Abstract
Undifferentiated carcinoma (UC) of the pancreas is a rare subtype of pancreatic cancer displaying no definitive direction of differentiation. UC has been reported as a highly aggressive malignant neoplasm, with a median overall survival of <1 year, except for several surgical series. On the other hand, UC tissue sometimes contains non-neoplastic osteoclast-like giant cells (OGCs), and such cases have been reported to have relatively longer survival. Thus, the World Health Organization (WHO) classification histologically distinguishes UC with OGCs (UCOGCs) from UC, and UCs were subclassified into three subtypes: anaplastic UC, sarcomatoid UC and carcinosarcoma. However, still less is known about UC due to its rarity, and such situations lead to further difficulties in treatment for UC. To date, only surgical resection can offer curative treatment for patients with UC, and no clear evidence for chemotherapy exists for them. However, a retrospective cohort study and case reports showed that relatively promising results paclitaxel-containing regimens for treatment of patients with unresectable UC. Furthermore, high programmed cell death protein 1 expression has been reported in sarcomatoid UCs and UCOGCs, and promising responses to anti-programmed death-ligand 1 therapy have been described in case reports of UCOGCs. Recent advances in chemotherapeutic agents and molecular technologies are opening up the possibilities for expanded treatments.
Keywords: osteoclast-like giant cells, carcinosarcoma, sarcomatoid undifferentiated carcinoma, anaplastic carcinoma, undifferentiated carcinoma
Undifferentiated carcinoma is an aggressive epithelial neoplasm with a poor prognosis. However, recent advances in diagnostic modalities and genomic sequencing technologies are opening up the possibilities for diagnosis and expanded treatments.
Introduction
Epithelial malignant tumours of the pancreas are categorized into exocrine neoplasms arising from exocrine glands and neuroendocrine neoplasms arising from endocrine glands. Pancreatic cancer (PC) is classified as an exocrine neoplasm, and it accounts for nearly 90% of pancreatic neoplasms (1). The prognosis of PC is poor, and Siegel et al. reported that the 5-year survival rate of PC patients was only 12%, the lowest of all cancer types (2).
Undifferentiated carcinoma (UC) of the pancreas, also known as anaplastic carcinoma of the pancreas, is a rare subtype of PC. UC is a neoplasm displaying no definitive direction of differentiation. Unlike conventional PC (ductal adenocarcinoma), UC is poorly cohesive and hypercellular, with scant stroma (3). The frequency of UC in malignant neoplasms of the pancreas varied from 0.3 to 7% in the previous reports (3–6). UC has basically been considered more aggressive than PC (4, 5, 7). On the other hand, UC tissue sometimes contains non-neoplastic osteoclast-like giant cells (OGCs), and such cases have been reported to have relatively long survival (5, 8). Based on these findings, although UCs were classified into three subtypes (pleomorphic type, spindle cell type and anaplastic carcinoma with OGCs) in the Japanese classification of pancreatic cancer (9), the WHO classification histologically distinguishes UC with OGCs (UCOGC) from UC. Furthermore, UCs were subclassified into three subtypes based on recent reports of UC variants with molecular analysis: anaplastic UC, sarcomatoid UC and carcinosarcoma (10).
So far, though there have been relatively many case reports of UC (11), only a few studies have systematically reported its clinical and molecular features. Such situations lead to further difficulties in our understanding of UC. However, recent developments in molecular technologies such as next-generation sequencing open up the possibility of understanding and treatment for UC. In this review, the current knowledge of the epidemiology, clinical features, diagnosis, prognosis, histological findings and treatment of UC of the pancreas are outlined. Furthermore, the molecular features of UC, which may lead to new therapeutic strategies for the treatment of them, are considered.
Epidemiology
The frequency of UC in malignant neoplasms of the pancreas varied from 0.3 to 7% in the previous reports (3–6). Recent studies reported that the frequency of UC was 2–3%. The most reliable epidemiological data were reported based on SEER registry data by Clark et al (4). Of a total 6212 patients with PC, 353 patients (5.7%) were diagnosed with UC, and 11 patients (0.2%) were diagnosed with UCOGC. The age-adjusted incidence of UC was 0.027 per 100 000 persons [95% confidence interval (CI), 0.023–0.031]. There are many case reports of UC. However, epidemiological reports by Clark et al. indicate that these are biased data, and that the actual frequency is extremely rare.
In the incidence of UC, no racial difference has been reported (4), and the median age at diagnosis is around the 1960s, being slightly more common in male than in female patients. Clark et al reported that the proportion of male patients with UC was slightly higher (57.5%) than that of female patients, and the median age at diagnosis was 67 years, with no significant difference both in sex and age compared with PC (4). Paal et al. also reported 35 cases of UC using registry data retrieved from the Armed Forces Institute of Pathology (7). In their reports, the median age at diagnosis was 62.0 years, and UC showed male predominance (71.4%). Thus, UC shares characteristics with its counterpart PC. On the other hand, a younger, female predominance has been reported in UCOGCs. Muraki et al. reported 38 cases of UCOGCs (8), and the mean age at diagnosis was 57.9 years, with a range from 29 to 86 years, and UCOGCs showed slight female predominance (62.9%).
Clinical features
Size and tumour location
Reflecting its highly aggressive behaviour, UC tends to show larger size. The median tumour size was reported to be 60–90 mm, with larger tumours than PC (4, 7, 8), but there was no significant difference in tumour size amongst subtypes of UC (7). The tumour was less frequently located in the head of the pancreas in UC than in PC (43.4% vs. 67.5%, P < 0.001) (4).
Symptoms
Due to their large size, UCs are often accompanied by some clinical symptoms. These symptoms commonly include abdominal pain and weight loss, but no specific symptoms have been reported in both UC and UCOGCs. In the report by Paal et al., patients reported abdominal pain (51.4%), weight loss, loss of appetite (14.3%), nausea and/or vomiting (11.4%), fatigue (5.7%) and diarrhoea (5.7%) (7). In UCOGCs, Muraki et al. reported that presenting symptoms included abdominal or back pain (62%), weight loss (38%), nausea (28%), tarry stool and jaundice (14%) in four patients each and diabetes mellitus (10%) in three.
The patients with UC were in poor condition at the time of diagnosis due to its aggressive behaviour (12). We reported that the Eastern Cooperative Oncology Group performance status (ECOG PS) was ≥1 for 80% and ≥ 2 for 20% at the time of diagnosis.
Diagnosis
Tumour markers
Both carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are commonly used tumour markers for PC. However, they were mostly not elevated in UC. We reported CEA and CA19-9 levels in both UC and UCOGCs at an advanced stage (12). The median [interquartile range (IQR)] CEA and CA19-9 levels were 3.5 (2.0–6.9) and 35.7 (16.4–-556.0) U/ml in UC and 2.1 (1.7–3.1) ng/mL and 22.8 (5.0–131.0) U/mL in UCOGCs, respectively. Gao et al. reported 13 patients with UCOGCs (13), and the commonly used tumour markers of CEA, CA19-9 and AFP were mostly not elevated. The median (IQR) CEA, CA19-9 and AFP levels were 3.0 (2.4–11.8) ng/ml, 34.0 (11.5–62.0) U/ml and 2.0 (1.2–2.3) ng/ml, respectively. Notably, on the other hand, NSE levels were elevated in 66% of patients with UCOGC (median, 23.0 ng/ml; IQR, 21.5–26.0 ng/ml). On the other hand, other tumour markers such as DUPAN-2 and Span-1 are known to be useful in the diagnosis of PC, but there have been no reports of their usefulness for UC.
Radiological modalities
In most cases of UC, computed tomography (CT) is sufficient to identify the tumour. However, since UC is a variant of PC, it has similar imaging features to those of PC. Ishigami et al. reported that multidetector-row CT showed a hypovascular tumour with upstream main pancreatic duct dilatation (14). On the other hand, due to its highly aggressive behaviour, haemorrhagic necrosis was present within tumours. Furthermore, intraductal tumour growth in the main pancreatic duct and tumour thrombus were often observed, and these findings may support the imaging diagnosis of UC.
Endoscopic ultrasound-guided tissue acquisition
As previously described, there are no tumour markers or radiological findings useful for the diagnosis of UC. Thus, pathological examination is mandatory for diagnosis. One of the reasons why UC is reported mainly in surgical series is that it is difficult to obtain a tissue diagnosis of UC preoperatively. Chadha et al. reported the utility of pathological diagnosis of biopsy specimens in exploratory laparotomy or from liver metastases (15). However, a less invasive approach is demanded for the diagnosis of UC.
Recently, advances in diagnostic modalities are facilitating the diagnosis of pancreatic neoplasms (16–19). This enables us to acquire more accurate pathological diagnoses of UC even in cases of unresectable disease (12). We reported the clinical courses of advanced-stage UC patients retrospectively. Of the total 55 patients, 14 (25.5%) were diagnosed via Endoscopic ultrasound-guided tissue acquisition (EUS-TA) (20). Currently, EUS-TA is vital for the diagnosis of UC, especially in the advanced stage.
The diagnosis of UCOGC requires inclusion of OGCs in the small specimens obtained by EUS-TA. However, EUS-TA is a useful approach in the diagnosis of UCOGC. Reid et al. examined 15 cases of UCOGC that underwent EUS-TA, and 6 (40%) could be diagnosed (21). EUS-TA specimens showed specific components to UCOGC (neoplastic pleomorphic mononuclear cells, OGCs and spindle cells) and adenocarcinoma components in the sample. Furthermore, they reported that immunostaining of specimens obtained by EUS-TA for the macrophage and monocyte marker CD68 and the epithelial differentiation marker epithelial membrane antigen (EMA), may help in the diagnosis. Similarly, the utility of EUS-TA for the diagnosis of UCOGC has been reported in some case series (22, 23).
Regarding the diagnosis of UC via EUS-TA, the most important issue is the differentiation of UC from conventional PC. Therefore, obtaining a tissue sample including neoplastic cells specific to UC, such as neoplastic pleomorphic mononuclear cells and OGCs, is fundamental to making a diagnosis of UC with EUS-TA. Newer endoscopic ultrasound-guided fine needle biopsy (EUS-FNB) needles potentially allow higher histological yield and enable immunohistochemical staining and even next-generation sequencing (19, 24–26). The use of EUS-FNB needles may help in the diagnosis.
Prognosis
UC has been believed to be more aggressive than PC (Table 1). However, there are only a few systematic reports on its prognosis due to its rarity. Furthermore, these data were based primarily on surgical series. Considering that it has been reported that ~80% of PC patients were diagnosed in unresectable stages (27, 28), these data were potentially biased. However, most studies reported that median overall survival (OS) of UC was poor, at <1 year, except for several surgical series (Table 2). Clark et al. compared the prognosis between UC and PC in their population-based study (4). The median OS was 3 months in patients with UC and 11 months in patients with PC. OS was significantly worse for patients with UC than for patients with PC [hazard ratio (HR), 1.9; 95% CI, 1.7–2.1]. Strobel et al. also reported a case-control study of surgically resected cases of UC (5). In their report, there were no significant differences in OS between UC and PC (7.1 vs. 16.5 months, respectively). However, this is possibly due to the small sample size.
Table 1.
Overview of undifferentiated carcinoma
| Epidemiology | |
| • The frequency in malignant neoplasms of the pancreas is 0.3–7% | |
| • The median age at diagnosis is around the 60s. | |
| • There is a slight male predominance. | |
| Clinical features | |
| • Tend to show larger size (the median size was reported to be 60–90 mm). | |
| • Slightly more frequently seen in the pancreatic body/tail. | |
| • No specific symptoms are seen. | |
| Diagnosis | |
| • Both CEA and CA19-9 are mostly not elevated. | |
| • CT shows similar imaging features to pancreatic cancer. | |
| • EUS-TA is essential for the diagnosis, especially in the advanced stage. | |
| Prognosis | |
| • UC patients have significantly shorter survival compared with PC patients. | |
| • UCOGCs have been reported to have relatively long survival compared with UC. | |
| Pathological findings | |
| Anaplastic UCs • Pleomorphic mononuclear cells admixed with bizarre-appearing giant cells with eosinophilic cytoplasm are present. Neoplastic cells are non-cohesive and lack gland formation. |
|
| Sarcomatoid UCs • Spindle-shaped cells mimicking sarcomas are present. |
|
| Rhabdoid UCs • A rare subtype of sarcomatoid UCs. Neoplastic cells have rhabdoid inclusions. |
|
| Carcinosarcomas • Admixture of both roundish epithelioid cells and spindle sarcomatous cells. |
|
| UCOGCs • Admixture of neoplastic pleomorphic mononuclear cells and multinucleated OGCs. OGCs are positive for leucocyte markers and considered non-neoplastic. |
|
| Molecular features | |
| • Similar to pancreatic cancer, KRAS, TP53, CDKN2A/B and SMAD4 are commonly observed. | |
| Anaplastic UCs • EMT-related proteins are highly expressed. |
|
| Sarcomatoid UCs • PD-L1 is highly expressed. |
|
| Rhabdoid UCs • Genomic alterations in SWI/SNF complex subunits are frequently observed. |
|
| Carcinosarcomas • Both carcinomatous and sarcomatous components share identical alterations of KRAS and TP53. |
|
| UCOGCs • PD-L1 is highly expressed. |
|
| Treatment | |
| • Basically, only surgical resection can offer curative treatment for patients with UC. | |
| • To date, no clear evidence exists for chemotherapy for UC. | |
| Anaplastic UCs • A retrospective cohort study and case reports indicate that a paclitaxel-containing regimen is a reasonable option for the treatment of patients with unresectable UC. |
|
| UCOGCs • Promising responses to ICIs for UCOGCs have been described in case reports. |
|
CT, computed tomography; EUS-TA, endoscopic ultrasound-guided tissue acquisition; UC, undifferentiated carcinoma; OGCs, osteoclast-like giant cells; UCOGCs, UC with OGCs; EMT, epithelial–mesenchymal transition; PD-L1, programmed death-ligand 1; SWI/SNF, SWItch/Sucrose Non-Fermentable; ICIs, immune checkpoint inhibitors.
Table 2.
Summary of prognosis of undifferentiated carcinoma
| Author (year) | No. of patients | Median OS | Extent of disease | Initial treatment | |||
|---|---|---|---|---|---|---|---|
| Metastatic | Localized (resectable or unresectable) | Curative resection | Chemotherapy/ chemoradiotherapy | Best supportive care | |||
| Any subtype | |||||||
| Clark (2012) (4) | 353 | 3 months | 204 | 127 | 81 (2-year OS rate, 30.7%) |
– | – |
| Strobel (2011) (5) | 18 | 5.7 months | 5 | 13 | 13 (median OS, 7.1 months) |
– | – |
| Imaoka (2021) (12) | 55 | 3.95 months | 47 (median OS, 3.95 months) | 8a (median OS, 2.83 months) | 0 | 44 | 11 |
| Imaoka (2020) (20) | 50 | 4.08 months | 44 | 6 | 0 | 50 | 0 |
| Anaplastic UCs | |||||||
| Strobel (2011) (5) | 11 | 3.3 months | – | – | 8 (median OS, 3.9 months) |
– | – |
| Sarcomatoid UCs | |||||||
| Gkountakos (2022) (41) | 7 | 21 months | 2 | 5 | 5 | – | – |
| Rhabdoid UCs | |||||||
| Sano (2014) (30) | 6 | <3 months | 6 | 0 | 0 | 2 | 4 |
| Carcinosarcomas | |||||||
| Li (2020) (31) | 6 | 14 months | 0 | 6 | 6 | 0 | 0 |
| UCOGCs | |||||||
| Clark (2012) (4) | 11 | (2-year OS rate, 50.0%) | – | – | 10 | – | – |
| Strobel (2011) (5) | 7 | (2-year OS rate, 57.1%) | – | – | 5 (2-year OS rate, 80%) |
– | – |
| Muraki (2016) (8) | 38 | 7.67 years | 0 | 38 | 38 | 0 | 0 |
| Luchini (2017) (47) | 16 | 20 months | 0 | 16 | 16 | 0 | 0 |
| Imaoka (2021) (12) | 13 | 5.36 months | 10 | 3a | 0 | 11 | 2 |
| Gao (2022) (13) | 13 | 13 months | 0 | 13 | 13 | 0 | 0 |
OS, overall survival.
aAll patients were diagnosed with locally advanced disease.
On the other hand, UCOGCs have been reported to have relatively long survival compared with UC. Strobel et al. compared OS between UCOGC and UC (5), and survival was significantly longer for UCOGC patients than for UC patients (2-year OS rate in UCOGC patients, 57.1%; median OS, 3.3 months in UC patients; P = 0.0477). Muraki et al. compared OS of UCOGC and PC (8), and the median OS was significantly longer for UCOGC patients than for PC patients (7.67 vs. 1.59 years, P = 0.0009).
Meanwhile, the difference in OS was attenuated in the advanced stage (12, 21). We reported the prognosis of 55 UC patients with the advanced stage (12). The median OS in all patients with unresectable UC was 3.95 months, and there was no significant difference in OS between the UCOGC group and the UC without OGC group (5.36 vs. 3.58 months, respectively). The possible reasons for this poor prognosis of UC in the advanced stage have been suggested to be poor response to chemotherapy and publication bias. We also evaluated prognostic factors for unresectable UC (12), and age, ECOG PS and C-reactive protein were independent prognostic factors of OS.
Pathological findings
UC is also called anaplastic carcinoma. ‘Anaplastic’ denotes the characteristics of cells or tissues that have lost their mature or specialized features, and it is used to describe neoplastic cells that divide rapidly and show no similarity to normal cells. Thus, UC is defined as a malignant, epithelial neoplasm displaying no particular differentiation, such as glandular formation, mucin production or keratinization. Typically, neoplastic cells are poorly cohesive and hypercellular, and they have only scant stroma. According to the WHO classification 5th edition, UC is currently categorized into two different types: UC (with three subtypes: anaplastic UC, sarcomatoid UC and carcinosarcoma) and UCOGC (10, 29). The details follow.
Anaplastic UCs
Anaplastic UCs are composed of pleomorphic mononuclear cells admixed with bizarre-appearing giant cells with eosinophilic cytoplasm and lack gland formation. Neoplastic cells are non-cohesive, and a neutrophilic inflammatory infiltrate may be prominent, with emperipolesis of neutrophils within the cytoplasm of the cells (Fig. 1).
Figure 1.
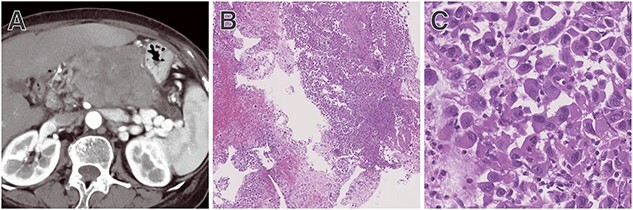
Representative case of anaplastic undifferentiated carcinoma (UC). (A) Computed tomography (CT) showing a hypovascular tumour at the body of the pancreas with upstream main pancreatic duct dilatation. (B) Tissue sample obtained by endoscopic ultrasound-guided tissue acquisition showing neoplastic cells, necrosis with neutrophil infiltration. In the high-powered field, (C) pleomorphic mononuclear neoplastic cells admixed with bizarre-appearing giant cells with eosinophilic cytoplasm were present.
Sarcomatoid UCs
On histological examination, sarcomatoid UCs are characterized by a predominance of spindle-shaped cells, accounting for ~80% of the cells without gland formation. These spindle-shaped cells mimic sarcomas, but they demonstrate epithelial derivation without specific mesenchymal differentiation.
Rhabdoid UCs
Rhabdoid UCs are a very rare subtype of sarcomatoid UCs. Much like sarcomatoid UCs, they have abundant pleomorphic neoplastic giant cells with abundant eosinophilic cytoplasm (Fig. 2). However, unlike sarcomatoid UCs, they have rhabdoid inclusions (30).
Figure 2.
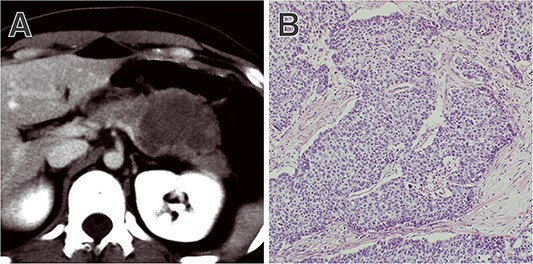
Representative case of rhabdoid UC. (A) CT showing a hypovascular tumour at the tail of the pancreas. In the surgical specimen, (B) abundant pleomorphic neoplastic giant cells with rhabdoid inclusion were present.
Carcinosarcomas
Carcinosarcoma refers to a neoplasm composed of an admixture of both roundish epithelioid cells and spindle sarcomatous cells. Each component should arbitrarily constitute 30% of the neoplasm to qualify as carcinosarcoma. Carcinosarcoma is found the most frequently in the uterus and has also been reported in other organs, including the pancreas (31).
Undifferentiated carcinoma with osteoclast-like giant cells
UCOGCs are pathologically characterized by the mixture of neoplastic pleomorphic mononuclear cells and multi-nucleated OGCs (Fig. 3). Neoplastic mononuclear cells have a high Ki-67 proliferation index. Immunohistochemically, they are positive for epithelial markers such as CEA, EMA and AE1/AE3, suggesting epithelial differentiation. On the other hand, OGCs show few mitoses, and immunohistochemistry shows low positivity for proliferation markers such as Ki-67. They are also positive for leucocyte markers CD45 and CD68 but negative for epithelial markers. These findings suggest that OGCs are not neoplastic but reactive cells derived from macrophages (histiocytes) (32).
Figure 3.
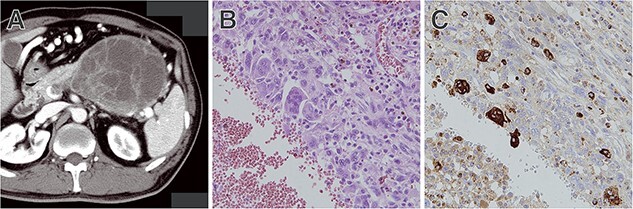
Representative case of UC with osteoclast-like giant cells. (A) CT showing a large tumour at the tail of the pancreas. (B) Tissue sample obtained by EUS-TA showing a mixture of neoplastic pleomorphic mononuclear cells and multinucleated OGCs. (C) Multinucleated OGCs were immunohistochemically positive for CD68.
Molecular features
Pathologically, UC is an epithelial tumour that originates from the pancreas, but the direction of differentiation is unknown. However, genomic alterations KRAS, TP53, CDKN2A/B and SMAD4 are also commonly observed in UCs (33–35). Of these, KRAS mutations are the most frequently observed genomic alterations, in 60–80%. This fact supports the concept that UCs are subtypes of PC. Molecular features of UCs are summarized in Table 3.
Table 3.
Summary of molecular features of undifferentiated carcinoma
| Author (year) | KRAS mutation | KRAS amplification | TP53 mutation | SMAD4 | CDKN2A | EMT-related marker expression | Predictive biomarkers for ICIs | SWI/SNF complex subunits | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loss of E-cadherin | Snai2 | Twist1 | PD-L1 | MSI-high | TMB-high | SMARCB1 (INI1) | ARID1A (SMARCF1) | ||||||
| Anaplastic UCs | |||||||||||||
| Hoorens (1998) (33) | 59% | ||||||||||||
| Paal E (2001) (7) | 67%a | 71% | |||||||||||
| Mattilo (2021) (40) | 60%a | 100%a | 30%a | ||||||||||
| Sakakida (2023) (35) | 76% | 68% | 8% | 40% | 0% | 0% | 8% | ||||||
| Sarcomatoid UCs | |||||||||||||
| Gkountakos (2022) (41) | 100% | 30% | 90% | 10% | 60% | ||||||||
| Faber (2023) (42) | 86% | 7% | 86% | 10% | 18% | 63%a | 2% | 2% | |||||
| Rhabdoid UCs | |||||||||||||
| Agaimy (2015) (43) | 54% | 38% | 29%a | ||||||||||
| UCOGCs | |||||||||||||
| Hoorens (1998) (33) | 50% | ||||||||||||
| Lehrke (2017) (49) | 80%a | ||||||||||||
| Hrudka (2020) (50) | 69.2%a | 76.9%a | 0%a | ||||||||||
| Mattilo (2021) (40) | 31.3%a | 50%a | 25%a | ||||||||||
EMT, epithelial–mesenchymal transition; ICIs, immune checkpoint inhibitors; SWI/SNF, SWItch/Sucrose Non-Fermentable; PD-L1, programmed death-ligand 1; MSI, microsatellite instability; TMB, tumour mutation burden; UC, undifferentiated carcinoma.
aAssessed by immunohistochemistry.
Anaplastic UCs
Previously, associations between UC and epithelial–mesenchymal transition (EMT) have been suggested. EMT is a complex process by which epithelial cells lose their cell polarity and cell–cell adhesion via inhibition of E-cadherin, and they gain migratory and invasive properties of mesenchymal cells. By EMT, cancer cells in a primary tumour break through the basement membrane with increased invasive properties and enter the bloodstream through intravasation. EMT is known to be associated with a poor prognosis in various cancers, and this fact may contribute to the aggressive clinical course of UCs. Furthermore, some research suggests that pleomorphic pathological findings of UC similar to mesenchymal phenotype are the result of EMT (36). It has been well known that loss of E-cadherin is frequently observed in UC. Yonemasu et al. compared UC with PC, and E-cadherin expression was completely lost in seven of eight UC cases, whereas half the PCs showed strong reactivity for E-cadherin immunohistochemically (37). Winter et al. evaluated E-cadherin protein expression by immunohistochemistry in pancreatobiliary cancers with a non-cohesive histological phenotype, predominantly UC (38). Interestingly, most UCs showed complete loss of E-cadherin expression, and they were characterized by either abnormal cytoplasmic accumulation or complete loss of β-catenin expression, suggesting a deficient adherens junction. They showed that E-cadherin inactivation via hypermethylation of CDH1, encoding for E-cadherin, may be involved in the process of dedifferentiation in PC, based on its known function as a transmembrane protein within the adherens junction and its central role in the EMT of tumour progression. Ishida et al. immunohistochemically assessed E-cadherin and three other EMT-related proteins, Slug (zinc finger protein SNAI2), Twist1 (Twist-related protein 1) and Zeb1 (zinc finger E-box-binding homeobox 1), in resected specimens. Increased Zeb1 expression and the complete loss of E-cadherin expression affect the progression of UC through the development of EMT (39). On the other hand, Mattiolo et al. compared the immunohistochemical expressions of three EMT-related markers (E-cadherin, Snai2 and Twist1) between UC and UCOGCs. They found that EMT was more frequently activated in UC than in UCOGC (40). Furthermore, among UCOGCs, EMT was more frequently activated in cases with PC components.
Sarcomatoid UCs
Advances in sequencing technology have shown that the genomic profile of sarcomatoid UCs is similar to that of PC. Gkountakos et al. reported that the most frequently observed genomic mutations were KRAS (100%), TP53 (90%) and CDKN2A (60%) (41). They reported that, unlike in PC, SMAD4 mutations were rare (10%), and KRAS amplification was more frequent in sarcomatoid UCs. Faber et al. also reported the genomic profiling of sarcomatoid UCs compared with PC patient samples (42). The most frequently observed genomic mutations were KRAS (86%), TP53 (86%) and CDKN2A (18%), with similar prevalences to PC. Programmed death-ligand 1 (PD-L1) was significantly highly expressed in sarcomatoid UCs compared with PC (63% vs. 16%, respectively), but there were no significant differences both in the frequency of microsatellite instability-high/mismatch repair (MMR)-deficient (2% vs. 1%, respectively) and tumour mutation burden–high (2% vs. 2%, respectively).
Rhabdoid UCs
Rhabdoid UCs are considered a rare subtype of sarcomatoid UCs. Unlike UCs and UCOGCs, the rhabdoid subtype is unique in having a low frequency of KRAS mutation and a high frequency of KRAS amplification. Agaimy et al. reported that the frequencies of KRAS amplification and KRAS mutation in this rare subtype were 38 and 54%, respectively (43).
On the other hand, high frequencies of SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodelling complex subunit deficiencies were reported in UCs of the gastrointestinal tract, especially with rhabdoid features (44). The SWI/SNF complex plays significant roles in the regulation of gene expression. On the other hand, alterations in SWI/SNF complex subunits, such as SMARCA4, SMARCB1 (INI1) and ARID1A (SMARCF1), can cause a loss of function of the subunit as a tumour suppressor. Furthermore, SWI/SNF subunits can also be required for tumour maintenance or even play an oncogenic role in certain disease contexts (45). Agaimy et al. reported that strong correlation between KRAS alterations and intact SMARCB1 expression, and loss of SMARCB1 expression correlated with the absence of KRAS alterations (43). Based on KRAS alterations and immunohistochemical SMARCB1 expression status, they also classified sarcomatoid UCs into two subtypes: the pleomorphic subtype (KRAS alterations and intact SMARCB1) and the monomorphic subtype (KRAS wild-type and loss of SMARCB1). Recently, SWI/SNF complex subunits have been highlighted as therapeutic targets, and multiple novel agents are currently being tested in several phase I–II trials (46): tazemetostat for loss of SMARCB1 (NCT02601950) and berzosertib for ARID1A (NCT02059265).
Carcinosarcoma
Although carcinosarcoma is composed of an admixture of both epithelial and sarcomatous components, each component is immunophenotypically similar to its pure counterpart. Li et al. reported the result of genomic and molecular analyses of carcinosarcoma, using laser-captured microdissection both in carcinomatous and sarcomatous components (31). These two components shared identical alterations of KRAS and TP53. In addition, the expression statuses of TP53, ARID1A and KDM6A were consistent between carcinomatous and sarcomatous tissues. In this study, genomic and molecular analyses showed the monoclonal origins of the carcinomatous and sarcomatous components.
Undifferentiated carcinoma with osteoclast-like giant cells
The results of whole exome sequencing of UCOGCs have been reported, and the genetic alterations of UCOGCs are quite similar to those of PC, including KRAS, CDKN2A, TP53 and SMAD4 (47). Despite unique pathological findings of UCOGCs, these results support the classification of UCOGC as a PC variant and suggest that somatic mutations are not the determinants of its phenotype.
Recently, a predictive biomarker for immune checkpoint inhibitors (ICIs), PD-L1, has been highlighted. Currently approved ICIs target immune checkpoint proteins: cytotoxic T-lymphocyte-associated antigen 4, programmed cell death protein 1 (PD-1) and PD-L1. The anti-PD-1 antibody pembrolizumab binds to PD-1 on the T cells and inhibits the interaction of PD-1 with the PD-L1 expressed on the cancer cells (48). This causes immunosuppressive signals to T cells to be blocked, which allows T cells to attack cancer cells. In UCOGCs, Lehrke et al. examined the expressions of PD-L1, CD3, CD20, CD68 and DNA MMR proteins in 24 patients with UC immunohistochemically (16 cases were anaplastic UC, 5 were UCOGCs, 1 was sarcomatoid UC and 5 were rhabdoid UC) (49). In their comparison with PC, PD-L1 expression was more frequently observed in UC than in PC (63% vs. 15%, P < 0.05). Of the total five UCOGCs, four expressed PD-L1. On the other hand, 8.3% (2/24) UCs were MMR-deficient, and they were positive for PD-L1, with more than 10% positive tumour cells. Hrudka et al. also compared PD-L1 expression between UCOGC and PC, and PD-L1 was significantly highly expressed in UCOGC compared with PC (76.9% vs. 12.5%, P < 0.001) (50). Among UCOGC patients with high or low expression levels of PD-L1, the median OS was 5.7 months and not reached, respectively. However, the difference was not significant mainly due to the small sample size, and high expression of PD-L1 may possibly impact negatively on survival of UCOGC patients. On the other hand, high expression of PD-L1 can potentially be a therapeutic target for ICIs (see Treatment).
Treatment
Surgical resection
Only surgical resection can offer curative treatment for patients with UC, and surgical resection such as the Whipple procedure, pylorus-preserving pancreatoduodenectomy, distal pancreatectomy or total pancreatectomy is performed depending on the primary site and extension of the lesion, as in the case of PC. In the population-based study by Clark et al., pancreatic resection was an independent prognostic factor for OS (HR = 0.3; 95% CI, 0.2–0.5; P = 0.001) (4). Strobel et al. also reported the prognosis of UC based on a surgical database (5). The study showed that curative resection significantly improved OS compared with palliative surgery (7.1 vs. 2.2 months, P = 0.0094). This difference was even more evident in terms of the presence of OGCs, and median OS in patients with curatively resected UCOGCs was significantly longer than in those without OGCs (not reached vs. 3.9 months, P = 0.0104). They also reported that adjuvant chemotherapy was not associated with longer OS in UC. Recent progress in adjuvant chemotherapy for PC may potentially benefit such UC patients treated with surgical resection (51–53), but there is no evidence for its use.
Chemotherapy
To date, no clear evidence exists for chemotherapy for UC. Furthermore, treatment data for patients with unresectable UC are lacking, as previous reports were based primarily on surgical series or registry data. However, a retrospective cohort study and case reports indicate that a paclitaxel-containing regimen is a reasonable option for treatment of patients with unresectable UC. We analysed a total of 50 UC patients treated with chemotherapy (20). The efficacy of chemotherapy was limited, with an objective response rate (ORR) of 10% and a median progression-free survival (PFS) of 1.84 months for first-line chemotherapy. However, gemcitabine plus nab-paclitaxel as first-line chemotherapy showed relatively promising results, with an ORR of 33.3% and a median PFS of 4.60 months. A paclitaxel-containing, first-line regimen significantly improved OS compared with a non-paclitaxel-containing regimen (6.94 vs. 3.75 months, P = 0.041). After adjustment, use of a paclitaxel-containing regimen in any line was still an independent predictor of OS (HR, 0.221; 95% CI, 0.076–0.647; P = 0.006). They also reported that the presence of OGCs did not affect chemotherapeutic effects. King et al. published a case report of rhabdoid UC with complete response achieved by gemcitabine plus nab-paclitaxel after failure of FOLFIRINOX (54). Wakatsuki et al. also reported a case of complete response to paclitaxel based on chemosensitivity testing (55). These reports suggest that gemcitabine plus nab-paclitaxel is a reasonable option in the treatment for UC.
ICIs have emerged as a new treatment paradigm for patients with many types of cancer (56, 57). ICIs bind to immune checkpoint proteins or their ligands and block these immunosuppressive signals in cancer cells (58). Only a limited number of cancer patients have been shown to derive benefit from ICIs; however, promising responses to ICIs for UCOGCs have been described in case reports (59, 60). Although biomarkers for predicting the efficacy of ICIs are not fully understood, the utility of PD-L1 expression has been demonstrated in non-small-cell lung cancer (57). Several studies showed high PD-L1 expression in UCOGCs, and ICIs may potentially benefit such patients with UCOGCs.
The most problematic issue is treatment for UC. Such rare cancers are generally under-represented, resulting in delays in the establishment of treatments, with little progress in research and development. As a result, UC patients lack access to appropriate treatment, resulting in poor treatment outcomes (61). However, because of their rarity, there are few ongoing clinical trials, and we do not expect any company-led therapeutic developments in the future. Based on previous retrospective data (20), we are currently conducting a phase II clinical trial of gemcitabine plus nab-paclitaxel for UC (jRCTs031220099).
Conclusion
UC of the pancreas is an aggressive epithelial neoplasm with a poor prognosis, but still less is known about it due to its rarity. However, recent advances in diagnostic modalities and genomic sequencing technologies are opening up the possibilities for diagnosis and expanded treatments. Deepening our understanding and accumulating knowledge of UCs are necessary to establish treatment for them.
Conflict of Interest statement
Hiroshi Imaoka has received honoraria from Yakult Honsha. Masafumi Ikeda has received research funding from Eli Lilly Japan, Yakult, Pfizer and Takeda and honoraria from Eli Lilly Japan, Yakult Honsha, Taiho Pharmaceutical and Takeda. Kumiko Umemoto has received honoraria from Yakult Honsha. Makoto Ueno has received research funding from Taiho Pharmaceutical, AstraZeneca, Merck Biopharma, MSD, Astellas Pharma, Eisai, Ono Pharmaceutical, Incyte, CHUGAI PHARMACEUTICAL, DFP, Daiichi Sankyo, Novartis, Boehringer Ingelheim and J-pharma and honoraria from Taiho Pharmaceutical, AstraZeneca, Yakult Honsha, MSD, Nihon Servier, Ono Pharmaceutical, Incyte, CHUGAI PHARMACEUTICAL, Boehringer Ingelheim and J-pharma. Masato Ozaka has received honoraria from Taiho Pharmaceutical, Yakult Honsha, MSD, Ono Pharmaceutical, Nihon Servier, Bayer and Pfizer. Naohiro Okano has received honoraria from Taiho Pharmaceutical, Eli Lilly Japan, Eisai, Bayer Yakuhin, Chugai Pharma, Ono Pharmaceutical, Takeda, Daiichi Sankyo and AstraZeneca and is a member of Data Safety Monitoring Board or advisory board for GlaxoSmithKline. Kenji Ikezawa has received research funding from ASKA Pharmaceutical and honoraria from Taiho Pharmaceutical, Yakult Honsha, Ono Pharmaceutical, MSD, Myriad Genetics, Asahi Kasei Pharma and Incyte Biosciences Japan. Junji Furuse has received research funding from Taiho Pharmaceutical and Daiichi Sankyo and honoraria from Eli Lilly, Yakult Honsha, Taiho Pharmaceutical and Daiichi Sankyo and is a member of Data Safety Monitoring Board or advisory board for Taiho Pharmaceutical.
Contributor Information
Hiroshi Imaoka, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital East, Kashiwa, Japan.
Masafumi Ikeda, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital East, Kashiwa, Japan.
Kumiko Umemoto, Department of Clinical Oncology, St. Marianna University School of Medicine, Kawasaki, Japan.
Yu Sunakawa, Department of Clinical Oncology, St. Marianna University School of Medicine, Kawasaki, Japan.
Makoto Ueno, Department of Gastroenterology, Kanagawa Cancer Center, Yokohama, Japan.
Hideki Ueno, Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Masato Ozaka, Hepato-Biliary-Pancreatic Medicine Department, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan.
Takamichi Kuwahara, Department of Gastroenterology, Aichi Cancer Center Hospital, Nagoya, Japan.
Naohiro Okano, Department of Medical Oncology, Kyorin University Faculty of Medicine, Tokyo, Japan.
Masashi Kanai, Department of Medical Oncology, Kyoto University Hospital, Kyoto, Japan.
Terumasa Hisano, Department of Hepato-Biliary-Pancreatology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan.
Yuko Suzuki, Department of Gastroenterology, Saitama Cancer Center, Saitama, Japan.
Akinori Asagi, Department of Gastrointestinal Medical Oncology, National Hospital Organization Shikoku Cancer Center, Matsuyama, Japan.
Kazuhiko Shioji, Department of Internal Medicine, Niigata Cancer Center Hospital, Niigata, Japan.
Akiko Todaka, Division of Gastrointestinal Oncology, Shizuoka Cancer Center, Shizuoka, Japan.
Kunihiro Tsuji, Department of Gastroenterology, Ishikawa Prefectural Central Hospital, Kanazawa, Japan.
Kenji Ikezawa, Department of Hepatobiliary and Pancreatic Oncology, Osaka International Cancer Institute, Osaka, Japan.
Ikuya Miki, Department of Gastroenterological Oncology, Hyogo Cancer Center, Akashi, Japan.
Yoshito Komatsu, Department of Gastroenterology, Hokkaido University Hospital, Sapporo, Japan.
Noriyuki Akutsu, Department of Gastroenterology and Hepatology, Sapporo Medical University School of Medicine, Sapporo, Japan.
Tatsuya Yamashita, Department of Gastroenterology, Kanazawa University Hospital, Kanazawa, Japan.
Hiroyuki Okuyama, Department of Medical Clinical Oncology, Kagawa University Hospital, MikiKagawa, Japan.
Junji Furuse, Department of Gastroenterology, Kanagawa Cancer Center, Yokohama, Japan.
Hiroaki Nagano, Department of Gastroenterological, Breast and Endocrine Surgery, Yamaguchi University Graduate School of Medicine, Ube, Japan.
References
- 1. Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas 2008;37:134–8. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. [DOI] [PubMed] [Google Scholar]
- 3. Tschang TP, Garza-Garza R, Kissane JM. Pleomorphic carcinoma of the pancreas: an analysis of 15 cases. Cancer 1977;39:2114–26. [DOI] [PubMed] [Google Scholar]
- 4. Clark CJ, Graham RP, Arun JS, Harmsen WS, Reid-Lombardo KM. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. J Am Coll Surg 2012;215:627–34. [DOI] [PubMed] [Google Scholar]
- 5. Strobel O, Hartwig W, Bergmann F, et al. Anaplastic pancreatic cancer: presentation, surgical management, and outcome. Surgery 2011;149:200–8. [DOI] [PubMed] [Google Scholar]
- 6. Morohoshi T, Held G, Klöppel G. Exocrine pancreatic tumours and their histological classification. A study based on 167 autopsy and 97 surgical cases. Histopathology 1983;7:645–61. [DOI] [PubMed] [Google Scholar]
- 7. Paal E, Thompson LD, Frommelt RA, Przygodzki RM, Heffess CS. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol 2001;5:129–40. [DOI] [PubMed] [Google Scholar]
- 8. Muraki T, Reid MD, Basturk O, et al. Undifferentiated carcinoma with osteoclastic giant cells of the pancreas: clinicopathologic analysis of 38 cases highlights a more protracted clinical course than currently appreciated. Am J Surg Pathol 2016;40:1203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Japan Pancreas Society . Classification of pancreatic carcinoma, 4th English edn. Kanehara & Co., Ltd., Tokyo, 2017. [Google Scholar]
- 10. Hruban RH, Adsay NV, Esposito I, et al. Pancreatic ductal adenocarcinoma. World Health Organization classification of tumours digestive system tumours, 5th edn. IARC Press, Lyon, 2019; 322–32. [Google Scholar]
- 11. Shiihara M, Higuchi R, Izumo W, Furukawa T, Yamamoto M. A comparison of the pathological types of undifferentiated carcinoma of the pancreas. Pancreas 2020;49:230–5. [DOI] [PubMed] [Google Scholar]
- 12. Imaoka H, Ikeda M, Maehara K, et al. Risk stratification and prognostic factors in patients with unresectable undifferentiated carcinoma of the pancreas. Pancreatology 2021;21:738–45. [DOI] [PubMed] [Google Scholar]
- 13. Gao Y, Cai B, Yin L, et al. Undifferentiated carcinoma of pancreas with osteoclast-like Giant cells: one Center's experience of 13 cases and characteristic pre-operative images. Cancer Manag Res 2022;14:1409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishigami K, Nishie A, Yamamoto T, et al. Imaging features of undifferentiated carcinoma of the pancreas. J Med Imaging Radiat Oncol 2019;63:580–8. [DOI] [PubMed] [Google Scholar]
- 15. Chadha MK, LeVea C, Javle M, Kuvshinoff B, Vijaykumar R, Iyer R. Anaplastic pancreatic carcinoma. A case report and review of literature. JOP 2004;5:512–5. [PubMed] [Google Scholar]
- 16. Hebert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology 2013;24:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012;75:319–31. [DOI] [PubMed] [Google Scholar]
- 18. Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: a meta-analysis and systematic review. Pancreas 2013;42:20–6. [DOI] [PubMed] [Google Scholar]
- 19. Imaoka H, Sasaki M, Hashimoto Y, Watanabe K, Ikeda M. New era of endoscopic ultrasound-guided tissue acquisition: next-generation sequencing by endoscopic ultrasound-guided sampling for pancreatic cancer. J Clin Med 2019;8:1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imaoka H, Ikeda M, Maehara K, et al. Clinical outcomes of chemotherapy in patients with undifferentiated carcinoma of the pancreas: a retrospective multicenter cohort study. BMC Cancer 2020;20:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reid MD, Muraki T, HooKim K, et al. Cytologic features and clinical implications of undifferentiated carcinoma with osteoclastic giant cells of the pancreas: an analysis of 15 cases. Cancer Cytopathol 2017;125:563–75. [DOI] [PubMed] [Google Scholar]
- 22. Khashab MA, Emerson RE, DeWitt JM. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of anaplastic pancreatic carcinoma: a single-center experience. Pancreas 2010;39:88–91. [DOI] [PubMed] [Google Scholar]
- 23. Sekulic M, Gilles S, Amin K, Stewart J III. Undifferentiated (anaplastic) carcinoma with osteoclast-like giant cells of the pancreas: a series of 5 cases with clinicopathologic correlation and cytomorphologic characterization. J Am Soc Cytopathol 2016;5:321–30. [DOI] [PubMed] [Google Scholar]
- 24. Facciorusso A, Wani S, Triantafyllou K, et al. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: a network meta-analysis. Gastrointest Endosc 2019;90:893–903 e7. [DOI] [PubMed] [Google Scholar]
- 25. Crino SF, Le Grazie M, Manfrin E, et al. Randomized trial comparing fork-tip and side-fenestrated needles for EUS-guided fine-needle biopsy of solid pancreatic lesions. Gastrointest Endosc 2020;92:648–58 e2. [DOI] [PubMed] [Google Scholar]
- 26. Karsenti D, Palazzo L, Perrot B, et al. 22G acquire vs. 20G Procore needle for endoscopic ultrasound-guided biopsy of pancreatic masses: a randomized study comparing histologic sample quantity and diagnostic accuracy. Endoscopy 2020;52:747–53. [DOI] [PubMed] [Google Scholar]
- 27. Latenstein AEJ, van der Geest LGM, Bonsing BA, et al. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer 2020;125:83–93. [DOI] [PubMed] [Google Scholar]
- 28. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. The Lancet 2011;378:607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sano M, Homma T, Hayashi E, et al. Clinicopathological characteristics of anaplastic carcinoma of the pancreas with rhabdoid features. Virchows Arch 2014;465:531–8. [DOI] [PubMed] [Google Scholar]
- 31. Li J, Wei T, Zhang J, et al. Carcinosarcoma of the pancreas: comprehensive clinicopathological and molecular characterization. HPB 2020;22:1590–5. [DOI] [PubMed] [Google Scholar]
- 32. Hruban RH, Pitman MB, Klimstra DS, Adenocarcinoma variants. Tumors of the pancreas. Washington, DC: American Registry of Pathology, 2007; 165–90. [Google Scholar]
- 33. Hoorens A, Prenzel K, Lemoine NR, Kloppel G. Undifferentiated carcinoma of the pancreas: analysis of intermediate filament profile and Ki-ras mutations provides evidence of a ductal origin. J Pathol 1998;185:53–60. [DOI] [PubMed] [Google Scholar]
- 34. Bazzichetto C, Luchini C, Conciatori F, et al. Morphologic and molecular landscape of pancreatic cancer variants as the basis of new therapeutic strategies for precision oncology. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sakakida T, Ishikawa T, Doi T, et al. Genomic landscape and clinical features of rare subtypes of pancreatic cancer: analysis with the national database of Japan. J Gastroenterol 2023;58:575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Demetter P, Maréchal R, Puleo F, et al. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: what do we know so far? Front Oncol 2021;11:630086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yonemasu H, Takashima M, Nishiyama KI, et al. Phenotypical characteristics of undifferentiated carcinoma of the pancreas: a comparison with pancreatic ductal adenocarcinoma and relevance of E-cadherin, alpha catenin and beta catenin expression. Oncol Rep 2001;8:745–52. [DOI] [PubMed] [Google Scholar]
- 38. Winter JM, Ting AH, Vilardell F, et al. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res 2008;14:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ishida K, Yamashita R, Osakabe M, et al. Expression of epithelial-mesenchymal transition proteins in pancreatic anaplastic (undifferentiated) carcinoma. Pancreas 2019;48:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mattiolo P, Fiadone G, Paolino G, et al. Epithelial-mesenchymal transition in undifferentiated carcinoma of the pancreas with and without osteoclast-like giant cells. Virchows Arch 2021;478:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gkountakos A, Mafficini A, Lou E, et al. Genomic characterization of undifferentiated sarcomatoid carcinoma of the pancreas. Hum Pathol 2022;128:124–33. [DOI] [PubMed] [Google Scholar]
- 42. Faber E, Krause H, Walker P, et al. Genomic profiling of rare undifferentiated sarcomatoid subtypes of pancreatic carcinomas for potential response to immunotherapy. J Clin Oncol 2023;41:741. [Google Scholar]
- 43. Agaimy A, Haller F, Frohnauer J, et al. Pancreatic undifferentiated rhabdoid carcinoma: KRAS alterations and SMARCB1 expression status define two subtypes. Mod Pathol 2015;28:248–60. [DOI] [PubMed] [Google Scholar]
- 44. Chang B, Sheng W, Wang L, et al. SWI/SNF complex-deficient undifferentiated carcinoma of the gastrointestinal tract: Clinicopathologic study of 30 cases with an emphasis on variable morphology, immune features, and the prognostic significance of different SMARCA4 and SMARCA2 subunit deficiencies. Am J Surg Pathol 2022;46:889–906. [DOI] [PubMed] [Google Scholar]
- 45. Andrades A, Peinado P, Alvarez-Perez JC, et al. SWI/SNF complexes in hematological malignancies: biological implications and therapeutic opportunities. Mol Cancer 2023;22:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mittal P, Roberts CWM. The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat Rev Clin Oncol 2020;17:435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luchini C, Pea A, Lionheart G, et al. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells is genetically similar to, but clinically distinct from, conventional ductal adenocarcinoma. J Pathol 2017;243:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206:3015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lehrke HD, Graham RP, McWilliams RR, et al. Undifferentiated pancreatic carcinomas display enrichment for frequency and extent of PD-L1 expression by tumor cells. Am J Clin Pathol 2017;148:441–9. [DOI] [PubMed] [Google Scholar]
- 50. Hrudka J, Lawrie K, Waldauf P, Ciprová V, Moravcová J, Matěj R. Negative prognostic impact of PD-L1 expression in tumor cells of undifferentiated (anaplastic) carcinoma with osteoclast-like giant cells of the pancreas: study of 13 cases comparing ductal pancreatic carcinoma and review of the literature. Virchows Arch 2020;477:687–96. [DOI] [PubMed] [Google Scholar]
- 51. Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473–81. [DOI] [PubMed] [Google Scholar]
- 52. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395–406. [DOI] [PubMed] [Google Scholar]
- 53. Unno M, Motoi F, Matsuyama Y, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol 2019;37:189. [DOI] [PubMed] [Google Scholar]
- 54. King DA, Rahalkar S, Bingham DB, Fisher GA. Pancreatic INI1-deficient undifferentiated rhabdoid carcinoma achieves complete clinical response on gemcitabine and nab-paclitaxel following immediate progression on FOLFIRINOX: a case report. J Gastrointest Oncol 2021;12:874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wakatsuki T, Irisawa A, Imamura H, et al. Complete response of anaplastic pancreatic carcinoma to paclitaxel treatment selected by chemosensitivity testing. Int J Clin Oncol 2010;15:310–3. [DOI] [PubMed] [Google Scholar]
- 56. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 58. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- 59. Obayashi M, Shibasaki Y, Koakutsu T, et al. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells curatively resected after pembrolizumab therapy for lung metastases: a case report. BMC Gastroenterol 2020;20:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Besaw RJ, Terra AR, Malvar GL, Chapman TR, Hertan LM, Schlechter BL. Durable response to PD-1 blockade in a patient with metastatic pancreatic undifferentiated carcinoma with osteoclast-like giant cells. J Natl Compr Cancer Netw 2021;19:247–52. [DOI] [PubMed] [Google Scholar]
- 61. Casali PG, Bruzzi P, Bogaerts J, Blay JY. Rare Cancers Europe Consensus P. Rare Cancers Europe (RCE) methodological recommendations for clinical studies in rare cancers: a European consensus position paper. Ann Oncol 2015;26:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


