Abstract
Introduction and importance:
Budd–Chiari Syndrome (BCS) is a rare disorder that affects the liver and is caused by blockage of the hepatic veins. Coronavirus disease 2019 (COVID-19) has been linked to an increased risk of developing BCS due to its ability to cause inflammation in the body, which can lead to clotting disorders.
Case presentation:
A 43-year-old female presented to the emergency department complaining of severe epigastric and right upper quadrant pain and progressive abdominal distention. Upon examination, investigation, and triphasic liver computed tomography with contrast, the patient was diagnosed with BCS.
Clinical discussion:
The patient was started on anticoagulant therapy with low-molecular-weight heparin and supportive treatment. She was hospitalized for 3 weeks and discharged on oral warfarin 5 mg/day after showing clinical improvement.
Conclusion:
Hepatosplenomegaly and abdominal distention after COVID-19 infection raise suspicion for BCS. Therefore, early detection of these signs is essential for immediate management.
Keywords: Budd–Chiari Syndrome, case report, COVID-19, liver, thrombosis
Introduction
Highlights
Budd–Chiari Syndrome (BCS) is a rare but serious disorder that can be caused by coronavirus disease 2019 (COVID-19) infection due to the inflammation and clotting disorders associated with it.
It is important for people who have had COVID-19 infection to be aware of potential symptoms of BCS and seek medical attention if they experience any of these symptoms.
Early diagnosis and treatment are crucial for improving outcomes and preventing further complications.
The few studies that have been conducted suggest that BCS may be a rare complication of COVID-19 infection, but more research is needed to confirm this association.
Coronavirus disease 2019 (COVID-19) is a contagious respiratory illness caused by a novel coronavirus that was first identified in Wuhan, China, in December 2019. The virus has since spread to over 200 countries and territories around the world, leading to a global pandemic. Symptoms of COVID-19 include fever, cough, shortness of breath, and difficulty in breathing. In severe cases, the virus can cause pneumonia and even death1. Previous studies showed that coagulopathy incidences might increase in severe COVID-19 cases, which is subsequently linked to a poor prognosis2,3.
Recently, COVID-19 has been linked to an increased risk of developing Budd–Chiari Syndrome (BCS) due to its ability to cause inflammation in the body, which can lead to clotting disorders. Generally, the exact cause of BCS in COVID-19 patients is not yet known, but it is believed to be related to the inflammatory response triggered by the virus. It is thought that this response may lead to thrombosis (blood clot formation) in the hepatic veins, resulting in obstruction of blood flow from the liver. In addition, some studies have suggested that certain medications used to treat COVID-19 may also contribute to BCS development3,4,5.
In this study, we presented a case of a female diagnosed with BCS after an acute COVID-19 infection. This would help healthcare providers to be aware of this potential complication of the COVID-19 infection so that they can recognize it early and provide appropriate treatment for their patients. This case has been reported in line with the Surgical CAse REport (SCARE) Criteria6 (Supplemental Digital Content 1, http://links.lww.com/MS9/A189).
Case presentation
We reported a case of a 43-year-old female who presented to the emergency department (ER) complaining of severe epigastric and right upper quadrant (RUQ) pain along with progressive abdominal distention, which had been ongoing for 1 month. The pain intensified 2 weeks before her ER visit and radiated to her back. The pain was associated with a documented fever and vomiting after meals. In addition, the patient experienced a 5 kg weight loss during these 2 weeks.
Her medical history is remarkable for a COVID-19 infection, which was confirmed with a PCR test; she recovered a month ago. She had symptoms of moderate severity, such as cough, fever, dyspnea, fatigue, decreased appetite, and relapsing fever but did not require hospitalization. The patient does not take any contraceptives, has no history of abortions, and has no family or personal medical history concerning thrombotic events. She does not have any comorbidities.
There was mild abdominal distention and tenderness over the RUQ and epigastric areas on abdominal examination. Her vitals on presentation were the following: blood pressure (BP) of 110/60 mmHg, pulse rate was 95 beats/min, temperature 37.8°C, and arterial oxygen saturation (SaO2) of 97% on room air.
Hematological, biochemical, and immunological investigations were done at the time of hospital admission. The results of these investigations are demonstrated in Table 1. In addition, the Antinuclear Antibody test was done to exclude autoimmune disorders such as Systemic lupus erythematosus, autoimmune hepatitis, and vasculitis that may lead to the mentioned clinical manifestations.
Table 1.
Shows the results of laboratory tests at the time of hospital admission.
| Test name | Patient value | Normal valuea |
|---|---|---|
| Bilirubin, direct (µmol/l) | 21.0 | 0.0–5.1 |
| Bilirubin, total (µmol/l) | 27.1 | 3.4–20.5 |
| Aspartate aminotransferase – AST (U/l) | 1124 | 8–33 |
| Alanine aminotransferase – ALT (U/l) | 1311.9 | 4–36 |
| Alkaline phosphatase (U/l) | 151 | 40–150 |
| Gamma-glutamyl transpeptidase – GGT (U/l) | 95 | 5–36 |
| Serum albumin (g/dl) | 3.47 | 3.4–4.8 |
| Prothrombin time (INR) | 1.37 | 0.85–1.15 |
| APTT (seconds) | 24.8 | 30–40 |
| Prothrombin time (seconds) | 17.9 | 12–16 |
| Hemoglobin (g/dl) | 8.4 | 12–16 |
| Leukocytes (109/l) | 10.3 | 4–11 |
| Platelets (109/l) | 318 | 150–450 |
| Reactive C-protein (mg/l) | 71.1 | 0–5 |
| Creatinine (µmol/l) | 60.40 | 45–98 |
| Infectious diseases | ||
| SARS-CoV-2 RT-PCR | Negative | Negative |
| Hepatitis B surface antigen (HBsAg) | Negative | Negative |
| Antihepatitis C virus antibody (HCV) | Negative | Negative |
| Antihepatitis A serology IgM | Negative | Negative |
| Epstein–Barr virus serology IgM | 15.7 | Negative <20 |
| Cytomegalovirus serology IgM | 0.4 | Negative <0.9 |
| HIV 1/2 antibody | Negative | Negative |
| Autoimmune antibodies | ||
| Antinuclear antibodies (ANA) | Negative | Negative |
| Hypercoagulable states | ||
| Anticardiolipin IgG (pg/ml) | <1.8 | Negative <10 |
| Anticardiolipin IgM (pg/ml) | <1.8 | Negative <10 |
| Antiphospholipid IgG (U/ml) | 1.67 | Negative <10 |
| Antiphospholipid IgM (U/ml) | 0.87 | Negative <10 |
| Protein C (%) | 55 | 65–150 |
| Protein S (%) | 79 | 50–130 |
| Lupus anticoagulant (LA) | Negative | Negative |
| Factor V (%) | 62 | 70–140 |
| Antithrombin III (%) | 73 | 80–120 |
The normal ranges of the lab values were according to the electronic database of the hospital.
Abdominal ultrasound showed hepatosplenomegaly with moderate free fluid in the pelvis. A triphasic liver computed tomography (CT) with contrast revealed the presence of hepatosplenomegaly and occlusion in the hepatic veins, confirming the diagnosis of BCS. The inferior vena cava (IVC) and portal vein appeared to be patent. Furthermore, with no remarkable findings, upper and lower endoscopies with biopsies were done. A second Doppler abdominal ultrasound was done 10 days after the first one. The second ultrasound demonstrated hepatomegaly (19.3 cm) span with a nonhomogeneous echo pattern.
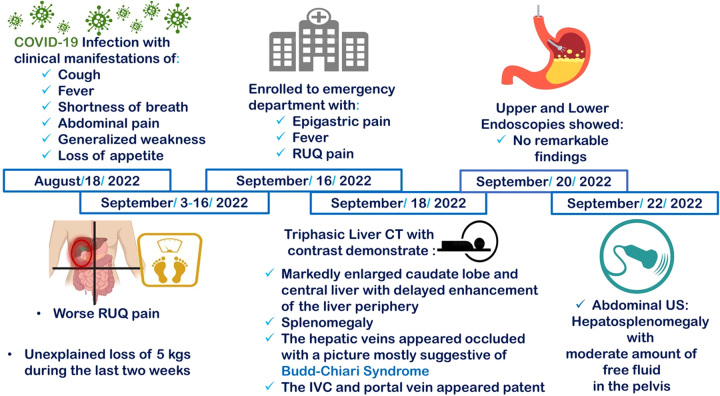
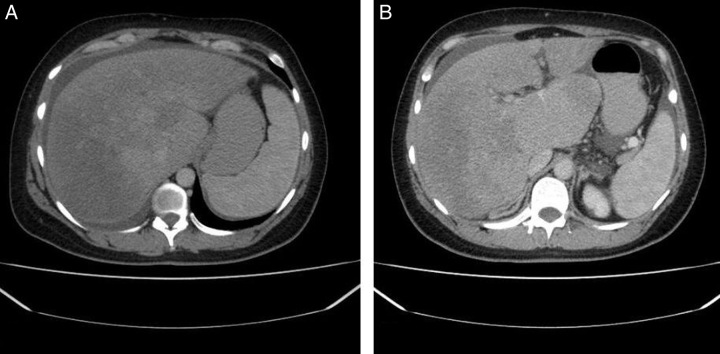
Moreover, the IVC, left, and middle hepatic veins were patent. However, the right hepatic and portal veins could not be elicited, suggesting occlusion. Figure 1 demonstrates the sequence of events in this case. Figure 2 demonstrates the nutmeg appearance and the enlarged caudate lobe of the liver on a CT scan.
Figure 1.
Demonstrates the sequence of events from the time of COVID-19 infection till the diagnosis of Budd–Chiari Syndrome. CT, computed tomography; RUQ, right upper quadrant; US, ultrasound.
Figure 2.
Shows the triphasic liver computed tomography (CT) with contrast which revealed the presence of hepatosplenomegaly and occlusion in the hepatic veins, confirming the diagnosis of BCS. (A) Demonstrates ‘nutmeg’ appearance of the liver; (B) Demonstrates enlarged caudate lobe of the liver.
A comprehensive thrombophilia screen was performed, excluding any inherited anticoagulant deficiency. Therefore, anticoagulant therapy with low-molecular-weight heparin (LMWH) was initiated with supportive treatment. After showing clinical improvement, the patient was discharged on oral warfarin 5 mg/day after staying in the hospital for 3 weeks. Her labs on discharge were as shown in Supplementary Table 1 (Supplemental Digital Content 2, http://links.lww.com/MS9/A190).
She had biweekly follow-up appointments for 3 months in the outpatient clinic and all showed an improvement in her lab results. She had no abdominal pain, ascites, or fever, and all subsequent abdominal ultrasounds were clear. She then stopped going to her follow-up appointments and was not compliant with her medications anymore. One month later, after fasting for a few days, her clinical picture deteriorated. She presented to the hospital with hepatomegaly and refractory ascites that were not responding to diuretics. Her lab results were as shown in Supplementary Table 2 (Supplemental Digital Content 3, http://links.lww.com/MS9/A191).
Thrombosis in the portal vein, splenic vein, and IVC was confirmed by Doppler ultrasound. She was then referred to another hospital where they used a surgical approach (portal vein recanalization followed by intrahepatic portosystemic shunt (PVR-DIPS)), and she showed clinical improvement in the following days.
Discussion
BCS is a rare disorder characterized by hepatic venous outflow obstruction in the absence of pericardial disease, cardiac disease, and sinusoidal obstruction syndrome as the causes of the obstruction. The exact cause of BCS is unknown, but it is believed to be related to clotting disorders or other conditions that affect blood flow in the veins of the liver. In some cases, BCS can be caused by an underlying medical condition such as cancer, cirrhosis, or thrombophilia. In other cases, it may be due to an infection or trauma7,8.
BCS can cause a variety of manifestations including abdominal pain, ascites, jaundice, fatigue, nausea, and vomiting. If left untreated, BCS can lead to liver failure and death. Treatment for BCS typically involves anticoagulant therapy (blood thinners) to prevent further clot formation and surgery or other interventions to remove existing clots. In severe cases, a liver transplant may be necessary7,9.
COVID-19 is known to cause blood clots in some patients, which can lead to serious complications such as stroke, heart attack, and pulmonary embolism. It is believed that this increased risk of blood clots may also contribute to the development of BCS in some patients. The exact mechanism by which COVID-19 leads to BCS is not yet fully understood. However, it is thought that the virus may cause inflammation and damage to the liver, leading to the formation of blood clots in the hepatic veins3,4,5. The most common finding in individuals with COVID-19 and coagulopathy is an elevated D-dimer concentration, a moderate reduction in platelet count, and a prothrombin time elongation. Elevated D-dimer (>05 mg/l) was detected in 260 (46%) of 560 individuals in a series of 1099 COVID-19 patients from China10.
The first case of COVID-19-associated BCS was reported in Saudi Arabia for a 48-year-old female suffering from diffuse abdominal pain for 3 days associated with abdominal distention. The patient was a confirmed COVID-19 case11. She has a medical history of well-controlled long-standing hypertension and takes no oral contraceptives. Contrast-enhanced abdominal CT revealed filling defects in the IVC and hepatic vein with an enlarged nonhomogeneous liver, leading to the diagnosis of the patient with BCS. Anticoagulant therapy with LMWH (1 mg/kg/day) and supportive treatment, including diuretics, were immediately initiated. She was discharged on oral warfarin after staying in the hospital for 10 days. In the follow-up visit 1 month after discharge, the patient was asymptomatic11. Our case also suffered from an occluded hepatic vein and was diagnosed with BCS following the COVID-19 infection. In addition, LMWH was initiated, and the patient was released on warfarin (5 mg/day). Contrary to the mentioned case, the patient in our case stayed in the hospital for 3 weeks and suffered from a relapse 4 months after discharge due to being noncompliant with her medications and fasting, leading to her relapse.
Another case was reported for a 50-year-old woman that presented with atypical symptoms, including severe RUQ pain, jaundice, hepatomegaly, and splenomegaly without biliary tract obstruction and ascites5. After 2-days of waiting for a differential diagnosis at the ER, the clinical manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) started to appear. Partial thrombosis and echogenic material were detected in the left suprahepatic vein by abdominal ultrasound imaging and MRI. Based on clinical, laboratory, and radiological imaging, she was diagnosed with BCS correlated to COVID-19 infection5. The patient received anticoagulation with rivaroxaban 20 mg/day. A new MRI was performed 3 months later, showing complete resolution of the left suprahepatic vein thrombosis5. The patient in our case also suffered from an occluded hepatic vein and was diagnosed with BCS following a COVID-19 infection. Furthermore, the patient in our case was administered LMWH; however, the patient in the mentioned case was administered rivaroxaban (factor Xa inhibitor), both improving the patient’s symptoms through different mechanisms. Moreover, the patient in the mentioned case showed complete resolution of the thrombus in the left suprahepatic vein; however, as mentioned previously, our patient suffered from a relapse. Alongside the venous and arterial thrombosis accidents related to SARS-CoV-2 infection, spontaneous hemorrhagic incidents have also been reported as in the case of a 44-year-old woman in Algeria presenting with body aches and fever for more than 10 days that was confirmed to be a giant subcapsular hematoma of the liver despite not having any trauma12.
Strengths and limitations of the study
The diagnosis was confirmed by an accurate imaging modality: a triphasic liver CT with contrast. In addition, the study involved face-to-face patient communication and there was no barrier to feedback between the investigators and the patient. However, unfortunately, we could not obtain images of the hepatic vein occlusion.
Conclusion
Ultimately, BCS is a rare but serious disorder that can be caused by COVID-19 infection due to the inflammation and clotting disorders associated with it. Generally, it is unclear what factors may increase the risk of developing BCS following COVID-19 infection or how best to diagnose and treat this condition in patients with COVID-19. Further research is needed to better understand the potential link between BCS and COVID-19 infection and to develop effective treatments for this rare complication.
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written informed consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
Not applicable.
Author contribution
S.S.S.: conceptualization and methodology; H.M.U., A.-T., B.-H., O.B.M., and A.M.: writing – original draft; R.A.G. and H.H.: software and visualization; Y.J.A. and A.K.: writing – review and editing.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
Name of the registry: not applicable.
Unique identifying number or registration ID: not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): not applicable.
Guarantor
All authors.
Data availability statement
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
Not applicable.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/annals-of-medicine-and-surgery.
Published online 21 July 2023
Contributor Information
Seri S. Sawaqed, Email: seris@hu.edu.jo.
Heba M. Urabi, Email: hebaurabi98@gmail.com.
Mohammad H. Al-thnaibat, Email: moh1.thunibat@gmail.com.
Anas Bani-Hani, Email: Anas.banihani@gmail.com.
Omar B. Mohd, Email: ob.jamil2001@gmail.com.
Ahmed B. Mohd, Email: abj.m00hd@gmail.com.
Reem A. Ghannam, Email: Reem.gh125@gmail.com.
Hanan Hasan, Email: hananyalu97@gmail.com.
Yasmeen J. Alabdallat, Email: abdallat.01@gmail.com.
Abdulrhman Khaity, Email: abdulrhman.marwan.khaity@gmail.com.
References
- 1.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Published 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- 2. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becker RC. COVID-19 update: COVID-19-associated coagulopathy. J Thromb Thrombolysis 2020;50:54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iba T, Levy JH, Levi M, et al. Coagulopathy in COVID-19. J Thromb Haemost 2020;18:2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Espinoza JAL, Júnior JE, Miranda CH. Atypical COVID-19 presentation with Budd–Chiari Syndrome leading to an outbreak in the emergency department. Am J Emerg Med 2021;46:e5–800.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agha RA, Franchi T, Sohrabi C, et al. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg 2020;84:226–230. [DOI] [PubMed] [Google Scholar]
- 7. Zu M, Xu H, Zhang Q, et al. Review of Budd–Chiari Syndrome. J Interv Med 2020;3:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grus T, Lambert L, Grusová G. Budd–Chiari Syndrome. Prague Med Rep 2017;118:69–80. [DOI] [PubMed] [Google Scholar]
- 9. Hernández-Gea V, De Gottardi A, Leebeek FWG, et al. Current knowledge in pathophysiology and management of Budd–Chiari Syndrome and non-cirrhotic non-tumoral splanchnic vein thrombosis. J Hepatol 2019;71:175–199. [DOI] [PubMed] [Google Scholar]
- 10. Aydinli M, Bayraktar Y. Budd–Chiari Syndrome: etiology, pathogenesis and diagnosis. World J Gastroenterol 2007;13:2693–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sh Hassan AA, Alsaleh ME, Alsaleh ME, et al. Budd–Chiari Syndrome: a case report of a rare presentation of COVID-19. Cureus 2021;13:e12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tidjane A, Laredj A, Boudjenan-Serradj N, et al. A giant spontaneous subcapsular hematoma of the liver revealing a COVID-19 infection, a coincidence? (a case report). Pan Afr Med J 2021;38:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.