Abstract
Cognitive control plays a pivotal role in guiding human goal-directed behavior. While existing studies have documented an inverted U-shaped trajectory of cognitive control both behaviorally and anatomically, little is known about the corresponding changes in functional brain activation with age. To bridge this gap, we conducted a comprehensive meta-analysis of 129 neuroimaging studies using conflict tasks, encompassing 3,388 participants aged from 5 to 85 years old. We have three major findings: 1) The inverted U-shaped trajectory is the predominant pattern; 2) Cognitive control-related brain regions exhibit heterogeneous lifespan trajectories: the frontoparietal control network follows inverted U-shaped trajectories, peaking between 24 and 40 years, while the dorsal attention network demonstrates no clear trajectories; 3) Both the youth and the elderly show weaker brain activities and greater left laterality than young to middle-aged adults. These results reveal the lifespan trajectories of cognitive control, highlighting heterogeneous fluctuations in brain networks with age.
Classification: Biological Sciences/Psychological, Cognitive Sciences
Keywords: Cognitive control, Lifespan, Brain activity, Neuroimaging meta-analysis, Heterogeneity
Introduction
The cognitive abilities of human beings dynamically change throughout the entire lifespan, experiencing rapid development in the early stages, and gradual decline in the later period of life. As one of the most fundamental cognitive functions, cognitive control is deeply engaged in various domains of high-level capabilities that humans greatly outperform other species, such as decision making, planning and problem solving1. Cognitive control refers to the cognitive processes that enable individuals to manage and regulate their attention, thoughts, and actions, which plays a vital role in goal-directed behavior, allowing us to focus on the target and ignore distractors2,3. For instance, cognitive control enables us to concentrate on reading in a library despite the presence of people chatting nearby.
Cognitive control provides fundamental support for normal human behaviors. Young adults typically maintain an optimal mature level of cognitive control4. However, the youth (including children and adolescents, less than 18 years) and elderly (60 years and older) individuals may struggle with behavioral problems because of their suboptimal cognitive control system5–10. While the state-of-art progress of cognitive/behavioral changes has been well-documented and shaped how the diagnosis of developmental/ageing related disorders11, the change of related neural system has been under-investigated. Understanding how the neural underpinning of cognitive control changes over the lifespan can yield valuable insights into the developmental and aging mechanisms of the human brain. This knowledge may assist in customizing cognitive training strategies based on related brain regions and their activities12.
Researchers generally believe that cognitive control ability follows an inverted U-shaped trajectory across the lifespan5,13,14,17. This inverted U-shaped trajectory has been generally supported by behavioral and anatomical evidence. The Eriksen Flanker task (requiring participants to respond to central stimuli while ignoring flanking distractions) has been widely utilized to detect cognitive control across the lifespan15, and results suggest a clear U-shape trajectory of conflict cost (measured by worsened behavioral performance, e.g., reaction time, in incongruent compared to congruent conditions) with age16–18. Similar results have been observed in other conflict tasks, such as color-word Stroop (requiring participants to name the ink color of a word that is incongruent with the word’s semantic meaning)16. Recent large-cohort studies have also found that gray and white matter volumes across all brain regions exhibit overall inverted U-shaped trajectories with age, with the gray matter volume peaking at early adolescence and the white matter volume peaking at young adulthood19,20. During late adulthood, normal ageing yields a protracted decline of brain structure, with the volumes of both gray matter and white matter reduced14. Consistently, the gray matter volumes of the frontal and parietal regions, which are essential in cognitive control tasks21, have also been found to increase during early childhood and atrophy in early elderly age22.
However, it remains largely unknown how brain activities related to cognitive control change over the lifespan. Previous research has primarily focused on brain activities in either youths or elderly adults, rather than examining changes across the entire lifespan. With conflict paradigms, children and adolescents are often found to have lower brain activity than young adults in frontoparietal regions (refs.23–28, but see Bunge et al.29). For example, a study utilizing the Flanker task revealed that children aged 8–12 years had reduced activation in dorsolateral prefrontal regions compared to young adults, suggesting an immature cognitive control system27. However, brain activity differences in elderly adults as compared to young adults during cognitive control tasks have been less consistently reported14. Some studies have found that elderly adults have lower neural activity in frontoparietal regions than young adults30–32, possibly because elderly adults may be unable to engage in an equal level of control-related activity due to functional decline. On the other hand, other studies have found that elderly adults may exhibit greater brain activity in frontoparietal regions than young adults33,34, possibly because they have recruited additional brain regions to compensate for their decreased efficiency in utilizing control resources. Adding to the debate, it has been proposed that the cognitive control function in elderly adults might not decline at all35,36. These conflicting findings underscore the complexity of understanding age-related differences in cognitive control.
Few studies have directly tested the change of brain activities related to cognitive control across the lifespan. One existing study observed a positive association between the activation of the bilateral prefrontal cortex and age37. Given the relatively small sample size (N = 30), the reliability of these findings is somewhat limited. As a result, it is difficult to draw a clear conclusion about how brain activities related to cognitive control change across the entire age range.
One direct way to test the lifespan trajectory of brain activation related to cognitive control is to conduct a large cohort of neuroimaging study with participants covering a wide age range. To the best of our knowledge, such studies have not yet been conducted. An alternative approach is to utilize meta-analyses to combine the results of existing studies targeting different age groups. Compared to the large cohort studies, meta-analyses are more accessible and resource-saving. In addition, meta-analyses can increase the statistical power and generalizability by combining various studies, reducing heterogeneity and bias from individual studies’ methods, populations, or confounding variables38. Importantly, meta-analyses can reveal patterns not evident in individual studies, like nonlinear effects or interactions38. Several neuroimaging meta-analyses have examined age-related changes of cognitive control brain activity31,39,40, but limitations such as incomplete age coverage and insufficient studies in certain age ranges prevent them from appropriately answering questions about the lifespan trajectory of cognitive control functions31,39,40. These studies have primarily focused on spatial convergence and/or diversity of coordinates across different age ranges, offering limited insights into lifespan trajectories of activity strength. Although a few studies have attempted parametric meta-regression to examine the age-related differences in cognitive control-related brain activity39,41, they have been constrained by utilizing linear models that may overlook non-linear trajectory patterns, such as the inverted U-shaped trend.
The goal of this study is to provide a comprehensive examination to reveal the lifespan trajectory of brain activities responsible for cognitive control. Instead of encompassing various aspects of cognitive control, we focus on conflict processing for several reasons. First, conflict processing reflects the fundamental cognitive control ability to maintain a goal while avoiding distractions2. Second, its mechanisms in young adults are relatively well-known, with the frontoparietal and cingulo-opercular networks engaged21,42, providing a baseline reference for our study. Third, conflict tasks with neuroimaging data have been widely applied to both younger and elderly groups, making a systematic meta-analysis feasible. Lastly, different conflict tasks share key components of cognitive control, such as conflict monitoring43 and inhibitory control8, which enables us to conduct effect-size-based meta-analyses using the congruency effect (i.e., the contrast between incongruent and congruent/neutral conditions). Including other sub-processes of cognitive control may introduce heterogeneity and make effect sizes incomparable.
Previous research suggests that better cognitive control-related performance is often associated with greater brain activity, especially in the prefrontal cortex44. Therefore, we hypothesized that brain activities related to cognitive control might follow an inverted U-shaped trajectory like that of behavior patterns. Additionally, we hypothesized that different brain networks may show some heterogeneity in their lifespan trajectories.
Results
Sample Description
A total of 3,611 articles were identified including 3,484 articles searched from the database, 111 articles adopted from a previous study21, and 16 articles searched from the references of crucial articles. After excluding duplicates and applying exclusion criteria, 119 articles including 129 studies with 3,388 participants and 1,579 brain activation foci, were included in this meta-analytic study (Supplementary Fig. S1 and Table S1). The average age of participants ranged from 8 to 74 years, with the individual age ranging from 5 to 85 years. A demonstration of the age distribution for each included study is presented in Supplementary Fig. S2.
Regions Related to Cognitive Control Identified by both ALE and SDM
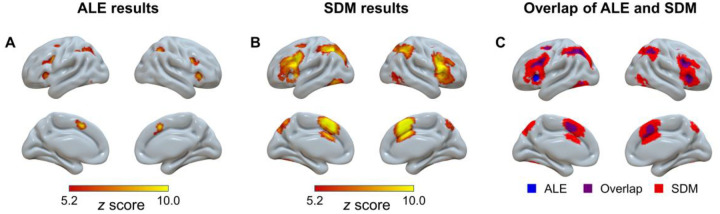
To enhance the replicability and robustness of our finding and enable direct comparison with a previous study21, we conducted the mean analysis across all studies with the activation likelihood estimation (ALE) and seed-based d mapping (SDM) approaches separately. First, we performed the single dataset analysis with the GingerALE software to explore the brain regions consistently reported in all the included studies (see section “Activation Likelihood Estimation (ALE)” in Methods). Results showed significant activation in the frontoparietal regions, including the left dorsolateral prefrontal cortex, bilateral frontal eye field, right inferior frontal gyrus and bilateral inferior parietal lobule; the cingulo-opercular regions, including the supplementary motor area and bilateral insula; and other regions, including the left inferior temporal gyri (Fig. 1A and Supplementary Table S2). Second, we calculated the average brain activation based on the effect sizes reported in all studies using the SDM with permutation of subject images (SDM-PSI) software (see section “Mean Analyses Across all Studies” in Methods). Similar to the ALE results, we found significant activation in the frontoparietal regions (left inferior parietal lobule, right inferior frontal gyrus, and right middle frontal gyrus), the cingulo-opercular regions (left anterior cingulate cortex), and other regions including bilateral inferior temporal gyrus, right caudate nucleus, right cerebellum, and left anterior thalamic projections (Fig. 1B and Supplementary Table S3). The count of voxels revealed that the overlapped area (3,496 voxels, Fig. 1C) accounted for 96.3% of the regions from the ALE analysis (3,635 voxels), and 15.0% of the regions from the SDM analysis (23,240 voxels). This result suggested that SDM analysis could be a reliable approach for detecting brain regions, thereby laying a solid foundation for using this approach in subsequent analyses. In addition, a robustness analysis suggested that age does not influence the mean results of SDM analysis (Supplementary Fig. S3 and Table S2).
Fig. 1.
Overview of significant clusters across all studies regardless of age in the ALE meta-analysis (A), the SDM meta-analysis (B), and their overlap (C). ALE = activation likelihood estimation; SDM = seed-based d mapping.
Trajectories of Cognitive Control Regions Identified in the Mean Analysis
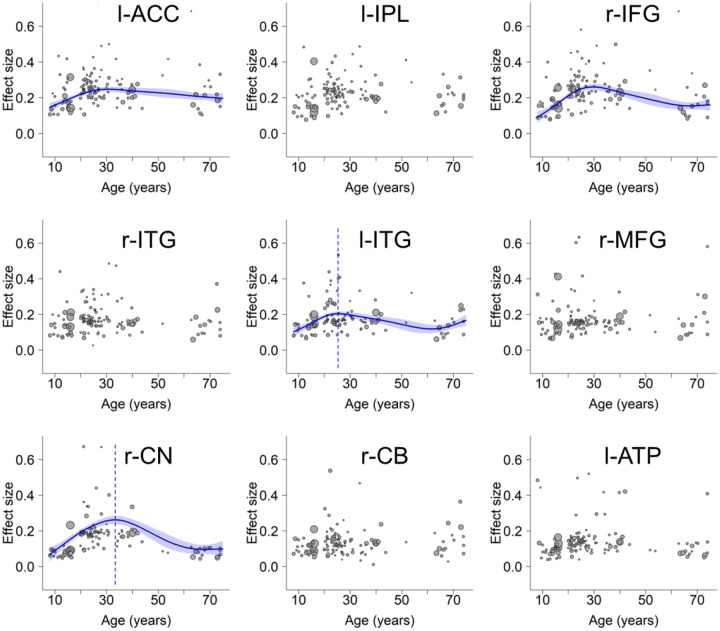
Having identified nine brain regions in the mean analysis using SDM-PSI, we proceeded to explore how the activation levels of these regions change with age. To this end, we extracted brain activity data from all studies for each identified region. Before performing the meta-regression, we verified the effectiveness of using mean age as a predictor with a simulation approach (Supplementary Note S1 and Fig. S4). We then subjected the extracted data to separate generalized additive model (GAM) analyses, factoring in the confounding covariates (see section “Generalized Additive Model (GAM) Fitting” in Methods). The analyses (Supplementary Table S4) revealed significant age-related changes in the activation levels of 4 out of the 9 regions (Fig. 2, l-ACC, r-IFG, l-ITG, and r-CN). Visualization of the trajectories suggests that these regions showed inverted U-shaped patterns. The significance of their inverted U-shaped trajectories was further examined using a two-line test approach45. Results suggest that all four regions involve an increase in activity from childhood to young adulthood (approximately up to 30 years of age), and three of them (r-IFG, l-ITG and r-CN) showed consistent decrease in the later stages of age (Supplementary Note S2). The other five regions (Fig. 2, l-IPL, r-ITG, r-MFG, r-CB and l-ATP) showed no significant age-related changes. Notably, none of the clusters showed significant publication bias based on Egger’s test (ps > 0.86), and they all showed low between-study heterogeneity (τs < 0.13, Qs < 12.11, I2s < 25%). This indicates that the observed results are not likely influenced by biased reporting or substantial variability in the included studies. A robustness analysis further ruled out the potential influence of including unpublished and non-English studies in this study (Supplementary Note S4 and Fig. S5).
Fig. 2.
Lifespan trajectories within regions identified in the mean analysis. l-ACC: left anterior cingulate cortex, l-IPL: left inferior parietal lobule, r-IFG: right inferior frontal gyrus, r-ITG: right inferior temporal gyrus, l-ITG: left inferior temporal gyrus, r-MFG: right middle frontal gyrus, r-CN: right caudate nucleus, r-CB: right cerebellum, l-ATP: left anterior thalamic projections. Scattered plots are the effect sizes as a function of age, with curves fitted by GAM. The sizes of the scattered dots show the square root of model weights (1/variance) for each study. Shaded areas around the curves represent standard errors. Dashed lines indicate peak ages. Panels l-ACC and l-IPL do not show the peak age due to an insignificant decrease at the later stage (Supplementary Note S2).
Note that some regions are large, containing up to over 9,000 voxels. This could obscure the distinct trajectories specific to different subregions, which are crucial in understanding the hierarchical patterns of lifespan trajectories. We address this by using a more granular approach below.
Detecting Different Trajectories of Whole Brain Activities
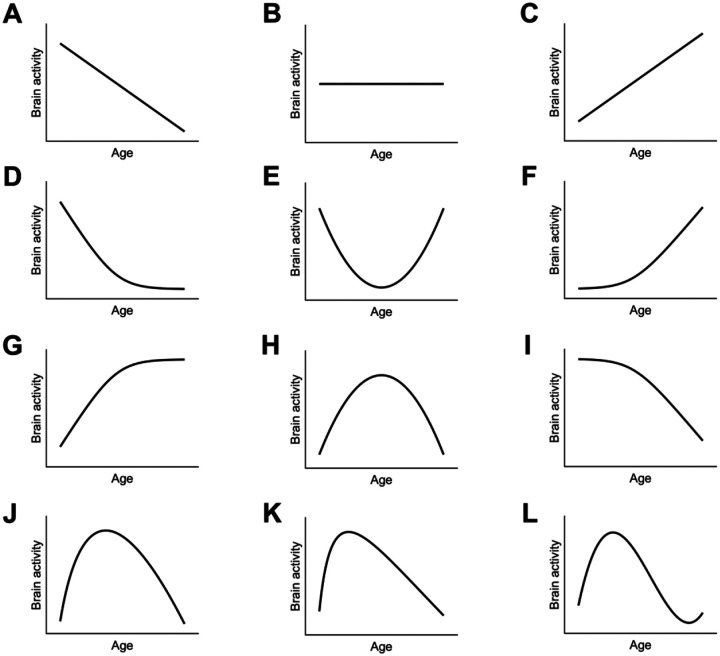
To explore various possibilities of lifespan trajectories, we grouped studies by their mean age into the youth, young to middle-aged, and elderly groups, and then conducted several contrast analyses (see section “Contrast Analysis” in Methods). These analyses did not reveal any regions that exhibited significantly higher or lower brain activity in the youth compared to others (the combination of young to middle-aged and older adults) (Table 1). Similarly, we did not observe any regions with higher or lower brain activity in older adults compared to others (the combination of the youth and young to middle-aged adults) (Table 1). These results excluded the possibilities of increase/decrease-then-stable and stable-then-increase/decrease trajectories (Fig. 3, panels D, F, G, and I). In addition, we failed to observe any region showing lower activity in young to middle-aged adults than others (the combination of the youth and older adults), and thereby ruled out the possibility of the upright U-shaped trajectory (Table 1, Fig. 3E).
Table 1.
Brain areas activated in the contrast of one age group versus others (voxel-wise FWE-corrected, p < 0.001, with minimum cluster size ≥ 10 voxels) with the SDM-PSI.
| Order | # Voxels | Z | p | L/R | MNI coordinate | Anatomical location | BA | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Young to middle-aged adults > Others | |||||||||
| 1 | 1165 | 4.713 | < 0.001 | R | 52 | 16 | 4 | inferior frontal gyrus | 48 |
| 2 | 607 | 4.895 | < 0.001 | L | −46 | 16 | 26 | inferior frontal gyrus | 48 |
| 3 | 569 | 4.289 | < 0.001 | R | 54 | −48 | 42 | inferior parietal lobule | 40 |
| 4 | 215 | 3.405 | < 0.001 | L | −8 | 0 | 58 | supplementary motor area | 6 |
| 5 | 202 | 3.830 | < 0.001 | L | −36 | −54 | 42 | inferior parietal lobule | 40 |
| 6 | 106 | 3.245 | < 0.001 | R | 10 | 4 | 6 | caudate nucleus | / |
| 7 | 76 | 3.231 | < 0.001 | L | −38 | 24 | 0 | insula | 47 |
| 8 | 33 | 3.119 | < 0.001 | L | −36 | 12 | −6 | insula | 48 |
| 9 | 10 | 2.794 | < 0.001 | R | 4 | −16 | 44 | middle cingulate cortex | 23 |
| Young to middle-aged adults < Others | |||||||||
| None | |||||||||
| The youth > Others | |||||||||
| None | |||||||||
| The youth < Others | |||||||||
| None | |||||||||
| The elderly > Others | |||||||||
| None | |||||||||
| The elderly < Others | |||||||||
| None | |||||||||
Note. The brain regions in the table correspond to the regions in Fig. 4. MNI = Montreal Neurological Institute, BA = Brodmann area, L = left, R = right.
Fig. 3.
The lifespan trajectories explored in our study. Panels A-C show linear decrease, flat, and linear increase patterns, respectively, and were modelled with the linear function. Panels E and H show the upright and inverted U-shapes, respectively, and were tested with the contrast between young to middle-aged adults and others, as well as with the quadratic function. Panels D, F, G, and I show combinations of a stable period and an increase/decrease period across the lifespan, and were tested with the contrast between the youth and others, or between the elderly and others. Panels J, K and L show the variants of inverted U-shaped trajectories, which capture the possibly early peak feature. They were tested with square root, quadratic logarithmic, and cubic functions, respectively. See Methods for detailed models.
However, we identified greater activity in young to middle-aged adults compared to others in the frontoparietal regions, including bilateral inferior frontal gyrus and bilateral inferior parietal lobule; the cingulo-opercular regions, including left supplementary motor area, left insula, and right middle cingulate cortex; and a subcortical region—right caudate nucleus (Fig. 4 and Table 1). This result essentially supports an inverted U-shaped trajectory. Notably, none of the clusters showed significant publication bias based on Egger’s test (ps > 0.79), and they all showed low between-study heterogeneity (τs < 0.17, Qs < 12.21, I2s < 25%). This indicates that the observed results are not likely influenced by biased reporting or substantial variability in the included studies. Consistently, further contrast analyses revealed that the young to middle-aged adults showed greater activity than both the youth (Supplementary Fig. S6) and the elderly (Supplementary Fig. S7).
Fig. 4.
Brain regions showing inverted U-shaped trajectory patterns. Scattered plots are the effect size as a function of age, with curves fitted by GAM (blue color) and the best simplified model (red color). Shaded areas around the curves represent standard errors. Dashed vertical lines show peak ages estimated from GAM (blue) and simplified model (red). The sizes of the scattered dots show the square root of model weights (1/variance) for each study. r-IFG: right inferior frontal gyrus, l-IFG: left inferior frontal gyrus, r-IPL: right inferior parietal lobule, l-SMA: left supplementary motor area, l-IPL: left inferior parietal lobule, r-CN: right caudate nucleus, l-Insula: left insula, r-MCC: right middle cingulate cortex.
Fitting the Lifespan Trajectories with the GAM
For each region identified from the contrast between young to middle-aged adults and others, the GAM could fit the data significantly with a smooth curve (Fig. 4), with degrees of freedom varying from 2.9 to 6.7. Peak ages of the inverted U-shaped trajectories were between 24.5 and 39.4 years. Detailed statistics are shown in Supplementary Table S4.
Model Simplification of the Lifespan Trajectories
Considering the GAM may overfit the data, we fitted the results with simpler models commonly adopted in lifespan developmental literature46–48, including the quadratic, cubic, square root and quadratic logarithmic models, and estimated the goodness of fit by comparing their Akaike information criterion (AIC) (see section “Model Simplification and Model Comparison” in Methods). Results showed that the quadratic model provided the best fit for capturing the age-related changes in left inferior parietal lobule, while the square root model demonstrated the best goodness of fit for all the other regions (Supplementary Table S5). We also calculated the peak age for each region based on the optimal model, and results showed that the peak ages ranged from 33.3 to 40.0 years. In addition, we found the peak ages obtained from the above optimal model (i.e., square root or quadratic models) and the GAM are consistent, r = 0.71, p = 0.032, 95% CI = [0.09 0.93]. See Fig. 4 and Supplementary Table S6 for details.
Fitting the Whole Brain with Square Root and Linear Models
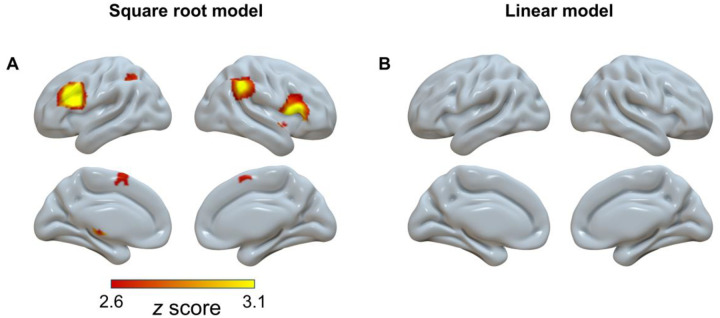
Based on model comparisons, we found that the square root model provided the best goodness of fit for the age-related change of the brain activities. To supplement the inverted U-shaped results from the contrast analysis, the square root function was then submitted to whole-brain meta-regression analyses in SDM-PSI (see section “Meta-regression Analyses” in Methods).
By fitting the activation over the whole brain, we found seven significant brain regions, including the bilateral inferior frontal gyrus, right angular, left inferior parietal lobule, right insula, right caudate nucleus, and left anterior thalamic projections (Fig. 5A and Supplementary Table S7). These results further supported the existence of the inverted U-shaped regions we initially identified (Fig. 4).
Fig. 5.
Significant clusters (voxel-wise FWE-corrected, p < 0.001, voxels ≥ 10) showing square root pattern (A) and linear pattern (B) with age in the model fitting.
In addition, we explored the whole-brain trajectories with a linear meta-regression (see section “Meta-regression Analyses” in Methods). However, no significant regions were observed (Fig. 5B), even under a more tolerant threshold of uncorrected p < 0.01.
Dissociated Brain Networks with Distinct Lifespan Trajectories
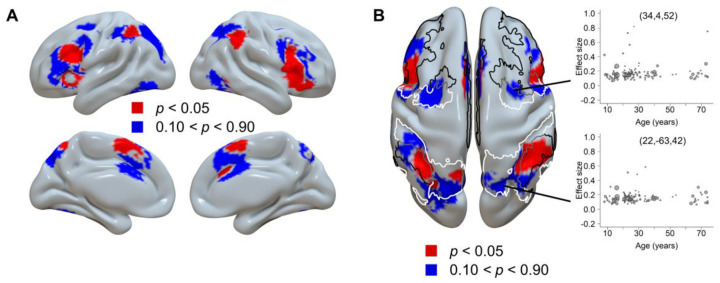
We note that the inverted U-shaped regions constitute only part of the cognitive control-related regions (Fig. 2), and the remaining regions show a less clear trajectory. To further elucidate the spatial distribution of brain regions following these different trajectory patterns, we used the results from the mean SDM analysis (Fig. 1B) as the mask and replotted the results of the contrast analysis between young to middle-aged adults and others with two different thresholds, and then compared them with the Yeo’s 7-network atlas49 (Fig. 6). Visualization of the spatial distribution patterns revealed a dissociation between the middle frontal gyrus and its adjacent rostral and caudal areas (Fig. 6A). Moreover, the distribution of inverted U-shaped regions was more consistent with the frontoparietal control network (FPCN), and the distribution of non-U-shaped regions was more closely related to the dorsal attention network (DAN, Fig. 6B). The count of voxels revealed that a numerically larger portion of the inverted U-shaped regions overlapped with the FPCN (2,538 voxels) than with the DAN (932 voxels), while the overlap with the cingulo-opercular network (CON) was in between (1,867 voxels). Conversely, a numerically larger portion of the non-U-shaped regions overlapped with the DAN (3,009 voxels) than with the FPN (2,447 voxels), while the overlap with the CON was lower (1,594 voxels).
Fig. 6.
Dissociated brain regions based on their trajectory patterns. A) The regions following inverted U-shaped trajectories (red color) and non-U-shaped trajectories (blue color). B) The axial view of the same results. The border lines display the frontoparietal control network (black), dorsal attention network (white), and their boundary (gray) from Yeo’s 7-network atlas49. Cingulo-opercular network was not plotted due to its less clear dissociation among the two maps. The two scatter plots show two example regions showing the non-U-shaped trajectory, one ([34, 4, 52]) representing a peak region from the average brain activity analysis (Supplementary Table S3), and the other ([22, −63, 42]) representing a region displaying a weak age-related change from the contrast analysis with p between 0.49 and 0.51. The GAM analysis showed that neither coordinate could be adequately fitted by a smoothed curve, with ps > 0.22.
To further investigate the potential functional difference between the two sets of brain regions, we decoded the related terms with the Neurosynth decoder41 (see section “Neurosynth Decoding Analysis” in Methods). Results showed that the inverted U-shaped regions were related to the term “cognitive control”(r = 0.187), but were less so to “attentional” (r = 0.100) and “monitoring” (r = 0.072). Note the keyword “monitoring” refers to the major function of the cingulo-opercular network21. On the other hand, the non-U-shaped regions were related to the term “attentional” (r = 0.240) but were less so to “monitoring” (r = 0.090) and “control” (r = 0.062) (Supplementary Table S8). This result suggests that the inverted U-shaped and non-U-shaped regions may be associated with cognitive control and attention, respectively, which is consistent with the frontoparietal control network and dorsal attention network as identified in the atlas overlapping analysis.
Lifespan Trajectory of the Laterality
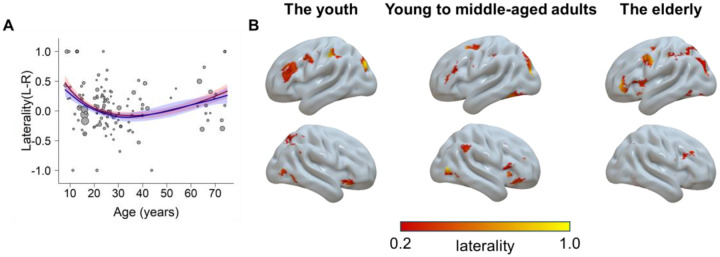
We also tested how the laterality of the brain activity changes with age (see section “Laterality Analysis” in Methods). We first modeled the laterality trajectory using the GAM. Results showed a significant model fitting, F(3.0, 3.7) = 3.49, p = 0.012, R2 = 0.24. Moreover, we fitted the data with the four simplified models (i.e., the quadratic, cubic, square root, and quadratic logarithmic models). The results showed that a square root function provided the best goodness of fit, with the βsqrt(age) = −0.68 (95% CI = [−1.02, −0.33]), p < 0.001. The two-line test suggests the hypothetical peaks from both models did not reach significance (Supplementary Note S2). A visually upright U-shaped trajectory indicated the youth and elderly adults tended to be more left-lateralized across the whole brain. A further comparison of the relative levels of laterality across different age groups revealed that both the youth and the elderly groups exhibited greater left lateralization than the young to middle-aged adult group (Supplementary Note S3). This left-lateralized pattern could be illustrated by the brain map estimated with voxel-wise laterality calculation (Fig. 7).
Fig. 7.
The laterality as a function of age. A) The trajectory fitted with a square root model (red) and the GAM (blue). Higher values mean more left-lateralized and lower values mean more right-lateralized. B) Visualization of the laterality for each group. Regions in the left hemisphere show the left laterality, and vice versa.
Discussion
The present study yielded three primary findings: 1) Among different possible trajectories, only the inverted U-shaped trajectories were reliably observed across the whole brain; 2) The cognitive control-related brain regions exhibit heterogeneous lifespan trajectories: the frontoparietal control network (such as the inferior frontal gyrus and inferior parietal lobule) follows inverted U-shaped trajectories, peaking between 24 and 40 years, while the dorsal attention network (such as the frontal eye field and superior parietal lobule) demonstrates less clear trajectories with age; 3) The youth and the elderly demonstrate weaker brain activities and a relatively greater extent of left laterality compared to the young to middle-aged adults. These results provide strong evidence for the existence of cognitive control regions exhibiting inverted U-shaped trajectory, and also show the heterogenous lifespan trajectories in different brain regions.
The Inverted U-shaped Trajectory of Brain Activity Related to Cognitive Control
The main finding is that a wide range of cognitive control regions follow inverted U-shaped lifespan trajectories, but no regions showed decrease-then-stable (Fig. 3D), upright U-shaped (Fig. 3E), stable-then-increase (Fig. 3F), increase-then-stable (Fig. 3G), stable-then-decrease (Fig. 3I), or linear trajectories (Fig. 3A and 3C).
The greater activation in the frontoparietal control network among young to middle-aged adults compared to the youth and the elderly supports the notion that cognitive control abilities may not be fully developed in children and may decline in the elderly. This finding is consistent with the idea that the cognitive control system is most effective in young adulthood, suggesting a possible correlation between the higher functional activations in the brain and the superior performance of young adults on cognitive control tasks50. Consistently, a previous study29 showed that behavioral performance (success of interference suppression) is positively correlated with the activity in frontal regions. Similar patterns have been repeatedly reported51,52, although the opposite results have also been observed53.
The inverted U-shaped trajectory of brain activation might be associated with the development of brain structure and functional changes. First, it may reflect the well-documented structural changes that occur in these regions across the lifespan, which include synaptic pruning, myelination, cortical thinning, and white matter maturation19,54. For example, the density of dopamine receptors increases during adolescence and young adulthood and subsequently declines with age55. These changes can affect the efficiency and connectivity of neural circuits within the frontoparietal control network13,56. Second, the inverted U-shaped trajectory may also arise from functional changes resulting from the modulation of neurotransmitters, hormones, and environmental factors57. Understanding change patterns of brain structure and function is critical for developing interventions and treatments aimed at improving cognitive control abilities across the lifespan.
The present results further revealed that the inverted U-shaped lifespan trajectories of cognitive control regions are not uniform. The GAM fitting results (Fig. 4, Supplementary Table S4) showed that the subregions exhibited varying degrees of association with age. Moreover, the model simplification demonstrated different underlying trajectory curves, with most regions showing a skewed shape that could best be fitted with a square root model, except that one region showed a symmetric quadratic shape. The quadratic lifespan trajectory has been well-documented in previous studies19,46,48,58, while the application of the square root model has been relatively rare59. The square root model can better capture the early peak in the trajectory. We also identified different peak ages for those regions, ranging from 24 to 40 years, suggesting that cognitive control regions may not develop at the same rate.
Hierarchical development trajectories in different brain networks
Previous research has indicated that the attentional orientation function is preserved during ageing35,60. Consistently, we found that the dorsal attention network regions underlying the attentional orientation showed no significant age-related change, in contrast to the inverted U-shaped trajectory in frontoparietal control network regions. In addition, we observed that the supporting regions mediating top-down control with motor61 (right cerebellum, Fig. 2) and sensory62 (left anterior thalamic projections, Fig. 2) functions also lack the sensitivity to age. The dissociation across regions may reflect hierarchical associations with age on brain function.
The brain regions are organized in a functional hierarchy, with the frontoparietal control network at the highest level. It acts as a hub that interacts with other systems, including the dorsal attention network63. The cingulo-opercular network did not present a clear dissociation of trajectory patterns, possibly suggesting its intermediate position between the frontoparietal control and dorsal attention networks64. During conflict tasks, these networks function in a hierarchical manner. The frontoparietal control network maintains task goals and resolves conflicts, the cingulo-opercular network monitors conflict, and the dorsal attention network directs attention towards task-relevant stimuli65. Even within the prefrontal cortex itself, a hierarchical organization exists, with middle frontal areas occupying the peak position66. This is in line with our finding that the middle frontal cortex is dissociated from rostral and caudal frontal regions (Fig. 6A). In addition, previous research suggests that the frontoparietal control network can be further divided67, with the rostral and caudal frontal regions observed in our study aligning closely with the sub-network that connects more strongly with the dorsolateral attention network. This may explain why some areas within the frontal region do not show age-related changes.
Furthermore, different brain regions exhibit different age-related changes. Higher-order regions typically have more complex lifespan trajectories58 and reach peaks during later periods56,68. Specifically, prefrontal control regions are among the last to mature and one of the earliest to decline5,13,69. Therefore, the different lifespan trajectory patterns among different networks likely reflect their hierarchical positions of age-related changes.
Implications for the Compensatory and Asymmetry Reduction Theories
Critically, there was no region showing higher activity in the elderly compared to the young to middle-aged adults, but we observed several regions showing the opposite (Supplementary Note S3 and Table S9). The results persisted after we controlled the behavioral congruency effect (Supplementary Note S4 and Fig. S8), thereby ruling out the possibility of weaker brain activity associated with poor behavioral performance in the elderly. The observed decrease in brain activation among the elderly might be attributed to several interrelated factors. First, cognitive control regions, especially the frontal area, tend to shrink with age, leading to a reduction in overall brain volume and potential loss of synaptic integrity70. This shrinkage can impair the brain’s ability to effectively process and manage complex tasks. Another significant factor is the impairment of neurovascular coupling, the relationship between neuronal activity, synaptic function, and subsequent blood flow, which disrupts the brain’s ability to maintain optimal function during cognitive tasks71,72. Furthermore, the decrease in cerebral blood flow with age can diminish the delivery of essential nutrients and oxygen to the brain, impairing its overall functionality73. These changes could lead to regional abnormalities, such as blood flow, blood volume, metabolic rate, or BOLD-derived physiologic proxies like the fractional amplitude of low-frequency fluctuation and regional homogeneity74. Future studies may validate and extend our study by adopting the age-sensitive regions we observed and testing other measurements, such as resting-state data, which are more easily collected in large-scale studies involving children and the elderly compared to task-based activations.
The compensatory theory10 proposes that the elderly recruit additional brain regions to compensate for age-related cognitive decline, but our results did not show this pattern. We suggest that the absence of compensatory upregulation in frontoparietal regions among the elderly observed in our study might be attributed to limited available resources when cognitive control related brain regions are already fully engaged75,76. Previous research has shown that younger adults recruit lower activity in frontoparietal regions during the congruent condition but significantly greater activities during the incongruent condition. In contrast, older adults already show a relatively higher activation during the congruent condition, leaving limited capacity for further increases in activation during the incongruent condition32. This is consistent with our findings, which are based on the contrast between incongruent and congruent conditions. Moreover, the nature of the task investigated might influence whether there is an upregulation in cognitive control regions with age. Upregulation in the frontal regions usually compensates for memory and sensory declination due to deficits in the hippocampus and sensory cortices77. Semantic cognition78 might also be a target of compensation. However, conflict tasks seem to rely minimally on memory, and involve relatively simple sensory stimuli (e.g., colors and locations) and simple semantic processing (e.g., reading a word). As such, conflict tasks may not necessitate compensation in these functions.
In addition, compensation in older adults may manifest as increased recruitment of bilateral regions and homologues with age79. For example, the hemispheric asymmetry reduction in older adults (HAROLD) theory80 suggests that older adults typically exhibit less lateralization, either as a compensatory response to functional deficits or as a reflection of neural dedifferentiation. However, we observed that the elderly showed greater left lateralization compared to young to middle-aged adults. This finding is inconsistent with the assumption of HAROLD but aligns with the right hemi-ageing model81, which posits that the right hemisphere is more vulnerable to age-related decline. Prior research has shown that functional connectivity within the frontoparietal control network is more disrupted in the right hemisphere than in the left during ageing82. This suggests that neural resources in the right hemisphere might be more limited for the elderly, reducing its capacity to compensate for cognitive demands. Stronger patterns of left laterality were also identified in childhood in the current study, primarily noticeable within the prefrontal region (Fig. 7), which may reflect the earlier development of the left hemisphere compared to the right83. In contrast, we found lower lateralization in young adults. It is possible that previous studies showing stronger laterality in young adults may have been biased by too small sample sizes and the use of non-quantitative methods for calculation of laterality84,85. Moreover, because both left and right lateralized results were reported in the literature on laterality81, it is reasonable to observe low laterality for young to middle-aged adults in the current meta-analysis.
Methodology Implications
By incorporating all the studies, our results demonstrate that the SDM can reliably identify brain regions as the ALE. However, the SDM has the added advantage of fully utilizing existing effect size data and coordinates86, allowing us to compare the relative activity strength among various age groups, such as the contrast between young to middle-aged adults and elderly groups. More importantly, this approach allows for meta-regressions to examine parametric relationships between brain region activity and age, providing insights into the lifespan trajectories of cognitive control regions.
Limitation of Results
One caveat to consider in this study is the non-uniform distribution of age among the included studies. Specifically, there is a noticeable gap in the age range of 45 to 60 years. Consequently, the observed age distribution could potentially influence the results of the regression analysis. We hope that future research could allocate more attention to the middle-aged period, considering the significant cognitive and neural changes during this stage, such as the onset of cognitive decline15,17,87. In addition, it is crucial to avoid the occurrence of ecological fallacy88 (associations observed at the group level are erroneously assumed to apply to individuals) when interpreting the results of meta-regression analyses. Therefore, associations between brain activities and age across various studies do not provide direct insights into the specific age-related changes at the individual level. Future research incorporating individual-level investigations (e.g., longitudinal follow-up studies) is crucial to obtaining a more comprehensive understanding of these relationships.
Conclusions
Our meta-analysis adopted advanced meta-regression approaches to chart the lifespan trajectories of cognitive control brain activities. We observed inverted U-shaped changing patterns in regions aligned with the frontoparietal control network, with the peaks occurring between 24 and 40 years. In contrast, the dorsal attention network does not present a clear age-related trajectory. This dissociation may reflect the hierarchy of brain development in different regions. No other trajectory patterns were observed, highlighting the predominance of the inverted U-shaped pattern in the lifespan trajectory of cognitive control. Furthermore, we found the youth and elderly showed a more asymmetric brain distribution than young to middle-aged adults. In sum, these results demonstrate the multifaceted nature of age-related changes in cognitive control brain function.
Methods
Literature Preparation
Literature Search
We report how we determined all data exclusions (if any), all manipulations, and all measures in the study. We first searched both English and Chinese articles on the youth and the elderly from PUBMED, Web of Science and CNKI (China National Knowledge Infrastructure) till 2022. The following search terms were applied in titles, abstracts, table of contents, indexing, and key concepts: (“Stroop” OR “Flanker” OR “Simon” OR “SNARC” OR “Navon” OR “interference” OR “cognitive conflict”) AND (“fMRI” OR “functional resonance imaging” OR “functional imaging” OR “neuroimaging” OR “PET”) AND (“children” OR “kids” OR “adolescents” OR “teenagers” OR “underage” OR “aged” OR “old” OR “older” OR “elder” OR “elderly” OR “senior” OR “development” OR “developmental” OR “aging” OR “life span”). The above process yielded 3,484 articles. In addition, 111 studies on young to middle-aged adults from a previous meta-analysis study21 were included in the literature pool, 40 of which were excluded according to the current literature exclusion criteria (see below). Moreover, we screened 16 articles citing or being cited by the crucial literature. After removing duplicates, the literature search identified 2,930 articles.
Exclusion Criteria
We excluded any articles that met one or more of the following predefined exclusion criteria89: 1) not in English or Chinese; 2) not including healthy human participants; 3) case study; 4) not empirical study; 5) not functional resonance imaging (fMRI) or positron emission tomography (PET) study; 6) not whole-brain results (i.e., not have covered the whole gray matter); 7) not in Talairach or Montreal Neurological Institute (MNI) space; 8) not reflecting the congruency effect (i.e., contrasts between incongruent and congruent or between incongruent and neutral conditions); 9) not reporting exact mean age of participants.
A total of 119 articles were identified as eligible for inclusion in our meta-analyses. No statistical methods were used to pre-determine sample sizes, but our sample sizes are similar to or larger than those reported in previous publications31,90. Supplementary Fig. S1 shows the preferred reporting items for systematic reviews and meta-analyses (PRISMA)91 flow chart for the literature screening process. The 119 articles included 129 studies (individual contrasts reported in the articles) with 3,388 participants and 1,579 activation foci reported. All studies were published or completed between 1994 and 2022. None of the experiments share the same group of participants. The included studies are written in English (124 studies) and Chinese (5 studies). Of the studies included, 125 were published in peer-reviewed journals, and 4 were master’s theses. All included studies reported the task type used, including 74 studies utilizing Stroop-like task (57%), 25 studies utilizing Simon task (19%), 25 studies utilizing Flanker task (19%), 2 studies utilizing a combination of Simon and Flanker tasks (2%), 1 study utilizing a combination of Simon and Stroop tasks (1%), and 3 studies utilizing multisource interference task (2%). In addition, the contrasts conducted to reveal brain activations were also reported, with 98 studies (76%) resulting from the contrast of Incongruent trials > Congruent trials, 25 studies (19%) resulting from the contrast of Incongruent trials > Neutral trials, and 6 studies (5%) resulting from the union contrast of Incongruent trials > Congruent trials and Incongruent trials > Neutral trials. Regarding the handedness of participants in the included studies, 89 studies (69%) included right-handed participants only, 6 studies (5%) included both left and right-handed participants, while 34 studies (26%) did not report this information. Furthermore, 78 studies (60%) included only correct response trials, 2 studies (2%) included both correct and incorrect response trials, while 49 studies (38%) did not report this information. A detailed description of these features for each study is available in the Supplementary Table S1. To eliminate the influence of these confounding factors, we included them as covariates in the modeling analyses.
Coding Procedure
A coding manual was formulated to record pertinent study information, including authors, publication dates, experimental tasks, contrasts, and sample demographics (such as the average age and sample size). To ensure coding accuracy, two authors independently coded all studies, with discrepancies resolved through discussion or reference to the original studies. In instances where studies lacked essential information, such as peak coordinates for relevant contrasts, participant age averages, or data for specific age groups, efforts were made to contact the authors via e-mail to obtain the relevant data. In addition, both coordinates and effect sizes (i.e., Hedge’s g) were extracted from each study. Further, Talairach space coordinates were transformed to MNI coordinates using the Lancaster transform92.
Meta-Analytic Procedure
Activation Likelihood Estimation (ALE)
In order to obtain a comprehensive understanding of cognitive control-related brain activity across all age groups and to replicate a prior study21, we initially conducted a single dataset meta-analysis using BrainMap GingerALE software93 (version 3.0.2, http://www.brainmap.org). This meta-analytical approach, known as activation likelihood estimation, utilizes the spatial convergence of brain activity across multiple studies to determine the probability of activation in specific regions. Foci from individual studies were transformed into a standardized coordinate space and modeled as Gaussian probability values that accounted for variability in the number of participants in each study. In situations where foci overlapped across studies, multiple Gaussians were associated with a single focus, and ALE selected the Gaussian with maximum probability for each focus93. Subsequently, ALE score maps were generated by comparing these modeled Gaussian distributions with a null distribution that simulated random brain effects. The null distribution was generated using the same sample size and number of foci groups as the experimental dataset for 1,000 times94. ALE scores were then used to calculate p-values, which were based on the proportion of values higher than a certain threshold in the null distribution. This resulted in a statistical ALE map that differentiated true brain effects from random effects. A cluster-defining threshold of p < 0.001 and a minimum cluster size of 10 voxels (80 mm3) were utilized to compute ALE maps, consistent with the threshold applied in the seed-based d mapping (SDM) approach (see below).
Seed-based d (Effect Size) Mapping
SDM is an alternative approach to statistically synthesize results from multiple neuroimaging experiments86. Similar to ALE, SDM employs a coordinate-based random-effect approach to amalgamate peak coordinate information into a standard space across several experiments. However, while ALE solely considers the binary feature (i.e., active versus inactive) of peak coordinates, SDM takes into account the quantitative effect size (can be positive or negative) connected to each peak and reconstructs the initial parametric maps of individual experiments before amalgamating them into a meta-analytic map95. Therefore, the use of a distinct algorithm in SDM from ALE allows us to scrutinize the robustness and replicability of the outcomes obtained via ALE. More importantly, SDM enables the inclusion of covariates in the meta-regression analyses to reflect the changes in brain function across the lifespan.
We conducted three types of analyses using the SDM approach. Firstly, we estimated the mean activation across all age groups and compared the results with ALE’s single dataset meta-analysis results. Secondly, we conducted contrasts between two groups of studies (e.g., between young to middle-aged adults and a combination of the youth and elderly groups) to identify brain regions that showed different levels of activity across age. This analysis method served to investigate the hypothesized lifespan trajectories, such as the inverted U-shaped pattern by elucidating neural activity variations linked to age. Thirdly, we defined specific models (e.g., linear and square root models) to fit the whole brain to validate brain regions adhering to the hypothetical lifespan changing patterns. This type of analysis aimed to explore various lifespan trajectories, recognizing that different brain regions might follow distinct model functions. See below for the details.
These analyses were conducted using the software of SDM with permutation of subject images (SDM-PSI) (version 6.22, https://www.sdmproject.com). Effect size maps were built for the 129 individual experiments. This was accomplished by (a) converting the statistical value of each peak coordinate into an estimate of effect size (Hedge’s g) using standard formulas96 and (b) convolving these peaks with a fully anisotropic unnormalized Gaussian kernel (α = 1, FWHM = 20 mm) within the boundaries of a gray matter template (voxel size = 2×2×2 mm3). Imputation (50 times) was conducted for each study separately to obtain a reliable estimate of brain activation maps95. In addition, the individual effect size maps were combined using a random-effect general linear model. To assess the statistical significance of activations in the resulting meta-analytic effect size map, 1,000 random permutations of activation peaks within the gray matter template were compared. Finally, the meta-analytic maps were thresholded using a voxel-wise family-wise error (FWE) corrected threshold of p < 0.001 and a cluster-wise extent threshold of 10 voxels97.
Mean Analyses Across all Studies
This analysis aimed to characterize the activation distributions of cognitive control-related brain regions across all studies, which was conducted utilizing the “Mean” function in SDM-PSI software. In order to verify the reliability of the SDM analysis results, we compared the results with the single dataset meta-analysis using ALE. Results from this analysis were further used as regions of interest (ROIs) in the subsequent model fitting analyses (see section “Generalized Additive Model (GAM) Fitting” below). The possibility of publication bias for resultant clusters was examined using Egger’s test98, in which any result showing p < 0.05 was regarded as having significant publication bias. Heterogeneity was evaluated using the I2 index, which quantifies the proportion of total variability attributable to heterogeneity between studies. A value less than 25% indicates low heterogeneity among the included studies99.
Contrast Analyses
To test our hypothesis that cognitive control related brain activities follow an inverted U-shaped trajectory with age, we categorized each study based on the mean age of participants into youth (< 18 years), young to middle-aged adults (18–59 years), and elderly (>= 60 years) groups. The age boundaries were determined to minimize age distribution overlap. We utilized SDM-PSI to perform a contrast analysis between the group of young to middle-aged adults and the combination of other groups in order to examine whether there are brain regions that exhibit an inverted U-shaped lifespan trajectory. This was achieved by assigning studies from the young to middle-aged adult group as 1 and all other studies as −1. This analysis yielded two results, one showing higher activity in young to middle-aged adults than the youth and elderly groups, and the other showing the opposite. Like the mean analysis, results from this analysis were used as ROIs in the subsequent model fitting analyses (see sections “Generalized Additive Model (GAM) Fitting” and “Model Simplification and Model Comparison”).
In addition, to explore other possible trajectories, such as the increase-and-stable pattern5, we conducted contrast analyses between the youth and the combined group of young to middle-aged and the elderly, as well as between the elderly and the combined group of youth and young to middle-aged adults. Furthermore, to address the controversies in previous studies, we conducted contrast analyses between older and young to middle-aged adult groups, and between the youth and young to middle-aged adult groups, respectively.
Meta-regression Analyses
To better describe the possible lifespan trajectories of the whole brain, we carried out meta-regression analyses with the age and/or its derivatives as regressors. Two regressions were conducted across the whole-brain, including a linear regression (with only age as the regressor) and a square root regression (with age and its square root as separate regressors). The linear regression aimed to test regions with increasing/decreasing activity with age, and the square root regression aimed to test regions with the inverted U-shaped trajectories based on the model fitting analyses (see below).
Data Extraction
Masks were generated for each ROI derived from the mean and contrast analyses in SDM-PSI as described above. Subsequently, we extracted the effect sizes for each mask. Fifty values were obtained from the SDM iterations and subsequently averaged for each study in each region. The iterated variances were also averaged in a similar way. Additionally, we removed the outliers (beyond 3 standard deviations from the mean) in the following model fitting analyses.
Generalized Additive Model (GAM) Fitting
To precisely estimate the inverted U-shaped trajectories, we adopted the GAM to fit the curves. The GAM allows for flexible, nonparametric smoothing of predictor variables100, and has been widely used to depict the lifespan trajectories101,102. We implemented GAMs using the “mgcv” package100 in R. For each ROI, we fitted a GAM with the following formula:
where g is the effect size (dependent variable), s(age) represents a smoothing spline of age (predictor variable), and covariates represent the dummy-coded categorical covariate regressors. These regressors correspond to six aspects of the included studies: (1) the presence of various conflict types (e.g., Stroop or Simon), (2) the mixed subject samples based on handedness (e.g., right handed only or both handed), (3) the different contrasts in reporting congruency effects (e.g., incongruent – congruent or incongruent – neutral), (4) different trial types regarding whether they excluded error trials, (5) the use of different types of experimental design (i.e., event-related or block designs), and (6) the behavioral congruency effects measured by reaction time. Notably, we adopted median imputation103 for 9 studies (accounting for 6.98% of the total included studies) not reporting the behavioral congruency effects, and included an indicator regressor to account for the potential impact of imputation104. The validity of this imputation approach was confirmed through a robustness analysis (Supplementary Note S4). We incorporated these covariates to control for their potential confounding effects related to age, which could otherwise influence our results. We also adjusted the estimate with a weight parameter, which was the reciprocal of variance. We used penalized regression splines, with the amount of smoothing determined automatically based on generalized cross validation.
For each ROI, we quantified the peak age by choosing the highest prediction of a fine-grained age scale (1,000 points from 8 to 74 years old). We also calculated the estimated degree of freedom (EDF) for the smooth curve by summing up the degree of freedom for each penalized term (i.e., s(age).1 to s(age).9).
Model Simplification and Model Comparison
While the GAM analysis may yield good fitting results on the data, it is important to acknowledge its potential limitations. One concern is that it can fit the data with high degree of freedoms (up to 7.0, Supplementary Table S4), which makes it susceptible to over-fitting and harder to generalize. Another issue is its poor interpretability. Therefore, we next sought to fit the data with simpler models.
To this end, we used the “metafor” package in R to fit these effect sizes with the age and its derivatives as predictors. Specifically, we tested four non-linear models (see below formulas and Fig. 3). Quadratic and cubic models were included based on previous studies46,47; quadratic logarithmic and square-root models were included to capture the possible skewed trajectory, which would reflect the asymmetric trajectory of development and decline of cognitive control5. Each model was fitted to each ROI separately. In addition, we included the same covariates as the GAM analysis in each regression model. We calculated the Akaike information criterion (AIC) to evaluate the goodness of fit for each model.
- Quadratic model
- Cubic model
- Quadratic logarithmic model
- Square root model
Neurosynth Decoding Analysis
To investigate the potential functional difference between the two sets of brain regions reported in section “Dissociated Brain Networks with Distinct Lifespan Trajectories” of Results, we generated two binary maps from the contrast analysis between young to middle-aged adults and the combination of other groups, and then submitted them to the Neurosynth decoding system41. As the non-U-shaped map is defined as the converse of the inverted U-shaped map, they were obtained by applying thresholds of 0.1 < p < 0.9 and p < 0.05, respectively. These lenient threshold boundaries were used to minimize the influence of sparsity on the decoding results. The two-boundary threshold was used in generating the non-U-shaped map, as the original statistical map from the SDM-PSI analysis was one-tailed, which means the threshold of p > 0.9 indicates the upright U-shaped trend instead of a non-U-shaped trajectory. Additionally, to focus on the brain regions specifically related to cognitive control, the two maps were masked by using results from the mean SDM analysis (Fig. 1B). In the decoding results, we deleted terms that were related to brain regions (e.g., “frontal”), not functional specific (e.g., “task”), and duplicated (e.g., “attention” was removed if there was already “attentional”).
Laterality Analysis
We calculated the laterality based on the effect size of reported brain coordinates from each study. We computed the sum of effect sizes across coordinates for the left and right hemisphere, respectively, yielding one global effect size each (i.e., gL and gR). Then, we calculated the index of brain laterality with the following equation105:
which was then submitted to the GAM and simplified models (i.e., the quadratic, cubic, square root, and quadratic logarithmic models).
To illustrate the contribution of different brain regions to the age-related change of laterality, we calculated the laterality for each voxel78. We first used the SDM-PSI to conduct a mean analysis for each age group (i.e., the youth, young to middle-aged adult and elderly), yielding three z-maps. Then the laterality was computed with the above equation for each voxel from the left hemisphere. The opposite values were calculated for the right hemisphere. To avoid the bias due to asymmetric brain hemispheres, we removed voxels without corresponding mirror coordinates. The results were visualized confining to the brain regions estimated from the grand mean analyses (Fig. 1B).
Supplementary Material
Acknowledgement
We thank Fergus I.M. Craik, Beatriz Luna, and Haiyan Wu for valuable suggestions on this manuscript. We also thank Jing Yang and Yifan Zhang for the data check of extracted coordinates. Z.L. discloses support for the research of this work from Scientific Research Fund of Zhejiang Provincial Education Department (Y202249966), Starting Research Fund from Hangzhou Normal University (2021QDL079), and STI 2030-Major Projects (2021ZD0201705). I.T.P discloses support for the research of this work from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HD098235). G.Y. discloses support for the research of this work from Jiefeng Jiang, and China Postdoctoral Science Foundation (2019M650884). X.L. discloses support for the research of this work from the Ministry of Science and Technology of the People’s Republic of China (2021ZD0200505). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Code Availability
All codes conducting the ROI analyses are available in Zenodo with the identifier doi: 10.5281/zenodo.12727621106.
Competing Interests Statement
The authors declare no competing interests.
Inclusion & Ethics
All collaborators of this study fulfilled the criteria for authorship required by Nature Portfolio journals have been included as authors, as their participation was essential for implementation of the study and improvement of the manuscript. Roles and responsibilities were agreed among collaborators ahead of the research. This work does not include findings that are locally relevant. As a meta-analysis, this study does not involve direct interactions with human participants or the collection of new data. Each of the original studies included in this meta-analysis obtained ethical approval from their respective institutions, and we confirm that we complied with all applicable ethical guidelines and regulations.
Data Availability
The meta-data that support the findings of this study are available in Zenodo with the identifier doi: 10.5281/zenodo.12727621106.
References
- 1.Cohen JD. Cognitive control: Core constructs and current considerations. In: The Wiley handbook of cognitive control (ed Egner T) (2017). [Google Scholar]
- 2.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24, 167–202 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Goschl F, Yang G. Dissociated Neural Mechanisms of Target and Distractor Processing Facilitated by Expectations. J Neurosci 40, 1997–1999 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantinidis C, Luna B. Neural Substrates of Inhibitory Control Maturation in Adolescence. Trends Neurosci 42, 604–616 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends Cogn Sci 10, 131–138 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Kar BR. Development of attentional processes in ADHD and normal children. Prog Brain Res 176, 259–276 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev 28, 62–77 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond A. Executive functions. Annu Rev Psychol 64, 135–168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanto TP, Gazzaley A. Attention and ageing. In: The Oxford handbook of attention.). Oxford University Press; (2014). [Google Scholar]
- 10.Reuter-Lorenz PA, Cappell KA. Neurocognitive Aging and the Compensation Hypothesis. Current Directions in Psychological Science 17, 177–182 (2008). [Google Scholar]
- 11.Association AP. Diagnostic and statistical manual of mental disorders : DSM-5.). 5th edn. American Psychiatric Association; (2013). [Google Scholar]
- 12.Adnan A, Chen AJW, Novakovic-Agopian T, D’Esposito M, Turner GR. Brain Changes Following Executive Control Training in Older Adults. Neurorehabil Neural Repair 31, 910–922 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 9, 104–110 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci 13, 491–505 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weintraub S, et al. Cognition assessment using the NIH Toolbox. Neurology 80, S54–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson HJ, Brunsdon VEA, Bradford EEF. The developmental trajectories of executive function from adolescence to old age. Sci Rep 11, 1382 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SC, Hammerer D, Muller V, Hommel B, Lindenberger U. Lifespan development of stimulus-response conflict cost: similarities and differences between maturation and senescence. Psychol Res 73, 777–785 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erb CD, Germine L, Hartshorne JK. Cognitive control across the lifespan: Congruency effects reveal divergent developmental trajectories. J Exp Psychol Gen 152, 3285–3291 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Brouwer RM, et al. Genetic variants associated with longitudinal changes in brain structure across the lifespan. Nat Neurosci 25, 421–432 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethlehem RAI, et al. Brain charts for the human lifespan. Nature, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Yang G, Li Z, Qi Y, Cole MW, Liu X. Conflict detection and resolution rely on a combination of common and distinct cognitive control networks. Neurosci Biobehav Rev 83, 123–131 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2, 861–863 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci 13, 636–650 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS One 6, e21598 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veroude K, Jolles J, Croiset G, Krabbendam L. Changes in neural mechanisms of cognitive control during the transition from late adolescence to young adulthood. Dev Cogn Neurosci 5, 63–70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilk HA, Morton JB. Developmental changes in patterns of brain activity associated with moment-to-moment adjustments in control. Neuroimage 63, 475–484 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Konrad K, et al. Development of attentional networks: an fMRI study with children and adults. Neuroimage 28, 429–439 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Marsh R, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp 27, 848–863 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron 33, 301–311 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milham MP, et al. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn 49, 277–296 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Yaple ZA, Stevens WD, Arsalidou M. Meta-analyses of the n-back working memory task: fMRI evidence of age-related changes in prefrontal cortex involvement across the adult lifespan. Neuroimage 196, 16–31 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Prakash RS, Erickson KI, Colcombe SJ, Kim JS, Voss MW, Kramer AF. Age-related differences in the involvement of the prefrontal cortex in attentional control. Brain Cogn 71, 328–335 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez NB, Hars M, Trombetti A, Vuilleumier P. Age-related changes in attention control and their relationship with gait performance in older adults with high risk of falls. Neuroimage 189, 551–559 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Sebastian A, et al. Differential effects of age on subcomponents of response inhibition. Neurobiology of aging 34, 2183–2193 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neurosci Biobehav Rev 26, 849–857 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Verhaeghen P. The elements of cognitive aging: Meta-analyses of age-related differences in processing speed and their consequences. OUP USA (2014). [Google Scholar]
- 37.Wood G, Ischebeck A, Koppelstaetter F, Gotwald T, Kaufmann L. Developmental trajectories of magnitude processing and interference control: an FMRI study. Cereb Cortex 19, 2755–2765 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA psychiatry 70, 185–198 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Peng P, Eickhoff SB, Lin X, Zhang D, Wang Y. Neural substrates of the executive function construct, age-related changes, and task materials in adolescents and adults: ALE meta-analyses of 408 fMRI studies. Dev Sci 24, e13111 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Long J, Song X, Wang Y, Wang C, Huang R, Zhang R. Distinct neural activation patterns of age in subcomponents of inhibitory control: A fMRI meta-analysis. Front Aging Neurosci 14, 938789 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8, 665–670 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7, 1–17 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8, 539–546 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–1026 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Simonsohn U. Two Lines: A Valid Alternative to the Invalid Testing of U-Shaped Relationships With Quadratic Regressions. Advances in Methods and Practices in Psychological Science 1, 538–555 (2018). [Google Scholar]
- 46.Zuo XN, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 30, 15034–15043 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coupe P, Catheline G, Lanuza E, Manjon JV, Alzheimer’s Disease Neuroimaging I. Towards a unified analysis of brain maturation and aging across the entire lifespan: A MRI analysis. Hum Brain Mapp 38, 5501–5518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amlien IK, Sneve MH, Vidal-Pineiro D, Walhovd KB, Fjell AM. The Lifespan Trajectory of the Encoding-Retrieval Flip: A Multimodal Examination of Medial Parietal Cortex Contributions to Episodic Memory. J Neurosci 38, 8666–8679 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Luca CR, Leventer RJ. Developmental trajectories of executive functions across the lifespan. In: Executive functions and the frontal lobes). Psychology Press; (2010). [Google Scholar]
- 51.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. The American journal of psychiatry 162, 1605–1613 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robertson BD, Hiebert NM, Seergobin KN, Owen AM, MacDonald PA. Dorsal striatum mediates cognitive control, not cognitive effort per se, in decision-making: An event-related fMRI study. Neuroimage 114, 170–184 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Hsu CL, et al. Aerobic exercise promotes executive functions and impacts functional neural activity among older adults with vascular cognitive impairment. Br J Sports Med 52, 184–191 (2018). [DOI] [PubMed] [Google Scholar]
- 54.(!!! INVALID CITATION !!!).
- 55.Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev 35, 1687–1703 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101, 8174–8179 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annu Rev Neurosci 38, 151–170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28, 3586–3594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grimm KJ, Ram N, Estabrook R. Growth Modeling: Structural Equation and Multilevel Modeling Approaches. Guilford Publications; (2016). [Google Scholar]
- 60.Verissimo J, Verhaeghen P, Goldman N, Weinstein M, Ullman MT. Evidence that ageing yields improvements as well as declines across attention and executive functions. Nat Hum Behav 6, 97–110 (2022). [DOI] [PubMed] [Google Scholar]
- 61.D’Mello AM, Gabrieli JDE, Nee DE. Evidence for Hierarchical Cognitive Control in the Human Cerebellum. Curr Biol 30, 1881–1892 e1883 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shine JM, Lewis LD, Garrett DD, Hwang K. The impact of the human thalamus on brain-wide information processing. Nat Rev Neurosci 24, 416–430 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multitask connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16, 1348–1355 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Y, Friston KJ, Zeidman P, Chen J, Li S, Razi A. The Hierarchical Organization of the Default, Dorsal Attention and Salience Networks in Adolescents and Young Adults. Cereb Cortex 28, 726–737 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci 35, 73–89 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Badre D, Nee DE. Frontal Cortex and the Hierarchical Control of Behavior. Trends Cogn Sci 22, 170–188 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixon ML, et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci U S A 115, E1598–E1607 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sydnor VJ, et al. Intrinsic activity development unfolds along a sensorimotor-association cortical axis in youth. Nat Neurosci 26, 638–649 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raz N, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 7, 268–282 (1997). [DOI] [PubMed] [Google Scholar]
- 70.Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci 21, 187–221 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Kaplan L, Chow BW, Gu C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci 21, 416–432 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lipecz A, et al. Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience 41, 341–349 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12, 723–738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montala-Flaquer M, Canete-Masse C, Vaque-Alcazar L, Bartres-Faz D, Pero-Cebollero M, Guardia-Olmos J. Spontaneous brain activity in healthy aging: An overview through fluctuations and regional homogeneity. Front Aging Neurosci 14, 1002811 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 46, 462–473 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanto TP, Gazzaley A. Cognitive Control and the Ageing Brain. In: The Wiley Handbook of Cognitive Control (ed Egner T) (2017). [Google Scholar]
- 77.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 60, 173–196 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoffman P, Morcom AM. Age-related changes in the neural networks supporting semantic cognition: A meta-analysis of 47 functional neuroimaging studies. Neurosci Biobehav Rev 84, 134–150 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. NeuroImage 21, 192–200 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17, 85–100 (2002). [DOI] [PubMed] [Google Scholar]
- 81.Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev 26, 819–825 (2002). [DOI] [PubMed] [Google Scholar]
- 82.Zhang HY, Chen WX, Jiao Y, Xu Y, Zhang XR, Wu JT. Selective vulnerability related to aging in large-scale resting brain networks. PLoS One 9, e108807 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science 236, 1110–1113 (1987). [DOI] [PubMed] [Google Scholar]
- 84.Cabeza R, et al. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci 17, 391–400 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychol Aging 17, 7–23 (2002). [DOI] [PubMed] [Google Scholar]
- 86.Albajes-Eizagirre A, Solanes A, Vieta E, Radua J. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage 186, 174–184 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist 10, 372–392 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 21, 1559–1573 (2002). [DOI] [PubMed] [Google Scholar]
- 89.Cumpston M, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10, ED000142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu J, Yao J, Zhou Z, Wang XT. Age effects on delay discounting across the lifespan: A meta-analytical approach to theory comparison and model development. Psychological Bulletin 149, 447–486 (2023). [Google Scholar]
- 91.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. 88, 105906 (2021). [DOI] [PubMed] [Google Scholar]
- 92.Lancaster JL, et al. Bias between MNI and talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping 28, 1194–1205 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping 33, 1–13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage 59, 2349–2361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Radua J, Mataix-Cols D. Meta-analytic methods for neuroimaging data explained. Biol Mood Anxiety Disord 2, 6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics 6, 107–128 (1981). [Google Scholar]
- 97.Radua J, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiat 27, 605–611 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ (Clinical research ed) 315, 1533–1537 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wood SN. mgcv: GAMs and generalized ridge regression for R. J R news 1, 20–25 (2001). [Google Scholar]
- 101.Zuo XN, He Y, Betzel RF, Colcombe S, Sporns O, Milham MP. Human Connectomics across the Life Span. Trends Cogn Sci 21, 32–45 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Bethlehem RAI, et al. Brain charts for the human lifespan. Nature 604, 525–533 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ochieng’ Odhiambo F. Comparative Study of Various Methods of Handling Missing Data. Mathematical Modelling and Applications 5, (2020). [Google Scholar]
- 104.Sandhu AT, Kohsaka S, Turakhia MP, Lewis EF, Heidenreich PA. Evaluation of Quality of Care for US Veterans With Recent-Onset Heart Failure With Reduced Ejection Fraction. JAMA Cardiol 7, 130–139 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging 26, 594–601 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang G. guochun-yang/Cognitive_Control_Developmental_Trajectory: Release_20240711 (Lifespan).). Zenodo (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The meta-data that support the findings of this study are available in Zenodo with the identifier doi: 10.5281/zenodo.12727621106.