Abstract
Epilepsy surgery consists of surgical resection of the epileptic focus and is recommended for patients with drug-resistant focal epilepsy. However, focal brain lesions can lead to effects in distant brain regions. Similarly, the focal resection in temporal lobe epilepsy surgery has been shown to lead to functional changes distant from the resection. Here we hypothesize that there are changes in brain function caused by temporal lobe epilepsy surgery in regions distant from the resection that are due to their structural disconnection from the resected epileptic focus. Therefore, the goal of this study was to localize changes in brain function caused by temporal lobe epilepsy surgery and relate them to the disconnection from the resected epileptic focus. This study takes advantage of the unique opportunity that epilepsy surgery provides to investigate the effects of focal disconnections on brain function in humans, which has implications in epilepsy and broader neuroscience.
Changes in brain function from pre- to post-epilepsy surgery were quantified in a group of temporal lobe epilepsy patients (n = 36) using a measure of resting state functional MRI activity fluctuations. We identified regions with significant functional MRI changes that had high structural connectivity to the resected region in healthy controls (n = 96) and patients based on diffusion MRI. The structural disconnection from the resected epileptic focus was then estimated using presurgical diffusion MRI and related to the functional MRI changes from pre- to post-surgery in these regions.
Functional MRI activity fluctuations increased from pre- to post-surgery in temporal lobe epilepsy in the two regions most highly structurally connected to the resected epileptic focus in healthy controls and patients—the thalamus and the fusiform gyrus ipsilateral to the side of surgery (PFWE < 0.05). Broader surgeries led to larger functional MRI changes in the thalamus than more selective surgeries (P < 0.05), but no other clinical variables were related to functional MRI changes in either the thalamus or fusiform. The magnitude of the functional MRI changes in both the thalamus and fusiform increased with a higher estimated structural disconnection from the resected epileptic focus when controlling for the type of surgery (P < 0.05). These results suggest that the structural disconnection from the resected epileptic focus may contribute to the functional changes seen after epilepsy surgery. Broadly, this study provides a novel link between focal disconnections in the structural brain network and downstream effects on function in distant brain regions.
Keywords: fMRI, structural connectivity, epilepsy, surgery, ALFF
Epilepsy surgery aims to alleviate seizures through the removal of the epileptic focus. Sainburg et al. provide evidence that this focal resection leads to functional changes in distant brain regions that are related to these regions’ structural disconnection from the resected epileptic focus.
Introduction
Epilepsy surgery is an effective treatment option for patients with drug-resistant focal epilepsy.1 The goal of epilepsy surgery is to eliminate seizures and retain healthy function in the rest of the brain through the removal of the epileptic focus, i.e. the brain tissue from which seizures originate. However, focal epilepsy is now well established as a network disorder as opposed to a solely focal disorder,2 suggesting that recurrent abnormal localized activity can have widespread effects. Similarly, focal brain lesions can have functional and behavioural effects that are not fully explained by the location of the lesioned tissue.3 This has led to recent studies mapping the functional deficits of lesions to brain networks as opposed to a single brain region, known as ‘lesion network mapping’.4–7 Both the view of focal epilepsy as a network disorder and the observation that focal brain lesions may cause alterations in the function of distributed brain networks imply that the focal resection in epilepsy surgery may have both focal and widespread effects on brain function.
Studies using functional MRI (fMRI) and PET in patients with temporal lobe epilepsy (TLE), the most common form of focal epilepsy, have shown that epilepsy surgery leads to changes in brain activity in regions distant from, but ipsilateral to the resection.8,9 Furthermore, functional connectivity changes have been observed after TLE surgery in distant regions both ipsilateral and contralateral to the resection using fMRI.10,11 It has been proposed that some of these distant changes in function may be due to disconnections in the structural network,8 however, this hypothesis has yet to be tested directly.
The goal of this work was to localize functional changes caused by TLE surgery and relate these changes to disconnections from the resected epileptic focus, i.e. the anterior hippocampus. Towards this goal, we used a measure of fMRI activity [amplitude of low frequency fluctuations (ALFF)] shown to be altered in epileptic foci and networks12 to quantify changes in brain function caused by TLE surgery. Using diffusion MRI (dMRI), we then identified regions highly structurally connected to the anterior hippocampus that overlapped with regions found to have significant functional changes after TLE surgery. Next, we determined which clinical variables contributed to the pre to post-surgical ALFF changes in these identified regions. The structural disconnections from the identified regions to the resected epileptic focus were then estimated using presurgical dMRI. Finally, we related these estimates of structural disconnection to the functional changes in ALFF from pre- to post-surgery in these identified regions, controlling for the effects of clinical variables. This study takes advantage of the unique opportunity that epilepsy surgery provides to investigate the effects of focal disconnections on brain function in humans, which has implications in epilepsy and broader neuroscience.
Materials and methods
Participants
This study included 36 unilateral drug-resistant TLE patients who underwent surgical treatment for their epilepsy along with 96 healthy control subjects (Table 1). TLE patients underwent either an anterior temporal lobectomy (ATL) (n = 7) or a selective amygdalohippocampectomy (SAH) (n = 29) to treat their seizures. Presurgical clinical assessments for patients included structural MRI, scalp EEG, seizure semiology and PET. Patients with signs of bilateral TLE or who had significant structural abnormalities aside from mesial temporal sclerosis were excluded from the study. All patients included in the study had confirmed mesial temporal sclerosis or gliosis based on postoperative pathology. The Vanderbilt University Institutional Review Board approved the use of human participants for this study.
Table 1.
Participant demographics
| TLE (n = 36) |
Controls (n = 96) |
P-value | |
|---|---|---|---|
| Age, years | 41.8 ± 12.3 | 37.9 ± 13.4 | 0.09a |
| Sex, % female) | 50.0 | 47.9 | 0.83b |
| Side of surgery, % left | 27.8 | ||
| Duration of epilepsy, years | 22.9 ± 16.7c | ||
| Mesial temporal sclerosis on MRI, % | 66.7 | ||
| Postoperative pathology, % | |||
| Mesial temporal sclerosis | 88.9 | ||
| Mesial temporal gliosis | 11.1 | ||
| PET hypometabolism localization, % | 77.8 | ||
| Interictal EEG localization, % | 69.4 | ||
| Ictal EEG localization, % | 91.7 | ||
| Type of surgery, % ATL | 19.4 | ||
| Time between surgery and post-surgical scan, months | 26.7 ± 17.2 | ||
| Engel outcome at time of post-surgical scan, % | |||
| Engel I | 75.0 | ||
| Engel II | 8.3 | ||
| Engel III | 16.7 | ||
| Engel IV | 0.0 |
ATL = anterior temporal lobectomy; TLE = temporal lobe epilepsy.
Wilcoxon rank-sum test.
Chi-squared test.
One patient reported an unknown duration of epilepsy (n = 35).
Imaging
All imaging was carried out on a Philips 3 T MRI scanner using a 32-channel head coil. All TLE patients underwent one scanning session prior to surgery and one following surgery, while healthy controls all underwent a single session. Each scanning session comprised a T1-weighted 3D scan for anatomy (1 mm isotropic), a T1-weighted 2D scan in the same slice orientation as the functional images for spatial normalization and segmentation (1 × 1 × 3.5 mm with a 0.5 mm gap), two consecutive 10-min T2*-weighted eyes-closed resting-state fMRI scans (3 × 3 × 3.5 mm with a 0.5 mm gap, repetition time = 2 s, echo time = 35 ms), and a diffusion-weighted scan for tractography (50 slices, 2.5 mm isotropic, one b = 0 volume acquisition and a single-shell of b = 1600 s/mm2 with 92 directions). A pulse oximeter and respiratory belt collected cardiac and respiratory fluctuations during fMRI scans for preprocessing.
Segmentation
T1-weighted images were segmented into white matter, grey matter and CSF masks using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). For presurgical images, 107 cortical and subcortical brain regions were obtained using a multi-atlas segmentation method.13,14 FreeSurfer 615 was used to segment the hippocampi into subfields, which were then merged into one anterior and posterior hippocampal region per side,16 resulting in an atlas of 111 total cortical and subcortical brain regions. In patients, regional fMRI and dMRI metrics described below were converted to ipsilateral and contralateral to the resection side using this atlas.
Surgical lacunae were segmented on post-surgical T1-weighted images using ResectVol17 (Supplementary Fig. 1). To obtain atlases for post-surgical scans, presurgical atlases were first registered to post-surgical T1-weighted images using SPM12's non-rigid body registration. Resected voxels were then removed from these atlases using each patient's individual lacuna mask to obtain a post-surgical atlas for each patient with the lacuna removed (Supplementary Fig. 2).
Functional MRI preprocessing and ALFF calculation
Functional MRI data were first corrected for physiological noise with RETROICOR18 using the pulse oximeter and respiratory belt signals. This was followed by preprocessing using SPM12 that included slice-timing correction, motion correction, normalization to Montreal Neurological Institute (MNI) coordinates and spatial smoothing with a 6 mm full-width at half-maximum (FWHM) Gaussian kernel. Next, the mean white matter and CSF signals, along with the six translational and rotational motion parameters were regressed out of the fMRI time courses. The two consecutive fMRI scans were then concatenated in time to obtain one 20-min scan.
To localize regions of functional change that may be due to the direct effects of surgery, we computed the ALFF, which is thought to reflect the magnitude of resting brain activity.19 ALFF was chosen since it can capture changes in brain activity in regions directly disconnected from the resection. Our group and others have shown that epileptic tissue presents with abnormally high ALFF,12,20,21 while other regional fMRI measures, such as regional homogeneity do not show abnormality specific to epileptic tissue.20,22 Moreover, interpretations of edge measures between regions such as functional connectivity are complicated by the edges associated with the resected region. ALFF was calculated as described previously,12 but is reiterated here. The voxel-wise power spectra of the preprocessed fMRI timeseries were obtained using Welch's method of estimating power spectral density with a Hamming window of length 120 s and an overlap of 50%. The square root of the power spectrum was then summed over the 0.0083–0.1 Hz band to obtain ALFF. ALFF was then Z-standardized across grey matter voxels within each subject to give units of standard deviations (Z) from the mean ALFF within each subject since Z-standardized ALFF has been shown to have high test-retest reliability.23,24 Since the fMRI data were collected before and after a scanner upgrade, resulting in different magnetic resonance signal scales, the variability in regional ALFF metrics due to this upgrade was linearly modelled in the healthy controls and subsequently regressed out in all participants.
Diffusion MRI preprocessing
Diffusion MRI data were processed according to the PreQual pipeline,25 which included denoising,26 eddy current and motion correction,27 bias correction of B1 field inhomogeneity,28 and synthetic B0 diffusion distortion correction.29 Next, the response function was estimated for spherical deconvolution to estimate the fibre orientation distribution (FOD).30 One control and one patient did not undergo dMRI scans and one patient's dMRI tractography failed, therefore, these three participants were not included in dMRI analyses. These FOD maps were then used to derive the anterior hippocampus structural connectivity profiles and the estimated structural disconnection due to surgery as described below.
Anterior hippocampus structural connectivity profiles
Anterior hippocampus structural connectivity profiles were derived for both the healthy control and TLE groups to assess which regions have the strongest structural connection to the anterior hippocampus, given that the anterior hippocampus is resected in these patients. To obtain this structural connectivity profile, we first computed whole-brain tractography from healthy control and presurgical TLE dMRI data. For whole-brain tractography, the grey matter-white matter interface was generated from the T1-weighted and B0 images using SPM12 and FOD maps were used to generate 2 × 107 anatomically constrained probabilistic streamlines through the white matter from this interface31 using MRtrix3.28 The streamlines were then reduced to 1 × 107 using spherical deconvolution-informed filtering of tractograms (SIFT) and then given weights using SIFT2—both of which attempt to better quantify the fibre cross-sectional area.32 From this, streamlines connecting each of the 111 regions of interest were identified. Streamline count (SC) was computed as the number of streamlines between each pair of regions weighted by streamline length and the inverse of the region size. For the healthy control group, the SC profile from the anterior hippocampus to the rest of the brain was averaged between right and left anterior hippocampi and then across participants to obtain a healthy control anterior hippocampus structural connectivity profile. For the TLE group, the SC profiles of the ipsilateral anterior hippocampi were averaged across participants to obtain a presurgical TLE ipsilateral anterior hippocampus structural connectivity profile. The regions with the highest structural connectivity to the anterior hippocampus were qualitatively compared to the regions with significant changes in ALFF from pre- to post-surgery.
Pre- to post-surgical ALFF changes
Pre- to post-surgical ALFF changes were first assessed regionally. TLE patients’ regional ALFF values were flipped to be ipsilateral and contralateral to the side of resection. All regions with >50% of their voxels removed from surgery in any of the patients were discarded from the analysis, removing most of the regions resected in an ATL. The regions discarded were the anterior hippocampus, posterior hippocampus, entorhinal cortex, inferior temporal gyrus, middle temporal gyrus, parahippocampal gyrus, temporal pole and amygdala, all ipsilateral to the resection. Pre- to post-surgical changes in ALFF were then tested in the remaining 103 regions using paired t-tests with Bonferroni correction and Cohen's d was computed to display effect size from the tests. In addition, pre- to post-surgical ALFF changes in each region across the brain were then related to the anterior hippocampus structural connectivity profiles described above using two-tailed Spearman correlations. This was performed separately for healthy controls and patients with TLE.
Voxel-wise pre- to post-surgical changes in ALFF were then assessed in the regions highly structurally connected to the anterior hippocampus that also showed changes in ALFF from pre- to post-surgery, i.e. the thalamus and fusiform gyrus, using threshold-free cluster enhancement (TFCE)33 for family-wise error (FWE) correction. To avoid including any resected voxels in this analysis, an ipsilateral fusiform mask was created by taking a group fusiform mask and removing all voxels that were included in any of the patients’ lacuna masks. This ensured that any voxels included in the analysis were not resected in any of the patients. The clusters of voxels identified in the thalamus and fusiform gyrus found here were then used as seeds in the dMRI tractography described below to estimate structural disconnection and investigate cluster specific structure-function relationships.
In addition, presurgical and post-surgical ALFF in the ipsilateral thalamic and fusiform clusters were compared to controls using two-sample t-tests. Difference from the control mean was measured prior to the ALFF t-tests between patients and controls to avoid baseline differences in ALFF between right and left sides of the brain. Furthermore, we expected that type of surgery would affect the magnitude of the ALFF changes after surgery, therefore pre- to post-surgical ALFF changes were compared between patients who underwent ATL and SAH in both the thalamic and fusiform cluster using two-sample t-tests.
Relationships between pre- to post-surgical ALFF change and other clinical variables in both the thalamic and fusiform cluster were carried out using partial two-tailed Spearman correlations controlling for surgery type. Other variables tested included age at the time of presurgical scan, age of epilepsy onset, duration of epilepsy, time between surgery and post-surgical scan, and seizure outcome at the time of post-surgical scan graded on the Engel scale.34
Estimated structural disconnection and relation to ALFF changes
The structural connectivity from the thalamic and fusiform clusters to the anterior hippocampus were computed using seeded tractography of both TLE patients’ presurgical dMRI data and healthy controls’ dMRI data. Using MRtrix3,28 FOD maps were used to generate 5 × 104 anatomically constrained probabilistic streamlines through the white matter31 from each of the thalamic and fusiform clusters identified in the above voxel-wise fMRI analysis. Streamlines were constrained to be >10 mm to minimize the number of streamlines starting and ending within the seed region. From this, streamlines connecting the seeds to the ipsilateral anterior hippocampus were identified to compute SC between each seed and the anterior hippocampus as in the healthy structural connectivity analysis above. This resulted in the presurgical structural connectivity from the thalamic and fusiform clusters to the anterior hippocampus. This presurgical structural connectivity was then taken as each cluster's estimated structural disconnection from the epileptic focus, since all these tracts should be disconnected after the surgical removal of the epileptic focus.
Presurgical SC between each cluster (thalamic and fusiform) and the anterior hippocampus was compared between TLE patients and controls using Wilcoxon rank-sum tests. Finally, relationships between the pre- to post-surgical ALFF change and the structural disconnection from the anterior hippocampus were tested for both the thalamic and fusiform clusters using partial right-tailed Spearman correlations controlling for surgery type. These relationships were also tested specifically within patients who underwent SAH using right-tailed Spearman correlations.
Data availability
Data supporting the findings of this article are available from the corresponding author upon reasonable request.
Results
Post-surgical ALFF changes overlap with high structural connectivity to the anterior hippocampus
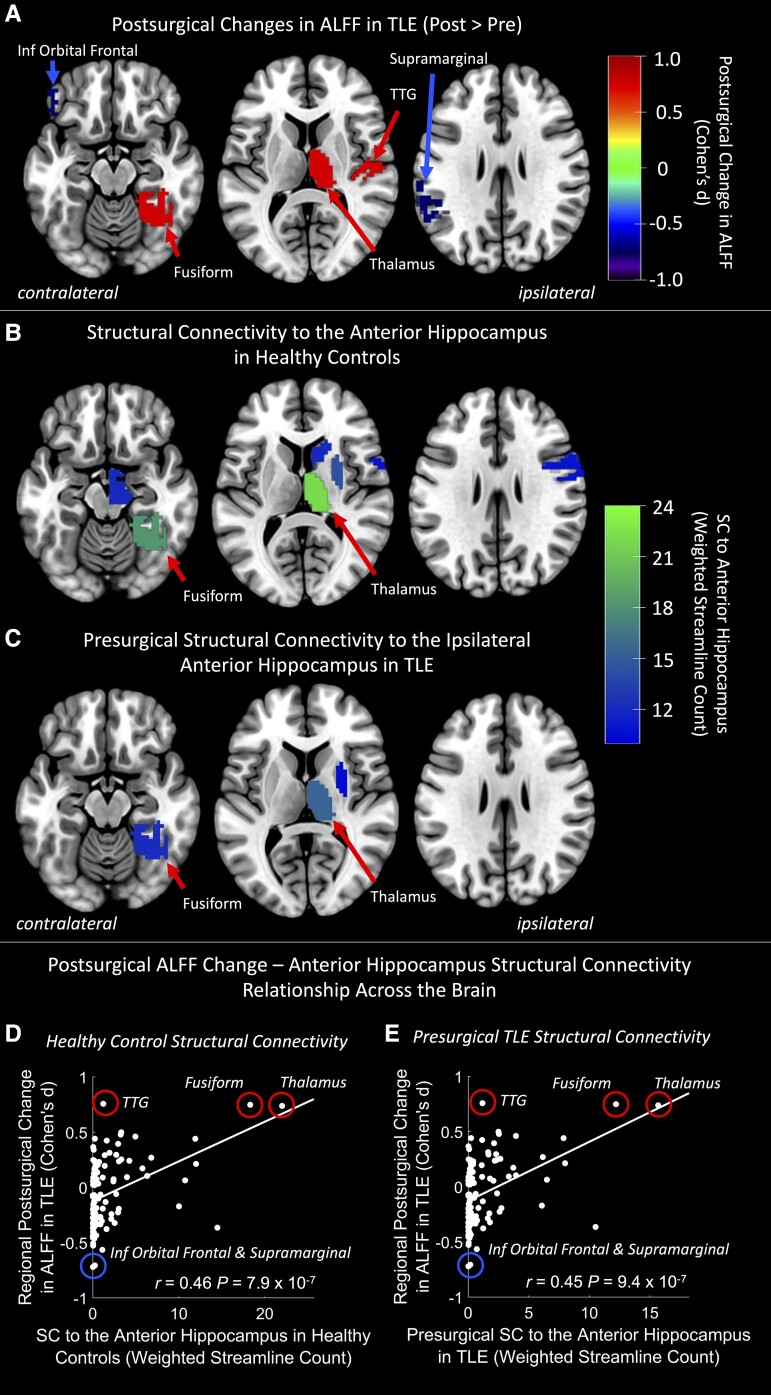
Regional ALFF increases from pre- to post-surgery were found in the ipsilateral thalamus, fusiform gyrus and transverse temporal gyrus (PFWE < 0.05, paired t-tests, Fig. 1A). Regional ALFF decreases from pre- to post-surgery were found in the contralateral inferior orbital frontal and supramarginal gyri (PFWE < 0.05, paired t-tests, Fig. 1A).
Figure 1.
Regional ALFF changes from pre- to post-TLE surgery overlap with high structural connectivity to the anterior hippocampus in the ipsilateral thalamus and fusiform gyrus. (A) Region-wise changes in amplitude of low frequency fluctuations (ALFF) from pre- to post-surgery were tested using paired t-tests. Paired t-test effect size (Cohen's d) of regions with PFWE < 0.05 are shown. Red regions depict post > pre ALFF while blue regions depict pre > post ALFF. Ipsilateral and contralateral are respective to the side of the epileptic focus. (B) Regions with the highest mean structural connectivity to the anterior hippocampus in healthy controls. Ipsilateral and contralateral are respective to the side of the anterior hippocampus. (C) Regions with the highest mean structural connectivity to the anterior hippocampus before surgery in patients with temporal lobe epilepsy (TLE). Ipsilateral and contralateral are respective to the side of the epileptic focus. (D and E) Relationship between pre- to post-surgical change in ALFF and structural connectivity to the anterior hippocampus across all brain regions with (D) depicting the structural connectivity to the anterior hippocampus in healthy controls, while (E) depicts the presurgical structural connectivity to the anterior hippocampus in patients with TLE. Statistics are from Spearman correlations. Regions with significant post-surgical ALFF changes from (A) are circled in red (increases) and blue (decreases). Ipsilateral anterior and medial temporal regions that were largely removed in some patients during surgery were removed from these analyses. Trend lines are based on a linear model and are shown for visualization purposes only. Inf = inferior; SC = streamline count; TTG = transverse temporal gyrus.
SC from whole-brain dMRI tractography was used to assess the structural connectivity between the anterior hippocampus and all other brain regions in both healthy controls and patients with TLE. The mean SC across controls from the anterior hippocampus was highest to the ipsilateral thalamus, fusiform gyrus, putamen, ventral diencephalon/midbrain, caudate and precentral gyrus in descending order (Fig. 1B). The mean SC across TLE patients from the anterior hippocampus was highest to the ipsilateral thalamus, fusiform gyrus and putamen in descending order (Fig. 1C), although this SC was lower than that of healthy controls. Pre- to post-surgical changes in ALFF overlapped with the two regions most highly structurally connected to the anterior hippocampus in both healthy controls and patients with TLE, the ipsilateral thalamus and fusiform gyrus (Fig. 1A–C). Pre- to post-surgical changes in ALFF were also positively correlated with the SC to the anterior hippocampus of both healthy controls (r = 0.46, P = 7.9 × 10−7, Spearman correlation, Fig. 1D) and TLE patients (r = 0.45, P = 9.4 × 10−7, Spearman correlation, Fig. 1E) across all regions of the brain.
ALFF and structural connectivity differences from controls in the thalamic and fusiform clusters
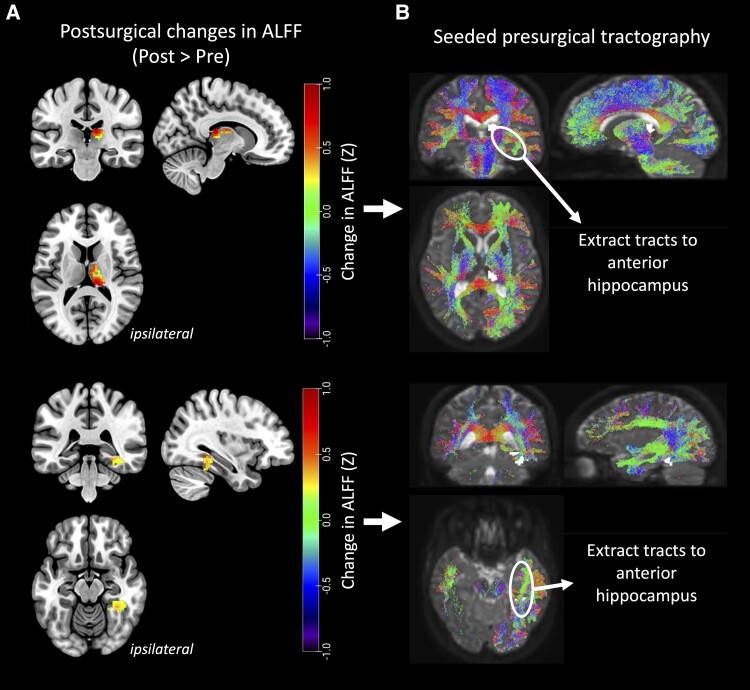
Using voxel-wise analyses, we identified clusters of voxels with ALFF increases from pre- to post surgery in both the ipsilateral thalamus and fusiform gyrus (PFWE < 0.05, paired t-tests, Fig. 2A). Ipsilateral thalamic ALFF increases from pre- to post-surgery were largely in the medial, dorsal and posterior portions of the thalamus. We observed ipsilateral fusiform ALFF increases from pre- to post-surgery in the posterior temporal portion of the fusiform gyrus.
Figure 2.
Pre- to post-surgical ALFF increases in thalamus and fusiform gyrus and estimation of structural disconnection from the resected epileptic focus. (A) Clusters of amplitude of low frequency fluctuations (ALFF) increases from pre- to post-surgery were localized within the ipsilateral thalamus (top) and fusiform gyrus (bottom) (PFWE < 0.05, paired t-tests). (B) These clusters were seeded for tractography using presurgical dMRI data. The structural connectivity to the anterior hippocampus was extracted as an estimate of structural disconnection from the resected epileptic focus for each cluster.
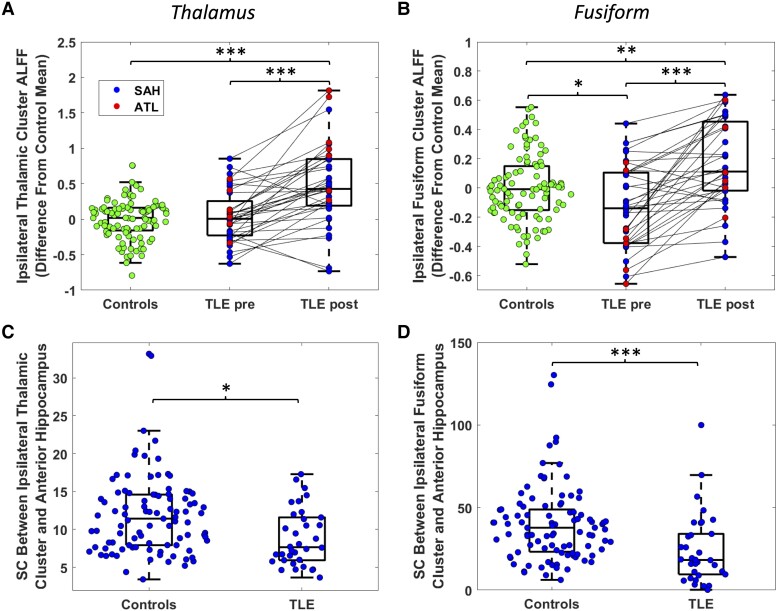
ALFF in the thalamic cluster was not different from controls prior to surgery but was higher than controls after surgery (P = 1.9 × 10−9, two-sample t-test, Fig. 3A). ALFF in the fusiform cluster was lower than controls prior to surgery (P = 0.0086, two-sample t-test, Fig. 3B) and was higher than controls after surgery (P = 6.5 × 10−4, two-sample t-test, Fig. 3B).
Figure 3.
ALFF and structural connectivity differences from controls in the thalamic and fusiform clusters. Amplitude of low frequency fluctuations (ALFF) of healthy controls and temporal lobe epilepsy (TLE) both pre- and post-surgery are shown for the thalamic (A) and fusiform (B) clusters. Control distributions reflect mean between left and right regions. Comparisons to controls were performed with two-sample t-tests, while comparisons from pre- to post-surgery were performed with paired t-tests. TLE and healthy control structural connectivity are shown for the thalamic (C) and fusiform (D) clusters, tested with Wilcoxon rank-sum tests. Control depicts mean between right and left hemisphere, while TLE measures are from the hemisphere ipsilateral to the epileptic focus. *P < 0.01, **P < 0.001, ***P < 0.0001, uncorrected. ATL = anterior temporal lobectomy; SAH = selective amygdalohippocampectomy; SC = streamline count.
We then seeded these clusters for tractography using presurgical dMRI data to obtain the structural connectivity between each of these regions and the ipsilateral anterior hippocampus (Fig. 2B). Presurgical structural connectivity between the thalamic cluster and the anterior hippocampus was decreased in TLE compared to controls (P = 0.0016, Wilcoxon rank-sum, Fig. 3C). Presurgical structural connectivity was also decreased between the fusiform cluster and the anterior hippocampus in TLE compared to controls (P = 7.9 × 10−5, Wilcoxon rank-sum, Fig. 3D).
Pre- to post-surgical ALFF changes are related to the structural disconnection from the resected focus
ALFF increases in the thalamic cluster were found to be significantly higher in patients who underwent ATL than those who underwent SAH (P = 0.0038, two-sample t-test), but no significant difference was detected in the fusiform cluster (P = 0.20, two-sample t-test). Therefore, surgery type was used as a covariate in all subsequent correlational analyses that included pre- to post-surgical ALFF changes. No relationships were found between pre- to post-surgical ALFF changes in either the thalamic or fusiform cluster and age, duration of epilepsy, age of epilepsy onset, or time between surgery and post-surgical scan (P > 0.05, partial Spearman correlation). Furthermore, no relationship was found between pre- to post-surgical ALFF changes in either the thalamic or fusiform cluster and seizure outcomes at the time of the post-surgical scan graded on the Engel scale (P > 0.05, partial Spearman correlation).
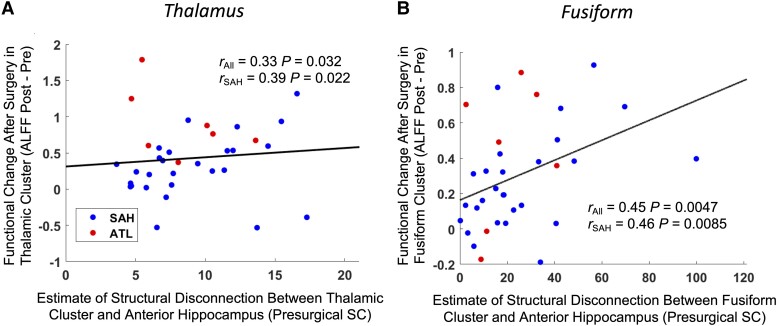
The change in ALFF from pre- to post-surgery was increased with a higher estimated structural disconnection from the ipsilateral anterior hippocampus in both the thalamic (r = 0.33, P = 0.032, partial right-tailed Spearman correlation, Fig. 4A) and fusiform (r = 0.45, P = 0.0047, partial right-tailed Spearman correlation, Fig. 4B) clusters when controlling for surgery type. This relationship remained when only considering patients who underwent a SAH in both the thalamic (r = 0.39, P = 0.022, right-tailed Spearman correlation, Fig. 4A) and fusiform (r = 0.46, P = 0.0085, right-tailed Spearman correlation, Fig. 4B) clusters.
Figure 4.
Pre- to post-surgical ALFF changes in the thalamus (A) and fusiform (B) are related to the estimated structural disconnection from the epileptic focus. Right-tailed Spearman correlation coefficients are shown for all patients together as well as for only patients who underwent a selective amygdalohippocampectomy (SAH). Surgery type was used as a covariate for the correlations across all patients. Trend lines are based on a linear model and are shown for visualization purposes only.
ALFF = amplitude of low frequency fluctuations; ATL = anterior temporal lobectomy; SC = streamline count.
Discussion
In this study, we identified functional MRI changes after TLE surgery in regions distant (thalamus) and near (fusiform gyrus) to the resection of the anterior hippocampus. We then showed that the increases in functional change after resection were significantly associated with increases in the structural disconnection between the resected region and the regions with functional changes. Not surprisingly, change in function was also related to the type of surgery, with smaller surgeries (SAH) leading to lesser changes than larger surgeries (ATL). However, no other clinical measures were related to the pre- to post-surgical functional changes in these regions.
Previous studies have identified widespread functional changes from pre- to post-TLE surgery,8–11 in line with the observation that focal brain lesions can have effects on remote areas of the brain.3,6 In this study we focused on the functional changes after TLE surgery in the ipsilateral thalamus, distant from the resection, and the ipsilateral fusiform gyrus, near the resection, since they were both found to be highly structurally connected to the resected region. There is strong evidence that the regions of the thalamus and fusiform with increased ALFF after surgery are anatomically connected to the hippocampus. The ALFF increases in the ipsilateral thalamus were localized to regions overlapping with the anterior, mediodorsal and pulvinar nuclei. This aligns with a previous study that found ALFF increases after TLE surgery in the anterior and mediodorsal regions of the ipsilateral thalamus.9 The anterior nucleus of the thalamus has direct connections with the hippocampus through the fornix, both of which are main nodes in the circuit of Papez.35,36 Because of its known connectivity with the hippocampus, the anterior nucleus is a common target for deep brain stimulation to treat seizures in TLE.37 Both the anterior and mediodorsal nuclei have been shown to be connected with the hippocampus using dMRI.38,39 The pulvinar is also known to be connected to the hippocampus, yet indirectly through the entorhinal cortex.36 Furthermore, these regions of the thalamus show consistent structural and pathological abnormalities in TLE prior to surgery,40 suggesting that they may be damaged by recurrent seizures propagating from the hippocampus. The fusiform gyrus has also been shown to be connected to the hippocampus using dMRI.41 Another study found that the fusiform gyrus was functionally connected to the hippocampus in TLE and was part of the interictal epileptic network using intracranial EEG recordings.42 Overall, these studies provide evidence of anatomical connections between the regions of the thalamus and fusiform seen to have increased ALFF in this study and the hippocampus, providing an anatomical grounding to our claim that the removal of these connections may give rise to the observed functional changes.
We directly evaluated these anatomical connections with dMRI and showed that the ipsilateral thalamus and fusiform gyrus were the two regions most highly structurally connected to the anterior hippocampus in healthy controls and before surgery in patients with TLE. Presuming that structural disconnections influence functional changes; these results suggest that the removal of the anterior hippocampus would be expected to have the largest impact on these two regions. In further support of this hypothesis, we found that higher structural connectivity to the anterior hippocampus was associated with a larger increase in ALFF after surgery across all regions of the brain. However, the ipsilateral transverse temporal gyrus increased in ALFF from pre- to post-surgery and had very low structural connectivity to the anterior hippocampus, straying from this brain-wide trend. Moreover, the regions with decreased ALFF from pre- to post-surgery also had very low structural connectivity to the anterior hippocampus, indicating that these changes did not arise from direct structural disconnections. These ALFF changes not explained by a direct structural disconnection could instead be due to indirect, multi-step effects of the disconnection on these regions. However, further studies are needed to determine what these changes represent and their potential causes.
The pre- to post-surgical ALFF changes increased in the thalamus and fusiform with greater presurgical structural connectivity to the anterior hippocampus. This relationship held both when controlling for surgery type and when only considering patients with very selective surgeries (SAH). These increases in ALFF from pre- to post-surgery resulted in higher ALFF than controls after surgery in TLE in both the thalamic and fusiform clusters. In the thalamic cluster, ALFF was not different from controls prior to surgery, while in the fusiform cluster, ALFF was slightly decreased from controls prior to surgery. Additionally, the structural connectivity between the anterior hippocampus and both clusters in TLE was decreased prior to surgery compared to the relatively strong connectivity between these regions in healthy controls. Taken together, higher, healthier presurgical structural connectivity is associated with greater ALFF changes after surgery. However, the interpretation of increased ALFF is unclear in this context and it is important to note that a few patients had decreases in ALFF from pre- to post-surgery.
Prior to surgery, the epileptic focus is atrophied,43,44 hypometabolic45 and has increased ALFF12,21 in TLE. This increased ALFF in the epileptic focus is thought to reflect hyperactivity,12,21 while the glucose hypometabolism is thought to reflect decreased activity from neuronal loss or decreased synaptic density.45 These contradictory interpretations are not well understood. However, Dienel et al.46 recently proposed that presurgical glucose hypometabolism in the epileptic focus may reflect changes in the mechanisms of metabolism as opposed to decreased activity. Interestingly, TLE surgery leads to a similar pattern of atrophy9,47 and decreased glucose metabolism8,48 in the ipsilateral thalamus and fusiform gyrus, both of which increase in ALFF after surgery. This may suggest the development of an epileptogenic process involving the thalamus and/or fusiform after surgery, however we did not find a relationship between increased ALFF and seizure outcome in this study. More investigations are needed to explore this idea along with other potential physiological mechanisms underlying these changes.
Limitations and considerations
An important consideration of this study is that we included both patients who received ATLs and SAHs. ATLs remove a significant portion of the anterior and lateral temporal cortex in addition to the mesial temporal structures resected in a SAH.49 Although these surgeries are different, we believe that the inclusion of both in this study is appropriate since we identified changes in function across the combined group, such that they were common to both ATL and SAH patients. However, patients who received an ATL did have larger increases in ALFF in the thalamus than those who received a SAH, which we suspect may be due to the thalamus being disconnected from more regions. To mitigate the effects of combining these two groups, we controlled for surgery type in our correlational analyses. In addition, we provided correlational results including only patients who underwent a SAH to show that the functional change—structural disconnection relationship held in these patients alone.
Another limitation of this study was that we only established a correlational relationship between the structural disconnection and functional changes. Additionally, the neural and physiological underpinnings of the fMRI signal are complex and remain poorly understood,50 limiting interpretations of fMRI studies including this one. Further multimodal studies including electrophysiological recordings may be able to advance our understanding of these post-surgical fMRI changes and their functional implications. Moreover, the small sample size in this study reduces its generalizability and statistical power. Due to this small sample size, we combined right and left TLE patients into a single group to increase statistical power, however, structural connectivity can vary based on the side of epilepsy.51 Finally, structural connectivity measures derived from dMRI are not exact representations of the underlying biological anatomy,52 which affects our estimates of structural disconnection.
Conclusions
In this study we leveraged a generally homogeneous population of patients with similar resections to study the functional effects of structural disconnection in humans. Our results show that the surgical resection of the epileptogenic brain tissue in the hippocampus leads to increases in functional MRI activity in regions both near and distant to the resection. We found that these functional changes linearly related to the structural disconnection from the resection. These changes may be related to clinically significant behavioural and neuropsychological deficits or seizure recurrence, not directly explained by the location of the resection. Broadly, this study provides a novel link between focal disconnections in the structural brain network and downstream effects on function in distant brain regions.
Supplementary Material
Contributor Information
Lucas E Sainburg, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232, USA; Institute of Imaging Science, Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN 37232, USA.
Andrew P Janson, Institute of Imaging Science, Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN 37232, USA.
Graham W Johnson, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232, USA; Institute of Imaging Science, Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN 37232, USA.
Jasmine W Jiang, Institute of Imaging Science, Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN 37232, USA; Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN 37232, USA.
Baxter P Rogers, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232, USA; Institute of Imaging Science, Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN 37232, USA.
Catie Chang, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232, USA; Institute of Imaging Science, Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN 37232, USA; Department of Electrical and Computer Engineering, Vanderbilt University, Nashville, TN 37212, USA.
Dario J Englot, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232, USA; Institute of Imaging Science, Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN 37232, USA; Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN 37232, USA; Department of Electrical and Computer Engineering, Vanderbilt University, Nashville, TN 37212, USA.
Victoria L Morgan, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232, USA; Institute of Imaging Science, Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN 37232, USA; Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN 37232, USA.
Funding
This work was supported by the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health (T32EB021937 to L.E.S.) and the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (R01 NS075270, R01 NS108445, and R01 NS110130 to V.L.M.; R00 NS097618 to D.J.E.).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: An update. Neurosurg Rev. 2014;37:389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis KA, Jirsa VK, Schevon CA. Wheels within wheels: Theory and practice of epileptic networks. Epilepsy Curr. 2021;21:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrera E, Tononi G. Diaschisis: Past, present, future. Brain. 2014;137:2408–2422. [DOI] [PubMed] [Google Scholar]
- 4. Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138:3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen AL, Soussand L, Corrow SL, Martinaud O, Barton JJS, Fox MD. Looking beyond the face area: Lesion network mapping of prosopagnosia. Brain. 2019;142:3975–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siddiqi SH, Kording KP, Parvizi J, Fox MD. Causal mapping of human brain function. Nat Rev Neurosci. 2022;23:361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ganos C, Al-Fatly B, Fischer JF, et al. A neural network for tics: Insights from causal brain lesions and deep brain stimulation. Brain. 2022;145:4385–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joo EY, Hong SB, Han HJ, et al. Postoperative alteration of cerebral glucose metabolism in mesial temporal lobe epilepsy. Brain. 2005;128:1802–1810. [DOI] [PubMed] [Google Scholar]
- 9. Li W, Jiang Y, Qin Y, et al. Dynamic gray matter and intrinsic activity changes after epilepsy surgery. Acta Neurol Scand. 2021;143:261–270. [DOI] [PubMed] [Google Scholar]
- 10. Li W, Jiang Y, Qin Y, et al. Structural and functional reorganization of contralateral hippocampus after temporal lobe epilepsy surgery. NeuroImage Clin. 2021;31:102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morgan VL, Rogers BP, González HFJ, Goodale SE, Englot DJ. Characterization of postsurgical functional connectivity changes in temporal lobe epilepsy. J Neurosurg. 2019;133:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sainburg LE, Little AA, Johnson GW, et al. Characterization of resting functional MRI activity alterations across epileptic foci and networks. Cereb Cortex. 2022;32:5555–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asman AJ, Landman BA. Non-local statistical label fusion for multi-atlas segmentation. Med Image Anal. 2013;17:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huo Y, Plassard AJ, Carass A, et al. Consistent cortical reconstruction and multi-atlas brain segmentation. NeuroImage. 2016;138:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fischl B. Freesurfer. NeuroImage. 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McHugo M, Talati P, Woodward ND, Armstrong K, Blackford JU, Heckers S. Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. NeuroImage Clin. 2018;20:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casseb RF, de Campos BM, Morita-Sherman M, et al. Resectvol: A tool to automatically segment and characterize lacunas in brain images. Epilepsia Open. 2021;6:720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. [DOI] [PubMed] [Google Scholar]
- 19. Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. [DOI] [PubMed] [Google Scholar]
- 20. Tang Y, Choi JY, Alexopoulos A, et al. Individual localization value of resting-state fMRI in epilepsy presurgical evaluation: A combined study with stereo-EEG. Clin Neurophysiol. 2021;132:3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Z, Lu G, Zhong Y, et al. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp. 2010;31:1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng H, Pizarro R, Nair VA, La C, Prabhakaran V. Alterations in regional homogeneity of resting-state brain activity in mesial temporal lobe epilepsy. Epilepsia. 2013;54:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: Complex and reliable. NeuroImage. 2010;49:1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia XZ, Sun JW, Ji GJ, et al. Percent amplitude of fluctuation: A simple measure for resting-state fMRI signal at single voxel level. PLoS One. 2020;15:e0227021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai LY, Yang Q, Hansen CB, et al. Prequal: An automated pipeline for integrated preprocessing and quality assurance of diffusion weighted MRI images. Magn Reson Med. 2021;86:456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cordero-Grande L, Christiaens D, Hutter J, Price AN, Hajnal JV. Complex diffusion-weighted image estimation via matrix recovery under general noise models. NeuroImage. 2019;200:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016;125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tournier JD, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage. 2019;202:116137. [DOI] [PubMed] [Google Scholar]
- 29. Schilling KG, Blaber J, Huo Y, et al. Synthesized b0 for diffusion distortion correction (Synb0-DisCo). Magn Reson Imaging. 2019;64:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage. 2007;35:1459–1472. [DOI] [PubMed] [Google Scholar]
- 31. Smith RE, Tournier JD, Calamante F, Connelly A. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage. 2012;62:1924–1938. [DOI] [PubMed] [Google Scholar]
- 32. Smith RE, Tournier JD, Calamante F, Connelly A. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. NeuroImage. 2015;119:338–351. [DOI] [PubMed] [Google Scholar]
- 33. Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 34. Engel J. Surgical treatment of the epilepsies. Raven Press; 1993. [Google Scholar]
- 35. Bubb EJ, Kinnavane L, Aggleton JP. Hippocampal–diencephalic–cingulate networks for memory and emotion: An anatomical guide. Brain Neurosci Adv. 2017;1:2398212817723443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Majtanik M, Gielen F, Coenen VA, Lehtimäki K, Mai JK. Structural connectivity of the ANT region based on human ex-vivo and HCP data. Relevance for DBS in ANT for epilepsy. NeuroImage. 2022;262:119551. [DOI] [PubMed] [Google Scholar]
- 37. Li MCH, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. 2018;59:273–290. [DOI] [PubMed] [Google Scholar]
- 38. Grodd W, Kumar VJ, Schüz A, Lindig T, Scheffler K. The anterior and medial thalamic nuclei and the human limbic system: Tracing the structural connectivity using diffusion-weighted imaging. Sci Rep. 2020;10:10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O’Muircheartaigh J, Keller SS, Barker GJ, Richardson MP. White matter connectivity of the thalamus delineates the functional architecture of competing thalamocortical systems. Cereb Cortex. 2015;25:4477–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Middlebrooks EH, He X, Grewal SS, Keller SS. Neuroimaging and thalamic connectomics in epilepsy neuromodulation. Epilepsy Res. 2022;182:106916. [DOI] [PubMed] [Google Scholar]
- 41. Smith CD, Lori NF, Akbudak E, et al. MRI Diffusion tensor tracking of a new amygdalo-fusiform and hippocampo-fusiform pathway system in humans. J Magn Reson Imaging. 2009;29:1248–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karunakaran S, Rollo MJ, Kim K, et al. The interictal mesial temporal lobe epilepsy network. Epilepsia. 2018;59:244–258. [DOI] [PubMed] [Google Scholar]
- 43. Bonilha L, Rorden C, Castellano G, et al. Voxel-Based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol. 2004;61:1379–1384. [DOI] [PubMed] [Google Scholar]
- 44. Li J, Zhang Z, Shang H. A meta-analysis of voxel-based morphometry studies on unilateral refractory temporal lobe epilepsy. Epilepsy Res. 2012;98:97–103. [DOI] [PubMed] [Google Scholar]
- 45. Kumar A, Chugani HT. The role of radionuclide imaging in epilepsy, part 1: Sporadic temporal and extratemporal lobe epilepsy. J Nucl Med. 2013;54:1775–1781. [DOI] [PubMed] [Google Scholar]
- 46. Dienel GA, Gillinder L, McGonigal A, Borges K. Potential new roles for glycogen in epilepsy. Epilepsia. 2023;64:29–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li W, Jiang Y, Qin Y, et al. Cortical remodeling before and after successful temporal lobe epilepsy surgery. Acta Neurol Scand. 2022;146:144–151. [DOI] [PubMed] [Google Scholar]
- 48. Güvenç C, Dupont P, Van den Stock J, et al. Correlation of neuropsychological and metabolic changes after epilepsy surgery in patients with left mesial temporal lobe epilepsy with hippocampal sclerosis. EJNMMI Res. 2018;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mathon B, Clemenceau S. Chapter 29 - Surgery procedures in temporal lobe epilepsies. In: Miceli G, Bartolomeo P, Navarro V, eds. Handbook of clinical neurology. Vol. 187.Elsevier; 2022:531–556. [DOI] [PubMed] [Google Scholar]
- 50. Ekstrom AD. Regional variation in neurovascular coupling and why we still lack a Rosetta stone. Philos Trans R Soc B Biol Sci. 2021;376:20190634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Besson P, Dinkelacker V, Valabregue R, et al. Structural connectivity differences in left and right temporal lobe epilepsy. NeuroImage. 2014;100:135–144. [DOI] [PubMed] [Google Scholar]
- 52. Schilling KG, Nath V, Hansen C, et al. Limits to anatomical accuracy of diffusion tractography using modern approaches. NeuroImage. 2019;185:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this article are available from the corresponding author upon reasonable request.