw double mutant increased the amount of secreted AβPP and Aβ [22]. Similarly, Sun and colleagues demonstrated that a cell line expressing BACE2 and Swedish mutant APP695 also considerably reduced Aβ production [43], with the same group recapitulating these findings in transgenic AD mice and identifying a novel BACE2 θ-cleavage site on AβPP to generate a non-amyloidogenic C80 fragment that is further processed by γ-secretase to yield a truncated Aβ42 peptide [19]. Notably, individuals harboring the Swedish mutation produce about 6–8 times more Aβ than normal individuals [44]. More recently, Huentelman and colleagues demonstrated that overexpressing BACE2 in BE(2)-M17 human neuroblastoma cells that normally express BACE2 at a low level led to decreased Aβ40 and Aβ42 concentrations in cell culture media [45]. Taken together, these various studies strongly support a non-amyloidogenic role of BACE2 that is protective against Aβ42-induced pathology.
Interestingly, BACE2 can also degrade Aβ itself. This was discovered in a functional screen of 352 proteases where BACE2 emerged as the top Aβ-lowering protease [18]. In this study, recombinant BACE2 degraded synthetic Aβ at an optimal pH of 3.5 at a rate of about 150 times faster than BACE1. Furthermore, BACE2 was found to cleave Aβ at three different sites: Phe19-Phe20, Phe20-Ala21, and Leu34-Met35, where the latter was identified to be the initial and main cleavage site. Cells overexpressing APP alone generated aggregation-prone Aβ species Aβ42, Aβ40, Aβ37, Aβ38 and Aβ39, whilst cells that co-expressed APP and BACE2 decreased the levels of the aforementioned Aβ species and produced non-AD related species Aβ19, Aβ20, and Aβ34 instead [18]. As an acidic pH is needed for BACE2 to exhibit its Aβ-degradation activity, it is likely to involve the lysosome. Indeed, Alic et al. found that the degradation of Aβ by BACE2 occurs in LAMP2-positive compartments of neurons as exemplified by the high degree of colocalization between BACE2, its substrate Aβ40, and degradation product Aβ34 with the components of the chaperone-mediated autophagy pathway [13]. BACE2 may also be degraded via the macroautophagy-lysosome pathway [46] and the ubiquitin-proteasome pathway [30]. Consistent with this, the inhibition of BACE2 degradation via lysosomal inhibitor treatment with chloroquine or NH4Cl in 4EB2 cells, which express both BACE2 and the human APP Swedish mutant, led to an increase in BACE2 levels and non-amyloidogenic C80 levels [46].
INSIGHTS FROM HUMAN BRAIN ORGANOID MODELS OF AD
Although the promising results above collectively suggest that BACE2 cleavage activity precludes the generation of aggregation prone Aβ species synonymous with AD, they still beg the question whether this phenomenon is mirrored in the human brain because these studies involve the artificial manipulation of the gene dose of BACE2 and were carried out in cultured cells or in rodents. Hence, the use of human cerebral organoids has started to gain traction as the new frontier of neurodevelopmental and disease modelling in a bid to address the inadequate translational potential of current preclinical models to better recapitulate the physiological context of the human brain.
Cerebral organoids are three-dimensional aggregates of cells that develop organized and distinct brain regions composed of various neuronal and non-neuronal subtypes of cells. This can be achieved by using the seminal method first described by Lancaster and colleagues [47, 48]. In this approach, pluripotent stem cells (PSCs) are seeded onto low attachment 96-well plates to generate embryoid bodies (EB). Over the next few days, each well will yield an EB that will undergo germ layer differentiation followed by neural ectodermal induction, which is an important step in embryogenesis as brain tissue is derived from the ectoderm. After which, EBs are embedded in Matrigel to promote the development of neuroepithelial rosettes composed of radially organized neuroprogenitors. These EBs will mature into cerebral organoids that can be maintained in culture on an orbital shaker or bioreactor for extended periods of time. Subsequently, these cerebral organoids should stain positively for hindbrain, midbrain, and forebrain markers. Over time, cerebral organoids will express a rich diversity of cell types including those from all the six layers of the cerebral cortex, as well as hippocampal neurons, ventral forebrain neurons, dopaminergic neurons, glutamatergic neurons, GABAergic neurons, astrocytes, and oligodendrocytes. Sometimes, other regions such as the choroid plexus and retinal pigmented epithelium can also be observed [47, 49, 50]. Emerging evidence has established similarities between cerebral organoids and human fetal brains in terms of epigenomics [51] and proteomics [52]. Remarkably, brain organoids can produce coordinated electrical oscillations in groups of neurons resembling brain waves observed in newborns [53]. Therefore, the cytoarchitectural and functional resemblance shared with some degree of fidelity between brain organoids and the human brain render the use of organoids as an attractive option to model neurological disorders, especially neurodevelopmental disorders. As these organoids may be cultured for prolonged periods, several groups including ours have also used the system to model age-related neurodegenerative diseases.
The first organoids modelling AD utilized ReNcell VM human neural stem cells that overexpressed APP and presenilin-1 to mimic familial AD (FAD) mutations. These FAD organoids exhibit enhanced levels of Aβ deposits and phosphorylated tau (p-tau), where the latter was significantly reduced by the treatment of these disease-associated organoids with β- or γ- secretase inhibitors [54]. Not only did this study demonstrate that organoids can recapitulate disease-specific hallmarks, it also illustrated that organoids are amenable to pharmacological intervention and have utility for drug development. In another study, brain organoids were generated from individuals with dupAPP or PSEN1 FAD mutations, both of which are associated with AD. These disease-associated organoids exhibited a time-dependent increase in the size and quantity of Aβ aggregates, as well as the concentration of Aβ oligomers. A significant difference in p-tau levels between disease-associated and control organoids was only observed at a later timepoint as compared to Aβ, which was observed earlier, suggesting that these organoids recapitulate the trajectory of AD pathologies as outlined in the amyloid cascade hypothesis. Moreover, these AD-associated organoids also contained enlarged endosomes with abnormalities typical of those observed in mouse AD models and AD patients [55]. Other cerebral organoids generated from cell lines with a missense mutation (A243E) in presenilin 1 and individuals with DS similarly exhibit elevated level of Aβ deposits, p-tau pathology, and cell death compared to their normal controls [56]. Not surprisingly, the same trend is observed in APOE4-bearing organoids derived from CRISPR/Cas9-edited APOE3 induced pluripotent stem cells (iPSCs) relative to APOE3-containing organoids. This is expected as the APOE4 allele is the strongest risk factor for sporadic AD. Conversely, in the same study, APOE3 organoids generated from the APOE4 iPSCs attenuated the AD pathologies observed in the APOE4 organoids [57]. Thereafter,
Zhao et al. used ELISA, immunostaining, and transcriptomic profiling with AD organoids to demonstrate that AD status has an effect on boosting Aβ40 and Aβ42 levels independently of APOE4, as well as increasing cell death, synaptic loss, and stress granule formation. APOE4 status was related to p-Tau generation, which suggests that APOE4 may drive p-Tau generation without Aβ. Consistent with this, isogenic conversion of APOE4 to APOE3 decreased the AD phenotypes observed earlier in APOE4 organoids, which corroborates with the fact that APOE4 is a significant AD risk factor [58]. Another recent paper also performed transcriptomic analyses utilizing brain organoids exposed to human serum to simulate the serum components associated with the malfunctioning of the blood brain barrier in AD. In this study, serum-exposed organoids showed a decline in synaptic function in neurons and an increase in insoluble Aβ [59].
With regards to BACE2, several groups have generated cerebral organoids from individuals with DS whose BACE2 and APP are naturally triplicated due to their position on Chromosome 21. In one of these studies, Murray and colleagues generated an isogenic DS iPSC model [60] that was used in a follow-up study that analyzed conditioned media from organoids grown from these iPSCs. The study revealed that the amount of non-amyloidogenic BACE2 cleavage products Aβ19, Aβ20, and Aβ34 increased in these DS organoids. This phenomenon was corroborated in cerebrospinal fluid samples obtained from individuals with DS. Subsequently, a copy of the triplicated BACE2 allele in these DS organoids was eliminated through CRISPR-Cas9 technology. As anticipated, DS organoids harboring BACE2 in a diploid state exhibited early amyloid and tau pathology as compared to unedited trisomic organoids. The subsequent treatment of these CRISPR-edited DS organoids with β-secretase and γ-secretase inhibitors prevented the formation of amyloid and tau pathology observed previously [13]. This supports a neuroprotective role for BACE2 in AD pathogenesis. In a similar vein, recent research findings from a group in China revealed rare BACE2 loss-of-function variants in patients with Hirschsprung disease and found that brain organoids generated from these individuals exhibit increased amounts of Aβ oligomers and cell death. In contrast, they found that BACE2 overexpression in brain organoids carrying the familial AD APP Swedish/Indiana mutation promoted a dual reduction of Aβ and cell death [21].
SINGLE NUCLEOTIDE POLYMORPHISMS IN BACE2 CORRELATE WITH THE AGE OF DEMENTIA ONSET
Although the evidence presented above collectively supports a neuroprotective role for BACE2 in AD pathogenesis, it remains intriguing as to why not all individuals with DS harboring an extra copy of BACE2 are protected from AD. Single nucleotide polymorphisms (SNPs) for BACE2 exist in the population of people with DS, suggesting that genetic background may determine the predisposition of DS individuals to AD. The premise that BACE2 genetic variations may modify the risk of individuals for AD has certainly been examined previously and has gained considerable traction recently. In 2005, Myllykangas and colleagues have reported the association of BACE2 haplotype with AD [61]. In particular, the rs2252576 (T allele) from the Myllykangas study is also associated with an earlier age of dementia onset in individuals with DS as reported in a separate study that also identified other significant SNPs in the population of DS individuals [62]. More recently, Huentelman and colleagues have also identified SNPs within the BACE2 locus that are associated with altered AD risk preferentially in APOE4 non-carriers, of which a subset with mild cognitive impairment or AD contained the rs2012050 SNP that is correlated with decreased BACE2 gene expression and increased Aβ42 cerebrospinal fluid levels [45]. Further, as mentioned above, Luo and colleagues have recently also reported rare variants of BACE2 that occur in individuals with Hirschsprung disease [21]. Perhaps the most direct evidence supporting a role of BACE2 in AD is the identification of a patient with early-onset AD (EOAD) who harbours a mutation in BACE2. In a screen for de novo variants that might participate in the genetic determinism of sporadic EOAD (typically with disease onset before 65 years), Rovelet-Lecrux and colleagues have identified two de novo copy number variations in two EOAD patients, with one (Patient EXT 804) harboring a 12 kb deletion within intron 1 of BACE2 (while the other harboring an APP duplication) [63]. The BACE2 intronic deletion overlaps with enriched H3K4Me3 and H3K27Ac histone marks, which are epigenetic modifications that correlate with promoters and enhancers, respectively, suggesting the likelihood of reduced BACE2 expression in Patient EXT 804. Supporting this, reverse transcription quantitative multiplex PCR performed by the group on cultured fibroblasts of the patient and 10 control individuals revealed that the abundance of BACE2 transcripts in the patient are among the lowest of the samples [63]. Although the large inter-individual variability among controls precluded a firm conclusion of the putative pro-amyloidogenic effect of the BACE2 mutation, the premise is consistent with several reported studies supporting a neuroprotective role of BACE2 as discussed above [13, 18–22]. Nonetheless, the disease causality and mechanism related to this index BACE2 intronic mutation remain to be clarified. In our attempt to address this, our preliminary (unpublished) results have revealed that cerebral organoids generated from this patient produced more amyloid plaques and phosphorylated tau than those generated from his asymptomatic parental control. Importantly, we documented that the appearance of amyloid plaques preceded that of phosphorylated tau, which aligns with the order of pathology proposed by the amyloid cascade hypothesis (Yeap et al., unpublished observations).
BEYOND AD: THE ROLE OF BACE2 IN DIABETES AND ITS IMPLICATIONS FOR THE DEVELOPMENT OF BACE2 THERAPEUTICS
Given the promise of BACE2 in offering neuroprotection, it seems intuitive to develop BACE2-based therapeutics for AD. In reality, the development of BACE2-specific drugs for AD has yet to catch on. The bulk of BACE2 drugs in development are actually BACE2 inhibitors designed mainly for the treatment of Type 2 diabetes (T2D) and not AD [64, 65] (Table 1). An earlier review has documented the patents for these inhibitors from 2010 to 2012 [66] and a recent search on SureChEMBL has yielded a few filed patents for BACE2 inhibitors from 2014 to date [67–73], but it seems that clinical trials have yet to be conducted using these inhibitors. Nonetheless, the use of BACE2 inhibitors for diabetes is important for us to appreciate given the emerging link between diabetes and AD, and the potential consequence that such an approach may pose to the brain. Indeed, there is a growing appreciation that poor sugar control, insulin resistance, and cognitive decline are all intertwined [74, 75], and that insulin resistance in the brain can cause disruptions in glucose metabolism and contribute to the pathogenesis of AD [76, 77]. For this reason, AD is also known as the “diabetes of the brain” or “Type 3 diabetes”.
Table 1.
Patents of BACE2 inhibitors published between 2013-present
| Year of Publication | Title & (Patent Number) | Target(s) | Reference(s) |
| 2017 | Bace-2 inhibitory compounds and related methods of use (WO2017066742A1) | BACE2 | [73] |
| 2016 | Compounds for inhibition of memapsin 1 (US9512099B2) | BACE2 | [64, 65, 72] |
| 2016 | 1,4 oxazines as BACE1 and/or BACE2 inhibitors (US9242943B2) | BACE1 or BACE2 | [71] |
| 2015 | Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as BACE1 and/or BACE2 inhibitors (US9079919B2) | BACE1 or BACE2 | [70] |
| 2015 | Halogen-alkyl-1,3 oxazines as BACE1 and/or BACE2 inhibitors (US8987255B2) | BACE1 or BACE2 | [69] |
| 2015 | Cyclopropyl-fused-1,3-thiazepines as BACE1 and/or BACE2 inhibitors (US8927535B2) | BACE1 or BACE2 | [68] |
| 2013 | N-[3-(5-amino-3,3a,7,7a-tetrahydro-1H-2,4-dioxa-6-aza-inden-7-yl)-phenyl]-amides as BACE1 and/or BACE2 inhibitors (US8404680B2) | BACE1 or BACE2 | [67] |
It is noteworthy to highlight that BACE2 mRNA expression is highest in pancreatic islets [78, 79] that lends its role in diabetes. Supporting this, mice with an in-frame bi-allelic deletion of exon 6 of Bace2 exhibited lower blood glucose levels, better intraperitoneal glucose tolerance, higher β-cell mass and numbers of Ki67-positive β-cells as compared to their wild-type counterparts [40]. Moreover, islet cells harvested from these mutant mice did not show any signs of apoptosis and released more insulin when stimulated with glucose as compared to those derived from wild-type mice. Similarly, the proliferation of MIN6 cells, a pancreatic β-cell line, increased upon treatment with a Bace2 inhibitor. On the contrary, overexpressing Bace2 led to a decrease in cell proliferation that was restored after treatment with the same Bace2 inhibitor. This phenomenon is attributed to the ability of Bace2 inhibition to prevent the cleavage of Tmem27, a β-cell-transmembrane protein and a Bace2-specific substrate that stimulates both β-cell growth and insulin activity. Alongside this, human islet cells exposed to this inhibitor also exhibit enhanced insulin secretion, although the proliferative capacity of the human islets did not change by much [78]. Interestingly, most individuals with type 2 diabetes (T2D) are presented with islet amyloid deposits [80] that are long considered as a classical pathological feature of T2D [81]. The deposits contain aggregates of amylin, also known as islet amyloid polypeptide (IAPP) [82], that promotes pancreatic islet amyloidosis upon its fibrillization, eventually leading to β-cell death [83]. Under normal circumstances, soluble IAPP functions as a neuro-pancreatic hormone that stabilizes blood glucose levels by decreasing the release of glucagon [84]. It is co-secreted with insulin to augment the latter’s function to trigger glucose uptake [85]. In rat pancreatic β-cell line INS1E overexpressing human IAPP, BACE2 expression was found to be upregulated by 2.2-fold. Subsequent overexpression of BACE2 in these cells decreased β-cell proliferation by 60%, increased reactive oxygen species levels by three-fold, and decreased insulin secretion by 25% as compared to controls. Once BACE2 was silenced, β-cell function was restored [79]. Intriguingly, IAPP can cross the blood-brain barrier and its amyloid deposits can cause brain damage [86]. Indeed, the presence of IAPP amyloid deposits has been documented in AD brains [87], even in the absence of clinical signs of diabetes in these individuals [88]. Furthermore, a recent report revealed that an AD-associated tau species directly interacts with IAPP, whereby the oligomerization of this tau fragment increased in the presence of IAPP [89].
Taking the findings above into account, the inhibition of BACE2 is expected to promote the function of pancreatic β-cells, which fueled the proposition that BACE2 may be a potential therapeutic target for diabetes. However, the complication is that IAPP is apparently a substrate of BACE2 as reported by Rulifson and colleagues [82], who further demonstrated that cleavage of hIAPP at the F15 and F23 residues reduces its propensity to aggregate. They proposed that enhancing, rather than inhibiting, BACE2 may provide translational benefits. Along the same vein, Diaz-Catalan and colleagues showed that Bace2 knockout mice fed with a high-fat diet led to increased weight gain, hyperinsulinemia, and insulin resistance, despite increased β-cell proliferation and higher levels of insulin and leptin. The authors concluded that these mice had damaged leptin and insulin signaling, which raised caution when using BACE2 inhibitors for the treatment of T2D [90]. Notwithstanding the controversies surrounding the outcomes of BACE2 inhibition in diabetic models, an important additional caveat to note is that BACE2 inhibition, when applied systemically, runs the danger of promoting amyloidosis in the brain and may prove to be catastrophic. Clearly, whether BACE2 inhibitors would exacerbate or ameliorate the pathology present in individuals with both AD and diabetes is a question that begs to be resolved. All in all, the link between BACE2, AD, and diabetes remains an enigma.
CONCLUDING REMARKS
To date, AD-related clinical trials have primarily leveraged on the inhibition of Aβ generation via β-secretase inhibitors. Unfortunately, current β-inhibitors are non-selective and inhibit both BACE1 and BACE2 [13, 91, 92], which might in part explain why drug trials have failed throughout the years [93]. This strategy thus seems counterproductive, given the multiple lines of evidence supporting a neuroprotective role for BACE2 in AD that we have presented above. Although the development of selective BACE1 inhibitors would certainly be useful, we propose that targeting BACE2 for therapeutic purposes represents a viable alternative strategy that merits consideration. Besides pharmacological approaches, BACE2 expression may be upregulated by genetic means involving microRNAs such as let-7c, which binds to BACE2 and triggers its expression via RNAa to release C83 or C80 fragments instead of C99 that greatly decrease Aβ generation. Notably, let-7c is downregulated in AD mice and individuals with DS [94]. Using exogenously introduced miRNAs such as let-7c to boost BACE2 gene expression therefore represents an intuitive approach. In addition, a recent study revealed that BACE2 can cleave Kv2.1, the main potassium efflux channel involved in neuronal apoptosis [95], which provides yet another reason why augmenting BACE2 function may be beneficial for AD. However, given the role of BACE2 in diabetes, the systemic effects of AD-targeting BACE2-enhancing drugs particularly in pancreatic β-cells need to be carefully assessed. Taken together, our review has highlighted the important role of BACE2 in the pathogenesis of AD and emphasized the need to focus more attention on BACE2 as a potential therapeutic target for AD.
ACKNOWLEDGMENTS
This work was supported by grants from the Singapore Ministry of Education (MOE2017-T3-1-002) (LKL) and the Lee Kong Chian School of Medicine (YYJ).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0867r1).
REFERENCES
- [1]. Patterson C (2018) World Alzheimer Report 2018. The state of the art of dementia research: New frontiers. Alzheimer’s Disease International, London.
- [2]. Gauthier S, Rosa-Neto P, Morais JA, Webster C (2021) World Alzheimer Report 2021. Journey through the diagnosis of dementia. Alzheimer’s Disease International, London.
- [3]. Alzheimer’s Association (2018) 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 14, 367–429. [Google Scholar]
- [4]. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1, a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Hardy JA, Higgins GA (1992) Alzheimer’s disease: The amyloid cascade hypothesis. Science 256, 184–185. [DOI] [PubMed] [Google Scholar]
- [7]. Selkoe DJ (1991) The molecular pathology of Alzheimer’s disease. Neuron 6, 487–498. [DOI] [PubMed] [Google Scholar]
- [8]. Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jonsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K (2012) A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99. [DOI] [PubMed] [Google Scholar]
- [9]. Arnsten AFT, Datta D, Del Tredici K, Braak H (2021) Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimers Dement 17, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerrière A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D (2006) APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38, 24–26. [DOI] [PubMed] [Google Scholar]
- [11]. Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, Del-Favero J, Cruts M, van Duijn CM, Van Broeckhoven C (2006) APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain 129, 2977–2983. [DOI] [PubMed] [Google Scholar]
- [12]. Wiseman FK, Al-Janabi T, Hardy J, Karmiloff-Smith A, Nizetic D, Tybulewicz VL, Fisher EM, Strydom A (2015) A genetic cause of Alzheimer disease: Mechanistic insights from Down syndrome. Nat Rev Neurosci 16, 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Alic I, Goh PA, Murray A, Portelius E, Gkanatsiou E, Gough G, Mok KY, Koschut D, Brunmeir R, Yeap YJ, O’Brien NL, Groet J, Shao X, Havlicek S, Dunn NR, Kvartsberg H, Brinkmalm G, Hithersay R, Startin C, Hamburg S, Phillips M, Pervushin K, Turmaine M, Wallon D, Rovelet-Lecrux A, Soininen H, Volpi E, Martin JE, Foo JN, Becker DL, Rostagno A, Ghiso J, Krsnik Z, Simic G, Kostovic I, Mitrecic D, LonDown SC, Francis PT, Blennow K, Strydom A, Hardy J, Zetterberg H, Nizetic D (2021) Patient-specific Alzheimer-like pathology in trisomy 21 cerebral organoids reveals BACE2 as a gene dose-sensitive AD suppressor in human brain. Mol Psychiatry 26, 5766–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Acquati F, Accarino M, Nucci C, Fumagalli P, Jovine L, Ottolenghi S, Taramelli R (2000) The gene encoding DRAP (BACE2), a glycosylated transmembrane protein of the aspartic protease family, maps to the down critical region. FEBS Lett 468, 59–64. [DOI] [PubMed] [Google Scholar]
- [15]. Solans A, Estivill X, de La Luna S (2000) A new aspartyl protease on 21q22.3, BACE2, is highly similar to Alzheimer’s amyloid precursor protein beta-secretase. Cytogenet Cell Genet 89, 177–184. [DOI] [PubMed] [Google Scholar]
- [16]. Vassar R, Kandalepas PC (2011) The β-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimers Res Ther 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741. [DOI] [PubMed] [Google Scholar]
- [18]. Abdul-Hay SO, Sahara T, McBride M, Kang D, Leissring MA (2012) Identification of BACE2 as an avid ß-amyloid-degrading protease. Mol Neurodegener 7, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Sun X, He G, Song W (2006) BACE2, as a novel APP theta-secretase, is not responsible for the pathogenesis of Alzheimer’s disease in Down syndrome. FASEB J 20, 1369–1376. [DOI] [PubMed] [Google Scholar]
- [20]. Fluhrer R, Capell A, Westmeyer G, Willem M, Hartung B, Condron MM, Teplow DB, Haass C, Walter J (2002) A non-amyloidogenic function of BACE-2 in the secretory pathway. J Neurochem 81, 1011–1020. [DOI] [PubMed] [Google Scholar]
- [21]. Luo J, Zou H, Guo Y, Huang K, Ngan ES, Li P (2022) BACE2 variant identified from HSCR patient causes AD-like phenotypes in hPSC-derived brain organoids. Cell Death Discov 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Basi G, Frigon N, Barbour R, Doan T, Gordon G, McConlogue L, Sinha S, Zeller M (2003) Antagonistic effects of beta-site amyloid precursor protein-cleaving enzymes 1 and 2 on beta-amyloid peptide production in cells. J Biol Chem 278, 31512–31520. [DOI] [PubMed] [Google Scholar]
- [23]. Bennett BD, Babu-Khan S, Loeloff R, Louis JC, Curran E, Citron M, Vassar R (2000) Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem 275, 20647–20651. [DOI] [PubMed] [Google Scholar]
- [24]. Stockley JH, O’Neill C (2007) The proteins BACE1 and BACE2 and beta-secretase activity in normal and Alzheimer’s disease brain. Biochem Soc Trans 35, 574–576. [DOI] [PubMed] [Google Scholar]
- [25]. Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC (2005) BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci 25, 11693–11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Ahmed RR, Holler CJ, Webb RL, Li F, Beckett TL, Murphy MP (2010) BACE1 and BACE2 enzymatic activities in Alzheimer’s disease. J Neurochem 112, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Holler CJ, Webb RL, Laux AL, Beckett TL, Niedowicz DM, Ahmed RR, Liu Y, Simmons CR, Dowling AL, Spinelli A, Khurgel M, Estus S, Head E, Hersh LB, Murphy MP (2012) BACE2 expression increases in human neurodegenerative disease. Am J Pathol 180, 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Hussain I, Powell DJ, Howlett DR, Chapman GA, Gilmour L, Murdock PR, Tew DG, Meek TD, Chapman C, Schneider K, Ratcliffe SJ, Tattersall D, Testa TT, Southan C, Ryan DM, Simmons DL, Walsh FS, Dingwall C, Christie G (2000) ASP1 (BACE2) cleaves the amyloid precursor protein at the beta-secretase site. Mol Cell Neurosci 16, 609–619. [DOI] [PubMed] [Google Scholar]
- [29]. Stockley JH, Ravid R, O’Neill C (2006) Altered beta-secretase enzyme kinetics and levels of both BACE1 and BACE2 in the Alzheimer’s disease brain. FEBS Lett 580, 6550–6560. [DOI] [PubMed] [Google Scholar]
- [30]. Qiu K, Wang S, Wang X, Wang F, Wu Y (2020) RCAN1 inhibits BACE2 turnover by attenuating proteasome-mediated BACE2 degradation. Biomed Res Int 2020, 1920789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Wang T, Liu H, Wang Y, Liu C, Sun X (2014) RCAN1 increases Aβ generation by promoting N-glycosylation via oligosaccharyltransferase. Curr Alzheimer Res 11, 332–339. [DOI] [PubMed] [Google Scholar]
- [32]. Wang Z, Xu Q, Cai F, Liu X, Wu Y, Song W (2019) BACE2, a conditional beta-secretase, contributes to Alzheimer’s disease pathogenesis. JCI Insight 4, e123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, Heun R, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J (2009) Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41, 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanché H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alpérovitch A, Lathrop M, Amouyel P (2009) Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41, 1094–1099. [DOI] [PubMed] [Google Scholar]
- [35]. Hendriks L, van Duijn CM, Cras P, Cruts M, Van Hul W, van Harskamp F, Warren A, McInnis MG, Antonarakis SE, Martin JJ, Hofman A, Van Broeckhoven C (1992) Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the beta-amyloid precursor protein gene. Nat Genet 1, 218–221. [DOI] [PubMed] [Google Scholar]
- [36]. Farzan M, Schnitzler CE, Vasilieva N, Leung D, Choe H (2000) BACE2, a beta -secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proc Natl Acad Sci U S A 97, 9712–9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Motonaga K, Itoh M, Becker LE, Goto Y, Takashima S (2002) Elevated expression of beta-site amyloid precursor protein cleaving enzyme 2 in brains of patients with Down syndrome. Neurosci Lett 326, 64–66. [DOI] [PubMed] [Google Scholar]
- [38]. Azkona G, Amador-Arjona A, Obradors-Tarrago C, Varea E, Arque G, Pinacho R, Fillat C, de la Luna S, Estivill X, Dierssen M (2010) Characterization of a mouse model overexpressing beta-site APP-cleaving enzyme 2 reveals a new role for BACE2. Genes Brain Behav 9, 160–172. [DOI] [PubMed] [Google Scholar]
- [39]. Azkona G, Levannon D, Groner Y, Dierssen M (2010) In vivo effects of APP are not exacerbated by BACE2 co-overexpression: Behavioural characterization of a double transgenic mouse model. Amino Acids 39, 1571–1580. [DOI] [PubMed] [Google Scholar]
- [40]. Dominguez D, Tournoy J, Hartmann D, Huth T, Cryns K, Deforce S, Serneels L, Camacho IE, Marjaux E, Craessaerts K, Roebroek AJ, Schwake M, D’Hooge R, Bach P, Kalinke U, Moechars D, Alzheimer C, Reiss K, Saftig P, De Strooper B (2005) Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem 280, 30797–30806. [DOI] [PubMed] [Google Scholar]
- [41]. Yan R, Munzner JB, Shuck ME, Bienkowski MJ (2001) BACE2 functions as an alternative alpha-secretase in cells. J Biol Chem 276, 34019–34027. [DOI] [PubMed] [Google Scholar]
- [42]. Shi XP, Tugusheva K, Bruce JE, Lucka A, Wu GX, Chen-Dodson E, Price E, Li Y, Xu M, Huang Q, Sardana MK, Hazuda DJ (2003) Beta-secretase cleavage at amino acid residue 34 in the amyloid beta peptide is dependent upon gamma-secretase activity. J Biol Chem 278, 21286–21294. [DOI] [PubMed] [Google Scholar]
- [43]. Sun X, Wang Y, Qing H, Christensen MA, Liu Y, Zhou W, Tong Y, Xiao C, Huang Y, Zhang S, Liu X, Song W (2005) Distinct transcriptional regulation and function of the human BACE2 and BACE1 genes. FASEB J 19, 739–749. [DOI] [PubMed] [Google Scholar]
- [44]. Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ (1992) Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 360, 672–674. [DOI] [PubMed] [Google Scholar]
- [45]. Huentelman M, De Both M, Jepsen W, Piras IS, Talboom JS, Willeman M, Reiman EM, Hardy J, Myers AJ (2019) Common BACE2 polymorphisms are associated with altered risk for Alzheimer’s disease and CSF amyloid biomarkers in APOE epsilon4 non-carriers. Sci Rep 9, 9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Liu X, Wang Z, Wu Y, Wang J, Song W (2013) BACE2 degradation mediated by the macroautophagy-lysosome pathway. Eur J Neurosci 37, 1970–1977. [DOI] [PubMed] [Google Scholar]
- [47]. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA (2013) Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Lancaster MA, Knoblich JA (2014) Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9, 2329–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, Boyden ES, Lichtman JW, Williams ZM, McCarroll SA, Arlotta P (2017) Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Matsui TK, Matsubayashi M, Sakaguchi YM, Hayashi RK, Zheng C, Sugie K, Hasegawa M, Nakagawa T, Mori E (2018) Six-month cultured cerebral organoids from human ES cells contain matured neural cells. Neurosci Lett 670, 75–82. [DOI] [PubMed] [Google Scholar]
- [51]. Luo C, Lancaster MA, Castanon R, Nery JR, Knoblich JA, Ecker JR (2016) Cerebral organoids recapitulate epigenomic signatures of the human fetal brain. Cell Rep 17, 3369–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Nascimento JM, Saia-Cereda VM, Sartore RC, da Costa RM, Schitine CS, Freitas HR, Murgu M, de Melo Reis RA, Rehen SK, Martins-de-Souza D (2019) Human cerebral organoids and fetal brain tissue share proteomic similarities. Front Cell Dev Biol 7, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, Wang A, Wu W, Haddad GG, Chaim IA, Domissy A, Vandenberghe M, Devor A, Yeo GW, Voytek B, Muotri AR (2019) Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, 558–569.e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY (2014) A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 515, 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, Seo J, Tsai LH (2016) Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PLoS One 11, e0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Gonzalez C, Armijo E, Bravo-Alegria J, Becerra-Calixto A, Mays CE, Soto C (2018) Modeling amyloid beta and tau pathology in human cerebral organoids. Mol Psychiatry 23, 2363–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, Cam HP, Gjoneska E, Raja WK, Cheng J, Rueda R, Kritskiy O, Abdurrob F, Peng Z, Milo B, Yu CJ, Elmsaouri S, Dey D, Ko T, Yankner BA, Tsai LH (2018) APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98, 1141–1154.e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Zhao J, Fu Y, Yamazaki Y, Ren Y, Davis MD, Liu CC, Lu W, Wang X, Chen K, Cherukuri Y, Jia L, Martens YA, Job L, Shue F, Nguyen TT, Younkin SG, Graff-Radford NR, Wszolek ZK, Brafman DA, Asmann YW, Ertekin-Taner N, Kanekiyo T, Bu G (2020) APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat Commun 11, 5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Chen X, Sun G, Tian E, Zhang M, Davtyan H, Beach TG, Reiman EM, Blurton-Jones M, Holtzman DM, Shi Y (2021) Modeling sporadic Alzheimer’s disease in human brain organoids under serum exposure. Adv Sci (Weinh) 8, e2101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Murray A, Letourneau A, Canzonetta C, Stathaki E, Gimelli S, Sloan-Bena F, Abrehart R, Goh P, Lim S, Baldo C, Dagna-Bricarelli F, Hannan S, Mortensen M, Ballard D, Syndercombe Court D, Fusaki N, Hasegawa M, Smart TG, Bishop C, Antonarakis SE, Groet J, Nizetic D (2015) Brief report: Isogenic induced pluripotent stem cell lines from an adult with mosaic down syndrome model accelerated neuronal ageing and neurodegeneration. Stem Cells 33, 2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Myllykangas L, Wavrant-De Vrièze F, Polvikoski T, Notkola IL, Sulkava R, Niinistö L, Edland SD, Arepalli S, Adighibe O, Compton D, Hardy J, Haltia M, Tienari PJ (2005) Chromosome 21 BACE2 haplotype associates with Alzheimer’s disease: A two-stage study. J Neurol Sci 236, 17–24. [DOI] [PubMed] [Google Scholar]
- [62]. Mok KY, Jones EL, Hanney M, Harold D, Sims R, Williams J, Ballard C, Hardy J (2014) Polymorphisms in BACE2 may affect the age of onset Alzheimer’s dementia in Down syndrome. Neurobiol Aging 35, 1513.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Rovelet-Lecrux A, Charbonnier C, Wallon D, Nicolas G, Seaman MN, Pottier C, Breusegem SY, Mathur PP, Jenardhanan P, Le Guennec K, Mukadam AS, Quenez O, Coutant S, Rousseau S, Richard AC, Boland A, Deleuze JF, Frebourg T, Hannequin D, Campion D, CNR-MAJ collaborators (2015) De novo deleterious genetic variations target a biological network centered on Abeta peptide in early-onset Alzheimer disease. Mol Psychiatry 20, 1046–1056. [DOI] [PubMed] [Google Scholar]
- [64]. Ghosh AK, Brindisi M, Yen YC, Lendy EK, Kovela S, Cárdenas EL, Reddy BS, Rao KV, Downs D, Huang X, Tang J, Mesecar AD (2019) Highly selective and potent human β-secretase 2 (BACE2) inhibitors against type 2 diabetes: Design, synthesis, x-ray structure and structure-activity relationship studies. ChemMedChem 14, 545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Ghosh AK, Reddy BS, Yen YC, Cardenas E, Rao KV, Downs D, Huang X, Tang J, Mesecar AD (2016) Design of potent and highly selective inhibitors for human β-secretase 2 (Memapsin 1), a target for type 2 diabetes. Chem Sci 7, 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Southan C (2013) BACE2 as a new diabetes target: A patent review (2010 - 2012). Expert Opin Ther Pat 23, 649–663. [DOI] [PubMed] [Google Scholar]
- [67]. Hilpert H, Humm R (2013) N-[3-(5-amino-3,3a,7,7a-tetrahydro-1H-2,4-dioxa-6-aza-inden-7-yl)-phenyl]-amides as BACE1 and/or BACE2 inhibitors. Patent No. US8404680B, 2–. [Google Scholar]
- [68]. Woltering T (2015) Cyclopropyl-fused-1,3-thiazepines as BACE1 and/or BACE2 inhibitors. Patent No. US8927535B2. [DOI] [PMC free article] [PubMed]
- [69]. Woltering T (2015) Halogen-alkyl-1,3 oxazines as BACE1 and/or BACE2 inhibitors. Patent No. US8987255B2.
- [70]. Narquizian R, Pinard E, Wostl W (2015) Spiro-[1,3]-oxazines and spiro-[1,4]-oxazepines as BACE1 and/or BACE2 inhibitors. Patent No. US9079919B2.
- [71]. Andreini M, Gabellieri E, Guba W, Hilpert H, Mayweg AV, Narquizian R, Power E, Travagli M, Woltering T, Wostl W, Mauser H (2016) 1,4 oxazines as BACE1 and/or BACE2 inhibitors. Patent No. US9242943B2.
- [72]. Ghosh AK (2016) Compounds for inhibition of memapsin 1. Patent No. US9512099B2.
- [73]. Ankala SV, Lilly JC, Peddapuddi G (2017) Bace-2 inhibitory compounds and related methods of use. Patent No. WO2017066742A1.
- [74]. Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis 7, 63–80. [DOI] [PubMed] [Google Scholar]
- [75]. Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM (2005) Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J Alzheimers Dis 8, 247–268. [DOI] [PubMed] [Google Scholar]
- [76]. Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2011) Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol 225, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122, 1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Esterházy D, Stützer I, Wang H, Rechsteiner MP, Beauchamp J, Döbeli H, Hilpert H, Matile H, Prummer M, Schmidt A, Lieske N, Boehm B, Marselli L, Bosco D, Kerr-Conte J, Aebersold R, Spinas GA, Moch H, Migliorini C, Stoffel M (2011) Bace2 is a β cell-enriched protease that regulates pancreatic β cell function and mass. Cell Metab 14, 365–377. [DOI] [PubMed] [Google Scholar]
- [79]. Alcarraz-Vizán G, Casini P, Cadavez L, Visa M, Montane J, Servitja JM, Novials A (2015) Inhibition of BACE2 counteracts hIAPP-induced insulin secretory defects in pancreatic β-cells. FASEB J 29, 95–104. [DOI] [PubMed] [Google Scholar]
- [80]. Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC (1988) Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: Quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 9, 151–159. [PubMed] [Google Scholar]
- [81]. Hull RL, Westermark GT, Westermark P, Kahn SE (2004) Islet amyloid: A critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab 89, 3629–3643. [DOI] [PubMed] [Google Scholar]
- [82]. Rulifson IC, Cao P, Miao L, Kopecky D, Huang L, White RD, Samayoa K, Gardner J, Wu X, Chen K, Tsuruda T, Homann O, Baribault H, Yamane H, Carlson T, Wiltzius J, Li Y (2016) Identification of human islet amyloid polypeptide as a BACE2 substrate. PLoS One 11, e0147254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, Carr DB, Westermark P, Westermark GT, Kahn SE, Hull RL (2011) β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol 178, 2632–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. Panagiotidis G, Salehi AA, Westermark P, Lundquist I (1992) Homologous islet amyloid polypeptide: Effects on plasma levels of glucagon, insulin and glucose in the mouse. Diabetes Res Clin Pract 18, 167–171. [DOI] [PubMed] [Google Scholar]
- [85]. James JH, Wagner KR, King JK, Leffler RE, Upputuri RK, Balasubramaniam A, Friend LA, Shelly DA, Paul RJ, Fischer JE (1999) Stimulation of both aerobic glycolysis and Na(+)-K(+)-ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol 277, E176–186. [DOI] [PubMed] [Google Scholar]
- [86]. Srodulski S, Sharma S, Bachstetter AB, Brelsfoard JM, Pascual C, Xie XS, Saatman KE, Van Eldik LJ, Despa F (2014) Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Fawver JN, Ghiwot Y, Koola C, Carrera W, Rodriguez-Rivera J, Hernandez C, Dineley KT, Kong Y, Li J, Jhamandas J, Perry G, Murray IV (2014) Islet amyloid polypeptide (IAPP): A second amyloid in Alzheimer’s disease. Curr Alzheimer Res 11, 928–940. [DOI] [PubMed] [Google Scholar]
- [88]. Jackson K, Barisone GA, Diaz E, Jin LW, DeCarli C, Despa F (2013) Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol 74, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89]. Arya S, Claud SL, Cantrell KL, Bowers MT (2019) Catalytic prion-like cross-talk between a key Alzheimer’s disease tau-fragment R3 and the type 2 diabetes peptide IAPP. ACS Chem Neurosci 10, 4757–4765. [DOI] [PubMed] [Google Scholar]
- [90]. Díaz-Catalán D, Alcarraz-Vizán G, Castaño C, de Pablo S, Rodríguez-Comas J, Fernández-Pérez A, Vallejo M, Ramírez S, Claret M, Parrizas M, Novials A, Servitja JM (2021) BACE2 suppression in mice aggravates the adverse metabolic consequences of an obesogenic diet. Mol Metab 53, 101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Evin G (2016) Future therapeutics in Alzheimer’s disease: Development status of BACE inhibitors. BioDrugs 30, 173–194. [DOI] [PubMed] [Google Scholar]
- [92]. Neumann U, Ufer M, Jacobson LH, Rouzade-Dominguez ML, Huledal G, Kolly C, Lüönd RM, Machauer R, Veenstra SJ, Hurth K, Rueeger H, Tintelnot-Blomley M, Staufenbiel M, Shimshek DR, Perrot L, Frieauff W, Dubost V, Schiller H, Vogg B, Beltz K, Avrameas A, Kretz S, Pezous N, Rondeau JM, Beckmann N, Hartmann A, Vormfelde S, David OJ, Galli B, Ramos R, Graf A, Lopez Lopez C (2018) The BACE-1 inhibitor CNP520 for prevention trials in Alzheimer’s disease. EMBO Mol Med 10, e9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Cummings J (2018) Lessons learned from Alzheimer disease: Clinical trials with negative outcomes. Clin Transl Sci 11, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Liu H, Chen S, Sun Q, Sha Q, Tang Y, Jia W, Chen L, Zhao J, Wang T, Sun X (2022) Let-7c increases BACE2 expression by RNAa and decreases Abeta production. Am J Transl Res 14, 899–908. [PMC free article] [PubMed] [Google Scholar]
- [95]. Liu F, Zhang Y, Liang Z, Sun Q, Liu H, Zhao J, Xu J, Zheng J, Yun Y, Yu X, Song W, Sun X (2018) Cleavage of potassium channel Kv2.1 by BACE2 reduces neuronal apoptosis. Mol Psychiatry 23, 1542–1554. [DOI] [PubMed] [Google Scholar]