Abstract
Clinical features applicable to the entire spectrum of viral meningitis are limited, and prognostic factors for adverse outcomes are undetermined.
This nationwide population-based prospective cohort study included all adults with presumed and microbiologically confirmed viral meningitis in Denmark from 2015 until 2020. Prognostic factors for an unfavourable outcome (Glasgow Outcome Scale score of 1–4) 30 days after discharge were examined by modified Poisson regression.
In total, 1066 episodes of viral meningitis were included, yielding a mean annual incidence of 4.7 episodes per 100 000 persons. Pathogens were enteroviruses in 419/1066 (39%), herpes simplex virus type 2 in 171/1066 (16%), varicella-zoster virus in 162/1066 (15%), miscellaneous viruses in 31/1066 (3%) and remained unidentified in 283/1066 (27%). The median age was 33 years (IQR 27–44), and 576/1066 (54%) were females. In herpes simplex virus type 2 meningitis, 131/171 (77%) were females. Immunosuppression [32/162 (20%)] and shingles [90/149 (60%)] were frequent in varicella-zoster virus meningitis. The triad of headache, neck stiffness and hyperacusis or photophobia was present in 264/960 (28%). The median time until lumbar puncture was 3.0 h (IQR 1.3–7.1), and the median CSF leucocyte count was 160 cells/µl (IQR 60–358). The outcome was unfavourable in 216/1055 (20%) 30 days after discharge. Using unidentified pathogen as the reference, the adjusted relative risk of an unfavourable outcome was 1.34 (95% CI 0.95–1.88) for enteroviruses, 1.55 (95% CI 1.00–2.41) for herpes simplex virus type 2, 1.51 (95% CI 0.98–2.33) for varicella-zoster virus and 1.37 (95% CI 0.61–3.05) for miscellaneous viruses. The adjusted relative risk of an unfavourable outcome was 1.34 (95% CI 1.03–1.75) for females. Timing of acyclovir or valacyclovir was not associated with the outcome in meningitis caused by herpes simplex virus type 2 or varicella-zoster virus.
In summary, the outcome of viral meningitis was similar among patients with different aetiologies, including those with presumed viral meningitis but without an identified pathogen. Females had an increased risk of an unfavourable outcome. Early antiviral treatment was not associated with an improved outcome in meningitis caused by herpes simplex virus type 2 or varicella-zoster virus.
Keywords: virology, aseptic meningitis, herpesviridae, toscana virus, tick-borne encephalitis
In a nationwide study of 1066 Danish adults with viral meningitis, Petersen et al. report that incomplete recovery persists in one in five patients 30 days after discharge. Female patients in particular have an increased risk of an unfavourable outcome, whereas the type of virus is not associated with the prognosis.
Introduction
Viruses are the leading cause of meningitis in adults in the western world, and enteroviruses (EVs), herpes simplex virus type 2 (HSV-2) and varicella-zoster virus (VZV) constitute the most common pathogens.1-3 The aetiology, however, remains unidentified in 50–60% of patients with presumed viral meningitis.4,5 Although viral meningitis is often considered a benign disease, incomplete recovery is observed in a substantial proportion of patients.1,6-9 In addition, the prognosis of presumed viral meningitis without an identified pathogen is unclear, which may cause concern among patients and physicians and lead to unnecessary examinations and prolonged treatment and hospitalization.1 Moreover, the benefit of antiviral treatment with acyclovir in meningitis caused by HSV-2 or VZV is unproven. As most previous studies on viral meningitis have been restricted to small study populations, selected pathogens or laboratory data incapable of discriminating between meningitis and encephalitis, clinical data applicable to the entire spectrum of the disease are limited. Here, we present clinical features and prognostic factors of all adults hospitalized for viral meningitis with and without an identified pathogen in Denmark from 2015 until 2020.
Materials and methods
Design, setting and participants
This nationwide population-based prospective observational cohort study was based on the Danish Study Group of Infections of the Brain (DASGIB) database. The DASGIB database has previously been described and contains data on prospectively included episodes of CNS infections hospitalized at the departments of infectious diseases in Denmark since 1 January 2015.10 Viral meningitis was defined as a clinical presentation consistent with viral meningitis (e.g. headache, neck stiffness, hyperacusis or photophobia, fever) combined with either: (i) detected viral DNA/RNA in the CSF; (ii) CSF leucocyte count >10 cells/µl and positive intrathecal synthesis of antibodies specific for herpes simplex virus type 1 (HSV-1), HSV-2 or VZV; (iii) CSF leucocyte count >10 cells/µl and detected DNA from HSV-1, HSV-2 or VZV from skin lesions or positive serology of a neurotropic virus; or (iv) CSF leucocyte count >10 cells/µl and no other diagnosis considered more likely at last follow up (i.e. categorized as presumed viral meningitis without an identified pathogen). In the present study, we included all episodes of viral meningitis in adults (≥18 years) in the DASGIB database hospitalized between 1 January 2015 and 31 December 2019. Patients with encephalitis (fulfilment of the diagnostic criteria for infectious encephalitis proposed by the International Encephalitis Consortium11) were not included, and patients with herpes zoster oticus were excluded. We have previously used the DASGIB database for studies on HSV-2 meningitis12 and enteroviral meningitis13 separately. Eleven episodes of enteroviral meningitis in patients aged 15–18 years included in the study on enteroviral meningitis were not included in the present study. Conversely, 11 episodes of enteroviral meningitis were retrospectively identified through quality control of the database and only included in the present study. The DASGIB database was approved by the Danish Health and Medicines Authority (record nos. 3-3013-2579/1 and 3-3013-3168/1). Danish legislation does not require consent from patients in this type of study.
Variables
Data on clinical features were obtained from medical records during hospitalization and registered in an online database using the Research Electronic Data Capture (REDCap) software. Immunosuppression was alcohol abuse, intravenous substance abuse, organ transplantation, cancer, diabetes mellitus, asplenia, HIV infection, primary immunodeficiency, prednisolone >7.5 mg/day or other immunosuppressive therapy.
Outcomes
Outcomes were categorized using the Glasgow Outcome Scale (GOS) (1, death; 2, vegetative state; 3, severe disability; 4, moderate disability; 5, good recovery) and assessed at discharge, and at follow-up visits 30, 90 and 180 days after discharge. In case of missing values, the last observation was carried forward if patients had a good recovery (GOS score of 5) at the most recent contact. An unfavourable outcome was defined as a GOS score of 1–4.
Statistical methods
Mean and standard deviation (SD) or median and interquartile range (IQR) were reported for quantitative variables. Counts and percentages were reported for categorical variables. Data were compared using the χ2 test, Fisher's exact test or a Mann-Whitney U-test. Annual incidences were calculated by dividing the number of episodes of viral meningitis by the total adult Danish population of each study year. Population data were obtained from Statistics Denmark.14 Multiple ln-linear regression was used to assess associations between brain imaging prior to lumbar puncture (yes, no) and admission directly to a department of infectious diseases (yes, no) and time from admission until lumbar puncture (ln-hours). A referral diagnosis of CNS infection (yes, no) was included as a covariable. To obtain easily interpretable parameters of differences in the probability of an unfavourable outcome 30 days after discharge between potential prognostic factors, a modified Poisson regression was used to estimate crude and adjusted relative risks (RRs) with 95% confidence intervals (95% CI).15 Potential prognostic factors and their classifications were prespecified and based on general knowledge and previous research,16,17 and constituted age (18–30, 31–50, ≥51 years), sex (male, female), immunosuppression (yes, no), duration of symptoms until admission (0–1, ≥2 days), the triad of headache, neck stiffness and hyperacusis or photophobia (yes, no), CSF leucocyte count (0–100, 101–500, ≥501 cells/µl), CSF protein (0.0–0.5, 0.6–1.0, ≥1.1 g/l), aetiology (unidentified pathogen, EVs, HSV-2, VZV, miscellaneous viruses) and treatment with dexamethasone for acute bacterial meningitis (yes, no). The analysis was repeated post hoc stratified according to aetiology, except for miscellaneous viruses due to a limited number of episodes. As the occupational dimension of the GOS can most reliably be assessed in patients with work or study before the event,18 a subgroup analysis was done in patients with premorbid full-time occupations. A subgroup analysis using only GOS scores that had not been carried forward was also done. Modified Poisson regression was used to estimate RRs of an unfavourable outcome 30 days after discharge for time until treatment with acyclovir or valacyclovir (≤8, 8–16, ≥17 h from admission) in meningitis caused by HSV-2 or VZV. The analysis was adjusted for all previously described factors as well as aetiology (HSV-2, VZV). Post hoc, patients were stratified according to immune status, and associations between time until acyclovir or valacyclovir and outcome were examined with χ2 test or Fisher's exact test. A P-value <0.05 was considered statistically significant, except in comparisons of signs and symptoms among aetiologies where a Bonferroni adjusted significance level of 0.005 was used. SAS Enterprise Guide v.7.11 was used for all statistical analyses.
Data availability
Data are only available with permission from the Danish health and legal authorities.
Results
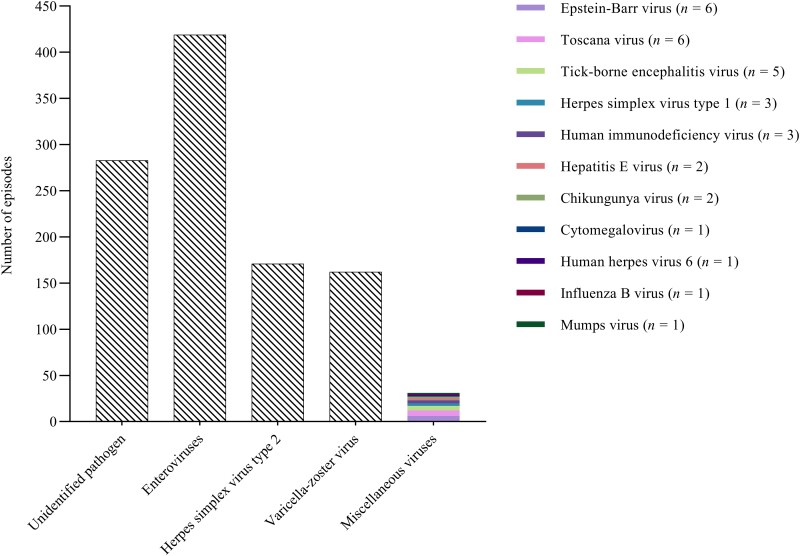
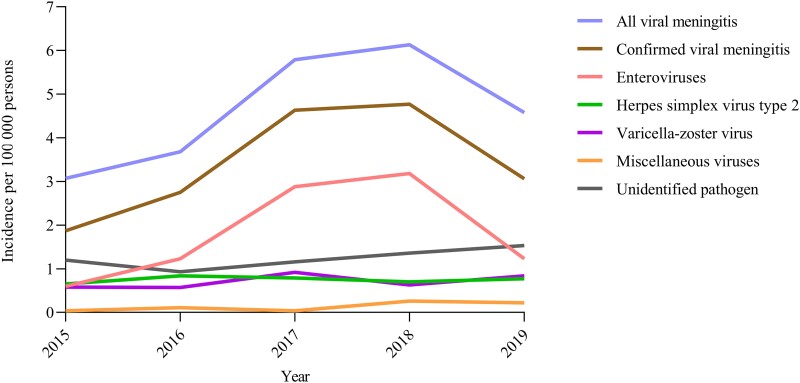
A total of 1120 episodes of presumed and microbiologically confirmed viral meningitis in adults were identified in the DASGIB database (Supplementary Fig. 1). Next, episodes with herpes zoster oticus (n = 54) were excluded, leaving 1066 episodes of viral meningitis in 1045 individuals for further analyses. EVs were detected in 419 (39%) of 1066 episodes, HSV-2 in 171 (16%) of 1066 episodes and VZV in 162 (15%) of 1066 episodes (Fig. 1). Eleven miscellaneous viruses were detected in 31 (3%) of 1066 episodes, whereas a pathogen was not identified in the remaining 283 (27%) of 1066 episodes. In the 5-year study period, the total mean annual incidence of presumed and microbiologically confirmed viral meningitis was 4.7 episodes (SD 1.3) per 100 000 persons, with an annual variation from 3.1 to 6.1 episodes due to fluctuation in episodes of enteroviral meningitis (Fig. 2).
Figure 1.
Aetiologies of viral meningitis in adults.
Figure 2.
Annual incidences of viral meningitis in adults.
The referral diagnosis was CNS infection in 435 (47%) of 917 episodes, and 474 (45%) of 1065 episodes were admitted directly to a department of infectious diseases (Table 1). The median age was 33 years (IQR 27–44) in the total study population and 46 years (IQR 27–69) in VZV meningitis (Table 2). Females constituted 576 (54%) of all 1066 episodes and 131 (77%) of 171 episodes of HSV-2 meningitis. Immunosuppression was present in 83 (8%) of all 1066 episodes, 32 (20%) of 162 episodes of VZV meningitis and 6 (19%) of 31 episodes of meningitis caused by miscellaneous viruses. In enteroviral meningitis, prodromal gastrointestinal or respiratory symptoms were reported in 66 (17%) of 385 episodes and 60 (16%) of 384 episodes, respectively. In viral meningitis of all other causes, prodromal gastrointestinal or respiratory symptoms were reported in 67 (11%) of 606 episodes and 81 (13%) of 601 episodes, respectively. Shingles were present in 90 (60%) of 149 episodes of VZV meningitis. At admission, headache was reported in 1005 (95%) of 1061 episodes, a history of fever in 691 (71%) of 971 episodes and hyperacusis or photophobia in 660 (67%) of 987 episodes. Neck stiffness was found in 371 (36%) of 1028 episodes and was most frequent in HSV-2 meningitis [92 (57%) of 162 episodes]. The triad of headache, neck stiffness and hyperacusis or photophobia was present in 264 (28%) of 960 episodes. At least two of four classic signs and symptoms of viral meningitis (headache, neck stiffness, hyperacusis or photophobia and a history of fever or measured fever) were present in 835 (87%) of 955 episodes and were less frequent in VZV meningitis [105 (74%) of 142 episodes] than in enteroviral meningitis [361 (94%) of 383 episodes; P < 0.001] and HSV-2 meningitis [138 (92%) of 150 episodes; P < 0.001]. At least two classic signs and symptoms were also less frequent in meningitis without an identified pathogen [211 (82%) of 257 episodes] than in enteroviral meningitis (P < 0.001) and HSV-2 meningitis (P = 0.003).
Table 1.
Management and treatment of adults with viral meningitis
| Management and treatment | All viral meningitis | Confirmed viral meningitis | EVs | HSV-2 | VSV | Miscellaneous viruses | Unidentified pathogen |
|---|---|---|---|---|---|---|---|
| n = 1066 | n = 783 | n = 419 | n = 171 | n = 162 | n = 31 | n = 283 | |
| CNS infection as the referral diagnosis | 435/917 (47) | 354/703 (50) | 220/401 (55) | 82/149 (55) | 42/126 (33) | 10/27 (37) | 81/214 (38) |
| Direct admission to a department of ID | 474/1065 (45) | 372/782 (48) | 221/419 (53) | 85/170 (50) | 63/162 (39) | 13/31 (42) | 102/283 (36) |
| Time until lumbar puncture, h | 3.0 (1.3–7.1) | 2.7 (1.2–6.3) | 2.2 (1.0–4.7) | 1.9 (1.0–4.7) | 6.2 (2.1–17.8) | 4.4 (2.0–15.9) | 4.3 (2.0–11.0) |
| CSF culture | 985/1066 (92) | 732/783 (93) | 400/419 (95) | 155/171 (91) | 148/162 (91) | 29/31 (94) | 253/283 (89) |
| CSF PCR for EVs/HSV/VZV | 1054/1066 (99) | 779/783 (99) | 419/419 (100) | 169/171 (99) | 162/162 (100) | 29/31 (94) | 275/283 (97) |
| ITS of antibodies for HSV/VZV | 240/1066 (23) | 167/783 (21) | 74/419 (18) | 41/171 (24) | 43/162 (27) | 9/31 (29) | 73/283 (26) |
| Blood culture | 781/1055 (74) | 591/775 (76) | 344/419 (82) | 135/170 (79) | 89/157 (57) | 23/29 (79) | 190/280 (68) |
| Brain imaging | 544/1066 (51) | 356/783 (45) | 168/419 (40) | 69/171 (40) | 100/162 (62) | 19/31 (61) | 188/283 (66) |
| Brain imaging prior to lumbar puncture | 408/1058 (39) | 270/780 (35) | 142/418 (34) | 48/171 (28) | 69/161 (43) | 11/30 (37) | 138/278 (50) |
| Acyclovir or valacyclovir | 743/1060 (70) | 552/782 (71) | 215/418 (51) | 163/171 (95) | 158/162 (98) | 16/31 (52) | 191/278 (69) |
| Antibiotics for bacterial meningitis | 504/1058 (48) | 365/776 (47) | 212/415 (51) | 96/171 (56) | 44/161 (27) | 13/29 (45) | 139/281 (49) |
| Dexamethasone for bacterial meningitis | 380/1062 (36) | 283/780 (36) | 168/418 (40) | 78/171 (46) | 27/162 (17) | 10/29 (34) | 97/282 (34) |
| Length of hospitalization, days | 3 (1–5) | 2 (1–4) | 2 (1–2) | 3 (2–6) | 5 (3–11) | 5 (3–8) | 4 (2–6) |
Quantitative data are presented as median (IQR) and categorical data are presented as n/N (%). ID = infectious diseases; ITS = intrathecal synthesis.
Table 2.
Clinical features of adults with viral meningitis
| Clinical features | All viral meningitisa | Confirmed viral meningitis | EVs | HSV-2 | VZV | Miscellaneous viruses | Unidentified pathogen |
|---|---|---|---|---|---|---|---|
| n = 1066 | n = 783 | n = 419 | n = 171 | n = 162 | n = 31 | n = 283 | |
| Age, years | 33 (27–44) | 33 (27–43) | 32 (28–35) | 36 (27–49) | 46 (27–69) | 41 (27–51) | 33 (26–46) |
| Sex, female | 576/1066 (54) | 419/783 (54) | 195/419 (47) | 131/171 (77) | 81/162 (50) | 12/31 (39) | 157/283 (55) |
| Full-time occupation | 852/1023 (83) | 630/747 (84) | 380/405 (94) | 128/162 (79) | 95/151 (63) | 27/29 (93) | 222/276 (80) |
| Immunosuppression | 83/1066 (8) | 62/783 (8) | 11/419 (3) | 13/171 (8) | 32/162 (20) | 6/31 (19) | 21/283 (7) |
| Duration of symptoms, days | 2 (1–5) | 2 (1–4) | 2 (1–3) | 1 (1–3) | 4 (2–6) | 5 (3–14) | 3 (1–6) |
| Prodromal gastrointestinal symptoms | 133/991 (13) | 96/722 (13) | 66/385 (17) | 13/158 (8) | 12/152 (8) | 5/27 (19) | 37/269 (14) |
| Prodromal airway symptoms | 141/985 (14) | 94/718 (13) | 60/384 (16) | 18/157 (11) | 15/150 (10) | 1/27 (4) | 47/267 (18) |
| Shingles | 104/897 (12) | 100/670 (15) | 4/350 (1) | 6/145 (4) | 90/149 (60) | 0/26 (0) | 4/227 (2) |
| GCS score <15b | 65/1029 (6) | 40/755 (5) | 13/401 (3) | 8/168 (5) | 19/157 (12) | 0/29 (0) | 25/274 (9) |
| History of fever | 691/971 (71) | 522/707 (74) | 308/379 (81) | 113/153 (74) | 77/146 (53) | 24/29 (83) | 169/264 (64) |
| Temperature ≥38°C | 432/1031 (42) | 337/763 (44) | 198/409 (48) | 75/167 (45) | 51/159 (32) | 13/28 (46) | 95/268 (35) |
| Headache | 1005/1061 (95) | 740/780 (95) | 414/418 (99) | 161/170 (95) | 139/162 (86) | 26/30 (87) | 265/281 (94) |
| Hyperacusis or photophobia | 660/987 (67) | 508/721 (70) | 303/394 (77) | 120/157 (76) | 73/146 (50) | 12/24 (50) | 152/266 (57) |
| Neck stiffness | 371/1028 (36) | 299/755 (40) | 156/408 (38) | 92/162 (57) | 42/156 (27) | 9/29 (31) | 72/273 (26) |
| Triad of signs and symptomsc | 264/960 (28) | 215/702 (31) | 118/385 (31) | 68/151 (45) | 24/143 (17) | 5/23 (22) | 49/258 (19) |
| ≥2 signs and symptomsd | 835/955 (87) | 624/698 (89) | 361/383 (94) | 138/150 (92) | 105/142 (74) | 20/23 (87) | 211/257 (82) |
| Blood leucocytes, cells × 109/l | 8.3 (6.6–10.4) | 8.2 (6.6–10.1) | 8.5 (6.9–10.1) | 8.4 (6.9–10.5) | 7.7 (6.2–9.5) | 8.1 (6.4–10.8) | 8.8 (6.6–11.2) |
| C-reactive protein, mg/l | 5 (2–15) | 5 (2–13) | 8 (3–17) | 3 (1–6) | 3 (1–8) | 6 (3–30) | 4 (1–25) |
| CSF leucocyte count, cells/µl | 160 (60–358) | 180 (68–417) | 135 (59–278) | 374 (162–670) | 233 (76–443) | 104 (35–296) | 117 (42–234) |
| CSF neutrophil percentage | 7 (1–31) | 8 (1–30) | 24 (6–55) | 3 (1–9) | 1 (0–4) | 4 (0–19) | 4 (0–33) |
| CSF to blood glucose ratio | 0.55 (0.48–0.62) | 0.54 (0.48–0.61) | 0.57 (0.51–0.62) | 0.52 (0.45–0.57) | 0.50 (0.45–0.57) | 0.54 (0.48–0.61) | 0.58 (0.52–0.64) |
| CSF lactate, mmol/l | 2.3 (2.0–2.8) | 2.4 (2.1–2.9) | 2.2 (2.0–2.6) | 3.1 (2.6–3.9) | 2.5 (2.2–3.0) | 2.3 (1.8–2.9) | 2.0 (1.7–2.4) |
| CSF protein, g/l | 0.70 (0.50–1.00) | 0.73 (0.51–1.07) | 0.60 (0.48–0.78) | 1.10 (0.78–1.50) | 1.00 (0.67–1.49) | 0.80 (0.50–1.38) | 0.59 (0.44–0.82) |
Quantitative data are presented as median (IQR) and categorical data are presented as n/N (%).
Including presumed viral meningitis without an identified pathogen.
Glasgow Coma Scale (GCS) score <15 for <24 h.
Triad of headache, neck stiffness and hyperacusis or photophobia.
Signs and symptoms were headache, neck stiffness, hyperacusis or photophobia and a history of fever or measured fever.
The median c-reactive protein was 5 mg/l (IQR 2–15) at admission, and 1010 (97%) of 1036 episodes had a c-reactive protein <100 mg/l (Table 2). The median CSF leucocyte count was 160 cells/µl (IQR 60–358) in the total population and 374 cells/µl (IQR 162–670) in HSV-2 meningitis (Supplementary Fig. 2). Among 38 patients with a CSF leucocyte count ≥1000 cells/µl, meningitis was caused by HSV-2 (n = 20), EVs (n = 11), VZV (n = 3), unidentified pathogen (n = 3) and Toscana virus (n = 1). The median CSF neutrophil percentage was 7 (IQR 1–31) in the total study population and 24 (IQR 6–55) in enteroviral meningitis (Supplementary Fig. 2).
Brain imaging was done in 544 (51%) of 1066 episodes during admission, of which 16 (3%) had new intracranial pathological findings (Table 1). The radiological diagnoses comprised benign brain tumours (n = 6), brain cysts (n = 5), brain infarction (n = 3), brain haemorrhage (n = 1) and brain oedema (n = 1). The median time from admission until brain imaging was 2.8 h (IQR 1.5–7.5). Brain imaging preceded lumbar puncture in 408 (39%) of 1058 episodes. A lumbar puncture was done in all episodes. The median time from admission until lumbar puncture was 3.0 h (IQR 1.3–7.1). The median time from admission until lumbar puncture was shorter in episodes admitted directly to a department of infectious diseases [2.0 h (IQR 0.9–4.7)] than in those admitted elsewhere [3.9 h (IQR 1.8–11.2); P < 0.001] and longer in episodes where brain imaging preceded lumbar puncture [6.2 h (IQR 3.6–15.2)] than in those where it did not [1.7 h (IQR 0.9–3.5); P < 0.001]. In multiple ln-linear regression, admission directly to a department of infectious diseases was associated with a 28% (95% CI: 16–39) decrease in time to lumbar puncture, whereas brain imaging prior to lumbar puncture was associated with a 184% (95% CI: 140–235) increase.
At least one dose of acyclovir or valacyclovir was administered in 743 (70%) of 1060 episodes (Table 1). In meningitis caused by HSV-2 or VZV, acyclovir or valacyclovir was administered in 321 (96%) of 333 episodes at a median time from admission of 6.4 h (IQR 2.7–18.0) and with a median duration of treatment of 10 days (IQR 7–14). The route of administration was exclusively oral in 35 (11%) of 321 episodes, exclusively intravenous in 45 (14%) of 321 episodes and a combination of oral and intravenous in 241 (75%) of 321 episodes. The median length of hospitalization was 3 days (IQR 1–5), and within 30 days of discharge, 93 (9%) of 1037 episodes were readmitted. Causes of readmission were headache (n = 38, 41%), post-dural puncture headache (n = 21, 23%), non-CNS infections (n = 8, 9%), other neurological symptoms (n = 7, 8%) and miscellaneous (n = 19, 20%).
An unfavourable outcome was observed in 216 (20%) of 1055 episodes 30 days after discharge, of which only three episodes had a GOS score of <4 (Table 3). At 180 days after discharge, an unfavourable outcome was observed in 62 (6%) of 957 episodes.
Table 3.
Outcome assessed on the GOS in adults with viral meningitis
| GOS score | Days after discharge | |||
|---|---|---|---|---|
| At discharge | 30 days | 90 days | 180 days | |
| n = 1062 | n = 1055 | n = 1010 | n = 957 | |
| 1 (death) | 0 (0) | 0 (0) | 0 (0) | 1 (<1) |
| 2 (vegetative state) | 0 (0) | 1 (<1) | 0 (0) | 0 (0) |
| 3 (severe disability) | 3 (<1) | 2 (<1) | 0 (0) | 0 (0) |
| 4 (moderate disability) | 304 (29) | 213 (20) | 136 (13) | 61 (6) |
| 5 (good recovery) | 755 (71) | 839 (80) | 874 (87) | 895 (94) |
Data are presented as n (%). GOS scores of 5 were carried forward for 121 (11%) of 1055 episodes at 30 days after discharge, 597 (59%) of 1010 episodes at 90 days after discharge and 779 (81%) of 957 episodes at 180 days after discharge.
Prognostic factors for an unfavourable outcome 30 days after discharge were examined by modified Poisson regression. Using meningitis without an identified pathogen as the reference, the adjusted RR of an unfavourable outcome was 1.34 (95% CI: 0.95–1.88) for enteroviral meningitis, 1.55 (95% CI 1.00–2.41) for HSV-2 meningitis, 1.51 (95% CI: 0.98–2.33) for VZV meningitis and 1.37 (95% CI: 0.61–3.05) for meningitis caused by miscellaneous viruses (Table 4). In two subgroup analyses restricted to episodes with premorbid full-time occupation or where GOS scores had not been carried forward, the adjusted RR for HSV-2 meningitis was 1.78 (95% CI: 1.06–2.98) and 1.56 (95% CI: 1.01–2.39), respectively (Supplementary Table 1).
Table 4.
Prognostic factors for an unfavourable outcome (GOS score of 1–4) 30 days after discharge in adults with viral meningitis
| Prognostic factor | Unfavourable outcome | Modified Poisson regression | |
|---|---|---|---|
| n/N (%) | Crude RR (95% CI) | Adjusteda RR (95% CI) | |
| Age, years | |||
| 18–30 | 71/417 (17) | Reference | Reference |
| 31–50 | 95/446 (21) | 1.25 (0.95–1.65) | 1.30 (0.98–1.74) |
| ≥51 | 50/192 (26) | 1.53 (1.11–2.10) | 1.26 (0.85–1.87) |
| Sex | |||
| Male | 82/486 (17) | Reference | Reference |
| Female | 134/569 (24) | 1.40 (1.09–1.79) | 1.34 (1.03–1.75) |
| Immunosuppression | |||
| No | 193/973 (20) | Reference | Reference |
| Yes | 23/82 (28) | 1.41 (0.98–2.05) | 1.19 (0.76–1.86) |
| Duration of symptoms, days | |||
| 0–1 | 78/396 (20) | Reference | Reference |
| ≥2 | 137/655 (21) | 1.06 (0.83–1.36) | 1.05 (0.80–1.37) |
| Triad of signs and symptomsb | |||
| No | 144/690 (21) | Reference | Reference |
| Yes | 50/259 (19) | 0.93 (0.69–1.23) | 0.88 (0.66–1.17) |
| CSF leucocyte count, cells/µl | |||
| 0–100 | 87/381 (23) | Reference | Reference |
| 101–500 | 98/502 (20) | 0.85 (0.66–1.10) | 0.81 (0.60–1.09) |
| ≥501 | 31/172 (18) | 0.79 (0.55–1.14) | 0.85 (0.54–1.32) |
| CSF protein, g/l | |||
| 0.0–0.5 | 77/392 (20) | Reference | Reference |
| 0.6–1.0 | 94/424 (22) | 1.13 (0.86–1.48) | 1.08 (0.81–1.45) |
| ≥1.1 | 44/213 (21) | 1.05 (0.76–1.46) | 0.90 (0.59–1.38) |
| Aetiology | |||
| Unidentified pathogen | 49/281 (17) | Reference | Reference |
| EVs | 82/416 (20) | 1.13 (0.82–1.56) | 1.34 (0.95–1.88) |
| HSV-2 | 38/166 (23) | 1.31 (0.90–1.92) | 1.55 (1.00–2.41) |
| VZV | 40/162 (25) | 1.42 (0.98–2.05) | 1.51 (0.98–2.33) |
| Miscellaneous viruses | 7/30 (23) | 1.34 (0.67–2.69) | 1.37 (0.61–3.05) |
| Dexamethasone for bacterial meningitis | |||
| No | 138/675 (20) | Reference | Reference |
| Yes | 77/376 (20) | 1.00 (0.78–1.28) | 1.16 (0.88–1.54) |
Owing to missing values, 922 episodes were used in the adjusted analysis.
Adjusted for all prognostic factors listed in the table.
Triad of headache, neck stiffness and hyperacusis or photophobia.
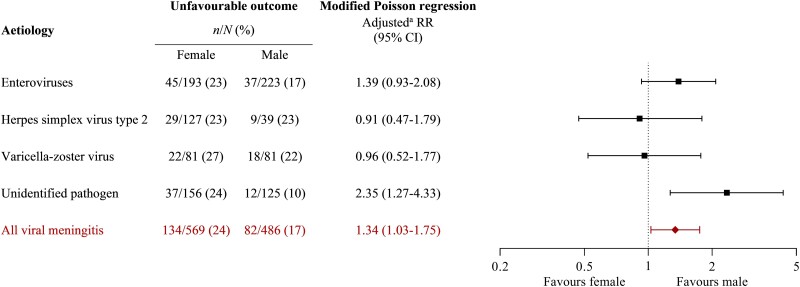
The adjusted RR of an unfavourable outcome 30 days after discharge was 1.34 (95% CI: 1.03–1.75) for females compared with males (Table 4). In analyses stratified according to the aetiology, the adjusted RR for females was 2.35 (95% CI: 1.27–4.33) in meningitis without an identified pathogen, 1.39 (95% CI 0.93–2.08) in enteroviral meningitis, 0.91 (95% CI: 0.47–1.79) in HSV-2 meningitis and 0.96 (95% CI: 0.52–1.77) in VZV meningitis (Fig. 3).
Figure 3.
Stratified analyses of sex-based differences in unfavourable outcome (GOS score 1–4) 30 days after discharge in adults with viral meningitis. aStratified analyses were adjusted for age (18–30, 31–50, ≥51 years), sex (male, female), immunosuppression (yes, no), triad of headache, neck stiffness and hyperacusis or photophobia (yes, no), CSF leucocyte count (0–100, 101–500, ≥501 cells/µl), CSF protein (0.0–0.5, 0.6–1.0, ≥1.1 g/l), and treatment with dexamethasone for bacterial meningitis (yes, no). The number of episodes used in adjusted analyses were 251 in unidentified pathogen, 369 in EVs, 140 in HSV-2, 140 in VZV and 922 in all viral meningitis.
The association between timing of antiviral treatment and an unfavourable outcome 30 days after discharge was examined by modified Poisson regression among patients with meningitis caused by HSV-2 or VZV. Using administration of acyclovir or valacyclovir ≤8 h from admission as the reference, the adjusted RR of an unfavourable outcome was 0.61 (95% CI: 0.30–1.23) for administration between 8 h and 16 h and 0.86 (95% CI: 0.51–1.43) for administration ≥17 h (Supplementary Table 2). When stratified according to immune status, post hoc testing did not disclose any associations between time until acyclovir or valacyclovir and outcome in either immunocompetent or immunosuppressed patients (Supplementary Table 3).
Discussion
The nationwide study design allowed us to estimate incidences of viral meningitis based on the total adult Danish population. Including presumed viral meningitis without an identified pathogen, the mean annual incidence was 4.7 episodes per 100 000 persons. In a study from the UK,1 the annual incidence of proven viral meningitis was 2.73 episodes per 100 000 persons, whereas annual incidences of aseptic meningitis in studies from Finland2 and Sweden3 were 7.6 and 7.4 episodes per 100 000 people, respectively. In contrast to our study, the Scandinavian studies included patients with verified non-viral aseptic meningitis.
A correct tentative diagnosis based on signs and symptoms is a prerequisite for timely lumbar puncture and appropriate management of viral meningitis. In our study, only one in four patients presented with the triad of headache, neck stiffness and hyperacusis or photophobia, and 1 in 10 patients had less than two of four classic signs and symptoms (triad and fever). Thus, clinicians should remain aware that viral meningitis may present atypically. Still, the median time from admission until lumbar puncture was short in our study compared with a previous study on viral meningitis (3 h versus 13 h).1 Consistent with other studies, we found that brain imaging before lumbar puncture was a potential amendable factor associated with increased time until lumbar puncture in meningitis.1,19,20 In addition, we found that admission directly to a department of infectious disease was associated with decreased time until lumbar puncture.
In agreement with most previous studies, we observed that short-term outcome after viral meningitis was frequently unfavourable but improved during extended follow up.6,21,22 Reassuringly, patients with presumed viral meningitis but without an identified pathogen had a similar risk of an unfavourable outcome compared with those with an identified virus. Of interest, the risk of an unfavourable outcome was also not associated with the type of virus, which contrasts with bacterial meningitis, where the prognosis varies between different bacteria.16 It could be hypothesized that the host immune response rather than the causative pathogen itself is relatively more important for the course of recovery in viral meningitis than in bacterial meningitis, as similarly proposed for some post-infectious syndromes.23 Correspondingly, viral meningitis is a relatively homogenous disease in terms of demographics, initial presentation and severity and CSF characteristics. Still, unaccounted factors such as serotypes and cross-immunity in enteroviral meningitis, as well as differences between de novo infections and reactivation in HSV-2 meningitis, could have affected our results. In a study from the UK, quality of life 6 weeks after discharge was lower among patients with HSV-2 meningitis than among those with meningitis caused by other viruses.1 However, a direct comparison with our results is limited due to the different outcome measures (EQ-5D-3L versus GOS).
In our study, female sex was independently associated with an increased risk of an unfavourable outcome 30 days after discharge. However, when the study population was stratified according to aetiology, the sex-based differences were not present in meningitis caused by HSV-2 or VZV. In meningitis without an identified pathogen and in enteroviral meningitis, female sex was associated with an increased risk of an unfavourable outcome, although not statistically significant in the latter strata. In a previous analysis of enteroviral meningitis, we found that female sex was associated with an increased risk of an unfavourable outcome at discharge.13 Similarly, in an analysis based on combined data from two randomized controlled trials of treatment with pleconaril in enteroviral meningitis, females had a longer time to resolution of headaches during admission.24 The importance of sex-based differences has also been recognized in other CNS infections,25,26 and an enhanced immune response to certain pathogens among females has been proposed as a theoretical framework for these observations.27-29 Since unaccounted factors probably remain in our analyses, these results serve as leverage to further explore the impact of sex and gender on the outcome of viral meningitis.
Although Danish guidelines on viral meningitis recommend withholding antiviral treatment until HSV-2 or VZV have been detected,30 most patients in our study were treated with acyclovir or valacyclovir. In analyses of time until treatment with aciclovir or valaciclovir among patients with meningitis caused by HSV-2 or VZV, early administration was not associated with an improved outcome 30 days after discharge. Similarly, previous observational studies have not found an apparent effect of antivirals in HSV-2 meningitis,31,32 except in immunosuppressed patients, where no or delayed treatment has been linked to complications.33,34 In the present study, time until acyclovir or valacyclovir was not associated with the outcome when examining immunosuppressed patients separately, but the analysis was not adjusted for potential confounders due to the limited number of episodes. Although at risk of residual confounding, these results together indicate that it is safe to withhold antivirals for 1–2 days until a microbiological diagnosis is established in immunocompetent patients without signs or symptoms of encephalitis.
Limitations
First, although the Danish Board of Health requires that adults with CNS infections be managed in specialized departments of infectious disease, some patients might have been admitted elsewhere, which would underestimate incidences and potentially introduce selection bias. Second, a CSF leucocyte count >10 cells/µl was chosen as the cut-off to indicate meningitis if no viral DNA/RNA was detected in the CSF. This may limit the generalizability in the presumed small subpopulation of patients with viral meningitis and lower CSF leucocyte counts. Third, patients without an identified pathogen were included in this study if viral meningitis was otherwise considered the most likely diagnosis, given all available information, including information on the post-discharge course of the disease, which was equal to patients with an identified pathogen. Although the aetiology is not microbiologically confirmed, we believe that it is important to account for this group of patients in research on viral meningitis to cover the entire spectrum of the disease. Fourth, as the completeness of the DASGIB database is ensured by annual searches of ICD-10 code of CNS infections or review of patients with CSF pleocytosis at local sites,10 a few patients may have been retrospectively identified. Fifth, information on the character of impairments was unavailable for the total study population, but in previous studies on enteroviral meningitis and HSV-2 meningitis, we observed that neurological (headache, photophobia and phonophobia, vertigo and tinnitus), cognitive (concentration and memory difficulties) and more general complaints (fatigue and sleep disturbances) were common.12,13 The GOS used to assess the outcome in the present study was developed to evaluate recovery after brain injury35 and is—although frequently used16,17,36—not validated for CNS infections. The GOS may be too crude to capture subtle neurocognitive impairments in patients with an otherwise favourable outcome (i.e. ceiling effect). Most patients with an unfavourable outcome had moderate disabilities (GOS score of 4), meaning they could not resume premorbid social or occupational activities. Therefore, the results on prognostic factors were validated by repeating the analysis in patients with premorbid full-time occupations. Sixth, data on long-term outcomes were missing for some patients, which could introduce bias. In Denmark, hospital follow up is usually discontinued if full recovery is achieved 30 days after discharge. Thus, GOS scores of 5 were carried forward if there was no subsequent follow up. Similarly, in the minority of patients without any post-discharge follow up, GOS scores of 5 at discharge were carried forward, assuming that full recovery would most probably persist. Importantly, results on prognostic factors for an unfavourable outcome 30 days after discharge were overall consistent when the analysis was restricted to patients where GOS scores had not been carried forward.
Conclusions
The outcome of viral meningitis was similar among patients with different aetiologies, including those with presumed viral meningitis but without an identified pathogen. Females had an increased risk of an unfavourable outcome, and future studies should explore causes of these sex-based differences. Stressing the need for randomized control trials, nearly all patients with meningitis caused by HSV-2 or VZV received antiviral treatment, but early administration was not associated with an improved outcome.
Supplementary Material
Contributor Information
Pelle Trier Petersen, Department of Pulmonary and Infectious Diseases, Nordsjællands Hospital, 3400 Hillerød, Denmark; Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen, Denmark.
Jacob Bodilsen, Department of Infectious Diseases, Aalborg University Hospital, 9000 Aalborg, Denmark; Department of Clinical Medicine, Aalborg University, 9000 Aalborg, Denmark.
Micha Phill Grønholm Jepsen, Department of Pulmonary and Infectious Diseases, Nordsjællands Hospital, 3400 Hillerød, Denmark.
Lykke Larsen, Department of Infectious Diseases, Odense University Hospital, 5000 Odense, Denmark.
Merete Storgaard, Department of Infectious Diseases, Aarhus University Hospital, 8200 Aarhus, Denmark.
Birgitte Rønde Hansen, Department of Infectious Diseases, Hvidovre Hospital, 2650 Hvidovre, Denmark.
Jannik Helweg-Larsen, Department of Infectious Diseases, Rigshospitalet, 2100 Copenhagen, Denmark.
Lothar Wiese, Department of Medicine, Sjællands University Hospital, 4000 Roskilde, Denmark.
Hans Rudolf Lüttichau, Department of Infectious Diseases, Herlev Hospital, 2730 Herlev, Denmark.
Christian Østergaard Andersen, Department of Clinical Microbiology, Hvidovre Hospital, 2650 Hvidovre, Denmark.
Henrik Nielsen, Department of Infectious Diseases, Aalborg University Hospital, 9000 Aalborg, Denmark; Department of Clinical Medicine, Aalborg University, 9000 Aalborg, Denmark.
Christian Thomas Brandt, Department of Medicine, Sjællands University Hospital, 4000 Roskilde, Denmark.
Funding
This work was supported by grants from Helsefonden (grant number 21-B-0437), Helen Rudes Fond (grant number 60988), A & J C Tvergaards Fond and Minister Erna Hamiltons Legat for Videnskab og Kunst (grant number 24-2022) to P.T.P.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. McGill F, Griffiths MJ, Bonnett LJ, et al. Incidence, aetiology, and sequelae of viral meningitis in UK adults: A multicentre prospective observational cohort study. Lancet Infect Dis. 2018;18:992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kupila L, Vuorinen T, Marttila RJ, Kotilainen P. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology. 2006;66:6. [DOI] [PubMed] [Google Scholar]
- 3. Franzen-Röhl E, Larsson K, Skoog E, et al. High diagnostic yield by CSF-PCR for entero- and herpes simplex viruses and TBEV serology in adults with acute aseptic meningitis in Stockholm. Scand J Infect Dis. 2008;40:914–921. [DOI] [PubMed] [Google Scholar]
- 4. de Ory F, Avellón A, Echevarría Je, et al. Viral infections of the central nervous system in Spain: A prospective study. J Med Virol. 2013;85:554–562. [DOI] [PubMed] [Google Scholar]
- 5. Calleri G, Libanore V, Corcione S, Rosa FGD, Caramello P. A retrospective study of viral central nervous system infections: Relationship amongst aetiology, clinical course and outcome. Infection. 2017;45:227–231. [DOI] [PubMed] [Google Scholar]
- 6. Quist-Paulsen E, Ormaasen V, Kran AMB, et al. Encephalitis and aseptic meningitis: Short-term and long-term outcome, quality of life and neuropsychological functioning. Sci Rep. 2019;9:16158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidt H, Heimann B, Djukic M, et al. Neuropsychological sequelae of bacterial and viral meningitis. Brain. 2006;129:333–345. [DOI] [PubMed] [Google Scholar]
- 8. Schmidt H, Cohrs S, Heinemann T, et al. Sleep disorders are long-term sequelae of both bacterial and viral meningitis. J Neurol Neurosurg Psychiatry. 2006;77:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sittinger H, Müller M, Schweizer I, Merkelbach S. Mild cognitive impairment after viral meningitis in adults. J Neurol. 2002;249:554–560. [DOI] [PubMed] [Google Scholar]
- 10. Bodilsen J, Larsen L, Brandt CT, et al. Existing data sources for clinical epidemiology: The Danish Study Group of Infections of the Brain Database (DASGIB). Clin Epidemiol. 2021;13:921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: Consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jakobsen A, Skov MT, Larsen L, et al. Herpes simplex virus 2 meningitis in adults: A prospective, nationwide, population-based cohort study. Clin Infect Dis. 2022;75:753–760. [DOI] [PubMed] [Google Scholar]
- 13. Bodilsen J, Mens H, Midgley S, et al. Enterovirus meningitis in adults: A prospective nationwide population-based cohort study. Neurology. 2021;97:e454–e463. [DOI] [PubMed] [Google Scholar]
- 14. Statistics Denmark. Accessed 20 April 2023. https://www.dst.dk/en/
- 15. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 16. van de Beek D, Spanjaard L, Reitsma JB. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849–1859. [DOI] [PubMed] [Google Scholar]
- 17. Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. Community-acquired bacterial meningitis in adults in The Netherlands, 2006–14: A prospective cohort study. Lancet Infect Dis. 2016;16:339–347. [DOI] [PubMed] [Google Scholar]
- 18. Wilson JTL, Pettigrew LEL, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J Neurotrauma. 1998;15:573–585. [DOI] [PubMed] [Google Scholar]
- 19. Michael BD, Sidhu M, Stoeter D, et al. Acute central nervous system infections in adults--a retrospective cohort study in the NHS North West region. QJM. 2010;103:749–758. [DOI] [PubMed] [Google Scholar]
- 20. Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345:1727–1733. [DOI] [PubMed] [Google Scholar]
- 21. Hansen ABE, Vestergaard HT, Dessau RB, et al. Long-term survival, morbidity, social functioning and risk of disability in patients with a herpes simplex virus type 1 or type 2 central nervous system infection, Denmark, 2000–2016. Clin Epidemiol. 2020;12:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Omland LH, Holm-Hansen C, Lebech AM, et al. Long-term survival, health, social functioning, and education in patients with an enterovirus central nervous system infection, Denmark, 1997–2016. J Infect Dis. 2020;222:619–627. [DOI] [PubMed] [Google Scholar]
- 23. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28:911–923. [DOI] [PubMed] [Google Scholar]
- 24. Desmond RA, Accortt NA, Talley L, Villano SA, Soong SJ, Whitley RJ. Enteroviral meningitis: Natural history and outcome of pleconaril therapy. Antimicrob Agents Chemother. 2006;50:2409–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dharmarajan L, Salazar L, Hasbun R. Gender differences in community-acquired meningitis in adults: Clinical presentations and prognostic factors. J Meningitis. 2016;1:106. [PMC free article] [PubMed] [Google Scholar]
- 26. Dias SP, Brouwer MC, Bijlsma MW, van der Ende A, van de Beek D. Sex-based differences in adults with community-acquired bacterial meningitis: A prospective cohort study. Clin Microbiol Infect. 2017;23:121.e9–121.e15. [DOI] [PubMed] [Google Scholar]
- 27. Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and therapeutic treatments for viral diseases. Bioessays. 2012;34:1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischer J, Jung N, Robinson N, Lehmann C. Sex differences in immune responses to infectious diseases. Infection. 2015;43:399–403. [DOI] [PubMed] [Google Scholar]
- 29. Jacobsen H, Klein SL. Sex differences in immunity to viral infections. Front Immunol. 2021;12:720952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lebech AM, Hansen BR, Brandt C, Wiese L, Larsen L, Mogensen T.. Viral meningitis. 2018. https://infmed.dk/guidelines
- 31. Omland LH, Vestergaard BF, Wandall JH. Herpes simplex virus type 2 infections of the central nervous system: A retrospective study of 49 patients. Scand J Infect Dis. 2008;40:59–62. [DOI] [PubMed] [Google Scholar]
- 32. Moon SM, Kim T, Lee EM, Kang JK, Lee SA, Choi SH. Comparison of clinical manifestations, outcomes and cerebrospinal fluid findings between herpes simplex type 1 and type 2 central nervous system infections in adults. J Med Virol. 2014;86:1766–1771. [DOI] [PubMed] [Google Scholar]
- 33. Noska A, Kyrillos R, Hansen G, Hirigoyen D, Williams DN. The role of antiviral therapy in immunocompromised patients with herpes simplex virus meningitis. Clin Infect Dis. 2015;60:237–242. [DOI] [PubMed] [Google Scholar]
- 34. Momméja-Marin H, Lafaurie M, Scieux C, Galicier L, Oksenhendler E, Molina JM. Herpes simplex virus type 2 as a cause of severe meningitis in immunocompromised adults. Clin Infect Dis. 2003;37:1527–1533. [DOI] [PubMed] [Google Scholar]
- 35. Jennett B. Assessment of outcome after severe brain damage a practical scale. Lancet. 1975;305:480–484. [DOI] [PubMed] [Google Scholar]
- 36. Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are only available with permission from the Danish health and legal authorities.