Abstract
A ductus arteriosus aneurysm is a rare congenital lesion with a localized saccular or tubular dilation of the ductus arteriosus. This lesion usually appears in all ages. Some case reports suggest the most common age of diagnosis is less than 2 months. We reported a case of an asymptomatic ductus arteriosus aneurysm in neonates. Echocardiography at 2 days of age revealed a tubular dilation of the ductus arteriosus connected to the pulmonary artery. Computed tomography angiogram showed a ductus arteriosus aneurysm with thrombus at the pulmonary end. It resolved spontaneously in the six months of life without serious complications.
Keywords: Computed tomography angiogram, Ductus arteriosus aneurysm, Echocardiography, Patent ductus arteriosus, Thrombosis
Introduction
A ductus arteriosus aneurysm (DAA) is a rare lesion with a localized saccular or tubular dilation of the ductus arteriosus. Congenital DAA may be identified in infants, children, and adults; published case reports suggest the most common age of diagnosis is less than 2 months. DAA may be observed in patients with connective tissue disorders such as Marfan [6], Ehlers-Danlos and Larsen syndromes [5,10], Loeys-Dietz syndrome [12], Smith-Lemli-Opitz, Trisomy 13, and Trisomy 18 [7], MYH11 mutation [3]. We diagnosed a case of asymptomatic neonatal DAA. It resolved spontaneously in the six months of life without serious complications.
Case report
We present the case of an asymptomatic 2-day-old full-term boy. Fetal ultrasound noted the great vessel disproportion. Family history was uncredited congenital anomalies and genetic disorders. The baby was delivered by elective cesarean section. A physical examination at birth demonstrated a neonate with no respiratory distress, normal vital signs, and no dysmorphic features. The lungs were clear. There were normal heart sounds and no murmurs. Echocardiography showed a normal intracardiac structure and good biventricular function. However, a DAA was detected on a suprasternal long-axis view with a left aortic arch (Fig. 1, Video 1).
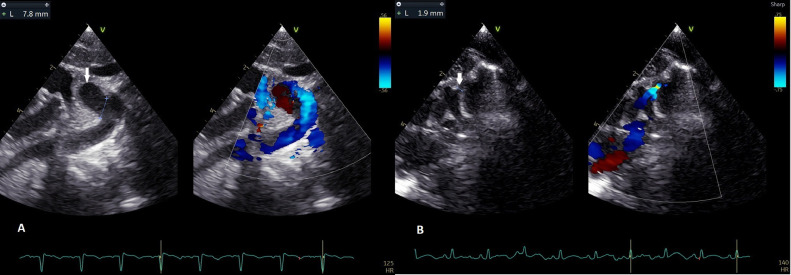
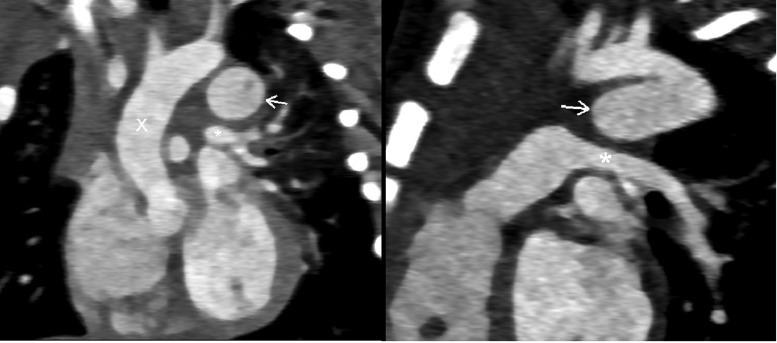
Fig. 1.
(A) The suprasternal long-axis view showed a 10 × 17 mm of the ductus arteriosus aneurysm (arrow) from the proximal descending aorta and toward to anterosuperior. (B) That sac connects to the main pulmonary artery (arrow).
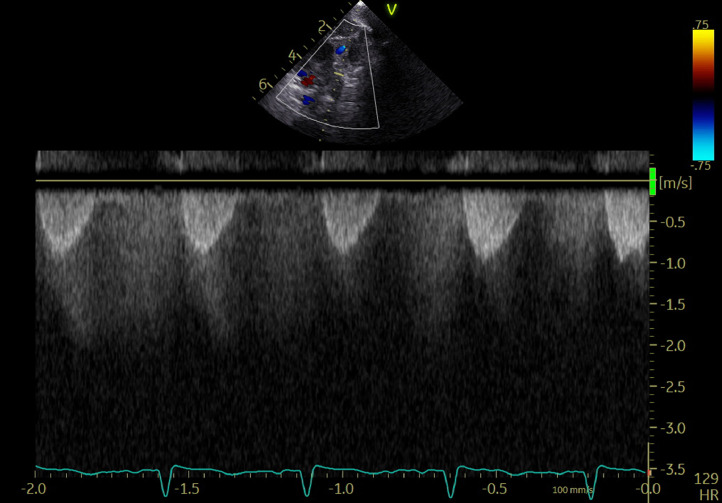
The DAA diameters were 6.1 mm at the aortic end, 10.7 mm at the midportion, and 1.9 mm at the pulmonary end. The flow by color Doppler across the ductus arteriosus was restrictive with continuous left-to-right shunt (Fig. 2, Video 2). 2D imaging showed spontaneous contrast in the ductal arteriosus aneurysm (Video 3), but no thrombus was seen.
Fig. 2.
Continuous left-to-right shunt at the pulmonary end of patent ductus arteriosus.
At 3 days of age, the neonate underwent MSCT to confirm the diagnosis and exclude thrombus. MSCT showed a normal intracardiac structure and a DAA (Fig. 3). The DAA diameters were 8 mm at the aortic end, 9.5 mm at the midportion, and without communication with the pulmonary artery. MSCT confirmed a thin thrombus layer at the bottom of the DAA at the pulmonary end (Fig. 4). The DAA did not compress adjacent structures (Fig. 5).
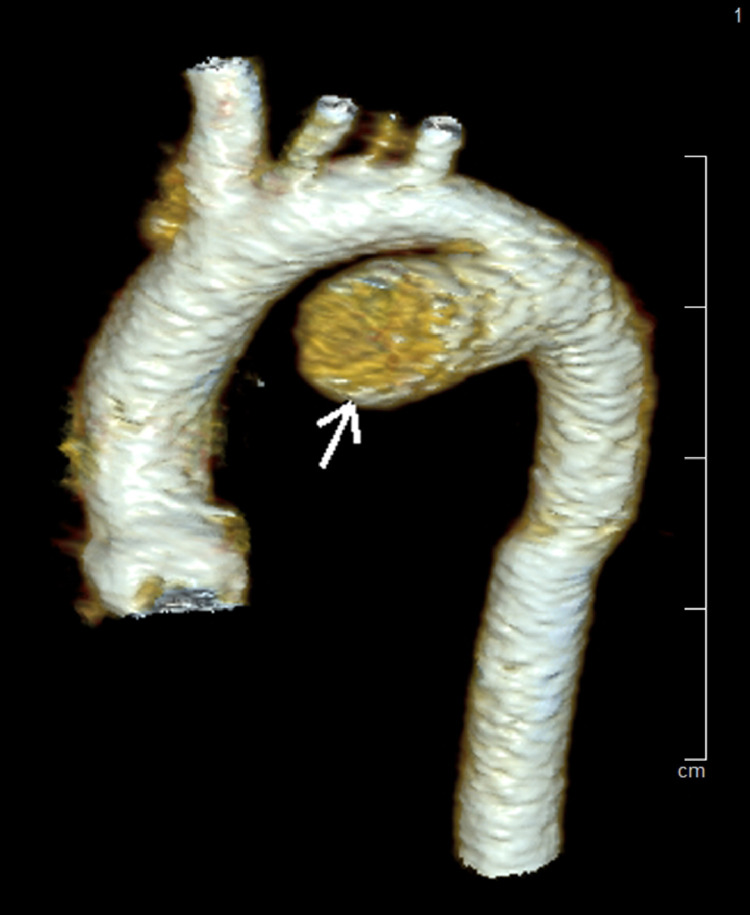
Fig. 3.
The ductus arteriosus aneurysm (arrow) in 3D reconstruction.
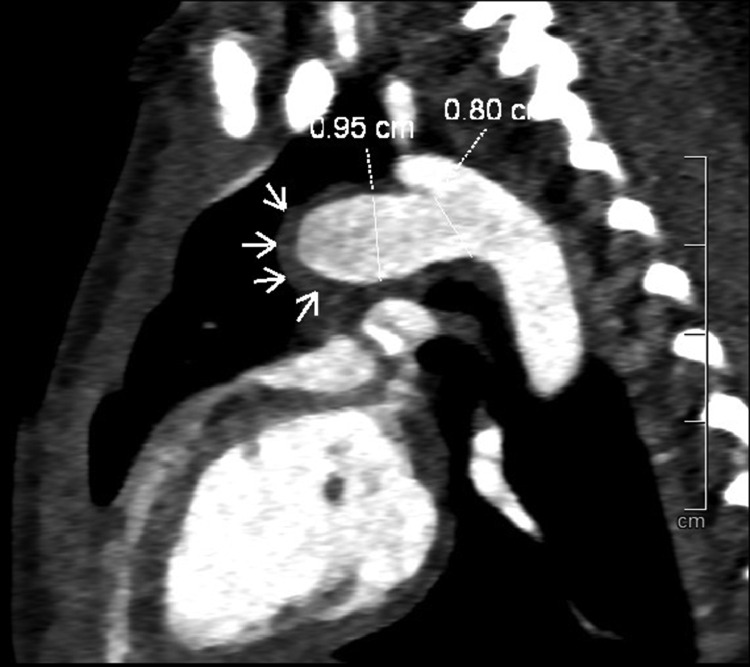
Fig. 4.
A thin thrombus layer (arrow) at the bottom of the saccular (pulmonary end), without communication between the ductus arteriosus aneurysm and the pulmonary artery.
Fig. 5.
The ductus arteriosus aneurysm (arrow) did not compress adjacent structures, such as the left pulmonary artery (asterisk) or trachea.
Repeat the echocardiogram at 3 days of life; there was no communication between the DAA and the pulmonary artery. But spontaneous echocardiographic contrast was still seen in DAA. After discussion, aspirin was used to reduce the risk of thrombosis. The patient was discharged and on regular follow-up.
An echocardiogram at 6 weeks of age demonstrated no evidence of communication between DAA and pulmonary artery, the DAA with in-complete resolution and normal aortic dimensions. At 6 months of age, the DAA was total nature closure and had no thrombus. The prophylactic aspirin therapy was discontinued.
Discussion
The DAA is when the ductus arteriosus becomes abnormally enlarged and forms a bulge or sac. The incidence of DAA is not precise, but it is estimated to be rare. According to different sources, the incidence of DAA ranges from 1.5% to 8.8% in neonates and is even rarer in adults [7,8]. Congenital DAA is often an incidental finding, and most cases have a benign course with spontaneous regression. Jan [8] reported 48 asymptomatic cases. However, Koneti [9] reported DAA presenting symptoms of respiratory distress, stridor, intercostal retraction, and weak crying. That resulted from DAA compression on the surrounding tissues, such as the phrenic nerve, the left main bronchi, and the recurrent laryngeal nerve, was reported. Lund [10] said the rate of dangerous DAA complications was 30%, such as thromboembolism, spontaneous rupture, dissection, airway erosion, and infection. However, all reports were case reports or retrospective data. Maybe a lot of asymptomatic or not serious complication DAAs would be missed. DAA may be observed in patients with connective tissue disorders such as Marfan, Ehlers-Danlos, and Larsen syndromes, Smith-Lemli-Opitz, Trisomy 13, and Trisomy 18, MYH11 mutation [3,5–7,10,12].
Imaging appearances of DAAs were various, depending on variable morphologic features of DAAs. Echocardiography is the first-line modality for the diagnosis and follow-up of DAAs. It is usually indicated when having abnormal heart sounds, congenital heart disease, or respiratory distress symptoms. There is no universally accepted definition of DAA, and different studies may use different cutoff values for the diameter or length of the ductus arteriosus to diagnose DAA. Tseng said ductus arteriosus, if dilated, is greater than 95% the size of the transverse arch or descending aorta [13]. Some authors reported that DDA had 6.5-24 mm in size, and large DAA is greater than 10 mm in size [7,8]. Bannan reported that some cases of DAA were mistaken at echocardiography for an anomalous pulmonary vein or a pulmonary sling [4]. If the echocardiogram does not provide sufficient information, CT or MRI can give more detailed information about the morphology of DAA and the presence of thrombus, calcification, or compression of surrounding structures. CT or MRI can also help to differentiate DAA from other lesions in the same region, such as aortic arch aneurysm, coarctation of the aorta, or vascular ring. CT or MRI can also be useful for planning the treatment and follow-up of DAA patients.
Although DAA is known to regress spontaneously following the closure of the ductus arteriosus in infants with asymptomatic ductal aneurysms, the asymptomatic patient would wait at least 4-6 weeks before considering surgical resection. Thrombus in the pulmonary arteries or aorta and thromboembolic events has been described in DAA [1,2,7]. The presence of spontaneous echocardiographic contrast in DAA is the risk for thrombus. In this patient, spontaneous echocardiographic contrast in DAA is clearly in echocardiography. CT shows a mural thrombus at the pulmonary end. In this case, waiting may lead to a thrombus in the aorta, thromboembolic events, and DAA rupture, but it is unclear whether asymptomatic DAA would be lethal. DAA would undergo spontaneous resolution in some reports [7,8].
There is limited evidence on the use of aspirin for thromboprophylaxis in DAA. Aspirin is a well-known antiplatelet agent that can prevent arterial thrombosis, but its efficacy in preventing DAA thrombosis is unclear. Masood reported one successful use of aspirin in DAA patients with thrombus formation [11]. Therefore, the role of aspirin in DAA remains uncertain and should be individualized based on the risk of thrombosis, bleeding, and preferences.
Conclusion
DAA is a rare congenital abnormality of the ductus arteriosus. Although most DAA are asymptomatic and have a benign course, the complications of DAA can be dangerous. Echocardiography and MSCT have an essential role in the diagnosis and monitoring of DAA, as well as in deciding the timing of surgical intervention. Prophylactic aspirin therapy in the presence of thromboembolism needs further investigation.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Our institution does not require ethical approval for reporting individual cases or case series.
Consent for publication
Not applicable.
Patient consent
I confirm that that written, informed consent for publication of their case was obtained from the patient's mother, allowing us to use the patient's photographs and medical information in this article.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2023.08.050.
Appendix. Supplementary materials
Video 1: TEE showed a DAA on a suprasternal long-axis view
Video 2: Left-to-right shunt at the pulmonary end of ductus arteriosus aneurysm.
Video 3: Spontaneous echocardiographic contrast in ductus arteriosus aneurysm.
References
- 1.Aly S.A., Contreras J, Honjo O, Villemain O. Antenatal occlusion of a ductal arteriosus aneurysm: a potential postnatal surgical emergency. Case report and literature review. Cardiol Young. 2020;30(11):1750–1752. doi: 10.1017/S1047951120002711. [DOI] [PubMed] [Google Scholar]
- 2.Amato J.J., Cardarelli M.G., Bierman F.Z. Aneurysm of the arterial duct—a case report and review of the literature. Cardiol Young. 1994;4(1):87–89. doi: 10.1017/S1047951100010969. [DOI] [Google Scholar]
- 3.Ardhanari M., Swaminathan S. Congenital ductus arteriosus aneurysm in association with MYH11 mutation: a case report. Cardiol Young. 2020;30(1):123–125. doi: 10.1017/s1047951119003287. [DOI] [PubMed] [Google Scholar]
- 4.Bannan B., Aly S, Yoo SJ, Seed M, Lam CZ. The many faces of neonatal ductus arteriosus aneurysms: multimodality imaging with an emphasis on CT and MRI appearance. Radiol Cardiothorac Imaging. 2021;3(3) doi: 10.1148/ryct.2021210017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang J.P., Chang C.H., Sheih M.J. Aneurysmal dilatation of patent ductus arteriosus in a case of Ehlers-Danlos syndrome. Ann Thorac Surg. 1987;44(6):656–657. doi: 10.1016/s0003-4975(10)62157-1. [DOI] [PubMed] [Google Scholar]
- 6.Crisfield R.J. Spontaneous aneurysm of the ductus arteriosus in a patient with Marfan's syndrome. J Thoracic Cardiovasc Surgery. 1971;62(2):243–247. [PubMed] [Google Scholar]
- 7.Dyamenahalli U., Smallhorn JF, Geva T, Fouron JC, Cairns P, Jutras L, et al. Isolated ductus arteriosus aneurysm in the fetus and infant: a multi-institutional experience. J Am Coll Cardiol. 2000;36(1):262–269. doi: 10.1016/S0735-1097(00)00707-5. [DOI] [PubMed] [Google Scholar]
- 8.Jan S.L., Hwang B, Fu YC, Chai JW, Chi CS. Isolated neonatal ductus arteriosus aneurysm. J Am Coll Cardiol. 2002;39(2):342–347. doi: 10.1016/s0735-1097(01)01736-3. [DOI] [PubMed] [Google Scholar]
- 9.Koneti N.R., Kanchi V, Kandraju H, Jaishankar S. Symptomatic aneurysm of ductus arteriosus in neonates. Ann Pediatr Cardiol. 2011;4(2):159–163. doi: 10.4103/0974-2069.84659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lund J.T., Hansen D, Brocks V, Jensen MB, Jacobsen JR. Aneurysm of the ductus arteriosus in the neonate: three case reports with a review of the literature. Pediatr Cardiol. 1992;13(4):222–226. doi: 10.1007/bf00838780. [DOI] [PubMed] [Google Scholar]
- 11.Masood S.A., Bokowski JW, Kazmouz S, Amin Z. Ductus arteriosus aneurysm with organized thrombus in a neonate: echocardiograms from diagnosis to resolution. Texas Heart Inst J. 2015;42(3):298–299. doi: 10.14503/thij-14-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan G.J., Yim DL, Hayes AM, Martin RP, Hamilton MC, Stuart G. Imaging and percutaneous occlusion of a large aneurysm of the ductus arteriosus in an infant with Loeys-Dietz syndrome. Congenit Heart Dis. 2013;8(6):E192–E195. doi: 10.1111/chd.12041. [DOI] [PubMed] [Google Scholar]
- 13.Tseng J.J., Jan S.L. Fetal echocardiographic diagnosis of isolated ductus arteriosus aneurysm: a longitudinal study from 32 weeks of gestation to term. Ultrasound Obstetr Gynecol. 2005;26(1):50–56. doi: 10.1002/uog.1859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: TEE showed a DAA on a suprasternal long-axis view
Video 2: Left-to-right shunt at the pulmonary end of ductus arteriosus aneurysm.
Video 3: Spontaneous echocardiographic contrast in ductus arteriosus aneurysm.
Data Availability Statement
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.