Abstract
Cognitive control plays a pivotal role in guiding human goal-directed behavior, and revealing its lifespan trajectory is crucial for optimizing cognitive functioning at different ages, especially for stages of rapid development and decline. While existing studies have shed light on the inverted U-shaped trajectory of cognitive control function both behaviorally and anatomically, little is known about the corresponding changes in functional brain activation with age. To bridge this gap, we conducted a comprehensive meta-analysis of 129 neuroimaging studies using conflict tasks, encompassing 3,388 participants whose age spanned from 5 to 85 years old. We applied the seed-based d mapping (SDM), generalized additive model (GAM) and model comparison approaches to investigate age-related changes of brain activity, chart the lifespan trajectories and pinpoint peaks of cognitive control brain activity. The present study have three major findings: 1) The inverted U-shaped lifespan trajectory is the predominant pattern; 2) Cognitive control related brain regions exhibit heterogeneous lifespan trajectories: the frontoparietal control network (such as the inferior frontal gyrus and inferior parietal lobule) follows inverted U-shaped trajectories, peaking between 24 and 41 years, while the dorsal attention network (such as the frontal eye field and superior parietal lobule) demonstrates flatter trajectories with age; 3) Both the youth and the elderly show weaker brain activities and greater left laterality than young adults. These results collectively reveal the lifespan trajectories of cognitive control, highlighting heterogeneous fluctuations in brain networks with age.
Keywords: Cognitive control, Lifespan, Brain activity, Neuroimaging meta-analysis, Heterogeneity
Introduction
The cognitive abilities of human beings dynamically change throughout the entire lifespan, experiencing rapid development in the early stages, and gradual decline in the later period of life. As one of the most fundamental cognitive functions, cognitive control is deeply engaged in various domains of high-level capabilities that human greatly outperform other species, such as decision making, planning and problem solving (Jonathan D Cohen, 2017). Cognitive control refers to the cognitive processes that enable individuals to manage and regulate their attention, thoughts, and actions, which plays a vital role in goal-directed behavior, allowing us to focus on the target and ignore distractors (Z. Li, Goschl, & Yang, 2020; Miller & Cohen, 2001). For instance, cognitive control enables us to concentrate on reading in a library despite the presence of people chatting nearby.
Cognitive control could be examined through various experimental paradigms, with conflict paradigms considered as essential for understanding its processes. For example, in the color-word Stroop task, the performance of color naming can be influenced by the congruency between the ink color and the word meaning, such as when the word “BLUE” is printed in red (incongruent) versus when the word “RED” is printed in red (congruent) (MacLeod, 1991; Stroop, 1935). The worsened performance in incongruent compared to congruent conditions is known as the congruency effect, and its magnitude is commonly interpreted as an indicator of the level of cognitive control exerted, with a higher congruency effect reflecting weaker cognitive control (J. D. Cohen, Dunbar, & McClelland, 1990). Beyond the Stroop task, several other paradigms are widely employed in cognitive control research. These include the Eriksen Flanker task (Eriksen & Eriksen, 1974), which measures the ability to maintain focused attention and respond to central stimuli while ignoring flanking distractions; the Simon task (Simon, 1969), which assesses spatial conflict and response inhibition; and the multi-source interference task (MSIT) (Bush & Shin, 2006), which is used to evaluate dual conflict processing and cognitive flexibility.
Cognitive control provides fundamental support for normal human behaviors. The young adults typically maintain an optimal mature level of cognitive control (Constantinidis & Luna, 2019). On the other hand, the youth (including children and adolescents) and elderly individuals may struggle with behavioral problems because of their suboptimal cognitive control system (Craik & Bialystok, 2006). For instance, school-age children may suffer from attentional deficits that are related to a noticeable deficit of control system (Gupta & Kar, 2009). The adolescents are characterized by more risk-taking or even susceptive to addiction, which is related to the underdeveloped cognitive control system (Casey, Getz, & Galvan, 2008; Diamond, 2013a) or insufficient top-down regulation on the limbic emotional system (Casey, Heller, Gee, & Cohen, 2019; Constantinidis & Luna, 2019). Elders may have difficulty focusing on target information as a result of decay in cognitive control (Zanto & Gazzaley, 2014) or a less efficient engagement of neural resources (Reuter-Lorenz & Cappell, 2008). While the state-of-art progress of cognitive/behavioral changes has been well-documented and shaped how the diagnosis of developmental/ageing related disorders (Association, 2013), the change of related neural system has been under-investigated. Understanding how the neural underpinning of cognitive control changes over the lifespan can yield valuable insights into the developmental and aging mechanisms of the human brain. This knowledge may lead to the identification of potential biomarkers for age-related disorders before any behavioral symptoms become apparent (Ing et al., 2019), and may assist in customizing cognitive training strategies based on brain activities (Adnan, Chen, Novakovic-Agopian, D’Esposito, & Turner, 2017).
Inverted U-shaped Lifespan Trajectory of Cognitive Control: Behavioral and Anatomical Evidence
Researchers have generally proposed that the cognitive control ability follows an inverted U-shaped trajectory across the lifespan (Casey, Tottenham, Liston, & Durston, 2005; Craik & Bialystok, 2006; C. Grady, 2012). Specifically, infants initially possess limited cognitive control that gradually develops as the brain anatomy matures. These functions continue to progress, reaching their peaks during young adulthood and maintaining relative stability until middle age, after which they begin to decline in elderly age (De Luca & Leventer, 2010). This inverted U-shaped trajectory has been generally supported by ample behavioral evidence. The Eriksen Flanker task, with its validity of detecting cognitive control across the lifespan (Weintraub et al., 2013), has been widely utilized, and results suggest a clear U-shape trajectory of conflict cost (measured by worsened behavioral performance, e.g., reaction time, in incongruent compared to congruent conditions) with age (Erb, Germine, & Hartshorne, 2023; Ferguson, Brunsdon, & Bradford, 2021; S. C. Li, Hammerer, Muller, Hommel, & Lindenberger, 2009). Similar results have been observed in other conflict tasks, such as color-word Stroop (Ferguson, et al., 2021). However, it is notable that a recent massive cross-sectional study (Erb, et al., 2023) found a weaker decline in performance on the Stroop and Simon tasks compared to the Flanker task, highlighting the possible variations in the lifespan trajectory across different conflict tasks.
Recent large-cohort studies have also found that grey and white matter volumes across all brain regions exhibit overall inverted U-shaped trajectories with age, with the grey matter peaks at early adolescence and the white matter peaks at young adulthood (Bethlehem et al., 2022; Brouwer et al., 2022). Gray matter volume exhibits a rapid increase during the early childhood, reflecting the growth and branching of dendrites and synaptogenesis; however, following its peak at early adolescent, there is a gradual volume thinning and density reduction due to synaptic pruning, where unused neural connections are eliminated, making brain networks more efficient (Beatriz Luna, 2009). White matter, on the other hand, undergoes continuous increase throughout adolescence until young adulthood due to myelination, a process that can enhance the efficiency of neural transmission (Giedd & Rapoport, 2010). During late adulthood, normal ageing yields protracted decline of brain structure, with the volumes of both gray matter and white matter reduced (C. Grady, 2012). These potentially provide the anatomical foundations for the lifespan trajectory of cognitive control, among other functions (Craik & Bialystok, 2006; Hammerer, Muller, & Li, 2014). Consistently, the gray matter volumes of the frontal and parietal regions, which are essential in cognitive control tasks (Q. Li et al., 2017), have also been found to increase during early childhood and atrophy in early elderly age (Giedd et al., 1999).
However, it remains largely unknown how brain activities during the performance of cognitive control tasks change over the lifespan.
Age Matters in Cognitive Control Related Brain Activities
Previous research has primarily focused on brain activities in either youths or elderly adults, rather than examining changes across the entire lifespan. With conflict paradigms, children and adolescents are often found to have lower brain activity than young adults in frontoparietal regions (Andrews-Hanna et al., 2011; Crone & Dahl, 2012; Konrad et al., 2005; Marsh et al., 2006; Veroude, Jolles, Croiset, & Krabbendam, 2013; Wilk & Morton, 2012; but see Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002). For example, Konrad, et al. (2005) found that children aged 8–12 years had reduced activation of dorsolateral prefrontal regions compared to young adults during the Flanker task, suggesting an immature cognitive control system. However, brain activity differences in elderly adults as compared to young adults during cognitive control tasks have been less consistently reported (C. Grady, 2012). Some studies have found that elderly adults have lower neural activity in frontoparietal regions than young adults (Milham et al., 2002; Prakash et al., 2009; Yaple, Stevens, & Arsalidou, 2019), possibly because elderly adults may be unable to engage in an equal level of control-related activity due to functional decline. On the other hand, other studies have found that elderly adults may exhibit greater brain activity in frontoparietal regions than young adults (Fernandez, Hars, Trombetti, & Vuilleumier, 2019; Sebastian et al., 2013), possibly because they have recruited additional brain regions to compensate for their decreased efficiency in utilizing control resources. Adding to the debate, it has been proposed that the cognitive control function in elderly adults might not decline at all (Paul Verhaeghen, 2014; P. Verhaeghen & Cerella, 2002). These conflicting findings underscore the complexity of understanding age-related differences in cognitive control.
Few studies have directly tested the change of cognitive control related brain activities across the lifespan. One existing study (G. Wood, Ischebeck, Koppelstaetter, Gotwald, & Kaufmann, 2009) observed a positive association between the activation within the bilateral prefrontal cortex and age. Given the relatively small sample size, the reliability of these findings is somewhat limited. As a result, it is difficult to draw a clear conclusion about how brain activities related to cognitive control change across the entire age range.
In addition to understanding how the activations of individual brain regions change with age, previous research has also suggested that the overall distribution of brain activity changes with age. Two notable phenomena have emerged as significant findings in the field of aging research: compensatory recruitment of additional brain regions and a decrease in hemispheric asymmetry in brain activities. The concept of compensatory recruitment posits that, while aging may lead to reduced functionality in specific brain areas, older adults can mitigate this decline by engaging alternative neural circuits or regions. This compensatory mechanism serves as a form of neural adaptation to the challenges of aging, allowing for the preservation of cognitive function despite age-related physiological changes (Reuter-Lorenz & Cappell, 2008). Similarly, some developmental studies have also reported compensatory brain recruitment in young children (see review Crone & Steinbeis, 2017). In parallel, previous studies have reported a decrease in hemispheric asymmetry in older populations. This phenomenon, often referred to as the hemispheric asymmetry reduction in older adults (HAROLD) model, proposes that older adults exhibit a reduced laterality of brain activation compared to younger individuals (Cabeza, 2002). This phenomenon might reflect a form of neural compensation, where both hemispheres are utilized to perform tasks that once predominantly engaged only one hemisphere (Park & Reuter-Lorenz, 2009).
The Hierarchical Developmental Trajectories across Different Brain Regions
Neurodevelopment follows a hierarchical trajectory, characterized by distinct rates of development and decline across various brain networks. Research has revealed a clear developmental hierarchy, where higher-order cortical regions tend to undergo more complex lifespan trajectories compared with the lower-order subcortical regions (Shaw et al., 2008). Additionally, it has been observed that higher-order associative regions typically reach the peak later than the lower-order primary areas (Gogtay et al., 2004; Sydnor et al., 2023; Teissier & Pierani, 2021). Notably, the frontal region is among the last regions to reach their peaks and one of the earliest to show declination (Casey, et al., 2005; Craik & Bialystok, 2006; Raz et al., 1997).
Importantly, the brain regions associated with cognitive control have been proposed to exhibit a hierarchical organization. At the apex of this hierarchy is the frontoparietal control network (FPCN), which is crucial for supporting multiple higher-order functions (Dixon et al., 2018; Duncan, 2010). During conflict resolution, FPCN is closely associated with central executive control processes, which involve maintaining goals and modulating sensory inputs in a top-down manner (Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013). The dorsal attention network (DAN), responsible for directing selective attention allocation (Corbetta & Shulman, 2002), is generally thought to be modulated by the FPCN (Dixon, et al., 2018; Spreng, et al., 2013). Another network pertinent to conflict processing is the cingulo-opercular network (CON), which is primarily involved in detecting salient stimuli (Seeley et al., 2007) and monitoring conflicts (Q. Li, et al., 2017). Research suggests that the CON and DAN occupy lower positions in the hierarchy compared to the FPCN (Margulies et al., 2016), with some evidence indicating that the CON may be situated at a higher level than the DAN (Zhou et al., 2018). Previous investigations into the morphological and connectivity-based characteristics of these networks have demonstrated that they follow distinct lifespan trajectories (Betzel et al., 2014; DuPre & Spreng, 2017). However, the specific lifespan trajectories of engagement of these networks during cognitive control tasks remain to be fully elucidated.
The Necessity of Conducting Meta-analyses
One direct way to test the trajectory of brain activation related to cognitive control is to conduct a large cohort of neuroimaging study with participants covering a wide age range. To the best of our knowledge, such studies have not yet been conducted. An alternative approach is to utilize meta-analyses to combine the results of existing studies targeting different age groups. Compared to the large cohort studies, meta-analyses are more readily available and resource-saving. In addition, meta-analyses show several advantages over individual studies. By combining results from various studies, meta-analyses can increase the statistical power and generalizability of the findings and thus result in more precise conclusions. Meta-analyses can also reduce the heterogeneity and bias that may exist in individual studies because of different methods, populations, or confounding variables (Hart, Radua, Nakao, Mataix-Cols, & Rubia, 2013). Importantly, meta-analyses can reveal patterns or trends that may not be immediately evident in individual studies, such as nonlinear effects or interactions (Hart, et al., 2013). There have been several neuroimaging meta-analyses that attempted to examine the changes of cognitive control brain activity with age (Long et al., 2022; Yaple, et al., 2019; Zhang et al., 2021), but a few limitations prevent them from appropriately answering questions about the lifespan trajectory of cognitive control functions. These limitations include not covering the full range of age across the lifespan (Yaple, et al., 2019; Zhang, et al., 2021), or having too few studies in some age ranges (e.g., elderly adults) (Long, et al., 2022). In addition, these studies have primarily focused on the spatial convergence and/or diversity of reported coordinates across different age ranges using methods like the activation likelihood estimation (ALE), which provides limited insights into lifespan trajectory of activity strength. Although a few studies have attempted parametric meta-regression with the seed-based d mapping (SDM) approach to examine the age-related differences in cognitive control (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011; Zhang, et al., 2021), they have been constrained by utilizing linear models that may overlook non-linear trajectory patterns, such as the inverted U-shaped trend.
The Present Study
The goal of this study is to reveal the lifespan trajectory of brain activities responsible for cognitive control. Instead of conducting a comprehensive meta-analysis encompassing various aspects of cognitive control such as working memory, cognitive flexibility, and conflict processing, we choose to focus on conflict processing as an indicator of cognitive control for several reasons. First, conflict processing reflects the ability to maintain a goal in mind while avoiding distractions, which is a fundamental aspect of cognitive control (Miller & Cohen, 2001). Second, the mechanisms underlying conflict processing in young adults are relatively well-known, with the frontoparietal and cingulo-opercular networks repeatedly reported as the most involved brain regions (Q. Li, et al., 2017; Nee, Wager, & Jonides, 2007), providing a baseline reference for our study. Third, conflict tasks with neuroimaging data have been widely applied to both younger and elderly groups, making a systematic meta-analysis feasible. Lastly, different conflict tasks share components on key aspects of cognitive control, such as conflict monitoring (Botvinick, Cohen, & Carter, 2004) and inhibitory control (Diamond, 2013b). Consequently, different conflict tasks can be measured using a clearly defined congruency effect (i.e., the contrast between incongruent and congruent/neutral conditions), which enables us to conduct effect-size based meta-analyses. Including other sub-processes of cognitive control may introduce heterogeneity and make the effect sizes incomparable.
Based on previous research, we hypothesized that brain activities related to cognitive control increase rapidly from childhood to adolescence, reach a peak in young adulthood, and then gradually decline with increasing age, following an inverted U-shaped trajectory. Additionally, we hypothesized that different brain networks may show some heterogeneity in their lifespan trajectories.
Methods
Literature Preparation
Literature Search
We report how we determined our sample size, all data exclusions (if any), all manipulations, and all measures in the study. We first searched both English and Chinese articles on the youth and the elderly from PUBMED, Web of Science and CNKI (China National Knowledge Infrastructure) till 2022. The following search terms were applied in titles, abstracts, table of contents, indexing, and key concepts: (“Stroop” OR “Flanker” OR “Simon” OR “SNARC” OR “Navon” OR “interference” OR “cognitive conflict”) AND (“fMRI” OR “functional resonance imaging” OR “functional imaging” OR “neuroimaging” OR “PET”) AND (“children” OR “kids” OR “adolescents” OR “teenagers” OR “underage” OR “aged” OR “old” OR “older” OR “elder” OR “elderly” OR “senior” OR “development” OR “developmental” OR “aging” OR “life span”). The above process yielded 3,484 articles. Besides, 111 studies on the young adult from a previous meta-analysis study (Q. Li, et al., 2017) were adopted, which had applied very similar recruitment criteria. Moreover, we screened 16 articles citing or being cited by the crucial literature. After removing duplicates, the literature search identified 2,930 articles.
Exclusion Criteria
We excluded any articles that met one or more of the following predefined exclusion criteria: 1) not in English or Chinese; 2) not including healthy human participants; 3) case study; 4) not empirical study; 5) not functional resonance imaging (fMRI) or positron emission tomography (PET) study; 6) not whole-brain results (i.e., not have covered the whole gray matter); 7) not in Talairach or Montreal Neurological Institute (MNI) space; 8) not reflecting the congruency effect (i.e., contrasts between incongruent and congruent or between incongruent and neutral conditions); 9) not reporting exact mean age of participants.
A total of 119 articles were identified as eligible for inclusion in our meta-analyses. Supplementary Figure S1 shows the preferred reporting items for systematic reviews and meta-analyses (PRISMA) (Page et al., 2021) flow chart for the literature screening process. The 119 articles included 129 studies (individual contrasts reported in the articles) with 3,388 participants and 1,579 activation foci reported. None of the experiments share the same group of participants.
Coding Procedure
A coding manual was formulated to record pertinent study information, including authors, publication dates, experimental tasks, contrasts, and sample demographics (such as the average age and sample size). To ensure coding accuracy, two authors independently coded all studies, with discrepancies resolved through discussion or reference to the original studies. In instances where studies lacked essential information, such as peak coordinates for relevant contrasts, participant age averages, or data for specific age groups, efforts were made to contact the authors via e-mail to obtain the relevant data. In addition, both coordinates and effect sizes (i.e., Hedge’s g) were extracted from each study. Further, Talairach space coordinates were transformed to MNI coordinates using the Lancaster transform (Lancaster et al., 2007).
Meta-Analytic Procedure
Activation Likelihood Estimation (ALE)
In order to obtain a comprehensive understanding of cognitive control-related brain activity across all age groups and to replicate a prior study (Q. Li, et al., 2017), we initially conducted a single dataset meta-analysis using BrainMap GingerALE software (Turkeltaub et al., 2012) (version 3.0.2, http://www.brainmap.org). This meta-analytical approach, known as activation likelihood estimation, utilizes the spatial convergence of brain activity across multiple studies to determine the probability of activation in specific regions. Foci from individual studies were transformed into a standardized coordinate space and modeled as Gaussian probability values that accounted for variability in the number of participants in each study. In situations where foci overlapped across studies, multiple Gaussians were associated with a single focus, and ALE selected the Gaussian with maximum probability for each focus (Turkeltaub, et al., 2012). Subsequently, ALE score maps were generated by comparing these modeled Gaussian distributions with a null distribution that simulated random brain effects. The null distribution was generated using the same sample size and number of foci groups as the experimental dataset for 1,000 times (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012). ALE scores were then used to calculate p-values, which were based on the proportion of values higher than a certain threshold in the null distribution. This resulted in a statistical ALE map that differentiated true brain effects from random effects. A cluster-defining threshold of p < 0.001 and a minimum cluster size of 10 voxels (80 mm3) were utilized to compute ALE maps, which matches the threshold applied in the seed-based d mapping (SDM) approach (see below).
Seed-based Effect Size Mapping
SDM is an alternative approach to statistically synthesize results from multiple fMRI experiments (Albajes-Eizagirre, Solanes, Vieta, & Radua, 2019). Similar to ALE, SDM employs a coordinate-based random-effects approach to amalgamate peak coordinate information into a standard space across several experiments. However, while ALE solely considers the binary feature (i.e., active versus inactive) of peak coordinates, SDM takes into account the quantitative effect size (can be positive or negative) connected to each peak and reconstructs the initial parametric maps of individual experiments before amalgamating them into a meta-analytic map (Radua & Mataix-Cols, 2012). Therefore, the use of a distinct algorithm in SDM from ALE allows us to scrutinize the robustness and replicability of the outcomes obtained via ALE. More importantly, SDM enables the inclusion of covariates in the meta-regression analyses to reflect the changes in brain function across the lifespan.
We conducted three types of analyses using the SDM approach. Firstly, we estimated the mean activation across all age groups and compared the results with ALE’s single dataset meta-analysis results. Secondly, we conducted contrasts between two groups of studies (e.g., between young adults and a combination of the youth and elderly groups) to identify brain regions that showed different levels of activity across age. This analysis method served to investigate the hypothesized lifespan trajectories, such as the inverted U-shaped pattern by elucidating neural activity variations linked to age. Thirdly, we defined specific models (e.g., quadratic and square root models) to fit the whole brain to validate brain regions adhering to the hypothetical lifespan changing patterns. This part of the analysis aimed to explore various lifespan trajectories, recognizing that different brain regions might follow distinct model functions.
These analyses were conducted using the software of SDM with permutation of subject images (SDM-PSI) (version 6.22, https://www.sdmproject.com). Effect size maps were built for the 129 individual experiments. This was accomplished by (a) converting the statistical value of each peak coordinate into an estimate of effect size (Hedge’s g) using standard formulas (Hedges, 1981) and (b) convolving these peaks with a fully anisotropic unnormalized Gaussian kernel (α = 1, FWHM = 20 mm) within the boundaries of a gray matter template (voxel size = 2×2×2 mm3). Imputation (50 times) was conducted for each study separately to obtain an reliable estimate of brain activation maps (Radua & Mataix-Cols, 2012). Besides, the individual effect size maps were combined using a random-effect general linear model. To assess the statistical significance of activations in the resulting meta-analytic effect size map, 1,000 random permutations of activation peaks within the gray matter template were compared. Finally, the meta-analytic maps were thresholded using a voxel-wise family-wise error (FWE) corrected threshold of p < 0.001 and a cluster-wise extent threshold of 10 voxels (Radua et al., 2012a).
Mean analyses across all studies
This analysis aimed to characterize the activation distributions of cognitive control-related brain regions across all studies, which was conducted utilizing the “Mean” function in SDM-PSI software. In order to verify the reliability of the SDM analysis results, we compared the results with the single dataset meta-analysis using ALE. Results from this analysis were further used as regions of interest (ROIs) in the subsequent model fitting analyses (see the section “Generalized Additive Model (GAM) Fitting”).
Contrast analyses
To test our hypothesis that cognitive control related brain activities follow an inverted U-shaped trajectory with age, we categorized each study based on the mean age of participants into youth (0–17 years), young adults (18–59 years), and elderly (60–100 years) groups. The age boundaries were determined to minimize age distribution overlap. We utilized SDM-PSI to perform a contrast analysis between the group of young adults and the combination of other groups in order to examine whether there are brain regions that exhibit an inverted U-shaped lifespan trajectory. This was achieved by assigning studies from the young adult group as 1 and all other studies as −1. This analysis yielded two results, one showing higher activity in young adults than the youth and elderly groups, and the other showing the opposite. Like the mean analysis, results from this analysis were used as ROIs in the subsequent model fitting analyses (see sections “Generalized Additive Model (GAM) Fitting” and “Model Simplification and Model Comparison”). The possibility of publication bias for resultant clusters was examined using Egger’s test (Egger, Smith, & Phillips, 1997), in which any result showing p < 0.05 was regarded as having significant publication bias. Heterogeneity was evaluated using the I2 index, which quantifies the proportion of total variability attributable to heterogeneity between studies. A value less than 25% indicates low heterogeneity among the included studies (Higgins, Thompson, Deeks, & Altman, 2003).
In addition, to explore other possible trajectories, such as the increase-and-stable pattern (Craik & Bialystok, 2006), we conducted contrast analyses between the youth and the combined group of young and old adults, as well as between old adults and the combined group of youth and young adults. Furthermore, to address the controversies in previous studies, we conducted contrast analyses between old and young adult groups, and between the youth and young adult groups, respectively.
Meta-regression analyses
To better describe the possible lifespan trajectories of the whole brain, we carried out meta-regression analyses with the age and/or its derivatives as regressors. In total three regressions were conducted across the whole-brain, including a linear regression (with only age as the regressor), a quadratic regression (with age and its square as separate regressors) and a square root regression (with age and its square root as separate regressors). The linear regression aimed to test regions with increasing/decreasing activity with age, and the other two non-linear regressions aimed to test regions with the inverted U-shaped trajectories based on the model fitting results (see below).
Data Extraction
Masks were generated for each ROI derived from the mean and contrast analyses in SDM-PSI as described above. Subsequently, we extracted the effect sizes for each mask. Fifty values were obtained from the SDM iterations and subsequently averaged for each study in each region. The iterated variances were also averaged in a similar way. Additionally, we removed the outliers (beyond 3 standard deviations from the mean) in the following model fitting analyses.
Generalized Additive Model (GAM) Fitting
To precisely estimate the inverted U-shaped trajectories, we adopted the GAM to fit the curves. The GAM allows for flexible, nonparametric smoothing of predictor variables (S. N. Wood, 2001), and has been widely used to depict the lifespan trajectories (Bethlehem, et al., 2022; Zuo et al., 2017). We implemented GAMs using the “mgcv” package (S. N. Wood, 2001) in R. For each ROI, we fitted a GAM with the following formula:
where is the effect size (dependent variable), s(age) represents a smoothing spline of age (predictor variable), and covariates represent the dummy-coded categorical covariate regressors. These regressors correspond to four aspects of the included studies: (1) the presence of various conflict types (e.g., Stroop or Simon), (2) the mixed subject samples based on handedness (e.g., right handed only or both handed), (3) the different contrasts in reporting congruency effects (e.g, incongruent – congruent or incongruent – neutral), and (4) different trial types regarding whether they excluded error trials. We incorporated these covariates to control for their potential confounding effects related to age, which could otherwise influence our results. We also adjusted the estimate with a weight parameter, which was the reciprocal of variance. We used penalized regression splines, with the amount of smoothing determined automatically based on generalized cross validation.
For each ROI, we quantified the peak age by choosing the highest prediction of a fine-grained age scale (1,000 points from 8 to 74 years old). We also calculated the estimated degree of freedom (EDF) for the smooth curve by summing up the degree of freedom for each penalized term (i.e., s(age).1 to s(age).9).
Model Simplification and Model Comparison
While the GAM analysis may yield good fitting results on the data, it is important to acknowledge its potential drawbacks. One concern is that it can fit the data with a high degree of freedoms (up to 7.0, supplementary Table S4), which makes it susceptible to over-fitting and harder to generalize. Another issue is its poor interpretability. Therefore, we next sought to fit the data with simpler models.
To this end, we used the “metafor” package in R to fit these effect sizes with the age and its derivatives as predictors. Specifically, we tested four non-linear models (see below formulas and Figure 7). Quadratic and cubic models were included based on previous studies (Coupe, Catheline, Lanuza, Manjon, & Alzheimer’s Disease Neuroimaging, 2017; Zuo et al., 2010); quadratic logarithmic and square-root models were included to capture the possible skewed trajectory, which would reflect the asymmetric trajectory of development and decline of cognitive control (Craik & Bialystok, 2006). Each model was fitted to each ROI separately. In addition, we included the same covariates as the GAM analysis in each regression model. We calculated the Akaike information criterion (AIC) to evaluate the goodness of fit for each model.
Figure 7.

The lifespan trajectories explored in our study. Panels A-C show linear decrease, flat, and linear increase patterns, respectively, and were modelled with the linear function. Panels E and H show the upright and inverted U-shapes, respectively, and were tested with the contrast between young adults and others, as well as with the quadratic function. Panels D, F, G, and I show combinations of a stable period and an increase/decrease period across the lifespan, and were tested with the contrast between the youth and others, or between the older adults and others. Panels J, K and L show the variants of inverted U-shaped trajectories, which capture the possibly early peak feature. They were tested with square root, quadratic logarithmic, and cubic functions, respectively. See Methods for detailed models.
- Quadratic model
- Cubic model
- Quadratic logarithmic model
- Square root model
Neurosynth Decoding Analysis
To investigate the potential functional difference between the two sets of brain regions reported in section “Dissociated Brain Networks with Distinct Lifespan Trajectories”, we generated two binary maps from the contrast analysis between young adults and the combination of other groups, and then submitted them to the Neurosynth decoding system (Yarkoni, et al., 2011). As the non-U-shaped map is defined as the converse of the inverted U-shaped map, they were obtained by applying thresholds of 0.1 < p < 0.9 and p < 0.05, respectively. These lenient threshold boundaries were used to minimize the impact of sparsity on the decoding results. The two-boundary threshold was used in generating the non-U-shaped map, as the original statistical map from the SDM-PSI analysis was one-tailed, which means the threshold of p > 0.9 indicates the upright U-shaped trend instead of a flat trajectory. Additionally, to focus on the brain regions specifically related to cognitive control, the two maps were masked by using results from the mean SDM analysis (see section “Regions Related to Cognitive Control Identified by both ALE and SDM” in Results). In the decoding results, we deleted terms that were related to brain regions (e.g., “frontal”), not functional specific (e.g., “task”), and duplicated (e.g., “attention” was removed if there was already “attentional”).
Laterality Analysis
We calculated the laterality based on the effect size of reported brain coordinates from each study. We computed the sum of effect sizes across coordinates for left and right hemisphere, respectively, yielding one global effect size each (i.e., and ). Then, we calculated the index of brain laterality with the following equation (Seghier, 2008):
which was then submitted to the GAM and simplified models (i.e., the quadratic, cubic, square root, and quadratic logarithmic models). Once again, we included covariates in our analysis to control for potential confounding factors, such as variations in task types.
To illustrate the contribution of different brain regions to the age-related change of laterality, we calculated the laterality for each voxel (Hoffman & Morcom, 2018). We first used the SDM-PSI to conduct a mean analysis for each age group (i.e., the youth, young adult and elderly), yielding three z-maps. Then the laterality was computed with the above equation for each voxel from the left hemisphere. The opposite values were calculated for the right hemisphere. To avoid the bias due to asymmetric brain hemispheres, we removed voxels without corresponding mirror coordinates. The results were visualized confining to the brain regions estimated from the grand mean analyses (see section “Seed-based Effect Size Mapping” in Results).
Transparency and Openness
We adhered to the MARS guidelines for meta-analytic reporting (Appelbaum et al., 2018). All meta-analytic data and analysis code are available at https://anonymous.4open.science/r/Cognitive_Control_Developmental_Trajectory-66E5. Data were analyzed using R, version 4.3.0 (R Core Team, 2018), and the packages mgcv, version (1.8.42) (S. N. Wood, 2011) and metafor, version 3.0–2 (Viechtbauer, 2010). This review project was not preregistered. It should be noted that ethics committee approval was not obtained for this study because it involved the analysis of existing data rather than the collection of new data from human participants. However, all datasets used in the analysis were obtained from studies that had undergone ethical review and approval.
Results
Sample Description
A total of 3,611 articles were identified including 3,484 articles searched from the database, 111 articles adopted from a previous study (Q. Li, et al., 2017), and 16 articles manually searched from the references of crucial articles. After excluding duplicates and applying exclusion criteria, 119 articles including 129 studies with N = 3,388 participants and 1,579 brain activation foci, were included in this meta-analytic review (supplementary Table S1). Of the studies included, 125 were published in peer-reviewed journals, and 4 were master’s theses. All studies were published or completed between 1994 and 2022. The average age of participants ranged from 8 to 74 years, with the individual age ranged from 5 to 85 years. Included studies are written in English (115 articles) and Chinese (4 articles). All included studies reported the task type used, including 73 studies utilizing Stroop-like task (57%), 26 studies utilizing Simon task (20%), 25 studies utilizing Flanker task (19%), 2 studies utilizing a combination of Simon and Flanker tasks (2%), 1 study utilizing a combination of Simon and Stroop tasks (1%), and 2 studies utilizing multi-source interference task (2%). Besides, the contrasts conducted to reveal brain activations were also reported, with 98 studies (76%) resulting from the contrast of Incongruent trials > Congruent trials, 25 studies (19%) resulting from the contrast of Incongruent trials > Neutral trials, and 6 studies (5%) resulting from the union contrast of Incongruent trials > Congruent trials and Incongruent trials > Neutral trials. Regarding the handedness of participants in the included studies, 89 studies (69%) included right-handed participants only, 5 studies (4%) included both left and right-handed participants, while 35 studies (27%) did not report this information. Furthermore, 76 studies (59%) included only correct response trials, 2 studies (2%) included both correct and incorrect response trials, while 51 studies (39%) did not report this information. To eliminate the influence of these confounding factors, we included them as covariates in the modeling analyses. A detailed description of these features for each study is available in the Supplemental Table S1.
Regions Related to Cognitive Control Identified by both ALE and SDM
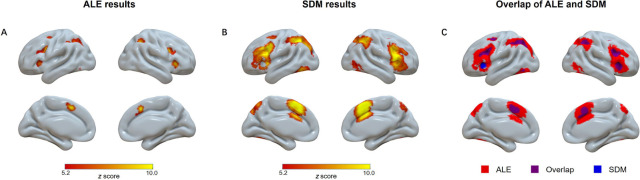
To enhance the replicability and robustness of our finding and enable direct comparison with a previous study (Q. Li, et al., 2017), we conducted the mean analysis across all studies with the ALE and SDM approaches separately. First, we performed the single dataset analysis with the GingerALE software to explore the consistently of brain regions reported in all the included studies. Results showed significant activation in the frontoparietal regions, including the left dorsolateral prefrontal cortex, bilateral frontal eye field, right inferior frontal gyrus and bilateral superior parietal lobule; the cingulo-opercular regions, including the pre-supplementary motor area and bilateral insula; and other regions, including left inferior temporal gyri (Figure 1A and supplementary Table S2). Second, we calculated the average brain activation based on the effect sizes reported in all studies using the SDM-PSI software. Similar to the ALE results, we found significant activation in the frontoparietal regions (left inferior parietal lobule, right inferior frontal gyrus, and right middle frontal gyrus), the cingulo-opercular regions (left anterior cingulate cortex), and other regions like bilateral inferior temporal gyrus, right caudate nucleus, right cerebellum, and left anterior thalamic projections (Figure 1B and supplementary Table S3). Despite their methodological distinction, that is, the SDM approach additionally incorporates the statistical values of brain activation compared to the ALE, these two approaches yielded very similar results (Figure 1C), both replicating a previous study including only the young adult studies (Q. Li, et al., 2017). The count of voxels revealed that the overlapped area (3,496 voxels) accounted for 96.3% of the regions from the ALE analysis (3,635 voxels), and 15.0% of the regions from the SDM analysis (23,240 voxels). This result also suggested that SDM analysis showed a greater sensitivity than the ALE analysis under the same threshold (voxel-wise FWE corrected p < 0.001, cluster ≥ 10 voxels).
Figure 1.
Overview of significant clusters across all studies regardless of age in the ALE meta-analysis (A), the SDM meta-analysis (B), and their overlap (C). ALE = activation likelihood estimation; SDM = seed-based d mapping.
Trajectories of Cognitive Control Regions Identified in the Mean Analysis
Having identified nine brain regions in the mean analysis using SDM-PSI, we proceeded to examine how the activation levels of these regions change with age. To this end, we extracted brain activity data from all studies for each identified region. Before performing the meta-regression, we verified the effectiveness of using mean age as a predictor with a simulation approach (see Supplementary Note S1). We then subjected the extracted data to separate GAM analyses, factoring in the confounding covariates. We did not conduct simplified model fitting (e.g., quadratic model) as we did not have pre-defined hypotheses about the trajectory shapes for these regions. The GAM analyses revealed significant age-related changes in the activation levels of 5 out of the 9 regions, ps < .05, with false discovery rate (FDR) corrected (Figure 2, panels A, B, C, E and G). The other four regions showed no significant age-related changes. Notably, none of the clusters showed significant publication bias based on Egger’s test (ps > 0.86), and they all showed low between-study heterogeneity (τs < 0.13, Qs < 12.11, I²s < 25%). This indicates that the observed results are not likely influenced by biased reporting or substantial variability in the included studies.
Figure 2.
Lifespan trajectories within regions identified in the mean analysis. Panels A-I correspond to left anterior cingulate cortex, left inferior parietal lobule, right inferior frontal gyrus, right inferior temporal gyrus, left inferior temporal gyrus, right middle frontal gyrus, right caudate nucleus, right cerebellum and left anterior thalamic projections, respectively. Scattered plots are the effect sizes as a function of age, with curves fitted by GAM. The sizes of the scattered dots show the square root of model weights (1/variance) for each study. Shaded areas around the curves represent standard errors. Dashed lines indicate peak ages. Panels A and B do not show the peak age due to an insignificant decrease at the later stage (see supplementary Note S2).
To test whether the visually inverted U-shaped trajectories reach significance, we applied the two-line test approach proposed by Simonsohn (2018) (see supplementary Note S2). Results suggest that all five regions (panels A, B, C, E, and G in Figure 2) involve an increase in activity from childhood to young adulthood (approximately up to 30 years of age). Three of them (panels C, E and G) showed consistent decrease in the later stages of age, and the other two (panels A and B) showed no significant decrease (supplementary Note S2).
A notable limitation of the model fitting using regions identified from the mean analysis is the considerable sizes of some regions, with the maximum region (i.e., the pre-supplementary motor area) encompassing over 9,000 voxels. This vastness means that the average trajectory of voxels within these regions could obscure the distinct trajectories specific to different subregions, which are crucial in understanding the hierarchical patterns of brain development and decline. Recognizing this issue, our next step was to delve deeper and examine the brain trajectory with a more granular approach.
Detecting Different Trajectories of Brain Activities
The present study aimed to investigate the lifespan trajectories of cognitive control brain activities. To explore various possibilities, we conducted several contrast analyses (Figure 7, also see Methods for details). However, our analyses did not reveal any regions that exhibited significantly higher or lower brain activity in the youth compared to others (the combination of young and older adults) (Table 1). Similarly, we did not observe any regions with higher or lower brain activity in older adults compared to others (the combination of the youth and young adults) (Table 1). These results excluded the possibilities of increase/decrease-then-stable and stable-then-increase/decrease trajectories (Figure 7, panels D, F, G, and I). In addition, we failed to observe any region showing lower activity in young adults than others (the combination of the youth and older adults), and thereby ruled out the possibility of the upright U-shaped trajectory (Table 1, Figure 7E).
Table 1.
Brain areas activated in the contrast of one age group versus others (voxel-wise FWE-corrected,p < 0.001, with minimum cluster size ≥ 10 voxels) with the SDM-PSI.
| Order | # Voxels | Z | p | L/R | MNI coordinate |
Anatomical location | BA | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
|
| |||||||||
| Young adults > Others | |||||||||
| 1 | 1165 | 4.713 | < 0.001 | R | 52 | 16 | 4 | inferior frontal gyrus | 48 |
| 2 | 607 | 4.895 | < 0.001 | L | −46 | 16 | 26 | inferior frontal gyrus | 48 |
| 3 | 569 | 4.289 | < 0.001 | R | 54 | −48 | 42 | inferior parietal lobule | 40 |
| 4 | 215 | 3.405 | < 0.001 | L | −8 | 0 | 58 | supplementary motor area | 6 |
| 5 | 202 | 3.830 | < 0.001 | L | −36 | −54 | 42 | inferior parietal lobule | 40 |
| 6 | 106 | 3.245 | < 0.001 | R | 10 | 4 | 6 | caudate nucleus | / |
| 7 | 76 | 3.231 | < 0.001 | L | −38 | 24 | 0 | insula | 47 |
| 8 | 33 | 3.119 | < 0.001 | L | −36 | 12 | −6 | insula | 48 |
| 9 | 10 | 2.794 | < 0.001 | R | 4 | −16 | 44 | anterior cingulate cortex | 23 |
| Others > Young adults | |||||||||
| None | |||||||||
| The youth > Others | |||||||||
| None | |||||||||
| Others > The youth | |||||||||
| None | |||||||||
| The elderly > Others | |||||||||
| None | |||||||||
| Others > The elderly | |||||||||
| None | |||||||||
Note. The orders in the table correspond to the numbers of each plot in Figure 3. ALE = activation likelihood estimation; MNI = Montreal Neurological Institute; BA = Brodmann area; L = left; R = right.
However, we identified greater activity in young adults compared to others in the frontoparietal regions, including bilateral inferior frontal gyrus and bilateral inferior parietal lobule; the cingulo-opercular regions, including left supplementary motor area, left insula, and right anterior cingulate cortex; and other regions, such as right caudate nucleus (Figure 3 and Table 1). Notably, none of the clusters showed significant publication bias based on Egger’s test (ps > 0.79), and they all showed low between-study heterogeneity (τs < 0.17, Qs < 12.21, I²s < 25%). This indicates that the observed results are not likely influenced by biased reporting or substantial variability in the included studies.
Figure 3.
Brain regions showing inverted U-shaped trajectory patterns. Scattered plots are the effect size as a function of age, with curves fitted by GAM (blue color) and the best simplified model (red color). Shaded areas around the curves represent standard errors. Dashed vertical lines show peak ages estimated from GAM (blue) and simplified model (red). The sizes of the scattered dots show the square root of model weights (1/variance) for each study. The numbers at the up-left corner of each plot correspond to the orders in Table 1. 1: right inferior frontal gyrus, 2: left inferior frontal gyrus, 3: right inferior parietal lobule, 4: left supplementary motor area, 5: left inferior parietal lobule, 6: right caudate nucleus, 7: left insula, 8: left insula, 9: right anterior cingulate cortex.
Fitting the Lifespan Trajectories with the Generalized Additive Model (GAM)
Results of the GAM applied to the regions identified from the contrast between young adults and others are shown in Figure 3 and supplementary Table S4. For each region, the GAM could fit the data significantly with a smooth curve, Fs > 4.7, ps < 0.002, FDR corrected, with degrees of freedom varying from 3.2 to 7.0. Peak ages of the inverted U-shaped trajectories were between 24.2 and 38.5 years.
Model Simplification of the Lifespan Trajectories
Considering the GAM may overfit the data, we fitted the results with simpler models commonly adopted in lifespan developmental literature (Amlien, Sneve, Vidal-Pineiro, Walhovd, & Fjell, 2018; Coupe, et al., 2017; Zuo, et al., 2010), including the quadratic, cubic, square root and quadratic logarithmic models, and estimated the goodness of fit by comparing their Akaike information criterion (AIC) (see section “Model Simplification and Model Comparison” in Methods). Weights of each model based on AIC values for each region are presented in supplementary Table S5. Results showed that the quadratic model provided the best fit for capturing the age-related changes in left inferior temporal gyrus, while the square root model demonstrated the best goodness of fit for all the other regions. We also calculated the peak age for each region based on the optimal model, and results showed that the peak ages ranged from 33.4 to 40.6 years. In addition, we found the peak ages obtained from the above optimal model (i.e., square root or quadratic models) and the GAM are consistent, r = 0.69, p < 0.05. The detailed results of the optimal model fitting are shown in Figure 3 and supplementary Table S6.
Fitting the Whole Brain with Quadratic, Square Root, and Linear Models
Based on model comparisons, we found that the quadratic and square root models provided the best goodness of fit for the age-related change of the brain activities. To supplement the inverted U-shaped results from the contrast analysis, these two models were then submitted to whole-brain meta-regression analyses in SDM-PSI.
By fitting the activation over the whole brain using the quadratic model, we found 4 significant brain regions, including the bilateral inferior frontal gyrus and bilateral inferior parietal lobule (Figure 4A and supplementary Table S7). Similarly, the square root model revealed 7 significant brain regions, including the bilateral inferior frontal gyrus, right superior longitudinal fasciculus, left inferior parietal lobule, right insula, right caudate nucleus, and left anterior thalamic projections (Figure 4B and supplementary Table S7). These results further supported the existence of the inverted U-shaped regions we initially identified, while also indicating that the contrast analysis exhibited a higher sensitivity compared to these functions.
Figure 4.
Significant clusters (voxel-wise FWE-corrected, p < 0.001, voxels ≥ 10) showing quadratic pattern (A) and square root pattern (B) with age in the model fitting.
In addition, we explored the whole-brain trajectories with a linear meta-regression. However, no significant regions were observed, even under a more tolerant threshold of uncorrected p < 0.01.
Dissociated Brain Networks with Distinct Lifespan Trajectories
We note that the inverted U-shaped regions constitute only part of the cognitive control-related regions (Figure 2), and the remaining regions appear to show a flat trajectory. To further elucidate the spatial distribution of brain regions following these two trajectory patterns, we used the results from the mean SDM analysis (see section “Regions Related to Cognitive Control Identified by both ALE and SDM”) as the mask and replotted the results of the contrast analysis between young adults and others with two different thresholds, and then compared them with the Yeo’s (2011) 7-network atlas (Figure 5). Results showed that the distribution of inverted U-shaped regions was more consistent with the frontoparietal control network, and the distribution of non-U-shaped regions was more closely related to the dorsal attention network (Figure 5B). The count of voxels revealed that a larger portion of the inverted U-shaped regions overlapped with the frontoparietal control network (2,538 voxels) than with the dorsal attention network (932 voxels), while the overlap with the cingulo-opercular network was in between (1,867 voxels). Conversely, a larger portion of the non-U-shaped regions overlapped with the dorsal attention network (3,009 voxels) than with the frontoparietal control network (2,447 voxels), while the overlap with the cingulo-opercular network was lower (1,594 voxels). In addition, the visualization of spatial distribution patterns revealed a dissociation between the middle frontal gyrus and its adjacent rostral and caudal areas (Figure 5A).
Figure 5.
Dissociated brain regions based on their trajectory patterns. A) The regions following inverted U-shaped trajectories (red color) and non-U-shaped trajectories (blue color). B) The axial view of the same results. The border lines display the frontoparietal control network (black), dorsal attention network (white), and their boundary (gray) from Yeo’s 7-network atlas (Yeo, et al., 2011). Cingulo-opercular network was not plotted due to its less clear dissociation among the two maps. The two scatter plots show two example regions showing the non-U-shaped trajectory.
To further investigate the potential functional difference between the two sets of brain regions, we decoded the related terms with the Neurosynth decoder (Yarkoni, et al., 2011) (see section “Neurosynth Decoding Analysis” in Methods). Results showed that the inverted U-shaped regions were related to the term “cognitive control” with a relatively high correlation (r = 0.187), but were less related to “attentional” (r = 0.100) and “monitoring” (r = 0.072). Note the key word “monitoring” refers to the major function of the cingulo-opercular network (Q. Li, et al., 2017). On the other hand, the non-U-shaped regions were related to the term “attentional” (r = 0.240) but were less related to “monitoring” (r = 0.090) and “control” (r = 0.062) (supplementary Table S8). This result suggests that the inverted and non-U-shaped regions may be associated with cognitive control and attention, respectively, which is consistent with the frontoparietal control network and dorsal attention network as identified in the atlas overlapping analysis.
To provide additional insight into the non-U-shaped trajectory, we specifically chose two coordinates for analysis (Figure 5B), one ([34, 4, 52]) representing a peak region from the average brain activity analysis using SDM-PSI (supplementary Table S3), and the other ([22, −63, 42]) representing a region displaying a null effect from the contrast analysis with p between 0.49 and 0.51, indicating a trajectory that does not align with either an inverted or upright U-shaped pattern. The GAM analysis showed that neither coordinate could be adequately fitted by a smoothed curve, with ps > 0.27. These results elucidate that the non-U-shaped regions exhibit relatively stable brain activities across the lifespan compared to regions showing inverted U-shaped patterns.
Lifespan Trajectory of the Laterality
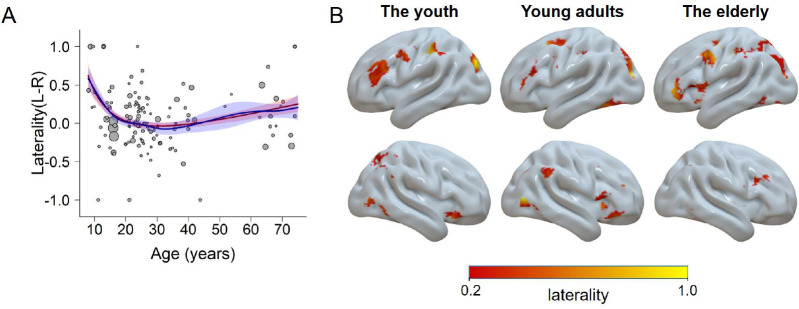
We also tested how the laterality of the brain activity changes with age (see section “Laterality Analysis” in Methods). We first modeled the laterality trajectory using the GAM. Results showed a significant model fitting, F(4.3, 5.3) = 3.38, p = 0.013. Moreover, we fitted the data with the four simplified models (i.e., the quadratic, cubic, square root, and quadratic logarithmic models). The results showed that a quadratic logarithmic function provided the best goodness of fit, with the βlog(age) = −2.48 and β(log(age))2 = 0.36, both ps < 0.001. The two-line test suggests the hypothetical peaks from both models did not reach significance (ps > .22), however. An upright U-shaped trajectory indicated the youth and elderly adults tended to be more left lateralized across the whole brain. This left lateralized pattern could be illustrated by the brain map estimated with voxel-wise laterality calculation (Figure 6B). In addition, the prefrontal regions appeared to be the major area driving the U-shaped trajectory, with the youth and elderly adults showing more left lateralized regions than the young adult group.
Figure 6.
The laterality as a function of age. A) The trajectory fitted with a quadratic logarithmic model (red) and the GAM (blue). Higher values mean more left-lateralized and lower values mean more right-lateralized. B) Visualization of the laterality for each group. Regions in the left hemisphere show the left laterality, and vice versa.
A previous study (Q. Li, et al., 2017) showed that the semantic conflicts (e.g., Stroop) are left-lateralized and the response conflicts (e.g., Simon) are right lateralized. However, the inclusion of conflict type as nuisance covariables in our model fitting analysis could essentially rule out the possibility that the laterality trajectory results were driven by different conflict types.
Discussion
The objective of this meta-analysis was to investigate the age-related changes in brain regions associated with cognitive control across the lifespan. Using data from 129 studies that encompass participants aged 8 to 74 years on average, we examined how the activities of cognitive control regions vary with age. Our analyses yielded three primary findings: 1) Among different possible trajectories, only the inverted U-shaped trajectories were reliably observed across the whole brain; 2) The cognitive control related brain regions exhibit heterogeneous lifespan trajectories: the frontoparietal control network (such as the inferior frontal gyrus and inferior parietal lobule) follows inverted U-shaped trajectories, peaking between 24 and 41 years, while the dorsal attention network (such as the frontal eye field and superior parietal lobule) demonstrates flatter trajectories with age; 3) The youth and the elderly groups demonstrate weaker brain activities and a relatively greater extent of left laterality compared to the young adult group. These results provide strong evidence for the existence of cognitive control regions exhibiting inverted U-shaped trajectory, and also show the heterogenous lifespan trajectories for different brain regions.
The Inverted U-shaped Trajectory of Brain Activity Related to Cognitive Control
The main result is that a wide range of cognitive control regions follow inverted U-shaped lifespan trajectories, but no regions showed decrease-then-stable (Figure 7D), upright U-shaped (Figure 7E), stable-then-increase (Figure 7F), increase-then-stable (Figure 7G), stable-then-decrease (Figure 7I), or linear trajectories (Figure 7, panels A, B, and C).
The greater activation in the frontoparietal control network among young adults compared to the youth and the elderly supports the notion that cognitive control abilities may not be fully developed in children and may decline in the elderly. This finding is consistent with the idea that the cognitive control system is most effective in young adulthood, suggesting a possible correlation between the higher functional activations in the brain and the superior performance of young adults on cognitive control tasks (De Luca & Leventer, 2010). The inverted U-shaped trajectories of cognitive control regions, instead of an increase-then-stable trajectory, are also consistent with the idea that cognitive control is an important aspect of fluid intelligence, which is known to peak in early to mid-adulthood and decline with age (Craik & Bialystok, 2006). Hence, our findings may reflect the general rise-and-fall patterns in the higher-order brain functions.
The inverted U-shaped trajectory of brain activation might be associated with the development of brain structure and functional changes. First, it may reflect the well-documented structural changes that occur in these regions across the lifespan, which include synaptic pruning, myelination, cortical thinning, and white matter maturation (Bethlehem, et al., 2022; Brouwer, et al., 2022). For example, the density of dopamine receptors increases during adolescence and young adulthood and subsequently declines with age (Brenhouse & Andersen, 2011). These changes can affect the efficiency and connectivity of neural circuits within the frontoparietal control network (Casey, et al., 2005; Gogtay, et al., 2004). Second, the inverted U-shaped trajectory may also arise from functional changes resulting from the modulation of neurotransmitters, hormones, and environmental factors (B. Luna, Marek, Larsen, Tervo-Clemmens, & Chahal, 2015). Understanding change patterns of brain structure and function is critical for developing interventions and treatments aimed at improving cognitive control abilities across the lifespan.
The present results further revealed that the inverted U-shaped lifespan trajectories of cognitive control regions are not uniform. The GAM fitting results (Figure 3, supplementary Table S4) showed that the subregions exhibited varying degrees of modulation effects by age. Moreover, the model simplification demonstrated different underlying trajectory curves, with most regions showing a skewed shape that could best be fitted with a square root model, except that one region showed a symmetric quadratic shape. The quadratic lifespan trajectory has been well-documented in previous studies (Amlien, et al., 2018; Brouwer, et al., 2022; Shaw, et al., 2008; Zuo, et al., 2010), while the application of the square root model has been relatively rare (Grimm, Ram, & Estabrook, 2016). The square root model can better capture the early peak in the trajectory, which aligns with the hypothesis. We also identified different peak ages for those regions, ranging from 24 to 41 years, suggesting that cognitive control regions may not develop at the same rate. Hence, the developmental peaks of cognitive control occur at distinct time points across various brain regions.
Hierarchical development trajectories in different brain networks
Previous research has indicated that the attentional orientation function is preserved during aging (P. Verhaeghen & Cerella, 2002; Verissimo, Verhaeghen, Goldman, Weinstein, & Ullman, 2022). Consistently, we found that the dorsal attention network regions underlying the attentional orientation showed a relatively flat pattern, in contrast to the inverted U-shaped trajectory in frontoparietal control network regions. The dissociation between these two sets of regions may reflect hierarchical age effects on brain function.
The brain regions are organized in a functional hierarchy, with the frontoparietal control network to be at the highest level. It acts as a connectivity hub, receiving inputs from and providing feedback to other systems, including the dorsal attention network (Cole et al., 2013). Within the cingulo-opercular network, we did not observe a clear dissociation between different trajectory patterns, which may correspond with its intermediate position in the hierarchy between the frontoparietal control and dorsal attention networks (Zhou, et al., 2018). During conflict tasks, the three sets of regions are coordinated in a hierarchical manner. The frontoparietal control network maintains task goals and resolves conflicts, the cingulo-opercular network monitors the existence of conflict, and the dorsal attention network directs attention towards task-relevant stimuli (Petersen & Posner, 2012). Even within the prefrontal cortex itself, a hierarchical organization exists, with middle frontal areas occupying the peak position (Badre & Nee, 2018). This is in line with our finding that the middle frontal cortex is dissociated from rostral and caudal frontal regions.
Furthermore, different brain regions exhibit different age effects. Higher-order regions typically have more complex lifespan trajectories (Shaw, et al., 2008) and reach peaks during later periods (Gogtay, et al., 2004; Sydnor, et al., 2023). Specifically, prefrontal control regions are among the last to mature and one of the earliest to decline (Casey, et al., 2005; Craik & Bialystok, 2006; Raz, et al., 1997). Therefore, the different lifespan trajectory patterns among frontoparietal control, cingulo-opercular and dorsal attention regions likely reflect their different positions within the functional hierarchy and the specific patterns of age-related changes.
No Evidence for the Compensatory and Asymmetry Reduction Theories
Critically, there was no region showing higher activity in the elderly compared to the young adults, but we observed several regions showing the opposite (see supplementary Note S3). This finding is inconsistent with the compensatory theory (Reuter-Lorenz & Cappell, 2008), which proposes that the elderly recruit additional brain regions to compensate for age-related cognitive decline. Interestingly, we did not observe a strong laterality in young adults, diverging from the findings from previous studies (Park & Reuter-Lorenz, 2009). In contrast, we found that elderly adults showed a tendency of left laterality, which diverges from the hemispheric asymmetry reduction in older adults (HAROLD) theory (Cabeza, 2002). The HAROLD theory suggests that older adults typically exhibit less lateralization, either as a compensatory response to functional deficits or as a reflection of neural dedifferentiation. Stronger patterns of left laterality were also identified in childhood, primarily driven by the prefrontal region (Figure 6). The left laterality at both ends of the age spectrum reflects differential age effects on the cerebral hemispheres. The left hemisphere is known to undergo earlier development (Thatcher, Walker, & Giudice, 1987) and later decline (Dolcos, Rice, & Cabeza, 2002) compared to the right hemisphere. Consequently, a stronger imbalance in activity favoring the left hemisphere may occur in both childhood and elderly adulthood. Notably, it is likely that previous studies showing stronger laterality in young adults may have been biased by too small sample sizes and the use of non-quantitative methods for calculation of laterality (Cabeza et al., 1997; C. L. Grady, Bernstein, Beig, & Siegenthaler, 2002). Moreover, since both left and right lateralized results were reported in the literature on laterality (Dolcos, et al., 2002), it is reasonable to observe a low laterality for the young adults in the current meta-analysis.
Implications for Other Cognitive Control Subdomains
A more comprehensive understanding of the lifespan trajectory of cognitive control relies on studying different subdomains of cognitive control, such as conflict processing, working memory, and cognitive flexibility (Diamond, 2013b). Previous studies have suggested that different subdomains of cognitive control exhibit both similar and separable neural mechanisms (He et al., 2021). Moreover, these subdomains might follow different lifespan trajectories (Ferguson, et al., 2021). Findings from our study may contribute valuable insights into the potential differences in brain activity across different components of cognitive control during development.
Consistent with the inverted U-shaped trajectory, we confirmed that the young adults exhibit higher levels of cognitive control brain activities compared to the youth during conflict processing but not otherwise (supplementary Figure S3, Table S9). This observation is in line with previous studies (Andrews-Hanna, et al., 2011; Konrad, et al., 2005; Marsh, et al., 2006; Veroude, et al., 2013; Wilk & Morton, 2012). To the best of our knowledge, previous research using conflict tasks has rarely reported findings that contradict this direction (except Bunge, et al., 2002). However, more controversial patterns have emerged in studies focusing on other subdomains of cognitive control, such as working memory (Geier, Garver, Terwilliger, & Luna, 2009; Olesen, Macoveanu, Tegner, & Klingberg, 2007; Scherf, Sweeney, & Luna, 2006) and response inhibition (Booth et al., 2003; Durston et al., 2006; Rubia et al., 2006). For instance, with visual-spatial working memory task, Scherf, et al. (2006) found an age-related increase in the recruitment of brain regions in the lateral prefrontal cortex, whereas Geier, et al. (2009) reported both age-related increase and decrease in different prefrontal regions. These studies employed similar paradigms but different contrast methods (i.e., delay vs. non-delay and long-delay vs. short-delay), which exemplifies the many diversities within the working memory domain (Rottschy et al., 2012). Similarly, in the subdomain of response inhibition, studies have shown discrepancies in brain engagement with different designs and different measurements (Criaud & Boulinguez, 2013; Simmonds, Pekar, & Mostofsky, 2008). On the contrary, conflict processing provides a more consistent framework for measuring brain activities across age groups. Given these differences, it is prudent to be cautious in generalizing our current findings to the lifespan trajectories of other subdomains of cognitive control, although an inverted U-shaped trajectory could be a good candidate hypothesis to begin with. Future research is necessary to develop more precise cross-age comparisons within these other cognitive control subdomains.
Methodology Implications
By incorporating all the studies, our results demonstrate that the SDM has relatively higher sensitivity than that of the ALE in identifying involved brain regions, consistent with the conclusion from a previous study (Radua et al., 2012b). Moreover, the SDM also has the added advantage of fully utilizing existing effect size data and coordinates (Albajes-Eizagirre, et al., 2019), allowing us to compare the relative activity strength among various age groups, such as the contrast between young adults and elderly groups. More importantly, this approach allows for meta-regressions to examine parametric relationships between brain region activity and age, providing insights into the lifespan trajectories of cognitive control regions. Our simulation analysis also confirms the efficacy of using mean variables as predictors for such regression. Future studies could implement the meta-regression approach to better link cognitive-behavioral measurements with brain functions. However, it is important to acknowledge that the coordinate based SDM analysis is an approximation of the actual statistical images. As such, image-based approaches (e.g., Luijten, Schellekens, Kuhn, Machielse, & Sescousse, 2017) are preferred whenever feasible (Muller et al., 2018; Salimi-Khorshidi, Smith, Keltner, Wager, & Nichols, 2009).
Limitation of Results
One caveat to consider in this study is the non-uniform distribution of age among the included studies. Specifically, most of the young adult studies (80 out of 84) had average ages lower than 45 years, resulting in a noticeable gap in the age range of 45 to 60 years. While these studies may have recruited an adequate number of individuals within this age range, the focus on the mean age may limit our study to capture this variation. Consequently, it is important to acknowledge that the observed age distribution could potentially impact the results of the regression analysis. We hope that future research could allocate more attention to the middle-aged period, considering the significant cognitive and neural changes that may occur during this stage, such as the onset of cognitive decline (S. C. Li, et al., 2009; Sowell, Thompson, & Toga, 2004; Weintraub, et al., 2013). Another noteworthy caveat is the potential influence of individual behavioral differences on neuroimaging brain activation results, especially when comparing brain activation across age groups (Mumford et al., 2023). Future studies should either ensure comparable behavioral performance or statistically control for these differences when comparing brain activities across age groups (Crone & Steinbeis, 2017). Besides, it is crucial to avoid the occurrence of ecological fallacy (Thompson & Higgins, 2002) when interpreting the results of meta-regression analyses. The ecological fallacy arises when associations observed at the group level are erroneously assumed to apply to individuals within those groups. Therefore, while our analysis reveals significant associations between brain activities and age across various studies, it does not provide direct insights into the specific age effects at the individual level. Future research incorporating individual-level investigations (e.g., longitudinal follow-up studies) is crucial to obtaining a more comprehensive understanding of these relationships. Furthermore, the disparity in brain activity levels between youth and young adults may be influenced by various physiological factors, such as differences in baseline glucose consumption, blood flow and amount of artifacts caused by motion, respiration, or cardiac activity. Lastly, the lifespan trajectory of cognitive control was only examined with conflict processing, which might limit the generalization of our conclusion to other aspects of cognitive control, such as working memory and cognitive flexibility.
Conclusions
Our meta-analysis adopted advanced meta-regression approaches to chart the lifespan trajectories of cognitive control brain activities. We observed inverted U-shaped changing patterns in regions aligned with the frontoparietal control network, with the peaks occurring between 24 and 41 years. In contrast, the dorsal attention network presented a relatively flat trajectory. This dissociation may reflect the hierarchy of brain development in different regions. No other trajectory patterns were observed, highlighting the predominance of the inverted U-shaped pattern in the lifespan trajectory of cognitive control. Furthermore, we found older adults showed lower brain activity strength and a more asymmetric brain distribution than young adults, in contrast to the hypotheses from the compensatory and asymmetric reduction theories of aging. In sum, these results demonstrate the multifaceted nature of age-related changes in cognitive control brain function.
Public significance statements.
This meta-analysis aims to reveal the lifespan trajectory of brain activities related to cognitive control, and shows that part of the brain regions related to cognitive control exhibit inverted U-shaped trajectory across the lifespan, while other regions show flatter trajectory patterns. Importantly, no other trajectory patterns are observed. We also found the elderly show weaker brain activities and more asymmetric brain activities than young adults, inconsistent with the hypotheses from existing theories.
Reference
- Adnan A., Chen A. J. W., Novakovic-Agopian T., D’Esposito M., & Turner G. R. (2017). Brain Changes Following Executive Control Training in Older Adults. Neurorehabil Neural Repair, 31(10–11), 910–922. doi: 10.1177/1545968317728580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albajes-Eizagirre A., Solanes A., Vieta E., & Radua J. (2019). Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage, 186, 174–184. doi: 10.1016/j.neuroimage.2018.10.077 [DOI] [PubMed] [Google Scholar]
- Amlien I. K., Sneve M. H., Vidal-Pineiro D., Walhovd K. B., & Fjell A. M. (2018). The Lifespan Trajectory of the Encoding-Retrieval Flip: A Multimodal Examination of Medial Parietal Cortex Contributions to Episodic Memory. J Neurosci, 38(40), 8666–8679. doi: 10.1523/JNEUROSCI.1702-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Mackiewicz Seghete K. L., Claus E. D., Burgess G. C., Ruzic L., & Banich M. T. (2011). Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS One, 6(6), e21598. doi: 10.1371/journal.pone.0021598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum M., Cooper H., Kline R. B., Mayo-Wilson E., Nezu A. M., & Rao S. M. (2018). Journal Article Reporting Standards for Quantitative Research in Psychology: The APA Publications and Communications Board Task Force Report. American Psychologist, 73(1), 3–25. doi: 10.1037/amp0000191 [DOI] [PubMed] [Google Scholar]
- Association A. P. (2013). Diagnostic and statistical manual of mental disorders : DSM-5 (5th ed.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Badre D., & Nee D. E. (2018). Frontal Cortex and the Hierarchical Control of Behavior. Trends Cogn Sci, 22(2), 170–188. doi: 10.1016/j.tics.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem R. A. I., Seidlitz J., White S. R., Vogel J. W., Anderson K. M., Adamson C., . . . Alexander-Bloch A. F. (2022). Brain charts for the human lifespan. Nature, 604(7906), 525–533. doi: 10.1038/s41586-022-04554-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel R. F., Byrge L., He Y., Goni J., Zuo X. N., & Sporns O. (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage, 102 Pt 2, 345–357. doi: 10.1016/j.neuroimage.2014.07.067 [DOI] [PubMed] [Google Scholar]
- Booth J. R., Burman D. D., Meyer J. R., Lei Z., Trommer B. L., Davenport N. D., . . . Mesulam M. M. (2003). Neural development of selective attention and response inhibition. Neuroimage, 20(2), 737–751. doi: 10.1016/S1053-8119(03)00404-X [DOI] [PubMed] [Google Scholar]
- Botvinick M. M., Cohen J. D., & Carter C. S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci, 8(12), 539–546. doi: 10.1016/j.tics.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Brenhouse H. C., & Andersen S. L. (2011). Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev, 35(8), 1687–1703. doi: 10.1016/j.neubiorev.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer R. M., Klein M., Grasby K. L., Schnack H. G., Jahanshad N., Teeuw J., . . . Hulshoff Pol H. E. (2022). Genetic variants associated with longitudinal changes in brain structure across the lifespan. Nat Neurosci, 25(4), 421–432. doi: 10.1038/s41593-022-01042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S. A., Dudukovic N. M., Thomason M. E., Vaidya C. J., & Gabrieli J. D. (2002). Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron, 33(2), 301–311. doi: 10.1016/s0896-6273(01)00583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., & Shin L. M. J. N. p. (2006). The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. 1(1), 308–313. [DOI] [PubMed] [Google Scholar]
- Cabeza R. (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging, 17(1), 85–100. doi: 10.1037//0882-7974.17.1.85 [DOI] [PubMed] [Google Scholar]
- Cabeza R., Grady C. L., Nyberg L., McIntosh A. R., Tulving E., Kapur S., . . . Craik F. I. (1997). Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci, 17(1), 391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. J., Getz S., & Galvan A. (2008). The adolescent brain. Dev Rev, 28(1), 62–77. doi: 10.1016/j.dr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. J., Heller A. S., Gee D. G., & Cohen A. O. (2019). Development of the emotional brain. Neurosci Lett, 693, 29–34. doi: 10.1016/j.neulet.2017.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. J., Tottenham N., Liston C., & Durston S. (2005). Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci, 9(3), 104–110. doi: 10.1016/j.tics.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Cohen J. D. (2017). Cognitive control: Core constructs and current considerations. In Egner T. (Ed.), The Wiley handbook of cognitive control (pp. 1–28). [Google Scholar]
- Cohen J. D., Dunbar K., & McClelland J. L. (1990). On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol Rev, 97(3), 332–361. doi: 10.1037/0033-295x.97.3.332 [DOI] [PubMed] [Google Scholar]
- Cole M. W., Reynolds J. R., Power J. D., Repovs G., Anticevic A., & Braver T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci, 16(9), 1348–1355. doi: 10.1038/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C., & Luna B. (2019). Neural Substrates of Inhibitory Control Maturation in Adolescence. Trends Neurosci, 42(9), 604–616. doi: 10.1016/j.tins.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., & Shulman G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci, 3(3), 201–215. doi: 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Coupe P., Catheline G., Lanuza E., Manjon J. V., & Alzheimer’s Disease Neuroimaging I. (2017). Towards a unified analysis of brain maturation and aging across the entire lifespan: A MRI analysis. Hum Brain Mapp, 38(11), 5501–5518. doi: 10.1002/hbm.23743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik F. I., & Bialystok E. (2006). Cognition through the lifespan: mechanisms of change. Trends Cogn Sci, 10(3), 131–138. doi: 10.1016/j.tics.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Criaud M., & Boulinguez P. (2013). Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev, 37(1), 11–23. doi: 10.1016/j.neubiorev.2012.11.003 [DOI] [PubMed] [Google Scholar]
- Crone E. A., & Dahl R. E. (2012). Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci, 13(9), 636–650. doi: 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Crone E. A., & Steinbeis N. (2017). Neural Perspectives on Cognitive Control Development during Childhood and Adolescence. Trends Cogn Sci, 21(3), 205–215. doi: 10.1016/j.tics.2017.01.003 [DOI] [PubMed] [Google Scholar]
- De Luca C. R., & Leventer R. J. (2010). Developmental trajectories of executive functions across the lifespan Executive functions and the frontal lobes (pp. 57–90): Psychology Press. [Google Scholar]
- Diamond A. (2013a). Executive Functions. Annual Review of Psychology, Vol 64, 64, 135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. (2013b). Executive functions. Annu Rev Psychol, 64, 135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. L., De la Vega A., Mills C., Andrews-Hanna J., Spreng R. N., Cole M. W., & Christoff K. (2018). Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences of the United States of America, 115(7), E1598–E1607. doi: 10.1073/pnas.715766115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., Rice H. J., & Cabeza R. (2002). Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev, 26(7), 819–825. doi: 10.1016/s0149-7634(02)00068-4 [DOI] [PubMed] [Google Scholar]
- Duncan J. (2010). The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci, 14(4), 172–179. doi: 10.1016/j.tics.2010.01.004 [DOI] [PubMed] [Google Scholar]
- DuPre E., & Spreng R. N. (2017). Structural covariance networks across the life span, from 6 to 94 years of age. Netw Neurosci, 1(3), 302–323. doi: 10.1162/NETN_a_00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S., Davidson M. C., Tottenham N., Galvan A., Spicer J., Fossella J. A., & Casey B. J. (2006). A shift from diffuse to focal cortical activity with development. Dev Sci, 9(1), 1–8. doi: 10.1111/j.1467-7687.2005.00454.x [DOI] [PubMed] [Google Scholar]
- Egger M., Smith G. D., & Phillips A. N. (1997). Meta-analysis: principles and procedures. Bmj, 315(7121), 1533–1537. doi: 10.1136/bmj.315.7121.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S. B., Bzdok D., Laird A. R., Kurth F., & Fox P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage, 59(3), 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb C. D., Germine L., & Hartshorne J. K. (2023). Cognitive control across the lifespan: Congruency effects reveal divergent developmental trajectories. J Exp Psychol Gen, 152(11), 3285–3291. doi: 10.1037/xge0001429 [DOI] [PubMed] [Google Scholar]
- Eriksen B. A., & Eriksen C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. J Perception Psychophysics, 16(1), 143–149. [Google Scholar]
- Ferguson H. J., Brunsdon V. E. A., & Bradford E. E. F. (2021). The developmental trajectories of executive function from adolescence to old age. Sci Rep, 11(1), 1382. doi: 10.1038/s41598-020-80866-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez N. B., Hars M., Trombetti A., & Vuilleumier P. (2019). Age-related changes in attention control and their relationship with gait performance in older adults with high risk of falls. Neuroimage, 189, 551–559. doi: 10.1016/j.neuroimage.2019.01.030 [DOI] [PubMed] [Google Scholar]
- Geier C. F., Garver K., Terwilliger R., & Luna B. (2009). Development of working memory maintenance. J Neurophysiol, 101(1), 84–99. doi: 10.1152/jn.90562.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J. N., Blumenthal J., Jeffries N. O., Castellanos F. X., Liu H., Zijdenbos A., . . . Rapoport J. L. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci, 2(10), 861–863. doi: 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Giedd J. N., & Rapoport J. L. (2010). Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron, 67(5), 728–734. doi: 10.1016/j.neuron.2010.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J. N., Lusk L., Hayashi K. M., Greenstein D., Vaituzis A. C., . . . Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A, 101(21), 8174–8179. doi: 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. (2012). The cognitive neuroscience of ageing. Nat Rev Neurosci, 13(7), 491–505. doi: 10.1038/nrn3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. L., Bernstein L. J., Beig S., & Siegenthaler A. L. (2002). The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychol Aging, 17(1), 7–23. doi: 10.1037//0882-7974.17.1.7 [DOI] [PubMed] [Google Scholar]
- Grimm K. J., Ram N., & Estabrook R. (2016). Growth Modeling: Structural Equation and Multilevel Modeling Approaches: Guilford Publications. [Google Scholar]
- Gupta R., & Kar B. R. (2009). Development of attentional processes in ADHD and normal children. Prog Brain Res, 176, 259–276. doi: 10.1016/S0079-6123(09)17614-8 [DOI] [PubMed] [Google Scholar]
- Hammerer D., Muller V., & Li S. C. (2014). Performance monitoring across the lifespan: still maturing post-conflict regulation in children and declining task-set monitoring in older adults. Neurosci Biobehav Rev, 46 Pt 1, 105–123. doi: 10.1016/j.neubiorev.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Hart H., Radua J., Nakao T., Mataix-Cols D., & Rubia K. (2013). Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry, 70(2), 185–198. doi: 10.1001/jamapsychiatry.2013.277 [DOI] [PubMed] [Google Scholar]
- He L., Zhuang K., Chen Q., Wei D., Chen X., Fan J., & Qiu J. (2021). Unity and diversity of neural representation in executive functions. J Exp Psychol Gen, 150(11), 2193–2207. doi: 10.1037/xge0001047 [DOI] [PubMed] [Google Scholar]
- Hedges L. V. (1981). Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 6(2), 107–128. [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J., & Altman D. G. (2003). Measuring inconsistency in meta-analyses. Bmj, 327(7414), 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P., & Morcom A. M. (2018). Age-related changes in the neural networks supporting semantic cognition: A meta-analysis of 47 functional neuroimaging studies. Neurosci Biobehav Rev, 84, 134–150. doi: 10.1016/j.neubiorev.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Ing A., Samann P. G., Chu C., Tay N., Biondo F., Robert G., . . . Consortium I. (2019). Identification of neurobehavioural symptom groups based on shared brain mechanisms. Nat Hum Behav, 3(12), 1306–1318. doi: 10.1038/s41562-019-0738-8 [DOI] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Thiel C. M., Specht K., Hanisch C., Fan J., . . . Fink G. R. (2005). Development of attentional networks: an fMRI study with children and adults. Neuroimage, 28(2), 429–439. doi: 10.1016/j.neuroimage.2005.06.065 [DOI] [PubMed] [Google Scholar]
- Lancaster J. L., Tordesillas-Gutierrez D., Martinez M., Salinas F., Evans A., ZilleS K., . . . Fox, P. T. (2007). Bias between MNI and talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping, 28(11), 1194–1205. doi: 10.1002/hbm.20345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yang G., Li Z., Qi Y., Cole M. W., & Liu X. (2017). Conflict detection and resolution rely on a combination of common and distinct cognitive control networks. Neurosci Biobehav Rev, 83, 123–131. doi: 10.1016/j.neubiorev.2017.09.032 [DOI] [PubMed] [Google Scholar]
- Li S. C., Hammerer D., Muller V., Hommel B., & Lindenberger U. (2009). Lifespan development of stimulus-response conflict cost: similarities and differences between maturation and senescence. Psychol Res, 73(6), 777–785. doi: 10.1007/s00426-008-0190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Goschl F., & Yang G. (2020). Dissociated Neural Mechanisms of Target and Distractor Processing Facilitated by Expectations. J Neurosci, 40(10), 1997–1999. doi: 10.1523/JNEUROSCI.2562-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J., Song X., Wang Y., Wang C., Huang R., & Zhang R. (2022). Distinct neural activation patterns of age in subcomponents of inhibitory control: A fMRI meta-analysis. Front Aging Neurosci, 14, 938789. doi: 10.3389/fnagi.2022.938789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M., Schellekens A. F., Kuhn S., Machielse M. W., & Sescousse G. (2017). Disruption of Reward Processing in Addiction : An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry, 74(4), 387–398. doi: 10.1001/jamapsychiatry.2016.3084 [DOI] [PubMed] [Google Scholar]
- Luna B. (2009). Developmental Changes in Cognitive Control through Adolescence. In Bauer P. (Ed.), Advances in Child Development and Behavior (Vol. 37, pp. 233–278): JAI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Marek S., Larsen B., Tervo-Clemmens B., & Chahal R. (2015). An integrative model of the maturation of cognitive control. Annu Rev Neurosci, 38, 151–170. doi: 10.1146/annurev-neuro-071714-034054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C. M. (1991). Half a century of research on the Stroop effect: an integrative review. Psychol Bull, 109(2), 163–203. doi: 10.1037/0033-2909.109.2.163 [DOI] [PubMed] [Google Scholar]
- Margulies D. S., Ghosh S. S., Goulas A., Falkiewicz M., Huntenburg J. M., Langs G., . . . Smallwood J. (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A, 113(44), 12574–12579. doi: 10.1073/pnas.1608282113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R., Zhu H., Schultz R. T., Quackenbush G., Royal J., Skudlarski P., & Peterson B. S. (2006). A developmental fMRI study of self-regulatory control. Hum Brain Mapp, 27(11), 848–863. doi: 10.1002/hbm.20225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M. P., Erickson K. I., Banich M. T., Kramer A. F., Webb A., Wszalek T., & Cohen N. J. (2002). Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn, 49(3), 277–296. doi: 10.1006/brcg.2001.1501 [DOI] [PubMed] [Google Scholar]
- Miller E. K., & Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annu Rev Neurosci, 24(1), 167–202. doi: 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Muller V. I., Cieslik E. C., Laird A. R., Fox P. T., Radua J., Mataix-Cols D., . . . Eickhoff S. B. (2018). Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev, 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford J. A., Bissett P. G., Jones H. M., Shim S., Rios J. A. H., & Poldrack R. A. (2023). The response time paradox in functional magnetic resonance imaging analyses. Nat Hum Behav. doi: 10.1038/s41562-023-01760-0 [DOI] [PubMed] [Google Scholar]
- Nee D. E., Wager T. D., & Jonides J. (2007). Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci, 7(1), 1–17. doi: 10.3758/cabn.7.1.1 [DOI] [PubMed] [Google Scholar]
- Olesen P. J., Macoveanu J., Tegner J., & Klingberg T. (2007). Brain activity related to working memory and distraction in children and adults. Cereb Cortex, 17(5), 1047–1054. doi: 10.1093/cercor/bhl014 [DOI] [PubMed] [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., . . . Brennan S. E. J. I. j. o. s. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. 88, 105906. [DOI] [PubMed] [Google Scholar]
- Park D. C., & Reuter-Lorenz P. (2009). The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol, 60, 173–196. doi: 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. E., & Posner M. I. (2012). The attention system of the human brain: 20 years after. Annu Rev Neurosci, 35, 73–89. doi: 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R. S., Erickson K. I., Colcombe S. J., Kim J. S., Voss M. W., & Kramer A. F. (2009). Age-related differences in the involvement of the prefrontal cortex in attentional control. Brain Cogn, 71(3), 328–335. doi: 10.1016/j.bandc.2009.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J., & Mataix-Cols D. (2012). Meta-analytic methods for neuroimaging data explained. Biol Mood Anxiety Disord, 2, 6. doi: 10.1186/2045-5380-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D., Phillips M. L., El-Hage W., Kronhaus D. M., Cardoner N., & Surguladze S. (2012a). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry, 27(8), 605–611. doi: 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D., Phillips M. L., El-Hage W., Kronhaus D. M., Cardoner N., & Surguladze S. (2012b). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry, 27(8), 605–611. doi: 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Raz N., Gunning F. M., Head D., Dupuis J. H., McQuain J., Briggs S. D., . . . Acker J. D. (1997). Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex, 7(3), 268–282. doi: 10.1093/cercor/7.3.268 [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A., & Cappell K. A. (2008). Neurocognitive Aging and the Compensation Hypothesis. Current Directions in Psychological Science, 17(3), 177–182. doi: 10.1111/j.1467-8721.2008.00570.x [DOI] [Google Scholar]
- Rottschy C., Langner R., Dogan I., Reetz K., Laird A. R., Schulz J. B., . . . Eickhoff S. B. (2012). Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage, 60(1), 830–846. doi: 10.1016/j.neuroimage.2011.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A. B., Woolley J., Nosarti C., Heyman I., Taylor E., & Brammer M. (2006). Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp, 27(12), 973–993. doi: 10.1002/hbm.20237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Smith S. M., Keltner J. R., Wager T. D., & Nichols T. E. (2009). Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage, 45(3), 810–823. doi: 10.1016/j.neuroimage.2008.12.039 [DOI] [PubMed] [Google Scholar]
- Scherf K. S., Sweeney J. A., & Luna B. (2006). Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci, 18(7), 1045–1058. doi: 10.1162/jocn.2006.18.7.1045 [DOI] [PubMed] [Google Scholar]
- Sebastian A., Baldermann C., Feige B., Katzev M., Scheller E., Hellwig B., . . . Kloppel S. (2013). Differential effects of age on subcomponents of response inhibition. Neurobiol Aging, 34(9), 2183–2193. doi: 10.1016/j.neurobiolaging.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Seeley W. W., Menon V., Schatzberg A. F., Keller J., Glover G. H., Kenna H., . . . Greicius M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 27(9), 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M. L. (2008). Laterality index in functional MRI: methodological issues. Magn Reson Imaging, 26(5), 594–601. doi: 10.1016/j.mri.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N. J., Lerch J. P., Eckstrand K., Lenroot R., Gogtay N., . . . Wise S. P. (2008). Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci, 28(14), 3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds D. J., Pekar J. J., & Mostofsky S. H. (2008). Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia, 46(1), 224–232. doi: 10.1016/j.neuropsychologia.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. R. (1969). Reactions toward the source of stimulation. J Exp Psychol, 81(1), 174–176. doi: 10.1037/h0027448 [DOI] [PubMed] [Google Scholar]
- Simonsohn U. (2018). Two Lines: A Valid Alternative to the Invalid Testing of U-Shaped Relationships With Quadratic Regressions. Advances in Methods and Practices in Psychological Science, 1(4), 538–555. doi: 10.1177/2515245918805755 [DOI] [Google Scholar]
- Sowell E. R., Thompson P. M., & Toga A. W. (2004). Mapping changes in the human cortex throughout the span of life. Neuroscientist, 10(4), 372–392. doi: 10.1177/1073858404263960 [DOI] [PubMed] [Google Scholar]
- Spreng R. N., Sepulcre J., Turner G. R., Stevens W. D., & Schacter D. L. (2013). Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci, 25(1), 74–86. doi: 10.1162/jocn_a_00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643–662. doi: 10.1037/h0054651 [DOI] [Google Scholar]
- Sydnor V. J., Larsen B., Seidlitz J., Adebimpe A., Alexander-Bloch A. F., Bassett D. S., . . . Satterthwaite T. D. (2023). Intrinsic activity development unfolds along a sensorimotor-association cortical axis in youth. Nat Neurosci, 26(4), 638–649. doi: 10.1038/s41593-023-01282-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier A., & Pierani A. (2021). Wiring of higher-order cortical areas: Spatiotemporal development of cortical hierarchy. Semin Cell Dev Biol, 118, 35–49. doi: 10.1016/j.semcdb.2021.05.010 [DOI] [PubMed] [Google Scholar]
- Thatcher R. W., Walker R. A., & Giudice S. (1987). Human cerebral hemispheres develop at different rates and ages. Science, 236(4805), 1110–1113. doi: 10.1126/science.3576224 [DOI] [PubMed] [Google Scholar]
- Thompson S. G., & Higgins J. P. (2002). How should meta-regression analyses be undertaken and interpreted? Stat Med, 21(11), 1559–1573. doi: 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- Turkeltaub P. E., Eickhoff S. B., Laird A. R., Fox M., Wiener M., & Fox P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping, 33(1), 1–13. doi: 10.1002/hbm.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P. (2014). The elements of cognitive aging: Meta-analyses of age-related differences in processing speed and their consequences: OUP USA. [Google Scholar]
- Verhaeghen P., & Cerella J. (2002). Aging, executive control, and attention: a review of meta-analyses. Neurosci Biobehav Rev, 26(7), 849–857. doi: 10.1016/s0149-7634(02)00071-4 [DOI] [PubMed] [Google Scholar]
- Verissimo J., Verhaeghen P., Goldman N., Weinstein M., & Ullman M. T. (2022). Evidence that ageing yields improvements as well as declines across attention and executive functions. Nat Hum Behav, 6(1), 97–110. doi: 10.1038/s41562-021-01169-7 [DOI] [PubMed] [Google Scholar]
- Veroude K., Jolles J., Croiset G., & Krabbendam L. (2013). Changes in neural mechanisms of cognitive control during the transition from late adolescence to young adulthood. Dev Cogn Neurosci, 5, 63–70. doi: 10.1016/j.dcn.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. (2010). Conducting Meta-Analyses in R with the metafor Package. Journal of Statistical Software, 36(3), 1–48. doi: DOI 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Weintraub S., Dikmen S. S., Heaton R. K., Tulsky D. S., Zelazo P. D., Bauer P. J., . . . Gershon R. C. (2013). Cognition assessment using the NIH Toolbox. Neurology, 80(11 Suppl 3), S54–64. doi: 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk H. A., & Morton J. B. (2012). Developmental changes in patterns of brain activity associated with moment-to-moment adjustments in control. Neuroimage, 63(1), 475–484. doi: 10.1016/j.neuroimage.2012.06.069 [DOI] [PubMed] [Google Scholar]
- Wood G., Ischebeck A., Koppelstaetter F., Gotwald T., & Kaufmann L. (2009). Developmental trajectories of magnitude processing and interference control: an FMRI study. Cereb Cortex, 19(11), 2755–2765. doi: 10.1093/cercor/bhp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. N. (2001). mgcv: GAMs and generalized ridge regression for R. J R news, 1(2), 20–25. [Google Scholar]
- Wood S. N. (2011). Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. Journal of the Royal Statistical Society Series B: Statistical Methodology, 73(1), 3–36. doi: 10.1111/j.1467-9868.2010.00749.x [DOI] [Google Scholar]
- Yaple Z. A., Stevens W. D., & Arsalidou M. (2019). Meta-analyses of the n-back working memory task: fMRI evidence of age-related changes in prefrontal cortex involvement across the adult lifespan. Neuroimage, 196, 16–31. doi: 10.1016/j.neuroimage.2019.03.074 [DOI] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R. A., Nichols T. E., Van Essen D. C., & Wager T. D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nat Methods, 8(8), 665–670. doi: 10.1038/nmeth.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B. T., Krienen F. M., Sepulcre J., Sabuncu M. R., Lashkari D., Hollinshead M., . . . Buckner R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol, 106(3), 1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto T. P., & Gazzaley A. (2014). Attention and ageing The Oxford handbook of attention. (pp. 927–971). New York, NY, US: Oxford University Press. [Google Scholar]
- Zhang Z., Peng P., Eickhoff S. B., Lin X., Zhang D., & Wang Y. (2021). Neural substrates of the executive function construct, age-related changes, and task materials in adolescents and adults: ALE meta-analyses of 408 fMRI studies. Dev Sci, 24(6), e13111. doi: 10.1111/desc.13111 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Friston K. J., Zeidman P., Chen J., Li S., & Razi A. (2018). The Hierarchical Organization of the Default, Dorsal Attention and Salience Networks in Adolescents and Young Adults. Cereb Cortex, 28(2), 726–737. doi: 10.1093/cercor/bhx307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X. N., He Y., Betzel R. F., Colcombe S., Sporns O., & Milham M. P. (2017). Human Connectomics across the Life Span. Trends Cogn Sci, 21(1), 32–45. doi: 10.1016/j.tics.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Zuo X. N., Kelly C., Di Martino A., Mennes M., Margulies D. S., Bangaru S., . . . Milham M. P. (2010). Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci, 30(45), 15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]