Abstract
Alzheimer’s disease (AD) is a major form of dementia. Abnormal amyloidogenic event-mediated degeneration of cholinergic neurons in the cognitive centers of the brain has been attributed to neuropathological sequelae and behavioral deficits in AD. Besides, impaired adult neurogenesis in the hippocampus has experimentally been realized as an underlying cause of dementia regardless of neurodegeneration. Therefore, nourishing the neurogenic process in the hippocampus has been considered an effective therapeutic strategy to mitigate memory loss. In the physiological state, the Wnt pathway has been identified as a potent mitogenic generator in the hippocampal stem cell niche. However, downstream components of Wnt signaling have been noticed to be downregulated in AD brains. Resveratrol (RSV) is a potent Sirtuin1 (SIRT1) enhancer that facilitates neuroprotection and promotes neurogenesis in the hippocampus of the adult brain. While SIRT1 is an important positive regulator of Wnt signaling, ample reports indicate that RSV treatment strongly mediates the fate determination of stem cells through Wnt signaling. However, the possible therapeutic roles of RSV-mediated SIRT1 enhancement on the regulation of hippocampal neurogenesis and reversal of memory loss through the Wnt signaling pathway have not been addressed yet. Taken together, this review describes RSV-mediated effects on the regulation of hippocampal neurogenesis via the activation of SIRT1 in synergy with the Wnt signaling. Further, the article emphasizes a hypothesis that RSV treatment can provoke the activation of quiescent neural stem cells and prime their neurogenic capacity in the hippocampus via Wnt signaling in AD.
Keywords: Adult neurogenesis, Alzheimer’s disease, cell cycle, hippocampus, resveratrol, Sirtuin1, Wnt pathway
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that accounts for the widespread prevalence of dementia in the elderly population [1]. A recent estimate from the World Health Organization indicates that around 55 million individuals have been affected by dementia worldwide. Nearly 10 million new AD cases are diagnosed with dementia every year. The global burden of AD has been predicted to be doubled by 2050 [2]. Thus, it necessitates an unmet need for potential therapeutic interventions to alleviate the clinical symptoms of AD. Defects in the processing and clearance mechanism of amyloid-β protein precursor (AβPP) responsible for the accumulation of soluble amyloid-β 40 (Aβ 40), insoluble Aβ 42, and C-terminal fragments in the brain have been linked to the pathogenies of AD. Besides, the amyloid plaques mediated abnormal biochemical, metabolic, molecular, and cellular events appear to induce neurodegeneration and render deleterious impacts on the ongoing neurogenic processes in the hippocampus [3]. Notably, impaired adult neurogenesis resulting from defects in cell cycle events of neural stem cells (NSCs) and apoptosis in newly developed neurons in the hippocampus has been identified as the potential cause of dementia regardless of neurodegeneration [4]. Thus, insight into the pathogenic determinants that hinder neural regenerative plasticity in AD appears to be a crucial scientific quest.
Notably, the regulation of adult neurogenesis in the hippocampus has been reported to be altered in AD subjects [5]. For example, a transgenic mouse model of AD expressing the mutant APP has been reported to display a drastic reduction in hippocampal neurogenesis [6]. Similarly, studies of autopsy brains from AD victims revealed prominent indications of reduced neurogenesis in the hippocampus [7]. However, the underlying pathogenic mechanisms responsible for the abnormal hippocampal neurogenesis in AD remain obscure. While the neurogenic failure has been attributed to impairments in the proliferative potentials of NSCs in many neurodegenerative conditions, cell cycle reentry of post-mitotic neurons in the brain resulting from elevated mitogenic stimuli has been linked to reactive neurogenic events [4, 8]. The Wnt signaling, a pleiotropic molecular pathway has been identified as a potent inducer and regulator of NSCs proliferation, cell fate determination, tissue remodeling, and neural plasticity in the physiological brain [8, 9]. Growing scientific evidence supports a strong association between dysfunctional Wnt signaling and pathogenic cell cycle events in the brains of subjects with various neuropathological conditions, including AD [9, 10]. Thus, it can be proposed that the aberrant Wnt signaling pathway might be responsible for the dysregulation of hippocampal neurogenesis in the brains of experimental animal models and human AD subjects, making it a valid therapeutic target in AD. Therefore, a fundamental understanding of a potential link between Wnt signaling-mediated regulation of neural regenerative plasticity and the pathogenic progression of AD would be of great aid in establishing a neural restorative strategy.
Of several therapeutic strategies proposed for neural regeneration, a potential drug that could exhibit modulatory effects over gene regulation or extend epigenetic modifications in reverting pathogenesis, gains major interest in treating various comorbid disease conditions like AD [11]. Epigenetic changes are likely to be involved in the learning and memory processes in association with active neurogenesis [12]. Epigenetic changes in some key molecular determinants of pathogenesis such as acetylcholinesterase have been identified as potential druggable targets for AD therapeutics [13]. Interestingly, neurogenesis can be regulated by intrinsic epigenetic mechanisms along the neuronal lineage of NSCs and extrinsically by surrounding niche signaling cells, such as neurons, astrocytes, and endothelial cells [14, 15]. In turn, many of the intrinsic factors are epigenetically regulated by the prominent homeostatic switch like Sirtuin1 (SIRT1). Sirtuins are a group of histone deacetylase class III proteins, widely involved in epigenetic regulation that maintain homeostasis of different types of cells and tissues during various stages of life [16]. SIRT1 appears to be involved in the regulation of multiple metabolic and molecular pathways associated with the regulation of neuroregenerative processes in the adult brain [17]. SIRT1 has been reported to promote adult neurogenesis via the regulation of different intrinsic factors like sonic hedgehog, sex-determining region Y (SRY)-box (Sox), nuclear receptor subfamily 2 group E member 1 (NR2E), and bone morphogenetic protein (BMP) in the brain of various experimental animal models [18–21].
Various in vitro experimental reports revealed that the enchantment of SIRT1 signaling facilitates the differentiation of stem cells and their cell survival mechanisms. For example, SIRT1 activation has been reported to be involved in the fate determination of mesenchymal stem cells [21]. Through decades, research evidence reported the loss of SIRT1 activity during aging as well as diseases, particularly AD [22, 23]. Considering the facts, therapeutic interventions that activate in vivo SIRT1 appear to be crucial. Resveratrol (RSV), a polyphenolic phytochemical present mainly in grapes, red wine, and peanuts has been identified as an effective SIRT1-activating compound [24, 25]. RSV has been known to facilitate various bioregulatory events in different types of stem cells. RSV is recognized to play a key role in the fate determination of stem cells including NSCs [26]. RSV treatment appears to boost neuronal differentiation in the brain via the SIRT1 signaling pathway. Moreover, RSV has been proven for its homeostasis role and neuroprotective effect acting via SIRT1 in the brain and therefore, it prevents memory loss in AD [23, 27, 28]. Though there are studies and theories on the action of RSV in promoting neurogenesis, its promising pro-neurogenic effect on cell cycle activation of NSCs in the brain still needs to be fully elucidated. While SIRT1 has been reported as an important positive regulator of Wnt signaling, ample reports indicate that RSV treatment facilitates the fate determination of stem cells via Wnt signaling [30, 31]. However, the possible therapeutic roles of RSV-mediated SIRT1 activation on the regulation of hippocampal plasticity and enhancement of cognitive functions in synergy with the Wnt signaling pathway have not been addressed yet. Given the valid scientific reports indicating the mutual association between the Wnt signaling and SIRT1 pathways, RSV could be a potent regulator of the Wnt signaling cascade acting at the level of neurogenic events in the hippocampus of the adult brain. Hence, this review has been intended to describe the recent and important research findings related to the possible role of the Wnt signaling pathway in the neuroregenerative plasticity in the brain of subjects with AD. Further, this article emphasizes a hypothesis that RSV treatment can override the quiescent state of NSCs and facilitate their pro-neurogenic capacity in the hippocampus acting via the Wnt signaling in synergy with SIRT1 in AD.
TYPES AND KEY PATHOLOGICAL SIGNATURES OF AD
In general, AD has been classified into two types: familial (early onset) and sporadic (late onset) forms. More than 95% of AD cases occur sporadically, which can stand up to interactions among numerous known and unknown genetic elements and environmental factors. Epidemiological data suggest that hypertension, cardiovascular diseases, hypercholesterolemia, diabetes, obesity, inflammation, and viral infections are the potential risk factors for the development of the sporadic form of AD [31, 32]. Genotypic versions of the apolipoprotein E (ApoE) have also been considered as a principal disease-causing molecular determinant of sporadic forms of AD, as they play a key role in the regulation of lipid metabolism [33]. Whereas the familial forms of AD are very rare and are linked to mutations in various genes involved in AβPP processing [34]. Besides, mutations in the presenilin-1 and γ-secretase have also long been linked to familial forms of AD [35].
The pathogenic events of AD have been reported to induce alterations primarily in the cholinergic systems of the brain thereby contributing to neuronal dysfunction and synaptic vulnerability [36, 37]. Moreover, decreased choline acetyltransferase activity-dependent selective loss of cholinergic neurons has been established as a prominent neuropathological hallmark in AD [38]. Further, the destructive intracellular signaling cascades like the mitogen-activated protein kinase (MAPK), peroxisome proliferator-activated receptor– γ, and nuclear factor-kappa B originated from activated astrocytes and microglia responsible for neuroinflammation have increasingly been evident in AD [39]. The neuropathogenic process of AD resulting in the altered inflammatory response, apoptotic and cell cycle events appear to induce progressive neurodegeneration and aberrant neurogenic events in the affected brain regions including hippocampus.
ABNORMAL CELL CYCLE EVENTS AND DYSREGULATION OF HIPPOCAMPAL NEUROGENESIS DUE TO IMPAIRED WNT SIGNALING IN AD
The Wnt signaling pathway has been considered an evolutionarily conserved signal transduction pathway that regulates various aspects of the stem cells in embryonic development and adulthood [40]. In the presence of Wnt ligand, a receptor complex is generated by joining a seven-pass transmembrane Frizzled receptor with phosphorylated forms of co-receptors like lipoprotein receptor-related protein (LRP5/6) followed by the recruitments of disheveled (Dvl) and axin [41]. This event disrupts and inactivates the molecular complex that exists among axin, adenomatosis polyposis coli, glycogen synthase kinase 3 (GSK3), and casein kinase 1 responsible for the proteasomal degradation of β-catenin in the cytoplasm of the cell. Thus, it promotes the translocation of β-catenin from the cytoplasm into the nucleus that acts as a transcriptional coactivator for the T-cell factor (TCF)/Lymphoid enhancer factor (LEF) leading to various cellular processes [42, 43]. Notably, Wnt signaling has been demonstrated to play a role in stem cell maintenance, brain development, and dorsoventral patterning during embryogenesis [44].
While Wnt signaling is important for the self-renewal of stem cells and immune cells in a physiological state, mutations in the Wnt signaling components lead to the progression of cancer in various tissues [45]. In the sub-granular zone of the hippocampus, the Wnt pathway regulates the proliferation of NSCs, migration, terminal differentiation, and synaptogenesis of existing and newborn neurons [43, 46]. Recent experimental evidence suggests that astrocytes-derived Wnt signaling components promote neuronal fate determination of NSCs and regulate neurogenesis in the hippocampus of the adult brain [47]. Besides, Wnt signaling has been identified to regulate the generation of astrocytes via BMPs in the adult brain [48]. Moreover, the activation of Wnt signaling pathways provokes cell cycle re-entry in the quiescent stem cells in the brain [49]. Thus, insight into the mechanisms by which the Wnt pathway promotes the proliferation of quiescent NSCs can be highly beneficial for neural replacement in AD [50]. While astrocytes and microglia are in active cell cycle phases in the brain, neurons in the central nervous system exit the cell cycle as soon as they differentiate from proliferative neuroepithelial cells and NSCs during embryonic neurogenesis and enter a post-mitotic state that lasts the rest of the life [51, 52]. Neurons in later stages of morphogenesis remain in permanent cell cycle arrest by expressing several cell cycle regulators required to stop cell cycle progression [53]. As a result, the terminally differentiated neurons remain in their postmitotic state through constitutive expression of cell checkpoint regulators like cyclin-dependent kinase 4 (CDK4) [4, 54, 55]. However, in neurodegenerative conditions like Huntington’s disease (HD) and AD, the pathogenic events trigger the cell cycle reentry in neurons [54, 56]. Yang et al. (2003) have indicated that aberrant cell cycle events in mitotically inactive neurons might lead to neuronal cell death responsible for the pathogenic progression of AD [57]. These abnormal cell cycle events in the brain have been linked to the upregulation of cell cycle proteins such as proliferating cell nuclear antigen (PCNA), cyclin D, and cyclin B [54]. In support of the above evidence, a higher expression level of cell cycle proteins was evident, particularly in the Cornu Ammonis-1 region of the hippocampus in AD subjects [57]. Experimental evidence suggests that neurons are at risk of resuming the cell cycle process due to abnormal cell cycle protein expression in AD, while the neurons with proliferative signals have been identified to be driven towards apoptosis accounting for neurodegenerations followed by memory impairment [57].
However, a substantial number of experimental studies conducted using transgenic animals and the human brain revealed abnormal cell cycle events along the neurogenic process as the early pathogenic sign of AD [58, 59]. Neurogenesis is the cellular process by which new neurons are generated from NSC through the development of neuroblasts or immature neurons in the brain [56, 60–62]. While robust neurogenesis is important for developing the brain, it appears to be gradually reduced upon aging but the generation of a considerable number of new neurons has become evident in the stem cell niches of the brain throughout the lifespan [62, 63]. Notably, regulation of neurogenesis has been linked to learning and memory in adulthood, while aging and neurodegenerative processes have been reported to negatively modulate the neurogenic process in the brain [64–66]. Eventually, failure in the neurogenic process has been regarded as a prominent underlying cause of the neurocognitive decline in various brain diseases including AD [56]. Tobin et al. (2019) reported a significant decrease in the doublecortin (DCX), a marker for immature neurons, and PCNA double-positive immature neurons in the postmortem brain samples of AD [67]. Another landmark study by Moreno-Jimenez and colleagues in 2019 reported that the impairment in the maturation of the DCX-expressing immature neurons in the hippocampus occurs during the progression of AD prior to the noticeable hyperphosphorylation of tubulin-associated unit (Tau) and accumulation of Aβ plaque [5]. Notably, AD brains have been characterized by abnormal hippocampal neurogenesis and defects in Wnt signaling [68]. As adult neurogenesis can be regulated by various cell cycle regulators and signaling pathways, investigation of the Wnt signaling responsible for the cell cycle events of NSCs has been a long-standing scientific interest [69]. Thus, it can be strongly presumed that abnormal Wnt signaling might be associated with impaired neurogenesis and memory loss in AD (Fig. 1). Choi et al. (2018) observed an improvement against AD pathology and cognitive behaviors by increasing hippocampal neurogenesis via induced Wnt3 using P7C3, a substance that increases the survival of NSC pathways in an animal model of AD [70]. Notably, the dysfunction of Wnt signaling has been reported to cause the prominent neuropathological events in AD as follows: 1) production and deposition of Aβ plaques, 2) phosphorylation of Tau proteins causing neurofibrillary tangles; 3) cognitive impairment linked to the aberrant hippocampal plasticity; and 4) dysregulation of neurogenesis [71, 72]. Aβ mediated reduced level of Wnt signaling has been reported in both the medial and temporal lobe of the hippocampus resulting in AD-like pathogenesis [73]. While activation of Wnt signaling promotes adult neurogenesis in the brain, many studies suggest that the Aβ mediated neurodegeneration in the AD brain is caused by a loss of the Wnt signaling [74, 75]. Therefore, scientific strategies for the restoration of Wnt signaling have become important to facilitate neurogenesis and memory enhancement in ADconditions.
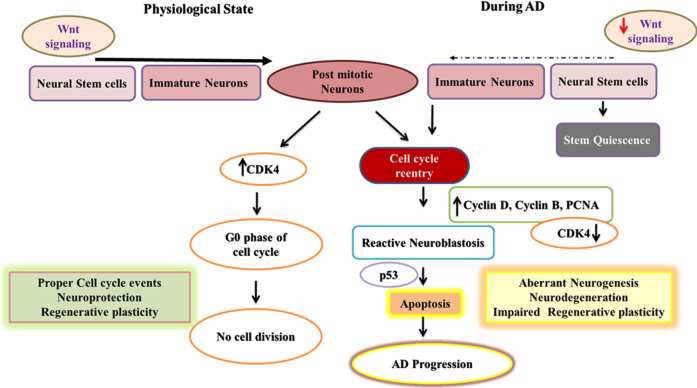
Fig. 1.
Adult neurogenesis in physiological state and mechanisms of cell cycle reentry of post-mitotic neurons in AD. This fig depicts the flowchart describing neurogenic process and the cell cycle reentry of post-mitotic neurons in association with Wnt signaling in healthy and AD conditions. In the normal brain, expression of CDK4 maintains the post-mitotic neurons at the G0 cell cycle phase whereas in the pathogenic process of AD the elevated expression of cyclin D, Cyclin B, and PCNA and downregulation of CDK4 results in the cell cycle reentry of post-mitotic neurons resulting in reactive neuroblastosis through the activation of Wnt signaling. Further, the activation of p53 in neurons can lead to apoptosis accounting for neurodegeneration in AD.
Aβ-MEDIATED ABERRANT WNT SIGNALING IN AD
Various studies implicate that Aβ-mediated defects in Wnt/β-catenin signal potentiate patho mechanisms of AD. During the generation of Aβ, multiple sequences of events are observed that includes a) inactivation of Wnt ligands, hence activating the GSK3β, b) induced degradation of β-catenin thus preventing its translocation into the nucleus, c) downregulation of the Wnt-mediated gene expression responsible for neuronal activities, and d) promotion of the deleterious effects through the production of reactive oxygen species accounting for the neuronal oxidative stress [72, 74, 76]. Experimental evidence supports that Aβ directly interacts with a Frizzled receptor, enabling Dickkopf (Dkk)-1 and impeding the activation of downstream transduction of Wnt [71, 74]. In AD, the overexpression of LRP5/6 ligand, Dkk-1 has been linked to the pathogenic cascade leading to neurodegeneration due to the antagonistic influence of Aβ on Wnt/β-catenin signaling [71]. Initial events with TCF/LEF-induced transcription of genes ensure β-catenin translocation into the nucleus that drives the cell to the S phase of the cell cycle [76]. Therefore, stimulation of Wnt signaling by external factors in the neurogenic niches may lead to a healthy cell cycle entry in quiescent NSCs in AD. Ample gene knockout/in studies suggests proper regulation of Wnt signaling events could result in the formation of new functional neurons in the brain.
In conditions like AD, the cell cycle reentry in mature neurons results in apoptosis, accounting for neurodegeneration rather than dedifferentiation. It has been reported that reduced β-catenin in the cytoplasm fails to activate the transcription levels of engrailed-1, a process that facilitates p53 activation and stimulates apoptosis accounting for neuronal death and impaired neural plasticity in the brain [77, 78]. The cell cycle reentry of neurons resulting in apoptosis is supported by recent evidence stating that Aβ reduces β-catenin levels in the cytoplasm of the neurons in the brains of AD subjects [79]. On the other hand, different in vitro and in vivo experiments revealed that overexpression of Wnt3 enhances neurogenesis in the hippocampus of the brain [80, 81]. However, enhanced Wnt signaling has been reported to result in the development of cancer [82]. Therefore, regulated expression of the Wnt ligand remains mandatory to elicit positive effects by promoting neural regeneration. Therefore, the current scenario demands suitable Wnt regulators rather than enhancers facilitating beneficial effects. Several pharmaceutical and natural agents are proposed to enhance Wnt activity and are recommended as a possible therapeutic method to promote the regeneration in AD [83, 84] (Fig. 2).
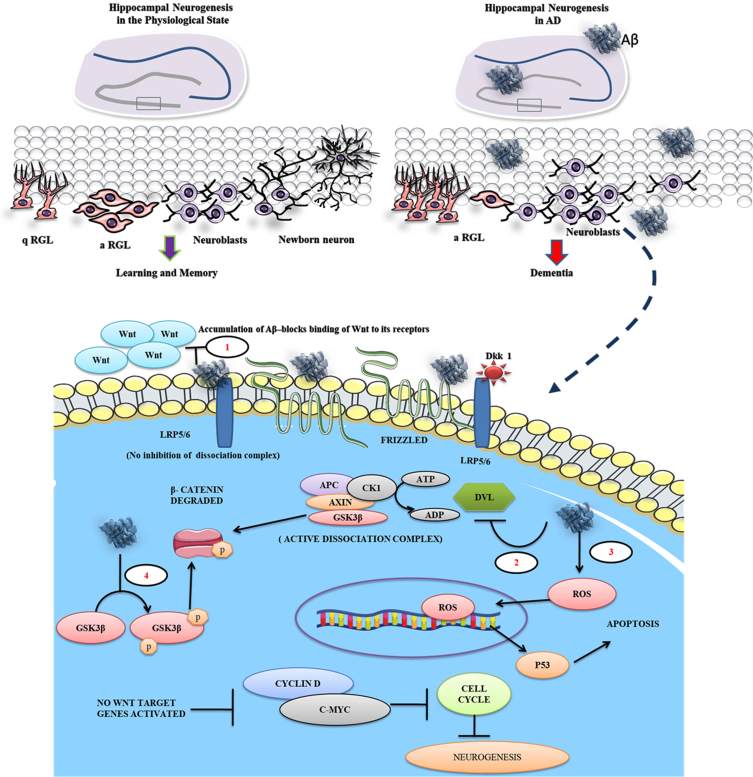
Fig. 2.
Aβ inhibits binding of Wnt ligand to the frizzled receptor and promotes β-catenin degradation. The figure illustrates that the quiescent radial glial cells (q-RGL) can be activated (a-RGL) which can give rise to newborn neurons through the generation of neuroblasts in the hippocampus of the normal brain responsible for learning and memory. In AD, the pathogenic Aβ plaques and their toxicity suppress the neurogenic process leading to dementia. The enlarged view represents the Aβ mediated proposed pathomechanism in a neuronal cell during AD at different levels. 1) Aβ antagonizes the Wnt signaling by binding to LRP5/6 and Frizzled receptors in the cell surface of the neurons, 2) Aβ deactivates Dvl, which in turn facilitates β-catenin degradation in the cytoplasm, 3) simultaneously, Aβ promotes the generation of reactive oxygen species (ROS), driving the cell toward apoptosis via p53 expression, and 4) Further, Aβ promotes phosphorylating of GSK3β and degrades β-catenin.
Moreover, the aberrant levels of cytokines and cell signaling regulators have been linked to altered SIRT1 pathways in conditions like aging and AD. Further, regulation of stem cell proliferation by the epigenetic modifications of some genes has been reported to be influenced by Wnt signaling [36]. SIRT1 is a nicotinamide adenine dinucleotide (NAD) + dependent lysine deacetylase, recognized to be involved in multiple metabolic events in association with Wnt signaling [85]. However, SIRT1 levels display an inverse proportionality with an increase in age [23, 91]. SIRT1 deficiency in abnormal aging and AD has been reported to be associated with impairment of synaptic plasticity and memory loss [87]. Therefore, targeting the transcriptional regulation of candidate genes through the activation of SIRT1 appears to be the most effective way of defending and managing the cellular, molecular, and biochemical defects in variousdiseases.
SIRT1-mediated histone modifications have been reported to be involved in multiple cellular events such as stress response, cellular differentiation, metabolism, and cell proliferation [88]. Alternatively, an age-dependent decrease in SIRT1 activity appears to be associated with abnormalities in the function of cell survival and trophic factors viz., brain-derived neurotrophic factor (BDNF), cAMP response element-binding protein (CREB), Dkk1, and Wnt that results in an aberrant cell cycle events leading to apoptosis, neurodegeneration, and impaired neurogenesis. Interestingly, recruiting H3K4 trimethyl transferase by SET Domain Containing 1A (SETD1A, Histone Lysine Methyltransferase), a chromatin remodeler of H3-Lys-4 by mono,- di- and trimethylation influencing its gene expression, stimulates β-catenin activity in NSCs, thereby promoting neuronal differentiation [89]. While the failure of the canonical Wnt pathway could be regarded as a causative factor for aberrant cell cycle events leading to AD pathogenesis, considering the use of SIRT1 activators to restore and promote the activity of the Wnt pathway may hold a therapeutic key to mitigate the pathogenic signature of AD [72]. Therefore, it necessitates unraveling the role of SIRT1’s epigenetic action on stem cell fate in AD.
EPIGENETIC ROLES OF SIRT1 IN FATE DETERMINATION OF NSCs ALONG THE NEUROGENIC PROCESS IN THE ADULT BRAIN
Neuronal commitment and differentiation of NSCs during development are tightly controlled by regulatory mechanisms responsible for the cytoarchitecture of the brain. Epigenetic mechanisms involving changes in DNA methylation, non-coding RNA expression, and histone modification determines the fate of NSCs in the brain [96]. During the embryonic development of the brain, the epigenetic regulation of stem cell fate has been known to be carried out by sirtuins, while the prominent expression of SIRT1is highly evident in several areas of the brain [91, 92]. The deacetylase activity of SIRT1 has recently been identified to influence the final fate of neural precursor cells [93]. SIRT1 recruitment at the hairy and enhancer of split (Hes)-5 promoter modulates histone acetylation, which promotes neurogenesis via SIRT1-dependent epigenetic suppression of specific notch targets [94]. The function of SIRT1 is associated with restricted calorie intake and is also vital for preserving neurophysiological functions upon ageing. Notably, SIRT1 has been identified as a therapeutic target in numerous neurodegenerative illnesses, including AD and Parkinson’s disease (PD). It has been reported that in many experimental models of brain pathologies, SIRT1 regulates the fate of NSCs [95, 96]. In a reducing environment, SIRT1 does not allow to bind to the mammalian achaete-scute homolog (Mash)-1, a neuronal fate determination factor of NSCs in the brain whereas in oxidative conditions, SIRT1 shifts the cell fate of NSCs toward the astroglial lineage [97, 98]. SIRT1 is expressed by proliferating NSCs and regulates their neurogenic potential in the sub-ventricular zone and hippocampus of the adult brain [99]. It has been reported that the expression of SIRT1 improves neurite outgrowth and prevents synaptic loss in association with a transient expression of miR-134 in AD models [100]. Taken together, the above evidence suggests the epigenetic activity of SIRT1 and its significant role in the fate determination of NSCs in the brain. However, additional experiments are required to reveal the functional roles of SIRT1 in the regulation of NSCs in the brain. Eventually, unraveling the therapeutic efficacy of SIRT1 can be vital in neurogenic stimulation for treating neurodegenerative diseases like AD [101]. Therefore, consideration of drugs like RSV that promote SIRT1 expression could be a promising therapeutic modality for differentdiseases.
RESVERATROL IS A POTENT THERAPEUTIC DRUG TO MITIGATE THE PATHOGENESIS OF AD
Decades of research have revealed that diet and nutritional factors play a major role in maintaining homeostasis, and proved to be standard therapy for managing numerous disorders. Polyphenols are phytochemicals found largely in many vegetables, fruits, coffee, chocolate, legumes, and beverages. There are over 8000 naturally existing polyphenolic compounds that mainly function as antioxidants. Polyphenols, flavonoids, caloric restriction, and physical activity are the inducers of homeostasis and exhibit positive effects on neurogenesis in the brain [102–104]. A variety of plant-derived polyphenols exhibit pleiotropic properties and show beneficial neuroprotective effects by improving cognition and memory in various models by interacting with a wide number of pathways involved in neuronal damage [102, 105]. Plants that contain antioxidants and secondary metabolites like RSV [106], curcumin [107], and epigallocatechin gallate [108], can exhibit positive effects in reverting AD pathology. Of which, RSV, a natural stilbene has been a drug of interest to many researchers around the world to investigate its protective benefits in neurodegenerative disorders [109]. Interestingly, RSV is present in a large number of plant species with the capability of mimicking calorie restriction, anti-oxidative effects, anti-inflammatory, anti-diabetogenic, anti-cancer, and anti-aging properties [110]. Initially, RSV has been recognized for its potential anti-oxidant effect against cardiovascular disease [111]; however, its applications later expanded to anti-cancer and anti-inflammatory activities [112]. RSV has been found to protect the nervous system from ischemic stroke, traumatic brain injury, PD, HD, and AD [113, 114]. Taken together, the role of RSV as a pro-neurogenic drug and its neuroregenerative potential in various conditions through SIRT1 action on the Wnt signaling and cell cycle events needs to be considered for the effective management of AD.
Interestingly, RSV has been found to interact with multi-targeted pathophysiological pathways associated with diseases like AD, thereby serving as a better therapeutic candidate. With respect to optimum dosage and effective administration of RSV, 300 mg to 1000 mg of RSV/kilogram body weight has been considered safe without any side effects. In particular, no obvious toxicity to the kidney and liver was observed in mice up to 1000 mg/kg body weight [115]. Further, ingestion of RSV up to 1 gram per day in a short-term dose did not exhibit any side effects among patients with non-alcoholic fatty liver disease [116]. Also, no significant adverse effect has been reported in long-term clinical trials using RSV upon treating various conditions [117]. RSV appears to reduce the effect of Caspase-8-mediated apoptosis via p38MAPK signaling [118, 119]. Despite the dose and age-dependent effect, several clinical trials suggest that the optimum dosage of RSV is highly beneficial against cancer, stroke, cardiovascular diseases, obesity, diabetes, and AD [117]. Thus, exploring the role of RSV with special reference to neurogenesis could gain more insight into the treatment value of RSV against AD. RSV in general activates various factors that are involved in regulation of neurogenesis like Wnt, NR2E1, Sox, and BMP via modulating SIRT1 levels [20, 93, 120]. RSV is a well-known polyphenolic compound that is well-reported for its sirtuin-enhancing capability in vivo conditions. Various experimental data suggest that RSV administration may promote sirtuin levels that could help maintain homeostasis in vivo and can extend its cytoprotective effect over a wide range of metabolic disorders and AD. RSV exhibits anti-neuroinflammatory properties by inhibiting activated microglia involved in the priming of neurodegenerative events [121]. Likewise, RSV ameliorates the expression of tumor necrosis factor-α, which reduces microglial activation, neuroinflammation, and oxidative stress-mediated neuronal degeneration [122, 123]. Aβ 1 - 42 accumulation in intercellular regions stimulates the phosphorylation of SIRT1 protein and thereby preventing the binding of CREB to Hes1, while RSV administration has been reported to reverse this adverse event and promote neurogenesis in the hippocampus [124, 125]. Modulation of SIRT1 levels may have a prominent role in treating geriatric disorders, which also alters the epigenetic regulation of genes in an age-independent manner. RSV administration regulates the apoptotic functions of both p53 and forkhead box O (FOXO), a transcription factor involved in multiple cell signaling pathways via SIRT1 expression in both in vivo and in vitro circumstances and confers neuroprotection in AD [126]. RSV-mediated upregulation of SIRT1 activity influences many cell survival and proliferation pathways like AMP-activated protein kinase (AMPK), phosphatidylinositol 3-kinase (PI3K), protein kinase B (PKB), and BDNF [127, 128]. Our research group over a decade has been working on the cognitive efficacy of RSV in normal-aged rats and various rat models of AD [23, 27, 129]. It was evidenced that RSV exhibits a neuroprotective effect and positively influences cognitive ability through SIRT1 enhancement in rodents [27, 129]. Interestingly, a considerable amount of experimental data suggests that RSV elicits its effects through upregulating SIRT1 to promote cell proliferation and improve neurogenesis in the hippocampus in different neurodegenerative conditions as well as normal aging, but the cell fate-determining role of SIRT1 mediated by RSV in the stem cell niches of the adult brain remains less explored.
THE BENEFIT OF RSV ON THE REGULATION OF NEUROREGENERATIVE PLASTICITY
RSV appears to elicit profound positive biological effects on hippocampal neurons. It is effective against prenatal stress-induced neuronal dysfunction, by increasing the expression of BDNF [27, 130]. RSV protects the neuronal cells from environmental toxicity and promotes proliferation, fate determination, and differentiation and survival of NSCs in vitro and in vivo in normal physiology and during pathology [131, 132]. Pretreatment with RSV has rescued ethanol-induced decline of granule cells and impairments in their dendritic spines in the hippocampus during early postnatal stages of experimental animals [133]. Besides, RSV has been reported to facilitate the proliferation and differentiation of NSC via SIRT1 enhancement in the hippocampus [133, 134]. Moreover, the intraperitoneal administration of RSV has been reported to protect hippocampal glutamatergic neurons, by increasing the phosphorylation of PKB responsible for inducing the neuronal differentiation pathway in NSCs [135]. The neuroprotective and neurogenic effects of RSV in the hippocampus have been linked to enhanced spatial learning and memory in aging and experimental animal models of neurodegenerative disorders [23, 136, 137].
RSV is a well-proven calorific restriction-mimicking drug that appears to minimize the metabolic defect of stem cells leading to their improved self-renewal and regenerative capacity. Calorific restriction acts on the stem cell niche through numerous mechanisms, including activating diverse targets such as FOXO, SIRT1, and AMPK, that promote genes involved in cell growth, proliferation, differentiation, and longevity [138]. Similarly, switching diets to RSV-rich foods has resulted in ideal body weight and fat loss in 6 weeks and considerable upregulation in the production of newborn neurons in the brain [139]. RSV treatment in late middle age is beneficial for improving cognition and mood swings later in old age, likely through positive alteration of the hippocampal neural circuitry and inhibition of chronic inflammation, which collectively accounts for the physiological functions mediated by RSV [140]. RSV treatment has been reported to enhance neurogenesis and memory in experimental models with lead-induced neurotoxicity [141]. However, the exact molecular action of RSV in enhancing memory via promoting hippocampal plasticity remains unclear and needs further experimentalassessment.
THE POSSIBLE THERAPEUTIC EFFECT OF RSV ACTING VIA THE WNT PATHWAY IN SYNERGY WITH SIRT1
SIRT1 is a transient and constitutive regulator of Wnt signaling as it interacts with Dvl proteins which are involved in the degradation of β-catenin in the cytoplasm [142]. In addition, SIRT1 inhibits GSK3β activity that promotes synaptogenesis in cultured hippocampal neurons by deacetylating PKB [143]. In other aspects, SIRT1 can also deacetylate Dkk1 protein thereby positively regulating the Wnt pathway. In contrast to the above findings, SIRT1 gene knockout increased the Dkk1 activity in cancer cell lines [144]. Thus, it is evident that SIRT1 could exhibit a modulatory effect on the Wnt signaling events in a context-dependent manner. It has been reported that several polyphenols promote the Wnt/β-catenin-related transcripts, thereby protecting neurons against oxidative stress and inflammation. The RSV-like compound exhibits its neuroprotective efficiency through SIRT1 enhancing ability. Increased SIRT1 activity appears to promote nuclear accumulation of β-catenin due to its deacetylation process leading to endogenous Wnt activation [145]. Therefore, we hypothesize that RSV could modulate Wnt signaling in multiple ways, presumably by increasing SIRT1, which may act on Dvl proteins to prevent β-catenin degradation. Here, we propose that SIRT1 may facilitate the activity of Dvl proteins to prevent the proteasomal degradation of β-catenin independent of the Wnt ligand. Simultaneously, deacetylation of β-catenin by SIRT1 may promote its translocation into the nucleus thereby facilitating the transcription of Wnt target genes independent of the ligand (Fig. 3).
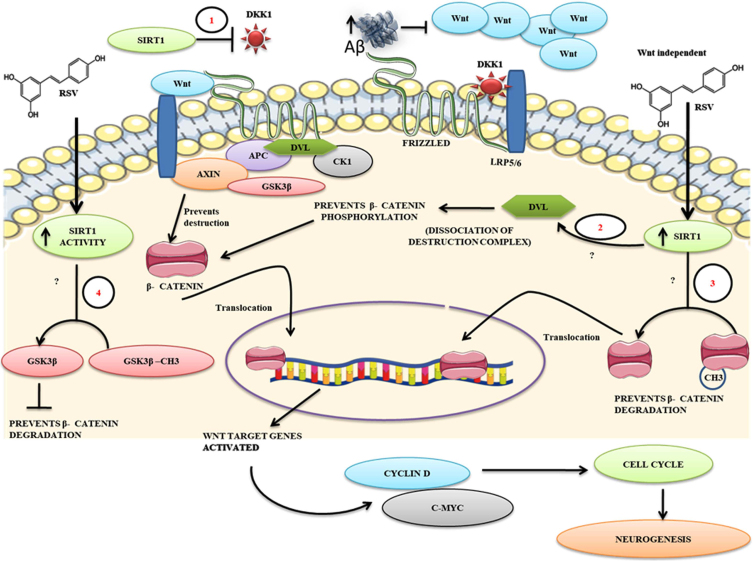
Fig. 3.
A proposed mechanism for RSV against Aβ mediated impaired neurogenesis. The figure describes the possibilities of how Aβ induces aberrant Wnt activity resulting in apoptosis. 1) RSV treatment-mediated intracellular increase of SIRT1 activity may decrease the expression of Dkk1 protein levels thereby facilitating positive Wnt signaling. 2) Blocked on Wnt signaling at the extracellular level by Aβ can be surpassed by RSV-mediated SIRT1 enhancement at the level of activation of Dvl and disassociate the β-catenin destruction complex β-catenin phosphorylation, 3) Enhanced SIRT1 levels in vivo may directly deacetylate β-catenin thereby promoting its translocation into the nucleus independent of the ligand, 4) RSV mediated SIRT1 activity might deacetylate GSK3β by which it may prevent the destruction of β-catenin. Overall, the action of SIRT1 on these key events that promote Wnt signaling to a positive cell cycle might enhance the neurogenic process in AD-like conditions thereby facilitating neural regeneration.
FUTURE PERSPECTIVES
Stem cell-based regenerative therapy has been a challenging approach in recent years due to the lack of defined therapeutic targets and scientific tools that positively modulate the proliferation and differentiation of stem cells. Current research fails to precisely characterize the beneficial effects of different stem cells in AD-like conditions. The concept of recruiting newly generated neurons from endogenous NSCs to treat AD and other neurodegenerative disorders opens novel therapeutic strategies. However, the cellular and molecular mechanisms that drive the progression of dividing NSCs into functional neurons in regions of neurodegeneration needs to be revealed. Finding a foregoing substance that elicits a positive impact on NSC proliferation and differentiation is crucial in managing and restoring functional loss in neurodegenerative conditions. Increasing neurogenic potentials of NSCs in the hippocampus of AD patients could be effective therapeutics to compensate for neurodegeneration thus preventing AD progression to memory loss. Among various drugs, RSV appears to be a promising naturally occurring candidate compound as it mitigates various pathogenic events and promotes tissue regeneration with no obvious side effects. Hence, considering RSV treatment to manage AD conditions may provide excellent support to enhance neurogenesis in the hippocampus. However, this notion raises many questions, like how activities of NSCs could be controlled by RSV and what could be the obvious role of RSV in regulating the stem cell niche. This review addressed the possible mechanism of RSV’s effects in boosting neuroregenerative aspects in the hippocampus via SIRT1 and Wnt pathways. The novel methods for characterization of the relevance and therapeutic efficacy of RSV on adult NSCs acting via SIRT1 and Wnt pathways using knockout model systems may demonstrate RSV as a promising supplement for neurodegenerative disease and dementia. Further, in silico approach to transcriptomic data applying SIRT1 and Wnt pathways on neurogenic events may help to address vital targets of neural regeneration in AD.
Thus, a continued study in this field is essential for identifying whether therapies that improve neurogenesis have the potential to help people with neurodegenerative disorders. This review is a suggestive of dynamic research on the much more detailed role of RSV in key events oriented to the Wnt signaling and cell cycle events that may promote the possibility of neuroregenerative plasticity in AD. Therefore, RSV may be a potent drug for individuals suffering from AD that may ensure healthy aging and prolonged survival and defend the memory loss to some extent.
CONCLUSION
RSV appears to be a promising natural drug in the fields of biomedical science and neuro-regenerative medicine. RSV has undergone a wide variety of experimental paradigms for its pleiotropic functions, particularly in neurodegenerative conditions. RSV is well known for its neurogenesis-stimulating characteristics in both physiological and diseased conditions. Under neurodegenerative conditions such as AD, RSV has been shown to generate new neurons from the quiescent NSCs in the hippocampus of the adult brain. However, over some decades of research, it is a quite unambiguous to know which pathway positively responds to RSV intervention in the cellular system. The upregulation of SIRT1 under RSV treatment appears to be the most promising to mitigate the pathogenesis of AD. Furthermore, RSV elicits its effects by promoting the transcription factors responsible for NSCs differentiation and integration into the central cognitive circuit through SIRT. SIRT1 has widely been recognized to regulate the differentiation and proliferation potential of various cultured pluripotent stem cells by upregulating the Wnt pathway. Similarly, SIRT1 effectively regulates the Wnt signaling components and its target genes in vivo conditions and several cell lines. The influence of SIRT1 in the Wnt pathway extends to various area of research that includes cancer biology, stem cell biology as well in neuroscience. Though attempt with transplantation of NSCs has been considered the potential advantage to rescue AD that involves invasive procedures and represent chances of potential adverse effects. Thus, the use of RSV as a non-invasive therapeutic option can be highly relevant to activate the endogenous neurogenic process to manage AD. Extending future research on RSV’s effects on cell cycle events of NSCs and neurogenesis will answer many unknown facts behind the current scenario of RSV treatment and neural regeneration.
ACKNOWLEDGMENTS
The authors acknowledge DST-FIST for the infrastructure of the Department of Biochemistry and Animal Science, Bharathidasan University. The authors thank DST- Cognitive science research initiative (CSRI) (DST/CSRI/2018/343(G)) for providing financial support and SK has been supported by DST-CSRI as JRF. MK has been supported by the Faculty Recharge Programme, University Grants Commission (UGC-FRP), New Delhi, India. MA, KSJ, and MK acknowledge RUSA (Rashtriya Uchchatar Shiksha Abhiyan) 2.0. The authors also express their gratitude towards DST-PURSE, ICMR (2019-2605/CMB/Adhoc-BMS) for funding. The authors extend sincere thanks to Ms. Mercy Priyadharshini B and Ms. Kamali A for their help in proofreading and language editing of the manuscript.
Parts of the all figures were created using Servier Medical Art, which was offered by Servier and is licensed under a Creative Commons Attribution 3.0 Unported license.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0559r2).
REFERENCES
- [1]. Botchway B, Iyer IC (2017) Alzheimer’s disease– the past, the present and the future. Science 6, 1–19. [Google Scholar]
- [2]. GBD 2019 Dementia Forecasting Collaborators (2022) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7, e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Babcock KR, Page JS, Fallon JR, Webb AE (2021) Adult hippocampal neurogenesis in aging and Alzheimer’s disease. Stem Cell Rep 16, 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Lee H, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Petersen RB, Bajic V, Smith MA (2009) Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer’s disease. Neurochem Int 54, 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M (2019) Adult hippocampal neurogenesis isabundant in neurologically healthy subjects and drops sharply inpatients with Alzheimer’s disease. Nat Med 25, 554–560. [DOI] [PubMed] [Google Scholar]
- [6]. Zeng Q, Zheng M, Zhang T, He G (2016) Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple transgenic mouse model of Alzheimer’s disease. Neuroscience 314, 64–74. [DOI] [PubMed] [Google Scholar]
- [7]. Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27, 1372–1384. [DOI] [PubMed] [Google Scholar]
- [8]. Nagy Z, Esiri MM, Smith AD (1997) Expression of cell division markers in the hippocampus in Alzheimer’s disease and other neurodegenerative conditions. Acta Neuropathol (Berl) 93, 294–300. [DOI] [PubMed] [Google Scholar]
- [9]. Arredondo SB, Valenzuela-Bezanilla D, Mardones MD, Varela-Nallar L (2020) Role of Wnt signaling in adult hippocampal neurogenesis in health and disease. Front Cell Dev Biol 8, 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Zheng R, Zhang Z-H, Chen C, Chen Y, Jia S-Z, Liu Q, Ni J-Z, Song G-L (2017) Selenomethionine promoted hippocampal neurogenesis via the PI3K-Akt-GSK3β-Wnt pathway in a mouse model of Alzheimer’s disease. Biochem Biophys Res Commun 485, 6–15. [DOI] [PubMed] [Google Scholar]
- [11]. Loera-Valencia R, Cedazo-Minguez A, Kenigsberg P a., Page G, Duarte A i., Giusti P, Zusso M, Robert P, Frisoni GB, Cattaneo A, Zille M, Boltze J, Cartier N, Buee L, Johansson G, Winblad B (2019) Current and emerging avenues for Alzheimer’s disease drug targets. J Intern Med 286, 398–437. [DOI] [PubMed] [Google Scholar]
- [12]. Kim S, Kaang B-K (2017) Epigenetic regulation and chromatin remodeling in learning and memory. Exp Mol Med 49, e281–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Nalivaeva NN, Turner AJ (2016) AChE and the amyloid precursor protein (APP)– Cross-talk in Alzheimer’s disease. Chem Biol Interact 259, 301–306. [DOI] [PubMed] [Google Scholar]
- [14]. Ma DK, Marchetto MC, Guo JU, Ming G, Gage FH, Song H (2010) Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci 13, 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Matsubara S, Matsuda T, Nakashima K (2021) Regulation of adult mammalian neural stem cells and neurogenesis by cell extrinsic and intrinsic factors. Cells 10, 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Satoh A, Imai S, Guarente L (2017) The brain, sirtuins, and ageing. Nat Rev Neurosci 18, 362–374. [DOI] [PubMed] [Google Scholar]
- [17]. Wątroba M, Dudek I, Skoda M, Stangret A, Rzodkiewicz P, Szukiewicz D (2017) Sirtuins, epigenetics and longevity. Ageing Res Rev 40, 11–19. [DOI] [PubMed] [Google Scholar]
- [18]. Lu Y, Zhou L, Wang L, He S, Ren H, Zhou N, Hu Z (2020) The role of SIRT1 in BMP2-induced chondrogenic differentiation and cartilage maintenance under oxidative stress. Aging 12, 9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Qu C, Qu C, Xu L, Shen J, Lv D, Li Y, Song H, Li T, Zheng J, Zhang J (2021) Nuclear receptor TLX may be through regulating the SIRT1/NF-κB pathway to ameliorate cognitive impairment in chronic cerebral hypoperfusion. Brain Res Bull 166, 142–149. [DOI] [PubMed] [Google Scholar]
- [20]. Yoon DS, Choi Y, Jang Y, Lee M, Choi WJ, Kim S-H, Lee JW (2014) SIRT1 directly regulates SOX2 to maintain self-renewal and multipotency in bone marrow-derived mesenchymal stem cells. Stem Cells 32, 3219–3231. [DOI] [PubMed] [Google Scholar]
- [21]. Wang X, Ma S, Meng N, Yao N, Zhang K, Li Q, Zhang Y, Xing Q, Han K, Song J, Yang B, Guan F (2016) Resveratrol exerts dosage-dependent effects on the self-renewal and neural differentiation of hUC-MSCs. Mol Cells 39, 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Zia A, Sahebdel F, Farkhondeh T, Ashrafizadeh M, Zarrabi A, Hushmandi K, Samarghandian S (2021) A review study on the modulation of SIRT1 expression by miRNAs in aging and age-associated diseases. Int J Biol Macromol 188, 52–61. [DOI] [PubMed] [Google Scholar]
- [23]. Moorthi P, Premkumar P, Priyanka R, Jayachandran KS, Anusuyadevi M (2015) Pathological changes in hippocampal neuronal circuits underlie age-associated neurodegeneration and memory loss: Positive clue toward SAD. Neuroscience 301, 90–105. [DOI] [PubMed] [Google Scholar]
- [24]. Iside C, Scafuro M, Nebbioso A, Altucci L (2020) SIRT1 activation by natural phytochemicals: An overview. Front Pharmacol 11, 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Moraes DS, Moreira DC, Andrade JMO, Santos SHS (2020) Sirtuins, brain and cognition: A review of resveratrol effects. IBRO Rep 9, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Safaeinejad Z, Kazeminasab F, Kiani-Esfahani A, Ghaedi K, Nasr-Esfahani MH (2018) Multi-effects of Resveratrol on stem cell characteristics: Effective dose, time, cell culture conditions and cell type-specific responses of stem cells to Resveratrol. Eur J Med Chem 155, 651–657. [DOI] [PubMed] [Google Scholar]
- [27]. Sathya M, Moorthi P, Premkumar P, Kandasamy M, Jayachandran KS, Anusuyadevi M (2017) Resveratrol intervenes cholesterol- and isoprenoid-mediated amyloidogenic processing of AβPP in familial Alzheimer’s disease. J Alzheimers Dis 60, S3–S23. [DOI] [PubMed] [Google Scholar]
- [28]. Karthick C, Nithiyanandan S, Essa MM, Guillemin GJ, Jayachandran SK, Anusuyadevi M (2019) Time-dependent effect of oligomeric amyloid-β (1-42)-induced hippocampal neurodegeneration in rat model of Alzheimer’s disease. Neurol Res 41, 139–150. [DOI] [PubMed] [Google Scholar]
- [29]. Xu S, Sun F, Ren L, Yang H, Tian N, Peng S (2017) Resveratrol controlled the fate of porcine pancreatic stem cells through the Wnt/β-catenin signaling pathway mediated by Sirt1, PloS One 12, e0187159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Liu H, Zhang S, Zhao L, Zhang Y, Li Q, Chai X, Zhang Y (2016) Resveratrol enhances cardiomyocyte differentiation of human induced pluripotent stem cells through inhibiting canonical WNT signal pathway and enhancing serum response factor-miR-1 axis. Stem Cells Int 2016, 2524092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Solfrizzi V, Panza F, Frisardi V, Seripa D, Logroscino G, Imbimbo BP, Pilotto A (2011) Diet and Alzheimer’s disease risk factors or prevention: The current evidence. Expert Rev Neurother 11, 677–708. [DOI] [PubMed] [Google Scholar]
- [32]. Silva MVF, Loures C de MG, Alves LCV, de Souza LC, Borges KBG, Carvalho M das G (2019) Alzheimer’s disease: Risk factors and potentially protective measures. J Biomed Sci 26, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Periyasamy S, Sathya M, Karthick C, Kandasamy M, Shanmugaapriya S, Tamilselvan J, Jayachandran KS, Anusuyadevi M (2017) Association studies of specific cholesterol related genes (APOE, LPL, and CETP) with lipid profile and memory function: A correlative study among rural and tribal population of Dharmapuri District, India, J Alzheimers Dis 60, S195–S207. [DOI] [PubMed] [Google Scholar]
- [34]. Tanzi RE (2012) The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2, a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Cacquevel M, Aeschbach L, Houacine J, Fraering PC (2012) Alzheimer’s disease-linked mutations in presenilin-1 result in a drastic loss of activity in purified γ-secretase complexes, PloS One 7, e35133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Hampel H, Mesulam M-M, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141, 1917–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Nguyen LD, Ehrlich BE (2020) Cellular mechanisms and treatments for chemobrain: Insight from aging and neurodegenerative diseases.EMBO Mol Med 12, e12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Bird TD, Stranahan S, Sumi SM, Raskind M (1983) Alzheimer’s disease: Choline acetyltransferase activity in brain tissue from clinical and pathological subgroups. Ann Neurol 14, 284–293. [DOI] [PubMed] [Google Scholar]
- [39]. Zhang F, Jiang L (2015) Neuroinflammation in Alzheimer’s disease. Neuropsychiatr Dis Treat 11, 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Chen X, Yang J, Evans PM, Liu C (2008) Wnt signaling: The good and the bad. Acta Biochim Biophys Sin 40, 577–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Nusse R (2012) Wnt signaling, Cold Spring Harb Perspect Biol 4, a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Minde DP, Radli M, Forneris F, Maurice MM, Rüdiger SG (2013) Large extent of disorder in Adenomatous Polyposis Coli offers a strategy to guard Wnt signalling against point mutations. PloS One 8, e77257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Lie D-C, Colamarino SA, Song H-J, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR (2005) Wntsignalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375. [DOI] [PubMed] [Google Scholar]
- [44]. Teo J-L, Kahn M (2010) The Wnt signaling pathway in cellular proliferation and differentiation: A tale of two coactivators. Adv Drug Deliv Rev 62, 1149–1155. [DOI] [PubMed] [Google Scholar]
- [45]. Mohammed MK, Shao C, Wang J, Wei Q, Wang X, Collier Z, Tang S, Liu H, Zhang F, Huang J (2016) Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis 3, 11–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Michaelidis TM, Lie DC (2008) Wnt signaling and neural stem cells: Caught in the Wnt web. Cell Tissue Res 331, 193–210. [DOI] [PubMed] [Google Scholar]
- [47]. Jagasia R, Song H, Gage FH, Lie DC (2006) New regulators in adult neurogenesis and their potential role for repair. Trends Mol Med 12, 400–405. [DOI] [PubMed] [Google Scholar]
- [48]. (2012) Mitotic and mitogenic Wnt signalling. EMBO J 31, 2705–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Bielen H, Houart C (2014) The Wnt cries many: Wnt regulation of neurogenesis through tissue patterning, proliferation, and asymmetric cell division. Dev Neurobiol 74, 772–780. [DOI] [PubMed] [Google Scholar]
- [50]. Arredondo SB, Valenzuela-Bezanilla D, Santibanez SH, Varela-Nallar L (2022) Wnt signaling in the adult hippocampal neurogenic niche. Stem Cells 40, 630–640. [DOI] [PubMed] [Google Scholar]
- [51]. Hardwick LJA, Ali FR, Azzarelli R, Philpott A (2015) Cell cycle regulation of proliferation versus differentiation in the central nervous system. Cell Tissue Res 359, 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Kato H, Takahashi A, Itoyama Y (2003) Cell cycle protein expression in proliferating microglia and astrocytes following transient global cerebral ischemia in the rat. Brain Res Bull 60, 215–221. [DOI] [PubMed] [Google Scholar]
- [53]. Yoshikawa K (2000) Cell cycle regulators in neural stem cells and postmitotic neurons. Neurosci Res 37, 1–14. [DOI] [PubMed] [Google Scholar]
- [54]. Manickam N, Radhakrishnan RK, Vergil Andrews JF, Selvaraj DB, Kandasamy M (2020) Cell cycle re-entry of neurons and reactive neuroblastosis in Huntington’s disease: Possibilities for neural-glial transition in the brain. Life Sci 263, 118569. [DOI] [PubMed] [Google Scholar]
- [55]. Schmetsdorf S, Gärtner U, Arendt T (2007) Constitutive expression of functionally active cyclin-dependent kinases and their binding partners suggests noncanonical functions of cell cycle regulators in differentiated neurons. Cereb Cortex 17, 1821–1829. [DOI] [PubMed] [Google Scholar]
- [56]. Kandasamy M, Anusuyadevi M, Aigner KM, Unger MS, Kniewallner KM, de Sousa DMB, Altendorfer B, Mrowetz H, Bogdahn U, Aigner L (2020) TGF-β signaling: A therapeutic target to reinstate regenerative plasticity in vascular dementia? Aging Dis 11, 828–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Yang Y, Mufson EJ, Herrup K (2003) Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neurosci 23, 2557–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Yoneyama M, Shiba T, Hasebe S, Ogita K (2011) Adult neurogenesis is regulated by endogenous factors produced during neurodegeneration. J Pharmacol Sci 115, 425–432. [DOI] [PubMed] [Google Scholar]
- [59]. Demars M, Hu Y-S, Gadadhar A, Lazarov O (2010) Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res 88, 2103–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Pino A, Fumagalli G, Bifari F, Decimo I (2017) New neurons in adult brain: Distribution, molecular mechanisms and theraies. Biochem Pharmacol 141, 4–22 . [DOI] [PubMed] [Google Scholar]
- [61]. Kandasamy M, Aigner L (2018) Reactive neuroblastosis in huntington’s disease: A putative therapeutic target for striatal regeneration in the adult brain. Front Cell Neurosci 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Kandasamy M, Aigner L (2018) Neuroplasticity, limbic neuroblastosis and neuro-regenerative disorders. Neural Regen Res 13, 1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Denoth-Lippuner A, Jessberger S (2021) Formation and integration of new neurons in the adult hippocampus. Nat Rev Neurosci 22, 223–236. [DOI] [PubMed] [Google Scholar]
- [64]. Shohayeb B, Diab M, Ahmed M, Ng DCH (2018) Factors that influence adult neurogenesis as potential therapy. Transl Neurodegener 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Marxreiter F, Nuber S, Kandasamy M, Klucken J, Aigner R, Burgmayer R, Couillard-Despres S, Riess O, Winkler J, Winner B (2009) Changes in adult olfactory bulb neurogenesis in mice expressing the A30P mutant form of alpha-synuclein. Eur J Neurosci 29, 879–890. [DOI] [PubMed] [Google Scholar]
- [66]. Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, Bogdahn U, Aigner L (2009) Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry 14, 856–864. [DOI] [PubMed] [Google Scholar]
- [67]. Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, Kim N, Dawe RJ, Bennett DA, Arfanakis K, Lazarov O (2019) Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 24, . , 974–982.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Choe Y, Pleasure SJ, Mira H (2016) Control of adult neurogenesis by short-range morphogenic-signaling molecules . Cold Spring Harb Perspect Biol 8, a018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Faigle R, Song H (2013) Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta 1830, 2435–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C (2018) Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361, eaan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Inestrosa NC, Toledo EM (2008) The role of Wnt signaling in neuronal dysfunction in Alzheimer’s disease. Mol Neurodegener 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Tapia-Rojas C, Inestrosa NC (2018) Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen Res 13, 1705–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Riise J, Plath N, Pakkenberg B, Parachikova A (2015) Aberrant Wnt signaling pathway in medial temporal lobe structures of Alzheimer’s disease. J Neural Transm 122, 1303–1318. [DOI] [PubMed] [Google Scholar]
- [74]. Magdesian MH, Carvalho MM, Mendes FA, Saraiva LM, Juliano MA, Juliano L, Garcia-Abreu J, Ferreira ST (2008) Amyloid-β binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/β-catenin signaling. J Biol Chem 283, 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Rosenbloom AB, Tarczyński M, Lam N, Kane RS, Bugaj LJ, Schaffer DV (2020) β-Catenin signaling dynamics regulate cell fate in differentiating neural stem cells. Proc Natl Acad Sci U S A 117, 28828–28837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Caricasole A, Copani A, Caruso A, Caraci F, Iacovelli L, Sortino MA, Terstappen GC, Nicoletti F (2003) The Wnt pathway, cell-cycle activation and β-amyloid: Novel therapeutic strategies in Alzheimer’s disease? Trends Pharmacol Sci 24, 233–238. [DOI] [PubMed] [Google Scholar]
- [77]. Boonen RA, van Tijn P, Zivkovic D (2009) Wnt signaling in Alzheimer’s disease: Up or down, that is the question. Ageing Res Rev 8, 71–82. [DOI] [PubMed] [Google Scholar]
- [78]. Fuentealba RA, Farias G, Scheu J, Bronfman M, Marzolo MP, Inestrosa NC (2004) Signal transduction during amyloid-β-peptide neurotoxicity: Role in Alzheimer disease. Brain Res Rev 47, 275–289. [DOI] [PubMed] [Google Scholar]
- [79]. Chi H, Chang H-Y, Sang T-K (2018) Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci 19, 3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Noureddini M, Bagheri-Mohammadi S (2021) Adult hippocampal neurogenesis and Alzheimer’s disease: Novel application of mesenchymal stem cells and their role in hippocampal neurogenesis. Int J Mol Cell Med 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Okamoto M, Inoue K, Iwamura H, Terashima K, Soya H, Asashima M, Kuwabara T (2011) Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J 25, 3570–3582. [DOI] [PubMed] [Google Scholar]
- [82]. Klaus A, Birchmeier W (2008) Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8, 387–398. [DOI] [PubMed] [Google Scholar]
- [83]. Rivera DS, Lindsay C, Codocedo JF, Morel I, Pinto C, Cisternas P, Bozinovic F, Inestrosa NC (2016) Andrographolide recovers cognitive impairment in a natural model of Alzheimer’s disease (Octodon degus). Neurobiol Aging 46, 204–220. [DOI] [PubMed] [Google Scholar]
- [84]. Tiwari SK, Agarwal S, Tripathi A, Chaturvedi RK (2016) Bisphenol-A mediated inhibition of hippocampal neurogenesis attenuated by curcumin via canonical Wnt pathway. Mol Neurobiol 53, 3010–3029. [DOI] [PubMed] [Google Scholar]
- [85]. Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Guan Y, Wang S-R, Huang X-Z, Xie Q, Xu Y-Y, Shang D, Hao C-M (2017) Nicotinamide mononucleotide, an NAD+precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J Am Soc Nephrol 28, 2337–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Michán S, Li Y, Chou MM-H, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W (2010) SIRT1 is essential for normalcognitive function and synaptic plasticity. J Neurosci 30, 9695–9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH (2012) Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol Rev 92, 1479–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89]. Li Y, Jiao J (2017) Histone chaperone HIRA regulates neural progenitor cell proliferation and neurogenesis via β-catenin. J Cell Biol 216, 1975–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Namihira M, Kohyama J, Abematsu M, Nakashima K (2008) Epigenetic mechanisms regulating fate specification of neural stem cells. Philos Trans R Soc B Biol Sci 363, 2099–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Lee O-H, Kim J, Kim J-M, Lee H, Kim EH, Bae S-K, Choi Y, Nam HS, Heo JH (2013) Decreased expression of sirtuin 6 is associated with release of high mobility group box-1 after cerebral ischemia. Biochem Biophys Res Commun 438, 388–394. [DOI] [PubMed] [Google Scholar]
- [92]. Sakamoto J, Miura T, Shimamoto K, Horio Y (2004) Predominant expression of Sir2α, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett 556, 281–286. [DOI] [PubMed] [Google Scholar]
- [93]. Cai Y, Xu L, Xu H, Fan X (2016) SIRT1 and neural cell fate determination. Mol Neurobiol 53, 2815–2825. [DOI] [PubMed] [Google Scholar]
- [94]. Tiberi L, van den Ameele J, Dimidschstein J, Piccirilli J, Gall D, Herpoel A, Bilheu A, Bonnefont J, Iacovino M, Kyba M, Bouschet T, Vanderhaeghen P (2012) BCL6 controls neurogenesis through Sirt1-dependent epigenetic repression of selective Notch targets. Nat Neurosci 15, 1627–1635. [DOI] [PubMed] [Google Scholar]
- [95]. Kumar V, Pandey A, Jahan S, Shukla RK, Kumar D, Srivastava A, Singh S, Rajpurohit CS, Yadav S, Khanna VK, Pant AB (2016) Differential responses of trans-resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci Rep 6, 28142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Ma C-Y, Yao M, Zhai Q, Jiao J, Yuan X, Poo M (2014) SIRT1 suppresses self-renewal of adult hippocampal neural stem cells. Development 141, 4697–4709. [DOI] [PubMed] [Google Scholar]
- [97]. Libert S, Cohen D, Guarente L (2008) Neurogenesis directed by Sirt1. Nat Cell Biol 10, 373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schröter F, Ninnemann O, Siegert E, Bendix I, Brüstle O, Nitsch R, Zipp F, Aktas O (2008) Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol 10, 385–394. [DOI] [PubMed] [Google Scholar]
- [99]. Saharan S, Jhaveri DJ, Bartlett PF (2013) SIRT1 regulates the neurogenic potential of neural precursors in the adult subventricular zone and hippocampus. J Neurosci Res 91, 642–659. [DOI] [PubMed] [Google Scholar]
- [100]. Abozaid OAR, Sallam MW, Ahmed ESA (2022) Mesenchymal stem cells modulate SIRT1/MiR-134/GSK3β signaling pathway in a rat model of Alzheimer’s disease. J Prev Alzheimers Dis 9, 458–468. [DOI] [PubMed] [Google Scholar]
- [101]. Herskovits AZ, Guarente L (2014) SIRT1 in neurodevelopment and brain senescence. Neuron 81, 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Dias GP, Cavegn N, Nix A, do Nascimento Bevilaqua MC, Stangl D, Zainuddin MSA, Nardi AE, Gardino PF, Thuret S (2012) The role of dietary polyphenols on adult hippocampal neurogenesis: Molecular mechanisms and behavioural effects on depression and anxiety. Oxid Med Cell Longev 2012, 541971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Stangl D, Thuret S (2009) Impact of diet on adult hippocampal neurogenesis. Genes Nutr 4, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104]. Kohl Z, Kandasamy M, Winner B, Aigner R, Gross C, Couillard-Despres S, Bogdahn U, Aigner L, Winkler J (2007) Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington’s disease. Brain Res 1155, 24–33. [DOI] [PubMed] [Google Scholar]
- [105]. Lephart ED (2015) Polyphenols and cognitive function. In Diet and Exercise in Cognitive Function and Neurological Diseases, Farooqui T, Farooqui AA, eds. Wiley; pp. 143–161. [Google Scholar]
- [106]. Brisdelli F, D’Andrea G, Bozzi A (2009) Resveratrol: A natural polyphenol with multiple chemopreventive properties (review). Curr Drug Metab 10, 530–546. [DOI] [PubMed] [Google Scholar]
- [107]. Namgyal D, Ali S, Mehta R, Sarwat M (2020) The neuroprotective effect of curcumin against Cd-induced neurotoxicity and hippocampal neurogenesis promotion through CREB-BDNF signaling pathway. Toxicology 442, 152542. [DOI] [PubMed] [Google Scholar]
- [108]. Wang Y, Li M, Xu X, Song M, Tao H, Bai Y (2012) Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol Nutr Food Res 56, 1292–1303. [DOI] [PubMed] [Google Scholar]
- [109]. Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: The in vivo evidence. . Nat Rev Drug Discov 5, 493–506. [DOI] [PubMed] [Google Scholar]
- [110]. Frémont L (2000) Biological effects of resveratrol. LifeSci 66, 663–673. [DOI] [PubMed] [Google Scholar]
- [111]. Lanz T, Schröder G, Schröder J (1990) Differential regulation of genes for resveratrol synthase in cell cultures ofArachis hypogaea L. Planta 181, 169–175. [DOI] [PubMed] [Google Scholar]
- [112]. Lange KW (2018) Red wine, resveratrol, and Alzheimer’s disease. J Dis Prev Health Promot 2. [Google Scholar]
- [113]. Ghazavi H, Shirzad S, Forouzanfar F, Negah SS, Rad MR, Vafaee F (2020) The role of resveratrol as a natural modulator in glia activation in experimental models of stroke. Avicenna J Phytomedicine 10, 557. [PMC free article] [PubMed] [Google Scholar]
- [114]. Pan S, Li S, Hu Y, Zhang H, Liu Y, Jiang H, Fang M, Li Z, Xu K, Zhang H (2016) Resveratrol post-treatment protects against neonatal brain injury after hypoxia-ischemia. Oncotarget 7, 79247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, Fokou PVT, Martins N, Sharifi-Rad J (2018) Resveratrol: A double-edged sword in health benefits. Biomedicines 6, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116]. Turner RS, Thomas RG, Craft S, Van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R, Aisen PS (2015) A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 85, 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117]. Berman AY, Motechin RA, Wiesenfeld MY, Holz MK (2017) The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis Oncol 1, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. Han G, Xia J, Gao J, Inagaki Y, Tang W, Kokudo N (2015) Anti-tumor effects and cellular mechanisms of resveratrol. Drug Discov Ther 9, 1–12. [DOI] [PubMed] [Google Scholar]
- [119]. Zhou X, Chen M, Zeng X, Yang J, Deng H, Yi L, Mi MT (2014) Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis 5, e1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120]. Casarin RC, Casati MZ, Pimentel SP, Cirano FR, Algayer M, Pires PR, Ghiraldini B, Duarte PM, Ribeiro FV (2014) Resveratrol improves bone repair by modulation of bone morphogenetic proteins and osteopontin gene expression in rats. Int J Oral Maxillofac Surg 43, 900–906. [DOI] [PubMed] [Google Scholar]
- [121]. Yang X, Xu S, Qian Y, Xiao Q (2017) Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun 64, 162–172. [DOI] [PubMed] [Google Scholar]
- [122]. Hoda U, Agarwal NB, Vohora D, Parvez S, Raisuddin S (2017) Resveratrol suppressed seizures by attenuating IL-1β, IL1-Ra, IL-6, and TNF-α in the hippocampus and cortex of kindled mice. Nutr Neurosci 20, 497–504. [DOI] [PubMed] [Google Scholar]
- [123]. Prabhakar O (2013) Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 386, 705–710. [DOI] [PubMed] [Google Scholar]
- [124]. Annunziata G, Sureda A, Orhan IE, Battino M, Arnone A, Jimenez-Garcia M, Capo X, Cabot J, Sanadgol N, Giampieri F (2021) The neuroprotective effects of polyphenols, their role in innate immunity and the interplay with the microbiota. Neurosci Biobehav Rev 128, 437–453. [DOI] [PubMed] [Google Scholar]
- [125]. Sanders O, Rajagopal L (2020) Phosphodiesterase inhibitors for Alzheimer’s disease: A systematic review of clinical trials and epidemiology with a mechanistic rationale. J Alzheimers Dis Rep 4, 185–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Pallàs M, Casadesús G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A (2009) Resveratrol and neurodegenerative diseases: Activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res 6, 70–81. [DOI] [PubMed] [Google Scholar]
- [127]. Chiang M-C, Nicol CJ, Cheng Y-C (2018) Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem Int 115, 1–10. [DOI] [PubMed] [Google Scholar]
- [128]. Zeng Y-H, Zhou L-Y, Chen Q-Z, Li Y, Shao Y, Ren W-Y, Liao Y-P, Wang H, Zhu J-H, Huang M (2017) Resveratrol inactivates PI3K/Akt signaling through upregulating BMP7 in human colon cancer cells. Oncol Rep 38, 456–464. [DOI] [PubMed] [Google Scholar]
- [129]. Karthick C, Periyasamy S, Jayachandran KS, Anusuyadevi M (2016) Intrahippocampal administration of ibotenic acid induced cholinergic dysfunction via NR2A/NR2B expression: Implications of resveratrol against Alzheimer disease pathophysiology. Front Mol Neurosci 9, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130]. Shen J, Xu L, Qu C, Sun H, Zhang J (2018) Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivoand in vitro. Behav Brain Res 349, 1–7. [DOI] [PubMed] [Google Scholar]
- [131]. Rigacci S, Stefani M (2015) Nutraceuticals and amyloid neurodegenerative diseases: A focus on natural phenols. Expert Rev Neurother 15, 41–52. [DOI] [PubMed] [Google Scholar]
- [132]. Valenti D, de Bari L, de Rasmo D, Signorile A, Henrion-Caude A, Contestabile A, Vacca RA (2016) The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model. Biochim Biophys Acta 1862, 1093–1104. [DOI] [PubMed] [Google Scholar]
- [133]. Xu L, Yang Y, Gao L, Zhao J, Cai Y, Huang J, Jing S, Bao X, Wang Y, Gao J, Xu H, Fan X (2015) Protective effects of resveratrol on the inhibition of hippocampal neurogenesis induced by ethanol during early postnatal life. Biochim Biophys Acta 1852, 1298–1310. [DOI] [PubMed] [Google Scholar]
- [134]. Tiwari V, Chopra K (2013) Resveratrol abrogates alcohol-induced cognitive deficits by attenuating oxidative– nitrosative stress and inflammatory cascade in the adult rat brain. Neurochem Int 62, 861–869. [DOI] [PubMed] [Google Scholar]
- [135]. Torres-Pérez M, Tellez-Ballesteros RI, Ortiz-López L, Ichwan M, Vega-Rivera NM, Castro-García M, Gómez-Sánchez A, Kempermann G, Ramirez-Rodriguez GB (2015) Resveratrol enhancesneuroplastic changes, including hippocampal neurogenesis, and memoryin Balb/C mice at six months of age. PLoS One 10, e0145687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136]. Moriya J, Chen R, Yamakawa J, Sasaki K, Ishigaki Y, Takahashi T (2011) Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull 34, 354–359. [DOI] [PubMed] [Google Scholar]
- [137]. Madhyastha S, Sekhar S, Rao G (2013) Resveratrol improves postnatal hippocampal neurogenesis and brain derived neurotrophic factor in prenatally stressed rats. Int J Dev Neurosci 31, 580–585. [DOI] [PubMed] [Google Scholar]
- [138]. Maharajan N, Vijayakumar K, Jang CH, Cho G-W (2020) Caloric restriction maintains stem cells through niche and regulates stem cell aging. J Mol Med 98, 25–37. [DOI] [PubMed] [Google Scholar]
- [139]. Safahani M, Aligholi H, Noorbakhsh F, Djalali M, Pishva H, Modarres Mousavi SM, Alizadeh L, Gorji A, Koohdani F (2019) Switching from high-fat diet to foods containing resveratrol as a calorie restriction mimetic changes the architecture of arcuate nucleus to produce more newborn anorexigenic neurons. Eur J Nutr 58, 1687–1701. [DOI] [PubMed] [Google Scholar]
- [140]. Kodali M, Parihar VK, Hattiangady B, Mishra V, Shuai B, Shetty AK (2015) Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature and reduced glial activation. Sci Rep 5, 8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141]. Wang R, Wu Z, Bai L, Liu R, Ba Y, Zhang H, Cheng X, Zhou G, Huang H (2021) Resveratrol improved hippocampal neurogenesis following lead exposure in rats through activation of SIRT1 signaling. Environ Toxicol 36, 1664–1673. [DOI] [PubMed] [Google Scholar]
- [142]. Holloway KR, Calhoun TN, Saxena M, Metoyer CF, Kandler EF, Rivera CA, Pruitt K (2010) SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc Natl Acad Sci U S A 107, 9216–9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143]. Li X, Chen C, Tu Y, Sun H, Zhao M, Cheng S, Qu Y, Zhang S (2013) Sirt1 promotes axonogenesis by deacetylation of Akt and inactivation of GSK3. Mol Neurobiol 48, 490–499. [DOI] [PubMed] [Google Scholar]
- [144]. Hussain M, Rao M, Humphries AE, Hong JA, Liu F, Yang M, Caragacianu D, Schrump DS (2009) Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res 69, 3570–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145]. Ma J, Fan H, Cai H, Hu Z, Zhou X, Li F, Chen H, Shen J, Qi S (2021) Promotion of Momordica Charantia polysaccharides on neural stem cell proliferation by increasing SIRT1 activity after cerebral ischemia/reperfusion in rats. Brain Res Bull 170, 254–263. [DOI] [PubMed] [Google Scholar]