Abstract
Object vision is commonly thought to involve a hierarchy of brain regions processing increasingly complex image features, with high-level visual cortex supporting object recognition and categorization. However, object vision supports diverse behavioral goals, suggesting basic limitations of this category-centric framework. To address these limitations, here we map a series of behaviorally-relevant dimensions derived from a large-scale analysis of human similarity judgments directly onto the brain. Our results reveal broadly-distributed representations of behaviorally-relevant information, demonstrating selectivity to a wide variety of novel dimensions while capturing known selectivities for visual features and categories. Behaviorally-relevant dimensions were superior to categories at predicting brain responses, yielding mixed selectivity in much of visual cortex and sparse selectivity in category-selective clusters. This framework reconciles seemingly disparate findings regarding regional specialization, explaining category selectivity as a special case of sparse response profiles among representational dimensions, and suggesting a behavior-centric view on visual processing in the human brain.
A central goal of visual neuroscience is to understand how the brain encodes and represents rich information about objects, allowing us to make sense of our visual world and act on it in meaningful ways. A widely studied and influential account posits that one central function of the visual system is to recognize objects by organizing them into distinct categories 1-4. According to this view, early visual cortex serves to analyze incoming visual information by representing basic visual features 5, which are then combined into more and more complex feature combinations, until higher-level visual regions in the inferior temporal cortex and beyond support the recognition of object identity and category 3. In line with this view, a number of category-selective clusters have been identified in occipitotemporal cortex that respond selectively to object classes such as faces, scenes, body parts, tools, or text 6-11. The functional significance of these regions is underscored by studies demonstrating that object category and identity as well as performance in some behavioral tasks can be read out from activity in occipitotemporal cortex 12-16 and that lesions to these regions can lead to selective deficits in object recognition abilities 17-21.
Despite the importance of object categorization and identification as crucial goals of object vision, it has been argued that these functions alone are insufficient for capturing how our visual system allows us to make sense of the objects around us 22. A more comprehensive understanding of object vision should account for the rich meaning associated with individual objects beyond discrete labels and incorporate the many visual and semantic properties of objects that are relevant to adaptive behavior 22-25. Indeed, others have proposed that visual cortex is organized based on continuous dimensions reflecting more general object properties, such as animacy 26-29, real-world size 27,30, aspect ratio 29,31, or semantics 32. In this framework, visual cortex is organized based on continuous topographic maps tuned to these dimensions that extend beyond category-selective clusters, and the behavioral relevance of objects is encoded in the combination of these tuning maps 33. Thus, according to this view, category-selective clusters may emerge from a more general organizing principle 32,34-36, reflecting cortical locations where these tuning maps encode feature combinations tied to specific object categories 32,33,37. Yet, while previously proposed dimensions have been shown to partially reflect activity patterns in category-selective clusters 38-43, they account for only part of the response profile and were largely inferior to category-selectivity in explaining the functional selectivity of the human visual cortex for objects 44,45.
To uncover the nature of behaviorally-relevant selectivity in human visual cortex, in the present study we built an encoding model of human visual cortex responses based on a large-scale functional MRI dataset with brain responses to thousands of object images, paired with representational dimensions underlying perceived similarity derived from millions of human similarity judgments. In contrast to much previous research that has focused on a small number of hypothesis-driven dimensions or that used small, selective image sets 27,46-48, here we carried out a comprehensive characterization of cortical selectivity in response to 66 representational dimensions of objects identified in a data-driven fashion from large-scale data of human similarity judgements 49,50. Perceived similarity has been used as a common proxy of mental object representations underlying various behavioral goals, including object identification and categorization according to diverse perceptual and conceptual criteria 50-54. As such, perceived similarity is ideally suited for revealing behaviorally relevant representational dimensions and how these dimensions are reflected in cortical patterns of brain activity.
Moving beyond the widely held view that mental object representations are primarily mirrored in higher-level visual cortex 55-58, we demonstrate that representations underlying core object dimensions are reflected throughout the entire visual cortex and capture its functional topography. Our results revealed that cortical tuning to these dimensions captures stimulus selectivity throughout the visual hierarchy. In this multidimensional representation, category selectivity stood out as a special case of sparse selectivity to a set of core behaviorally relevant representational object dimensions, while other parts of visual cortex reflected a more mixed selectivity. A direct model comparison revealed that continuous object dimensions provide a better model of brain responses than binary categories across the visual system, suggesting that dimension-related tuning maps offer more explanatory power than categories. Together, our findings reveal the importance of behaviorally-relevant object dimensions for understanding the functional organization of the visual system and offer a broader, comprehensive view of object representations that bridges the gap between regional specialization and domain-general topography.
Results
We first aimed at mapping core representational object dimensions to patterns of brain activity associated with visually-perceived objects. To model the neural representation of objects while accounting for their large visual and semantic variability 59,60, we used the THINGS-data collection 55, which includes densely sampled fMRI data for thousands of naturalistic object images from 720 semantically diverse objects, as well as 4.7 million behavioral similarity judgments of these objects (Fig. 1).
Fig. 1. Overview: An fMRI encoding model of object dimensions underlying human similarity judgements.
We linked core representational dimensions capturing the behavioral relevance of objects to spatially resolved neural responses to thousands of object images. For this, we used the THINGS-data collection 55 which includes fMRI and behavioral responses to objects from the THINGS object concept and image database 63. The behavioral data was used to train a computational model of core object dimensions underlying human similarity judgements to different object concepts. We extended this embedding to the level of individual object images based on the computer vision model CLIP-VIT 61. The fMRI data comprises three participants who each saw 8,740 unique object images. We used an encoding model of the object dimension embedding to predict fMRI responses to each image in each voxel. The estimated encoding model weights reflect the tuning of each voxel to each object dimension. X, B, and Y denote the design matrix, regression weights, and outcome of the encoding mode, respectively.
As core object dimensions, we used a recent similarity embedding of behaviorally-relevant object dimensions, which underlie the perceived similarity of 1,854 object concepts 50,55. In this embedding, each object image is characterized by 66 dimensions derived from the human similarity judgments in an odd-one-out task. We chose this embedding for several reasons: First, it provides highly reproducible dimensions that together are sufficient for capturing single trial object similarity judgments close to the noise ceiling. Second, the use of an odd-one-out task supports the identification of the minimal information required to distinguish between different objects, and as such is sensitive not only to conceptual information, such as high-level category (e.g., “is an animal”), but also to key visual-perceptual distinctions (e.g., “is round”). Indeed, the object dimensions capture external behavior such as categorization and typicality judgements, underscoring its behavioral relevance as a model of neural responses to objects 50. Third, the object dimensions are easily interpretable, thus simplifying the interpretation of neural activity patterns in relation to individual dimensions.
The fMRI dataset covers 8,740 unique images from 720 categories presented to three participants (2 female) over the course of 12 sessions 55. Given that the behavioral similarity embedding was trained only on one image per each of the 1,854 THINGS categories, these dimensions may only partially capture the visual richness of the entire image set, which may affect the potential for predicting image-wise brain responses. To address this challenge, we fine-tuned the artificial neural network model CLIP-VIT 61 to directly predict object dimensions for the 8,740 images in our fMRI dataset. This approach led to highly accurate cross-validated predictions of object similarity 62 and consistent improvements in BOLD signal predictions for all 66 dimensions (Suppl. Fig. 1).
Core object dimensions are reflected in widespread fMRI activity patterns throughout the human visual system
To test how these dimensions were expressed in voxel-wise brain responses, we fit an fMRI encoding model which predicts spatially resolved brain responses based on a weighted sum of these object dimensions. This allowed us to map out the contribution of the dimensions to the measured signal and thus link interpretable behaviorally-relevant dimensions to patterns of brain activity.
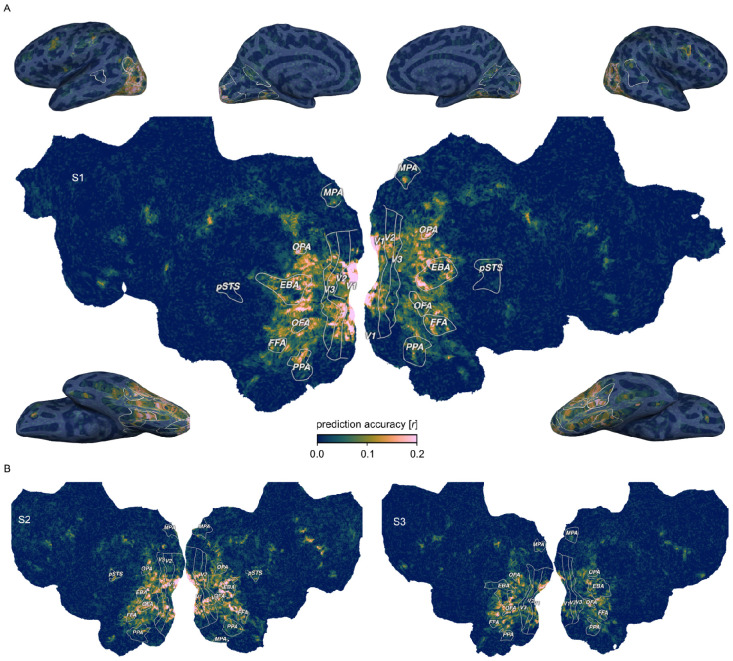
Across all 66 object dimensions, our results revealed a widely distributed cortical representation of these dimensions that spans much of visual cortex and beyond (Fig. 2). The spatial extent of these effects was consistent across all three subjects, underscoring the generality of these findings. Prediction accuracies peaked not only in lateral occipital and posterior ventral temporal regions, but also reached significant values in early visual, dorsal visual, and frontal regions (Suppl. Fig. 2). This suggests that, in contrast to previous work that indicates behaviorally-relevant representations to be confined to higher-level visual cortex 55,56,58,64, cortical representations convey behaviorally-relevant information about objects in a much more distributed fashion throughout the visual processing hierarchy, including the earliest cortical processing stages.
Fig. 2. Prediction accuracy of the fMRI voxel-wise encoding model based on 66 core object dimensions.
Colors indicate the Pearson correlation between predicted and held-out data in a 12-fold between-session cross-validation. White outlines indicate regions of interests defined in separate localizer experiments: FFA: Fusiform face area; OFA: Occipital face area; pSTS: Posterior superior temporal sulcus; EBA: Extrastriate body area; PPA: Parahippocampal place area; OPA: Occipitoparietal place area; MPA: Medial place area; V1-V3: Primary to tertiary visual cortex. A. Prediction accuracy for one example subject (S1) visualized on a cortical flat map (center) and inflated views of the cortical surface (corners). B. Results for the other two subjects visualized on cortical flat maps.
Behaviorally relevant object dimensions reflect the functional topography of the human visual system
Having identified where information about perceived similarity is encoded, we next explored the spatial layout of each individual dimension underlying this representation. By using a voxel-encoding model of interpretable object dimensions, it is possible to inspect the cortical distribution of the weights of each regressor separately and interpret them in a meaningful fashion. This has two benefits. First, it allows us to probe to what degree behaviorally-relevant dimensions alone can capture the known topography of visual cortex. Second, it allows us to identify novel topographic patterns across visual cortex. This promises important insights into how the topography of visual cortex reflects object information relevant to behavior and how functionally specialized regions are situated in this cortical landscape.
Visualizing the voxel-wise regression weights for each object dimension on the cortical surface (Fig. 3) revealed a clear correspondence between numerous dimensions and characteristic, known topographic patterns of the visual system. For example, the “animal-related” dimension mirrors the well established spoke-like tuning gradient for animate versus inanimate objects 27, while dimensions like “head-related” and “body-part related” differentiate the regional selectivity for faces and body parts in the fusiform face area (FFA), occipital face area (OFA), and extrastriate body area (EBA), respectively 6,7,65. Likewise, the implicit inclusion of natural scenes as object backgrounds revealed scene content-related dimensions (e.g. “house-/furnishing-related”, “transportation-/ movement-related”, and “outdoors”), which were found to be associated with scene-selective brain regions such as parahippocampal place area (PPA), medial place area (MPA), and occipital place area (OPA) 8,66-69. Our approach also independently identified a “food-related” dimension in areas adjacent to the fusiform gyrus, in line with recently reported clusters responding selectively to food stimuli 70-72. A dimension related to tools (“tool-related/handheld/elongated”) also matched expected activation patterns in middle temporal gyrus 11,73,74. Dimensions related to low- to mid-level visual features (e.g. “grid/grating-related”, “repetitive/spiky”) reflected responses primarily in early visual cortex.
Fig. 3. Functional tuning maps to individual object dimensions.
The figure shows example maps for 12 out of the 66 dimensions for Subject S1. Each panel shows the encoding model weights for one object dimension projected onto the flattened cortical surface. Numbers in the subtitles show the dimension number in the embedding.
Beyond these established topographies, the results also revealed numerous additional topographic patterns. For example, one dimension reflected small, non-mammalian animals (“bug-related / non-mammalian / disgusting”) that was clearly distinct from the “animal-related” dimension by lacking responses in face and body selective regions. Another dimension reflected a widely distributed pattern in response to thin, flat objects (“thin / flat / wrapping”). Thus, our approach allowed for the identification of candidate functional selectivities in visual cortex that might have gone undetected with more traditional approaches based on proposed categories or features 45,70. Importantly, the functional topographies of most object dimensions were also found to be highly consistent across the three subjects, suggesting that they may reflect general organizing principles rather than idiosyncratic effects (Extended Data Fig. 1-6).
Together, our results uncover cortical maps of object dimensions underlying the perceived similarity between objects. These maps span extensive portions of the visual cortex, capturing topographic characteristics such as tuning gradients of object animacy, lower-level visual feature tuning in early visual cortex, and category-selective, higher-level regions while uncovering new candidate selectivities. Thus, these findings support an organizing principle where multiple, superimposing cortical tuning maps for core object properties collectively represent behaviorally-relevant information of objects.
Cortical tuning to behaviorally-relevant object dimensions explains regional functional selectivity
Having delineated the multidimensional topographic maps across visual cortex, we next honed in on individual brain regions to determine their functional selectivity as defined by their response tuning across these behaviorally-relevant dimensions. To this end, we developed a high-throughput method to identify object images representative for specific brain regions. Specifically, we first determined a functional tuning profile across dimensions for each region of interest based on the region’s mean encoding model weights. Next, we aimed at identifying images for which their behavioral dimension profile best matched the functional tuning profile of the brain region. To this end, we used all 26,107 object images in the THINGS database 63, most of which were unseen by participants, and assessed the cosine similarity between the dimension profiles of brain regions and images. This enabled us to rank over 26,000 images based on their similarity to a given brain region's functional tuning profile.
Despite having been fitted solely on the 66-dimensional similarity embedding, our approach successfully identified diverse functional selectivities of visual brain regions (Fig. 4). For instance, the most representative images for early visual regions (V1, V2, V3) contained fine-scale, colorful, and repeating visual features, consistent with known representations of oriented edges and color in these areas 75,76. These patterns appeared more fine-grained in earlier (V1 or V2) compared to later retinotopic regions (hV4), potentially reflecting increased receptive field size along the retinotopic hierarchy 77-79. A similar finding is reflected in dimension selectivity profiles (Fig. 4), revealing increased color selectivity in hV4 compared to early retinotopic regions V1-V3 while yielding reductions in the “repetitive/spiky” dimension. Notably, tuning profiles in category-selective regions aligned with images of expected object categories: faces in face-selective regions (FFA, OFA), body parts in body-part selective regions (EBA), and scenes in scene-selective regions (PPA, OPA, MPA). Closer inspection of the tuning profiles revealed differences between regions that respond to the same basic object category, such as a stronger response to the “body-part related” dimension in OPA but not in other place-selective regions. Also, selectivity to faces (FFA, OFA) vs. body parts (EBA) appeared to be driven by the response magnitude to the “head-related” dimension, while tuning to the remaining dimensions was highly similar across these regions. Together, these findings demonstrate that the 66 object dimensions derived from behavior capture the selectivity across the visual processing hierarchy, highlighting the explanatory power of the dimensional framework for characterizing the functional architecture of the visual system.
Fig. 4. Regional tuning profiles across 66 object dimensions and representative images for selectivity of each region of interest in visual cortex.
Rose plots indicate the magnitude of tuning for each object dimension in a given visual brain region. Image panels show 8 images with the most similar model representation to the regional tuning profile. For copyright reasons, all original images have been replaced with visually similar images. For identity protection reasons, images containing human faces and body parts have replaced with verbal descriptions. Original images are available upon request.
Category-selective brain regions are sparsely tuned to behaviorally-relevant object dimensions
Given that dimensional tuning profiles effectively captured the selectivity of diverse visual regions, we asked what factors distinguish category-selective visual brain regions from non-category-selective regions in this dimensional framework. We reasoned that category-selectivity reflects a sparsely tuned representation, where activity in category-selective regions is driven by only a few dimensions, while non-category-selective regions reflect a more mixed selectivity, with activity related to a larger number of dimensions. In this way, functionally specialized, category-selective regions might stand-out as an extreme case of multidimensional tuning. As a consequence, this would also make it easier to identify category-selective regions due to their sparser selectivity.
To quantify this, we estimated a measure of sparseness over the encoding model weights in each voxel. Large sparseness indicates regions that are selective to very few dimensions, while lower sparseness indicates a dense representation in regions that respond broadly to diverse dimensions. Our results (Fig. 5A) indeed revealed sparser tuning in category-selective regions compared to other parts of the visual system. This effect was most pronounced in face and body part selective regions (FFA, OFA, EBA), with the sparsest tuning across all subjects. The face-selective posterior superior temporal sulcus exhibited particularly sparse representation in Subjects 1 and 2, while this region was not present in Subject 3 and, as expected, also yielded no increase in sparseness. Scene-selective regions (PPA, MPA, OPA) also exhibited sparseness, though the effects were more variable across subjects, which could arise from the representational dimensions being derived from objects within scenes, as opposed to isolated scene images without a focus on individual objects. Conversely, non-category-selective regions, such as early visual cortices, clearly exhibited dense representations. These findings suggest that category-selective regions, while responsive to multiple dimensions, may primarily respond to a small subset of behaviorally-relevant dimensions. Thus, in a multidimensional representational framework, category-selectivity may reflect a special case of sparse tuning within a broader set of distributed dimension tuning maps.
Fig. 5. Representational sparseness of behaviorally-relevant object dimensions in object category selective brain regions.
A. Inflated cortical surfaces for Subject 1 showing the sparseness over the encoding model weights in each voxel. Colors indicate z-values of sparseness compared to a noise pool of voxels. Statistical maps are thresholded at p < .05. B. Ventral view of the right hemisphere for all three subjects. Round outlines illustrate the location of two explorative, sparsely tuned regions of interest: One in the fusiform gyrus and one in orbitofrontal cortex. C. Functional selectivity of these explorative regions of interest demonstrated by their multidimensional tuning profiles and most representative object images. For copyright reasons, all original images have been replaced with visually similar images. Original images are available upon request.
Beyond the increased sparseness in functionally selective clusters, which had been defined in an independent localizer experiment 55, we explored to what degree we could use sparseness maps for revealing additional, potentially novel functionally selective regions. To this end, we identified two clusters with consistently high sparseness values across subjects (Fig. 5B). One cluster was located in the right hemisphere anterior to anatomically-defined area FG4 80 and between functionally-defined FFA and anterior temporal face patch 81, with no preferential response to human faces in 2 of 3 subjects in a separate functional localizer. The other cluster was located in orbitofrontal cortex, coinciding with anatomically defined Fo3 between the olfactory and medial orbital sulci 82. Having identified these clusters, we extracted regional tuning profiles and determined the most representative object images for each cluster. Inspection of the tuning profiles in these sparsely tuned regions revealed that their responses were best captured by images of animal faces for the region anterior to FFA and sweet food for orbitofrontal cortex (Fig. 5C). While the results in orbitofrontal cortex are in line with the motivational significance of rewarding foods and food representations in frontal regions 71,83-86, the selective response to animal faces in the cluster anterior to FFA deserves further study. By identifying regional response selectivity in a data-driven fashion 87, the results show that sparse tuning can aid in localizing functionally selective brain regions, corroborating the link between representational dimensions and regional selectivity.
Object dimensions offer a better account of visual cortex responses than categories
If representational dimensions offer a better account of the function of ventral visual cortex than categorization, this would predict that they have superior explanatory power for brain responses to visually-perceived objects in these regions 45,88. To compare these accounts formally, we compiled a multidimensional and a categorical model of object responses and compared the amount of shared and unique variance explained by these models. We first constructed a category model by assigning all objects appearing in the presented images into 50 common high-level categories (e.g. “animal”, “bird”, “body part”, “clothing”, “food”, “fruit”, “vehicle”) available as part of the THINGS metadata 89. Then, for each category, we determined the most diagnostic object dimension. Since some dimensions mapped to multiple categories, this resulted in a model of 30 object dimensions. Note that this approach is biased in favor of the object category model, representing a conservative test of our prediction. Based on the 50 categories and the 30 dimensions, we fit two encoding models to the fMRI single trial responses and performed variance partitioning to disentangle the relative contribution of the object category and dimension models to the cross-validated prediction.
The results (Fig. 6) demonstrate that both object dimensions and categories shared a large degree of variance in explaining brain responses, especially in higher-level ventro-temporal and lateral occipital cortices, suggesting that both models are well suited for predicting responses in the visual system. However, when inspecting the unique variance explained by either model, object dimensions explained a much larger amount of additional variance than object categories. This gain in explained variance was not only evident in higher-level regions, where both models performed well, but extended across large parts of visual cortex, suggesting that behaviorally-relevant dimensions captured information not accounted for by categories. Conversely, category membership added little unique explained variance throughout the visual system. Together, these results indicate that a multidimensional model offers an account with more explanatory value than a category model, supporting the idea that capturing behaviorally-relevant responses in the visual systems requires moving beyond categorization and suggesting object dimensions as a suitable model of encoding the behavioral significance of objects.
Fig. 6. Comparison of a continuous dimensional and a categorical model of object responses.
Flat maps show the left hemisphere of each subject. A. Shared variance in single-trial fMRI responses explained by both models. B. Variance explained uniquely by a multidimensional model. C. Variance explained uniquely by a model of object categories.
Discussion
Determining how the human brain represents object properties that inform our broad range of behaviors is crucial for understanding how we make sense of our visual world and act on it in meaningful ways. Here, we identified behaviorally-relevant brain representations by predicting fMRI responses to thousands of object images with 66 interpretable representational dimensions underlying millions of object similarity judgements. The results revealed that this behaviorally-relevant information is mirrored in activity patterns throughout the entire visual processing hierarchy in the form of distributed tuning maps, emphasizing the importance of considering the entire system for identifying the behavioral relevance of visual responses. The diverse image selectivity of different visual brain regions emerged from the multidimensional tuning profiles in this distributed representation. This suggests that behaviorally-relevant dimensions offer a broadly applicable model for understanding the architecture of the visual system in which category-selective regions stand out as a special case of sparse tuning. A direct model comparison confirmed that such a multidimensional account has more explanatory value than a focus on categories.
The behavioral relevance of object responses has often been linked primarily to higher-level visual cortex and to goals such as recognition and categorization 2,68,88. This perspective is consistent with the claim that these regions represent objects invariantly to factors such as viewpoint, background, and context that are non-essential for recognition and categorization 3,90,91. Our findings provide an alternative perspective onto the nature of cortical object representations that may offer larger explanatory power than this predominant view. By moving beyond a focus on categories and considering a richer representation of objects supporting broader behavioral domains 22, object information is no longer tied to invariance. It is also not restricted to the commonalities between objects based on how we label them. Instead, a behaviorally-relevant representation can also include low-level image features. In this framework, even responses in early visual cortex to images from high-level categories such as food 70,71, though likely driven by their texture or color, are treated as important information that supports the processing of food-related visual features.
While our results favor a distributed view of object representations, localized response selectivity for ecologically important object stimuli has been consistently replicated, underscoring the significance of specialized clusters. Regional specialization and distributed representations are traditionally seen as opposing levels of description 33,36. By demonstrating that category selective clusters differ from other visual regions by exhibiting highly sparse response tuning profiles, our study advances a framework for unifying these perspectives, treating regional specialization not as an isolated phenomenon, but rather a special case within a more general organizing principle. Specifically, in our framework, category-selectivity is manifested in representations that selectively emphasize only some of the dimensions encoding the behavioral relevance of objects.
One limitation of our study is that we did not identify behaviorally-relevant dimensions specific to each individual tested in the MRI. Instead, dimensions were based on a separate population of participants. However, our findings were highly replicable across the three participants, suggesting that the dimensions we used reflect general organizing principles rather than idiosyncratic effects. Second, there may be other behaviorally-relevant dimensions not captured by the dimensions underlying human similarity judgments on objects. Future work could extend these results based on alternative behavioral or computational approaches 15,58,64,92,93. Third, while our results reveal behaviorally-relevant information about objects throughout visual cortex, future work could establish a clear causal link that demonstrates that this information forms representations that are also directly read out in behavior 13,94. Finally, despite the large number of object categories used in the present study, our work was limited to images of isolated objects, excluding non-object images like scenes or text 63. While effects of representational sparseness were less pronounced in scene-selective regions and largely absent in text-selective regions 10, our encoding model did significantly predict brain responses in scene-selective regions (Suppl. Fig. 2), hinting at validity beyond isolated objects. Still, future research might explore additional image classes to extend these insights beyond the current domain.
In conclusion, our work provides a multidimensional framework that aligns with the rich and diverse behavioral relevance of objects. This behavior-centric approach promises increased explanatory power relative to a category-centric framework and integrates regional specialization within a broader organizing principle, thus offering a promising perspective for understanding how we make sense of our visual world.
Methods
THINGS-data
We relied on the openly available THINGS-data collection to investigate the brain representation of every-day objects 55. THINGS-data includes 4.7 million human similarity judgements as well as neural responses measured with functional magnetic resonance imaging (fMRI) to thousands of naturalistic and broadly sampled object images. The collection also includes a representational embedding of core object dimensions learned from the similarity judgments, which predicts unseen human similarity judgements with high accuracy and offers an interpretable account of the mental representation of objects 50,55. Here, we used these object dimensions to predict fMRI responses to object images. All data generation and processing methods are described in detail in the original data publication 55 and are only summarized here.
Participants
The MRI dataset in the THINGS-data collection comprises data from 3 healthy volunteers (2 female, 1 male, mean age: 25.33 years). Participants had normal or corrected-to-normal visual acuity, and were right-handed. The behavioral dataset in the THINGS-data collection was obtained from 12,340 participants through the crowdsourcing platform Amazon Mechanical Turk (6,619 female, 4,400 male, 56 other, 1,065 not reported; mean age: 36.71, std: 11.87, n=5,170 no age reported).
Stimuli
All images were taken from the THINGS database 63. The THINGS database contains 26,107 high-quality, colored images of 1,854 object concepts from a wide range of nameable living and non-living objects, including non-countable substances (e.g. “grass”), faces (e.g. “baby”, “boy”, “face”), and body parts (e.g. “arm”, “leg”, “shoulder”). The stimuli presented during functional MRI included 720 object concepts from the THINGS database, with the first 12 examples of each concept selected for a total of 8,640 images. In addition, 100 of the remaining THINGS images were presented repeatedly in each session for the purpose of estimating data reliability.
Experimental procedure
Participants of the THINGS-fMRI experiment took part in 15-16 scanning sessions, with the first 1-2 sessions serving to acquire individual functional localizers for retinotopic visual areas and category-selective clusters (faces, body parts, scenes, words, and objects). The main fMRI experiment comprised 12 sessions where participants were presented with the 11,040 THINGS images (8,740 unique images, catch trials excluded, 500 ms presentation followed by 4 s of fixation). For details on the procedure of the fMRI and behavioral experiments, please consult the original publication of the datasets 55.
Behavioral similarity judgements in the THINGS-data collection were collected in a triplet odd-one-out study using the online crowdsourcing platform Amazon Mechanical Turk. Participants were presented with three object images side by side and were asked to indicate which object they perceived to be the odd-one-out. Each task comprised 20 odd-one-out trials, and participants could perform as many tasks as they liked.
MRI data acquisition and preprocessing
Whole-brain functional MRI images were acquired with 2mm isotropic resolution and a repetition time of 1.5s. The MRI data was preprocessed with the standard pipeline fMRIPrep 95 which included slice time correction, head motion correction, susceptibility distortion correction, co-registration between functional and T1-weighted anatomical images, brain tissue segmentation, and cortical surface reconstruction. Additionally, cortical flat maps were manually generated 96. Functional MRI data was denoised with a semi-automated procedure based on independent component analysis (ICA) which was developed specifically for the THINGS-fMRI dataset. The retinotopic mapping data and functional localizer data were used to define retinotopic visual regions as well as the category-selective regions used in this study. Image-wise response estimates were obtained by fitting a single-trial model to the fMRI time series of each functional run while accounting for variation in hemodynamic response shape and mitigating overfitting 97-99.
Behavioral embedding
In order to predict the neural response to seen objects, we used a recent, openly available model of representational dimensions underlying human similarity judgements of objects 50. This model was trained to estimate a low-dimensional, sparse, and non-negative embedding predictive of individual trial choices in an odd-one-out task on 1,854 object images. The dimensions of this embedding have been demonstrated to be highly predictive of human similarity judgments while yielding human-interpretable dimensions reflecting both perceptual (e.g. “red”, “round”) as well as conceptual (e.g. “animal-related”) object properties. We used a recent 66-dimensional embedding trained on 4.7 million odd-one-out judgments on triplets of 1,854 object images 55.
While the original embedding was trained on one example image for each of the 1,854 object concepts, it may not account for differences between exemplars of the same object concept. For example, the color of the apple the model was trained on might have been red, while we also presented participants with images of a green apple. This may underestimate the model’s potential to capture variance in brain responses to visually presented object images. To address this, we extended the original embedding by predicting the 66 object dimensions for each individual image in the THINGS database 63. To this end, we used the neural network model CLIP-ViT, which is a multimodal model trained on image-text pairs and which was recently demonstrated to yield excellent prediction of human similarity judgments 62,100. For each of the 1,854 object images, we extracted the activity pattern from the final layer of the image encoder. Then, for each of the 66 dimensions, we fitted a ridge regression model to predict dimension values, using cross-validation to determine the regularization hyperparameter. Finally, we applied the learned regression model to activations for all images in the THINGS database. This resulted is a 66-dimensional embedding that captures the mental representation of all 26,107 THINGS images. We used these predicted dimension values to predict fMRI responses to the subset of 8,740 unique images presented in fMRI, which yielded consistent improvements in explained variance for all dimensions (see Supplementary Fig. 1).
Encoding model
We used a voxel-wise encoding model of the 66-dimensional similarity embedding to predict image-wise fMRI responses in order to test 1) how well the model predicts neural responses in different parts of the visual system and 2) how neural tuning to individual dimensions maps onto the topography of visual cortex.
Linear regression on fMRI single trial trial estimates
To test how well the core object dimensions predict brain responses in different parts of the visual system, we fit them to the fMRI single trial response estimates using ordinary least squares regression. While most analyses in this work rely on a more powerful parametric modulation model estimated on time-series data (see below), we used single trial responses for estimating the predictivity of the object dimensions, since this approach does not require extracting the contribution of the parametric modulators for estimating the explained variance of the general linear model. We evaluated the prediction performance of this encoding model in a leave-one-session-out cross-validation, using the average correlation between predicted and observed fMRI responses across folds. Within each cross-validation fold, we also computed a null distribution of correlation values based on 10,000 random permutations of the held-out test data. To assess statistical significance, we obtained voxel-wise p-values by comparing the estimated correlation with the generated null-distribution and corrected for multiple comparisons based on a false discovery rate of p < 0.01.
Parametric modulation on fMRI time series
In order to evaluate the contribution of individual object dimensions to the neural response in a given voxel, we used a parametric modulation model on the voxel-wise time series data. In this parametric modulation, a general onset regressor accounts for the average response across all trials, and a set of 66 parametric modulators account for the modulation of the BOLD signal by individual object dimensions. To compile the parametric modulation model, we constructed dimension-specific onset regressors and mean-centered each parametric modulator in order to make them orthogonal to the general onset regressor. We then convolved these regressors with a hemodynamic response function (HRF) to obtain predictors of the BOLD response. To account for variation in the shape of the HRF, we determined the best fitting HRF for each voxel based on a library of 20 HRFs 97,98. The resulting design matrix was then concatenated and fit to the fMRI time-series data. In order to mitigate overfitting, we regularized the regression weights using fractional ridge regression 99. We chose a range of regularization parameters from 0.10 to 0.90 in steps of 0.10 and from 0.90 to 1.00 in steps of 0.01 in order to sample values more densely which reflect less regularization. We determined the best hyperparameter combination (20 HRFs, 26 regularization parameters) for each voxel based on the amount of variance explained in a 12-fold between-session cross-validation. Finally, we fit the model with the best hyperparameter combination per voxel to the entire dataset, yielding 66 statistical maps of regression weights representing the voxel-wise contribution of individual object dimensions in predicting the fMRI signal. The regularization hyperparameter turned out to be small throughout visual cortex (Suppl. Fig. 3), demonstrating that regularization of regression weights had little impact on the absolute size of regression weights.
Regional tuning profiles and most representative object images
To explore the functional selectivity implied by regional tuning to core object dimensions, we extracted tuning profiles for different visual brain regions and related them to the multidimensional representation of all object images in the THINGS database 63 using a high-throughput approach. First, we extracted the regression weights resulting from the parametric modulation model in different visual brain regions: V1, V2, V3, human V4 (hV4), occipital face area (OFA), fusiform face area (FFA), extrastriate body area (EBA), parahippocampal place area (PPA), medial place are (MPA), and occipital place area (OPA). We then averaged these regional tuning profiles across participants and set negative weights to zero, given that the predicted dimensions reflect non-negative values, as well. We plotted the regional tuning profiles as rose plots to visualize the representation of core object dimensions in these brain regions. In order to explore the regional selectivity for specific object images, we determined the cosine similarity between each regional tuning profile and the model representation of all 26,107 images in the THINGS database. This allowed us to identify those THINGS images that are most representative of the local representational profile in different visual brain regions.
Representational sparseness
We estimated the sparseness of the representation of core object dimensions based on the regression weights from the parametric modulation model. Given our aim of identifying local clusters of similarly-tuned voxels, we performed spatial smoothing on the regression weight maps (FWHM = 4mm) to increase the spatial signal-to-noise ratio. We then took the vectors representing the 66-dimensional tuning profile for each voxel and removed negative vector elements, mirroring the analysis of the regional tuning profiles. We computed the sparseness of the resulting voxel-wise tuning vectors based on a previously introduced sparseness measure which is based on the normalized relationship between the L-1 and L-2 norm of a vector 101:
Where indicates the sparseness of the -dimensional input vector . A sparseness value of 1 indicates a perfectly sparse representation where all vector elements except one have the same value. In turn, a value of 0 indicates a perfectly dense representation where all elements have identical values. We computed this sparseness measure over the regression weights in each voxel which yielded a sparseness measure as a single value per voxel. To assess their statistical significance, we first identified the distribution of sparseness values in a noise pool of voxels. This noise pool included voxels where the parametric modulation model predicted the fMRI signal poorly in the cross-validation procedure (R2 < 0.0001). Since visual inspection of sparseness histograms suggested a log-normal distribution, we log-transformed all sparseness values to convert them to a normal distribution. Finally, we estimated the mean and standard deviation of the sparseness distribution in the noise pool, allowing us to obtain z- and p-values of the sparseness in each voxel.
Based on these results, we explored whether local clusters of representational sparseness are indicative of brain regions with high functional selectivity. To this end, we identified two regional clusters of high sparseness values which were present in all subjects and which had not yet been defined based on the functional localizer experiment (see MRI data preprocessing). Based on visual inspection of the sparseness maps, we defined two regions of interest. The first region of interest was located in the right ventro-temporal cortex, anterior to anatomically-defined area FG4 80 and functionally-defined FFA, but posterior to the anterior temporal face patch 81. The second region of interest was located in the orbitofrontal cortex. We probed the functional selectivity of these sparsely tuned regions by extracting regional tuning profiles and determining the most representative object images as described in the previous section.
Variance partitioning of object category vs. dimension based models
The aim of the variance partitioning was to test whether object dimensions or object categories offer a better model of neural responses to object images. To this end, we compiled a multidimensional and categorical model and compared the respective amount of shared and unique variance explained by these models.
We used 50 superordinate object categories provided in the THINGSplus metadata collection to construct a category encoding model 89. These high-level categories comprised: "animal", "bird", "body part", "breakfast food", "candy", "clothing", "clothing accessory", "condiment", "construction equipment", "container", "dessert", "drink", "electronic device", "farm animal", "food", "footwear", "fruit", "furniture", "game", "garden tool", "hardware", "headwear", "home appliance", "home decor", "insect", "jewelry", "kitchen appliance", "kitchen tool", "lighting", "mammal", "medical equipment", "musical instrument", "office supply", "outerwear", "part of car", "plant", "protective clothing", "safety equipment", "school supply", "scientific equipment", "sea animal", "seafood", "sports equipment", "tool", "toy", "vegetable", "vehicle", "watercraft", "weapon" , and "women’s clothing". To account for cases where images contained multiple objects (e.g. an image of “ring” might also contain a finger), we used the image annotations in the THINGSplus metadata 89 and manually matched these annotations to objects in the THINGS database for all images presented in the fMRI experiment. We then compiled an encoding model with 50 binary regressors encoding the superordinate categories of all respective objects.
Next, we compiled a corresponding encoding model of object dimensions. Note that we predicted that this model would outperform the categorical model in explaining variance in neural responses. To conservatively test this prediction, we biased our analysis in favor of the categorical model by selecting fewer dimensions than categories. To this end, for each category we identified the object dimension with the strongest relationship based on the area under the curve metric (AUC). Since some dimensions are diagnostic for multiple categories (e.g. “animal-related” might be the most diagnostic dimension for both “bird” and “insect”), this resulted in a one-to-many mapping between 30 dimensions and 50 categories. The selected dimensions comprised: "Metallic / artificial", "food-related", "animal-related", "textile", "plant-related", "house-related / furnishing-related", "valuable / precious", "transportation- / movement-related", "electronics / technology", "colorful / playful", "outdoors", "paper-related / flat", "hobby-related / game-related / playing-related", "tools-related / handheld / elongated", "fluid-related / drink-related", "water-related", "weapon-related / war-related / dangerous", "household-related", "feminine (stereotypical)", "body part-related", "music-related / hearing-related / hobby-related / loud", "construction-related / craftsmanship-related / housework-related", "spherical / voluminous", "flying-related / sky-related", "bug-related / non-mammalian / disgusting", "heat-related / fire-related / light-related", "foot-related / walking-related", "head-related", "medicine-related / health-related", and "sweet / dessert-related".
In order to compare the predictive potential of these two models, we fitted them to the fMRI single trial responses in a voxel-wise linear regression and performed variance partitioning. In order to estimate the uniquely explained variance, we first orthogonalize the target model and the data with respect to the other model 102. This effectively removes the shared variance from both the target model and the data. We then fit the residuals of the target model to the residuals of the data and calculated the coefficient of determination (R2) in a 12-fold between-session cross-validation as an estimate of the unique variance explained by the target model. We then estimated the overall variance explained by both models by concatenating the two models, fitting the resulting combined model to the data, and determining the cross-validated R2 estimate. Lastly, we computed an estimate of the shared variance explained by the two models by subtracting the uniquely explained variances from the overall explained variance.
Extended Data
Extended Data Figure 1. Dimension tuning maps 1-36 for Subject 1.
Colors indicate regression weights for each dimension predictor from the parametric modulation encoding model. Labels indicate regions of interest on the cortex: V1-V3: primary - tertiary visual cortex, OFA: occipital face area, FFA: fusiform face area, EBA: extrastriate body area, PPA: parahippocampal place area, MPA: medial place area, OPA: occipital place area.
Extended Data Figure 2. Dimension tuning maps 37-66 for Subject 1.
Colors indicate regression weights for each dimension predictor from the parametric modulation encoding model.
Extended Data Figure 3. Dimension tuning maps 1-36 for Subject 2.
Colors indicate regression weights for each dimension predictor from the parametric modulation encoding model.
Extended Data Figure 4. Dimension tuning maps 37-66 for Subject 2.
Colors indicate regression weights for each dimension predictor from the parametric modulation encoding model.
Extended Data Figure 5. Dimension tuning maps 1-36 for Subject 3.
Colors indicate regression weights for each dimension predictor from the parametric modulation encoding model.
Extended Data Figure 6. Dimension tuning maps 37-66 for Subject 3.
Colors indicate regression weights for each dimension predictor from the parametric modulation encoding model.
Supplementary Material
Acknowledgements
We thank Philipp Kaniuth for his help with image-wise dimension predictions and Magdalena Holzner for her help with identifying background objects in images and finding copyright free alternative images for publication. This work was supported by a doctoral student fellowship awarded to O.C. by the Max Planck School of Cognition, the Intramural Research Program of the National Institutes of Health (ZIA-MH-002909), under National Institute of Mental Health Clinical Study Protocol 93-M-1070 (NCT00001360), a research group grant by the Max Planck Society awarded to M.N.H., the ERC Starting Grant project COREDIM (ERC-StG-2021-101039712), and the Hessian Ministry of Higher Education, Science, Research and Art (LOEWE Start Professorship to M.N.H. and Excellence Program "The Adaptive Mind"). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Competing interests
There are no financial and non-financial competing interests.
Data availability
The data supporting our analyses were obtained from the publicly available THINGS-fMRI dataset. The fMRI dataset is accessible on OpenNeuro (https://doi.org/10.18112/openneuro.ds004192.v1.0.5) and Figshare (https://doi.org/10.25452/figshare.plus.c.6161151). The object dimensions embedding underlying behavioral similarity judgements which was used to predict the fMRI responses is available at the Open Science Framework repository (https://osf.io/f5rn6/). The higher-level object category labels which were used to construct a categorical model of object responses are part of the THINGSplus metadata and available at the Open Science Framework (https://osf.io/jum2f/).
Code availability
The code used for data processing, analysis, and visualization in this study is publicly available on GitHub (https://github.com/ViCCo-Group/dimension_encoding).
References
- 1.Mishkin M. & Ungerleider L. G. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav. Brain Res. 6, 57–77 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Goodale M. A. & Milner A. D. Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25 (1992). [DOI] [PubMed] [Google Scholar]
- 3.DiCarlo J. J., Zoccolan D. & Rust N. C. How Does the Brain Solve Visual Object Recognition? Neuron 73, 415–434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marr D. Vision: A Computational Investigation into the Human Representation and Processing of Visual Information. (MIT Press, 2010). [Google Scholar]
- 5.Hubel D. H. & Wiesel T. N. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 160, 106–154 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanwisher N. & Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 2109–2128 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downing P. E. & Kanwisher N. A cortical area specialized for visual processing of the human body. J. Vis. 1, 341–341 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Epstein R. A. & Kanwisher N. A cortical representation of the local visual environment. Nature 392, 598–601 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Kanwisher N., McDermott J. & Chun M. M. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. J. Neurosci. 17, 4302–4311 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puce A., Allison T., Asgari M., Gore J. C. & McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J. Neurosci. 16, 5205–5215 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin A., Wiggs C. L., Ungerleider L. G. & Haxby J. V. Neural correlates of category-specific knowledge. Nature 379, 649–652 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Cohen M. A., Alvarez G. A., Nakayama K. & Konkle T. Visual search for object categories is predicted by the representational architecture of high-level visual cortex. J. Neurophysiol. 117, 388–402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson T. A., Ritchie J. B., Kriegeskorte N., Durvasula S. & Ma J. Reaction time for object categorization is predicted by representational distance. J. Cogn. Neurosci. 26, 132–142 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Ritchie J. B., Tovar D. A. & Carlson T. A. Emerging Object Representations in the Visual System Predict Reaction Times for Categorization. PLoS Comput. Biol. 11, e1004316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritchie J. B. & Carlson T. A. Neural Decoding and “Inner” Psychophysics: A Distance-to-Bound Approach for Linking Mind, Brain, and Behavior. Front. Neurosci. 10, 190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung C. P., Kreiman G., Poggio T. & DiCarlo J. J. Fast Readout of Object Identity from Macaque Inferior Temporal Cortex. Science 310, 863–866 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Kanwisher N. & Barton J. J. S. The functional architecture of the face system: Integrating evidence from fMRI and patient studies. in The Oxford handbook of face perception (Oxford University Press; Oxford, UK, 2011). [Google Scholar]
- 18.Moro V. et al. The neural basis of body form and body action agnosia. Neuron 60, 235–246 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Wada Y. & Yamamoto T. Selective impairment of facial recognition due to a haematoma restricted to the right fusiform and lateral occipital region. J. Neurol. Neurosurg. Psychiatry 71, 254–257 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konen C. S., Behrmann M., Nishimura M. & Kastner S. The functional neuroanatomy of object agnosia: a case study. Neuron 71, 49–60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiltz C. et al. Impaired face discrimination in acquired prosopagnosia is associated with abnormal response to individual faces in the right middle fusiform gyrus. Cereb. Cortex 16, 574–586 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Bracci S. & Op de Beeck H. P. Understanding Human Object Vision: A Picture Is Worth a Thousand Representations. Annu. Rev. Psychol. 74, 113–135 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Krakauer J. W., Ghazanfar A. A., Gomez-Marin A., MacIver M. A. & Poeppel D. Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron 93, 480–490 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Peelen M. V. & Downing P. E. Category selectivity in human visual cortex: Beyond visual object recognition. Neuropsychologia 105, 177–183 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Cox D. D. Do we understand high-level vision? Curr. Opin. Neurobiol. 25, 187–193 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Caramazza A. & Shelton J. R. Domain-Specific Knowledge Systems in the Brain: The Animate-Inanimate Distinction. J. Cogn. Neurosci. 10, 1–34 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Konkle T. & Caramazza A. Tripartite Organization of the Ventral Stream by Animacy and Object Size. Journal of Neuroscience 33, 10235–10242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriegeskorte N. Relating Population-Code Representations between Man, Monkey, and Computational Models. Front. Neurosci. 3, 363–373 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao P., She L., McGill M. & Tsao D. Y. A map of object space in primate inferotemporal cortex. Nature 583, 103–108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konkle T. & Oliva A. A real-world size organization of object responses in occipitotemporal cortex. Neuron 74, 1114–1124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coggan D. D. & Tong F. Spikiness and animacy as potential organizing principles of human ventral visual cortex. Cereb. Cortex 33, 8194–8217 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huth A. G., Nishimoto S., Vu A. T. & Gallant J. L. A Continuous Semantic Space Describes the Representation of Thousands of Object and Action Categories across the Human Brain. Neuron 76, 1210–1224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Op de Beeck H. P., Haushofer J. & Kanwisher N. G. Interpreting fMRI data: maps, modules and dimensions. Nat. Rev. Neurosci. 9, 123–135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin A. The representation of object concepts in the brain. Annu. Rev. Psychol. 58, 25–45 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Mahon B. Z. & Caramazza A. What drives the organization of object knowledge in the brain? Trends Cogn. Sci. 15, 97–103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haxby J. V. et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293, 2425–2430 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Arcaro M. J. & Livingstone M. S. On the relationship between maps and domains in inferotemporal cortex. Nat. Rev. Neurosci. 22, 573–583 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasr S. & Tootell R. B. H. A cardinal orientation bias in scene-selective visual cortex. J. Neurosci. 32, 14921–14926 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nasr S., Echavarria C. E. & Tootell R. B. H. Thinking outside the box: rectilinear shapes selectively activate scene-selective cortex. J. Neurosci. 34, 6721–6735 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coggan D. D., Baker D. H. & Andrews T. J. Selectivity for mid-level properties of faces and places in the fusiform face area and parahippocampal place area. Eur. J. Neurosci. 49, 1587–1596 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Andrews T. J., Clarke A., Pell P. & Hartley T. Selectivity for low-level features of objects in the human ventral stream. Neuroimage 49, 703–711 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Coggan D. D., Liu W., Baker D. H. & Andrews T. J. Category-selective patterns of neural response in the ventral visual pathway in the absence of categorical information. Neuroimage 135, 107–114 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Rice G. E., Watson D. M., Hartley T. & Andrews T. J. Low-level image properties of visual objects predict patterns of neural response across category-selective regions of the ventral visual pathway. J. Neurosci. 34, 8837–8844 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yargholi E. & Op de Beeck H. Category Trumps Shape as an Organizational Principle of Object Space in the Human Occipitotemporal Cortex. J. Neurosci. 43, 2960–2972 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Downing P. E., Chan A. W.-Y., Peelen M. V., Dodds C. M. & Kanwisher N. Domain specificity in visual cortex. Cereb. Cortex 16, 1453–1461 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Bracci S. & Op de Beeck H. Dissociations and Associations between Shape and Category Representations in the Two Visual Pathways. J. Neurosci. 36, 432–444 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kriegeskorte N. et al. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron 60, 1126–1141 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almeida J. et al. Neural and behavioral signatures of the multidimensionality of manipulable object processing. bioRxiv (2023) doi: 10.1101/2023.03.29.534804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng C. Y., Pereira F., Baker C. I. & Hebart M. N. Revealing interpretable object representations from human behavior. 1–16 (2019) doi: 10.48550/arXiv.1901.02915. [DOI] [Google Scholar]
- 50.Hebart M. N., Zheng C. Y., Pereira F. & Baker C. I. Revealing the multidimensional mental representations of natural objects underlying human similarity judgements. Nature Human Behaviour 4, 1173–1185 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashby F. G. & Perrin N. A. Toward a unified theory of similarity and recognition. Psychol. Rev. 95, 124–150 (1988). [Google Scholar]
- 52.Nosofsky R. M. Choice, similarity, and the context theory of classification. J. Exp. Psychol. Learn. Mem. Cogn. 10, 104–114 (1984). [DOI] [PubMed] [Google Scholar]
- 53.Shepard R. N. Toward a universal law of generalization for psychological science. Science 237, 1317–1323 (1987). [DOI] [PubMed] [Google Scholar]
- 54.Edelman S. Representation is representation of similarities. Behav. Brain Sci. 21, 449–67; discussion 467–98 (1998). [DOI] [PubMed] [Google Scholar]
- 55.Hebart M. N. et al. THINGS-data, a multimodal collection of large-scale datasets for investigating object representations in human brain and behavior. Elife 12, e82580 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cichy R. M., Kriegeskorte N., Jozwik K. M., van den Bosch J. J. F. & Charest I. The spatiotemporal neural dynamics underlying perceived similarity for real-world objects. Neuroimage 194, 12–24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mur M. et al. Human Object-Similarity Judgments Reflect and Transcend the Primate-IT Object Representation. Front. Psychol. 4, 128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charest I., Kievit R. A., Schmitz T. W., Deca D. & Kriegeskorte N. Unique semantic space in the brain of each beholder predicts perceived similarity. Proc. Natl. Acad. Sci. U. S. A. 111, 14565–14570 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groen I. I. A., Silson E. H. & Baker C. I. Contributions of low- and high-level properties to neural processing of visual scenes in the human brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naselaris T., Allen E. & Kay K. Extensive sampling for complete models of individual brains. Current Opinion in Behavioral Sciences 40, 45–51 (2021). [Google Scholar]
- 61.Radford A. et al. Learning Transferable Visual Models From Natural Language Supervision. in Proceedings of the 38th International Conference on Machine Learning (eds. Meila M. & Zhang T.) vol. 139 8748–8763 (PMLR, 2021). [Google Scholar]
- 62.Hebart M. N., Kaniuth P. & Perkuhn J. Efficiently-generated object similarity scores predicted from human feature ratings and deep neural network activations. in Journal of Vision vol. 22 4057–4057 (The Association for Research in Vision and Ophthalmology, 2022). [Google Scholar]
- 63.Hebart M. N. et al. THINGS: A database of 1,854 object concepts and more than 26,000 naturalistic object images. PLoS One 14, e0223792 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magri C. & Konkle T. Comparing facets of behavioral object representation: implicit perceptual similarity matches brains and models. 2019 Conference on Cognitive Computational Neuroscience Preprint at 10.32470/ccn.2019.1395-0 (2019). [DOI] [Google Scholar]
- 65.Gauthier I., Skudlarski P., Gore J. C. & Anderson A. W. Expertise for cars and birds recruits brain areas involved in face recognition. Nat. Neurosci. 3, 191–197 (2000). [DOI] [PubMed] [Google Scholar]
- 66.O’Craven K. M. & Kanwisher N. Mental imagery of faces and places activates corresponding stiimulus-specific brain regions. J. Cogn. Neurosci. 12, 1013–1023 (2000). [DOI] [PubMed] [Google Scholar]
- 67.Epstein R. A., Higgins J. S. & Thompson-Schill S. L. Learning places from views: variation in scene processing as a function of experience and navigational ability. J. Cogn. Neurosci. 17, 73–83 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Grill-Spector K. The neural basis of object perception. Curr. Opin. Neurobiol. 13, 159–166 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Hasson U., Harel M., Levy I. & Malach R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron 37, 1027–1041 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Khosla M., Ratan Murty N. A. & Kanwisher N. A highly selective response to food in human visual cortex revealed by hypothesis-free voxel decomposition. Curr. Biol. 32, 4159–4171.e9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jain N. et al. Selectivity for food in human ventral visual cortex. Commun Biol 6, 175 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pennock I. M. L. et al. Color-biased regions in the ventral visual pathway are food selective. Curr. Biol. 33, 134–146.e4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin A. & Weisberg J. Neural foundations for understanding social and mechanical concepts. Cogn. Neuropsychol. 20, 575–587 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He C., Hung S.-C. & Cheung O. S. Roles of Category, Shape, and Spatial Frequency in Shaping Animal and Tool Selectivity in the Occipitotemporal Cortex. J. Neurosci. 40, 5644–5657 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hubel D. H. & Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. 195, 215–243 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Livingstone M. S. & Hubel D. H. Anatomy and physiology of a color system in the primate visual cortex. J. Neurosci. 4, 309–356 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tootell R. B. et al. Functional analysis of V3A and related areas in human visual cortex. J. Neurosci. 17, 7060–7078 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kastner S. et al. Modulation of sensory suppression: implications for receptive field sizes in the human visual cortex. J. Neurophysiol. 86, 1398–1411 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Smith A. T., Singh K. D., Williams A. L. & Greenlee M. W. Estimating receptive field size from fMRI data in human striate and extrastriate visual cortex. Cereb. Cortex 11, 1182–1190 (2001). [DOI] [PubMed] [Google Scholar]
- 80.Rosenke M. et al. A cross-validated cytoarchitectonic atlas of the human ventral visual stream. Neuroimage 170, 257–270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajimehr R., Young J. C. & Tootell R. B. H. An anterior temporal face patch in human cortex, predicted by macaque maps. Proceedings of the National Academy of Sciences 106, 1995–2000 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henssen A. et al. Cytoarchitecture and probability maps of the human medial orbitofrontal cortex. Cortex 75, 87–112 (2016). [DOI] [PubMed] [Google Scholar]
- 83.Simmons W. K., Martin A. & Barsalou L. W. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb. Cortex 15, 1602–1608 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Avery J. et al. Dissociable prefrontal and limbic brain networks represent distinct information about the healthiness and pleasantness of food. PsyArXiv (2023) doi: 10.31234/osf.io/9qswa. [DOI] [Google Scholar]
- 85.Small D. M. et al. The role of the human orbitofrontal cortex in taste and flavor processing. Ann. N. Y. Acad. Sci. 1121, 136–151 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Rolls E. T. The orbitofrontal cortex, food reward, body weight and obesity. Soc. Cogn. Affect. Neurosci. 18, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lashkari D., Vul E., Kanwisher N. & Golland P. Discovering structure in the space of fMRI selectivity profiles. Neuroimage 50, 1085–1098 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grill-Spector K. & Weiner K. S. The functional architecture of the ventral temporal cortex and its role in categorization. Nat. Rev. Neurosci. 15, 536–548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stoinski L. M., Perkuhn J. & Hebart M. N. THINGSplus: New norms and metadata for the THINGS database of 1854 object concepts and 26,107 natural object images. Behav. Res. Methods (2023) doi: 10.3758/s13428-023-02110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DiCarlo J. J. & Cox D. D. Untangling invariant object recognition. Trends Cogn. Sci. 11, 333–341 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Kanwisher N. Functional specificity in the human brain: a window into the functional architecture of the mind. Proc. Natl. Acad. Sci. U. S. A. 107, 11163–11170 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin C. B., Douglas D., Newsome R. N., Man L. L. & Barense M. D. Integrative and distinctive coding of visual and conceptual object features in the ventral visual stream. Elife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Devereux B. J., Tyler L. K., Geertzen J. & Randall B. The Centre for Speech, Language and the Brain (CSLB) concept property norms. Behav. Res. Methods 46, 1119–1127 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams M. A., Dang S. & Kanwisher N. G. Only some spatial patterns of fMRI response are read out in task performance. Nat. Neurosci. 10, 685–686 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esteban O. et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao J. S., Huth A. G., Lescroart M. D. & Gallant J. L. Pycortex: an interactive surface visualizer for fMRI. Front. Neuroinform. 9, 23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen E. J. et al. A massive 7T fMRI dataset to bridge cognitive neuroscience and artificial intelligence. Nat. Neurosci. 25, 116–126 (2022). [DOI] [PubMed] [Google Scholar]
- 98.Prince J. S. et al. Improving the accuracy of single-trial fMRI response estimates using GLMsingle. Elife 11, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rokem A. & Kay K. Fractional ridge regression: a fast, interpretable reparameterization of ridge regression. Gigascience 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muttenthaler L., Dippel J., Linhardt L., Vandermeulen R. A. & Kornblith S. Human alignment of neural network representations. (2022). [Google Scholar]
- 101.Hoyer P. O. Non-negative Matrix Factorization with Sparseness Constraints. J. Mach. Learn. Res. 5, 1457–1469 (2004). [Google Scholar]
- 102.Mumford J. A., Poline J.-B. & Poldrack R. A. Orthogonalization of regressors in FMRI models. PLoS One 10, e0126255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting our analyses were obtained from the publicly available THINGS-fMRI dataset. The fMRI dataset is accessible on OpenNeuro (https://doi.org/10.18112/openneuro.ds004192.v1.0.5) and Figshare (https://doi.org/10.25452/figshare.plus.c.6161151). The object dimensions embedding underlying behavioral similarity judgements which was used to predict the fMRI responses is available at the Open Science Framework repository (https://osf.io/f5rn6/). The higher-level object category labels which were used to construct a categorical model of object responses are part of the THINGSplus metadata and available at the Open Science Framework (https://osf.io/jum2f/).
The code used for data processing, analysis, and visualization in this study is publicly available on GitHub (https://github.com/ViCCo-Group/dimension_encoding).