Abstract
Objectives
To explore the course of lung function and RA disease activity and predictive factors for deteriorating lung function in patients with RA-interstitial lung disease (ILD).
Methods
The Korean Rheumatoid Arthritis–Interstitial Lung Disease cohort is a multicentre, prospective observational cohort. Patients with RA-ILD were enrolled and followed up annually for 3 years for RA disease activity and ILD status assessment. Group-based modelling was used to cluster a similar predicted percentage of forced vital capacity (FVC%) patterns into trajectories.
Results
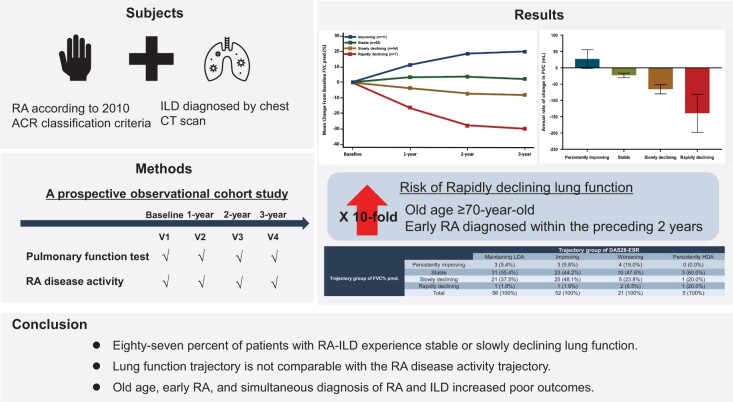
This study included 140 patients who underwent at least two pulmonary function tests. Four distinctive trajectories for predicted FVC% were ‘improving’ [n = 11 (7.9%)], ‘stable’ [n = 68 (38.4%)], ‘slowly declining’ [n = 54 (48.6%)] and ‘rapidly declining’ [n = 7 (5.0%)]. Most (77.7%) patients maintained or improved to low RA disease activity. The lung function trajectory was not comparable to the RA disease activity trajectory. Age ≥70 years [relative risk (RR) 10.8 (95% CI 1.30, 89.71)] and early RA diagnosed within the preceding 2 years [RR 10.1 (95% CI 1.22, 84.2)] were associated with increased risk for rapidly declining predicted FVC%. The risk for deterioration or mortality increased in patients with a simultaneous diagnosis of RA and ILD within 24 weeks [RR 9.18 (95% CI 2.05, 41.0)] and the extent of lung involvement [RR 3.28 (95% CI 1.12, 9.60)].
Conclusion
Most patients with RA-ILD experienced stable or slowly declining lung function. In 5% of patients, predicted FVC% deteriorated rapidly, especially in older adults with early RA. The lung function trajectory was not comparable to the RA disease activity trajectory.
Keywords: RA, interstitial lung disease, lung function trajectory, prospective cohort study
Graphical Abstract
Rheumatology key messages.
Overall, 87% of patients with RA-ILD experience stable or slowly declining lung function.
Lung function trajectory is not comparable with the RA disease activity trajectory.
Old age, early RA and simultaneous diagnosis of RA and ILD increased poor outcomes.
Introduction
Interstitial lung disease (ILD) is not uncommon in patients with RA. One in six to ten patients with RA is diagnosed with clinically symptomatic ILD [1–3]. Patients with RA are less active than healthy adults, thus an additional 20–50% of patients may have interstitial lung abnormalities without respiratory symptoms [4–6]. The mortality rate is 2–10 times higher in patients with RA-ILD than in patients with RA without ILD [3, 4, 7–12]. Patient-specific (age and sex) and ILD-specific factors (pulmonary function and extent or pattern of fibrosis) may predominantly influence mortality [2, 9, 10, 13–18]. Baseline pulmonary function impairment is an independent risk factor for mortality in RA-ILD [10]. Active RA was associated with an increased risk of RA-ILD [13] and was also an independent predictor of mortality in RA-ILD [15].
Most previous studies of RA-ILD were conducted in retrospective settings and lung-related items were not measured repeatedly at regular intervals, therefore the study outcome could not focus on worsening but focussed on mortality alone [19]. Risk factors were measured once at baseline in these mortality-related studies. Moreover, definitions of ILD were inconsistent; RA was diagnosed based on the 1987 ACR criteria and RA disease activity was not assessed simultaneously [11–16]. Thus we aimed to explore the course of lung function and RA disease activity and the predictive factors for deteriorating lung function using a prospective cohort.
Methods
Study design
The KOrean Rheumatoid Arthritis–Interstitial Lung Disease (KORAIL) cohort is a multicentre, prospective, observational cohort study conducted to assess the course of RA-ILD. This study was approved by the ethical committees of each participating centre (Supplementary Table S1, available at Rheumatology online). All patients provided written informed consent. All procedures followed the ethical standards outlined in the Declaration of Helsinki and Good Clinical Practice guidelines.
Patient recruitment
Patients ≥18 years of age who regularly attended an outpatient rheumatology clinic were recruited and enrolled if they had been diagnosed with RA using the 2010 ACR/EULAR RA classification criteria [20] and diagnosed with ILD via chest CT scan, regardless of respiratory symptoms [20, 21]. The chest CT patterns were determined by radiologists at each centre and further confirmed by two expert radiologists who were blinded to the patients’ clinical status and demographics. The chest CT patterns were classified using the 2018 clinical recommendations for diagnosing idiopathic pulmonary fibrosis (IPF) [22]. Patient recruitment began in January 2015 and ended in December 2018. The enrolled patients were followed up yearly for 3 years.
Data collection
Data on body weight, height and smoking history were collected annually. To assess the course of ILD, we evaluated pulmonary function tests (PFTs), including forced vital capacity (FVC) and diffusion capacity for carbon monoxide (DLCO), chest X-ray and chest CT annually. To assess RA disease activity, we evaluated radiographs of the hands and feet; 28-joint DAS (DAS28) score, including 28-joint swollen joint counts (SJC28) and tender joint counts (TJC28); patient global assessment scores; ESR; CRP level and HAQ Disability Index scores. The treating rheumatologist assessed the swollen and tender joints. We surveyed current or previous medications for RA treatment, including steroids and any DMARDs.
Definition of early RA and long-standing RA
Patients with early RA were diagnosed with RA within 2 years before enrolment and patients with long-standing RA were diagnosed with RA ≥5 years before enrolment.
Definition of ILD progression based on PFT findings
ILD progression was defined as a decline in FVC of ≥10% or a decline in FVC of 5–10% with a decline in DLCO of 15%, based on the definition used in a previous study [23].
Statistical analyses
A group‐based trajectory model approach was adopted using the trajectory procedure (Proc TRAJ) of SAS software version 9.4 (SAS Institute, Cary, NC) to determine lung function and RA disease activity trajectories over 3 years and categorize patients into the relevant trajectory groups [24, 25]. Briefly, this approach involved application of a finite mixture model, wherein the longitudinal lung function and RA disease activity data were fitted and grouped using a maximum likelihood method as a mixture of multiple latent trajectories in a censored normal model with a polynomial function of time [26].
The optimal number of groups was determined using the Bayesian information criterion comparing the 2Δ Bayesian information criterion between each number of groups and polynomial orders for time function. Each group was named according to the visual description of the trajectory.
Group-wise characteristics are presented as mean (s.d.) or median [interquartile range (IQR)] for continuous variables and frequencies (percentages) for categorical variables. Data were compared using the χ2 test, Fisher’s exact test, analysis of variance or Kruskal−Wallis test, as appropriate. Poisson regression was applied to calculate the relative risks (RRs) and their 95% CIs for FVC deterioration. A univariate analysis was performed to determine prognostic factors associated with deterioration.
The linear mixed-effects model was used to estimate each patient’s annual FVC change rate. The intercept and slope were fitted as random effects to address the interpatient differences at baseline and different rates of FVC change during the follow-up period. All statistical analyses were conducted using SAS software version 9.4. P-values <0.05 were considered statistically significant.
Results
Baseline patients’ characteristics
Among all KORAIL cohort patients (n = 168), 140 patients with at least two PFT results were included. Overall, 12 (8.6%) patients died before October 2021: 3 patients died between the 1- and 2-year follow-ups, 6 patients between the 2- and 3-year follow-ups and 3 patients after the 3-year follow-up. Supplementary Table S2 (available at Rheumatology online) presents the patients’ clinical information.
Overall, 69% of patients were female. The mean age at enrolment was 66.5 years (s.d. 13.9). The patients’ mean follow-up duration was 2.8 years (s.d. 0.6). Fifty (35.7%) patients were ≥70 years. Thirty-seven (26.4%) patients were ever-smokers. The mean RA duration was 7.9 (8.4) years. Forty-five (32.1%) patients had early RA and 77 (55.0%) patients had long-standing RA. The mean ILD duration was 7.8 years (s.d. 8.4). Fifty-one (36.4%) patients were diagnosed with RA and ILD simultaneously within 24 weeks.
Overall, 138 (98.6%) patients showed positive findings for either RF or anti-CCP antibodies. Only 2 (1.4%) patients showed negative findings for both RF and anti-CCP antibodies, whereas 119 (85.0%) patients showed positive findings for both. Most of the anti-CCP antibody-positive patients [50.0% (n = 70)] exhibited a high titre (≥200 IU/ml).
Eighty-four patients (61.7%) exhibited definite usual interstitial pneumonia (UIP) or probable UIP on chest CT scans.
Trajectory of RA disease activity over 3 years
The mean DAS28-ESR and DAS28-CRP were 4.0 (s.d. 1.4) and 3.1 (s.d. 1.4), respectively (Table 1). The proportion of patients in remission increased during the study period, based on the DAS28-ESR (from 20.0 to 29.8%) or DAS28-CRP (from 40.7 to 51.9%) (Supplementary Fig. S1, available at Rheumatology online).
Table 1.
Baseline characteristics of the entire cohort
| Characteristics | Values |
|---|---|
| Patients, N | 140 |
| Age at enrolment, years, mean (s.d.) | 66.5 (8.1) |
| >65 years, n (%) | 83 (59.3) |
| >70 years, n (%) | 50 (35.7) |
| >75 years, n (%) | 21 (15.0) |
| Male, n (%) | 44 (31.4) |
| BMI, kg/m2, mean (s.d.) | 23.9 (3.1) |
| Smoking, n (%) | |
| Never | 102 (72.9) |
| Ex-smoker | 23 (16.4) |
| Pack-years, median (range, IQR) | 30.0 (10.0–50.0, 10.0) |
| Current smoker | 14 (10.0) |
| Pack-years, median (range, IQR) | 22.0 (1.0 − 55.0, 14.0) |
| Unknown | 1 (0.7) |
| RF positive, n (%) | 124 (88.6) |
| Anti-CCP positive, n (%) | 133 (95.0) |
| ≥200 IU/mL | 70 (50.0) |
| RA duration, years, mean (s.d.) | 7.9 (8.4) |
| Early RA (<2 years of diagnosis), n (%) | 45 (32.1) |
| Duration ≥2–<5 years of diagnosis, n (%) | 18 (12.9) |
| Long-standing RA (≥5 years of diagnosis), n (%) | 77 (55.0) |
| ILD duration, years, mean (s.d.) | 7.8 (8.4) |
| Interval between RA diagnosis and ILD, n (%) | |
| RA before ILD at least 24 weeks | 78 (55.7) |
| Simultaneously (within 24 weeks) | 51 (36.4) |
| ILD before RA at least 24 weeks | 11(7.9) |
| RA disease activity (baseline), mean (s.d.) | |
| Tender joint count | 3.3 (4.8) |
| Swollen joint count | 2.6 (3.4) |
| Patient’s global assessment | 35.7 (26.4) |
| ESR | 39.5 (26.4) |
| CRP | 9.3 (14.4) |
| DAS28-ESR | 4.0 (1.4) |
| HRCT characters at baseline, n (%) | |
| HRCT patterns | |
| UIP | 43 (31.6) |
| Probable UIP | 41 (30.1) |
| Indeterminate UIP | 35 (25.7) |
| Non-specific interstitial pneumonia | 14 (10.3) |
| COP/BOOP | 3 (2.2) |
| RA medication until enrolment, n (%) | |
| Methotrexate | 77 (55.0) |
| Glucocorticoid | 123 (87.9) |
| Cumulative dose, mean (s.d.) | 2.8 (1.1) |
BOOP: bronchiolitis obliterans organizing pneumonia; COP: cryptogenic organizing pneumonia.
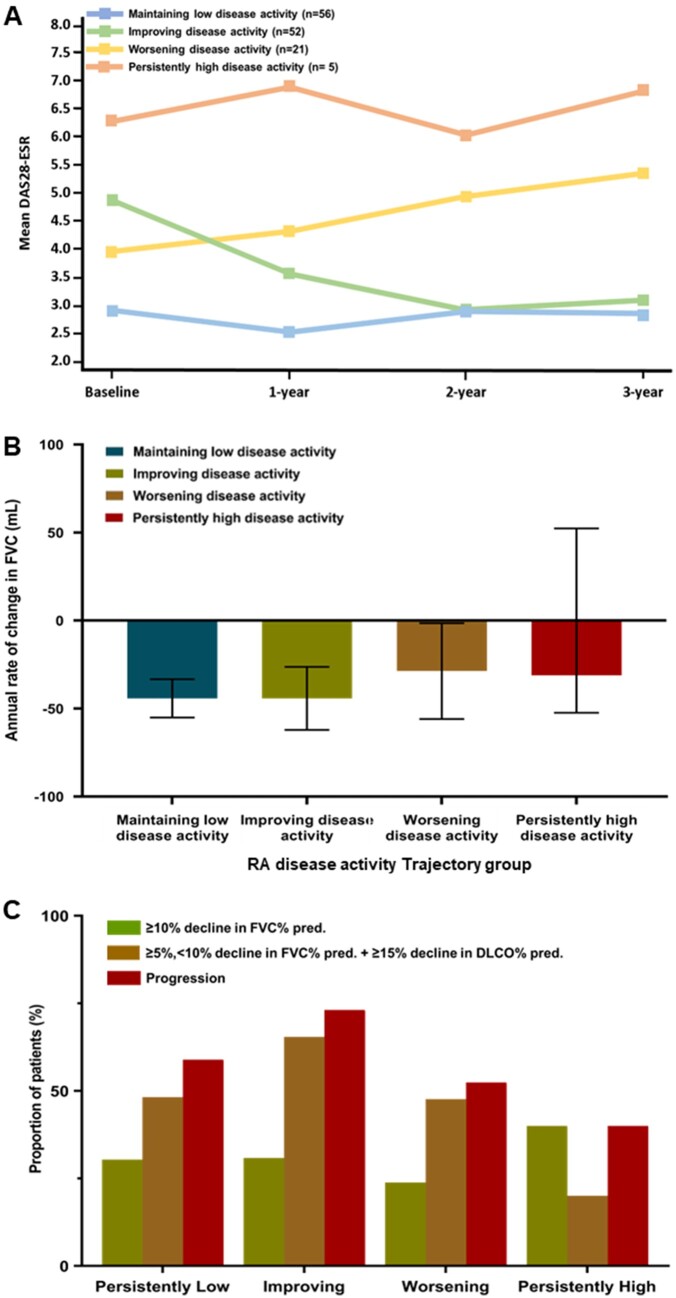
Among 140 patients included in PFT trajectory analysis, 134 had at least two DAS28 evaluations. Four distinctive trajectories were identified for RA disease activity (Fig. 1A and B). Overall, 108 (80.6%) patients exhibited maintenance or improved to low disease activity and 21 (15.7%) patients exhibited worsening disease activity, based on DAS28-ESR. The course of disease activity was similar based on DAS28-CRP. Clinical characteristics according of RA disease activity trajectory groups based on the DAS28-ESR are shown in Supplementary Table S3, available at Rheumatology online.
Figure 1.
The trajectory for RA disease activity using the DAS28-ESR and pulmonary function characteristics. (A) Distinctive four-group trajectory model for DAS28-ESR. (B) The annual rate of change in FVC (mL) according to the DAS28-ESR trajectories. (C) The proportion of patients who experienced ≥10% decline in the predicted FVC% from baseline (green), ≥5% or <10% decline in the predicted FVC% from baseline and ≥15% decline in the predicted DLCO% from baseline (yellow) and progression (red) according to the DAS28-ESR trajectories
PFT at baseline and during 3-year follow-up
Forty-seven (33.5%) patients showed a predicted percentage of FVC (FVC%) <80%. Only two patients had a predicted FVC% <50% at baseline. Seventy-six (54.3%) patients had a predicted percentage DLCO (DLCO%) <75%. The mean annual rate of decline in FVC over 3 years was −41.6 ml/year (95% CI −50.6, −32.5). Eighty-nine (63.6%) patients experienced ILD progression during the 3-year follow-up period. Table 2 summarizes these data.
Table 2.
Lung physiology according to trajectory group of the predicted FVC%
| Characteristics | Total | Persistently improving | Stable | Slowly declining | Rapidly declining | P-value |
|---|---|---|---|---|---|---|
| N | 140 | 11 | 68 | 54 | 7 | |
| FVC | ||||||
| Baseline | ||||||
| FVC (mL), mean (s.d.) | 2517.2 (756.3) | 2292.7 (490.8) | 2502.9 (817.4) | 2565.7 (745.4) | 2634.3 (590.4) | 0.71 |
| Predicted FVC%, mean (s.d.) | 84.7 (16.6) | 84.0 (13.9) | 81.6 (16.4) | 88.0 (16.7) | 89.0 (19.9) | 0.17 |
| Annual rate of change in FVC (mL), mean (95% CI) | −41.6 (−50.6, −32.5) | 26.8 (−1.8, −55.3) | −23.4 (−30.3, −16.5) | −65.7 (−79.8, −51.6) | −139.6 (−198.0, −81.2) | <0.01 |
| Change of relative FVC from baseline (predicted FVC%), n (%) | ||||||
| ≥10-point decline | 38 (27.1) | 0 (0.0) | 0 (0.0) | 31 (57.4) | 7 (100.0) | <0.01 |
| Relative ≥5% decline | 70 (50.0) | 0 (0.0) | 13 (19.1) | 50 (92.6) | 7 (100.0) | <0.01 |
| Relative ≥10% decline | 43 (30.7) | 0 (0.0) | 1 (1.5) | 35 (64.8) | 7 (100.0) | <0.01 |
| Change of absolute FVC from baseline (FVC in mL), n (%) | ||||||
| Relative ≥5% decline | 77 (55.0) | 2 (18.2) | 19 (27.9) | 49 (90.7) | 7 (100.0) | <0.01 |
| Relative ≥10% decline | 52 (37.1) | 1 (9.1) | 6 (8.8) | 38 (70.4) | 7 (100.0) | <0.01 |
| Diffusing capacity | ||||||
| N | 137 | 11.0000 | 67 | 52 | 7 | |
| Predicted DLCO% (baseline), mean (s.d.) | 71.6 (20.4) | 71.0 (20.2) | 69.9 (20.7) | 72.5 (20.7) | 81.3 (16.4) | 0.55 |
| Annual rate of change in predicted DLCO% | −1.48 (−1.78, −1.19) | −0.70 (−2.09, −0.69) | −1.13 (−1.51, −0.76) | −1.94 (−2.44, −1.44) | −2.62 (−4.39, −0.84) | <0.01 |
| Relative ≥15% decline, n (%) | 58 (42.3) | 4 (36.4) | 17 (25.4) | 31 (59.6) | 6 (85.7) | <0.01 |
| Progression, n (%) | ||||||
| A. Relative ≥10% decline in predicted FVC% | 43 (30.7) | 0 (0.0) | 1 (1.5) | 35 (64.8) | 7 (100.0) | <0.01 |
| B. Relative ≥5% (<10% decline in predicted FVC% + ≥15% decline in predicted DLCO% | 77 (55.0) | 4 (36.4) | 25 (36.8) | 42 (77.8) | 6 (85.7) | <0.01 |
| Progression (A + B) | 89 (63.6) | 4 (36.4) | 25 (36.8) | 53 (98.1) | 7 (100.0) | <0.01 |
Trajectory of lung function over 3 years
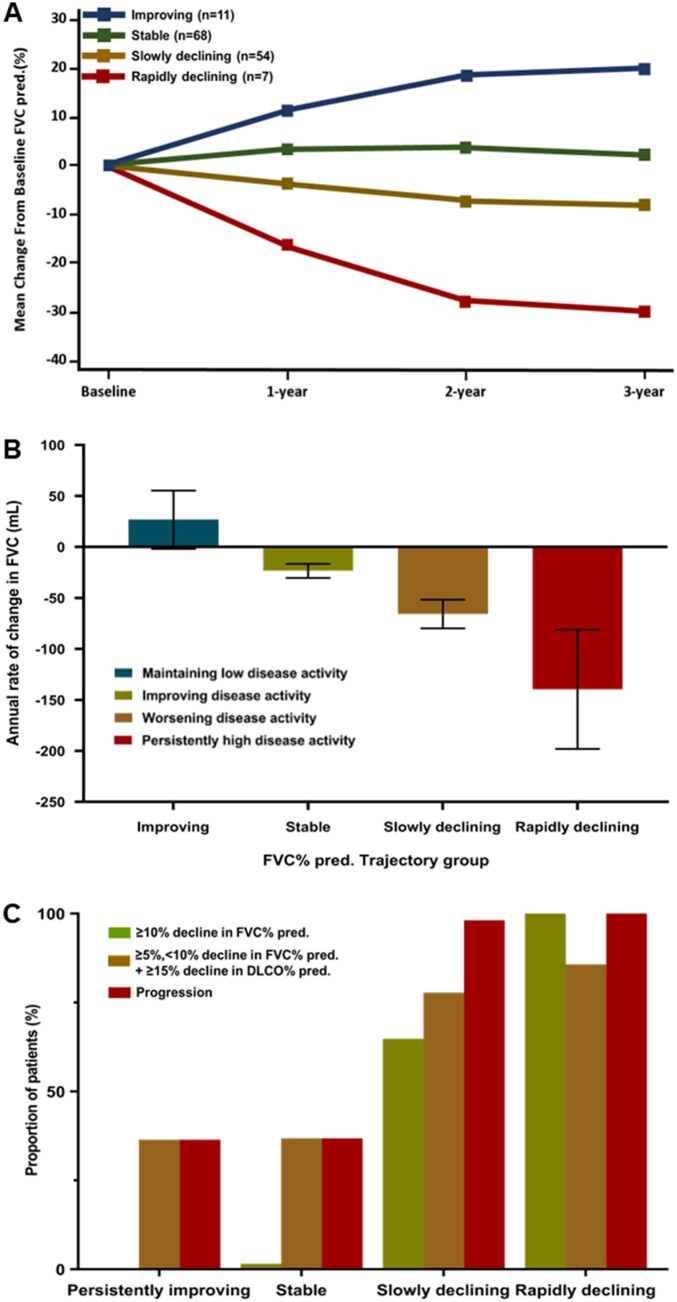
The four distinctive trajectories for change in predicted FVC% over 3 years (Fig. 2A) were ‘persistently improving’ [n = 11 (7.9%)], ‘stable’ [n = 68 (38.4%)], ‘slowly declining’ [n = 54 (48.6%)] and ‘rapidly declining’ [n = 7 (5.0%)]. The mean age at enrolment was the highest [73.0 years (s.d. 7.2)] and the duration of RA and ILD was the shortest in the rapidly declining predicted FVC% group [mean 2.1 (s.d. 3.8) and 1.9 (s.d. 3.8), respectively]. In the rapidly declining predicted FVC% trajectory group, six (85.7%) of seven patients had early RA and five patients were diagnosed with RA and ILD within 24 weeks. Supplementary Table S4 (available at Rheumatology online) summarizes the demographic characteristics and baseline RA disease activity. Between-group differences were not significant in high-resolution CT (HRCT) patterns (Supplementary Fig. S2, available at Rheumatology online).
Figure 2.
The trajectory for the predicted FVC% and pulmonary function characteristics. (A) A distinctive four-group trajectory model for predicted FVC%. (B) The annual rate of change in FVC (mL) according to the predicted FVC% trajectories. (C) The proportion of patients who experienced a ≥10% decline in predicted FVC% from baseline according to the FVC trajectories. (D) The proportion of patients who experienced a ≥15% decline in the predicted DLCO% from baseline according to the FVC trajectories
In the rapidly declining predicted FVC% trajectory group, the mean annual rate of change in FVC was as fast as −139.6 ml/year (95% CI −198.0, −81.2; Fig. 2B). All patients in the rapidly declining group and 98.1% of patients in the slowly progressive declining group experienced ILD progression during the follow-up period (Table 2). The proportion of patients experiencing a significant decline in predicted FVC% or diffusing capacity was comparable among the four trajectory groups, based on DAS28-ESR and DAS28-CRP (Fig. 2B–D).
Relationship between RA disease activity and FVC trajectories over 3 years
Most patients exhibited a stable or slowly declining predicted FVC% trajectory, regardless of the RA disease activity trajectory (Table 3). Similarly, most patients with stable or slow declining predicted FVC% trajectory maintained or improved to low RA disease activity over 3 years. The annual rate of change in FVC (mL) and the proportion of patients experiencing a significant decline in predicted FVC% or diffusing capacity were comparable among the four trajectory groups, based on DAS28-ESR and DAS28-CRP (Fig. 1B–D).
Table 3.
Distribution of patients according to trajectory over 3 years
| Trajectory group of predicted FVC% | Trajectory group of DAS28-ESR |
|||
|---|---|---|---|---|
| Maintaining LDA | Improving | Worsening | Persistently HDA | |
| Persistently improving, n (%) | 3 (5.4) | 3 (5.8) | 4 (19.0) | 0 (0.0) |
| Stable, n (%) | 31 (55.4) | 23 (44.2) | 10 (47.6) | 3 (60.0) |
| Slowly declining, n (%) | 21 (37.5) | 25 (48.1) | 5 (23.8) | 1 (20.0) |
| Rapidly declining, n (%) | 1 (1.8) | 1 (1.9) | 2 (9.5) | 1 (20.0) |
| Total, n (%) | 56 (100) | 52 (100) | 21 (100) | 5 (100) |
HDA: high disease activity; LDA: low disease activity.
P = 0.1137 by Fisher’s exact test.
Factors associated with rapidly declining predicted FVC%
The risk for rapidly declining predicted FVC% trajectory increased by 72% for each 5 year increment in age [RR 1.72 (95% CI 1.04, 2.83)]. The risk was 10.8 times higher in patients ≥70 years of age [RR 10.8 (95% CI 1.34, 87.2)] and 12.7 times higher in patients with early RA [RR 12.7 (95% CI 1.52, 105.21)]. Previous glucocorticoid use before enrolment was associated with a lower risk [RR 0.18 (95% CI 0.04, 0.82)], but the dose (mean ≥5 mg/day of prednisolone) was not associated with risk [RR 0.87 (95% CI 0.17, 4.50)] (Table 4).
Table 4.
RR of the trajectory group of rapidly declining predicted FVC%a
| Variables | Predicted FVC% trajectory, rapidly declining (n = 7) |
Predicted FVC% trajectory, rapidly declining + mortality (n = 15) |
||
|---|---|---|---|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Age (for every 5 years of age) | 1.72 (1.04, 2.83) | 0.03 | 1.54 (1.10, 2.16) | 0.01 |
| ≥70, n (%) | 10.80 (1.30, 89.71) | 0.03 | 3.60 (1.23, 10.53) | 0.02 |
| ≥75, n (%) | 4.25 (0.95, 18.99) | 0.06 | 2.83 (0.97, 8.29) | 0.06 |
| Male | 2.91 (0.65, 13.00) | 0.16 | ||
| BMI | ||||
| <18.5 (underweight) | 1 (Ref) | 1 (Ref) | ||
| 18.5–24.9 (normal) | 0.43 (0.05, 3.70) | 0.44 | 0.35 (0.07, 1.63) | 0.18 |
| 25–29.9 (overweight) | 0.15 (0.01, 2.38) | 0.18 | 0.37 (0.07, 1.92) | 0.24 |
| >30 (obese) | NA | NA | ||
| Ever smoker | 2.01 (0.45, −8.99) | 0.36 | 1.86 (0.66, 5.21) | 0.24 |
| RA disease duration (years) | ||||
| <2 | 1 (Ref) | 1 (Ref) | ||
| ≥2–<5 | NA | NA | ||
| ≥5 | 0.10 (0.01, 0.82) | 0.03 | 0.15 (0.04, 0.52) | <0.01 |
| Interval between diagnosis of RA and ILD | ||||
| Diagnosis of RA prior to ILD at least 24 weeks | 1 (Ref) | 1 (Ref) | ||
| Diagnosis of RA and ILD simultaneous within 24 weeks | 7.65 (0.89, 65.45) | 0.06 | 9.18 (2.05, 41.00) | 0.04 |
| Diagnosis of ILD prior to RA at least 24 weeks | 7.09 (0.44, 113.37) | 0.17 | 3.55 (0.32, 39.10) | 0.30 |
| High titre of anti-CCP (≥200 IU) | 1.68 (0.31, 9.18) | 0.55 | 1.89 (0.58, 6.14) | 0.29 |
| RA disease activity trajectory by DAS28-ESR | ||||
| Maintaining low disease activity | 1 (Ref) | 1 (Ref) | ||
| Improving disease activity | 0.74 (0.07, 8.13) | 0.80 | 0.49 (0.05, 4.72) | 0.54 |
| Worsening disease activity | 0.45 (0.06, 3.21) | 0.43 | 1.05 (0.27, 4.08) | 0.94 |
| Persistently high disease activity | 0.90 (0.13, 6.41) | 0.92 | 1.20 (0.27, 5.38) | 0.81 |
| RA disease activity transition (by DAS28-CRP) | ||||
| Maintaining low disease activity | 1 (Ref) | 1 (Ref) | ||
| Improving disease activity | NA | 1.06 (0.21, 5.23) | 0.95 | |
| Worsening disease activity | 0.28 (0.03, 2.50) | 0.25 | 0.75 (0.21, 2.64) | 0.65 |
| Persistently high disease activity | 2.04 (0.37, 11.11) | 0.41 | 2.04 (0.51, 8.14) | 0.31 |
| HRCT patterns at baseline | ||||
| UIP patterns | 1.67 (0.32, 8.59) | 0.54 | 2.67 (0.75, 9.45) | 0.13 |
| UIP or probable UIP | 1.69 (0.38, 7.56) | 0.49 | 1.13 (0.39, 3.30) | 0.83 |
| NSIP/COP/BOOP | 0.79 (0.15, 4.08) | 0.78 | 1.73 (0.63, 4.77) | 0.29 |
| HRCT extent at baseline | ||||
| CT extent >10% | 4.10 (0.80, 21.15) | 0.09 | 3.28 (1.12, 9.60) | 0.03 |
| RA medication until enrolment | ||||
| Methotrexate, ever (until enrolment) | ||||
| Yes | 1 (Ref) | 1 (Ref) | ||
| No | 7.45 (0.90, 61.89) | 0.06 | 8.07 (1.82, 35.77) | <0.01 |
| Glucocorticoid use | 0.18 (0.04, 0.82) | 0.03 | 0.28 (0.09, 0.81) | 0.02 |
| Glucocorticoid dose with cumulative dose ≥5.0 mg/day | 0.87 (0.17, 4.50) | 0.87 | 1.09 (0.37, 3.19) | 0.87 |
| Glucocorticoid dose with cumulative dose ≥7.5 mg/day | NA | 2.67 (0.75, 9.45) | 0.13 | |
The RR was calculated by univariate analysis because of a relatively small sample size.
BOOP: bronchiolitis obliterans organizing pneumonia; COP: cryptogenic organizing pneumonia; NA: not available.
Factors associated with rapidly declining predicted FVC% or all-cause mortality
Twelve patients died during the study period and within 1 year after the study period, four patients had a rapidly declining predicted FVC% trajectory, five had a slowly declining trajectory and three had a stable trajectory. The risk for the rapidly declining predicted FVC% trajectory or mortality increased by 54% for each 5 year increment [RR 1.54 (95% CI 1.10, 2.16)]. The risk was 3.6 times higher in patients ≥70 years of age [RR 3.60 (95% CI 1.23, 10.53)]. Patients with long-standing RA had a lower risk up to 85% [RR 0.15 (95% CI 0.04, 0.52)] than patients with early RA. The risk for a rapidly declining predicted FVC% trajectory or mortality was as high as 9.2 times in patients diagnosed with RA and ILD simultaneously within 24 weeks compared with that in patients diagnosed with RA before being diagnosed with ILD [RR 9.18 (95% CI 2.05, 41.0)]. Extensive involvement of ILD in >10% of the lung, based on CT imaging, increased the risk by 3.28 times [RR 3.28 (95% CI 1.12, 9.60)]. Patients who had never used methotrexate had 8.1 times higher risk than those who had ever used methotrexate [RR 8.07 (95% CI 1.82, 35.77)]. Previous use of glucocorticoid before enrolment was associated with a lower risk [RR 0.28 (95% CI 0.09, 0.81)], however, the dose (mean ≥5 mg/day of prednisolone) was not associated with risk [RR 1.09 (95% CI 0.37, 3.19)] (Table 4).
Discussion
This study analysed the lung function trajectory over 3 years, the effect of RA disease activity on the lung function trajectory and the risk factors associated with lung function deterioration using prospective cohort data. Lung function deteriorated rapidly in 5% of patients. Older age and early RA (i.e. ≤2 years of RA diagnosis) significantly increased the risk of deterioration. Nine patients died during the study period and three died within 1 year after the study period. Four of seven patients in the rapidly progressive predicted FVC% trajectory group died. Older age, extensive lung involvement and no use of methotrexate increased the mortality risk, whereas longer RA disease duration, simultaneous diagnosis of RA and ILD and previous glucocorticoid use reduced the risk.
Several studies [10, 13, 19, 27, 28] report the natural course of RA-ILD with regard to pulmonary physiology. Among 84 patients with RA-UIP [19, 27], 50% were stable, one-third progressed, 17% experienced acute exacerbation and 6% improved. Among 167 patients in the retrospective Mayo Clinic Cohort study [19], one-third of patients required supplemental oxygen, 40% developed a predicted DLCO% <40% and 22% developed a predicted FVC% <50% within 5 years after ILD diagnosis. Solomon et al. [10] reported that the slopes for both predicted FVC% and predicted DLCO% declined over time, and the HRCT patterns differed significantly. They demonstrated that pulmonary physiology predicts mortality, independent of HRCT patterns. Another retrospective study [28] revealed that ILD progressed in 55.1% (102/185) of patients, with progression defined as a combination of PFT and HRCT score worsening or clinical worsening. Untreated non-IPF ILD patients with progressive fibrosing phenotypes (14.2% had RA-ILD) experienced lung function decline that was as rapid as that of patients with untreated IPF [29, 30].
Herein, most patients showed stable or slowly declining predicted FVC% trajectories. Discrepancies among studies, including this study, might be due to the following reasons. First, ILD was less severe in our cohort of patients than in patients enrolled in other studies. The mean predicted FVC% of the entire cohort was >80% and 62.1% (82/140) showed ≤10% lung involvement on chest CT scans at enrolment. Our cohort was recruited from patients treated at rheumatology clinics, hence they had less severe conditions than patients treated by pulmonologists. Second, more patients with early RA were possibly included in our study. Our cohort recruitment started in January 2015, and 32.1% of patients had early RA and were diagnosed with RA within 2 years. Therefore a greater proportion of patients were diagnosed using the 2010 criteria that were established to diagnose early RA. However, patients were diagnosed using the 1987 criteria in almost all other studies [1, 2, 9, 10, 16, 28]. Of note, mortality occurred even if a patient exhibited a stable or slowly declining lung function trajectory. No patient with an improving predicted FVC% trajectory died during the study period or 1 year after the study period.
Previous use of glucocorticoids before enrolment decreased the risk of rapidly declining lung function in this study. However, the mean cumulative glucocorticoid dose of patients in the rapidly declining predicted FVC% trajectory group was low [2.8 mg/day (s.d. 1.1)] and the use of cumulative glucocorticoid dose of 5.0 mg/day was not associated with the risk. In general practice, low-dose steroids (<7.5 mg/day) are often used to treat arthritis, while higher doses are often used to treat the lung condition [27, 31, 32]. Glucocorticoid use until enrolment in our study likely reflects controlled RA disease activity or systemic inflammation. Another possible reason for this outcome is that patients may have enrolled in the study at the time of RA diagnosis and most of those patients may have no history of steroid use. The effect of glucocorticoid on the course of lung function and mortality should be studied further.
No significant association was noted between RA disease activity trajectory, using DAS28 scores, and lung function trajectory in our study. This study’s result contradicts those of previous research, indicating that high inflammatory burdens, including high RA disease activity, are associated with the incidence of RA-ILD or mortality in RA-ILD [9, 13, 14, 28, 33]. The discordance may be because only a limited number of patients had high disease activity in our study, which limited our data’s statistical power. A recently reported retrospective analysis of a large multicentre UK network cohort showed improved survival of patients with RA-ILD because of the increasing use of mycophenolate mofetil and rituximab. This finding suggests that controlling inflammation mitigates further deterioration of RA-ILD [9]. Our study population may reflect the real-world setting of a rheumatologic clinic, as patients are diagnosed with RA because of their arthritis and not because of their lung condition in most cases. Patients with RA-ILD are often treated intensively for arthritis using a treat-to-target strategy at rheumatologic clinics.
In this study, 5% of patients had a rapid FVC decline. Consistent with previous research findings, age was a significant risk factor: each 5 year increment in age and age ≥70 years increased the risk for rapid FVC decline of 1.7 times and 10.8 times, respectively. Patients with long-standing RA had a lower risk of FVC deterioration than patients with early RA. This result may be attributable to older age because 44.4% (22/45) of patients within 2 years of RA diagnosis were ≥70 years of age. The risk factors were further expanded when considering the mortality conjunction with the deterioration of lung function: simultaneous diagnosis of RA and ILD within 6 months, more extensive ILD and no previous methotrexate use before enrolment increased the risk 9 times, 3 times and 8 times, respectively.
Patients with a history of ever smoking had an RR of 2.0 for rapidly declining predicted FVC% and 1.86 for rapidly declining predicted FVC% or mortality, but it was not statistically significant in our study. Current smoking or smoking history is a risk factor for developing RA-ILD and increased mortality in patients with RA-ILD [1, 2, 34, 35]. However, the effect of smoking on ILD progression, especially pulmonary function, remains controversial. In a Mayo Clinic study [19], the hazard ratio for the association between ever-smoking history and DLCO progression was significant, while the HR for the association between ever-smoking history and FVC progression was not significant. Smoking was not a significant risk factor after multivariate analysis in other studies on acute exacerbation or progression of RA-ILD [1, 36–38]. The present study results, together with those mentioned previously, suggest that the effect of smoking was likely attenuated because of other factors, such as those associated with RA.
Our study demonstrated that 5% of patients experienced rapid lung function deterioration, regardless of RA disease activity, suggesting that mechanisms other than inflammation impact the deterioration of lung function in RA-ILD. Recent clinical trials [30] suggest the potential benefit of antifibrotic therapy in CTD-ILDs, including RA-ILD. In SSc-ILD, the most extensively studied CTD-ILD, antifibrotic agents (i.e. nintedanib) decreased the annual rate of FVC decline; these effects are further accentuated by background immunosuppressive therapy with mycophenolate mofetil [39]. In a subgroup analysis of the INBUILD trial [40], nintedanib significantly delayed the deterioration of lung function in RA-ILD patients. These findings and our findings suggest the involvement of mechanisms other than inflammation in RA-ILD progression, which implies that treatments other than those controlling systemic inflammation may be necessary.
Our study has several limitations. First, the follow-up interval was relatively long at 1 year. However, performing CT scans at intervals of <12 months for routine check-ups may be difficult because of the cost and radiation exposure. However, one strength of this study is that, among the well-organized prospective studies, our study included the largest number of patients assessed for RA disease activity and ILD status simultaneously. Second, most patients had relatively mild ILD with <10% lung involvement on CT scans; some of these patients were possibly classified with interstitial lung abnormalities. In particular, patients attending rheumatology clinics were recruited, and this population may represent real-world rheumatology patients. Third, most patients were seropositive, which may reflect selection bias. Seropositivity is a risk factor for RA-ILD development [17, 41–44], thus the finding that most patients with RA-ILD were seropositive in our cohort was not surprising. The association between seropositivity and the occurrence of RA-ILD is known; however, its association with prognosis is not well known. Therefore, further research may be necessary. Fourth, owing to the lack of an appropriate age-matched cohort, whether the patients’ decline in each trajectory was decreasing within the normal range was difficult to confirm. However, regardless of this fact, clinically significant changes were observed even in the slow declining and rapidly declining groups (Table 2). Fifth, the effect of medications for RA treatment was not fully analysed and is undergoing further analysis. Finally, this study had a relatively small sample size. Further studies with larger sample sizes are warranted to validate our results.
In conclusion, most patients with RA-ILD showed stable or slowly declining predicted FVC% trajectories and exhibited maintained or improved to low RA disease activity over 3 years. Nevertheless, in 5% of patients, predicted FVC% deteriorated rapidly, particularly in older adults with early RA (i.e. diagnosed with RA within the preceding 2 years). The risk factors were further expanded when considering the mortality with the deterioration of lung function, as simultaneous diagnosis of RA and ILD within 6 months and more extensive ILD increased the risk. Previous glucocorticoid or methotrexate treatment decreased the risk of rapid FVC deterioration in our study; however, additional studies may be necessary to validate our clinical interpretation.
Supplementary Material
Acknowledgements
We thank the patients who participated in the study and are grateful for the time and effort they have invested in the project. We thank the investigators and study nurses for their support.
Contributor Information
Sung Hae Chang, Division of Rheumatology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Republic of Korea; Division of Rheumatology, Department of Internal Medicine, Soonchunhyang University Hospital, Cheonan, Republic of Korea.
Ji Sung Lee, Department of Medical Statistics, Clinical Research Center, Asan Institute for Life Sciences, Asan Medical Center, Seoul, Republic of Korea.
You-Jung Ha, Division of Rheumatology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Republic of Korea.
Min Uk Kim, Department of Radiology, SMG-SNU Boramae Medical Center, Seoul, Republic of Korea.
Chan Ho Park, Department of Radiology, Soonchunhyang University College of Medicine, Cheonan, Republic of Korea.
Jeong Seok Lee, Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Republic of Korea.
Ji-Won Kim, Division of Rheumatology, Department of Internal Medicine, Catholic University of Daegu School of Medicine, Daegu, Republic of Korea.
Sang Wan Chung, Division of Rheumatology, Department of Internal Medicine, Kyung Hee University Hospital, Seoul, Republic of Korea.
Jung Yoon Pyo, Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea.
Sung Won Lee, Division of Rheumatology, Department of Internal Medicine, Soonchunhyang University Hospital, Cheonan, Republic of Korea.
Eun Ha Kang, Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Republic of Korea.
Yeon-Ah Lee, Division of Rheumatology, Department of Internal Medicine, Kyung Hee University Hospital, Seoul, Republic of Korea.
Yong-Beom Park, Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea.
Jung-Yoon Choe, Division of Rheumatology, Department of Internal Medicine, Catholic University of Daegu School of Medicine, Daegu, Republic of Korea.
Eun Young Lee, Division of Rheumatology, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ contributions
Conceptualization: S.H.C., J.S.L., and E.Y.L. Collection of data and critical review of the manuscript: S.H.C., Y.J.H., J.S.L., J.W.K., S.W.C., J.Y.P., S.W.L., E.H.K., Y.A.L., Y.B.P., J.Y.C., and E.Y.L. Data analysis and interpretation: J.S.L., S.H.C., and E.Y.L. Drafting of the manuscript: S.H.C., E.Y.L. All authors have read and approved the final manuscript.
Funding
This work was supported by Seoul National University Hospital (cohort project no. 2520160060) and Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant no. HI14C1277).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Bongartz T, Nannini C, Medina-Velasquez YF. et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62:1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelly CA, Saravanan V, Nisar M. et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics—a large multicentre UK study. Rheumatology 2014;53:1676–82. [DOI] [PubMed] [Google Scholar]
- 3. Koduri G, Norton S, Young A. et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology 2010;49:1483–9. [DOI] [PubMed] [Google Scholar]
- 4. Samhouri BF, Vassallo R, Achenbach SJ. et al. Incidence, risk factors, and mortality of clinical and subclinical rheumatoid arthritis-associated interstitial lung disease: a population-based cohort. Arthritis Care Res (Hoboken) 2022;74:2042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabbay E, Tarala R, Will R. et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997;156:528–35. [DOI] [PubMed] [Google Scholar]
- 6. Habib HM, Eisa AA, Arafat WR, Marie MA.. Pulmonary involvement in early rheumatoid arthritis patients. Clin Rheumatol 2011;30:217–21. [DOI] [PubMed] [Google Scholar]
- 7. Hyldgaard C, Hilberg O, Pedersen AB. et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017;76:1700–6. [DOI] [PubMed] [Google Scholar]
- 8. Kronzer VL, Huang W, Dellaripa PF. et al. Lifestyle and clinical risk factors for incident rheumatoid arthritis-associated interstitial lung disease. J Rheumatol 2021;48:656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelly CA, Nisar M, Arthanari S. et al. Rheumatoid arthritis related interstitial lung disease - improving outcomes over 25 years: a large multicentre UK study. Rheumatology (Oxford) 2021;60:1882–90. [DOI] [PubMed] [Google Scholar]
- 10. Solomon JJ, Chung JH, Cosgrove GP. et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016;47:588–96. [DOI] [PubMed] [Google Scholar]
- 11. Kim D, Cho SK, Choi CB. et al. Impact of interstitial lung disease on mortality of patients with rheumatoid arthritis. Rheumatol Int 2017;37:1735–45. [DOI] [PubMed] [Google Scholar]
- 12. Sparks JA, Jin Y, Cho SK. et al. Prevalence, incidence and cause-specific mortality of rheumatoid arthritis-associated interstitial lung disease among older rheumatoid arthritis patients. Rheumatology (Oxford) 2021;60:3689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sparks JA, He X, Huang J. et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis–associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol 2019;71:1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Assayag D, Lubin M, Lee JS. et al. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology 2014;19:493–500. [DOI] [PubMed] [Google Scholar]
- 15. Brooks R, Baker JF, Yang Y. et al. The impact of disease severity measures on survival in U.S. veterans with rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford) 2022;61:4667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacob J, Hirani N, Van Moorsel CHM. et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J 2019;53:1800869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duarte AC, Porter JC, Leandro MJ.. The lung in a cohort of rheumatoid arthritis patients—an overview of different types of involvement and treatment. Rheumatology (Oxford) 2019;58:2031–8. [DOI] [PubMed] [Google Scholar]
- 18. Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL.. Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology (Oxford) 2017;56:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL.. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 2017;69:542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aletaha D, Neogi T, Silman AJ. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 21. Travis WD, Costabel U, Hansell DM. et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raghu G, Remy-Jardin M, Myers JL. et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–68. [DOI] [PubMed] [Google Scholar]
- 23. Hoffmann-Vold A-M, Allanore Y, Alves M. et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021;80:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones BL, Nagin DS.. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociolog Methods Res 2007;35:542–71. [Google Scholar]
- 25. Man A, Davidyock T, Ferguson LT. et al. Changes in forced vital capacity over time in systemic sclerosis: application of group-based trajectory modelling. Rheumatology (Oxford) 2015;54:1464–71. [DOI] [PubMed] [Google Scholar]
- 26. Nagin DS, Odgers CL.. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–38. [DOI] [PubMed] [Google Scholar]
- 27. Song JW, Lee HK, Lee CK. et al. Clinical course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:103–12. [PubMed] [Google Scholar]
- 28. Li L, Liu R, Zhang Y. et al. A retrospective study on the predictive implications of clinical characteristics and therapeutic management in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 2020;39:1457–70. [DOI] [PubMed] [Google Scholar]
- 29. Brown KK, Martinez FJ, Walsh SLF. et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J 2020;55:2000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flaherty KR, Wells AU, Cottin V. et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019;381:1718–27. [DOI] [PubMed] [Google Scholar]
- 31. Raghu G, Collard HR, Egan JJ. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jang HJ, Yong SH, Leem AY. et al. Corticosteroid responsiveness in patients with acute exacerbation of interstitial lung disease admitted to the emergency department. Sci Rep 2021;11:5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robles-Pérez A, Luburich P, Bolivar S. et al. A prospective study of lung disease in a cohort of early rheumatoid arthritis patients. Sci Rep 2020;10:15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Doyle TJ, Patel AS, Hatabu H. et al. Detection of rheumatoid arthritis–interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med 2015;191:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kadura S, Raghu G.. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev 2021;30:210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Izuka S, Yamashita H, Iba A, Takahashi Y, Kaneko H.. Acute exacerbation of rheumatoid arthritis–associated interstitial lung disease: clinical features and prognosis. Rheumatology (Oxford) 2021;60:2348–54. [DOI] [PubMed] [Google Scholar]
- 37. Liu L, Fang C, Sun B, Bao R, Zhang H.. Predictors of progression in rheumatoid arthritis‐associated interstitial lung disease: a single‐center retrospective study from China. Int J Rheum Dis 2022;25:795–802. [DOI] [PubMed] [Google Scholar]
- 38. Mori S, Koga Y, Sugimoto M.. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med 2012;106:1591–9. [DOI] [PubMed] [Google Scholar]
- 39. Distler O, Highland KB, Gahlemann M. et al. Nintedanib for systemic sclerosis–associated interstitial lung disease. N Engl J Med 2019;380:2518–28. [DOI] [PubMed] [Google Scholar]
- 40. Wells AU, Flaherty KR, Brown KK. et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020;8:453–60. [DOI] [PubMed] [Google Scholar]
- 41. Kamiya H, Panlaqui OM.. Systematic review and meta-analysis of the risk of rheumatoid arthritis-associated interstitial lung disease related to anti-cyclic citrullinated peptide (CCP) antibody. BMJ Open 2021;11:e040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Natalini JG, Baker JF, Singh N. et al. Autoantibody seropositivity and risk for interstitial lung disease in a prospective male-predominant rheumatoid arthritis cohort of U.S. veterans . Ann Am Thorac Soc 2021;18:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang S, He X, Doyle TJ. et al. Association of rheumatoid arthritis-related autoantibodies with pulmonary function test abnormalities in a rheumatoid arthritis registry. Clin Rheumatol 2019;38:3401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie S, Li S, Chen B. et al. Serum anti-citrullinated protein antibodies and rheumatoid factor increase the risk of rheumatoid arthritis–related interstitial lung disease: a meta-analysis. Clin Rheumatol 2021;40:4533–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.