Abstract
BACKGROUND
Heartburn is identically the key symptom of both, gastroesophageal reflux disease (GERD) and functional heartburn (FHB), making the differential diagnosis resource-intensive. Oral manifestations of GERD can be easily examined; therefore, their exploration might be a cheap, widely available, and useful tool in the differentiation of GERD and FHB.
AIM
To evaluate the prevalence of dental erosions (DE) and periodontal diseases (PD) in patients with heartburn and their association with GERD and FHB.
METHODS
A total of 116 [M/F: 51/65, mean age: 54 (17-80) years] consecutive patients with heartburn were enrolled for detailed esophageal function and orodental examinations.
RESULTS
Dental disorders were detected in 89% (103/116). Patients with PD + DE had significantly more often pathologic reflux (90.0% vs 27.8%; P < 0.05), higher esophagitis scores (1.8 vs 0.9; P < 0.05), and a significantly different mean impedance curve (P = 0.04) than those without any dental diseases. The opposite approach established that patients with GERD had significantly higher prevalence of DE and PD, especially if both were present (28.9% vs 2.0%; P < 0.01), more severe PD (1.5 vs 1.0; P < 0.01), and longer history of heartburn (15 years vs 9 years; P < 0.01) than those with FHB.
CONCLUSION
The dental evaluation of patients with heartburn seems to be useful in the differential diagnosis of GERD and FHB. Among the studied parameters, the co-appearance of DE and PD seems to be the best predictor of GERD, whereas the absence of dental disorders was mostly observed in FHB.
Keywords: Gastroesophageal reflux disease, Gastric acid, Heartburn, Differential diagnosis, Epidemiology, Oral manifestations, Prevalence, Risk factors, Dental erosion, Periodontal diseases
Core Tip: Heartburn is identically the key symptom of gastroesophageal reflux disease (GERD) and functional heartburn (FHB), making the differential diagnosis resource-intensive. Oral manifestations of GERD can be easily examined to differentiate GERD and FHB. A total of 116 consecutive patients with heartburn were enrolled to evaluate the prevalence of dental erosions (DE) and periodontal diseases (PD). The dental evaluation of patients with heartburn seems to be useful in the differential diagnosis of GERD and FHB. The co-appearance of DE and PD seems to be the best predictor of GERD, while the absence of dental disorders was mostly observed in FHB.
INTRODUCTION
Heartburn is mostly considered a typical symptom of gastroesophageal reflux disease (GERD) and has a global prevalence of 11.9%. However, it cannot be diagnosed without performing detailed esophageal function tests based on the symptom of patients with functional heartburn (FHB)[1].
According to the Montreal definition, GERD may be associated with supraesophageal manifestations, including oropharyngeal symptoms[2]. Among various oropharyngeal symptoms (salivation, mouth burning, and tongue burning), dental erosion (DE) is considered to have a proven correlation with GERD. The association between DE and GERD was apparently first reported in 1933[3]. By definition, DE is a progressive loss of tough tissues of the teeth due to the action of extrinsic or intrinsic acids. Its median prevalence has been reported to be 24% in all patients with GERD and 32.5% in adult patients with GERD[4]. However, DE can be accompanied by other disorders, such as bulimia, rumination, and the consumption of acidic foods or drinks.
Much less data are available regarding other oral symptoms, especially periodontal diseases (PD), which have recently been suggested to be associated with GERD[5]. PD, which represent a group of oral inflammatory conditions caused by oral pathogens, lead to the destruction of tooth-supporting soft tissues. DE and PD may be considered as cumulative lesions, representing the long-term consequences of gastroesophageal reflux.
To the best of our knowledge, currently, no studies have been performed to assess hard and soft tissue injuries (DE and PD), and their relationship has never been investigated from dental and gastroenterological perspectives. Moreover, there are no studies about the possibility to distinguish GERD from FHB on the basis of oral manifestations.
Therefore, we aimed to obtain data on the prevalence of DE and PD in patients with heartburn and evaluate their association with GERD.
MATERIALS AND METHODS
One-hundred and sixteen consecutive patients (M/F: 51/65, mean age: 54.00 years ± 15.62 years) with heartburn were enrolled in our tertiary center for detailed esophageal function testing, including upper gastrointestinal endoscopy, high-resolution esophageal manometry [medical measurement systems (MMS) solar with a 22-channel, water-perfused catheter], and 24-h multi-channel intra-esophageal pH-impedance monitoring (MMS Ohmega®, with a pHersaflex Z61A pH probe). Any medications with any effect on gastrointestinal motility or gastric secretion were suspended one month before the esophageal testing. For gastroscopy, Olympus GIF-Q165 endoscopes were used, and the procedure was carried out under local, topical anesthesia. The presence of esophageal manifestations was recorded. Esophagitis was classified per the Los Angeles criteria[6]. On this basis, the following scoring system (no erosion = 0, LA-A = 1, LA-B = 2, LA-C = 3, LA-D = 4) was applied for quantitative comparison of the degree of esophagitis. Esophagogastric junction outflow obstruction and other major motility disorders were excluded via high-resolution esophageal manometry according to the Chicago classification 3.0[7]. During pH-impedance monitoring, the pH sensor was placed 5 cm above the lower esophageal sphincter as determined via manometry.
The significance of GERD was judged by the Lyon consensus[8]. The diagnosis of FHB was established according to the Rome IV criteria[9], including < 4% acid exposure time in the esophagus and the independence of symptoms of acidic and non-acidic reflux episodes. The occurrence of reflux hypersensitivity was also evaluated, but none of the studied patients fulfilled the accepted Rome IV criteria of this disease. Moreover, baseline impedance values were above 2000 Ω in this patient group[10]. We also calculated the mean 24-h impedance in all channels, the impedance values of the six channels during the 24-h measurement were exported to a .csv file and averaged.
Before dental examinations, general personal data, social and dental habits, and the presence, frequency, and appearance of typical and atypical reflux symptoms were assessed using standardized questionnaires that were collected by an interviewer (medical doctor and student). Among the enrolled subjects, 116 patients [M/F: 51/65, mean age: 56 (22-82) years] with heartburn were participating in further oral and dental examinations. Oral evaluations were carried out by a dentist who was blinded to the results of the esophageal function tests. The tooth wear index was evaluated and scored using the Smith and Knight’s criteria, while the clinical staging of periodontitis was performed according to some study[11,12]. To quantitatively compare the severity of periodontitis, the following score system was used: No sign = 0, mild = 1, moderate = 2, severe = 3). The plaque index was calculated via percentage of plaque area in relation to total area.
Based on the presence of DE and/or PD, subgroups were formed.
Statistical analyses (one-way analysis of variance, chi-squared test, and unpaired t-test) were performed using R program; the significance level was set at P = 0.05. Data are expressed using the mean ± SD. This study was approved by the Regional Human Research Ethics Committee of the University of Szeged (Ethical approval No. 4564).
RESULTS
Detailed esophageal testing identified 66 patients with GERD (56.9%) and 50 patients with FHB (43.1%) among the 116 enrolled patients. Dental disorders were detected in 89% (103/116) of the enrolled patients with heartburn. The global prevalence of DE among the enrolled patients was 23.3%. In the group of subjects with GERD, the mean DeMeester score (DMS) was 29.84 ± 27.06. In contrast, in the other group, the mean DMS was 3.34 ± 2.94. Fourteen subjects were diagnosed with Barrett’s esophagus. Among patients with GERD, LA-A in 12 (18.2%), LA-B in 15 (22.7%), LA-C in 20 (30.3%), and LA-D in 4 cases (6.1%) were detected, and 15 (22.7%) of them had no sign of esophagitis. In the group with FHB, there was no esophagitis on gastroscopy. Based on the results of pH-impedance monitoring, proximal reflux was found in 41 cases. Dental erosions were significantly more common among patients with GERD (66/116) than among those with FHB (21/66, 31.8% vs 6/50, 12.0%; P = 0.0312). The mean body mass index (BMI) in the GERD group was 27.8 kg/m2 ± 4.45 kg/m2 while that in the FHB group was 26.2 kg/m2 ± 4.53 kg/m2 (P = 0.0192). Eleven patients were toothless. Furthermore, we established significantly more severe periodontal problems in patients with GERD (P = 0.0253). However, instead of the fact that neither only DE nor only PD was significantly more common in any of the study groups, PD and DE together were significantly more prevalent among patients with GERD (P = 0.00008). DEs alone were less common among patients with GERD (3/8, 37.5%) than among those with FHB (5/8, 62.5%). Moreover, more patients were toothless in the GERD group (8/11, 72.7%). However, the most prominent difference is the presence of DE and PD together: 19/20 (95%) in the group of patients with GERD and 1/20 (5%) in the control group. The mean plaque index was 52 (0-100) in both groups. Fewer teeth were detected in the GERD group; however, the difference was not statistically significant (18 vs 21; P = 0.098). Patients with GERD had a longer history of symptoms than those with FHB (15 years vs 9 years, P = 0.0041) (Table 1).
Table 1.
Comparison of parameters between patients with gastroesophageal reflux disease and those with functional heartburn
|
|
GERD (n = 66)
|
FHB (n = 50)
|
P value
|
|
| Gender (male/female) | 32 (48.5%)/34 (51.5%) | 19 (38%)/31 (62%) | NS | |
| Age, yr (min-max) | 57 (22-82) | 51 (25-79) | NS | |
| BMI, kg/m2 (min-max) | 28 (16-37) | 26 (17-39) | < 0.05 | |
| Mean DMS | 29.84 | 3.34 | < 0.0001 | |
| Mean impedance ± SD | 2175 ± 650 | 2489 ± 731 | < 0.05 | |
| Number of teeth (min-max) | 18.3 (0-32) | 20.7 (0-32) | NS | |
| Toothless | 8 (12.1%) | 3 (6%) | NS | |
| DE all | 22 (33.3%) | 6 (12%) | < 0.01 | |
| DE only | 3 (4.5%) | 5 (10%) | NS | |
| PD all | 52 (78.8%) | 32 (64%) | NS | |
| PD only | 33 (50%) | 31 (62%) | NS | |
| DE and PD | 19 (28.9%) | 1 (2%) | < 0.01 | |
| Neither DE, nor PD | 3 (4.5%) | 10 (20%) | < 0.01 | |
| Periodontal scores (mean ± SD) | 1.45 ± 0.85 | 0.97 ± 0.84 | < 0.01 | |
| Drinking carbonated drinks | Nowadays | 8 (12.2%) | 10 (20%) | NS |
| Previously | 22 (33.3%) | 15 (30%) | ||
| Never | 36 (54.5%) | 25 (50%) | ||
| Eating sour foods | Nowadays | 15 (22.7%) | 9 (18%) | NS |
| Previously | 13 (19.7%) | 9 (18%) | ||
| Never | 38 (57.6%) | 32 (64%) | ||
| Bruxism/teeth grinding | 9 (13.6%) | 8 (16%) | NS | |
| Total duration of heartburn, mean years (range) | 15 (0-64) | 9 (0-35) | < 0.01 | |
| Duration of heartburn until diagnosis, mean years (range) | 5.3 (0-49) | 2.9 (0-30) | NS | |
GERD: Gastroesophageal reflux disease; FHB: Functional heartburn; BMI: Body mass index; DMS: DeMeester score; PD: Periodontal disease; DE: Dental erosion; DE all: All the patients who had DE, and some of them have associated PD as well; DE only: Such patients have only DE and have not PD; PD all: All the patients who had PD, and some of them have associated DE as well; PD only: Such patients have only PD and have not DE; SD: Standard deviation; NS: Not significant.
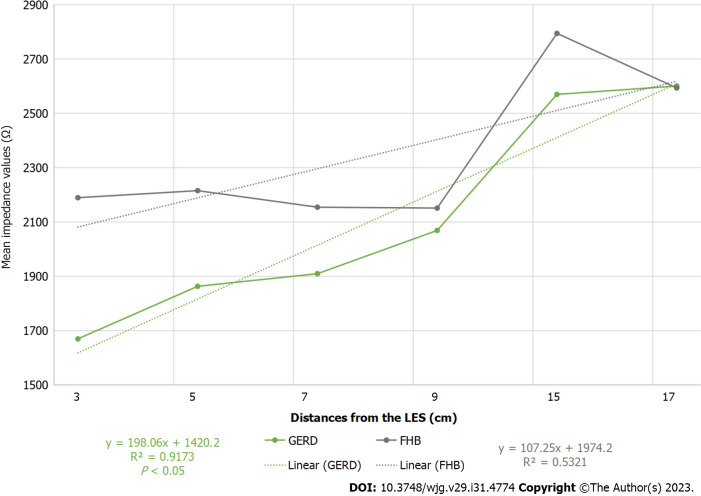
Mean impedance values were compared between the two study groups and found to be significantly lower among patients with GERD than among those with FHB, and a characteristic tendency of GERD was detected (Figure 1).
Figure 1.
Mean impedance values in patients with gastroesophageal reflux disease and those with functional heartburn. GERD: Gastroesophageal reflux disease; FHB: Functional heartburn; LES: Lower esophageal sphincter.
In the entire study population, the periodontal examination was possible in only 105 patients because 11 of them were toothless. Among the examined subjects, 17/105 (16.2%) had DE alone, 24/105 (22.9%) had PD alone, 10/105 (9.5%) had both, and 54/105 (51.4%) had neither. Patients with DE alone had no more pathologic reflux than those with intact teeth (41.2% vs 27.8%). Among patients with both PD and DE, pathologic reflux was significantly more prevalent (27.8% and 90.0%; P = 0.03) than among patients without DE and PD. Furthermore, patients with PD and DE had higher esophagitis scores (1.8 vs 0.9; P = 0.05) than those without any dental diseases, and there was a tendency for more proximal reflux (P = 0.08). The presence of PD causing tooth loss was more common than the presence of DE or both (18 vs 22 and 24, P = 0.11) On the other hand, the mean plaque index was significantly higher among patients with PD than among patients without PD and/or DE (72 vs 49, P < 0.0001; Table 2). Other oral, atypical symptoms were not significant in the studied group, such as burning sensation of the mouth and tongue, sore throat, bad breath, sour taste, and ageusia.
Table 2.
Comparison of parameters between patients with or without dental erosion and periodontal disease
|
|
DE (n = 17)
|
PD (n = 24)
|
Both (n = 10)
|
Neither (n = 54)
|
P value
|
|
| Gender (male/female) | 7 (41.2%)/10 (58.8%) | 12 (50%)/12 (50%) | 7 (70%)/3 (30%) | 21 (38.9%)/33 (61.1%) | NS | |
| Age, yr (min-max) | 50 (24-79) | 60 (41-82) | 62 (40-71) | 53 (22-80) | NS | |
| BMI, kg/m2 (min-max) | 27 (17-35) | 28 (16-39) | 29 (26-35) | 26 (18-37) | NS | |
| Heartburn | 17 (100%) | 24 (100%) | 10 (100%) | 54 (100%) | NS | |
| Nausea | 12 (70.6%) | 9 (37.5%) | 7 (70%) | 23 (42.6%) | NS | |
| Vomiting | 4 (23.6%) | 5 (20.8%) | 5 (50%) | 9 (16.7%) | NS | |
| Dysphagia | 11 (64.7%) | 10 (41.7%) | 5 (50%) | 21 (38.9%) | NS | |
| Regurgitation | 15 (88.2%) | 15 (62.5%) | 8 (80%) | 39 (72.2%) | NS | |
| Drinking carbonated drinks | Nowadays | 2 (11.8%) | 5 (20.8%) | 2 (20%) | 8 (14.8%) | NS |
| Previously | 6 (35.3%) | 10 (41.7%) | 1 (10%) | 17 (31.5%) | ||
| Never | 9 (52.9%) | 9 (37.5%) | 7 (70%) | 29 (53.7%) | ||
| Eating sour foods | Nowadays | 5 (29.4%) | 6 (25%) | 0 (0%) | 11 (20.4%) | NS |
| Previously | 2 (11.8%) | 6 (25%) | 4 (40%) | 7 (13%) | ||
| Never | 10 (58.8%) | 12 (50%) | 6 (60%) | 36 (66.7%) | ||
| Bruxism (teeth grinding) | 4 (23.6%) | 4 (16.7%) | 2 (20%) | 7 (13%) | NS | |
| Number of teeth (min-max) | 24 (13-31) | 18 (1-30) | 21 (13-28) | 22 (2-32) | NS | |
| Plaque Index (min-max) | 58 (15-100) | 72 (32-100) | 67 (35-97) | 49 (0-94) | < 0.01 | |
| Esophagitis score (mean ± SD) | 1.6 ± 1.4 | 1.5 ± 1.4 | 1.8 ± 1.2 | 0.9 ± 1.1 | 0.05 | |
| Mean DMS | 23.11 | 17.5 | 26.91 | 13.94 | NS | |
| Mean impedance ± SD | 2390 ± 878 | 2393 ± 714 | 1708 ± 249 | 2427 ± 690 | NS | |
| Pathological reflux | 7 (41.2%) | 9 (37.5%) | 9 (90%) | 15 (27.8%) | < 0.01 | |
| Any proximal reflux | 8 (47.1%) | 9 (37.5%) | 7 (70%) | 19 (35.2%) | NS | |
| Distal reflux | 11 (64.7%) | 13 (54.2%) | 10 (100%) | 21 (38.9%) | < 0.01 | |
PD: Periodontal disease; DE: Dental erosion; BMI: Body mass index; DMS: DeMeester score; SD: Standard deviation; NS: Not significant.
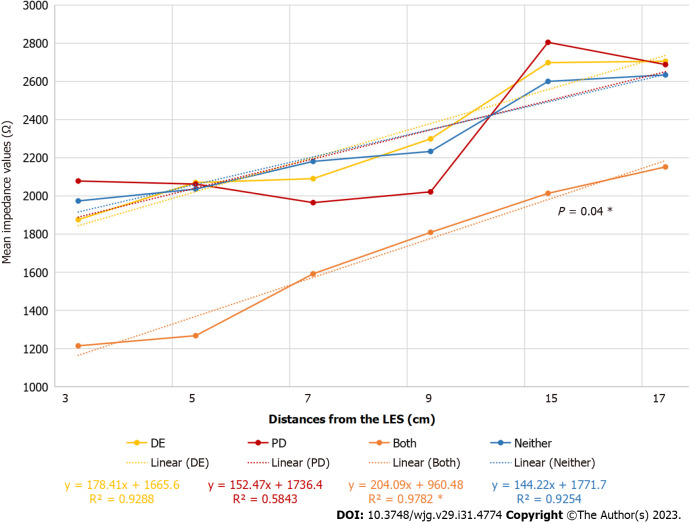
Evaluating the mean impedance values, the tendency in patients with DE and PD was similar to that in patients with GERD (Figure 2).
Figure 2.
Mean impedance values in patients with or without dental erosion and periodontal disease. The asterisk (*) the curve of “Both” group is significantly different from the others, and similar to the one in patients with gastroesophageal reflux disease. PD: Periodontal disease; DE: Dental erosion; LES: Lower esophageal sphincter.
Besides the abovementioned risk factors, no other ones were detected in the study. Furthermore, there was no difference between the four groups in terms of smoking (P = 0.36), alcohol consumption (P = 0.59), and coffee consumption (P = 0.86). There was also no significant difference in different habits resulting in DEs, such as drinking carbonated drinks (P = 0.58), teeth grinding (P = 0.71), and eating sour foods (P = 0.23).
DISCUSSION
The complete symptom similarity of GERD and FHB makes the differential diagnosis of heartburn complicated and resource-intensive. According to the Rome IV classification, it is not possible to differentiate the role of acid and hypersensitivity in the development of heartburn on the basis of the frequency and subjective severity of heartburn symptom. To confirm the diagnosis, detailed esophageal examinations are mandatory. That is why the necessity of comparative studies was also raised in the latest Rome IV criteria[13]; however, such studies had not been carried out. To the best of our knowledge, this study attempted the first differentiation between FHB and GERD based on oral manifestations. The rationale behind the use of oral evaluation is based on its low cost, wide availability, and the fact that the suggested parameters are not temporary symptoms but long-term consequences of GERD.
In the literature, there are many studies on the association between GERD and DE or PD. However, to the best of our knowledge, studies assessing the hard and soft tissue injuries, namely DE and PD, together have not been conducted yet. Furthermore, none of the previous studies examined their relations from both dental and gastroenterological perspectives.
Several studies discussed and concluded a clear but variable relationship between DE and GERD[14,15]. The proposed pathogenesis of DEs is attributed the direct contact of acid and the enamel, resulting in dissolution of the enamel crystals and destruction of the interprismatic matrix and subsequently, the dentin[16-19].
As a result of our research, 41.2% of those with DE had reflux, which did not prove to be a significant result. However, this result differed from the findings recorded in the literature. Pace et al[4] published a recent systematic review involving 17 eligible studies, mainly observational and case-control studies on GERD and DE, in which they reported a strong association between the two conditions. The median prevalence of DE among all patients with GERD was 24%, and the median prevalence of GERD among adults with DEs was 32.5% (21.0%-83.0%) However, in this population, there were wide percentage ranges and degrees of tooth tissue loss, and not all studies and evaluations of patients included esophageal endoscopy and/or 24 h esophageal pH-metry[4].
Another systematic review was carried out and used different references since 2007. From a total of 273 articles, the mean prevalence values of DE were 48.8% in GERD patients and 20.5% in non-GERD controls. The prevalence of DE among adults with GERD was 38.9%, compared to 98.1% among children with GERD[20].
The total prevalence of DE (23.3%) in all subjects was less than the known global prevalence of DE. It can be stated that in the patient group we examined, the prevalence of DEs was found to be significantly higher among patients with GERD (33.1%) than among patients with FHB (12.0%) (P < 0.01). Our findings differ from those of studies conducted in different parts of the world.
A recent study conducted in China in 2016, reported a 60.8% presence of DE among patients diagnosed with GERD[21]. Another study carried out in Italy could not establish a significant co-appearance in the association between GERD and DE[22].
Previous studies have confirmed the association between DE and GERD. However, other manifestations (xerostomia, halitosis, oral burning, altered taste, bruxism, and soft tissue injuries, such as mucositis/stomatitis, aphthous-like ulcerations, gingivitis, and periodontal disease) are less likely to be investigated. The relationship between these diseases and GERD could either be direct or indirect[23].
In the literature, the presence of extrinsic factors resulting in DE was uncertain. According to a cross-sectional study, there was a clear relationship between DE and extrinsic dietary factors in patients with GERD[24]. This result was supported by a systematic review that highlighted the etiological complexity of DE (dietary habits, lifestyle, abrasion, bruxism, etc.), and the importance of taking a detailed medical history[25]. In contrast, based on an Indian cross-sectional study, extrinsic factors were not related to DE in GERD. In our study, there was no significant difference between the different habits resulting in DE (P = 0.23)[26].
In contrast to Song et al[27], our results could not confirm a close association between GERD and such manifestations except PD. The mechanism by which PD develops in GERD is mainly attributed to the direct action of acid on the mucosa, although hyposalivation is also suggested to play a role[28,29]. Watanabe et al[30] reported significant presence of soft tissue symptoms (stinging, bad breath, and burning sensation), oral cavity symptoms (sour/sour taste sensation), and the presence of GERD.
Di Fede et al[22] assessed the occurrence of oral pathological changes and symptoms in patients with GERD. Two hundred patients with GERD and 100 matched healthy controls were enrolled and studied. Univariate analyses revealed that xerostomia, oral burning sensation, subjective halitosis, and soft, hard palate mucosa, and uvula erythema were more common among patients with GERD than among matched controls (P < 0.05). The main outcome of this study was that no significant association between GERD and DEs was found, whereas some other symptoms or objective oral mucosal changes were found to be significantly associated with GERD[22]. In contrast, based on the responses of the patients we interviewed and examined, we did not find any data indicating a significant occurrence of oral complaints (such as the mouth and tongue burning, unpleasant breath, taste perception problems, inflammation of the mucous membrane, hypersensitivity, and sensations of sour taste).
A Chinese study found that periodontal factors were significantly associated with the risk of GERD in the studied 50183 patients. Severe periodontitis (OR = 1.40, P < 0.001) and lower frequency of tooth brushing (OR = 2.01, P < 0.001) were significantly associated with GERD[31].
In our study, neither DE nor PD alone was predictive of the presence of pathological reflux. There is not significantly more reflux in these cases. However, if both are present, the simultaneous presence of pathological reflux is more likely, as evidenced by the characteristic impedance deviations following the reflux pattern.
Increased BMI is commonly mentioned as a predictor of GERD. In their population-based study, Locke et al[31] found a significant relationship between higher BMI and the presence of GERD compared to subjects without reflux disease. Conversely, Watanabe et al[30] failed to establish a significant correlation between an increase in BMI and the presence of GERD. Our results seem to support the suggested association because our patients with GERD had significantly higher BMI than those with FHB. However, the observed difference is not significant enough to allow the prediction of GERD on the basis of this parameter alone.
In our study, higher esophagitis scores were detected in patients with DE and PD together than those without any dental diseases. This result suggests that there is more severe esophagitis in case of DE and PD than in the other groups. The correlation between the degree of DE and the severity of esophagitis was barely studied. A study conducted among the Mexican population found that 3/4 of the patients with mild grade DE had normal esophageal mucosa or LA-A esophagitis, whereas patients with severe DE were associated with a higher frequency of esophagitis LA-C and -D (P = 0.021)[32].
There are limitations to our study: First, the study was carried out in a single tertiary referral center; therefore, the prevalence of GERD phenotypes is different from the values of the general population. Second, during the process of pH-MII, inpatients were examined under standard conditions that do not correspond to their everyday conditions at home. DEs could be considered as cumulative lesions, representing the long-term consequences of reflux. Therefore, the dental status does not necessarily correlate with the current reflux state, since the bolus exposure time is not always the same, and it may significantly vary day by day, especially in the proximal part of the esophagus.
CONCLUSION
The dental evaluation of patients with heartburn seems to be useful in the differential diagnosis of GERD and FHB. The co-appearance of dental erosions and periodontal diseases was present mostly in patients with GERD, whereas DE or PD (especially its mild forms) alone were not predictive for the disease. In contrast, the absence of dental disorders in patients with heartburn was predictive of FHB.
ARTICLE HIGHLIGHTS
Research background
Heartburn is a typical symptom of gastroesophageal reflux disease (GERD) and other functional gastrointestinal diseases. To diagnose them, detailed esophageal function tests are required. Oral manifestations are also common in patients with GERD. The dental evaluation is cheap and widely available.
Research motivation
This study raised the hypothesis that dental evaluation (dental erosions, periodontal diseases) in patients with heartburn may be useful in the differential diagnosis of GERD and functional heartburn (FHB).
Research objectives
To evaluate the prevalence of oral manifestations in patients with heartburn and their association with GERD and FHB.
Research methods
We enrolled 116 [M/F: 51/65, mean age: 54 (17-80) years] consecutive patients with heartburn for detailed esophageal function tests and dental evaluation.
Research results
The prevalence of dental diseases in patients with heartburn was about 89%. Compared with heartburn patients without any dental diseases, heartburn patients with both DE and PD had more significant pathological reflux, higher grade of esophagitis, and significantly different mean impedance curves. Compared to FHB, GERD had a higher prevalence of DE and PD, especially when they coexisted. When evaluating the mean impedance curve, the trend of patients with both PD and DE was similar to those with GERD. The results of the study confirmed the abovementioned hypothesis experimentally.
Research conclusions
The co-existence of PD and DE is more likely to have pathological reflux, and the severity of esophagitis is higher than that of other groups. This study provides a new, inexpensive, widely available, and useful method for the differential diagnosis of GERD and FHB.
Research perspectives
On the one hand, our aim is to expand the study multicentrally in the direction of patients with heartburn presenting in primary care, and thereby reach a larger number of cases. On the other hand, this would probably also enable the inclusion of patients with reflux hypersensitivity, which is defined as a special borderline area between GERD and FHB in the Rome IV criteria system, which may help to decide whether this group of patients is more similar to patients GERD or FHB based on the oral status.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of the University of Szeged.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors have no conflict of interest to disclose.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 20, 2023
First decision: July 10, 2023
Article in press: July 27, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Montasser IF, Egypt; Wang ZF, China S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
Contributor Information
Krisztina Helle, Department of Internal Medicine, Division of Gastroenterology, University of Szeged, Szeged 6725, Hungary.
Anna Zsófia Árok, Department of Operative and Esthetic Dentistry, University of Szeged, Szeged 6725, Hungary.
Georgina Ollé, Department of Internal Medicine, Division of Gastroenterology, University of Szeged, Szeged 6725, Hungary.
Márk Antal, Department of Operative and Esthetic Dentistry, University of Szeged, Szeged 6725, Hungary.
András Rosztóczy, Department of Internal Medicine, Division of Gastroenterology, University of Szeged, Szeged 6725, Hungary. rosztoczy.andras@med.u-szeged.hu.
Data sharing statement
No additional data are available.
References
- 1.Moraes-Filho JP, Chinzon D, Eisig JN, Hashimoto CL, Zaterka S. Prevalence of heartburn and gastroesophageal reflux disease in the urban Brazilian population. Arq Gastroenterol. 2005;42:122–127. doi: 10.1590/s0004-28032005000200011. [DOI] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20; quiz 1943. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 3.Bodecker CF. Dental erosion: its possible causes and treatment. Dental Cosmos. 1933;75:1056–1062. [Google Scholar]
- 4.Pace F, Pallotta S, Tonini M, Vakil N, Bianchi Porro G. Systematic review: gastro-oesophageal reflux disease and dental lesions. Aliment Pharmacol Ther. 2008;27:1179–1186. doi: 10.1111/j.1365-2036.2008.03694.x. [DOI] [PubMed] [Google Scholar]
- 5.Boyapati R, Vudathaneni VKP, Nadella SB, Bollepalli AC, Marella Y, Adurty C. Reflex Gastroesophageal Disorders and Functional Dyspepsia: Potential Confounding Variables for the Progression of Chronic Periodontitis: A Clinical Study. Int J Prev Med. 2020;11:138. doi: 10.4103/ijpvm.IJPVM_141_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, Vaezi M, Sifrim D, Fox MR, Vela MF, Tutuian R, Tack J, Bredenoord AJ, Pandolfino J, Roman S. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, Crowell MD, Keefer L, Lembo AJ, Parkman HP, Rao SS, Sperber A, Spiegel B, Tack J, Vanner S, Walker LS, Whorwell P, Yang Y. Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Zhong C, Duan L, Wang K, Xu Z, Ge Y, Yang C, Han Y. Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J Gastroenterol. 2013;48:601–610. doi: 10.1007/s00535-012-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BG, Knight JK. An index for measuring the wear of teeth. Br Dent J. 1984;156:435–438. doi: 10.1038/sj.bdj.4805394. [DOI] [PubMed] [Google Scholar]
- 12.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Functional Esophageal Disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett D. Intrinsic causes of erosion. Monogr Oral Sci. 2006;20:119–139. doi: 10.1159/000093359. [DOI] [PubMed] [Google Scholar]
- 15.Ranjitkar S, Kaidonis JA, Smales RJ. Gastroesophageal reflux disease and tooth erosion. Int J Dent. 2012;2012:479850. doi: 10.1155/2012/479850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannig C, Hannig M, Attin T. Enzymes in the acquired enamel pellicle. Eur J Oral Sci. 2005;113:2–13. doi: 10.1111/j.1600-0722.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 17.Meurman JH, Frank RM. Scanning electron microscopic study of the effect of salivary pellicle on enamel erosion. Caries Res. 1991;25:1–6. doi: 10.1159/000261335. [DOI] [PubMed] [Google Scholar]
- 18.Lussi A, Jäggi T, Schärer S. The influence of different factors on in vitro enamel erosion. Caries Res. 1993;27:387–393. doi: 10.1159/000261569. [DOI] [PubMed] [Google Scholar]
- 19.Cairns AM, Watson M, Creanor SL, Foye RH. The pH and titratable acidity of a range of diluting drinks and their potential effect on dental erosion. J Dent. 2002;30:313–317. doi: 10.1016/s0300-5712(02)00044-1. [DOI] [PubMed] [Google Scholar]
- 20.Picos A, Badea ME, Dumitrascu DL. Dental erosion in gastro-esophageal reflux disease. A systematic review. Clujul Med. 2018;91:387–390. doi: 10.15386/cjmed-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Liu J, Chen S, Wang Y, Zhang Z. Prevalence of dental erosion among people with gastroesophageal reflux disease in China. J Prosthet Dent. 2017;117:48–54. doi: 10.1016/j.prosdent.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Di Fede O, Di Liberto C, Occhipinti G, Vigneri S, Lo Russo L, Fedele S, Lo Muzio L, Campisi G. Oral manifestations in patients with gastro-oesophageal reflux disease: a single-center case-control study. J Oral Pathol Med. 2008;37:336–340. doi: 10.1111/j.1600-0714.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 23.Mahajan R, Kulkarni R, Stoopler ET. Gastroesophageal reflux disease and oral health: A narrative review. Spec Care Dentist. 2022;42:555–564. doi: 10.1111/scd.12726. [DOI] [PubMed] [Google Scholar]
- 24.Ortiz AC, Fideles SOM, Pomini KT, Buchaim RL. Updates in association of gastroesophageal reflux disease and dental erosion: systematic review. Expert Rev Gastroenterol Hepatol. 2021;15:1037–1046. doi: 10.1080/17474124.2021.1890030. [DOI] [PubMed] [Google Scholar]
- 25.Chauhan N, Manjunath BC, Malhotra P, Yadav V, Kumar JS, Muppalla L, Bhukal S. Dietary Practices as a Potential Predictor for Dental Erosion among Patients Having Gastroesophageal Reflux Disease: An Analytical Cross-sectional Study. J Int Soc Prev Community Dent. 2022;12:583–589. doi: 10.4103/jispcd.JISPCD_95_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Gao X, Liang L, Zhou X, Han X, Yang T, Huang K, Lin Y, Deng S, Wang Z, Wang C. Prevalence, General and Periodontal Risk Factors of Gastroesophageal Reflux Disease in China. J Inflamm Res. 2023;16:235–244. doi: 10.2147/JIR.S395777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song JY, Kim HH, Cho EJ, Kim TY. The relationship between gastroesophageal reflux disease and chronic periodontitis. Gut Liver. 2014;8:35–40. doi: 10.5009/gnl.2014.8.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauk L. Laparoscopic Roux-en-Y gastric bypass. AORN J. 2018;107:P12–P14. doi: 10.1002/aorn.12107. [DOI] [PubMed] [Google Scholar]
- 29.Jajam M, Bozzolo P, Niklander S. Oral manifestations of gastrointestinal disorders. J Clin Exp Dent. 2017;9:e1242–e1248. doi: 10.4317/jced.54008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe M, Nakatani E, Yoshikawa H, Kanno T, Nariai Y, Yoshino A, Vieth M, Kinoshita Y, Sekine J. Oral soft tissue disorders are associated with gastroesophageal reflux disease: retrospective study. BMC Gastroenterol. 2017;17:92. doi: 10.1186/s12876-017-0650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642–649. doi: 10.1016/s0002-9343(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 32.Roesch-Ramos L, Roesch-Dietlen F, Remes-Troche JM, Romero-Sierra G, Mata-Tovar Cde J, Azamar-Jácome AA, Barranca-Enríquez A. Dental erosion, an extraesophageal manifestation of gastroesophageal reflux disease. The experience of a center for digestive physiology in Southeastern Mexico. Rev Esp Enferm Dig. 2014;106:92–97. doi: 10.4321/s1130-01082014000200004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.