Abstract
Recent studies indicate that humans can taste starch hydrolysis products (i.e. maltooligosaccharides; MOS). However, the structural specificity of oligosaccharides that elicit such perception is not known. This study investigated taste perception of pullulan-derived oligosaccharides (PDOS) that are structurally similar to MOS, but differ in that every third glycosidic linkage in PDOS is α-1,6, rather than α-1,4. Three food-grade PDOS stimuli were produced by limited-enzyme hydrolysis of pullulan. The resulting products were stimuli with degree of polymerization (DP) of 3, 6, and 9. Subjects discriminated all 3 stimuli from blanks at a significant level (P < 0.00001) in the absence of lactisole, a sweet taste inhibitor. In the presence of lactisole, the subjects could not detect DP 3 at a significant level (P > 0.05), but were able to detect DP 6 and 9 (P < 0.005), although the degree of detectability dropped significantly (P < 0.05). In a follow-up qualitative study, subjects made the target stimuli and glucose into 2 groups (glucose/DP 3 vs. DP 6/DP 9) and characterized both groups as mostly “sweet” with having different sweetness intensity. With lactisole, they described glucose and DP 3 as “taste like blank” (lactisole water) and found it challenging to describe DP 6 and 9 stimuli due to their subtle nature. These results suggest that taste perception of PDOS primarily depends on the sweet taste receptor, although they may elicit other sensory attributes; this is strikingly different from the reported taste of MOS. The potential impact of structural configuration on taste perception is further discussed.
Keywords: glycosidic linkage, oligosaccharide, pullulan, structural configuration, sweet, taste perception
Introduction
It has long been accepted that humans can taste simple sugars (mono and disaccharides) but not complex carbohydrates (oligo and polysaccharides). This notion is understandable given the large size, complex structure, and limited solubility of complex carbohydrates (Lim and Pullicin 2019). Starch, which is the most common form of carbohydrates consumed in the human diet (Huber and BeMiller 2017), is a prime example. They consist of thousands of anhydrous glucose subunits, and have limited solubility in water (Takeda and Hanashiro 2003). It is generally accepted that starches per se do not elicit a taste. However, oral digestion of dietary starches result in the production of smaller saccharides [maltopolysaccharides (MPS; degree of polymerization (DP) > 20), maltooligosaccharides (MOS; DP 3-20), and maltose (DP 2)] due to the activity of salivary α-amylase (Hoebler et al. 1998; Peyrot des Gachons and Breslin 2016; Lim and Pullicin 2019). Of these oral processing byproducts, MOS and maltose are relatively small and soluble in water.
Previous work in our lab has shown that humans can taste MOS of varying chain lengths (Lapis et al. 2016; Pullicin et al. 2017; Martin et al. 2023). Lapis et al. (2016) reported that subjects can taste MOS of average DP 7 and 14, but not MPS of average DP 44, and that MOS detection is independent of the canonical sweet taste receptor hT1R2/hT1R3. It was also reported that subjects described the taste of the detected stimuli as “starchy,” while the same panel described the taste of sweeteners, such as sucrose and sucralose, as sweet (Lapis et al. 2016). Subsequent work by Pullicin et al. (2017) corroborated the earlier findings and demonstrated that subjects are able to taste MOS stimuli of DP 3–4, 5–6, and 6–7 independent of the sweet taste receptor. A more recent study Martin et al. (2023) determined that humans can taste the entire range of MOS samples [DP 4–6 (average DP 5.2), 7–12 (average DP 9.8), and 14–21 (average DP 17)] and that the degrees of taste detection are generally similar across the 3 target samples. All of these studies used acarbose, an α-amylase inhibitor, in conjunction with the MOS stimuli to prevent oral hydrolysis of MOS during tasting.
The MOS stimuli tested in the previous studies were all linear, with their glucose subunits exclusively linked via α-1,4 glycosidic bonds (see Fig. 1A). A pertinent question emanating from the previous studies relates to the structural specificity of MOS to elicit “starchy” taste. There is only 1 published study directly investigating the question of structural specificity; that study showed, unlike their linear analogs, cyclic MOS (i.e. α-, β-, and γ-cyclodextrins with DP 6, 7, and 8, respectively) are ligands of the human sweet taste receptor (Martin and Lim 2022). That finding established the importance of MOS linearity to elicit “starchy” taste.
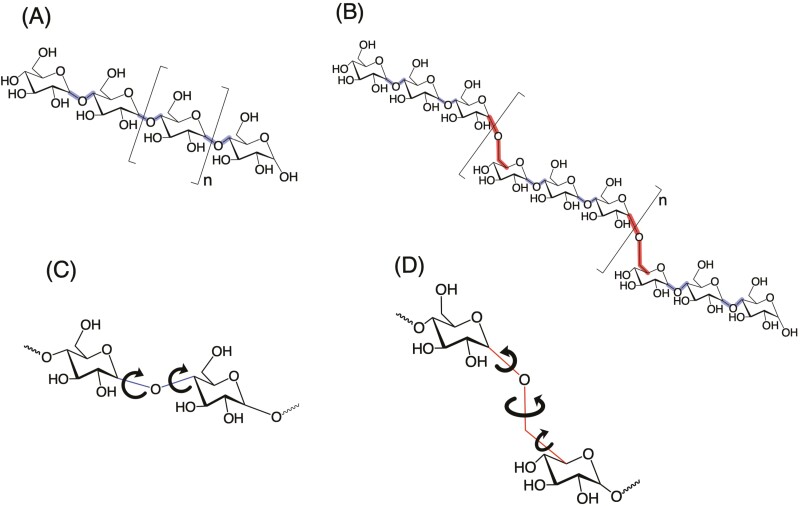
Fig. 1.
Structural representation of (A) linear MOS, (B) linear PDOS, (C) α-1,4 glycosidic linkage, and (D) α-1,6 glycosidic linkage. Linear MOS consists of glucose subunits linked via α-1,4 glycosidic bonds (shown in blue); PDOS consists of maltotriose units linked via α-1,6 glycosidic bonds (shown in red). Permissibly rotatable bonds linking glucose subunits are depicted with curved arrows.
The structural attributes of MOS necessary to elicit its starch-like taste were further tested in the present study; specifically, the sensory properties of oligosaccharides comprised of maltotriose units linked via α-1,6 glycosidic bonds were tested. These oligosaccharides are herein referred to as pullulan-derived oligosaccharides (PDOS; see Fig. 1B). Both MOS and PDOS are linear glucooligosaccharides (i.e. oligosaccharides comprised solely of glucose subunits). Critically, MOS and PDOS differ in that the number of rotatable bonds making up the bridges between 2 glucose subunits at α-1,4 versus α-1,6 linkages are different (i.e. 2 vs. 3, respectively; see Fig. 1C and D), thereby resulting in a difference in the spacing of glucose subunits involved in α-1,4 versus α-1,6 linkages and a greater flexibility in PDOS (Fig. 1D) as compared to MOS (Fig. 1C).
By necessity, the initial phase of this study focused on the development of a method to produce the target PDOS. This was achieved by limited pullulanase-catalyzed pullulan hydrolysis and subsequent chromatographic fractionation. Pullulan is a natural high molecular weight, linear, homo-polysaccharide comprised of repeating maltotriose units linked by α-1,6 glycosidic bonds (Singh et al. 2008; Cheng et al. 2011). Pullulanase specifically catalyzes the hydrolysis of α-1,6 linkages of pullulan (Hii et al. 2012). Thus, limited pullulanase-catalyzed pullulan hydrolysis produces a range of PDOS differing in the number of maltotriose units per molecule. The resulting PDOS mixture, the components of which differ with respect to DP, can be fractionated using adsorption chromatography.
The objective of this study was to investigate if the change in specific α-1,4 glycosidic linkages to α-1,6 glycosidic linkages within linear glucooligosaccharides impacts their taste perception. The answer to this question is important with respect to understanding the structural specificity of taste perception of MOS-like glucooligosaccharides. The target PDOS stimuli produced for this study were DP of 6 and 9; these have 1 and 2 α-1,6 glycosidic linkages, respectively. Additionally, PDOS DP 3, which was expected to have no α-1,6 glycosidic linkage, was also collected during the production process and included as a test stimulus. PDOS DP 3 is theoretically identical to MOS DP 3; the latter is reportedly a sweet taste receptor agonist (Pullicin et al. 2017, 2019). A series of psychophysical experiments was conducted to determine if the target PDOS stimuli could be tasted in the presence and absence of lactisole, a sweet taste inhibitor, and to further investigate the sensory attributes that contribute towards the taste perception of the stimuli.
Preparation and characterization of target stimuli
Preparation of PDOS DP 3, 6, and 9
Materials
All materials used for sample preparation were of food or pharmaceutical grade. Pullulan (NutriScience Innovations, LLC; Milford, CT) and pullulanase (EC 3.2.1.41; 2 U/ml, with 1 unit being that amount of enzyme that liberates 1.0 µmole of maltotriose, measured as glucose, from 1% pullulan solution per minute at pH 5.0 and 25℃; Creative Enzymes; Shirley, NY) were used as starting materials for the preparation of PDOS. ACS/USP-grade 100% ethanol (Pharmco-Aaper; Shelbyville, KT) and deionized (DI) water (18.2 Ω; purified using a Millipore Direct-Q 5 UV-R system) were used as solvents. Avicel PH-101 microcrystalline cellulose (Unicel MCC 101; Universal Preserv-A-Chem Inc.; Mebane, NC) was used as packing material for column chromatography.
Methods
Enzymatic hydrolysis and ethanol fractionation of pullulan.
Pullulan was enzymatically hydrolyzed to produce a mixture comprising relatively low molecular weight hydrolysis products (Wu et al. 2009). The hydrolysis medium contained pullulan (22% w/v) and pullulanase (2 U/ml) in DI water. This reaction mixture was incubated for 2 h at ambient temperature with constant stirring. After the 2 h incubation period, the reaction was terminated by adding 100% ethanol to the reaction mixture until a final ethanol concentration of 75% was reached. Termination of the reaction by the addition of ethanol resulted in a loss of soluble pullulanase activity, precipitation of the high molecular weight hydrolysis products, and enrichment of the supernatant with the desired low molecular weight PDOS (Miao et al. 2009; Talekar et al. 2013; Balto et al. 2016; Lu et al. 2018). The supernatant containing the PDOS mixture was centrifuged at 10,000 rpm for 15 min to further remove any suspended precipitate. The resulting supernatant was then reduced to ~10 ml using a rotary evaporator (Büchi Rotavapor R-300 Basic, Büchi Labortechnik AG) equipped with a 55°C water bath (Büchi B-300 Base) and a vacuum pump (Welch, Mt. Prospect, IL).
Separation of PDOS fractions using column chromatography.
Column chromatography was used to isolate the PDOS test stimuli (DP 3, 6, and 9) from the concentrated PDOS mixture. The procedure was adapted from Pullicin et al. (2017). A slurry made of 200 g microcrystalline cellulose (MCC) in 1 L 50% aqueous ethanol was gently poured into a glass column [73 mm I.D. × 305 mm L, with 1 L reservoir and coarse fritted disc (Synthware, Pleasant Prairie, WI)] that had been previously rinsed with 50% ethanol. The column was then immediately filled with an additional 1 L 50% ethanol and packed with the help of compressed air such that an eluate flow rate of 12 ml/min was achieved. The resulting height of the packed bed (MCC stationary phase) was about 17 cm. The packed column was then rinsed repeatedly with 75% ethanol until the eluate coming off the column was clear. The concentrated PDOS mixture was then gently loaded onto the top of the packed bed. Once the loaded sample seeped into the packed bed, a stepwise concentration gradient of aqueous ethanol was run through it in the following order: 1.5 L of 75% ethanol, 1.25 L of 65% ethanol, and 1.25 L of 60% ethanol. The eluates or fractions were then collected in 125 ml increments.
Thin layer chromatography.
The composition of the individual fractions was determined using 10 cm long 60 Å silica gel TLC plates (EMD Millipore; Billerica, MA). The solvent chamber for the thin layer chromatography (TLC) consisted of ethanol, water, and butanol in the ratio of 3.4:1.1:0.5, respectively. The developed plates were stained using the staining solution mentioned in Robyt and Mukerjea (1994). The stained plates were heated using a heat gun and visually examined; the spots where carbohydrate samples were present turned dark purple (Pullicin et al. 2017, 2018). The fractions found to contain indistinguishable PDOS compositions, based on the TLC analyses, were consolidated, and prepared for freeze drying.
Solvent removal and lyophilization.
Aqueous ethanol was removed from each PDOS fraction using the rotary evaporator. Once the ethanol was removed, the samples were solubilized in 100 ml DI water and rotary-evaporated again to ensure that there was no residual ethanol in the final product. This process was repeated twice. The rotary-evaporated samples were then lyophilized using a pharmaceutical freeze dryer (Harvest Right, Salt Lake City, UT). The final lyophilized products were stored in airtight glass containers at room temperature.
Characterization of PDOS DP 3, 6, and 9
Materials
The following chemical reagents were used for sample characterization: butyl alcohol, 1-napthol, and l-serine from Sigma-Aldrich (St. Louis, MO), sodium bicarbonate, sodium carbonate, and bicinchoninic acid disodium salt from Thermo Scientific (Rockford, IL), cupric sulphate pentahydrate from Avantor (Center Valley, PA), acetonitrile from Fisher Scientific (Fairlawn, NJ), sulfuric acid from EMD Millipore (Billerica, MA), 99.96% deuterium oxide (D2O) from Cambridge Isotope Laboratories (Tewksbury, MA), and maltose monohydrate from Spectrum Chemical Mfg. Corp. (New Brunswick, NJ). All chemicals were either ACS or ≥99% ReagentPlus grade.
Methods
High-performance liquid chromatography.
High-performance liquid chromatography (HPLC) chromatograms were used to determine the composition and purity of the PDOS fractions. HPLC was done using a Prominence UFLC-HPLC system (Shimadzu, Columbia, MD) which included a system controller (CBM-20A), autosampler (SIL-20A HT), degasser (DGU-20A3), solvent delivery system (LC-20AD), column oven (CTO-20A) kept at 40°C, and an evaporative light scattering detector (ELSD-LT II) kept at 60°C with a nitrogen gas pressure of 350 kPa. The columns used consisted of serially linked HILICpak VN-50G 4A guard and HILICpak VN-50 4D analytical column (Shodex, New York, NY). Aqueous acetonitrile with a concentration gradient of 70 to 50% was used as the mobile phase with a flow rate of 1 ml/min. The HPLC chromatograms were analyzed using the LCSolution software (Shimadzu, Columbia, MD).
Reducing ends assay.
Reducing end assays were performed to calculate the average DP of the prepared stimuli. The reagents for this assay were prepared fresh right before the analysis as mentioned in Garcia et al. (1993), and the procedure followed was according to the method described in Kongruang et al. (2004). Aqueous maltose solutions ranging in concentration from 0 to 50 µM were used to prepare the standard curves. The assay was performed in triplicate.
Proton nuclear magnetic resonance.
Nuclear magnetic resonance (NMR) analyses were used to verify that ethanol had been sufficiently removed from the prepared samples (Pullicin et al. 2017) and to quantify the α-1,4 to α-1,6 linkage ratios in the target stimuli (McIntyre and Vogel 1991; Hernandez-Tenorio and Giraldo-Estrada 2022). The test samples were solubilized in D2O and analyzed at 25℃ using a Bruker 700 MHz Avance III Spectrometer with a 5 mm proton/carbon cryoprobe with z-axis gradient. Bruker Topspin 4.1.1 software was used to process the spectra.
Results
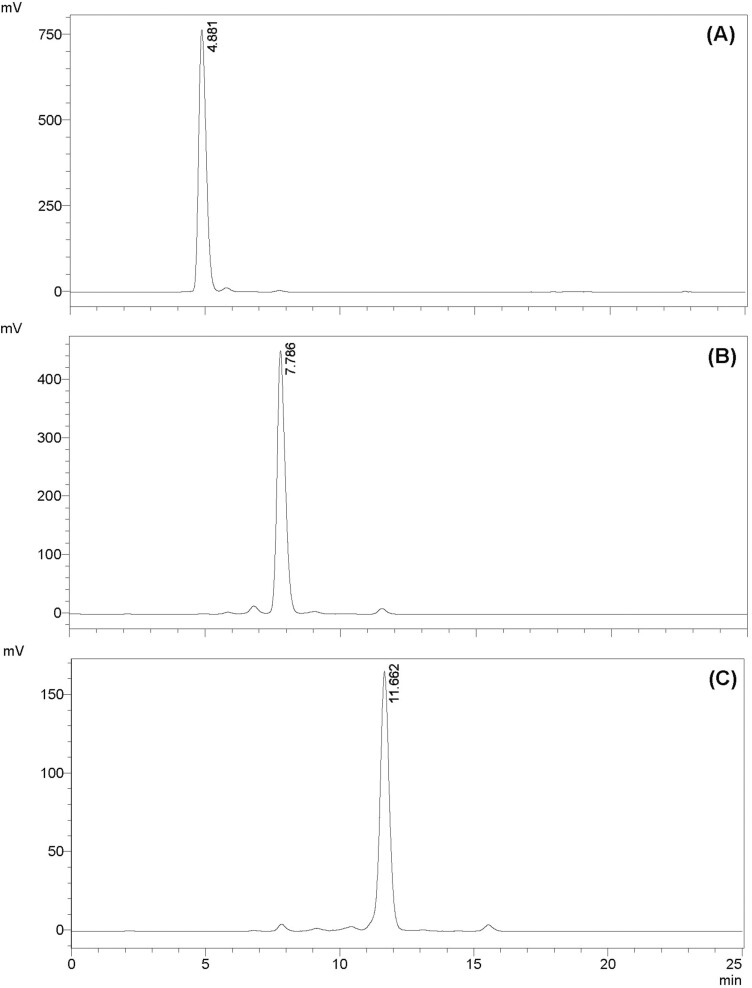
The PDOS-preparation technique described above resulted in 3 fractions: PDOS DP 3, 6, and 9. The DP values of these PDOS fractions, based on reducing ends per unit mass, were 2.77 ± 0.11 for DP 3, 6.28 ± 0.34 for DP 6, and 8.99 ± 0.35 for DP 9 (Table 1). The composition and purity of the PDOS fractions were determined from the HPLC chromatograms (see Fig. 2; retention peaks of PDOS DP 3, 6, and 9 are observed at 4.88 min, 7.78 min, and 11.66 min, respectively). NMR spectra confirmed the ratio of α-1,4 to α-1,6 glycosidic linkages (Table 1); the signals observed between 5.28 and 5.36 ppm (centered at 5.34 ppm) (Supplementary Fig. S1) characterized the α-1,4 glycosidic linkages (shown in blue in Fig. 1B), while the signal at 4.88 ppm (Supplementary Fig. S1) characterized the α-1,6 glycosidic linkages (shown in red in Fig. 1B). These results were consistent with those observed by Hernandez-Tenorio and Giraldo-Estrada (2022) and McIntyre and Vogel (1991) for pullulan samples. The concentration of residual ethanol in all fractions, characterized by a signal at 1.10 ppm in the NMR spectra (Pullicin et al. 2017; Hernandez-Tenorio and Giraldo-Estrada 2022) (see Supplementary Fig. S1), was less than 0.1%, which is lower than the oral detection limit (Mattes and DiMeglio 2001).
Table 1.
Chemical characterization of PDOS samples.
| Sample | Quantified DP value a,b | Linkage ratio (α-1,4:α-1,6) c |
|---|---|---|
| DP 3 | 2.77 ± 0.11 | 2:0.09 |
| DP 6 | 6.28 ± 0.34 | 4:1.11 |
| DP 9 | 8.99 ± 0.35 | 6:2.02 |
aValues are means of triplicates with corresponding standard deviations.
bDetermined using copper/BCA-based reducing end assay with maltose as standard.
cDetermined using NMR.
Fig. 2.
Chromatograms from HPLC-ELSD of PDOS samples (A) DP 3, (B) DP 6, and (C) DP 9. The chromatograms have a common x-axis representing the retention time in minutes and the y-axis as the signal intensity in millivolts. The numbers at the top of the peaks signify the retention time (min) of the sample.
Taste perception of PDOS DP 3, 6, and 9
Psychophysical study
Subjects
Twenty-eight individuals (16 female, 10 male, and 2 nonbinary; mean age ± SD = 34 ± 11.37) participated in the study. Eligibility criteria included (i) age between 18 and 60 years, (ii) nonsmokers, (iii) no taste or smell loss or other oral disorders, (iv) not pregnant, (v) had no oral lesions or piercings, (vi) no food allergies, and (vii) not on prescription pain or insulin medication. They were additionally asked to comply with the following restrictions: (i) no dental work within 48 h, (ii) no alcohol consumption within 12 h, (iii) no consumption of foods and beverages that are acidic or caffeinated and/or contain dairy within 4 h, (iv) no consumption of menthol-containing products, food or beverage of any kind except water within 1 h, and (v) no partaking in any strenuous physical activity within 1 h of their scheduled sessions. The experimental protocol was approved by the Oregon State University Institutional Review Board (#5373) and complied with the Declaration of Helsinki for Medical Research. Subjects agreed to the written informed consent and were paid for their time.
Stimuli
The 3 PDOS stimuli used in this experiment were prepared as aqueous solutions of 75 mM concentration. Prior reports (Lapis et al. 2016; Pullicin et al. 2017; Martin et al. 2023) confirmed that 75 mM MOS evoked a salient taste response in most humans. The stimuli were prepared with and without 1.4 mM lactisole (Domino Specialty Ingredients; Cypha lactisole; Yonkers, NY) to determine if their detection depended on the sweet taste receptor. Lactisole, an inverse agonist of the human sweet taste receptor, inhibits the taste perception of sweet-tasting compounds. Although lactisole itself is tasteless, it has been known to elicit a sweet “water-taste” when rinsed away from the receptor (Galindo-Cuspinera et al. 2006); data suggest this “water-taste” effect is attenuated in colder temperatures (Green and Nachtigal 2015; Lapis et al. 2016). Thus, all stimuli, blanks, and rinse water were presented at 10℃ to prevent a sweet “water-taste” (Green and Nachtigal 2015). To eliminate the confounding effect of taste potentially arising from oral hydrolysis products of the target stimuli, all solutions were prepared with acarbose (MuseChem; Fairfield, NJ), an α-amylase inhibitor. The concentration of acarbose used (5 mM) was below its taste detection limit and yet high enough to inhibit salivary α-amylase activity (Lim and Pullicin 2019). All stimuli were prepared at least the evening before the test session and placed in the refrigerator to allow for complete mutarotation of reducing end tautomers (Pangborn and Gee 1961). All samples were presented blind with random 3-digit codes.
Procedure
Each subject participated in a single session on a 1-on-1 basis in a psychophysics lab. At the beginning of the session, subjects were verbally instructed on the discrimination task that they were to perform.
Taste discrimination task.
The triangle test was used, where 1 target stimulus and 2 blanks were given during each trial. Before testing, subjects rinsed their mouth with DI water and spit into a sink; this rinse and spit process was repeated 3 times. Subjects then extended their tongue out of their mouth and held it steady between their lips. Next, the experimenter applied a sample by rolling a saturated cotton swab across the tip of the tongue (volume of stimulus absorbed by swab is ~0.25 ml). Subjects were instructed not to retract their tongue back into their mouth while tasting the sample in order to avoid touching the roof of their mouth. Once they had tasted the sample, the subjects immediately followed the rinse protocol once, as noted above, before tasting the next sample. After the 3 samples were tasted, subjects marked the odd sample on a paper ballot. To prevent potential olfactory cues, subjects wore nose clips while performing the task.
Practice/screening trials.
Three sets of practice/screening trials were performed. Each trial involved discriminating 75 mM maltose (DP 2) from water blanks. To advance to the test session, subjects were required to successfully discriminate at least 2 out of the 3 trials. To avoid fatigue and carry-over effects the subjects were given a 1 min break in between sets during which they rinsed their mouth thrice with cold DI water as described previously. Six out of the initial 34 participants did not pass this screener.
Test trials.
After a 5 min break, participants moved on to the test trials. The 3 stimuli were presented in 2 blocks, without and with lactisole in a counterbalanced block design. To avoid fatigue and carry-over effects the subjects were given a 1 min break between sets and a 5 min break between the 2 blocks, during which they followed the rinse and spit protocol 3 times.
Data analysis
The number of correct responses among all subjects for each stimulus and test condition was tallied and converted to a proportion correct (Pc). The proportions were further converted to corresponding d’ values by using precalculated d’ table for triangle test (see Ennis 1993). The d’ value is a measure of discriminability of signal (taste response from the stimulus) from noise (blank) in terms of the standard deviations of the distributions (Lawless and Heymann 2010). Note that a d’ value of zero is equivalent to chance performance (33% correct). To test if a given d’ value is significantly different from zero and further if there is a significant difference between 2 d’ values, Z-statistics were used (see Bi et al. 1997 for a step-by-step procedure). The alpha level was set at P < 0.05.
Results
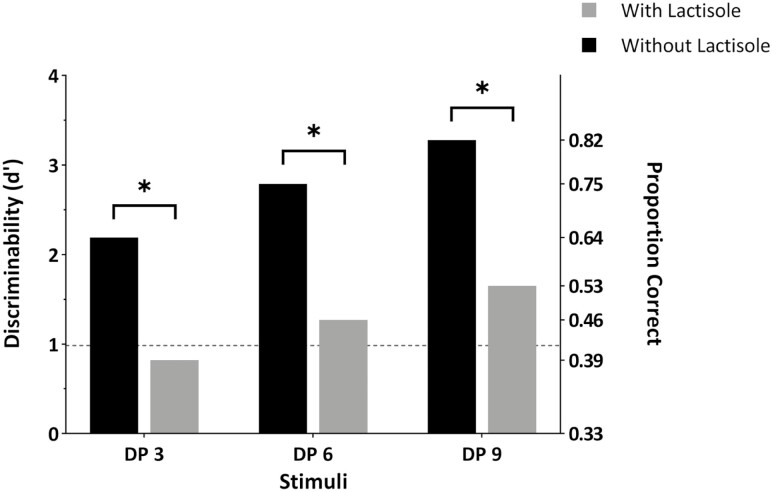
Figure 3 shows the detectability of the PDOS stimuli in the absence and presence of lactisole. The 3 stimuli were detectable at a significant level (P < 0.00001) in the absence of lactisole (see black bars). While detectability appeared to increase as a function of chain length, the differences in the d’ values between the stimuli were not statistically significant (Pc = 0.64, 0.75, and 0.82; d’ = 2.19, 2.79, and 3.28 for DP 3, 6, and 9, respectively; P > 0.05). This finding suggests that the stimuli at 75 mM concentration were detected at a similar degree.
Fig. 3.
Discriminability of PDOS DP 3, 6, and 9 from blanks. d’ values were determined from triangle tests performed under 2 conditions, with and without lactisole. The dashed horizontal line indicates the value above which the d’ values are statistically significant (P < 0.05). The asterisk (*) indicates statistically significant difference (P < 0.05) in d’ values for each target stimulus with and without lactisole.
In the presence of lactisole (see gray bars), the subjects could not discriminate PDOS DP 3 at a statistically significant level (Pc = 0.39; d’ = 0.82; P > 0.05), thus signifying that detection of PDOS DP 3 is dependent on the sweet taste receptor. In contrast, the subjects could still discriminate PDOS DP 6 (Pc = 0.46; d’ = 1.27; P < 0.005) and PDOS DP 9 (Pc = 0.53; d’ = 1.65; P < 0.005) from the blank, albeit their detectability being significantly reduced.
Qualitative study
The results of the initial study showed that the addition of lactisole significantly reduced the detection of PDOS DP 6 and 9, but that both stimuli were still detectable for some subjects in the presence of lactisole. These outcomes suggest that PDOS DP 6 and 9 are predominantly sweet but that they also elicit sensations other than sweetness. In order to determine the sensory qualities of the PDOS stimuli, a follow-up study was conducted. Subjects who correctly identified both PDOS DP 6 and 9 stimuli from blanks in the presence of lactisole (n = 7) were invited to participate in this study. Five of the 7 subjects responded to our invite.
Subjects
Five individuals (3 female and 2 male; mean age ± SD = 34 ± 7.92) participated in the study and were paid for their time. The physical and dietary restrictions were the same as section “Subjects.”
Stimuli
All 3 stimuli and blank were prepared as mentioned in section “Stimuli”. One exception was that the stimuli were presented at 150 mM concentration. In addition, 150 mM glucose solution was added as a sweet control. The higher concentration of the stimuli was used to elicit a more distinct sensation. For tests without lactisole, all samples and rinse water were presented at room temperature whereas samples with lactisole, along with the corresponding blank and rinse water, were presented at 10℃.
Procedure
The focus group study was conducted in 2 batches in a quiet conference room. Each batch had 3 and 2 participants, respectively and lasted for ~75 min. The samples were presented in 2 blocks, with and without lactisole. Within each block, subjects were asked to taste the stimuli in a random order by swabbing across the tip of the tongue with nose clips on. The subjects were asked to promptly note down the perceived sensory attributes of the tasted sample and were asked not to retract their tongue back in before writing down their notes. The subjects took a 1 min break and rinsed their mouth with the provided rinse water between samples. The subjects were encouraged to taste all stimuli multiple times over the course of the session. They were unaware of the identity of the stimuli. Once individual tasting was completed, they participated in a group discussion.
Results
The 5 subjects agreed that, in the absence of lactisole, all stimuli had a “sweet” taste. When asked, they made 2 groups based on similarity in sweetness intensity: glucose and PDOS DP 3 versus PDOS DP 6 and 9. Some subjects also mentioned that the PDOS DP 6 and 9 stimuli had a “starchy-like” or “thick taste” to it similar to either “potato-” or “rice-water.” Others described the samples as eliciting a “tingling” or “drying” sensation on the tongue reminiscent of “licking flour” or “dry residue on hand from cutting pumpkin.” These descriptors need to be considered with caution given the small sample size and the subjective nature of a focus group.
In the presence of lactisole, the subjects stated that glucose and PDOS DP 3 stimuli were similar to the blank reference and had an imperceptible taste or texture quality. Conversely, those that could detect the higher DP stimuli in the presence of lactisole noted that they had some sensations although they were hard to describe. When asked, they described them as being “maybe sweet,” “starchy,” and “slightly thick,” or being very similar to the reference but having a “drying” or “tingling” sensation. The sensations of “drying” or “tingling” could have originated from the rubbing of the tongue with cotton swabs. The varied oral perception of the PDOS DP 6 and 9 stimuli with lactisole led to inconsistent grouping. In most cases, glucose and PDOS DP 3 stimuli were grouped together, but were sometimes also paired with either the PDOS DP 6 or 9 stimuli.
Discussion
The aim of this study was to better understand the structural attributes of MOS that enable them to be tasted by humans. This aim was accomplished by investigating human taste perception of MOS-analogous PDOS and comparing these results with those reported previously for MOS (Lapis et al. 2016). PDOS and MOS differ only in that specific α-1,4 glycosidic linkages within MOS are replaced by α-1,6 glycosidic linkages within PDOS (see Fig. 1A and B).
Characterization of food-grade PDOS stimuli
The lack of commercially available short-chain PDOS made it imperative to produce them. This was achieved by limited enzyme (pullulanase)-catalyzed hydrolysis of food-grade pullulan followed by chromatographic fractionation of the resulting PDOS. The resulting samples were PDOS of DP 3, 6, and 9. PDOS DP 3 is equivalent to MOS DP 3; while PDOS DP 6 and 9 are analogous to MOS DP 6 and 9 with the exception that the maltotriose units within each molecule are linked by α-1,6 instead of α-1,4 glycosidic bonds (see Fig. 1A and B). The presented chromatograms combined with the reported DP values attest to the high purity of the preparations. We attribute the minor peaks in the chromatograms (see Fig. 2) to the incomplete specificity of pullulanase; while pullulanase is expected to catalyze the hydrolysis of the α-1,6 glycosidic linkages in pullulan, it has also been shown to hydrolyze α-1,4 glycosidic linkages to a limited extent (Hii et al. 2012). The products represented by these smaller peaks in the chromatograms make up less than 2% of the total fractions. These smaller peaks are also the likely source of the trivial amounts of α-1,6 glycosidic linkages in PDOS DP 3 (see Table 1).
PDOS DP 3 is sweet
The present data suggests that PDOS DP 3 is a ligand of the human sweet taste receptor, hT1R2/hT1R3. When tested in the absence of lactisole, PDOS DP 3 was discriminated from the blank at a significant level [d’ = 2.19 (64% correct responses); P < 0.00001]. However, in the presence of lactisole, the stimuli was not detectable at a significant level [d’ = 0.82 (39% correct responses); P > 0.05]. This result was further confirmed by the findings of the qualitative study; subjects grouped PDOS DP 3 with glucose and described them as “sweet,” but in the presence of lactisole, the subjects described the taste of glucose and PDOS DP 3 to be “like blank.” Overall, these results confirmed the previous report by Pullicin et al. (2017, 2019) showing that maltotriose (MOS DP 3), which is equivalent to PDOS DP 3, is a sweet tasting compound.
PDOS DP 6 and 9 are predominantly sweet
A notable finding of this study is that PDOS DP 6 and 9 are also ligands of the sweet taste receptor. Compared to the condition that did not have lactisole, the detectability of both PDOS DP 6 and 9 dropped significantly (d’ analysis, P < 0.05), when the sweet taste receptor was inhibited by lactisole (see Fig. 3). In addition, the subjects who participated in the qualitative study grouped PDOS DP 6 and 9 together and described them as primarily “sweet,” although their sweetness level was somewhat different from that of glucose and PDOS DP 3. Interestingly, PDOS DP 6 and 9 were still discriminable from the blank in the presence of lactisole at a significant level (P < 0.005). This result suggests that PDOS DP 6 and 9 may elicit sensations other than sweetness. During the qualitative study, certain attributes, mostly referring to texture-related terminologies, were brought up to describe these stimuli both in the presence and absence of lactisole. Nevertheless, the subjects found it difficult to clearly describe and agree upon the sensory attributes of these stimuli when presented with lactisole. Collectively, the findings of this study suggest that PDOS DP 6 and 9 are primarily sweet while they elicit other minor sensations that might be related to textural properties.
Structural configuration impacts oligosaccharide taste perception
The finding that PDOS DP 6 and 9 are primarily sweet is intriguing given that MOS with similar DP are perceived differently; the taste detection of MOS stimuli are not inhibited by lactisole and are described as “starchy” (Lapis et al. 2016). This brings up an interesting question of what causes such perceptual differences. To that end, it is worth comparing the structural similarities and differences between PDOS and MOS. First, both PDOS and MOS stimuli are solely comprised of glucose as their base monomer. Second, analogous PDOS and MOS have the same number of glucose units (e.g. MOS DP 6 and PDOS DP 6 are both comprised of 6 glucose units) and, accordingly, they have the same molecular weight (e.g. 990.86 g/mol for DP 6). Third, they are both linear molecules with a single reducing end (see Fig. 1A and B). What makes PDOS and MOS different is the configuration in terms of their glycosidic linkages: glucose units of MOS are linked solely via α-1,4 glycosidic bonds, whereas PDOS stimuli have an α-1,6 glycosidic bond at every third glucose subunit (see Fig. 1A vs. B). Importantly, this difference in the linkage configuration dictates the distinct spatial orientations of PDOS and MOS.
A structural consequence of substituting α-1,6 for α-1,4 glycosidic linkage is the incorporation of an additional permissibly-rotatable bond between the bridged glucose subunits (Fig. 1C vs. D). This results in a greater distance between the glucose subunits at α-1,6 glycosidic linkages compared to α-1,4 glycosidic linkages and considerably more rotational freedom at the 1,6 linkages; the latter, in turn, enhances molecular flexibility about α-1,6 compared to α-1,4 glycosidic linkages. The additional flexibility associated with α-1,6 glycosidic linkages tends to favor a more disordered conformation relative to an analogous α-1,4 glycosidic linkage. These differences clearly impact the solution properties of PDOS and MOS. MOS tend to form helical structures in solution (Naidoo and Kuttel, 2001; Hassan Khatami et al. 2021), while analogous PDOS tend to form random coils (Kato et al. 1982; Nishinari et al. 1991; Okada et al. 2002). Considering that maltotriose itself is a sweet receptor ligand (Pullicin et al. 2017, 2019), it seems plausible that the increased structural flexibility associated with α-1,6 glycosidic linkages improves the likelihood of at least 1 maltotriose subunit within the PDOS stimuli functioning as a sweet taste receptor ligand. The same structural arguments suggest that the enhanced flexibility at the α-1,6 glycosidic linkages serves to significantly decrease the association of PDOS DP 6 and 9 with the sensory mechanism responsible for the starch-like perception of analogous MOS [as reported in (Lapis et al. 2016)].
The oligosaccharide conformations that specifically serve to elicit tastes are not known and thus, at present, structural data must be interpreted at a very general level. Understanding how specific structural features dictate taste perception of glucooligosaccharides, like MOS and PDOS, will surely benefit from further experiments using alternative, well-defined, ligands. To the best of our knowledge, the only such study available thus far is the one that evaluated the taste perception of cyclic MOS, another glucooligosaccharides [i.e. cyclodextrins; (Martin and Lim 2022)]. In that study, it was shown that subjects could perceive cyclodextrins in the absence of lactisole (d’ = 1.77–2.39; P < 0.05) but were not able to detect them in the presence of lactisole (d’ = 0–0.88; P > 0.1), suggesting that they are, similar to PDOS, also ligands of the sweet taste receptor.
Conclusion
This study provides the first psychophysical and qualitative evidence of the impact of substituting α-1,6 glycosidic linkages on the taste perception of linear α-1,4 linked glucooligosaccharides (i.e. MOS). The experiments described herein required the development of a food-grade PDOS preparation protocol capable of producing highly purified PDOS samples of DP 3, 6, and 9 for human testing. The prepared PDOS samples of DP 6 and 9, containing 1 and 2 α-1,6 glycosidic linkages, respectively, were found to be primarily sweet compounds, with other slightly perceivable oral-sensory qualities. These results distinguish the sensory properties of PDOS from those previously reported for analogous MOS (Lapis et al. 2016); which points to the importance of molecular structure in dictating the ligand binding properties and subsequent human taste perception of linear glucooligosaccharides. An important aspect of this work is its contribution toward establishing a much-needed database covering the taste perception of structurally defined oligosaccharides.
Supplementary Material
Acknowledgments
The authors would like to thank Tucker Hamilton and Dr. Christopher M. Beaudry for their assistance with NMR.
Contributor Information
Shashwat Damani, Department of Food Science and Technology, Oregon State University, Corvallis, OR 97331, United States.
Michael H Penner, Department of Food Science and Technology, Oregon State University, Corvallis, OR 97331, United States.
Juyun Lim, Department of Food Science and Technology, Oregon State University, Corvallis, OR 97331, United States.
Author contributions
Shashwat Damani: investigation, formal analysis, visualization, and writing (original draft). Michael H. Penner: conceptualization, methodology, resources, and writing (review and editing). Juyun Lim: conceptualization, methodology, resources, supervision, and writing (review and editing).
Funding
This work was supported by National Institute on Deafness and Other Communication Disorders (NIDCD) Grant R01DC017555 “Oral Complex Carbohydrate Sensing” (to J.L.).
Conflict of interest
The authors declare no conflict of interest.
Data availability
Data available on request.
References
- Balto AS, Lapis TJ, Silver RK, Ferreira AJ, Beaudry CM, Lim J, Penner MH.. On the use of differential solubility in aqueous ethanol solutions to narrow the DP range of food-grade starch hydrolysis products. Food Chem. 2016:197(Pt A):872–880. 10.1016/j.foodchem.2015.10.120. [DOI] [PubMed] [Google Scholar]
- Bi J, Ennis DM, O’Mahony M.. How to estimate and use the variance of d’ from difference tests. J Sens Stud. 1997:12(2):87–104. 10.1111/j.1745-459X.1997.tb00055.x. [DOI] [Google Scholar]
- Cheng K-C, Demirci A, Catchmark JM.. Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol. 2011:92(1):29–44. 10.1007/s00253-011-3477-y. [DOI] [PubMed] [Google Scholar]
- Ennis DM. The power of sensory discrimination methods. J Sens Stud. 1993:8(4):353–370. 10.1111/j.1745-459X.1993.tb00225.x. [DOI] [Google Scholar]
- Galindo-Cuspinera V, Winnig M, Bufe B, Meyerhof W, Breslin PAS.. A TAS1R receptor-based explanation of sweet “water-taste.” Nature. 2006:441(7091):354–357. 10.1038/nature04765. [DOI] [PubMed] [Google Scholar]
- Garcia E, Johnston D, Whitaker JR, Shoemaker SP.. Assessment of endo-1,4-beta-d-glucanase activity by a rapid colorimetric assay using disodium 2,2’-bicinchoninate. J Food Biochem. 1993:17(3):135–145. 10.1111/j.1745-4514.1993.tb00463.x. [DOI] [Google Scholar]
- Green BG, Nachtigal D.. Temperature affects human sweet taste via at least two mechanisms. Chem Senses. 2015:40(6):391–399. 10.1093/chemse/bjv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan Khatami M, Barber W, de Haan HW.. Using geometric criteria to study helix-like structures produced in molecular dynamics simulations of single amylose chains in water. RSC Adv. 2021:11(20):11992–12002. 10.1039/D1RA00071C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Tenorio F, Giraldo-Estrada C.. Characterization and chemical modification of pullulan produced from a submerged culture of Aureobasidium pullulans ATCC 15233. 2022:114:107686. 10.1016/j.polymertesting.2022.107686. [DOI] [Google Scholar]
- Hii SL, Tan JS, Ling TC, Ariff AB.. Pullulanase: role in starch hydrolysis and potential industrial applications. Enzyme Res. 2012:2012:921362. 10.1155/2012/921362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebler C, Karinthi A, Devaux M-F, Guillon F, Gallant DJG, Bouchet B, Melegari C, Barry J-L.. Physical and chemical transformations of cereal food during oral digestion in human subjects. Br J Nutr. 1998:80(5):429–436. 10.1017/S0007114598001494. [DOI] [PubMed] [Google Scholar]
- Huber KC, BeMiller JN.. Carbohydrates. In: Damodaran S, Parkin KL, editors. Fennema’s food chemistry. 5th ed. Boca Raton: CRC Press; 2017. p. 91–170. [Google Scholar]
- Kato T, Okamoto T, Tokuya T, Takahashi A.. Solution properties and chain flexibility of pullulan in aqueous solution. Biopolymers. 1982:21(8):1623–1633. 10.1002/bip.360210812. [DOI] [Google Scholar]
- Kongruang S, Han MJ, Breton CIG, Penner MH.. Quantitative analysis of cellulose-reducing ends. Appl Biochem Biotechnol. 2004:113:213–232. 10.1385/ABAB:113:1-3:213. [DOI] [PubMed] [Google Scholar]
- Lapis TJ, Penner MH, Lim J.. Humans can taste glucose oligomers independent of the hT1R2/hT1R3 sweet taste receptor. Chem Senses. 2016:41(9):755–762. 10.1093/chemse/bjw088. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Heymann H.. Sensory evaluation of food: principles and practices. New York: Springer; 2010. 10.1007/978-1-4419-6488-5. [DOI] [Google Scholar]
- Lim J, Pullicin AJ.. Oral carbohydrate sensing: beyond sweet taste. Physiol Behav. 2019:202:14–25. 10.1016/j.physbeh.2019.01.021. [DOI] [PubMed] [Google Scholar]
- Lu Z-H, Belanger N, Donner E, Liu Q.. Debranching of pea starch using pullulanase and ultrasonication synergistically to enhance slowly digestible and resistant starch. Food Chem. 2018:268:533–541. 10.1016/j.foodchem.2018.06.115. [DOI] [PubMed] [Google Scholar]
- Martin LE, Andrewson TS, Penner MH, Lim J.. Taste detection of maltooligosaccharides with varying degrees of polymerization. J Agric Food Chem. 2023:71(17):6699–6705. 10.1021/acs.jafc.3c00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Lim J.. Taste perception of cyclic oligosaccharides: α, β, and γ cyclodextrins. Chem Senses. 2022:47:bjac006. 10.1093/chemse/bjac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD, DiMeglio D.. Ethanol perception and ingestion. Physiol Behav. 2001:72(1–2):217–229. 10.1016/S0031-9384(00)00397-8. [DOI] [PubMed] [Google Scholar]
- McIntyre DD, Vogel HJ.. Nuclear magnetic resonance studies of homopolysaccharides related to starch. Starch-Stärke 1991:43(2):69–76. 10.1002/star.19910430209. [DOI] [Google Scholar]
- Miao M, Jiang B, Zhang T.. Effect of pullulanase debranching and recrystallization on structure and digestibility of waxy maize starch. Carbohydr Polym. 2009:76(2):214–221. 10.1016/j.carbpol.2008.10.007. [DOI] [Google Scholar]
- Naidoo KJ, Kuttel M.. Water structure about the dimer and hexamer repeat units of amylose from molecular dynamics computer simulations. J Comput Chem. 2001:22(4):445–456. . [DOI] [Google Scholar]
- Nishinari K, Kohyama K, Williams PA, Phillips GO, Burchard W, Ogino K.. Solution properties of pullulan. Macromolecules. 1991:24(20):5590–5593. 10.1021/ma00020a017. [DOI] [Google Scholar]
- Okada R, Matsukawa S, Watanabe T.. Hydration structure and dynamics in pullulan aqueous solution based on 1H NMR relaxation time. J Mol Struct. 2002:602-603:473–483. 10.1016/S0022-2860(01)00728-1. [DOI] [Google Scholar]
- Pangborn RM, Gee SC.. Relative sweetness of alpha- and beta-forms of selected sugars. Nature. 1961:191(4790):810–811. 10.1038/191811a0. [DOI] [PubMed] [Google Scholar]
- Peyrot des Gachons C, Breslin PAS.. Salivary amylase: digestion and metabolic syndrome. Curr Diab Rep. 2016:16(10):102. 10.1007/s11892-016-0794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullicin AJ, Ferreira AJ, Beaudry CM, Lim J, Penner MH.. Preparation and characterization of isolated low degree of polymerization food-grade maltooligosaccharides. Food Chem. 2018:246:115–120. 10.1016/j.foodchem.2017.10.039. [DOI] [PubMed] [Google Scholar]
- Pullicin AJ, Penner MH, Lim J.. Human taste detection of glucose oligomers with low degree of polymerization. PLoS One. 2017:12(8):e0183008. 10.1371/journal.pone.0183008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullicin AJ, Penner MH, Lim J.. The sweet taste of acarbose and maltotriose: relative detection and underlying mechanism. Chem Senses. 2019:44(2):123–128. 10.1093/chemse/bjy081. [DOI] [PubMed] [Google Scholar]
- Robyt JF, Mukerjea R.. Separation and quantitative determination of nanogram quantities of maltodextrins and isomaltodextrins by thin-layer chromatography. Carbohydr Res. 1994:251:187–202. 10.1016/0008-6215(94)84285-X. [DOI] [Google Scholar]
- Singh RS, Saini GK, Kennedy JF.. Pullulan: microbial sources, production and applications. Carbohydr Polym. 2008:73(4):515–531. 10.1016/j.carbpol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Hanashiro I.. Examination of the structure of amylose and amylopectin by fluorescent labeling of the reducing terminal. J Appl Glycosci. 2003:50(2):163–166. 10.5458/jag.50.163. [DOI] [Google Scholar]
- Talekar S, Pandharbale A, Ladole M, Nadar S, Mulla M, Japhalekar K, Pattankude K, Arage D.. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-CLEAs): a tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour Technol. 2013:147:269–275. 10.1016/j.biortech.2013.08.035. [DOI] [PubMed] [Google Scholar]
- Wu S, Chen H, Tong Q, Xu X, Jin Z.. Preparation of maltotriose by hydrolyzing of pullulan with pullulanase. Eur Food Res Technol. 2009:229(5):821–824. 10.1007/s00217-009-1118-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request.