Abstract
Introduction and importance:
Condyloma acuminatum (CA) or genital warts, represents a rare sexually transmitted disease caused by the human papillomavirus. Infection occurs when host basal cells are exposed to viral infection through a damaged epithelial barrier, during sexual intercourse, or due to other minor skin abrasions. Giant condyloma acuminatum (GCA) has a higher rate of malignant transformation than CA.
Case presentation:
We are presenting a 44-year-old single gentleman known as a smoker, hepatitis B positive, vitiligo, and hypercholesteromia. He was an alcoholic and had multiple heterosexual relationships abroad. Referred from the dermatology clinic, complaining of genital warts that had increased in size for 10 years.
Clinical discussion:
CA management includes local applications like imiquimod and podophyllotoxin and clinician-administered treatments such as cryotherapy, surgical excision, electrosurgery, and CO2 laser therapy. Other options, such as interferon, radiotherapy, or chemotherapy, are available. The selection of therapy should be individualized and based upon consideration of the extent of the disease, patient preference, cost, adverse effects, treatment availability, and the response to previous treatments. Complete surgical excision is the treatment of choice for GCA.
Conclusion:
We present a patient with GCA in the perianal area that was surgically treated with an excellent outcome.
Keywords: genital warts condyloma acuminatum (genital warts), giant condyloma acuminatum, human papillomavirus, sexually transmitted disease
Introduction and importance
Highlights
A patient diagnosed with giant condyloma acuminatum (GCA) in the perianal region underwent surgical treatment successfully.
For extensive lesions, skin graft coverage or a flap can reduce the recovery period and lower the risk of severe anal stricture.
The histopathological examination revealed a GCA, characterized by significant irregular polypoid skin with hyperplastic papillomatous squamous epithelium, hyperkeratosis, and parakeratosis. Koilocytes and chronic inflammatory infiltration were also present. There was no evidence of dysplasia or malignancy.
Condyloma acuminatum (CA) and giant condyloma acuminatum (GCA), or Buschke–Löwenstein tumor (BLT), represent a rare sexually transmitted disease (STD) involving the anogenital region caused by human papillomavirus (HPV), especially genotype 6 or 11. The condition has numerous risk factors, including multiple sexual partners, homosexuality and anal sex, prostitution, chronic genital infections, smoking, immunodeficiencies, and a lack of proper hygiene. GCA has a higher rate of malignant transformation than CA. GCA also called BLT, is characteristically bulky (more than 10 cm in diameter), slow growing, and exophytic, with locally aggressive behavior that tends to invade and destroy the tissues on which it sits and form fistulas and abscesses1,2. Despite benign histology in most cases, malignant degeneration into a verrucous carcinoma or invasive squamous carcinoma is possible, especially in immunocompromised patients; foci of invasive carcinoma are noted in 50% of the reports, and carcinoma in situ in 8%.
Case presentation
A 44-year-old male, single, known as a smoker, hepatitis B positive, vitiligo, and hypercholesteromia. He was an alcoholic, without marriage, and had multiple heterosexual relationships abroad. Visited our general surgery clinic for the first time on 6 March 2022, with a referral from the dermatology clinic, complaining of genital warts that had been increasing in size for 10 years; in the last 4 years, they started causing him pruritus and a bloody, foul-smelling discharge. Dermatology cryotherapy failed. On examination: the patient was well built, with normal vital signs and giant cauliflower-shaped hard perianal circumferential warts, except for 1 cm at 6 o’clock. A lesion with a diameter of 22 cm also involves the lower scrotum skin. No intra-anal involvement by per rectal and proctoscopy. No prerenal involvement. The rest of the physical examination was normal. Laboratory wise, WBC (white blood cells count) 7.2×109/l, Hb (hemoglobin level) 12.3 g/dl, platelet 303×109/l, LFT (liver function tests) within normal limits, RFT (renal function tests) l, PT (prothrombin time), PTT (partial thromboplastin time), INR (international normalized ratio) are within normal limits, HBV (hepatitis B virus) <15. Venereal disease serology Treponema pallidum hemagglutination assay (TPHA) and indirect hemagglutination assay (IHA) tests non-reactive, Hepatitis Hbs Ag (hepatitis b surface antigen) + HBC (hepatitis B core antigen) plus positive, HIV Ag/Ab (human immunodeficiency virus antigen/antibody test) negative. With these giant warts, we decided with our plastic surgeon to excise them surgically after the patient’s informed consent. So sigmoid diverting colostomy was done, with total surgical anal sphincter preserving excision. Lower involved scrotal skin excised with it without tunica opening. We left the 1 cm normal skin bridge to the anus at 6 o’clock to prevent later anal stenosis. A vacuum dressing was applied for 3 days after the healthy wound’s skin graft was closed. Intravenous antibiotic coverage with cefuroxime and metronidazole lasted 7 days, starting the night before the first surgery. Enoxaparin is used for venous thromboembolism prophylaxis (VTE). The patient was followed in our clinic postoperatively for 4 months, with complete skin healing assured without recurrence and no anal stenosis. Then, closer to discharge, the sigmoid colostomy was done, and an extra 1-month follow-up was done with no complaints before discharge. Histopathology result: significant irregular polypoid skin, skin lined by hyperplastic papillomatous squamous epithelial with hyperkeratosis and parakeratosis, presence of koilocytes, and chronic inflammatory infiltration. GCA, no evidence of dysplasia or malignancy (Figs 1–6).
Figure 1.

Giant condyloma acuminatum post-excision, showing the preserved skin bridge to the anus.
Figure 6.

Koilocytes.
Figure 2.
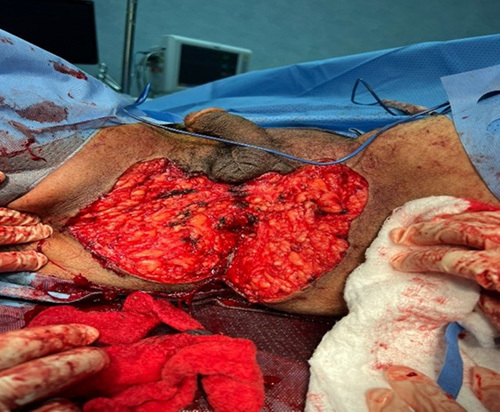
Giant condyloma acuminatum post total excision, sphincter preserving surgery.
Figure 3.

Giant condyloma acuminatum post-excision specimen.
Figure 4.

Post-skin graft uptake.
Figure 5.

Hyperplastic papillomatous squamous epithelium.
Discussion
A CA diagnosis can have adverse psychosocial effects and may be accompanied by symptoms such as pruritus, pain, bleeding, micturition or defecation difficulty, and sexual restriction. It is associated with concerns regarding cosmetic appearance, stigmatization, personal health, and sexual relationships. Educating patients about HPV infection and CA is a vital management component. HPV infection and CA are acquired through direct genital contact. Patients with anogenital warts are at risk for other STDs. Individuals with anogenital warts can transmit HPV to sexual partners. Patients should inform current sexual partners of their diagnosis. Sexual partners may benefit from an evaluation for anogenital warts and other STDs. Anogenital warts may spontaneously resolve, persist, or progress. Approximately one-third of anogenital warts are estimated to regress without treatment within 4 months3,4. Investigators have identified 200 types of HPV, more than 40 of which can be transmitted through sexual contact and infect the anogenital region. Genetically, HPV types are divided into low-risk and high-risk types based on the associated cancer risk in any body area. The low-risk types HPV 6 and HPV 11 are detected in around 90% of anogenital warts. HPV is transmitted through contact with infected skin or mucosa. The virus invades the cells of the epidermal basal layer through microabrasions. Anogenital HPV infection is almost always acquired through sexual contact. Warts are not required for transmission but are highly infectious because of their high viral load. Male circumcision may reduce the risk of HPV infection5. External anogenital warts are typically found on the vulva, penis, groin, perineum, anal skin, perianal skin, and suprapubic skin. Treatment options for external CA include patient-applied topical medications and clinician-administered treatments. First-line patient-applied (topically) therapies include imiquimod, podophyllotoxin, and sinecatechins. First-line clinician-administered therapies include topically applied trichloroacetic acid (TCA), and bichloroacetic acid (BCA). Surgical removal [excision, electrosurgery, or laser: mainly CO2 laser and the neodymium-doped yttrium aluminum garnet (Nd:YAG) laser focus on use for urethral warts]5. The therapy selection should be individualized and based upon consideration of the extent of the disease, patient preference, cost, adverse effects, treatment availability, and the response to previous treatments. Second-line therapy: Patients who do not respond to the first-line treatment may benefit from a trial of an alternative first-line treatment or combination treatment. Cryotherapy plus a topical agent is a common initial choice for combination treatment. Podophyllin resin and topical fluorouracil can be effective for external anogenital warts but are not recommended as first-line treatments because of side effects. The refractory disease can benefit from topical cidofovir and intralesional interferon (both expensive and used in combination therapy). Systemic treatments are used for complex cases. A benefit has been reported with low-dose oral cyclophosphamide. Hyperthermia6 and external beam radiotherapy7 use also used for extensive anogenital warts. Emerging therapies like Mycobacterium w vaccine8, topical nitric–zinc complex, virus-like particle immunotherapy, glycyrrhizinic acid plus an immunostimulant food supplement, and photodynamic therapy, have been documented in clinical studies, and resolution of recalcitrant giant CA after combination therapy with intralesional Mycobacterium indicus pranic immunotherapy and topical acitretin is written in a case report9. Further study is necessary before recommendations for the use of these treatments5. The differential diagnosis of CA includes common benign papular cutaneous conditions, such as seborrheic keratosis, acrochordon, pearly penile papules, squamous papilloma, and Fordyce spots. In addition, STDs such as molluscum contagiosum, which typically manifests as umbilicated small papules, and condyloma latum of syphilis, which manifests as single or multiple moist, gray-white papules or plaques. Another differential diagnosis is that inflammatory conditions, such as lichen nitidus and papulosquamous lichen planus lesions, may also be included. Lichen nitidus most commonly manifests as multiple flat-topped, pinhead-sized papules on the penis. Papulosquamous lichen planus often manifests as violaceous polygonal or annular pruritic papules. Premalignant and malignant disorders, such as Bowenoid papulosis and GCA (BLT), may enter the differential diagnosis. Bowenoid papulosis is a premalignant focal epidermal dysplasia that usually manifests as multiple red to brown papules on the genitals and is most often associated with HPV5. GCA is a rare low-grade form of squamous cell carcinoma. Immunosuppression favors the rapid growth of the condylomas and increases the risk of their malignant transformation, maybe favoring the oncogenetic mechanisms caused by HPV infection10. The patient’s immunological status must be checked, including screening test serology for STDs (HIV, syphilis, HBV), and hepatitis C virus (HCV). Complete surgical excision is the treatment for GCA. It allows histological examination of the entire specimen and evaluation for foci of squamous cell carcinoma. The cure rate with radical surgery is reportedly 61%, and recurrences of GCA can be successfully treated with radical surgery11,12. Primary excision can be safely performed in limited CA lesions, leaving wounds open to granulation. In more extensive lesions, skin graft coverage or flap is performed to decrease the length of recovery and minimize the risk of severe anal stricture. The experiences reported in the literature are heterogeneous and mainly derived from case reports. Skin grafting, advancement, or rotating flaps like circumferential sleeve rectal advancement, house advancement, S-plasty rotations, and V–Y advancement flaps are all effective alternative methods for covering wide wounds. Most authors do not perform colostomies for fecal diversion with an acceptable postoperative complication rate. A combination of bowel cleansing, a low-fiber diet, and loperamide can be administered to reduce early contamination with the feces of the wound13–15. In our case, we preferred and did colostomy for fecal diversion, and that helped us in postoperative care and the excellent graft uptake. In cases of anal canal involvement when mucosectomy extends beyond the dentate line, a colostomy must be performed to prevent potentially severe pelviperineal sepsis due to the high rate of suture dehiscence at this level. When performing an angioplasty, it is important to construct large flaps with a good blood supply and to obtain complete hemostasis of the raw surface to prevent hematomas. Avoiding tension during the much-needed cutaneous anastomosis is important but only sometimes technically simple. Partial or complete dehiscence is not uncommon, but anal stenosis is rare13,16. Abdominoperineal resection should be performed for more extensive lesions with deep invasion, malignant transformation, or tumor recurrence. Chemotherapy with 5-fluorouracil and focused radiation therapy may be used in certain cases of recurrence or extensive pelvic disease1,2,3,4,5,6,7,8,9,17,18,19.
Conclusion
Giant anal condyloma is a rare pathology with mainly sporadic single-center experience reported in the literature. Surgical complete excision remains the best treatment. A skin graft is as good as advancement or rotating flaps (performed in other centers’ case reports), which are effective alternative methods for covering wide wounds. Postoperative complications such as graft rejection, hematoma, or suture dehiscence are possible and should be avoided with accurate hemostasis, good mobilization, and a tension-free flap. Local wound cleansing and an adequate follow-up can avoid anal stenosis.
Learning points and recommendations
Surgical excision is the preferred treatment for giant condyloma acuminatum, aiming to achieve clear margins and prevent a recurrence.
Histopathological examination of the excised tissue is crucial for accurate diagnosis and to exclude the possibility of malignancy.
Adjuvant therapies such as electrocautery, cryotherapy, and topical agents may be beneficial in reducing the risk of recurrence, but further studies are needed to assess their effectiveness.
Ethical approval
IRB approval. The Dammam Medical Complex conducted a study on 3 March 2023, entitled ‘Surgical Management of Giant Condyloma Acuminatum: A Case Report and Literature Review,’ which received ethical approval from the DMC Ethical Committee in Saudi Arabia on 13 June 2023.
Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
None.
Author contribution
A.M.A.-G.: operating surgeon and wrote the manuscript; I.A.: a plastic surgeon who performed a graft; S.A., K.A., and A.A.A.: data collection and searching for reference; S.M.A.: wrote the highlights, structural abstract, learning points and recommendations, and revised the manuscript.
Conflicts of interest disclosure
The authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
None.
Guarantor
Ahmed M. Al-Ghamdi.
Data availability statement
None.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 31 July 2023
Contributor Information
Ahmed M. Al-Ghamdi, Email: ahmedgh999@gmail.com.
Seba Alfalah, Email: G3Z3L3@gmail.com.
Khurshid Anwer, Email: dranwer78@gmail.com.
Ibrahim Alzaher, Email: bibiars8@yahoo.com.
Ahmed A. Alsuhaimi, Email: A7meddd@gmail.com.
Sahar M. Aldhafeeri, Email: d-sahar44@hotmail.com.
References
- 1. Kreuter A, Wieland U. Giant condyloma acuminatum of Buschke and Löwenstein. New Engl J Med 2011;365:1624. [DOI] [PubMed] [Google Scholar]
- 2. Arajo Beatriz Lima. Case report: Buschke–Löwenstein tumor in a non-immunosuppressed patient. Clin Biomed Res 2021;41:1–261. [Google Scholar]
- 3. Handsfield HH. //www.uptodate.com/contents/condylomata-acuminate-anogenital-warts-management-of-external-condylomata-acuminata-in-adult-males/abstract/2
- 4.Yanofsky VR, Patel RV, Goldenberg G.http://www.uptodate.com/contents/condylomata-acuminata-anogenital-warts-management-of-external-condylomata-acuminata-in-adult-males/abstract/3
- 5. Rosen T. 9 February 2023. https://www.uptodate.com/contents/condylomata-acuminata-anogenital-warts-management-of-external-condylomata-acuminata-in-adult-males/contributors
- 6. Huo W, Li G-H, Qi R-Q, et al. Clinical and immunologic results of local hyperthermia at 44 °C for extensive genital warts in patients with diabetes mellitus. Int J Hyperthermia 2013;29:17–20. [DOI] [PubMed] [Google Scholar]
- 7. Dhadda AS, Anand A, Boynton C, et al. External beam radiotherapy for extensive genital condyloma acuminate: a role in selected patients? Clin Oncol (R Coll Radiol 2008;20:91. [DOI] [PubMed] [Google Scholar]
- 8. Kumar P, Dar L, Saldiwal S, et al. Intralesional injection of Mycobacterium w vaccine vs. imiquimod, 5%, cream in patients with anogenital warts: a randomized clinical trial. JAMA Dermatol 2014;150:1072. [DOI] [PubMed] [Google Scholar]
- 9. Khullar G, Narang T, De D, et al. Recalcitrant giant condyloma acuminatum were treated successfully with a novel combination of Mycobacterium indicus pranii immunotherapy and acitretin. Int J STD AIDS 2017;28:1155. [DOI] [PubMed] [Google Scholar]
- 10. Chu QD, Vezeridis MP, Libbey NP, et al. Giant condyloma acuminatum (Buschke–Löwenstein tumor) of the anorectal and perianal regions. Analysis of 42 cases. Dis Colon Rectum 1994;37:950–957. [DOI] [PubMed] [Google Scholar]
- 11. De Toma G, Cavallaro G, Bitonti A, et al. Surgical management of perianal giant condyloma acuminatum (Buschke–Löwenstein tumor): report of three cases. Eur Surg Res 2006;38:418–422. [DOI] [PubMed] [Google Scholar]
- 12. Millan UM, Flores J, Asencio F, et al. Excision and V–Y plasty reconstruction for giant condyloma acuminatum. Tech Coloproctol 2004;8:99–101. [DOI] [PubMed] [Google Scholar]
- 13. Abbas MA. Wide local excision for Buschke–Löwenstein tumor or circumferential carcinoma in situ. Tech Coloproctol 2011;15:313–318. [DOI] [PubMed] [Google Scholar]
- 14. Balik E, Eren T, Bugra D. A surgical approach to anogenital Buschke–Loewenstein tumors (giant condyloma acuminata). Acta Chir Belg 2009;109:612–616. [DOI] [PubMed] [Google Scholar]
- 15. Oh C, Albanese C. S-plasty for various anal lesions. Am J Surg 1992;163:606–608. [DOI] [PubMed] [Google Scholar]
- 16. Klaristenfeld D, Israelit S, Beart RW, et al. Surgical excision of extensive anal condylomata is not associated with risk of anal stenosis. Int J Colorectal Dis 2008;23:853–856. [DOI] [PubMed] [Google Scholar]
- 17. Trombetta LJ, Place RJ. Giant condyloma acuminatum of the anorectum: trends in epidemiology and management: report of a case and review of the literature. Dis Colon Rectum 2001;44:1878–1886. [DOI] [PubMed] [Google Scholar]
- 18. Paul S, Cheng CE, Kroshinsky D. Combination systemic fluorouracil and radiation for the treatment of recalcitrant condyloma with associated squamous cell carcinoma in an immunocompromised 15-year-old girl. Pediatr Dermatol 2015;32:e148–e150. [DOI] [PubMed] [Google Scholar]
- 19. Gilson R, Nugent D, Werner RN, et al. 2019 IUSTI-Europe guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol 2020;34:1644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


