Abstract
Objectives
Gastroesophageal reflux disease (GERD) occurs frequently in patients with SSc. We investigated whether the presence of GERD and/or the use of anti-acid therapy, specifically proton-pump inhibitors (PPIs), are associated with long-term outcomes, especially in SSc-associated interstitial lung disease (SSc-ILD).
Methods
We retrospectively analysed patients with SSc and SSc-ILD from the German Network for Systemic Sclerosis (DNSS) database (2003 onwards). Kaplan–Meier analysis compared overall survival (OS) and progression-free survival (PFS) in patients with GERD vs without GERD (SSc and SSc-ILD), and PPI vs no PPI use (SSc-ILD only). Progression was defined as a decrease in either percentage predicted forced vital capacity of ≥10% or single-breath diffusing capacity for carbon monoxide of ≥15%, or death.
Results
It was found that 2693/4306 (63%) registered patients with SSc and 1204/1931 (62%) with SSc-ILD had GERD. GERD was not associated with decreased OS or decreased PFS in patients in either cohort. In SSc-ILD, PPI use was associated with improved OS vs no PPI use after 1 year [98.4% (95% CI: 97.6, 99.3); n = 760 vs 90.8% (87.9–93.8); n = 290] and after 5 years [91.4% (89.2–93.8); n = 357 vs 70.9% (65.2–77.1); n = 106; P < 0.0001]. PPI use was also associated with improved PFS vs no PPI use after 1 year [95.9% (94.6–97.3); n = 745 vs 86.4% (82.9–90.1); n = 278] and after 5 years [66.8% (63.0–70.8); n = 286 vs 45.9% (39.6–53.2); n = 69; P < 0.0001].
Conclusion
GERD had no effect on survival in SSc or SSc-ILD. PPIs improved survival in patients with SSc-ILD. Controlled, prospective trials are needed to confirm this finding.
Keywords: anti-acid, reflux, interstitial lung disease, proton pump inhibitors, SSc
Rheumatology key messages.
We assessed the effects of gastroesophageal reflux disease (GERD) and proton-pump inhibitor (PPI) use on overall survival/progression-free survival (OS/PFS) in SSc and SSc-ILD.
PPI use was associated with improved OS and PFS in patients with SSc-ILD.
The presence of GERD had no effect on OS or PFS in SSc or SSc-ILD.
Introduction
SSc is a rare and complex autoimmune disease, characterized by immune dysregulation, microvascular damage and progressive fibrosis [1]. SSc affects multiple organ systems and can lead to interstitial lung disease (ILD), which is a leading cause of death among patients with SSc [2–5]. SSc-associated ILD (SSc-ILD) results in significant pulmonary symptoms, such as exertional dyspnoea and cough, as well as impaired quality of life [6, 7].
Current evidence suggests that 20–30% of patients with SSc-ILD will develop progressive disease, i.e. worsening lung function [8–10]. Certain patient characteristics and biomarkers are associated with disease progression in SSc-ILD, though predicting which patients will progress remains challenging. Risk factors for progression include male sex, older age, elevated CRP, positive anti-Scl-70 (anti-topo I) status, negative ACA status, pulmonary arterial hypertension, dcSSc, arthritis, lower peripheral oxygen saturation after exercise, lower diffusing capacity of the lung for carbon monoxide (DLco), exertional dyspnoea, and non-productive cough [11–14].
Gastroesophageal reflux disease (GERD) is frequent in SSc (prevalence range 30–96%) [15–17]. Increased oesophageal diameter on high-resolution CT (HRCT) is associated with more severe radiographic ILD, lower lung volume and worse lung function [18, 19]. In addition, oesophageal dysmotility and absent contractility are associated with worse lung function [20, 21]. In one study of 145 patients with SSc-ILD, oesophageal diameter and hiatal hernia were independently associated with disease severity and mortality, but not with progression of ILD [22]. GERD occurs with higher frequency and greater severity in SSc-ILD (both dcSSc and lcSSc subsets) than in SSc without ILD [23]. However, it is not known whether GERD is associated with an increased risk of disease progression or death in SSc or SSc-ILD.
GERD is due to an insufficiency of the lower oesophageal sphincter and can be managed through lifestyle modification, or pharmacologically with anti-acid therapy (AAT), which mainly consists of proton-pump inhibitors (PPIs) [24, 25]. No large-scale, randomized controlled trials of AATs in general, and PPIs in particular, in SSc have been conducted, and the limited outcome data available suggest that PPI use is associated with short-term relief of GERD symptoms but not with long-term benefits [26, 27]. Data on the effect of PPIs on disease progression in idiopathic pulmonary fibrosis (IPF) (the archetypal progressive fibrosing ILD) are conflicting [28–33], and in SSc-ILD are non-existent. Therefore, there is a need to investigate the potential association between PPI use and disease progression in patients with SSc-ILD.
The objectives of this analysis of the registry of the German Network for Systemic Sclerosis (DNSS) were to assess whether (1) the progression of SSc and SSc-ILD is associated with the presence of GERD, and (2) the progression of SSc-ILD is associated with the use of PPIs.
Methods
Study design and study population
The DNSS is an interdisciplinary collaboration of hospitals and research centres with a special interest in SSc as previously described [34]. In this study, we retrospectively analysed patients with SSc registered in the DNSS from 2003 onwards. Two sets of patients were analysed: Set 1 included all patients with SSc or SSc-ILD, and all visits since the initial diagnosis of SSc were analysed; Set 2 included all patients with SSc-ILD, and all visits prior to the diagnosis of ILD were excluded, and is a subset of Set 1. Set 1 was categorized by the presence of GERD (yes/no) (Objective 1), and Set 2 was categorized by the use of PPIs (yes/no) (Objective 2). GERD was defined as oesophageal dysphagia and reflux by patient-reported symptoms such as difficulty swallowing liquid or hard food as well as intermittent heartburn [34].
Following inclusion into the registry, a four-page disease- and organ-specific questionnaire containing >110 items was completed for each patient. These items included patient data on current signs and symptoms, gender, year of birth, characteristic laboratory data (autoantibodies, clinical chemistry), SSc subsets, symptoms, organ involvement, modified Rodnan Skin Score (mRSS), as well as treatment with CSs, immunosuppressants, vasoactive drugs and prescription of physical therapy. Follow-up visits and respective investigations [e.g. echocardiography, ECG, pulmonary function tests (PFTs)] were recommended at least once per year. No specific patient-reported outcome questionnaires were used; symptoms were either self- or physician-reported. At the time of the initiation of the registry in 2003, there was no generally accepted definition of ILD progression based on DLco and/or forced vital capacity (FVC) thresholds. While data on DLco were recorded in the initial case report form (CRF), data on FVC were not systematically included before 2014.
Outcome definition
Overall survival (OS) was defined as the time from initial SSc diagnosis (Set 1), or first visit with an ILD diagnosis (Set 2), to death from any cause (recorded on the CRF by the respective centre) or last visit (censoring). Progression-free survival (PFS) was defined as the time from first DNSS registry visit (Set 1), or first visit with an ILD diagnosis (Set 2), to either death or disease progression [defined as a decrease in predicted FVC (FVC pred) of ≥10% or a decrease in predicted single-breath DLco (DLco-SB pred) of ≥15%], in line with definitions of progression used in previous real-world studies [9, 35], and IPF where comparable data on PPI exist [29].
Ethics approval
All patients in the registry provided written informed consent, and the Ethics Committee of the coordinating centre, Cologne University Hospital, approved the patient information and consent form for the registry (No. 04–43). This was used as the basis for approval from local ethics committees by all participating centres prior to registering patients.
Inclusion and exclusion criteria
Eligible patients were aged ≥18 years and had a diagnosis of SSc according to classification criteria published by the ACR 1980 criteria [36] or, from 2014, the ACR/European League Against Rheumatism 2013 criteria [37] for SSc. Classification of patients with dcSSc vs lcSSc was informed by criteria established by Le Roy et al. [38]. SSc-overlap syndrome was defined as a disease occurring with clinical aspects of SSc (according to the ACR criteria) or main symptoms of SSc simultaneously with those of other CTDs/autoimmune diseases such as DM, SS or SLE, as previously described [1, 34, 38–41]. In the original inclusion criteria, patients were defined as having SSc-ILD if bilateral fibrosis was visible on chest X-ray or HRCT scans and other possible causes of lung fibrosis were excluded [34]. Following a revision of the CRF in 2014, only patients with ILD confirmed on HRCT were accepted. No minimum duration of PPI treatment was specified as part of the eligibility criteria (any use of PPIs was sufficient for inclusion).
Statistics
Statistical analyses were conducted using SPSS Statistics 23.0.0.3 64-Bit software (IBM Corp., Armonk, NY, USA). In the analysis of patient characteristics, P values were calculated using the Pearson’s Chi-squared test for categorical data. For continuous data, P values were calculated parametrically using the t test, since the sample distribution was assumed to be normal due to the large sample size. The requirement of homogeneous variance between the groups for performing the t test was checked using Levene’s test; if the assumption was violated, the degrees of freedom were adjusted accordingly. We did not adjust for multiple testing due to the exploratory nature of this study.
For Set 1, data recorded between the first and last visits were aggregated (in order to capture all follow-up data) using the following coding system: dichotomous variables (ever/never), ordinal and continuous variables (worst value: minimum or maximum value), and irreversible symptoms and characteristics (value at last visit). For Set 2, data were taken from the first visit with an ILD diagnosis. Kaplan–Meier analysis compared OS and PFS between (1) patients with SSc and SSc-ILD with vs without GERD (Set 1), and (2) patients with SSc-ILD receiving vs not receiving PPIs (Set 2). Missing data were imputed using available data from other visits, where possible. No further imputation of missing data was performed.
Cox regression analyses were performed to evaluate the relationship between GERD/PPI use and survival. A univariable analysis including the variables affecting survival in SSc-ILD (baseline FVC, age, sex, baseline mRSS) was also performed. To estimate the effect of GERD on OS and PFS, univariable and multivariable Cox regression models were performed. In addition to GERD, univariable models were fitted for clinically relevant covariates, i.e. age (years), gender, SSc subtype, Scl-70 positive, ACA positive, AAT use, immunosuppressive therapy, pulmonary hypertension (OS and PFS); DLco-SB%, FVC% (OS only), and time since SSc diagnosis (years; PFS only). As FVC pred was only included in the registry from 2014, only a smaller subset has FVC pred values. In univariable analysis, a higher FVC pred value showed improved survival (P = 0.005). FVC pred was not included in the multivariable analysis of OS. The remaining covariates were included in the original multivariable model along with GERD/PPI. While GERD/PPI was kept as the primary outcome variable in the model, covariates were selected using the backward elimination method based on P > 0.1 (Wald statistic). For Cox regression models, hazard ratios, corresponding 95% CIs, and P values (Wald test) are reported.
Results
Patient characteristics
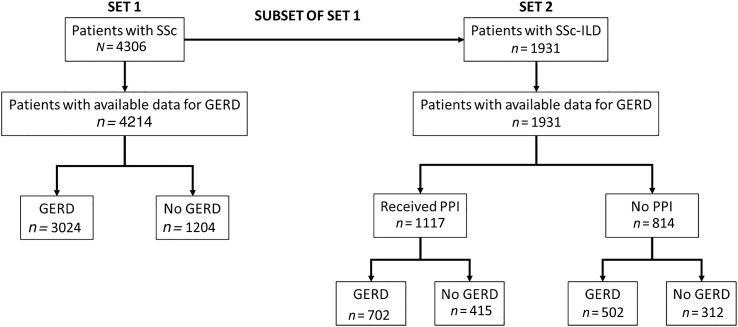
We identified 4306 patients with SSc, of whom 4214 had available data for GERD, with 3024 (71.8%) recorded as suffering from GERD at any point between their first and last DNSS visit (data extraction: 6 February 2019). Almost half of the patients with SSc (44.8%; n = 1931) had SSc-ILD, of which 62.3% (1204 out of 1931 patients) were recorded as having GERD at their first visit with an ILD diagnosis. In the SSc-ILD group, 1117 patients received PPIs and 814 patients did not (Table 1 and Fig. 1). Patients received only PPIs in this study; no other types of AAT were administered. GERD was recorded in 65.9% of patients in the PPI group and 58.1% of patients in the non-PPI group (P = 0.001). In patients with SSc and with at least two documented visits, the median follow-up time was 38 months [interquartile range (IQR) 18–86]. In patients with SSc-ILD and at least one follow-up visit after the first visit with an ILD diagnosis, the median follow-up time was 39 months (IQR 19–89). At baseline, mean (s.d.) FVC% pred was lower in the PPI group (n = 232) compared with the non-PPI group (n = 83) [77.8% (18.9) vs 84.9% (19.1); P = 0.003]. Mean (s.d.) DLCO%-SB pred was also lower in the PPI group (n = 824) compared with the non-PPI group (n = 442) [57.8% (19.4) vs 63.3% (22.7); P < 0.001]. The use of immunosuppressants (CYC, MTX, AZA, MMF or chloroquine/HCQ) was higher in the PPI group vs the non-PPI group [550/1109 (49.6%) vs 321/746 (43.0%); P = 0.005]. Statistically significant differences between the PPI and non-PPI group were also found for several other baseline characteristics, including gastrointestinal and renal involvement, as well as use of certain medications (AT1 receptor antagonists, endothelin receptor antagonists, prostanoids, analgesics) (Supplementary Table S1, available at Rheumatology online). There were no significant differences between the PPI and non-PPI groups for SSc subtype (lcSSc, dcSSc, overlap), age, sex, antibody status, BMI, mRSS, GERD, or steroid use (Table 1; Supplementary Table S1, available at Rheumatology online). A similar proportion of patients with dcSSc (65.3%; n = 620) and lcSSc (62.4%; n = 454) had GERD at baseline.
Table 1.
Baseline patient characteristics in the total SSc cohort, in patients with SSc-ILD and by PPI status
| SSc-ILD |
|||||
|---|---|---|---|---|---|
| SSc (n = 4306) | SSc-ILD (n = 1931) | Non-PPI (n = 814) | PPI (n = 1117) | P value (non-PPI vs PPI) | |
| SSc subtypes, n | 4306 | 1873 | 801 | 1072 | |
| lcSSc, n (%) | 2421 (56) | 729 (39) | 308 (39) | 421 (39) | 0.88 |
| dcSSc, n (%) | 1370 (32) | 945 (51) | 405 (51) | 540 (50) | |
| Overlap, n (%) | 515 (12) | 199 (11) | 88 (11) | 111 (10) | |
| Age at SSc diagnosis, n | 4140 | 1857 | 778 | 1079 | |
| Mean age, years (s.d.) | 48.61 (14.55) | 48.12 (14.91) | 48.36 (15.23) | 47.97 (14.67) | 0.58 |
| Time since diagnosis, n | 4189 | 1877 | 781 | 1096 | |
| Mean time, years (s.d.) | 7.06 (7.90) | 7.69 (8.08) | 8.29 (8.71) | 7.27 (7.58) | 0.08 |
| Males, n (%) | 799 (19) | 432 (23) | 168 (22) | 264 (25) | 0.13 |
| BMI, n | 3137 | 1072 | 248 | 824 | |
| Mean BMI, kg/m2 (s.d.) | 25 (5) | 25 (5) | 24 (5) | 25 (5) | 0.21 |
| Scl-70 positive, n (%) | 1287 (32) | 887 (48) | 368 (47) | 519 (48) | 0.76 |
| ACA positive, n (%) | 1530 (37) | 359 (20) | 142 (18) | 217 (20) | 0.39 |
| GERD, n (%) | 2693 (63) | 1204 (62) | 502 (62) | 702 (63) | 0.63 |
| Steroid use, n (%) | 1842 (44) | 864 (47) | 341 (46) | 523 (47) | 0.50 |
| Immunosuppressant use, n (%)a | 2109 (51) | 871 (47) | 321 (43) | 550 (49) | 0.005 |
| DLCO-SB% pred, n | 3196 | 1266 | 442 | 824 | |
| Mean DLCO-SB pred, % (s.d.) | 61 (21) | 60 (21) | 63 (23) | 58 (19) | <0.001 |
| FVC% pred, n | 1439 | 315 | 83 | 232 | |
| Mean FVC pred, % (s.d.) | 86 (22) | 80 (19) | 85 (19) | 78 (19) | 0.003 |
| FEV1% pred, n | 1514 | 346 | 87 | 259 | |
| Mean FEV1 pred, % (s.d.) | 84 (21) | 81 (19) | 84 (17) | 79 (20) | 0.004 |
| mRSS, n | 4019 | 1770 | 715 | 1055 | |
| Mean mRSS (s.d.) | 11 (9) | 12 (9) | 12 (10) | 12 (9) | 0.36 |
Aggregated data (first to last visit) presented for Set 1 (column 2); data on first visit with ILD diagnosis presented for Set 2 (columns 3–6). Valid n for each variable is indicated. P values were calculated using the Pearson’s Chi-squared test for categorical data, and the Mann–Whitney-U test for continuous data. aImmunosuppressant use does not include steroid use. GERD: gastroesophageal reflux disease; DLCO-SB: single-breath diffusing capacity of the lung for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; ILD: interstitial lung disease; IQR: interquartile range; mRSS: modified Rodnan skin score; PPI: proton pump inhibitor; pred: predicted; SSc-ILD: interstitial lung disease associated with SSc.
Figure 1.
Patient groups in the DNSS analysed in the study. For patients with SSc, data show GERD at any visit. For patients with SSc-ILD, data show GERD at first visit with ILD diagnosis. DNSS: German Network for Systemic Sclerosis; GERD: gastroesophageal reflux disease; PPI: proton pump inhibitor; SSc-ILD: SSc-associated interstitial lung disease
Effect of GERD on outcomes in SSc and SSc-ILD
In patients with SSc, GERD was not associated with a difference in 20-year OS: 86.2% (95% CI: 84.1, 88.3) in the GERD group (n = 429) vs 87.4% (83.6–91.4) in the non-GERD group (n = 93; P = 0.82). GERD was also not associated with a difference in 5-year PFS: 66.0% (63.5–68.6) in the GERD group (n = 672) vs 68.0% (62.2–74.2) in the non-GERD group (n = 94; P = 0.77) (Supplementary Fig. S1, available at Rheumatology online). Of 612 patients with progression in Set 1, 156 died and 456 had progression based on DLco and/or FVC thresholds.
In the subgroup of patients with SSc-ILD, GERD was not associated with a difference in 20-year OS: 83.4% (80.2–86.8) in the GERD group (n = 192) vs 79.4% (69.4–90.9) in the non-GERD group (n = 17; P = 0.36). GERD was also not associated with a difference in 5-year PFS: 62.2% (58.8–65.8) in the GERD group (n = 374) vs 67.9% (59.3–77.7) in the non-GERD group (n = 39; P = 0.57) (Supplementary Fig. S2, available at Rheumatology online). Of 353 patients with progression in Set 2, 129 died and 224 had progression based on DLco and/or FVC thresholds.
The number of events reported in Supplementary Figs S1B and S2B, available at Rheumatology online, is slightly lower, as only patients with additional information on GERD were included in the Kaplan–Meier analyses.
Effect of PPIs on outcomes in patients with SSc-ILD
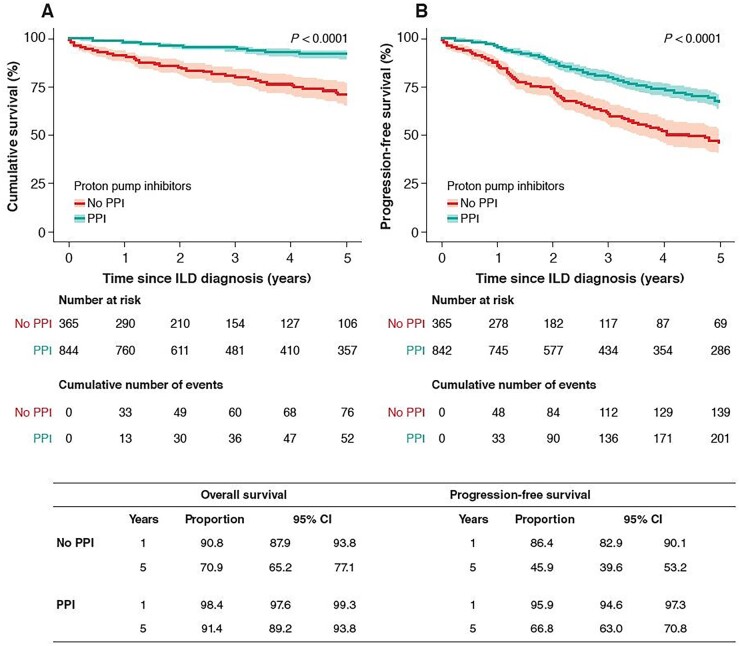
In patients with SSc-ILD, PPI use was associated with an improved OS and PFS (Fig. 2A and B). One year after the first ILD visit, the rate of OS (95% CI) was 90.8% (87.9, 93.8) in the non-PPI group (n = 290) vs 98.4% (97.6, 99.3) in the PPI group (n = 760); after 5 years, the rate of OS was 70.9% (65.2, 77.1) in the non-PPI group (n = 106) vs 91.4% (89.2–93.8) in the PPI group (n = 357) (P < 0.0001). One year after the first ILD visit, the rate of PFS (95% CI) was 86.4% (82.9, 90.1) in the non-PPI group (n = 278) vs 95.9% (94.6, 97.3) in the PPI group (n = 745) (P < 0.0001); after 5 years, the rate of PFS (95% CI) was 45.9% (39.6, 53.2) in the non-PPI group (n = 69) vs 66.8% (63.0, 70.8) in the PPI group (n = 286) (P < 0.0001).
Figure 2.
Kaplan–Meier curve for overall survival (A) and progression-free survival (B) according to use of PPIs in patients with SSc-ILD. Analysis of Set 2. Progression defined as decline in FVC of ≥10%, decline in DLCO of ≥15%, or death. Shading represents confidence intervals. DLCO: diffusing capacity of the lung for carbon monoxide; FVC: forced vital capacity; ILD: interstitial lung disease; PPI: proton-pump inhibitor; SSc-ILD: SSc-associated interstitial lung disease
Regression analyses
Using Cox regression models for OS or PFS, no additional role of potential confounders was identified. The effect of GERD and PPI on OS and PFS remained stable after adjusting for relevant covariates, including immunosuppressive therapy (Supplementary Tables S2–S5, available at Rheumatology online).
Discussion
While there is some debate around the potential effects of PPIs on IPF [42, 43], nothing is known about whether (or how) PPIs affect disease progression in SSc-ILD. Our study, the largest of its kind to investigate outcomes associated with PPI use in patients with SSc-ILD, suggests that PPIs may provide some survival benefit (in terms of both OS and PFS) over a 5-year period in this at-risk population. In addition, our findings suggest that the presence of GERD has no association with survival outcomes (20-year OS or 5-year PFS) in patients with SSc or SSc-ILD. Regression analyses confirmed that the effect of GERD and PPIs on OS and PFS remained stable after adjusting for relevant covariates, including immunosuppressive therapy. Thus, our results suggest that a potential survival benefit might be associated with PPI use rather than influenced by other comorbidities or perhaps immunosuppressive therapies.
Improved survival in the PPI group could have been influenced by the type of treatment centre visited by patients, i.e. specialist centres may have been more likely to prescribe PPIs to patients with SSc-ILD and may have provided better all-round care. However, since the type of treatment centre was not documented as part of the DNSS database, the potential influence of this factor is unknown. In addition, data on the specific doses of PPI received by patients are also unavailable, preventing a more granular interpretation of the effect of PPIs on survival. Higher doses of PPIs may have resulted not only in different survival outcomes, but also in different safety outcomes, e.g. higher infection rates. Lastly, the number of patients with available and consistent data from serial PFTs was too low to analyse any potential association between pulmonary function decline (change in FVC% pred and DLCO) and survival outcomes over the 5-year observation period.
It is also possible that the favourable survival outcomes in the PPI group were due to other, indirect effects of PPIs on acidic micro-aspiration (the unintentional aspiration of very small amounts of acidic reflux material) [44, 45]. It has been hypothesized that in IPF, persistent inflammation of the lung tissue infrastructure caused by micro-aspiration (discussed by Wang et al. [44]) could accelerate disease progression. However, this is yet to be proven, and no studies have investigated the possible effect of PPIs on the acidic component of micro-aspiration.
While it is possible that any use of PPIs could bias our findings, only regularly prescribed drugs were recorded in the CRF. Therefore, a single dose or 1-week course of therapy was not recorded. It should also be noted that GERD in patients with SSc is a chronic disease and shows no remission, therefore regular dosage is likely once initiated.
DLco was reduced both in patients with SSc-ILD and in those with SSc without ILD. This may be due to pulmonary hypertension, or indicate patients at risk of developing pulmonary hypertension [46, 47]. In the Cox regression models for OS or PFS, no additional role of potential confounders was found, and the effect of GERD and PPIs remained stable after adjusting for relevant covariates, including pulmonary hypertension.
Despite these interesting findings, this study is subject to several limitations. Patient characteristics varied between the PPI and non-PPI groups. In the PPI group, patients had worse lung function (lower FVC% pred and DLCO) and higher immunosuppressant use than patients in the non-PPI group. As such, patients in the PPI group (with more advanced disease) may have been less likely to cross the pre-specified thresholds of progression (FVC ≥10% or DLCO ≥15%), which could have biased the PFS results in favour of PPIs. However, this line of reasoning would also suggest that OS should be shorter in the PPI group, which is not what we observed. Furthermore, lack of standardization of PFTs is a typical limitation of registry data. Although the types of immunosuppressant used were recorded in the database, low patient numbers and changing patterns of use across the study period prevent stratification of our analysis by immunosuppressant type. The frequency of steroid and immunosuppressant therapy may be higher in Germany than in other countries. However, the frequencies are in line with those previously reported for this registry [48], or are similar or lower than those reported in a EUSTAR cohort analysis [49]. In addition, because the PPI subgroup in the SSc-ILD analysis set included patients both with (65.9%) and without (58.1%) GERD, it is not possible to make any conclusions about the association between PPI use and survival outcomes in patients with SSc-ILD and GERD specifically. Finally, at the time of data capture, novel therapies such as antifibrotic drugs were not used in the included patients. Their effect on OS and PFS cannot, therefore, be assessed.
Building on our research, prospective studies evaluating PPI alone vs in combination with other novel approaches to the management of GERD would be of clinical interest. One such approach that has recently shown promise in IPF is laparoscopic surgical treatment, which had a clinically meaningful impact on lung function in patients with IPF and GERD in a Phase II study [50]. To date, no such study has been conducted in patients with SSc-ILD.
Conclusion
Overall, our findings in this retrospective analysis of a large, prospective, observational cohort suggest that PPI use may provide a survival benefit over 5 years in patients with SSc-ILD. However, further randomized controlled trials are urgently needed to shed more light on the possible effects of PPIs on outcomes in SSc-ILD.
Supplementary Material
Acknowledgements
Writing support was provided by Chester Trinick and Helen Keyworth of Nucleus Global, which was contracted and funded by Boehringer Ingelheim.
Contributor Information
Michael Kreuter, Center for Interstitial and Rare Lung Diseases, Department of Pneumology, Thoraxklinik, University of Heidelberg, German Center for Lung Research, Heidelberg, Germany.
Francesco Bonella, Center for Interstitial and Rare Lung Diseases, Ruhrlandklinik, Pneumonology Department, University of Duisburg-Essen, Essen, Germany.
Norbert Blank, Division of Rheumatology, Department of Internal Medicine V, University Hospital Heidelberg, Heidelberg, Germany.
Gabriela Riemekasten, Clinic for Rheumatology and Clinical Immunology, University Hospital Schleswig-Holstein, University of Lübeck, Lübeck, Germany.
Ulf Müller-Ladner, Department of Rheumatology, Kerckhoff Clinic, Bad Nauheim, Germany.
Jörg Henes, Centre for Interdisciplinary Rheumatology, Immunology and Auto-inflammatory Diseases and Department of Internal Medicine 2, University Hospital Tübingen, Tübingen, Germany.
Elise Siegert, Department of Rheumatology and Clinical Immunology, Charité – Universitaetsmedizin Berlin, Berlin, Germany; Berlin Institute of Health, Berlin, Germany.
Claudia Günther, Department of Dermatology, University Hospital Carl Gustav Carus, TU Dresden, Dresden, Germany.
Ina Kötter, Division of Rheumatology and Systemic Inflammatory Diseases, University Hospital Hamburg, Rheumatology Clinic, Bad Bramstedt, Germany.
Christiane Pfeiffer, Department of Dermatology and Allergology, University Hospital of Munich (LMU), Munich, Germany.
Marc Schmalzing, Rheumatology/Clinical Immunology, Department of Internal Medicine II, University Hospital Würzburg, Würzburg, Germany.
Gabriele Zeidler, Department of Rheumatology, Osteology and Pain Therapy, Center for Rheumatology Brandenburg, Johanniter-Hospital Treuenbrietzen, Treuenbrietzen, Germany.
Peter Korsten, Department of Nephrology and Rheumatology, University Medical Center Göttingen, Göttingen, Germany.
Laura Susok, Department of Dermatology, Venereology and Allergology, St. Josef Hospital Bochum, Bochum, Germany.
Aaron Juche, Department of Rheumatology, Immanuel Hospital Berlin-Buch, Berlin, Germany.
Margitta Worm, Department of Dermatology, Venereology and Allergology, Charité – Universitaetsmedizin Berlin, Berlin, Germany.
Ilona Jandova, Rheumatology and Clinical Immunology, University Medical Center Freiburg, Freiburg, Germany.
Jan Ehrchen, Department of Dermatology, University Hospital Münster, Münster, Germany.
Cord Sunderkötter, Department of Dermatology, University Hospital Halle (Saale), Halle, Germany.
Gernot Keyßer, Department of Internal Medicine, Division of Rheumatology, University Hospital Halle (Saale), Halle, Germany.
Andreas Ramming, Department of Internal Medicine 3, Rheumatology & Immunology, Friedrich-Alexander-University (FAU) Erlangen-Nürnberg and University Hospital Erlangen, Erlangen, Germany.
Tim Schmeiser, Department for Rheumatology, Immunology and Osteology, St. Josef Hospital Wuppertal, Wuppertal, Germany.
Alexander Kreuter, Department of Dermatology, Venereology and Allergology, Helios St Elisabeth Hospital Oberhausen, University Witten/Herdecke, Oberhausen, Germany.
Kathrin Kuhr, Institute of Medical Statistics and Computational Biology (IMSB), University Hospital Cologne, Cologne, Germany.
Hanns-Martin Lorenz, Center for Interstitial and Rare Lung Diseases, Ruhrlandklinik, Pneumonology Department, University of Duisburg-Essen, Essen, Germany.
Pia Moinzadeh, Department of Dermatology and Venereology, University Hospital Cologne, Cologne, Germany.
Nicolas Hunzelmann, Department of Dermatology and Venereology, University Hospital Cologne, Cologne, Germany.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data presented in this study are derived from an independent German registry of patients with SSc (the DNSS network). For all data-related queries, please contact the last author, N.H.
Contribution statement
All authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; and contributed substantially to the study design, data analysis, and interpretation and writing of the manuscript.
Funding
Analysis of ILD-related data was supported by Boehringer Ingelheim International GmbH. No author received payment for the development of this manuscript. Writing support was provided by Chester Trinick and Helen Keyworth of Nucleus Global, which was contracted and funded by Boehringer Ingelheim.
Disclosure statement: M.K. reports grants and personal fees from Boehringer Ingelheim and Roche, and personal fees from Galapagos. F.B. reports grants and personal fees from Boehringer Ingelheim, Roche, Galapagos and Savara Pharma. N.B. reports personal fees from Actelion, Boehringer Ingelheim, Roche, Pfizer, MSD and AbbVie, and grants and personal fees from Novartis and SOBI. J.H. reports grants and personal fees from AbbVie, Boehringer Ingelheim, Roche/Chugai, Janssen, Novartis, Pfizer and UCB. C.G. reports fees from Novartis, Boehringer Ingelheim and Amgen. C.P. reports personal fees from Boehringer Ingelheim, Pfizer and Takeda, grants and personal fees from Actelion and Novartis, and grants from Corbus and Amgen. M.S. reports grants and personal fees from Roche/Chugai, Hexal/Sandoz, Janssen and BMS, and personal fees from AbbVie, Novartis, UCB, Boehringer Ingelheim and Gilead. GZ reports a lecture for Janssen. P.K. reports personal fees and non-financial support from AbbVie, Chugai, Novartis and Pfizer unrelated to the present manuscript, and personal fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline and Janssen-Cilag unrelated to the present manuscript. A.J. reports personal fees from Boehringer Ingelheim and AbbVie. J.E. reports personal fees for advisory board participation from Boehringer Ingelheim, and for lectures from Actelion, Janssen and Boehringer Ingelheim. N.H. reports personal fees for advisory participation from Boehringer Ingelheim, and lectures from Actelion, Boehringer Ingelheim and Roche. G.R., U.M.-L., E.S., I.K., L.S., M.W., I.J., C.S., G.K., A.R., T.S., A.K., K.K., H.M.-L. and P.M. have declared no conflicts of interest. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations.
References
- 1. Walker UA, Tyndall A, Czirjak L. et al. ; EUSTAR Co-authors. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis 2007;66:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubio-Rivas M, Royo C, Simeón CP, Corbella X, Fonollosa V.. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:208–19. [DOI] [PubMed] [Google Scholar]
- 3. Elhai M, Meune C, Boubaya M. et al. ; EUSTAR Group. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017;76:1897–905. [DOI] [PubMed] [Google Scholar]
- 4. Steen VD, Medsger TA.. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007;66:940–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tyndall AJ, Bannert B, Vonk M. et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 6. Beretta L, Santaniello A, Lemos A, Masciocchi M, Scorza R.. Validity of the Saint George’s Respiratory Questionnaire in the evaluation of the health-related quality of life in patients with interstitial lung disease secondary to systemic sclerosis. Rheumatology (Oxford) 2007;46:296–301. [DOI] [PubMed] [Google Scholar]
- 7. Tashkin DP, Volkmann ER, Tseng CH. et al. Improved cough and cough-specific quality of life in patients treated for scleroderma-related interstitial lung disease: results of Scleroderma Lung Study II. Chest 2017;151:813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jaeger VK, Wirz EG, Allanore Y. et al. ; EUSTAR co-authors. Incidences and risk factors of organ manifestations in the early course of systemic sclerosis: a longitudinal EUSTAR study. PLoS One 2016;11:e0163894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goh NS, Hoyles RK, Denton CP. et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017;69:1670–8. [DOI] [PubMed] [Google Scholar]
- 10. Hoffmann-Vold AM, Allanore Y, Alves M. et al. ; EUSTAR Collaborators. Progressive interstitial lung disease in patients with systemic sclerosis–associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021;80:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volkmann ER, Tashkin DP, Sim M. et al. ; SLS I and SLS II Study Groups. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann Rheum Dis 2019;78:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu W, Jordan S, Becker MO. et al. Prediction of progression of interstitial lung disease in patients with systemic sclerosis: the SPAR model. Ann Rheum Dis 2018;77:1326–32. [DOI] [PubMed] [Google Scholar]
- 13. Nihtyanova SI, Schreiber BE, Ong VH. et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014;66:1625–35. [DOI] [PubMed] [Google Scholar]
- 14. Morisset J, Vittinghoff E, Elicker BM. et al. Mortality risk prediction in scleroderma-related interstitial lung disease: the SADL model. Chest 2017;152:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ntoumazios SK, Voulgari PV, Potsis K. et al. Esophageal involvement in scleroderma: gastroesophageal reflux, the common problem. Semin Arthritis Rheum 2006;36:173–81. [DOI] [PubMed] [Google Scholar]
- 16. Ebert EC. Esophageal disease in scleroderma. J Clin Gastroenterol 2006;40:769–75. [DOI] [PubMed] [Google Scholar]
- 17. Forbes A, Marie I.. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii36–9. [DOI] [PubMed] [Google Scholar]
- 18. Richardson C, Agrawal R, Lee J. et al. A dilated esophagus is an independent risk factor for interstitial lung disease in SSc. Arthritis Rheumatol 2014;66:S319. [Google Scholar]
- 19. Salaffi F, Di Carlo M, Carotti M. et al. Relationship between interstitial lung disease and oesophageal dilatation on chest high-resolution computed tomography in patients with systemic sclerosis: a cross-sectional study. La Radiol Med 2018;123:655–63. [DOI] [PubMed] [Google Scholar]
- 20. Zhang XJ, Bonner A, Hudson M. et al. ; Canadian Scleroderma Research Group. Association of gastroesophageal factors and worsening of forced vital capacity in systemic sclerosis. J Rheumatol 2013;40:850–8. [DOI] [PubMed] [Google Scholar]
- 21. Kimmel JN, Carlson DA, Hinchcliff M. et al. The association between systemic sclerosis disease manifestations and esophageal high-resolution manometry parameters. Neurogastroenterol Motil 2016;28:1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winstone TA, Hague CJ, Soon J. et al. Oesophageal diameter is associated with severity but not progression of systemic sclerosis–associated interstitial lung disease. Respirology 2018;23:921–6. [DOI] [PubMed] [Google Scholar]
- 23. Savarino E, Bazzica M, Zentilin P. et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med 2009;179:408–13. [DOI] [PubMed] [Google Scholar]
- 24. Katz PO, Gerson LB, Vela MF.. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329. [DOI] [PubMed] [Google Scholar]
- 25. Hunt R, Armstrong D, Katelaris P. et al. ; Review Team. World Gastroenterology Organisation global guidelines: GERD global perspective on gastroesophageal reflux disease. J Clin Gastroenterol 2017;51:467–78. [DOI] [PubMed] [Google Scholar]
- 26. Pakozdi A, Wilson H, Black CM, Denton CP.. Does long term therapy with lansoprazole slow progression of oesophageal involvement in systemic sclerosis? Clin Exp Rheumatol 2009;27(3 Suppl 54):5–8. [PubMed] [Google Scholar]
- 27. Foocharoen C, Chunlertrith K, Mairiang P. et al. Effectiveness of add-on therapy with domperidone vs alginic acid in proton pump inhibitor partial response gastro-oesophageal reflux disease in systemic sclerosis: randomized placebo-controlled trial. Rheumatology (Oxford) 2017;56:214–22. [DOI] [PubMed] [Google Scholar]
- 28. Lee JS, Collard HR, Anstrom KJ. et al. ; IPFnet Investigators. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med 2013;1:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kreuter M, Wuyts W, Renzoni E. et al. Antacid therapy and disease outcomes in idiopathic pulmonary fibrosis: a pooled analysis. Lancet Respir Med 2016;4:381–9. [DOI] [PubMed] [Google Scholar]
- 30. Tran T, Suissa S.. The effect of anti-acid therapy on survival in idiopathic pulmonary fibrosis: a methodological review of observational studies. Eur Respir J 2018;51:1800376. [DOI] [PubMed] [Google Scholar]
- 31. Jo HE, Corte TJ, Glaspole I. et al. Gastroesophageal reflux and antacid therapy in IPF: analysis from the Australia IPF Registry. BMC Pulm Med 2019;19:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu B, Su F, Xu N. et al. Chronic use of anti-reflux therapy improves survival of patients with pulmonary fibrosis. Int J Clin Exp Med 2017;10:5805–10. [Google Scholar]
- 33. Costabel U, Behr J, Crestani B. et al. Anti-acid therapy in idiopathic pulmonary fibrosis: insights from the INPULSIS trials. Respir Res 2018;19:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hunzelmann N, Genth E, Krieg T. et al. ; Registry of the German Network for Systemic Scleroderma. The registry of the German Network for Systemic Scleroderma: frequency of disease subsets and patterns of organ involvement. Rheumatology (Oxford) 2008;47:1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu W, Jordan S, Graf N. et al. Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort. Ann Rheum Dis 2019;78:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masi AT, Rodnan GP, Medsger TA Jr. et al. ; Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 37. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LeRoy EC, Black C, Fleischmajer R. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 39. Balbir-Gurman A, Braun-Moscovici Y.. Scleroderma overlap syndrome. Isr Med Assoc J 2011;13:14–20. [PubMed] [Google Scholar]
- 40. Moinzadeh P, Aberer E, Ahmadi-Simab K. et al. ; All Participating DNSS Centers. Disease progression in systemic sclerosis—overlap syndrome is significantly different from limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis 2015;74:730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mierau R, Moinzadeh P, Riemekasten G. et al. Frequency of disease-associated and other nuclear autoantibodies in patients of the German Network for Systemic Scleroderma: correlation with characteristic clinical features. Arthritis Res Ther 2011;13:R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghebre YT, Raghu G.. Idiopathic pulmonary fibrosis: novel concepts of proton pump inhibitors as antifibrotic drugs. Am J Respir Crit Care Med 2016;193:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee JS, Ryu JH, Elicker BM. et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:1390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Z, Bonella F, Li W. et al. Gastroesophageal reflux disease in idiopathic pulmonary fibrosis: uncertainties and controversies. Respiration 2018;96:571–87. [DOI] [PubMed] [Google Scholar]
- 45. Lee AS, Ryu JH.. Aspiration pneumonia and related syndromes. Mayo Clin Proc 2018;93:752–62. [DOI] [PubMed] [Google Scholar]
- 46. Steen VD, Graham G, Conte C, Owens G, Medsger TA Jr. Isolated diffusing capacity reduction in systemic sclerosis. Arthritis Rheum 1992;35:765–70. [DOI] [PubMed] [Google Scholar]
- 47. Colaci M, Giuggioli D, Sebastiani M. et al. Predictive value of isolated DLCO reduction in systemic sclerosis patients without cardio-pulmonary involvement at baseline. Reumatismo 2015;67:149–55. [DOI] [PubMed] [Google Scholar]
- 48. Hunzelmann N, Moinzadeh P, Genth E. et al. ; The German Network for Systemic Scleroderma Centers. High frequency of corticosteroid and immunosuppressive therapy in patients with systemic sclerosis despite limited evidence for efficacy. Arthritis Res Ther 2009;11:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adler S, Huscher D, Allanore Y. et al. Use of immunosuppressants in SSc patients with interstitial lung disease – results of the DeSScipher project of the EUSTAR Group. Clin Exp Rheumatol 2014;32:S85–6.24528649 [Google Scholar]
- 50. Raghu G, Pellegrini CA, Yow E. et al. Laparoscopic anti-reflux surgery for the treatment of idiopathic pulmonary fibrosis (WRAP-IPF): a multicentre, randomised, controlled phase 2 trial. Lancet Respir Med 2018;6:707–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are derived from an independent German registry of patients with SSc (the DNSS network). For all data-related queries, please contact the last author, N.H.