Abstract
The incidence of type 2 diabetes mellitus is growing in epidemic proportions and has become one of the most critical public health concerns. Cardiovascular complications associated with diabetes are the leading cause of morbidity and mortality. The cardiovascular diseases that accompany diabetes include angina, myocardial infarction, stroke, peripheral artery disease, and congestive heart failure. Among the various risk factors generated secondary to hyperglycemic situations, advanced glycation end products (AGEs) are one of the important targets for future diagnosis and prevention of diabetes. In the last decade, AGEs have drawn a lot of attention due to their involvement in diabetic patho-physiology. AGEs can be derived exogenously and endogenously through various pathways. These are a non-homogeneous, chemically diverse group of compounds formed non-enzymatically by condensation between carbonyl groups of reducing sugars and free amino groups of protein, lipids, and nucleic acid. AGEs mediate their pathological effects at the cellular and extracellular levels by multiple pathways. At the cellular level, they activate signaling cascades via the receptor for AGEs and initiate a complex series of intracellular signaling resulting in reactive oxygen species generation, inflammation, cellular proliferation, and fibrosis that may possibly exacerbate the damaging effects on cardiac functions in diabetics. AGEs also cause covalent modifications and cross-linking of serum and extracellular matrix proteins; altering their structure, stability, and functions. Early diagnosis of diabetes may prevent its progression to complications and decrease its associated comorbidities. In the present review, we recapitulate the role of AGEs as a crucial mediator of hyperglycemia-mediated detrimental effects in diabetes-associated complications. Furthermore, this review presents an overview of future perspectives for new therapeutic interventions to ameliorate cardiovascular complications in diabetes.
Keywords: Type 2 diabetes mellitus, Cardiovascular complications, Hyperglycemia, Advanced glycation end products, Reactive oxygen species, Oxidative stress, Endothelial cells, Receptor of advanced glycation end products, Anti-advanced glycation end products strategies
Core tip: Cardiovascular diseases (CVDs) in type 2 diabetes mellitus impose a clinical and an economic burden on the healthcare system. Early diagnosis of diabetes may prevent its progression to complications and decrease its associated comorbidities. The present manuscript reports the clinical relevance of estimating advanced glycation end products (AGEs) in diabetes. The deleterious effects of AGEs include many important biochemical reactions central to the development and progression of cardiovascular complications in diabetes. Therefore, AGEs are one of the important targets for future diagnosis and prevention of diabetes. The epidemiology of CVD in diabetes, AGEs as a crucial mediator of diabetic CVD, and an overview of different strategies for countering the accumulation of AGEs is discussed along with new therapeutic interventions to ameliorate their effects.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a cluster of metabolic disturbances consequent to non-utilization of glucose due to insufficient production/secretion of insulin or its resistance. T2DM poses a major threat to global health. The number of people with T2DM is increasing at an alarming rate and has become one of the leading causes of death worldwide. The upsurge is corresponding with rising obesity, aging populations, increasing urbanization, calorie dense diets, economic development, and reduced physical activity. The global prevalence of diabetes as described by the International Diabetes Federation in 2021 was estimated to be 536.6 million (10.5%) and it is projected to reach 783.2 million (12.2%) by 2045[1]. Prevalence is expected to be higher in urban areas compared to rural ones. The estimated global cost of diabetes is slated to rise from 966 billion USD in 2021 to 1054 billion USD by 2045[1,2]. Consequently, T2DM imposes both a clinical and an economic burden on the health care system. DM is a complex pathophysiological process associated with several disabling and life-threatening health problems. Since DM basically affects blood vessels, it can affect almost any part of the body. People with diabetes are at risk of developing several complications affecting the heart, eyes, kidneys, and nerves. Vascular dysfunction is the single most serious consequence of long-standing DM[3,4] resulting in debilitating morbidity and mortality due to cardiovascular diseases (CVDs)[5,6]. The CVDs that accompany DM include stroke, myocardial infarction, peripheral artery disease, and coronary thrombosis[7].
Early diagnosis of DM may prevent its progression to CVD and decrease its associated comorbidities. Persistent hyperglycemia is considered to be an important factor in the development and the progression of diabetic complications and the exact mechanism of the deleterious effects of hyperglycemia on the onset of diabetic complications is still being explored[8]. Numerous hyperglycemia-induced mechanisms have been hypothesized to account for vascular complications in T2DM. These include the hexosamine pathway, polyADP-ribose polymerase activation, protein kinase C (PKC) activation, aldose reductase-mediated polyol pathway, and enhanced formation of advanced glycation end products (AGEs)[9-11]. Among these, the AGE-mediated pathways have been explored in the last decade because of mounting evidence that AGE accumulation is the crucial factor in the progression of diabetic complications[12,13]. AGEs are heterogeneous compounds resulting from nonenzymatic reactions of reducing sugars with other biomolecules such as lipids, proteins, and nucleic acid. This nonenzymatic glycation of proteins, lipids and nucleic acids is a slow and complicated process depending on the relative concentrations of the reactants. The moderate presence of AGEs has been notice in healthy individuals whereas, its formation increased under hyperglycemic conditions[14]. The severity of the complications in T2DM through AGEs corresponds with the quantum of hyperglycemia and varies with the structural and functional changes generated in most macromolecules. Also, AGEs interact with their receptors namely the receptor of AGEs (RAGE), and trigger the activation of multiple signals that can affect cellular functions and metabolism through upregulation of inflammation and oxidative stress[15,16].
The importance of AGEs in diabetic CVD is corroborated by the fact that the serum level of AGEs in T2DM CVD patients is higher compared to DM patients without CVD[17,18]. Studies have shown the association of AGEs with the prevalence as well as pathophysiological mechanisms of CVD in T2DM[19-21]. Jia et al[22] found that the tissue level of AGEs was independently associated with cardiac systolic dysfunction in T2DM patients with heart failure compared to T2DM patients without heart failure[22]. In vitro studies have shown that treatment of cardiomyocytes with AGEs for 24 h significantly reduces calcium transient in cells due to increased reactive species (RS) production[23]. Elevated serum AGEs predicted increased mortality due to CVD in Finnish women with DM who were followed up for 18 years[24]. In a recent review article by Dozio et al[25], the involvement of glycation in cardiovascular remodeling causing molecular, cellular and interstitial changes in the heart and vessels through different mechanisms has been demonstrated[25]. In a cross-sectional study carried out by De la Cruz-Ares et al[26] in 540 subjects, AGE levels and intima–media thickness of carotid arteries was consistently observed to be higher in CVD patients with T2DM[26]. Ninomiya et al[27] highlighted the importance of AGEs as a screening marker of atherosclerosis[27]. The AGE–RAGE axis further activates the pathological inflammation in plaques and atheromas[28]. Ren et al[29] identified the inhibition of prostacyclin in endothelial cells by the AGE–RAGE system, which promotes the formation of plasminogen activator inhibitor (PAI)-1 contributing to the stabilization of thrombus formation by inhibiting the fibrinolytic activity[29].
This review focuses on summarizing the clinical relevance of AGEs in CVD development and progression in T2DM. Different anti-AGE strategies are also being discussed that may become potential candidates for future preventive and therapeutic strategies in diabetic CVD.
EPIDEMIOLOGY OF CVD IN T2DM
Current trends in the epidemiology of CVD in T2DM present an underlying connection between chronic and un-controlled T2DM and vascular complications[30]. T2DM poses a major risk for the development of CVD and T2DM-associated mortality[5]. Prevalence of coronary artery diseases, peripheral vascular diseases, and carotid artery disease has been observed in different macrovascular complications in T2DM[31]. Numerous epidemiological studies suggested that T2DM can accelerate atherosclerosis and increase the incidence of heart attacks and strokes[31,32]. Patients with T2DM have a two- to six-times higher risk of heart failure than non-T2DM patients and heart failure accounts for > 50% of deaths in T2DM patients[6,33,34]. CVD is a major comorbidity affecting about one-third of all people with T2DM. A cohort study carried out on 1.9 million people by Dinesh et al[35] identified T2DM as a significant risk factor for CVD, including stroke, heart failure, atherosclerosis, and myocardial infarction[35]. T2DM patients are also prone to various cardiovascular risk factors, such as hypertension, dyslipidemia, and obesity that can directly promote the occurrence of cardiovascular complications in T2DM[36,37].
A cohort study carried out by Shah et al[33] demonstrated that the occurrence of peripheral artery diseases and heart failure was higher in T2DM by 16.2% and 14.7%, respectively[33]. Another cohort study carried out by National Health and Nutrition Examination Survey demonstrated that T2DM increases the risk of stroke by 26.3%, hemorrhagic stroke by 50% and ischemic stroke by 50%[32,38]. An American heart report of 2014 revealed a risk of heart failure of 40% in T2DM patients compared to patients without T2DM[39]. A prospective study showed that angina, coronary angioplasty, myocardial infarction, and congestive heart failure were among the predictors of all-cause mortality in T2DM[40]. A systematic review by Vaidya et al[41] has shown that 15%–81% of T2DM patients have at least one cardiovascular complication[41]. Einarson et al[42] confirmed that CVD imposes a substantial burden on the treatment of T2DM at both patient and population levels[42]. On an average patients treated for both CVD and T2DM resulted in an additional cost ranging from $3418 to $9705 compared to T2DM alone. Given the substantial economic and health burden of CVD in T2DM patients, there is a need to understand the mechanism of T2DM–CVD relationship and early diagnosis of T2DM to prevent its devastating complications.
DIFFERENT PATHWAYS FOR AGEs FORMATION
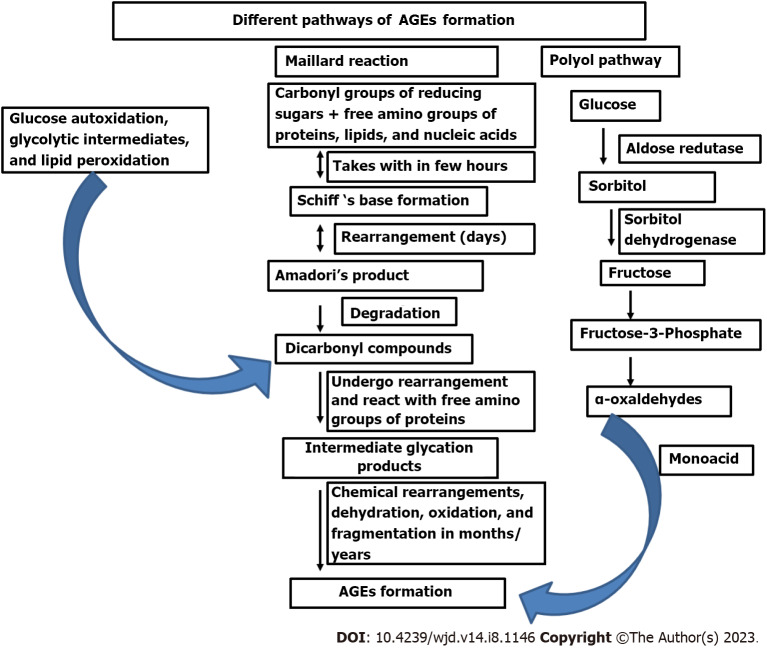
AGEs are chemically modified complex group of heterogeneous molecules formed either exogenously or endogenously by different pathways specifically, Maillard reaction, polyol pathway, and oxidation reactions (Figure 1). The Maillard reaction was first described in 1912 by French Scientist Louis Camille Maillard as “browning reaction” due to the associated yellow–brown color change when reducing sugar was heated with amino acid[43]. The AGEs formed through the Maillard reaction secondary to hyperglycemic condition is under intense investigation since a positive correlation is found with vascular complications like CVD, retinopathy, neurodegenerative diseases and other parameters of aging[44-46]. Maillard glycation reaction is different from enzymatic N-/O-linked glycosylation of proteins since they produce crosslinked products obtained from spontaneous and nonenymatic action of reducing sugars or their derivatives on other molecules, altering the structure and function of important cellular and extracellular components[47,48]. In healthy individuals AGEs are formed minimally and are cleared efficiently from the system. Formation and accumulation of AGEs becomes more rapid and pronounced under hyperglycemic conditions, oxidative stress, inflammatory conditions, and obesity[9,16]. AGE levels are higher in aged individuals, due to either overproduction or slower clearance indicative of their pathophysiological implications[49,50]
Figure 1.
Pathways for endogenous advanced glycation end products formation. Formation of AGEs occurs through different pathways. Maillard reaction which occurs at three stages: (1) Covalent binding of reducing sugars to free amino groups of proteins, lipids, and nucleic acid resulting in reversible Schiff base formation within hours; (2) it undergoes chemical rearrangement over a period of days to form a more stable Amadori product (the reaction is still reversible); and (3) Amadori’s products can be degraded into many reactive dicarbonyl compounds undergoing chemical rearrangements leading to the formation of irreversible AGEs. These spontaneous rearrangements are slow and often taking months to years but enhanced in presence of oxidative stress, and metal ions. Autoxidation of glucose and the peroxidation of lipids into dicarbonyl derivatives also results in AGEs formation. Monosaccharides glycolytic intermediates and dicarbonyl compounds formed during glycolysis also play an important role in AGEs formation. Polyol pathway, where glucose is converted to sorbitol by the enzyme aldose reductase and then sorbitol is converted to fructose by the action of sorbitol dehydrogenase. Fructose metabolites are converted into α-oxaldehydes and interact with monoacids to form AGEs. AGEs: Advanced glycation end products.
Accrual of AGEs is a multistage process starting with covalent binding of functional groups of monosaccharides to free amino groups of proteins, lipids, and nucleic acids forming labile reversible Schiff base intermediates under a hyperglycemic environment. This reaction is reversed if the hyperglycemia abates timeously. The initial Schiff’s base transforms over a period of days to a ketoamine, called Amadori’s product. The Amadori products are more stable, but the reaction is still reversible. The most well-recognized Amadori product is glycated hemoglobin, which is widely used as a reliable marker of glycemic control. Amadori products can be degraded into a variety of dicarbonyl compounds like 3-deoxy-glucosone, glyoxal and methyl-glyoxal, which can further react with proteins to form intermediate glycation products. Yellow–brown irreversible AGEs are formed after a sequence of chemical modifications including dehydration, oxidation, and fragmentation reactions (Figure 1). These spontaneous rearrangements are normally slow, often taking months to years. Nevertheless, the presence of oxidative stress, metal ions, and other catalysts can substantially increase the post-Amadori formation of AGEs. They are stable and accumulate inside and outside the cells and some of them have fluorescent properties[9,12,16].
Besides the Maillard reaction, other pathways such as the Hodge pathway, Namiki pathway and Wolff pathway can also result in AGEs formation, through autoxidation interactions of Amadori products, monosaccharides (glucose, fructose, ribose and glyceraldehyde) with amino acids and lipids[16,51-53]. Besides monosaccharides, the reactive products formed during glycolysis can also form AGEs by attacking proteins and other components. Some of the important glycolytic intermediates identified in AGEs formation are glyoxal, methylglyoxal, glucose-6-phosphate, triose phosphates, glyceraldehydes-3-phosphate and dihydroxy-acetone phosphate and 3-deoxyglucosone[54,55]. Auto-oxidation of glucose, reaction between glycolipid and arginine/lysine also results in AGEs formation through glyoxal and methyl-glyoxal production[56,57]. The Polyol pathway where, enzymatically formed metabolites of glucose like sorbitol and fructose also contributes significantly to AGEs formation[58,59]. The free ribose formed during the degradation of nucleic acid also represents the main source of pentosidine formation[60].
Also, sugars vary in their susceptibility to the Maillard reaction, where D-glucose is less reactive and D-fructose is more reactive sugar as demonstrated in both thermally processed food and in vivo conditions[53,61,62]. Temperature also has a significant effect on early glycation product formation, where high temperature (120–180C) accelerates the Maillard reaction in processed food, and the same reaction for Amadori’s product formation in vivo conditions require much longer time[63].
Exogenous formation of AGEs through glyco-oxidation and lipo-oxidation reactions formed from heating food at high temperature and chemical processing, tobacco smoke components and other pollutants also contributes to the chemical load of AGEs. Blood and tissue AGE levels have been consistently observed to be higher in smokers and in patients on high AGEs diets compared to non-smokers and controls on low AGE diets[64-67]. Ingestion of exogenous AGEs has been shown to exacerbate diabetic complications like CVD in animal models, hence their role needs further exploration[68,69].
TYPE OF AGEs
Due to variety of precursors and numerous pathways of nonenzymatic reactions, the AGEs are diverse in their chemical structure and properties. AGEs comprise a large number of chemical structures like N-carboxy-methyl-lysine (CML), pyrraline, pentosidine, cross-linked AGEs include GOLD [glyoxal-derived lysine dimer, 1,3-di(N_-lysino imidazolium salt], MOLD [methylglyoxal-derived lysine dimer, 1,3-di(N_-lysino)-4-(methyl-imidazolium salt], DOLD [3-deoxy-glucosone-derived lysine dimer, 1,3- di(N_-lysino)-4 (2,3,4-trihydroxybutyl)imidazolium salt], etc.[16,70-72]. The best biochemical and immunohistochemically characterized AGEs found in humans are pentosidine, carboxyl methyl lysine and methylglyoxal, which accumulate and can potentially be used as biomarkers[73,74]. CML is the most well-characterized AGE demonstrated in DM patients with CVD[75]. Structure and function of matrix proteins are modified with variable loss of function due to the aggregation of these adducts. Some of these AGEs have native fluorescence which can be used for their identification and quantification.
AGEs AND DIABETIC-CARDIOVASCULAR COMPLICATIONS
AGEs formed secondary to hyperglycemic conditions are gaining prominence as the underlying mechanism of CVD complications in T2DM. DM patients are known to have 20%–30% more circulating AGEs compared to controls, whereas DM patients with CVD complications have up to 40%–100% higher levels of AGEs[17,76]. The AGEs remain significantly high even after correction of variables such as duration of diabetes, sex, and age in T2DM patients with complications compared to those without complications[77,78]. Statistical analyses have also shown the association of AGEs level with the development and severity of atherosclerosis in DM patients[79,80]. Clinical reports have indicated that serum AGE levels can act as important marker or predictor of heart failure and CVD mortality in T2DM since their deposition has been detected in atherosclerotic plaques and heart muscles[81,82].
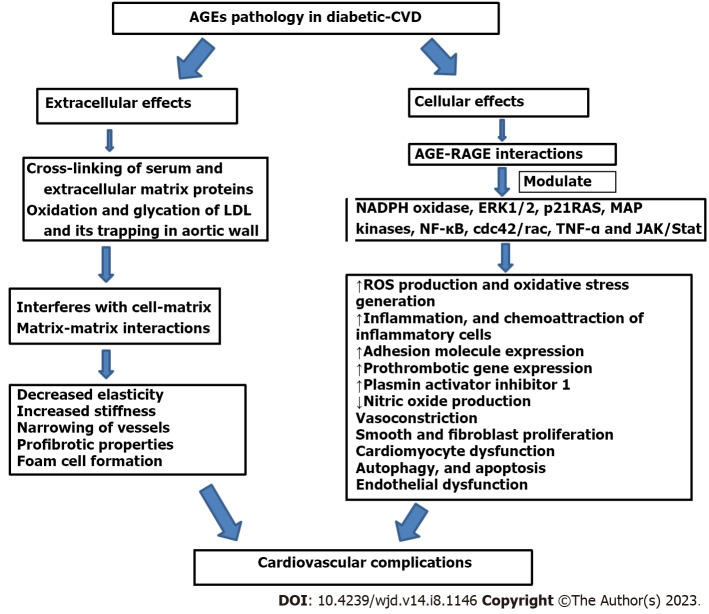
The deleterious effects of AGE-mediated cardiovascular complications in T2DM involve various pathological changes such as plaque formation, arterial stiffening, and generalized endothelial dysfunction aided by prothrombotic gene expression[83-85]. These detrimental effects of AGEs can be explained at the cellular and extracellular level as shown in Figure 2.
Figure 2.
Advanced glycation end product-mediated diabetic cardiovascular complications. AGEs mediate their pathological effects at the cellular and extracellular level by multiple pathways. At the cellular level, they activate signaling cascades via RAGE and initiate a complex series of intracellular signaling leading to reactive oxygen species generation, oxidative stress development, inflammation, adhesion molecule expression, endothelin-1, plasmin activator inhibitor 1, tumor necrosis factor alpha, chemoattraction of inflammatory cells, smooth muscle and fibroblast proliferation, autophagy, and apoptosis. AGE–RAGE interaction modulate the cellular properties through stimulation of signaling molecules such as ERK 1/2, p21RAS, MAPK, NF-B, cdc42/rac, and Janus kinase/STAT and adversely affects the cardiovascular health in diabetes. AGEs also causes covalent modifications and crosslinking of serum and ECM proteins, altering their structure, stability, and functions. Modification of ECM proteins and cross-linking interferes with cell–matrix and matrix–matrix interactions, affecting the matrix–cell signaling and leading to profibrotic action, decreased elasticity, increased stiffness, narrowing of vessels, and other hallmarks of atherosclerosis. VCAM1: Vascular cell adhesion molecules; JAK: Janus kinase; RAGE: Receptor for advanced glycation end products; NADPH: Nicotinamide adenine dinucleotide phosphate oxidase; NF-κB: Nuclear factor-B; AGEs: Advanced glycation end products; MAPK: Mitogen-activated protein kinase; ROS: Reactive oxygen species; TNF-α: Tumor necrosis factor ; ERK: Extracellular signal-regulated kinase; LDL: Low-density lipoprotein; ECM: Extracellular matrix.
AGE–RAGE axis in cardiovascular complications
At the cellular level, AGEs mediate their effects through interaction with their receptors, especially RAGE. RAGE is recognized by multiple ligands and has been localized on endothelial cells, vascular smooth muscle cells (VSMCs), immune cells and many others[86]. The presences of RAGE on multiple cells indicate its involvement in pathways affecting the vascular system in diabetes[87]. AGE–RAGE interaction activates signaling cascades leading to enhanced production of reactive oxygen species (ROS), oxidative stress, inflammation, adhesion molecule expression, endothelin-1, PAI-1, tumor necrosis factor (TNF)-α, chemoattraction of inflammatory cells, smooth muscle and fibroblast proliferation, autophagy, and apoptosis[88-90]. AGE–RAGE interaction modulates the cellular properties that possibly promote proinflammatory and procoagulant gene pathways through stimulation of signaling molecules such as extracellular signal-regulated kinase (ERK)1/2, p21RAS, mitogen-activated protein kinase (MAPK), nuclear factor (NF)-κB, cdc42/rac, and Janus kinase (JAK)/STAT and adversely affect the cardiovascular health in diabetes[91,92]. Cipollone et al[93] have studied the association of AGE–RAGE interaction and RAGE overexpression in human diabetic plaque macrophages by an increased inflammatory reaction, cyclooxygenase-2/prostaglandin E synthase-1 expression that may contribute to plaque destabilization through induction of metalloproteinase expression[93]. Also, the AGE–RAGE system activates inflammation in plaques and atheromas. Therefore, therapeutic approaches are now targeting the AGE-RAGE system to prevent the development of atherosclerosis[94].
Glycation of cellular and extracellular components in diabetic CVD
AGEs are also involved in the covalent modifications and crosslinking of serum and extracellular matrix (ECM) proteins, lipids and nucleic acid leading to perturbation of their structure and functions. Proteins of ECM have slow turnover rate and longer half-life which make them more prone to glycation reaction and crosslinking under hyperglycemic conditions. Modification of ECM proteins and crosslinking interferes with cell–matrix and matrix–matrix interactions, leading to profibrotic action, decreased elasticity, increased stiffness and narrowing of vessels and other hallmarks of atherosclerosis[14,95]. Cellular proteins also undergo the nonenzymatic glycation reaction by glucose and its derivatives like glucose-6-phosdphate, glyceraldehyde-3-phosphate, dihydroxyacetone-phosphate, GO, and MGO. Cellular AGEs have also been known to activate signaling pathways further impacting the diabetic vascular complications[96]. AGEs also induce crosslinking of intracellular proteins that participate in Ca2+ homeostasis resulting in cardiomyocyte dysfunction[97]. AGE–RAGE interaction is also found to be associated with decreased Ca2+ levels by upregulated ryanodine receptor which is involved in maintaining ionic balance during systolic and diastolic phases[98].
Development of cardiovascular complications in T2DM is also associated with increased incidence of low-density lipoprotein (LDL) oxidation, glycation of paraoxonase (PON)1, and high-density lipoprotein (HDL)[99]. Oxidation of LDL in arterial walls is the primary step in initiation and progression of atherosclerosis by foam cell formation. Recent studies have reported that glycated LDL can evade recognition by LDL receptors and can attach to arterial walls[100]. Non-enzymatic glycation of LDL is also responsible for impairment of hepatic receptor-mediated uptake and its removal. As a result, AGE-modified LDL is trapped in the subendothelium, causing its retention in the aortic wall where it is internalized by macrophages resulting in foam cell formation[101-103]. Glycation of LDL also makes it more vulnerable to crosslinking with collagen in the arterial wall. Elevated lipid-linked AGEs in LDL have also been noticed in T2DM patients[104]. Glycation of HDL also influences inflammation and affects the removal of cholesterol, leading to the development of atherosclerosis[105]. PON1 is an HDL-associated enzyme with antiatherogenic properties that protects LDL and cell membranes from oxidation. Glycation of PON1 is found to decrease its activity in DM, leading to the development of premature atherosclerosis[17,106,107].
AGEs and oxidative stress in diabetic-CVD
T2DM patients are exposed to high oxidative stress, increased reactive species (RS) generation, and decreased antioxidant defense mechanism. Hyperglycemia-induced ROS generation unveils the pathophysiology of CVD in T2DM and increased production of ROS triggers the inflammatory cascades responsible for the pathogenesis of cardiovascular complications[108,109]. The level of transcription factors such as TNF-α and NF-кB is modulated by increased RS production mediated signal transduction pathways enhancing the proinflammatory events including inflammatory adhesion molecules, interleukin (IL)-6, IL-1, and cytokines[110-112]. The AGE–RAGE interaction is also involved in increased RS generation through stimulation of certain signaling mediators like ERK, phospholipase A2, phophoinositide 3-kinase activation, activation of NADPH oxidase, inducible NO synthase (NOS), PKC and p38 MAPK[113-115]. Increased ROS production by mitochondria also triggers the inflammatory cascades in DM and prolonged exposure to high levels of ROS leads to oxidation, peroxidation and glyoxidation reactions resulting in increased oxidative stress markers such as protein carbonyl, oxidation of thiol group, lipid peroxidation, advanced oxidation protein products, and 8-OHdG[17,116]. Oxidative injury to biomolecules has also been observed in tissues and blood of diabetics with high AGEs concentration[117,118]. In vitro and in vivo studies have reported that increased ROS production by AGE–RAGE interaction causes DNA damage that induces endothelial cell death by triggering the apoptotic pathway[119,120].
AGEs and endothelial cell dysfunction
Endothelial dysfunction is the hallmark for the development of cardiovascular complications in T2DM. The presence of RAGE on the endothelial cell surface suggests its relevance in endothelial dysfunction by interacting with AGEs in T2DM. Lowered NO production, increased ROS generation, and enhanced expression of adhesion molecules, chemokines and cytokines are the hallmarks of endothelial dysfunction[121]. These conditions lead to inflammation, vasoconstriction, oxidative stress, myofibroblast migration, and proliferation inside the endothelial layer of vessels; all of which play a vital role in the development and progression of vascular complications in T2DM[122]. Under hyperglycemic condition endothelial cell proteins such as fibroblast growth factor and mitochondrial proteins undergo nonenzymatic glycation reactions affecting the vascular properties of cells by increased superoxide production, altering mitogenic and endothelial NOS (eNOS) activity[123,124].
Serum level of AGEs is negatively associated with the extent of endothelium-dependent vasodilation in T2DM patients[125]. NO acts as an antiatherogenic factor due to its effective vasodilatory, anti-inflammatory, and antiproliferative activities[110,126]. Increased ROS production by AGEs is one of the reasons for inactivation of NO as well their conversion to peroxynitrite form, thereby affecting the integrity of endothelial cells. Formation and accumulation of AGEs inside the endothelial cells is also found to be associated with reduced eNOS gene expression and increased eNOS mRNA degradation[126]. AGE–RAGE interaction on endothelial cells also results in enhanced production of asymmetric dimethylarginine, which is an endogenous inhibitor of eNOS and is one of the strongest marker of cardiovascular disease progression[127]. AGEs are also involved in NO quenching and inactivation of endothelium-derived NO[88]. Uhlmann et al[128] reported a significant reduction in NO production in AGE-treated cells in vitro. Their results implied that AGEs have a role in the modulation of NO activity in diabetic pathophysiology[128]. Ren et al[29] demonstrated the involvement of AGEs in reducing eNOS expression and NO bioavailability by increasing the oxidative stress development through activation of p38 and ERK1/2 in human coronary artery endothelial cells in vitro[29]. Therefore, accumulation of AGEs and AGE–RAGE interaction has an important impact on the pathogenesis of diabetic-CVD by affecting the vasodilating properties of endothelial cells. The AGE–RAGE axis also provokes the expression of p22hox and gp91hox, which are reduced form of NADPH oxidase in endothelial cells and causes its dysfunction[28].
Involvement of AGEs has also been noticed in the production of vascular endothelial growth factor (VEGF) by endothelial cells and thereby involved in atheroma formation. The activation of NF-кB by AGEs increases the secretion of VEGF (that prevent the repair of endothelial lesions resulting in atherogenesis), stimulates the differentiation of monocyte to macrophages and the accumulation of oxidized LDL in the vasculature leading to foam cell formation[29,129]. AGE–RAGE involvement has also been observed to inhibit the prostacyclin production and generation of PAI-1 in endothelial cells[130]. Formation and accumulation of AGEs have also been implicated in platelet activation and aggregation, stimulation of procoagulant activity, thrombus formation, and endothelial cell damage mediated by upregulation of protease-activated receptor-1 and -2 potentiates thrombin[131,132]. Decreased endothelial progenitor cell (EPC) function and mobilization poses a major risk for developing cardiovascular complications in T2DM[133]. AGE–RAGE interaction augments the apoptotic pathways and suppresses the migration and tube formation of late EPC by downregulation of Akt and cyclooxygenase-2[134]. Glycation of Arg-Gly-Asp motif of fibronectin by AGEs results in impairment of vascular repair by inhibiting EPC adhesion, migration, and spreading[134].
Vascular complications are also characterized by the adhesion and transmigration of monocyte into the subendothelial space. AGE–RAGE interactions enhance this process by activation of proinflammatory molecules such as NF-кB, which causes the overexpression of proinflammatory genes and adhesion proteins that aid monocyte adhesion to endothelial cells[103,135,136]. Foam cells and fatty streak formation take place in the vessel wall by monocyte and oxidized lipid at the adhesion site. These fatty streaks mature into advanced lesions with a fibrous cap that can dislodged resulting in an infarct or a stroke[137]. These observations suggest that AGEs have a definitive role in development and progression of vascular injuries observed in diabetes.
AGEs and VSMC modifications
Recently researchers have identified the phenotype transformation of VSMCs into macrophages during cardiovascular pathology[138]. In vitro studies have shown the effects of AGEs on increased proliferative activity and production of fibronectin in cultured SMCs. Transforming growth factor-β might act as a mediator in AGE-induced fibronectin production in SMC through AGE–RAGE interactions[139]. In vivo, the effect of AGEs on the growth of SMCs has also been noticed and is mediated by increased production of cytokines or growth factors[140]. Expansion of neointima is a unifying feature of atherosclerosis. Significant decreased in neointimal expansion, SMC proliferation, migration, and expression of ECM proteins have been demonstrated in homozygous RAGE-null mice. These data highlight the involvement of the AGE–RAGE axis in modulating the SMC properties and suggesting an important pharmaceutical target for suppression of neointima expansion[44,140]. VSMC phenotype transformation and calcification is one of the main pathological manifestations of atherosclerosis[141]. Recently Bao et al[142] showed the effect of AGEs on VSMC-derived foam cell formation and phenotype transformation. They identified the effect of CML on decreased expression of VSMC markers and increased expression of macrophage markers. They also noticed the involvement of AGEs in SMC migration and the secretion of proinflammatory factors[142]. Xing et al[143] explained the associated mechanism of phenotype transformation of VSMCs to macrophages by AGEs during atherosclerosis. They noticed that AGEs induced activation of RAGE/TLR4/FOXC2 signaling in macrophages with high expression of delta-like ligand (Dll)4 during M1 polarization. These altered macrophages promoted phenotype conversion of VSMC through Dll4/Notch pathway after cell-to-cell contact[143].
ANTI-AGEs THERAPIES
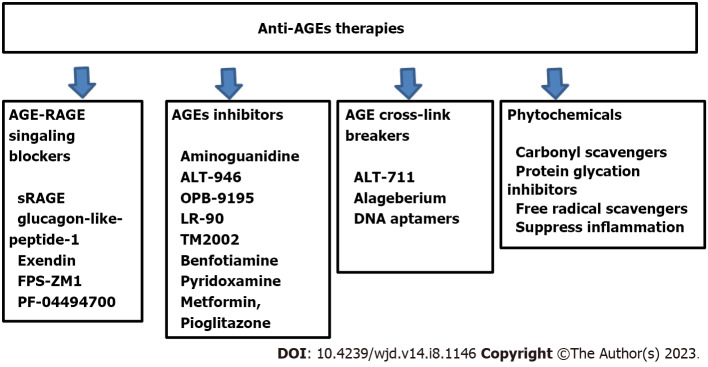
The deleterious effects of AGEs in the development and progression of diabetic vascular complications have driven the focus of pharmacological intervention towards attenuating the effects of AGEs. Although lifestyle modification, better glycemic control, regular physical activity, smoking cessation, restriction of AGE-rich diet have been reported to reduce the availability of precursors for glycation reactions and AGEs formation in T2DM[144-146]. A plethora of studies over the last few decades have been dedicated to in searching for pharmacological agents capable of interfering with glycation reactions and their sequelae. The underlying mechanism of action of these proposed drugs are based on AGEs inhibitors, AGEs crosslink breakers, detoxifying the dicarbonyls intermediates, and AGE–RAGE signaling blockers (Figure 3)[147,148]. No AGE-modifiers have been approved as drugs as yet, although some AGE-associated medications are in clinical and preclinical testing. Phytochemicals having antioxidant and anti-inflammatory properties have the potential to arrest the detrimental effects of AGEs and downstream consequences of the AGE–RAGE pathway[149].
Figure 3.
Anti-advanced glycation end product therapeutic strategies. Anti-AGE therapies target multiple pathways based on AGE-mediated effects in type 2 diabetes mellitus and associated complications. These include inhibitors of AGE formation, AGE crosslink breakers, and AGE–RAGE for AGE signaling blockers. The uses of phytochemicals having antioxidant and anti-inflammatory properties are also providing options to arrest the detrimental effects of AGEs by reducing peroxidative inflammatory reactions through carbonyl scavengers, protein glycation inhibitors and free radical scavengers which can reduce oxidative stress. RAGE: Receptor for advanced glycation end products; AGEs: Advanced glycation end products; sRAGE: Soluble RAGE.
Inhibition of endogenous AGEs formation
The first drug that was discovered to impede endogenous AGE formation was aminoguanidine with a guanidine group that is capable of trapping α-dicarbonyl product of early glycation reactions and thereby preventing the subsequent reactions with proteins[150,151]. Bolton et al[152] demonstrated the role of aminoguanidine in reducing proteinuria and progression to retinopathy, however due to its side effects, it is unlikely to be used for therapeutic purposes[152]. Compounds structurally related to aminoguanidine such as ALT-946 and OPB-9195 have been developed and tested as potential drugs. ALT-946 therapy was found to reduce renal AGE accumulation, and reduce albumin excretion in animal models[153]. OPB-9195 is an antagonist of peroxisome proliferator-activated receptor-γ and inhibits the glycoxidation and lipoxidation reactions. In animal models, OPB-9195 decreased the progression of nephropathy, lowered the blood pressure, and the serum level of AGEs[154,155]. LR-90 is another aromatic compound with anti-AGE properties due to its metal-chelating ability and its interaction with dicarbonyl compounds. It affords renoprotection such as improved renal albuminuria, reduction of connective tissue growth factors, fibronectin and collagen deposition in experimental model of type 1 and type 2 nephropathy[156]. TM2002 is a powerful AGE inhibitor that has transition metal-chelating properties and is nontoxic. It improves renal and cardiac lesions, and decreases infarct volume in different animal models[157,158]. Benfotiamine is a prodrug of thiamine monophosphate with AGE-lowering properties, mediated through preventing dicarbonyl formation[159,160]. In a pilot study, Brownlee et al[150] observed that treatment along with α-lipoic acid improved complications in patients with type 1 or type 2 DM. Pyridoxamine also intervenes in the glycation process by blocking the transformation of Amadori products into AGEs[161]. They have the ability to trap ROS, thereby blocking the oxidative degradation of Amadori intermediates and preventing the formation of AGEs[162,163].
Preformed AGEs breakers
Among the deleterious effects of AGE accumulation, crosslinking of ECM is of prominence and results in cardiovascular stiffness. Phenylthiazolium bromide was the first reported AGE crosslink breaker that is not stable in aqueous solution[164]. Several of its derivates have now been derived, such as ALT-711 or alageberium, and have the ability to break AGE crosslinks. The precise mechanism of their action relies on reaction with carbonyl groups present in AGE crosslinks and cleavage of carbon–carbon bonds. Application of alageberium in animal models has proved to be effective in reducing large artery stiffness and blood vessel fibrosis, attenuating atherosclerosis, diabetic nephropathy, and hypertension[165,166]. The role of aptamers has been explored in biomedical and pharmaceutical industries[167]. Aptamers are a group of short and single-stranded DNA or RNA molecules with the ability to bind with high affinity/specificity to a variety of proteins. DNA aptamers raised against AGEs bind and ameliorate AGE-associated effects[168]. These specific DNA aptamers can become novel therapeutic agents for AGE-related pathologies.
AGE–RAGE signaling blockers/RAGE antagonists
In vitro and in vivo studies have confirmed that AGE–RAGE axis is one of the major pathways for diabetic vascular complications. Therefore, it would be an ideal target to prevent the development and progression of complication in T2DM. Pharmacological agents that focus on the AGE–RAGE axis could function through different means such as inhibiting the RAGE expression, altering the AGE–RAGE signaling or by raising the blood level of soluble RAGE (sRAGE) to trap AGEs. sRAGEs are formed by alternative gene splicing of RAGE gene or proteolytic cleavage of membranous RAGE. Administration of sRAGE has shown to decrease albuminurea, glomerulosclerosis and diabetic CVD[169,170]. Statin and thiazolindinediones have been shown to ameliorate RAGE expression in conjugation with increased sRAGE[171,172]. The proposed underlying mechanisms of statin and thiazolindinediones have also been described. Activation of peroxisome proliferator-activated receptor-γ can inhibit the phosphorylation of ERK1/2 and downregulate NF-кB, thereby lowering the expression of inflammatory cytokines and RAGE[173,174]. Other molecules such as glucagon-like peptide (GLP)-1 and its analog exendin also decrease RAGE expression through suppressing NF-кB and decreasing ROS production by inhibiting NADPH oxidase activity[175,176]. Studies have also reported the involvement of GLP-1 and exendin in reducing activation of the AGE–RAGE axis and its associated complications such as atherosclerosis and diabetic cardiomyopathy etc[177,178]. RAGE inhibitors FPS-ZM1 and PF-04494700 had neuroprotective effects against ischemic brain injury in a rat model and β-amyloid structures in clinical trials for Alzheimer’s disease[179,180]. The effect of FPS-ZM1 as a RAGE inhibitor is associated with decreased inflammation and oxidative stress by targeting other ligands of RAGE such as S100, high-mobility group protein 1, and amyloid β-protein[180-183]. The promising effect of RAGE blockers such as FPS-ZM1 and PF-04494700 in neurodegenerative diseases provides the rationale to study their effects in T2DM patients.
AGEs and hypoglycemic drugs
The effects of many hypoglycemic drugs have also been studied in the context of decreasing AGE level and ameliorating the effects of AGE–RAGE axis. Prasad and Tiwari[169] have reported the effects of rosiglitazone in inhibiting the AGE–RAGE interaction and found elevated sRAGE levels[169]. Similar results have been reported in a randomized placebo-controlled study of 111 patients with T2DM CVD, where increased sRAGE and decreased inflammatory markers were reported after 6 mo of rosiglitazone treatment[184]. Effects of glimepiride beyond glycemic control have been reported in reduction of toxic glyceraldehyde-derived AGE levels and increased colony-stimulating factors to potentially repair tissue damage in T2DM patients[185]. Metformin treatment inhibits development of adverse myocardial structural and functional changes by inhibiting the production and accumulation of AGEs[186,187]. Metformin also inhibits the AGE-induced VSMC proliferation[188]. Animal and in vitro models have shown the efficacy of dipeptidyl peptidase-4 inhibitors such as sitagliptin, cilizytin, vildagliptin and linalgliptin in inhibiting glycosylation, downregulating the levels of AGEs, RAGE and oxidative stress markers, and decreasing the expression of VCAM-1, PAI-1, and ICAM-1[189-192]. GLP analog liraglutide was also found to ameliorate atherogenesis by inhibiting AGE-induced expression of RAGE in a mouse model[193].
CONCLUSION
T2DM imposes both clinical and economic burdens on the healthcare system. Recent reports have confirmed that CVD represents a substantial burden on the treatment of T2DM at both patient and population level. The pathophysiology of hyperglycemia in T2DM is closely associated with AGEs formation, accumulation, and their deleterious effects. The adverse effects of AGE accumulation include many important biochemical reactions that are central to the development and progression of cardiovascular complications in T2DM. AGE-mediated cardiovascular complications show many pathological changes such as plaque formation, arterial stiffening, neointimal proliferation, vasoconstriction, oxidation of LDL, and endothelial dysfunction. The probable mechanisms through which AGEs exert their detrimental effects include increased ROS generation, oxidative stress development, decreased NO production and its inactivation, inflammation, adhesion molecule expression, crosslinking of proteins, and prothrombotic gene expression. AGE–RAGE interactions also alter the cellular properties by promoting proinflammatory and procoagulant pathways acting through modulation of signaling molecules such as ERK1/2, cdc42/rac, p21RAS, TNF-α, MAPK, NF-κB, and JAK/STAT that adversely affect the cardiovascular health in T2DM. The AGE–RAGE axis is also involved in modulating SMC properties and neointima expansion, where it mediates SMC proliferation, phenotype transformation of VSMCs into macrophages during cardiovascular pathology. Therefore, clinical and experimental research is now focused on AGEs as new biomarkers or therapeutic target to prevent the development and progression of diabetic vascular complications. Based on AGE-mediated effects in pathogenesis of T2DM and its complications, pharmacological approaches are exploring combination therapies targeting multiple pathways based on inhibitors of AGE formation, AGE cross-ink breakers, free radical scavengers, and anti-inflammatory therapies, detoxifying the dicarbonyl intermediates and AGE–RAGE signaling blockers that may attenuate AGE-mediated effects in diabetic cardiovasculature. The use of phytochemicals with antioxidant and anti-inflammatory properties is promising for arresting the detrimental effects of AGEs. Also, there is a need to develop more specific and sensitive methods for the assay of circulatory AGEs. An epidemic of diabetes over the past half century has also been associated with increased consumption of modern heat-processed and highly palatable AGE-rich diet. Therefore, lifestyle modifications including dietary AGE restriction, regular exercise and cessation of smoking are some of the important interventions and practical ways to attenuate the effects of the AGE–RAGE axis and AGE-associated pathways.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 29, 2023
First decision: March 1, 2023
Article in press: May 22, 2023
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma JH, China; Rojas A, Chile S-Editor: Fan JR L-Editor: Kerr C P-Editor: Fan JR
Contributor Information
Savita Bansal, Department of Biochemistry, Institute of Home Sciences, University of Delhi, New Delhi 110016, India. savita.bansal@ihe.du.ac.in.
Archana Burman, Department of Biochemistry, Institute of Home Economics, University of Delhi, New Delhi 110016, India.
Asok Kumar Tripathi, Department of Biochemistry, University College of Medical Sciences, University of Delhi, New Delhi 110095, India.
References
- 1.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377–390. doi: 10.1038/s41581-020-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammedi K, Woodward M, Marre M, Colagiuri S, Cooper M, Harrap S, Mancia G, Poulter N, Williams B, Zoungas S, Chalmers J. Comparative effects of microvascular and macrovascular disease on the risk of major outcomes in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16:95. doi: 10.1186/s12933-017-0574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 6.Htay T, Soe K, Lopez-Perez A, Doan AH, Romagosa MA, Aung K. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. Curr Cardiol Rep. 2019;21:45. doi: 10.1007/s11886-019-1133-9. [DOI] [PubMed] [Google Scholar]
- 7.Sardu C, De Lucia C, Wallner M, Santulli G. Diabetes Mellitus and Its Cardiovascular Complications: New Insights into an Old Disease. J Diabetes Res. 2019;2019:1905194. doi: 10.1155/2019/1905194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61:21–28. doi: 10.1007/s00125-017-4390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells. 2022;11 doi: 10.3390/cells11081312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care. 2006;29:1420–1432. doi: 10.2337/dc05-2096. [DOI] [PubMed] [Google Scholar]
- 12.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 13.Indyk D, Bronowicka-Szydełko A, Gamian A, Kuzan A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci Rep. 2021;11:13264. doi: 10.1038/s41598-021-92630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018;24:59. doi: 10.1186/s10020-018-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asadipooya K, Uy EM. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J Endocr Soc. 2019;3:1799–1818. doi: 10.1210/js.2019-00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalid M, Petroianu G, Adem A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules. 2022;12 doi: 10.3390/biom12040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansal S, Chawla D, Siddarth M, Banerjee BD, Madhu SV, Tripathi AK. A study on serum advanced glycation end products and its association with oxidative stress and paraoxonase activity in type 2 diabetic patients with vascular complications. Clin Biochem. 2013;46:109–114. doi: 10.1016/j.clinbiochem.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Hartog JW, Voors AA, Bakker SJ, Smit AJ, van Veldhuisen DJ. Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. Eur J Heart Fail. 2007;9:1146–1155. doi: 10.1016/j.ejheart.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Chawla D, Bansal S, Banerjee BD, Madhu SV, Kalra OP, Tripathi AK. Role of advanced glycation end product (AGE)-induced receptor (RAGE) expression in diabetic vascular complications. Microvasc Res. 2014;95:1–6. doi: 10.1016/j.mvr.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Koska J, Saremi A, Howell S, Bahn G, De Courten B, Ginsberg H, Beisswenger PJ, Reaven PD VADT Investigators. Advanced Glycation End Products, Oxidation Products, and Incident Cardiovascular Events in Patients With Type 2 Diabetes. Diabetes Care. 2018;41:570–576. doi: 10.2337/dc17-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomis SJ, Chen Y, Sacks DB, Christenson ES, Christenson RH, Rebholz CM, Selvin E. Cross-sectional Analysis of AGE-CML, sRAGE, and esRAGE with Diabetes and Cardiometabolic Risk Factors in a Community-Based Cohort. Clin Chem. 2017;63:980–989. doi: 10.1373/clinchem.2016.264135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegab Z, Mohamed TMA, Stafford N, Mamas M, Cartwright EJ, Oceandy D. Advanced glycation end products reduce the calcium transient in cardiomyocytes by increasing production of reactive oxygen species and nitric oxide. FEBS Open Bio. 2017;7:1672–1685. doi: 10.1002/2211-5463.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Hanssen KF, Laakso M. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia. 2007;50:1409–1417. doi: 10.1007/s00125-007-0687-z. [DOI] [PubMed] [Google Scholar]
- 25.Dozio E, Massaccesi L, Corsi Romanelli MM. Glycation and Glycosylation in Cardiovascular Remodeling: Focus on Advanced Glycation End Products and O-Linked Glycosylations as Glucose-Related Pathogenetic Factors and Disease Markers. J Clin Med. 2021;10 doi: 10.3390/jcm10204792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Cruz-Ares S, Cardelo MP, Gutiérrez-Mariscal FM, Torres-Peña JD, García-Rios A, Katsiki N, Malagón MM, López-Miranda J, Pérez-Martínez P, Yubero-Serrano EM. Endothelial Dysfunction and Advanced Glycation End Products in Patients with Newly Diagnosed Versus Established Diabetes: From the CORDIOPREV Study. Nutrients. 2020;12 doi: 10.3390/nu12010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ninomiya H, Katakami N, Sato I, Osawa S, Yamamoto Y, Takahara M, Kawamori D, Matsuoka TA, Shimomura I. Association between Subclinical Atherosclerosis Markers and the Level of Accumulated Advanced Glycation End-Products in the Skin of Patients with Diabetes. J Atheroscler Thromb. 2018;25:1274–1284. doi: 10.5551/jat.44859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhee SY, Kim YS. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab J. 2018;42:188–195. doi: 10.4093/dmj.2017.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren X, Ren L, Wei Q, Shao H, Chen L, Liu N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc Diabetol. 2017;16:52. doi: 10.1186/s12933-017-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun JS, Ko SH. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism. 2021;123:154838. doi: 10.1016/j.metabol.2021.154838. [DOI] [PubMed] [Google Scholar]
- 31.Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep. 2019;21:21. doi: 10.1007/s11886-019-1107-y. [DOI] [PubMed] [Google Scholar]
- 32.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37:2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 33.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hangaard MH, Rossing P, Jensen JS, Jensen MT. [Heart failure often accompanies diabetes mellitus] Ugeskr Laeger. 2018;180 [PubMed] [Google Scholar]
- 35.Dinesh Shah A, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1·9 million people. Lancet. 2015;385 Suppl 1:S86. doi: 10.1016/S0140-6736(15)60401-9. [DOI] [PubMed] [Google Scholar]
- 36.Yang P, Feng J, Peng Q, Liu X, Fan Z. Advanced Glycation End Products: Potential Mechanism and Therapeutic Target in Cardiovascular Complications under Diabetes. Oxid Med Cell Longev. 2019;2019:9570616. doi: 10.1155/2019/9570616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mostaza-Prieto JM, Martín-Jadraque L, López I, Tranche S, Lahoz C, Taboada M, Mantilla T, Soler B, Monteiro B, Sanchez-Zamorano MA. Evidence-based cardiovascular therapies and achievement of therapeutic goals in diabetic patients with coronary heart disease attended in primary care. Am Heart J. 2006;152:1064–1070. doi: 10.1016/j.ahj.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McEwen LN, Karter AJ, Waitzfelder BE, Crosson JC, Marrero DG, Mangione CM, Herman WH. Predictors of mortality over 8 years in type 2 diabetic patients: Translating Research Into Action for Diabetes (TRIAD) Diabetes Care. 2012;35:1301–1309. doi: 10.2337/dc11-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaidya V, Gangan N, Sheehan J. Impact of cardiovascular complications among patients with Type 2 diabetes mellitus: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2015;15:487–497. doi: 10.1586/14737167.2015.1024661. [DOI] [PubMed] [Google Scholar]
- 42.Einarson TR, Acs A, Ludwig C, Panton UH. Economic Burden of Cardiovascular Disease in Type 2 Diabetes: A Systematic Review. Value Health. 2018;21:881–890. doi: 10.1016/j.jval.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 43.John WG, Lamb EJ. The Maillard or browning reaction in diabetes. Eye (Lond) 1993;7 ( Pt 2):230–237. doi: 10.1038/eye.1993.55. [DOI] [PubMed] [Google Scholar]
- 44.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Boehm BO, Schilling S, Rosinger S, Lang GE, Lang GK, Kientsch-Engel R, Stahl P. Elevated serum levels of N(epsilon)-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia. 2004;47:1376–1379. doi: 10.1007/s00125-004-1455-y. [DOI] [PubMed] [Google Scholar]
- 46.Wada R, Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci. 2005;1043:598–604. doi: 10.1196/annals.1338.067. [DOI] [PubMed] [Google Scholar]
- 47.Khalifah RG, Baynes JW, Hudson BG. Amadorins: novel post-Amadori inhibitors of advanced glycation reactions. Biochem Biophys Res Commun. 1999;257:251–258. doi: 10.1006/bbrc.1999.0371. [DOI] [PubMed] [Google Scholar]
- 48.Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53:131–142. [PubMed] [Google Scholar]
- 49.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 50.Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc Diabetol. 2002;1:1. doi: 10.1186/1475-2840-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyons TJ, Jenkins AJ. Glycation, oxidation, and lipoxidation in the development of the complications of diabetes: a carbonyl stress hypothesis. Diabetes Rev (Alex) 1997;5:365–391. [PMC free article] [PubMed] [Google Scholar]
- 52.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344 Pt 1:109–116. [PMC free article] [PubMed] [Google Scholar]
- 53.Bunn HF, Higgins PJ. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981;213:222–224. doi: 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- 54.Thornalley PJ. Dicarbonyl intermediates in the maillard reaction. Ann N Y Acad Sci. 2005;1043:111–117. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- 55.Miyata T, van Ypersele de Strihou C, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: origin and significance of "carbonyl stress" in long-term uremic complications. Kidney Int. 1999;55:389–399. doi: 10.1046/j.1523-1755.1999.00302.x. [DOI] [PubMed] [Google Scholar]
- 56.Kilhovd BK, Giardino I, Torjesen PA, Birkeland KI, Berg TJ, Thornalley PJ, Brownlee M, Hanssen KF. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism. 2003;52:163–167. doi: 10.1053/meta.2003.50035. [DOI] [PubMed] [Google Scholar]
- 57.Turk Z. Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiol Res. 2010;59:147–156. doi: 10.33549/physiolres.931585. [DOI] [PubMed] [Google Scholar]
- 58.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaneko M, Bucciarelli L, Hwang YC, Lee L, Yan SF, Schmidt AM, Ramasamy R. Aldose reductase and AGE-RAGE pathways: key players in myocardial ischemic injury. Ann N Y Acad Sci. 2005;1043:702–709. doi: 10.1196/annals.1333.081. [DOI] [PubMed] [Google Scholar]
- 60.Booth AA, Khalifah RG, Todd P, Hudson BG. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs). Novel inhibition of post-Amadori glycation pathways. J Biol Chem. 1997;272:5430–5437. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- 61.Suárez G, Rajaram R, Oronsky AL, Gawinowicz MA. Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. J Biol Chem. 1989;264:3674–3679. [PubMed] [Google Scholar]
- 62.Helsley RN, Moreau F, Gupta MK, Radulescu A, DeBosch B, Softic S. Tissue-Specific Fructose Metabolism in Obesity and Diabetes. Curr Diab Rep. 2020;20:64. doi: 10.1007/s11892-020-01342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R, Cerami A. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94:13915–13920. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A. 1997;94:6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicholl ID, Stitt AW, Moore JE, Ritchie AJ, Archer DB, Bucala R. Increased levels of advanced glycation endproducts in the lenses and blood vessels of cigarette smokers. Mol Med. 1998;4:594–601. [PMC free article] [PubMed] [Google Scholar]
- 67.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng F, He C, Cai W, Hattori M, Steffes M, Vlassara H. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev. 2002;18:224–237. doi: 10.1002/dmrr.283. [DOI] [PubMed] [Google Scholar]
- 69.Uribarri J, Tuttle KR. Advanced glycation end products and nephrotoxicity of high-protein diets. Clin J Am Soc Nephrol. 2006;1:1293–1299. doi: 10.2215/CJN.01270406. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed N, Thornalley PJ. Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochem Soc Trans. 2003;31:1417–1422. doi: 10.1042/bst0311417. [DOI] [PubMed] [Google Scholar]
- 71.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 72.Brownlee M. Negative consequences of glycation. Metabolism. 2000;49:9–13. doi: 10.1016/s0026-0495(00)80078-5. [DOI] [PubMed] [Google Scholar]
- 73.Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Cho SJ, Roman G, Yeboah F, Konishi Y. The road to advanced glycation end products: a mechanistic perspective. Curr Med Chem. 2007;14:1653–1671. doi: 10.2174/092986707780830989. [DOI] [PubMed] [Google Scholar]
- 75.Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000;26:163–176. [PubMed] [Google Scholar]
- 76.Sharp PS, Rainbow S, Mukherjee S. Serum levels of low molecular weight advanced glycation end products in diabetic subjects. Diabet Med. 2003;20:575–579. doi: 10.1046/j.1464-5491.2003.00973.x. [DOI] [PubMed] [Google Scholar]
- 77.Lapolla A, Piarulli F, Sartore G, Ceriello A, Ragazzi E, Reitano R, Baccarin L, Laverda B, Fedele D. Advanced glycation end products and antioxidant status in type 2 diabetic patients with and without peripheral artery disease. Diabetes Care. 2007;30:670–676. doi: 10.2337/dc06-1508. [DOI] [PubMed] [Google Scholar]
- 78.Anitha B, Sampathkumar R, Balasubramanyam M, Rema M. Advanced glycation index and its association with severity of diabetic retinopathy in type 2 diabetic subjects. J Diabetes Complications. 2008;22:261–266. doi: 10.1016/j.jdiacomp.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 79.Yoshida N, Okumura K, Aso Y. High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism. 2005;54:345–350. doi: 10.1016/j.metabol.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 80.Willemsen S, Hartog JW, Hummel YM, van Ruijven MH, van der Horst IC, van Veldhuisen DJ, Voors AA. Tissue advanced glycation end products are associated with diastolic function and aerobic exercise capacity in diabetic heart failure patients. Eur J Heart Fail. 2011;13:76–82. doi: 10.1093/eurjhf/hfq168. [DOI] [PubMed] [Google Scholar]
- 81.Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving HH, Tarnow L, Rossing P, Stehouwer CD. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care. 2011;34:442–447. doi: 10.2337/dc10-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koyama Y, Takeishi Y, Arimoto T, Niizeki T, Shishido T, Takahashi H, Nozaki N, Hirono O, Tsunoda Y, Nitobe J, Watanabe T, Kubota I. High serum level of pentosidine, an advanced glycation end product (AGE), is a risk factor of patients with heart failure. J Card Fail. 2007;13:199–206. doi: 10.1016/j.cardfail.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Zhou H, Tan KC, Shiu SW, Wong Y. Increased serum advanced glycation end products are associated with impairment in HDL antioxidative capacity in diabetic nephropathy. Nephrol Dial Transplant. 2008;23:927–933. doi: 10.1093/ndt/gfm631. [DOI] [PubMed] [Google Scholar]
- 84.Zeng C, Li Y, Ma J, Niu L, Tay FR. Clinical/Translational Aspects of Advanced Glycation End-Products. Trends Endocrinol Metab. 2019;30:959–973. doi: 10.1016/j.tem.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, Guo TL. Dietary advanced glycation end-products elicit toxicological effects by disrupting gut microbiome and immune homeostasis. J Immunotoxicol. 2021;18:93–104. doi: 10.1080/1547691X.2021.1959677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 89.Matsui T, Oda E, Higashimoto Y, Yamagishi S. Glyceraldehyde-derived pyridinium (GLAP) evokes oxidative stress and inflammatory and thrombogenic reactions in endothelial cells via the interaction with RAGE. Cardiovasc Diabetol. 2015;14:1. doi: 10.1186/s12933-014-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kobori T, Ganesh D, Kumano-Kuramochi M, Torigoe K, Machida S. Assay for advanced glycation end products generating intracellular oxidative stress through binding to its receptor. Anal Biochem. 2020;611:114018. doi: 10.1016/j.ab.2020.114018. [DOI] [PubMed] [Google Scholar]
- 91.Lee J, Yun JS, Ko SH. Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus. Nutrients. 2022;14 doi: 10.3390/nu14153086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 93.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–1077. doi: 10.1161/01.CIR.0000086014.80477.0D. [DOI] [PubMed] [Google Scholar]
- 94.Zhou Q, Cheng KW, Gong J, Li ETS, Wang M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem Pharmacol. 2019;166:231–241. doi: 10.1016/j.bcp.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 95.McNulty M, Mahmud A, Feely J. Advanced glycation end-products and arterial stiffness in hypertension. Am J Hypertens. 2007;20:242–247. doi: 10.1016/j.amjhyper.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 96.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 97.Bidasee KR, Zhang Y, Shao CH, Wang M, Patel KP, Dincer UD, Besch HR Jr. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes. 2004;53:463–473. doi: 10.2337/diabetes.53.2.463. [DOI] [PubMed] [Google Scholar]
- 98.Fischer TH, Herting J, Tirilomis T, Renner A, Neef S, Toischer K, Ellenberger D, Förster A, Schmitto JD, Gummert J, Schöndube FA, Hasenfuss G, Maier LS, Sossalla S. Ca2+/calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2+ leak in human cardiac pathology. Circulation. 2013;128:970–981. doi: 10.1161/CIRCULATIONAHA.113.001746. [DOI] [PubMed] [Google Scholar]
- 99.Wu L, Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002;39:809–814. doi: 10.1161/hy0302.105207. [DOI] [PubMed] [Google Scholar]
- 100.Nabi R, Alvi SS, Saeed M, Ahmad S, Khan MS. Glycation and HMG-CoA Reductase Inhibitors: Implication in Diabetes and Associated Complications. Curr Diabetes Rev. 2019;15:213–223. doi: 10.2174/1573399814666180924113442. [DOI] [PubMed] [Google Scholar]
- 101.Di Marco E, Gray SP, Jandeleit-Dahm K. Diabetes alters activation and repression of pro- and anti-inflammatory signaling pathways in the vasculature. Front Endocrinol (Lausanne) 2013;4:68. doi: 10.3389/fendo.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jamwal S, Sharma S. Vascular endothelium dysfunction: a conservative target in metabolic disorders. Inflamm Res. 2018;67:391–405. doi: 10.1007/s00011-018-1129-8. [DOI] [PubMed] [Google Scholar]
- 103.Hodgkinson CP, Laxton RC, Patel K, Ye S. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:2275–2281. doi: 10.1161/ATVBAHA.108.175992. [DOI] [PubMed] [Google Scholar]
- 104.Xu L, Wang YR, Li PC, Feng B. Advanced glycation end products increase lipids accumulation in macrophages through upregulation of receptor of advanced glycation end products: increasing uptake, esterification and decreasing efflux of cholesterol. Lipids Health Dis. 2016;15:161. doi: 10.1186/s12944-016-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Q, Jiang Z, Xu Y. HDL and Oxidation. Adv Exp Med Biol. 2022;1377:63–77. doi: 10.1007/978-981-19-1592-5_5. [DOI] [PubMed] [Google Scholar]
- 106.Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96:2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kinumi T, Ogawa Y, Kimata J, Saito Y, Yoshida Y, Niki E. Proteomic characterization of oxidative dysfunction in human umbilical vein endothelial cells (HUVEC) induced by exposure to oxidized LDL. Free Radic Res. 2005;39:1335–1344. doi: 10.1080/10715760500306695. [DOI] [PubMed] [Google Scholar]
- 108.Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, Ghirlanda G. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010;7:15–25. doi: 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hulsmans M, Van Dooren E, Holvoet P. Mitochondrial reactive oxygen species and risk of atherosclerosis. Curr Atheroscler Rep. 2012;14:264–276. doi: 10.1007/s11883-012-0237-0. [DOI] [PubMed] [Google Scholar]
- 110.Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. Int J Mol Sci. 2013;14:21525–21550. doi: 10.3390/ijms141121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perrone A, Giovino A, Benny J, Martinelli F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid Med Cell Longev. 2020;2020:3818196. doi: 10.1155/2020/3818196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 113.Chang PC, Chen TH, Chang CJ, Hou CC, Chan P, Lee HM. Advanced glycosylation end products induce inducible nitric oxide synthase (iNOS) expression via a p38 MAPK-dependent pathway. Kidney Int. 2004;65:1664–1675. doi: 10.1111/j.1523-1755.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 114.Rizvi AA. Cytokine biomarkers, endothelial inflammation, and atherosclerosis in the metabolic syndrome: emerging concepts. Am J Med Sci. 2009;338:310–318. doi: 10.1097/MAJ.0b013e3181a4158c. [DOI] [PubMed] [Google Scholar]
- 115.Bansal S, Siddarth M, Chawla D, Banerjee BD, Madhu SV, Tripathi AK. Advanced glycation end products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol Cell Biochem. 2012;361:289–296. doi: 10.1007/s11010-011-1114-9. [DOI] [PubMed] [Google Scholar]
- 116.Sarkar P, Kar K, Mondal MC, Chakraborty I, Kar M. Elevated level of carbonyl compounds correlates with insulin resistance in type 2 diabetes. Ann Acad Med Singap. 2010;39:909–904. [PubMed] [Google Scholar]
- 117.Al-Aubaidy HA, Jelinek HF. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur J Endocrinol. 2011;164:899–904. doi: 10.1530/EJE-11-0053. [DOI] [PubMed] [Google Scholar]
- 118.Pan HZ, Zhang H, Chang D, Li H, Sui H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br J Ophthalmol. 2008;92:548–551. doi: 10.1136/bjo.2007.130542. [DOI] [PubMed] [Google Scholar]
- 119.Nishikawa T, Sasahara T, Kiritoshi S, Sonoda K, Senokuchi T, Matsuo T, Kukidome D, Wake N, Matsumura T, Miyamura N, Sakakida M, Kishikawa H, Araki E. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care. 2003;26:1507–1512. doi: 10.2337/diacare.26.5.1507. [DOI] [PubMed] [Google Scholar]
- 120.Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960–966. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- 121.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. 2011;34 Suppl 2:S285–S290. doi: 10.2337/dc11-s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–418. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- 123.Giardino I, Edelstein D, Brownlee M. Nonenzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity. A model for intracellular glycosylation in diabetes. J Clin Invest. 1994;94:110–117. doi: 10.1172/JCI117296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosca MG, Mustata TG, Kinter MT, Ozdemir AM, Kern TS, Szweda LI, Brownlee M, Monnier VM, Weiss MF. Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am J Physiol Renal Physiol. 2005;289:F420–F430. doi: 10.1152/ajprenal.00415.2004. [DOI] [PubMed] [Google Scholar]
- 125.Tan KC, Chow WS, Ai VH, Metz C, Bucala R, Lam KS. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care. 2002;25:1055–1059. doi: 10.2337/diacare.25.6.1055. [DOI] [PubMed] [Google Scholar]
- 126.Soro-Paavonen A, Zhang WZ, Venardos K, Coughlan MT, Harris E, Tong DC, Brasacchio D, Paavonen K, Chin-Dusting J, Cooper ME, Kaye D, Thomas MC, Forbes JM. Advanced glycation end-products induce vascular dysfunction via resistance to nitric oxide and suppression of endothelial nitric oxide synthase. J Hypertens. 2010;28:780–788. doi: 10.1097/HJH.0b013e328335043e. [DOI] [PubMed] [Google Scholar]
- 127.Ishibashi Y, Matsui T, Ueda S, Fukami K, Okuda S, Yamagishi S. Irbesartan inhibits advanced glycation end product-induced increase in asymmetric dimethylarginine level in mesangial cells through its anti-oxidative properties. Int J Cardiol. 2014;176:1120–1122. doi: 10.1016/j.ijcard.2014.07.299. [DOI] [PubMed] [Google Scholar]
- 128.Uhlmann S, Rezzoug K, Friedrichs U, Hoffmann S, Wiedemann P. Advanced glycation end products quench nitric oxide in vitro. Graefes Arch Clin Exp Ophthalmol. 2002;240:860–866. doi: 10.1007/s00417-002-0548-x. [DOI] [PubMed] [Google Scholar]
- 129.Kosmopoulos M, Drekolias D, Zavras PD, Piperi C, Papavassiliou AG. Impact of advanced glycation end products (AGEs) signaling in coronary artery disease. Biochim Biophys Acta Mol Basis Dis. 2019;1865:611–619. doi: 10.1016/j.bbadis.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 130.Yamagishi S, Fujimori H, Yonekura H, Yamamoto Y, Yamamoto H. Advanced glycation endproducts inhibit prostacyclin production and induce plasminogen activator inhibitor-1 in human microvascular endothelial cells. Diabetologia. 1998;41:1435–1441. doi: 10.1007/s001250051089. [DOI] [PubMed] [Google Scholar]
- 131.Takenaka K, Yamagishi S, Matsui T, Nakamura K, Imaizumi T. Role of advanced glycation end products (AGEs) in thrombogenic abnormalities in diabetes. Curr Neurovasc Res. 2006;3:73–77. doi: 10.2174/156720206775541804. [DOI] [PubMed] [Google Scholar]
- 132.Ishibashi Y, Matsui T, Fukami K, Ueda S, Okuda S, Yamagishi S. Rivaroxaban inhibits oxidative and inflammatory reactions in advanced glycation end product-exposed tubular cells by blocking thrombin/protease-activated receptor-2 system. Thromb Res. 2015;135:770–773. doi: 10.1016/j.thromres.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 133.Chen Q, Dong L, Wang L, Kang L, Xu B. Advanced glycation end products impair function of late endothelial progenitor cells through effects on protein kinase Akt and cyclooxygenase-2. Biochem Biophys Res Commun. 2009;381:192–197. doi: 10.1016/j.bbrc.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 134.Bhatwadekar AD, Glenn JV, Li G, Curtis TM, Gardiner TA, Stitt AW. Advanced glycation of fibronectin impairs vascular repair by endothelial progenitor cells: implications for vasodegeneration in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:1232–1241. doi: 10.1167/iovs.07-1015. [DOI] [PubMed] [Google Scholar]
- 135.Salazar J, Navarro C, Ortega Á, Nava M, Morillo D, Torres W, Hernández M, Cabrera M, Angarita L, Ortiz R, Chacín M, D'Marco L, Bermúdez V. Advanced Glycation End Products: New Clinical and Molecular Perspectives. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18147236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen CY, Lu CH, Wu CH, Li KJ, Kuo YM, Hsieh SC, Yu CL. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules. 2020;25 doi: 10.3390/molecules25235591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 139.Sakata N, Meng J, Takebayashi S. Effects of advanced glycation end products on the proliferation and fibronectin production of smooth muscle cells. J Atheroscler Thromb. 2000;7:169–176. doi: 10.5551/jat1994.7.169. [DOI] [PubMed] [Google Scholar]
- 140.Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–972. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Johnson JL. Emerging regulators of vascular smooth muscle cell function in the development and progression of atherosclerosis. Cardiovasc Res. 2014;103:452–460. doi: 10.1093/cvr/cvu171. [DOI] [PubMed] [Google Scholar]
- 142.Bao Z, Li L, Geng Y, Yan J, Dai Z, Shao C, Sun Z, Jing L, Pang Q, Zhang L, Wang X, Wang Z. Advanced Glycation End Products Induce Vascular Smooth Muscle Cell-Derived Foam Cell Formation and Transdifferentiate to a Macrophage-Like State. Mediators Inflamm. 2020;2020:6850187. doi: 10.1155/2020/6850187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xing Y, Pan S, Zhu L, Cui Q, Tang Z, Liu Z, Liu F. Advanced Glycation End Products Induce Atherosclerosis via RAGE/TLR4 Signaling Mediated-M1 Macrophage Polarization-Dependent Vascular Smooth Muscle Cell Phenotypic Conversion. Oxid Med Cell Longev. 2022;2022:9763377. doi: 10.1155/2022/9763377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Prasad C, Davis KE, Imrhan V, Juma S, Vijayagopal P. Advanced Glycation End Products and Risks for Chronic Diseases: Intervening Through Lifestyle Modification. Am J Lifestyle Med. 2019;13:384–404. doi: 10.1177/1559827617708991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Waateringe RP, Mook-Kanamori MJ, Slagter SN, van der Klauw MM, van Vliet-Ostaptchouk JV, Graaff R, Lutgers HL, Suhre K, El-Din Selim MM, Mook-Kanamori DO, Wolffenbuttel BHR. The association between various smoking behaviors, cotinine biomarkers and skin autofluorescence, a marker for advanced glycation end product accumulation. PLoS One. 2017;12:e0179330. doi: 10.1371/journal.pone.0179330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim CS, Park S, Kim J. The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. J Exerc Nutrition Biochem. 2017;21:55–61. doi: 10.20463/jenb.2017.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Younus H, Anwar S. Prevention of non-enzymatic glycosylation (glycation): Implication in the treatment of diabetic complication. Int J Health Sci (Qassim) 2016;10:261–277. [PMC free article] [PubMed] [Google Scholar]
- 148.Abbas G, Al-Harrasi AS, Hussain H, Hussain J, Rashid R, Choudhary MI. Antiglycation therapy: Discovery of promising antiglycation agents for the management of diabetic complications. Pharm Biol. 2016;54:198–206. doi: 10.3109/13880209.2015.1028080. [DOI] [PubMed] [Google Scholar]
- 149.Matsui T, Nakamura N, Ojima A, Nishino Y, Yamagishi SI. Sulforaphane reduces advanced glycation end products (AGEs)-induced inflammation in endothelial cells and rat aorta. Nutr Metab Cardiovasc Dis. 2016;26:797–807. doi: 10.1016/j.numecd.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 150.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 151.Joglekar MM, Bavkar LN, Sistla S, Arvindekar AU. Effective inhibition of protein glycation by combinatorial usage of limonene and aminoguanidine through differential and synergistic mechanisms. Int J Biol Macromol. 2017;99:563–569. doi: 10.1016/j.ijbiomac.2017.02.104. [DOI] [PubMed] [Google Scholar]
- 152.Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE, Spinowitz BS, Whittier FC, Wuerth JP ACTION I Investigator Group. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 153.Forbes JM, Soulis T, Thallas V, Panagiotopoulos S, Long DM, Vasan S, Wagle D, Jerums G, Cooper ME. Renoprotective effects of a novel inhibitor of advanced glycation. Diabetologia. 2001;44:108–114. doi: 10.1007/s001250051587. [DOI] [PubMed] [Google Scholar]
- 154.Nakamura S, Makita Z, Ishikawa S, Yasumura K, Fujii W, Yanagisawa K, Kawata T, Koike T. Progression of nephropathy in spontaneous diabetic rats is prevented by OPB-9195, a novel inhibitor of advanced glycation. Diabetes. 1997;46:895–899. doi: 10.2337/diab.46.5.895. [DOI] [PubMed] [Google Scholar]
- 155.Mizutani K, Ikeda K, Tsuda K, Yamori Y. Inhibitor for advanced glycation end products formation attenuates hypertension and oxidative damage in genetic hypertensive rats. J Hypertens. 2002;20:1607–1614. doi: 10.1097/00004872-200208000-00024. [DOI] [PubMed] [Google Scholar]
- 156.Figarola JL, Scott S, Loera S, Tessler C, Chu P, Weiss L, Hardy J, Rahbar S. LR-90 a new advanced glycation endproduct inhibitor prevents progression of diabetic nephropathy in streptozotocin-diabetic rats. Diabetologia. 2003;46:1140–1152. doi: 10.1007/s00125-003-1162-0. [DOI] [PubMed] [Google Scholar]
- 157.Izuhara Y, Nangaku M, Takizawa S, Takahashi S, Shao J, Oishi H, Kobayashi H, van Ypersele de Strihou C, Miyata T. A novel class of advanced glycation inhibitors ameliorates renal and cardiovascular damage in experimental rat models. Nephrol Dial Transplant. 2008;23:497–509. doi: 10.1093/ndt/gfm601. [DOI] [PubMed] [Google Scholar]
- 158.Takizawa S, Izuhara Y, Kitao Y, Hori O, Ogawa S, Morita Y, Takagi S, van Ypersele de Strihou C, Miyata T. A novel inhibitor of advanced glycation and endoplasmic reticulum stress reduces infarct volume in rat focal cerebral ischemia. Brain Res. 2007;1183:124–137. doi: 10.1016/j.brainres.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 159.Karachalias N, Babaei-Jadidi R, Rabbani N, Thornalley PJ. Increased protein damage in renal glomeruli, retina, nerve, plasma and urine and its prevention by thiamine and benfotiamine therapy in a rat model of diabetes. Diabetologia. 2010;53:1506–1516. doi: 10.1007/s00125-010-1722-z. [DOI] [PubMed] [Google Scholar]
- 160.Thornalley PJ. The potential role of thiamine (vitamin B1) in diabetic complications. Curr Diabetes Rev. 2005;1:287–298. doi: 10.2174/157339905774574383. [DOI] [PubMed] [Google Scholar]
- 161.Metz TO, Alderson NL, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: a novel therapy for treatment of diabetic complications. Arch Biochem Biophys. 2003;419:41–49. doi: 10.1016/j.abb.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 162.Voziyan PA, Hudson BG. Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci. 2005;62:1671–1681. doi: 10.1007/s00018-005-5082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Deluyker D, Ferferieva V, Driesen RB, Verboven M, Lambrichts I, Bito V. Pyridoxamine improves survival and limits cardiac dysfunction after MI. Sci Rep. 2017;7:16010. doi: 10.1038/s41598-017-16255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vasan S, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan J, Ulrich P. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382:275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- 165.Bakris GL, Bank AJ, Kass DA, Neutel JM, Preston RA, Oparil S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am J Hypertens. 2004;17:23S–30S. doi: 10.1016/j.amjhyper.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 166.Kranstuber AL, Del Rio C, Biesiadecki BJ, Hamlin RL, Ottobre J, Gyorke S, Lacombe VA. Advanced glycation end product cross-link breaker attenuates diabetes-induced cardiac dysfunction by improving sarcoplasmic reticulum calcium handling. Front Physiol. 2012;3:292. doi: 10.3389/fphys.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guan B, Zhang X. Aptamers as Versatile Ligands for Biomedical and Pharmaceutical Applications. Int J Nanomedicine. 2020;15:1059–1071. doi: 10.2147/IJN.S237544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yamagishi S, Taguchi K, Fukami K. DNA-aptamers raised against AGEs as a blocker of various aging-related disorders. Glycoconj J. 2016;33:683–690. doi: 10.1007/s10719-016-9682-2. [DOI] [PubMed] [Google Scholar]
- 169.Prasad K, Tiwari S. Therapeutic Interventions for Advanced Glycation-End Products and its Receptor- Mediated Cardiovascular Disease. Curr Pharm Des. 2017;23:937–943. doi: 10.2174/1381612822666161006143032. [DOI] [PubMed] [Google Scholar]
- 170.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238–243. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xu L, Zang P, Feng B, Qian Q. Atorvastatin inhibits the expression of RAGE induced by advanced glycation end products on aortas in healthy Sprague-Dawley rats. Diabetol Metab Syndr. 2014;6:102. doi: 10.1186/1758-5996-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marx N, Walcher D, Ivanova N, Rautzenberg K, Jung A, Friedl R, Hombach V, de Caterina R, Basta G, Wautier MP, Wautiers JL. Thiazolidinediones reduce endothelial expression of receptors for advanced glycation end products. Diabetes. 2004;53:2662–2668. doi: 10.2337/diabetes.53.10.2662. [DOI] [PubMed] [Google Scholar]
- 173.Chen M, Li H, Wang G, Shen X, Zhao S, Su W. Atorvastatin prevents advanced glycation end products (AGEs)-induced cardiac fibrosis via activating peroxisome proliferator-activated receptor gamma (PPAR-γ) Metabolism. 2016;65:441–453. doi: 10.1016/j.metabol.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 174.Chiang MC, Cheng YC, Nicol CJ, Lin CH. The neuroprotective role of rosiglitazone in advanced glycation end product treated human neural stem cells is PPARgamma-dependent. Int J Biochem Cell Biol. 2017;92:121–133. doi: 10.1016/j.biocel.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 175.Chen S, Yin L, Xu Z, An FM, Liu AR, Wang Y, Yao WB, Gao XD. Inhibiting receptor for advanced glycation end product (AGE) and oxidative stress involved in the protective effect mediated by glucagon-like peptide-1 receptor on AGE induced neuronal apoptosis. Neurosci Lett. 2016;612:193–198. doi: 10.1016/j.neulet.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 176.Zhang SS, Wu Z, Zhang Z, Xiong ZY, Chen H, Huang QB. Glucagon-like peptide-1 inhibits the receptor for advanced glycation endproducts to prevent podocyte apoptosis induced by advanced oxidative protein products. Biochem Biophys Res Commun. 2017;482:1413–1419. doi: 10.1016/j.bbrc.2016.12.050. [DOI] [PubMed] [Google Scholar]
- 177.Dorecka M, Siemianowicz K, Francuz T, Garczorz W, Chyra A, Klych A, Romaniuk W. Exendin-4 and GLP-1 decreases induced expression of ICAM-1, VCAM-1 and RAGE in human retinal pigment epithelial cells. Pharmacol Rep. 2013;65:884–890. doi: 10.1016/s1734-1140(13)71069-7. [DOI] [PubMed] [Google Scholar]
- 178.Zhan Y, Sun HL, Chen H, Zhang H, Sun J, Zhang Z, Cai DH. Glucagon-like peptide-1 (GLP-1) protects vascular endothelial cells against advanced glycation end products (AGEs)-induced apoptosis. Med Sci Monit. 2012;18:BR286–BR291. doi: 10.12659/MSM.883207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xie J, Xu H, Wu X, Xie Y, Lu X, Wang L. Design, synthesis and anti-TNBC activity of Azeliragon triazole analogues. Bioorg Med Chem Lett. 2021;54:128444. doi: 10.1016/j.bmcl.2021.128444. [DOI] [PubMed] [Google Scholar]
- 180.Hong Y, Shen C, Yin Q, Sun M, Ma Y, Liu X. Effects of RAGE-Specific Inhibitor FPS-ZM1 on Amyloid-β Metabolism and AGEs-Induced Inflammation and Oxidative Stress in Rat Hippocampus. Neurochem Res. 2016;41:1192–1199. doi: 10.1007/s11064-015-1814-8. [DOI] [PubMed] [Google Scholar]
- 181.Yue Q, Song Y, Liu Z, Zhang L, Yang L, Li J. Receptor for Advanced Glycation End Products (RAGE): A Pivotal Hub in Immune Diseases. Molecules. 2022;27 doi: 10.3390/molecules27154922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Arumugam T, Ramachandran V, Gomez SB, Schmidt AM, Logsdon CD. S100P-derived RAGE antagonistic peptide reduces tumor growth and metastasis. Clin Cancer Res. 2012;18:4356–4364. doi: 10.1158/1078-0432.CCR-12-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li D, Lei C, Zhang S, Liu M, Wu B. Blockade of high mobility group box-1 signaling via the receptor for advanced glycation end-products ameliorates inflammatory damage after acute intracerebral hemorrhage. Neurosci Lett. 2015;609:109–119. doi: 10.1016/j.neulet.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 184.Gada E, Owens AW, Gore MO, See R, Abdullah SM, Ayers CR, Rohatgi A, Khera A, de Lemos JA, McGuire DK. Discordant effects of rosiglitazone on novel inflammatory biomarkers. Am Heart J. 2013;165:609–614. doi: 10.1016/j.ahj.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 185.Nakamura I, Oyama J, Komoda H, Shiraki A, Sakamoto Y, Taguchi I, Hiwatashi A, Komatsu A, Takeuchi M, Yamagishi S, Inoue T, Node K. Possible effects of glimepiride beyond glycemic control in patients with type 2 diabetes: a preliminary report. Cardiovasc Diabetol. 2014;13:15. doi: 10.1186/1475-2840-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Agarwal N, Rice SP, Bolusani H, Luzio SD, Dunseath G, Ludgate M, Rees DA. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 2010;95:722–730. doi: 10.1210/jc.2009-1985. [DOI] [PubMed] [Google Scholar]
- 187.Beisswenger PJ. Methylglyoxal in diabetes: link to treatment, glycaemic control and biomarkers of complications. Biochem Soc Trans. 2014;42:450–456. doi: 10.1042/BST20130275. [DOI] [PubMed] [Google Scholar]
- 188.Dziubak A, Wójcicka G. The pathophysiological basis of the protective effects of metformin in heart failure. Postepy Hig Med Dosw (Online) 2017;71:773–787. doi: 10.5604/01.3001.0010.3855. [DOI] [PubMed] [Google Scholar]
- 189.Jung E, Kim J, Kim SH, Kim S, Cho MH. Gemigliptin, a novel dipeptidyl peptidase-4 inhibitor, exhibits potent anti-glycation properties in vitro and in vivo. Eur J Pharmacol. 2014;744:98–102. doi: 10.1016/j.ejphar.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 190.Ishibashi Y, Matsui T, Maeda S, Higashimoto Y, Yamagishi S. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc Diabetol. 2013;12:125. doi: 10.1186/1475-2840-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Matsui T, Nishino Y, Takeuchi M, Yamagishi S. Vildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axis. Pharmacol Res. 2011;63:383–388. doi: 10.1016/j.phrs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 192.Lee TI, Kao YH, Chen YC, Huang JH, Hsu MI, Chen YJ. The dipeptidyl peptidase-4 inhibitor-sitagliptin modulates calcium dysregulation, inflammation, and PPARs in hypertensive cardiomyocytes. Int J Cardiol. 2013;168:5390–5395. doi: 10.1016/j.ijcard.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 193.Li P, Tang Z, Wang L, Feng B. Glucagon-like peptide-1 analogue liraglutide ameliorates atherogenesis via inhibiting advanced glycation end product-induced receptor for advanced glycosylation end product expression in apolipoprotein-E deficient mice. Mol Med Rep. 2017;16:3421–3426. doi: 10.3892/mmr.2017.6978. [DOI] [PubMed] [Google Scholar]