Abstract
Objective
To describe the risks and predictors of coronavirus disease 2019 (COVID-19) hospitalization and mortality among patients with early inflammatory arthritis (EIA), recruited to the National Early Inflammatory Arthritis Audit (NEIAA).
Methods
NEIAA is an observational cohort. We included adults with EIA from Feb 2020 to May 2021. Outcomes of interest were hospitalization and death due to COVID-19, using NHS Digital linkage. Cox proportional hazards were used to calculate hazard ratios for outcomes according to initial treatment strategy, with adjustment for confounders.
Results
From 14 127 patients with EIA, there were 143 hospitalizations and 47 deaths due to COVID-19, with incidence rates per 100 person-years of 0.93 (95% CI 0.79, 1.10) for hospitalization and 0.30 (95% CI 0.23, 0.40) for death. Increasing age, male gender, comorbidities and ex-smoking were associated with increased risk of worse COVID-19 outcomes. Higher baseline DAS28 was not associated with COVID-19 admissions [confounder adjusted hazard ratio (aHR) 1.10; 95% CI 0.97, 1.24] or mortality (aHR 1.11; 95% CI 0.90, 1.37). Seropositivity was not associated with either outcome. Higher symptom burden on patient-reported measures predicted worse COVID-19 outcomes. In unadjusted models, CS associated with COVID-19 death (HR 2.29; 95% CI 1.02, 5.13), and SSZ monotherapy associated with COVID-19 admission (HR 1.92; 95% CI 1.04, 3.56). In adjusted models, associations for CS and SSZ were not statistically significant.
Conclusion
Patient characteristics have stronger associations with COVID-19 than the initial treatment strategy in patients with EIA. An important limitation is that we have not looked at treatment changes over time.
Keywords: COVID-19, COVID-19 mortality, COVID-19 admissions, inflammatory arthritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, early inflammatory arthritis, DMARD, corticosteroids
Rheumatology key messages.
In patients with early inflammatory arthritis (EIA), no increased risk of coronavirus disease 2019 (COVID-19) mortality was observed relative to the general population.
Patients’ characteristics showed a strong association with an increased risk of COVID-19 admissions.
There were no significant associations between the initial treatment strategy and severe COVID-19 outcomes in EIA.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has led to significant morbidity and mortality worldwide, with over 190 million confirmed COVID-19 cases and over 4 million COVID-19-related deaths by July 2021 [1]. While the impact of the pandemic is declining, there remains an enormous amount that we can learn from the disease and in particular the impact of autoimmunity and immunosuppression on infectious outcomes. At the beginning of the pandemic, several countries identified patients with inflammatory arthritis as potentially vulnerable to adverse COVID-19 outcomes, with recommendations to shield [2]. There were concerns about the impact of COVID-19 infection in people with inflammatory arthritis, due to underlying immune dysregulation, immunosuppressant use, and a high prevalence of comorbidities. RA patients were known to have higher mortality and morbidity rates compared with the general population [3, 4], and an increased risk of serious infections in general, linked to the use of DMARDs and biologics [5, 6].
Studies have been performed to assess the impact of COVID-19 in people with inflammatory arthritis, albeit with conflicting results. A number of studies reported that the risk of COVID-19 admissions and mortality in people with rheumatological diseases were similar to those in the general population when adjusting for socioeconomic factors and comorbidities [7–9], while others reported higher COVID-19 admission and mortality rates [10–12]. Results could be explained by differing sample sizes and underlying disease burdens in these cohorts. Additionally, studies to date have mostly included patients with well-established disease, with exposure to different DMARDs and biologics. Outcomes from COVID-19 specifically in people with early inflammatory arthritis (EIA), and without prior DMARD exposure, are less clear.
To improve our understanding of COVID-19 outcomes in people with EIA, in this study we describe the incidences of hospitalization and mortality due to COVID-19 in patients enrolled in the National Early Inflammatory Arthritis Audit (NEIAA) in England. We analysed the risk of these outcomes according to individual risk factors and the initial treatment strategy using conventional synthetic DMARDs (csDMARDs) by 3 months.
Methods
Data source
NEIAA is an audit commissioned by the Health Quality Improvement Partnership (HQIP), on behalf of the National Health Services (NHS) England and the Welsh Government. The main aim of the audit is to promote quality improvement activity linked to EIA management by assessing care according to the six metrics defined by the National Institute for Health and Clinical Excellence (NICE) guidelines QS33 [13].
NEIAA started on May 2018 and collects data on adults (aged >16 years) referred to secondary care rheumatology services in England and Wales with a suspected inflammatory arthritis, irrespective of the ultimate diagnosis. Patients with a confirmed EIA diagnosis are eligible for further follow-up. Information on patient-reported and clinical outcomes are gathered at baseline, 3 months and 12 months, for those with confirmed EIA diagnosis. Full methodology on data collection has been described in NEIAA’s annual report [14].
Study population
In this study, we included patients in England with confirmed EIA diagnoses including RA, PsA, ankylosing spondylitis (axSpA), undifferentiated inflammatory arthritis and other arthritis (predominantly reflecting patients yet to be assigned a diagnosis subtype in the database) enrolled in NEIAA. Data for Wales were excluded from this report due to limitations of data availability. In this cohort, patients with EIA are defined as individuals prior to, or at the time of commencing, csDMARD therapy.
Exposures
Individuals were considered at risk from February 2020 (date of first case of COVID-19 in NEIAA) or the date of diagnosis (whichever was later), and censored at a COVID-19 event, May 2021 or death (whichever was sooner).
Data collection
Baseline characteristics
Including age, gender, smoking status (current smoker, ex-smoker and never smoked), working diagnosis, patient follow-up eligibility (as a result of a confirmed EIA diagnosis), symptom duration, associated comorbidities including diabetes, hypertension, lung diseases and the rheumatic disease comorbidity index (RDCI––a validated tool that gives a weighted measure upon the history of cardiovascular disease, hypertension, diabetes mellitus, chronic lung disease, peptic ulcer disease, depression and cancer [15]), seropositivity status for RF and anti-CCP and deprivation level [using index of multiple deprivation (IMD)], scores were then grouped into 10 categories according to the English IMD 2015 guidance [16]. Clinical and patient-reported measures collected at baseline.
Markers of disease activity, including tender joint count (0–28 joints), swollen joint count (0–28 joints), patient-reported global assessment score (0–100 scale, from best to worst), CRP (mg/l) and/or ESR (mm/h), were used to calculate a DAS 28 joints (DAS28) which is a validated tool to measure disease activity in RA [17, 18]. A DAS28 was calculated irrespective of the underlying diagnosis of EIA (i.e. RA, PsA, axSpA and undifferentiated arthritis). The score was categorized to reflect remission (0 to <2.6), low disease activity (2.6 to ≤ 3.2), moderate disease activity (> 3.2 to ≤5.1) and high disease activity (> 5.1).
Physical function was assessed using the Health Assessment Questionnaire version 2 (HAQ-II) [19]. The 10 items included in this scale are summed to provide a total score ranging from 0 to 3, where higher scores indicate worse function.
Mental health (MH) was assessed using the four-item Patient Health Questionnaire (PHQ-4) [20] which is the combined form of the two-item PHQ-2 depression scale and two-item Generalized Anxiety Disorder scale (GAD-2) [21]. Significant MH comorbidity (i.e. a likely diagnosis of anxiety or depression) is defined if a response is >2 on either PHQ-2 or GAD-2 subscales.
Disease impact was assessed using the Musculoskeletal Health Questionnaire (MSK-HQ). This 15-item questionnaire evaluates how musculoskeletal symptoms affect day to day life across a range of domains including pain, fatigue, emotional well-being, and work and social activities. Scores range from 0 to 56, with higher scores indicating better MSK health [22].
Information on the initial treatment strategies (i.e. csDMARDs monotherapy, csDMARDs combination therapy and concomitant steroids) were collected by the treating clinician at baseline and 3 months (steroids use was only collected at baseline). MTX, SSZ and HCQ monotherapy and combination therapies use up to 3 months were included in this report. Steroids use was considered as a separate adjacent therapy.
COVID-19 outcomes definition
COVID-19 admission and death were identified using linkage to NHS Digital Hospital Episodes Statistics (HES) and Office for National Statistics (ONS) datasets. COVID-19 diagnosis was coded according to the International Classification of Diseases-10 codes [23]. COVID-19-related death was defined according to Public Health England 28 mortality, which is death in a person with a confirmed positive COVID-19 test that occurred within (in or less than) 28 days of the first positive COVID-19 specimen [24]. COVID-19-related hospitalization is defined as a hospital admission with a duration of >24 h associated with a diagnosis of COVID-19 (either a primary infection or nosocomial acquisition) [25].
Statistical analysis
For continuous measures, data were described as medians and interquartile ranges (IQR). For categorical measures, absolute numbers and percentages were used. Treatment strategies were used as binary variables, as dose information was not collected. Inferential statistics (i.e. P-values) were not reported for differences in baseline characteristics due to the large sample sizes, and to avoid drawing inferences based upon multiple hypothesis tests.
COVID-19 mortality rates in EIA patients were compared with COVID-19 mortality rates in England’s general population, using age- and gender-standardized incidence rates per 100 person-years. Age was used as the underlining time scale in these analyses. General population data were downloaded from the gov.uk coronavirus dashboard [26].
Cox proportional hazards were used to calculate the risk of COVID-19 admission and deaths in the EIA cohort. Single failure models were used to examine time to first event (COVID-19 hospital admission and COVID-19 death analysed as separate models).
Risks were reported as hazard ratios (HRs) and 95% CI. Possible confounders were selected based upon clinical knowledge and available variables.
A confounder adjusted model included age, gender, smoking status, comorbidities (diabetes, hypertension, lung disease), deprivation (using IMD). A disease severity adjusted model included the above-mentioned variables plus disease factors [seropositivity for RF and/or anti-CCP and disease severity (DAS28) at baseline].
Missing baseline variables, including deprivation, comorbidities, baseline DAS28 and seropositivity, were imputed using multivariate sequential imputation models with 20 chained equations (at the end of each cycle, one imputed dataset was generated and then the process was repeated to generate 20 imputed datasets). All missing data were imputed regardless of the reason/second they were missing. The following variables with complete data were utilized for the imputation: age, gender and smoking status.
In the sensitivity analyses, we reported standardized differences in hazards ratios of COVID-19 outcomes for baseline DAS28, HAQ, MSK-HQ and MH as these variables are measured on different scales. The variables were rescaled to a standardized value with a mean of zero and s.d. of one, and then differences in s.d. were calculated.
For prediction models, statistical significance was assessed at the 5% level. No correction for multiple hypotheses was made. All statistical analyses were performed using Stata version 17.0 (StataCorp, College Station, TX, USA).
Ethical approval
Approval to conduct this research using NEIAA’s dataset was obtained from HQIP. Informed patient consent was not required, as NEIAA has permission from the UK Government Secretary of State for Health to collect data for the purposes of national audit. Ethical approval to undertake research in NEIAA has been granted (Clinical Advisory Group Reference: 19/CAG/0059; Research Ethics Committee reference: 19/EE/0082). Data access requests can be made through HQIP and are subject to data-sharing agreement approval.
Results
Baseline characteristics
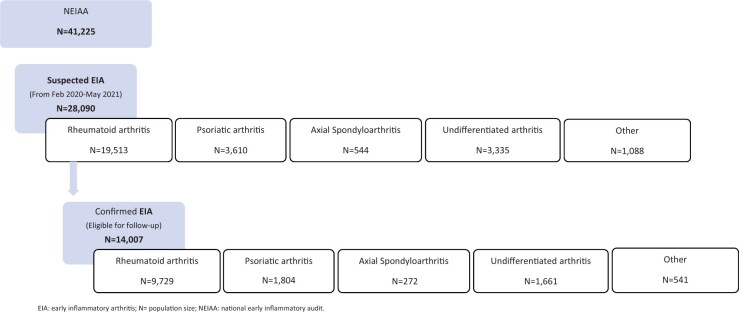
In total, 28 090 individuals were recruited to NEIAA between February 2020 and May 2021 (Fig. 1). Of those, 14 007 patients had a confirmed EIA diagnosis and were included in further analyses. RA was the most frequent diagnosis (n = 9792), followed by PsA (n = 1804), undifferentiated arthritis (n = 1661), other EIA (n = 541) and axSpA (n = 272).
Figure 1.
Population flow chart
Baseline characteristics by EIA diagnosis are shown in Table 1. The median age was 57 (IQR 45, 69) years and 8677 (62%) were female. Some 2664 (19%) patients were current smokers, while 4011 (29%) were ex-smokers. A total of 2563 (19%) had hypertension, 1187 (9%) had diabetes and 1280 (9%) had lung disease.
Table 1.
Baseline characteristics of patients with confirmed EIA in NEIAA
| Total | RA | PsA | axSpA | Undifferentiated arthritis | Other | |
|---|---|---|---|---|---|---|
| N = 14 007 | N = 9729 | N = 1804 | N = 272 | N = 1661 | N = 541 | |
| Age, years, median (IQR) | 57 (45, 69) | 60 (49, 71) | 47 (35, 58) | 37 (30, 49) | 55 (41, 68) | 53 (38, 65) |
| Age band, n (%) | ||||||
| 16–24 | 380 (2.7) | 163 (1.7) | 87 (4.8) | 30 (11.0) | 73 (4.4) | 27 (5.0) |
| 25–39 | 2120 (15.1) | 1039 (10.7) | 542 (30.0) | 124 (45.6) | 295 (17.8) | 120 (22.2) |
| 40–54 | 3540 (25.3) | 2285 (23.5) | 581 (32.2) | 78 (28.7) | 450 (27.1) | 146 (27.0) |
| 55–64 | 3114 (22.2) | 2325 (23.9) | 333 (18.5) | 23 (8.5) | 331 (19.9) | 102 (18.9) |
| 65–74 | 2859 (20.4) | 2277 (23.4) | 196 (10.9) | 11 (4.0) | 295 (17.8) | 80 (14.8) |
| ≥75 | 1994 (14.2) | 1640 (16.9) | 65 (3.6) | 6 (2.2) | 217 (13.1) | 66 (12.2) |
| Gender, n (%) | ||||||
| Male | 5330 (38.1) | 3547 (36.5) | 792 (43.9) | 159 (58.5) | 625 (37.6) | 207 (38.3) |
| Female | 8677 (61.9) | 6182 (63.5) | 1,012 (56.1) | 113 (41.5) | 1036 (62.4) | 334 (61.7) |
| Ethnicity, n (%) | ||||||
| White | 11 970 (85.5) | 8264 (84.9) | 1609 (89.2) | 232 (85.3) | 1418 (85.4) | 447 (82.6) |
| Black | 354 (2.5) | 283 (2.9) | 16 (0.9) | 7 (2.6) | 41 (2.5) | 7 (1.3) |
| Asian | 1039 (7.4) | 720 (7.4) | 113 (6.3) | 21 (7.7) | 135 (8.1) | 50 (9.2) |
| Mixed | 80 (0.6) | 50 (0.5) | 11 (0.6) | 0 (0.0) | 17 (1.0) | 2 (0.4) |
| Other | 371 (2.6) | 272 (2.8) | 27 (1.5) | 10 (3.7) | 43 (2.6) | 19 (3.5) |
| Smoking status, n (%) | ||||||
| Current smoker | 2664 (19.0) | 1940 (19.9) | 319 (17.7) | 58 (21.3) | 263 (15.8) | 84 (15.5) |
| Ex-smoker | 4011 (28.6) | 2894 (29.7) | 465 (25.8) | 51 (18.8) | 458 (27.6) | 143 (26.4) |
| Never smoked | 6336 (45.2) | 4240 (43.6) | 865 (47.9) | 132 (48.5) | 833 (50.2) | 266 (49.2) |
| Comorbidity, n (%) | ||||||
| None | 8175 (59.2) | 5407 (56.2) | 1234 (69.3) | 197 (73.8) | 981 (60.2) | 356 (68.2) |
| One | 2947 (21.3) | 2086 (21.7) | 358 (20.1) | 44 (16.5) | 355 (21.8) | 104 (19.9) |
| Two or more | 2698 (19.5) | 2128 (22.1) | 188 (10.6) | 26 (9.7) | 294 (18.0) | 62 (11.9) |
| Diabetes mellitus, n (%) | ||||||
| No | 12 633 (91) | 8732 (91) | 1664 (93) | 254 (95) | 1491 (91) | 492 (94) |
| Yes | 1187 (9) | 889 (9) | 116 (7) | 13 (5) | 139 (9) | 30 (6) |
| Hypertension, n (%) | ||||||
| No | 11 257 (81) | 7613 (79) | 1586 (89) | 248 (93) | 1353 (83) | 457 (88) |
| Yes | 2563 (19) | 2008 (21) | 194 (11) | 19 (7) | 277 (17) | 65 (12) |
| Lung disease, n (%) | ||||||
| No | 12 540 (91) | 8551 (89) | 1717 (96) | 259 (97) | 1512 (93) | 501 (96) |
| Yes | 1280 (9) | 1070 (11) | 63 (4) | 8 (3) | 118 (7) | 21 (4) |
| Disease characteristics Duration symptoms, n (%) | ||||||
| <1 month | 1125 (8.1) | 775 (8.0) | 96 (5.4) | 9 (3.3) | 190 (11.5) | 55 (10.4) |
| 1–3 months | 4489 (32.4) | 3377 (35.1) | 390 (22.0) | 37 (13.7) | 526 (31.8) | 159 (29.9) |
| 3–6 months | 3305 (23.8) | 2350 (24.4) | 419 (23.6) | 29 (10.7) | 391 (23.6) | 116 (21.8) |
| 6–12 months | 2578 (18.6) | 1770 (18.4) | 391 (22.0) | 46 (17.0) | 279 (16.9) | 92 (17.3) |
| >12 months | 2367 (17.0) | 1361 (14.1) | 479 (26.9) | 149 (55.1) | 269 (16.2) | 109 (20.5) |
| RF or anti-CCPA positive, n (%) | ||||||
| No | 5493 (43.7) | 2641 (29.1) | 1279 (89.3) | 137 (95.1) | 1141 (77.4) | 295 (67.8) |
| Yes | 7082 (56.3) | 6447 (70.9) | 154 (10.7) | 7 (4.9) | 334 (22.6) | 140 (32.2) |
| Baseline DAS28, mean (SD) | 4.6 (1.5) | 4.9 (1.4) | 4.2 (1.4) | 2.7 (1.4) | 4.1 (1.4) | 3.5 (1.5) |
| ESR mm/h, median (IQR) | 24.0 (10.0, 41.0) | 27.0 (12.0, 44.0) | 16.0 (7.0, 32.0) | 7.0 (2.0, 25.0) | 20.0 (8.0, 38.0) | 17.0 (6.0, 33.5) |
| CRP mg/L, median (IQR) | 10.0 (4.0, 26.0) | 11.0 (4.0, 29.0) | 7.0 (3.0, 17.0) | 5.0 (2.0, 16.0) | 8.0 (3.0, 22.0) | 6.0 (3.0, 18.0) |
| Patient-reported outcomes, median (IQR) | ||||||
| MSK-HQ score | 24.0 (17.0, 33.0) | 24.0 (16.0, 33.0) | 26.0 (18.0, 34.0) | 25.0 (17.0, 33.0) | 25.0 (17.5, 33.5) | 27.0 (17.0, 36.0) |
| HAQ | 1.0 (0.5,1.6) | 1.1 (0.6,1.6) | 0.9 (0.5,1.4) | 0.8 (0.5,1.4) | 0.9 (0.5,1.5) | 0.9 (0.4,1.4) |
| PHQ and GAD combined total (MH) | 4.0 (1.0, 8.0) | 4.0 (1.0, 8.0) | 4.0 (1.0, 7.0) | 4.0 (2.0, 7.0) | 4.0 (1.0, 8.0) | 4.0 (1.0, 7.0) |
Baseline characteristics of patients with EIA in England recruited to NEIAA. Individuals were considered at risk from February 2020 (date of first case of COVID-19 in NEIAA) or date of diagnosis (whichever was later) and censored at a COVID-19 event, May 2021 or death (whichever was sooner). EIA: early inflammatory arthritis; NEIAA: National Early Inflammatory Arthritis Audit; DAS28: DAS for 28 joints; GAD: Generalized Anxiety Disorder scale; HAQ: HAQ Disability Index; IQR: interquartile range; MH: Mental Health based on (PHQ-2) and (GAD-2) combined score, significant MH comorbidity (i.e. a likely diagnosis of anxiety or depression is present) is defined if a response is >2 on either PHQ or GAD; MSK-HQ: Musculoskeletal Health Questionnaire; PHQ: Patient Health Questionnaire; COVID-19: coronavirus disease 2019.
RF or anti-CCP antibodies were positive in 7082 (56%) patients. Some 8919 (64%) patients had arthritis symptoms for <6 months at the time of rheumatology assessment. Mean DAS28 at presentation was 4.6 (1.5), with a median baseline ESR of 24 (IQR 10.0, 41.0) and a median CRP of 10 (IQR 4.0, 26.0). The median HAQ score at baseline was 1.0 (IQR 0.5, 1.5) and median MSK-HQ score was 24 (IQR 17.0, 33.0). The median combined PHQ-2 and GAD score was 4 (IQR 1.0, 8.0).
Initial DMARD therapy by 3 months was known for 13 656/14 007 patients. Details on the monotherapy and combination csDMARDs can be seen in Table 2. MTX was the most common DMARD prescribed (54%), followed by HCQ (23%) then SSZ (11%). More than half of patients were taking steroids at baseline (70%).
Table 2.
Treatment strategy used in EIA patients by 3 months
| Total | RA | PsA | axSpA | Undifferentiated arthritis | Other | |
|---|---|---|---|---|---|---|
| N = 14 007 | N = 9729 | N = 1804 | N = 272 | N = 1661 | N = 541 | |
| No DMARD, n (%) | 2866 (21) | 1223 (13) | 452 (26) | 201 (80) | 689 (43) | 301 (60) |
| MTX, n (%) | ||||||
| MTX monotherapy | 6008 (44) | 4591 (48) | 954 (56) | 13 (5) | 385 (24) | 65 (13) |
| MTX combination | 1346 (10) | 1253 (13) | 25 (1) | 7 (3) | 54 (3) | 7 (1) |
| SSZ, n (%) | ||||||
| SSZ monotherapy | 1280 (9) | 798 (8) | 224 (13) | 10 (4) | 202 (13) | 46 (9) |
| SSZ combination | 244 (2) | 207 (2) | 15 (1) | 3 (1) | 17 (1) | 2 (0) |
| HCQ, n (%) | ||||||
| HCQ monotherapy | 1813 (13) | 1450 (15) | 27 (2) | 8 (3) | 254 (16) | 74 (15) |
| HCQ combination | 1352 (10) | 1282 (14) | 7 (0) | 4 (2) | 51 (3) | 8 (2) |
| Missing treatment information, n | 351 | 236 | 91 | 21 | 57 | 37 |
| Corticosteroids, n (%) | ||||||
| No | 4080 (30) | 2096 (22) | 882 (51) | 195 (79) | 632 (40) | 275 (55) |
| Yes | 9513 (70) | 7417 (78) | 851 (49) | 51 (21) | 968 (60) | 226 (45) |
| Missing steroid information, n | 414 | 216 | 71 | 26 | 61 | 40 |
Early treatment strategy used in patients with EIA in England recruited to NEIAA. Individuals were considered at risk from February 2020 (date of first case of COVID-19 in NEIAA) or date of diagnosis (whichever was later) and censored at a COVID-19 event, May 2021 or death (whichever was sooner). EIA: early inflammatory arthritis; NEIAA: National Early Inflammatory Arthritis Audit; n: number of patients; COVID-19: coronavirus disease 2019.
COVID-19 admissions and mortality
Details on COVID-19 admissions and mortality by EIA diagnosis can be seen in Table 3. In total, there were 143 COVID-19-related admissions in 14 007 patients with a confirmed EIA diagnoses during the follow-up period, with an incidence rate of 0.93 per 100 person-years (95% CI 0.79, 1.10). Six patients had COVID-19 admissions before they were diagnosed with EIA and those patients did not contribute to the estimates.
Table 3.
Incidence rate for COVID-19 admissions and deaths in patients with EIA using single failure Cox model
| Variable | All EIA | RA | PsA | axSpA | Undifferentiated arthritis | Other |
|---|---|---|---|---|---|---|
| COVID-19 admissions | ||||||
| Total patients | 14 007 | 9729 | 1804 | 272 | 1661 | 541 |
| n | 143 | 104 | 11 | 2 | 19 | 7 |
| PY | 15291.89 | 10611.01 | 1961.69 | 298.67 | 1828.88 | 591.64 |
| IR (95% CI) per 100 PY | 0.93 (0.79, 1.10) | 0.98 (0.80, 1.18) | 0.56 (0.31, 1.01) | 0.66 (0.16, 2.67) | 1.03 (0.66, 1.62) | 1.18 (0.56, 2.48) |
| COVID-19 deaths | ||||||
| Total patients | 14 007 | 9729 | 1804 | 272 | 1661 | 541 |
| n | 47 | 36 | 1 | 0 | 5 | 5 |
| PY | 15 295.43 | 10 613.52 | 1961.82 | 298.67 | 1829.45 | 591.97 |
| IR (95% CI) per 100 PY | 0.30 (0.23, 0.40) | 0.33 (0.24, 0.47) | 0.05 (0.00, 0.36) | 0 (0, 0.01) | 0.27 (0.11, 0.65) | 0.84 (0.35, 2.03) |
Incidence rates of COVID-19 hospital admissions and deaths in patients diagnosed with EIA in England. EIA: early inflammatory arthritis; COVID-19: coronavirus disease 2019; IR: incidence rate; n: number of patients with COVID-19 outcome; PY: person years.
There were 47 COVID-19 deaths in 14 007 patients with confirmed EIA diagnoses during our follow-up period, with an incidence rate of 0.30 per 100 person-years (95% CI 0.23, 0.40).
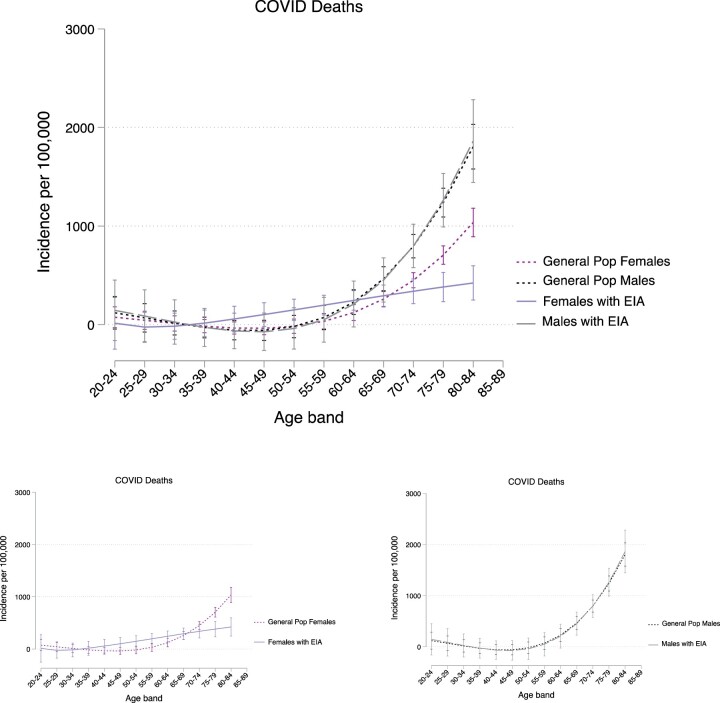
Overall, the standardized mortality ratio (SMR) for COVID-19 mortality in patients with EIA was not significantly different to the general population in England (matched by age and gender), as shown in Fig. 2. The SMR also did not vary by gender: males 1.05 (95% CI 0.65, 1.45) and females 1.04 (95% CI 0.58, 1.50).
Figure 2.
Smoothed graph of COVID-19 deaths in patients with EIA and the general population in males and females from Feb 2020 to May 2021. Smoothed graph of the standardized incidence rate of COVID-19 deaths in males and females diagnosed with EIA and the general population in England. COVID-19 mortality rates in EIA patients were compared with COVID-19 mortality rates in England’s general population, using age- and gender-standardized incidence rates per 100 person-years. Age was used as the underlining time scale in these analyses. General population data were downloaded from the gov.uk coronavirus dashboard [26]. COVID-19: coronavirus disease 2019; EIA: early inflammatory arthritis
Predictors of COVID-19 admissions and mortality
Details on factors associated with COVID-19 admissions and deaths in different adjusted models can be seen in Supplementary Table S1, available at Rheumatology online.
In confounder adjusted models, the risk of both COVID-19 admissions and deaths increased with increasing age: adjusted hazard ratio (aHR) 1.02, 95% CI 1.01, 1.04, and aHR 1.07, 95% CI 1.04, 1.10, for each year older, respectively. Female gender and living in a less deprived area significantly associated with fewer admissions: aHR 0.69, 95% CI 0.49, 0.97, and 0.92 per decile (95% CI 0.87, 0.98), respectively. There were no significant associations found between COVID-19 deaths and gender. Results were consistent when we adjusted for disease severity, except for the association between gender and COVID-19 admissions, where it was no longer significant.
Current smoking did not show any significant associations with COVID-19 admissions or deaths, whereas ex-smoking status associated with COVID-19 admissions (aHR 1.62, 95% CI 1.10, 2.38) but not mortality (aHR 1.68, 95% CI 0.85, 3.33).
Comorbidities (diabetes mellitus, hypertension and lung disease) were significantly associated with COVID-19 admissions. Diabetes and hypertension were significantly associated with COVID-19 deaths (aHR 3.66, 95% CI 1.99, 6.74) and (aHR 1.99, 95% CI 1.09, 3.62), respectively. Associations between lung disease and COVID-19 deaths were non-significant (aHR 1.85, 95% CI 0.92, 3.69).
Disease severity was not significantly associated with COVID-19 admissions (aHR 1.10, 95% CI 0.97, 1.24) or death (aHR 1.11, 95% CI 0.90, 1.37). Also, there were no significant associations between seropositivity for RF or anti-CCP and admissions (aHR 0.84, 95% CI 0.58, 1.20) or mortality (aHR 1.51, 95% CI 0.77, 2.95).
Higher HAQ was associated with both COVID-19 admissions (aHR 2.49, 95% CI 1.64, 3.80) and death (aHR 2.61, 95% CI 1.29, 5.25). Lower MSK-HQ scores (indicating greater functional impairment) and higher MH (the likelihood diagnosis of anxiety of depression) were associated with COVID-19 admissions (aHR 0.96, 95% CI 0.93, 0.98, and aHR 1.06, 95% CI 0.99, 1.14), respectively, but not mortality. To describe the impact of different predictors using a common scale, the standardized differences in HRs for baseline DAS28, HAQ, MSK-HQ and MH in predicting COVID-19 outcomes can be seen in the Supplementary Table S2, available at Rheumatology online.
COVID-19 admissions, mortality and early treatment strategy used in patients with EIA
In unadjusted models, CS use within 3 months of assessment associated with COVID-19 death (HR 2.29, 95% CI 1.02, 5.13). SSZ monotherapy associated with COVID-19 admission (HR 1.92, 95% CI 1.04, 3.56), but not COVID-19 mortality. In age/gender, confounder and disease severity-adjusted models, there were no significant associations between COVID-19 admissions or deaths and DMARD monotherapy (Table 4), combination therapy (Supplementary Table S3, available at Rheumatology online) or CS use (Table 4).
Table 4.
Hazard ratios for COVID-19 admissions and mortality in patients with EIA in relation to early monotherapy treatment (imputed analyses)
| Models | MTX monotherapy | SSZ monotherapy | HCQ monotherapy | Steroids | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| COVID-19 admissions | ||||||||
| Unadjusted | 1.23 (0.75, 2.01) | 0.39 | 1.92 (1.04, 3.56) | 0.03 | 1.43 (0.79, 2.61) | 0.23 | 1.36 (0.92, 2.02) | 0.11 |
| Age- and gender-adjusted | 1.10 (0.67, 1.80) | 0.68 | 1.73 (0.93, 3.20) | 0.08 | 1.37 (0.75, 2.49) | 0.30 | 1.04 (0.70, 1.54) | 0.83 |
| Confounder-adjusteda | 1.11 (0.68, 1.81) | 0.67 | 1.47 (0.78, 2.76) | 0.22 | 1.25 (0.68, 2.28) | 0.46 | 1.07 (0.71, 1.59) | 0.74 |
| Disease severity-adjustedb | 1.02 (0.60, 1.72) | 0.92 | 1.36 (0.70, 2.64) | 0.35 | 1.19 (0.63, 2.25) | 0.58 | 1.00 (0.64, 1.56) | 0.98 |
| COVID-19 deaths | ||||||||
| Unadjusted | 0.97 (0.42, 2.26) | 0.96 | 1.39 (0.45, 4.25) | 0.58 | 1.69 (0.65, 4.40) | 0.27 | 2.29 (1.02, 5.13) | 0.04 |
| Age- and gender-adjusted | 0.87 (0.37, 2.03) | 0.76 | 1.13 (0.37, 3.46) | 0.82 | 1.58 (0.61, 4.11) | 0.34 | 1.40 (0.62, 3.16) | 0.41 |
| Confounder-adjusteda | 0.89 (0.38, 2.08) | 0.80 | 1.02 (0.33, 3.13) | 0.97 | 1.49 (0.57, 3.89) | 0.41 | 1.49 (0.66, 3.36) | 0.33 |
| Disease severity-adjustedb | 0.78 (0.31, 1.95) | 0.60 | 0.88 (0.25, 3.05) | 0.84 | 1.24 (0.43, 3.58) | 0.68 | 1.14 (0.48, 2.67) | 0.76 |
Hazards ratios of COVID-19 hospital admissions and deaths in patients diagnosed with EIA in England based on early treatment strategies (monotherapy) compared with no DMARD start (0–3 months) and concomitant steroids use at baseline using single failure Cox regression models. Multiple imputation model using chained equations (20 imputed datasets) was used for missing data in comorbidities, deprivation, baseline DAS28 and seropositivity. The following variables with complete data were utilized for the imputation: age, gender and smoking.
Confounder adjusted model adjusted for age, gender, smoking status, comorbidities (hypertension, diabetes mellitus, lung disease) and social deprivation using deprivation index.
Adjusted for age and gender, confounders and baseline DAS28, RF or anti-CCP seropositivity. EIA: early inflammatory arthritis; COVID-19: coronavirus disease 2019; HR: hazard ratio; DAS28: DAS for 28 joints.
Discussion
To our knowledge, this is the first study to report COVID-19 deaths and admissions in patients with newly diagnosed inflammatory arthritis. We found no increased risk of COVID-19 deaths in patients with early inflammatory arthritis relative to the general population in England [SMR for males was 1.05 (95% CI 0.65, 1.45) and for females 1.04 (95% CI 0.58, 1.50)]. Age, male gender, social deprivation, ex-smoking, comorbidities and patient-reported outcomes (HAQ and MSK-HQ) were related to increased risk of COVID-19 hospitalization. In contrast, there were no associations between the initial treatment strategy used in patients with EIA by 3 months and severe COVID-19 outcomes.
Our findings were broadly in agreement with previous studies focusing on patients with established disease. A Danish cohort reported higher odds of COVID-19 deaths in patients with RA and CTDs, relative to the general population, in unadjusted analyses. However, when adjusted for age and gender, there was no increase in risk [27]. A large UK study using the OpenSAFELY database reported a slightly higher risk of deaths in patients with RA, psoriasis and SLE, compared with the general population [28]. However, despite the difference inference based on significance testing, the 95% CIs were consistent with this study. Other studies reported no substantial differences in clinical outcomes between patients with rheumatic diseases and the general population [11, 29].
Our findings demonstrated that older age, male sex, the presence of comorbidities, living in a more deprived area, worse physical function and frailty (i.e. worse HAQ, MSK-HQ and MH scores) were risk factors for severe COVID-19 outcomes.
This has been shown in previous studies on patients with rheumatic diseases [8, 10, 11, 29, 30]. In confounder-adjusted analyses, seropositivity and disease severity using the DAS28 joint score did not significantly associate with adverse COVID-19 outcomes. Other studies on patients with inflammatory arthritis and CTD had shown an association with higher disease activity and COVID-19 deaths [2, 31]. It is important to recognize that most previous studies included patients with well-established rheumatic disease, and these patients were therefore more likely to be older and may have had a substantially higher comorbidity burden, prior treatment exposure and different immune dysregulation than newly diagnosed EIA patients [32, 33].
The risk of being hospitalized due to COVID-19 was found to be increased in ex-smokers (aHR 1.62, 95% CI 1.10, 2.38) compared with non-smokers. There were no significant associations between severe COVID-19 outcomes and current smoking. These findings were reported in other observational studies [34–38]. The exact effect of smoking on the severity of COVID-19 outcomes is still ambiguous [39, 40]. There are a number of plausible explanations for the difference between current and ex-smokers including selection bias [41], unmeasured confounders, (such as smoking related comorbidities in ex-smokers) [37], and misclassification bias if smokers hospitalized with COVID-19 stopped during admission [38].
Of the four patient reported outcomes collected in NEIAA, using standardized difference in HRs, physical function seems to be a more important predictor for worse COVID-19 outcomes compared with disease impact measures or mental health. Only HAQ was associated with COVID-19 deaths in our model. Both HAQ and MSK-HQ assess the impact of musculoskeletal symptoms on the patient’s life and are highly correlated. HAQ is more sensitive tool in differentiating patients with extremely poor musculoskeletal health, while MSK-HQ is better in differentiating patients with better function [41].
In our model there was an association between worse mental health scores measured by the PHQ GAD combined score and COVID-19 admissions but not deaths. Psychological distress including depression and anxiety is considered a risk factor for COVID-19 hospitalization but not mortality [39]. It was reported previously that mood and anxiety disorders are not associated with a higher risk for COVID-19 mortality. Only schizophrenia spectrum disorders were linked to COVID-19 deaths; however, this is beyond our scope as we did not report details on the psychiatric diagnosis [40].
We also have demonstrated that there is no clear increased risk of adverse COVID-19 outcomes in patients with EIA from the use of csDMARDs monotherapy or combination therapy in our adjusted analyses compared with no DMARDs use. However, most of our patients were on csDMARDs monotherapy by 3 months, and this may affect our interpretation for the combination strategies risk. Similar findings on the use of csDMARDs were reported previously [42, 43]. In our analyses SSZ predicted COVID-19 admissions in the unadjusted model. However, this result was no longer significant when we adjusted for age, gender, confounders and disease severity.
There have been previous reports on the impact of SSZ monotherapy use and poor COVID-19 outcomes [31, 44, 45]. Results may be affected by the selection bias in these studies, different patient cohorts and/or by confounders. SSZ is a long established DMARD and is considered to have a low immunosuppressive effect [46], and this may have led to an increased number of patients started on SSZ due to this consideration at the beginning of the pandemic [31]. It is important to note that these other studies included patients with CTD, vasculitis and IBD [31, 45] while our study mainly reported on patients with EIA. In the context of inflammatory arthritis, our findings suggest that the link between SSZ and COVID-19 mortality may be driven by confounding factors rather than a true causal link.
There has also been considerable work surrounding the use of DMARDs, steroids and COVID-19 severity in long-lasting rheumatic diseases [31, 47].
In our cohort the use of CS was associated with increased risk of COVID-19 deaths in the unadjusted model, but this relation was no longer significant when we accounted for age, gender, confounders and disease severity. This finding was similar to another study [48]. Our result should be interpreted with several caveats as assessment of steroids exposure was imperfect. The steroid data are captured at one time point (which may reflect short-term induction therapy and not long-term use) and we do not collect steroid dose in NEIAA.
Other data from observational studies suggest the association between moderate to high dose of chronic steroids use and worse COVID-19 outcomes [31, 49, 50]. The COVID-19 Global Rheumatology Alliance (C19-GRA) in 2021 indicated that high-dose glucocorticoids (≥10 mg/day of prednisolone-equivalent) prior to infection increase the chance of adverse COVID-19 outcomes [31]. Another analysis suggested that the steroid risk maybe confounded by indication and that the underlying rheumatic disease activity affected the risk of COVID-19 death [51].
Strengths and limitations
NEIAA is one of the largest cohorts of patients with EIA. In our analyses we included patients with inflammatory arthritis only, and we adjusted for several important confounding factors including age, gender, comorbidities, disease severity, and RA and/or anti-CCP seropositivity. We acknowledge that our study has important limitations. The captured COVID-19 events were low in frequency, although our sample size was large, and thus power and precision were low for some analyses, particularly when considering subgroups. The information on treatment needs to be considered in the context that we did not capture any data on treatment changes over time, treatment adherence or dose. We did not capture information on biologics or targeted treatment. We also analysed patients with inflammatory arthritis as one group and have not accounted for disease-specific risk.
In conclusion, we have shown that in an EIA population, the risk factors for severe COVID-19 did not differ to the known predictors in the general population. Importantly, the initial treatment strategy did not appear to associate with COVID-19 hospitalization or mortality. Although the impacts of COVID-19 are reducing, there remains much that we can learn from the pandemic, including how immune modulation impacts upon viral hospitalization infection risk.
Supplementary Material
Contributor Information
Maryam A Adas, Centre for Rheumatic Disease, King’s College London, London, UK.
Mark D Russell, Centre for Rheumatic Disease, King’s College London, London, UK.
Emma Cook, Centre for Rheumatic Disease, King’s College London, London, UK.
Edward Alveyn, Centre for Rheumatic Disease, King’s College London, London, UK.
Jennifer Hannah, Centre for Rheumatic Disease, King’s College London, London, UK.
Sathiyaa Balachandran, Centre for Rheumatic Disease, King’s College London, London, UK.
Sarah Oyebanjo, British Society for Rheumatology, NEIAA, London, UK.
Paul Amlani-Hatcher, British Society for Rheumatology, NEIAA Patient Panel, London, UK.
Joanna Ledingham, Rheumatology Department, Portsmouth Hospitals University NHS Trust, Portsmouth, UK.
Sam Norton, Centre for Rheumatic Disease, King’s College London, London, UK; Psychology Department, Institute for Psychiatry, Psychology & Neuroscience, King’s College London, London, UK.
James B Galloway, Centre for Rheumatic Disease, King’s College London, London, UK.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Data used in this study were collected for the National Early Inflammatory Arthritis Audit and are available on request to the data controllers [the Healthcare Quality Improvement Partnership (HQIP)]. Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article. All figures and tables included in this article are original.
Contribution statement
J.B.G. and J.L. were involved in the design. J.B.G., M.A.A. and S.N. analysed the data. All authors interpreted the results. J.B.G., M.A.A., S.N., M.D.R., J.L. and E.C. wrote the report with contributions from all other authors. J.B.G. and S.N. accessed and verified the underlying data. M.A.A. and J.B.G. had final responsibility for the decision to submit for publication.
Funding
The National Early Inflammatory Arthritis Audit (NEIAA) is commissioned by the Healthcare Quality Improvement Partnership (HQIP), funded by National Health Services (NHS) England and NHS Improvement and the Welsh government, and carried out by the British Society for Rheumatology, King’s College London, King’s College Hospital and Net Solving. We used data provided by patients and staff within the NHS. HQIP had no involvement in designing this study, collecting, analysing and interpreting the data, or writing this report. Approval to submit the article for publication was obtained.
Disclosure statement: J.B.G. has received honoraria from AbbVie, Celgene, Chugai, Galapagos, Gilead, Janssen, Lilly, Pfizer, Roche and UCB. J.L. is a BSR trustee. M.D.R. has received honoraria from Pfizer, Lilly, Menarini, Janssen and UBC, and has received a research grant from the National Institute for Health Research (NIHR). All other authors declare no competing interests.
References
- 1. World Health Organization. WHO Coronavirus (COVID-19) Diagnosis. covid19.who.int (20 July 2021, date last accessed).
- 2. Grainger R, Kim AHJ, Conway R, Yazdany J, Robinson PC.. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat Rev Rheumatol 2022;18:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aviña‐Zubieta JA, Choi HK, Sadatsafavi M. et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Arthritis Care Res 2008;59:1690–7. [DOI] [PubMed] [Google Scholar]
- 4. Yoshida K, Lin TC, Wei MY. et al. Roles of postdiagnosis accumulation of morbidities and lifestyle changes in excess total and cause‐specific mortality risk in rheumatoid arthritis. Arthritis Care Res 2021;73:188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernatsky S, Hudson M, Suissa S.. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1157–60. [DOI] [PubMed] [Google Scholar]
- 6. Pawar A, Desai RJ, Gautam N, Kim SC.. Risk of admission to hospital for serious infection after initiating tofacitinib versus biologic DMARDs in patients with rheumatoid arthritis: a multidatabase cohort study. Lancet Rheumatol 2020;2:e84–98. [DOI] [PubMed] [Google Scholar]
- 7. Spila Alegiani S, Crisafulli S, Giorgi Rossi P. et al. ; ITA-COVID-19 Network. Risk of coronavirus disease 2019 hospitalization and mortality in rheumatic patients treated with hydroxychloroquine or other conventional disease-modifying anti-rheumatic drugs in Italy. Rheumatology (Oxford) 2021;60:Si25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bower H, Frisell T, Di Giuseppe D. et al. ; ARTIS Study Group. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis 2021;80:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saadoun D, Vieira M, Vautier M. et al. POS0055 SARS-COV-2 outbreak in autoimmune diseases: the Euro-Covimid study. Ann Rheum Dis 2021;80:233–4. [Google Scholar]
- 10. Gianfrancesco M, Hyrich KL, Al-Adely S. et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akiyama S, Hamdeh S, Micic D, Sakuraba A.. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 2021;80:384–91. [DOI] [PubMed] [Google Scholar]
- 12. Yang H, Xu J, Shi L, Duan G, Wang Y.. Correspondence on ‘Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis’. Ann Rheum Dis 2021;annrheumdis-2020-219821. [DOI] [PubMed] [Google Scholar]
- 13. Nice.org.uk. NICE Quality standard for rheumatoid arthritis in over 16s. Published 2020. https://www.nice.org.uk/guidance/qs33 (29 November 2022, date last accessed).
- 14. rheumatology.org.uk. UK: British Society for Rheumatology/clinical annual report: [updated 2022]. Available from: https://www.rheumatology.org.uk/Portals/0/Documents/Practice_Quality/Audit/NEIA/2022/NEIAA%20Fourth%20Annual%20Report_FINAL.pdf?ver=2022-10-13-110553-063 (29 November 2022, date last accessed).
- 15. England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K.. Validation of the rheumatic disease comorbidity index. Arthritis Care Res 2015;67:865–72. [DOI] [PubMed] [Google Scholar]
- 16. Assets.publishing.service.gov.uk. Published 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/464430/English_Index_of_Multiple_Deprivation_2015_-_Guidance.pdf (15 August 2022, date last accessed).
- 17. Wells G, Becker J, Teng J. et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fransen J, Creemers M, Van Riel P.. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford) 2004;43:1252–5. [DOI] [PubMed] [Google Scholar]
- 19. Wolfe F, Michaud K, Pincus T.. Development and validation of the health assessment questionnaire II: a revised version of the health assessment questionnaire. Arthritis Rheum 2004;50:3296–305. [DOI] [PubMed] [Google Scholar]
- 20. Kroenke K, Spitzer RL, Williams JB, Löwe B.. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009;50:613–21. [DOI] [PubMed] [Google Scholar]
- 21. Kroenke K, Spitzer RL, Williams JB, Monahan PO, Löwe B.. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146:317–25. [DOI] [PubMed] [Google Scholar]
- 22. Norton S, Ellis B, Santana Suárez B. et al. Validation of the Musculoskeletal Health Questionnaire in inflammatory arthritis: a psychometric evaluation. Rheumatology (Oxford) 2019;58:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emergency use ICD codes for COVID-19 disease outbreak. Who.int. Published 2021. https://www.who.int/standards/classifications/classification-of-diseases/emergency-use-icd-codes-for-covid-19-disease-outbreak (14 October 2021, date last accessed).
- 24.Gov.uk. UK: UKHSA data series on deaths in people with COVID-19 [updated 2022 February 4]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1052203/UKHSA-technical-summary-update-February-2022.pdf (29 November 2022, date last accessed).
- 25.England.nhs.uk. UK: Publication-definitions-1.pdf-NHS. England [updated 2020]. Available from: https://www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2020/12/Publication-definitions-1.pdf (29 November 2022, date last accessed).
- 26.Coronavirus.data.gov.uk. Published 2022. https://coronavirus.data.gov.uk/details/download (15 August 2022, date last accessed).
- 27. Reilev M, Kristensen KB, Pottegård A. et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol 2020;49:1468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williamson EJ, Walker AJ, Bhaskaran K. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Q, Liu J, Shao R. et al. Risk and clinical outcomes of COVID-19 in patients with rheumatic diseases compared with the general population: a systematic review and meta-analysis. Rheumatol Int 2021;41:851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi C, Wang L, Ye J. et al. Predictors of mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. BMC Infect Dis 2021;21:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strangfeld A, Schäfer M, Gianfrancesco MA. et al. ; COVID-19 Global Rheumatology Alliance. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raza K, Falciani F, Curnow SJ. et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther 2005;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yeo L, Adlard N, Biehl M. et al. Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis. Ann Rheum Dis 2016;75:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hippisley-Cox J, Young D, Coupland C. et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart 2020;106:1503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. González-Rubio J, Navarro-López C, López-Nájera E. et al. A systematic review and meta-analysis of hospitalised current smokers and COVID-19. Int J Environ Res Public Health 2020;17:7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kowall B, Nonnemacher M, Brune B. et al. A model to identify individuals with a high probability of a SARS-CoV-2 infection. J Infect 2021;82:e32–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saadatian-Elahi M, Amour S, Elias C. et al. Tobacco smoking and severity of COVID-19: experience from a hospital-based prospective cohort study in Lyon, France. J Med Virol 2021;93:6822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patanavanich R, Glantz SA.. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: a systematic review and meta-analysis. BMC Public Health 2021;21:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang S, Quan L, Ding M, Kang JH. et al. Depression, worry, and loneliness are associated with subsequent risk of hospitalization for COVID-19: a prospective study. Psychol Med 2022;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nemani K, Li C, Olfson M. et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry 2021;78:380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Norton S, Ellis B, Santana Suárez B. et al. Validation of the Musculoskeletal Health Questionnaire in inflammatory arthritis: a psychometric evaluation. Rheumatology (Oxford) 2019;58:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freites Nuñez DD, Leon L, Mucientes A. et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020;79:1393–9. [DOI] [PubMed] [Google Scholar]
- 43. Bower H, Frisell T, di Giuseppe D. et al. Effects of the COVID-19 pandemic on patients with inflammatory joint diseases in Sweden: from infection severity to impact on care provision. RMD Open 2021;7:e001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bower H, Frisell T, Di Giuseppe D. et al. ; ARTIS Study Group. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis 2021;80:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brenner EJ, Ungaro RC, Gearry RB. et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020;159:481–91.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ghasemnejad-Berenji M. Can sulfasalazine as an old drug with immunomodulatory and anti‐inflammatory effects be effective in COVID‐19? J Basic Clin Physiol Pharmacol 2022;33:113–5. [DOI] [PubMed] [Google Scholar]
- 47. Cano EJ, Fonseca Fuentes X, Corsini Campioli C. et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest 2021;159:1019–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gazzaruso C, Carlo Stella N, Mariani G. et al. Impact of anti-rheumatic drugs and steroids on clinical course and prognosis of COVID-19. Clin Rheumatol 2020;39:2475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raiker R, DeYoung C, Pakhchanian H. et al. Outcomes of COVID-19 in patients with rheumatoid arthritis: a multicenter research network study in the United States. Semin Arthritis Rheum 2021;51:1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis 2021;80:527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schäfer M, Strangfeld A, Hyrich KL. et al. Response to: ‘Correspondence on ‘Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician reported registry’’ by Mulhearn et al. Ann Rheum Dis 2021;annrheumdis-2021-220134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study were collected for the National Early Inflammatory Arthritis Audit and are available on request to the data controllers [the Healthcare Quality Improvement Partnership (HQIP)]. Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article. All figures and tables included in this article are original.