Abstract
The cancer-promoting ligand vascular endothelial growth factor-C (VEGF-C) activates VEGF receptor-3 (VEGFR-3). The VEGF-C/VEGFR-3 axis is expressed by a range of human tumor cells in addition to lymphatic endothelial cells. Activating the VEGF-C/VEGFR-3 signaling enhances metastasis by promoting lymphangiogenesis and angiogenesis inside and around tumors. Stimulation of VEGF-C/VEGFR-3 signaling promotes tumor metastasis in tumors, such as ovarian, renal, pancreatic, prostate, lung, skin, gastric, colorectal, cervical, leukemia, mesothelioma, Kaposi sarcoma, and endometrial carcinoma. We discuss and update the role of VEGF-C/VEGFR-3 signaling in tumor development and the research is still needed to completely comprehend this multifunctional receptor.
Keywords: Tumor, VEGFR-3, VEGF-C, Lymphangiogenesis, Angiogenesis
Introduction
The aggressive phenotype of human malignancies is mostly determined by the tumor's ability to invade and disseminate to other organs. More effective cancer treatments need a deeper knowledge of the molecular mechanisms that cause metastasis and the complicated interactions between metastatic cells and host factors. One of these processes involves the body's own synthesis of growth factors, which interact with and stimulate the activity of tumors' external receptors. The five polypeptide growth factors encoded by the vascular endothelial growth factor (VEGF) gene family, VEGF-A, B, C, D, and E, are especially significant due to their angiogenic and lymphangiogenic capabilities, which foster the progress and metastasis of cancers (Apte et al. 2019). However, the other isoforms also be involved in angiogenesis (Peach et al. 2018). New blood vessels are formed by a process known as angiogenesis. Endothelial cells, which line the interior of blood arteries, undergo migration, proliferation, and differentiation in this process. Lymphangiogenesis refers to the process by which new lymphatic vessels are formed from preexisting lymphatics; this process happens during embryonic development, wound healing, and different pathological situations, including cancer. To accomplish this, VEGFs activate the tyrosine kinase receptors VEGFR-1 [VEGF receptors (VEGFRs)] and VEGFR-2. Although endothelial cells are the primary source of expression of these receptors, they are also widely expressed in tumors (Apte et al. 2019). When it comes to coordinating the formation and ongoing health of lymphatic and blood vessels, VEGFRs are crucial (Monaghan et al. 2021). An autocrine effect is caused by the simultaneous expression of VEGFRs and their ligands. Exogenous VEGF-A, for instance, increases cell proliferation via the activation of VEGFR-2 in gastric cancer (Lin et al. 2017). Furthermore, VEGFR-2 is involved in the mitogenic effects of VEGF-A on human leukemic cells, including their ability to proliferate, migrate, and produce matrix metalloproteinase 9 (MMP9). Furthermore, VEGF-A enhances cell migration and adhesion via integrin AVB3 in skin cancer and stimulates cell proliferation in breast and pancreatic cells (Lian et al. 2019). These studies show that the well-described involvement of VEGFs/VEGFRs signaling in different tumor cells is crucial.
Additionally, VEGF and VEGF receptors are expressed on a wide variety of non-endothelial cells, including tumor cells. Endothelial cells are the only cells known to express VEGF and VEGF receptors. The importance of VEGF signaling in non-endothelial cells is also investigated, and the VEGFRs and their respective but specific legend VEGF-A, B, C, and D, which are associated with their respective designated physiological roles in the cardiovascular system, central nervous system (CNS), bone and hematopoietic cells, and hematological malignancies, are extensively reviewed by Onimaru and Yonemitsu (2011), Duffy et al. (2000).
Due to the intricate nature of VEGFR signaling, we direct the reader to refer to recent publications on VEGFR1/2 signaling (Yang and Li 2018; Uemura et al. 2021; Shah et al. 2021). The current review will concentrate on the known and hypothesized functions of VEGFR3, which is encoded by the FLT4 gene, in tumor progression. VEGFR3/FLT4 has a wide variety of developmental and physiological activities. VEGFR-3 and VEGFR-2 have been identified as the receptors through which VEGF-C transmits its signals as a lymphangiogenic and angiogenic growth factor (Yang and Li 2018; Uemura et al. 2021). Lymphangiogenesis susceptibility has also been linked to the expression of VEGFR-3. The study disproved the hypothesis that VEGFR-3 is exclusively expressed in lymphatic endothelial cells (LECs) and showed its widespread expression in various patient cancers (Monaghan et al. 2021). Significant correlations between VEGFR-3 expression and cervical carcinogenesis stages have also been documented (Van Trappen et al. 2003). In colorectal cancer, VEGFR-3 expression seems to be linked to a worse prognosis, suggesting that VEGF-C and VEGFR-3 form an axis (Martins et al 2013). Several mechanisms contribute to tumor development and progression through the VEGF-C/VEGFR-3 axis. For instance, stimulating the VEGF-C/VEGFR-3 axis encourages cancer cell invasion and metastasis (Su et al. 2007). Therefore, the VEGF-C/VEGFR-3 axis controls several cell processes that are important to the growth of cancer, such as proliferation, invasion, and resistance to chemotherapy.
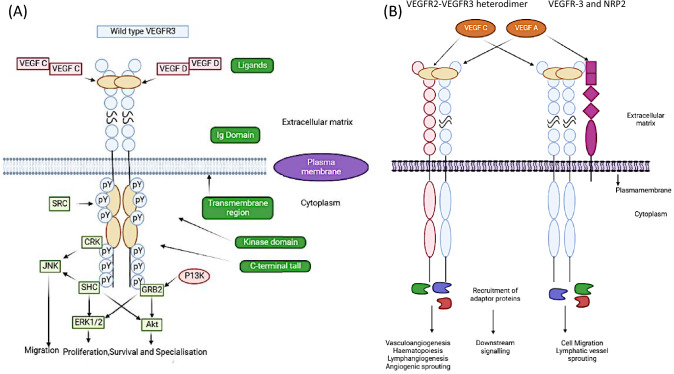
Signaling via VEGFR3 homodimers
An important role in lymphatic system development is played by the VEGF-R-3. The extracellular matrix-matured form of VEGFR-3 is generated by proteolytic cleavage at its fifth extracellular immunoglobulin-like domain, yielding two disulphide-linked peptides (Fig. 1A). Although the role of this cleavage is poorly understood, it is thought that it is essential for ligand binding and receptor stability at the cell membrane (Leppänen et al. 2013). The direct contact site for VEGFC is identified as the first three immunoglobulin-like domains of VEGFR3, although the remaining extracellular space is still necessary for efficient ligand-induced receptor stimulation and following downstream signaling (Leppänen et al. 2013). Humans express at least three distinct isoforms of VEGFR3, each of which has a somewhat different role in physiology. There are both a full-length isoform and a shortened variant of the receptor, with the shorter version lacking tyrosine residues that, when phosphorylated, may initiate downstream signaling of the receptor. The third form of VEGFR3, which is a soluble protein released by cells and acts to sequester VEGFC in the retina, is missing a large C-terminal coding region, a transmembrane domain (Dixelius et al. 2003; Hughes 2001). Homodimer detects and acts on the ligands VEGFC and VEGFD expressed by LECs (Sarkar et al. 2022). Generally, once a ligand is attached, the intracellular kinase domains become active, resulting in trans-autophosphorylation between the two kinase domains (Salameh et al. 2005). The VEGFR3 kinase domain, C-terminal tail, and juxtamembrane region all contain phosphotyrosine residues that activate intracellular signaling. Trans-autophosphorylation activates phosphatidylinositol-3-kinase (PI3K) and other members of the conserved PI3K/MAPK family, including AKT, ERK1/2, and JNK, which then recruit adaptor proteins such as Src homology and collagen (SHC), growth factor receptor bound protein 2 (GRB2), and CT10 Regulator of Kinase (CRK). The VEGFR3/VEGFC is essential for lymphatic system development when LECs first begin to branch from the cardinal vein (Su et al. 2007). Paracrine secretion of VEGFC at lymphangiogenesis hotspots results in a chemogradient that allows for spatial and temporal control of lymphatic artery development. CCBE1 is essential for the immobilization of Pro-VEGFC, which is required for its cofactor ADAMTS3 to proteolytically stimulate the ligand. Primary lymphoedema has been related to mutations in VEGFC, ADAMTS3, and CCBE1 because of the critical role each gene plays in VEGFC processing in humans (Le Guen et al. 2014). As a means of maintaining their endothelial cell identity, LECs rely on a positive feedback loop in which signaling through VEGFR3 keeps PROX1 expressed, which in turn regulates the expression of VEGFR3. For proper lymphatic valve development, localization, and maintenance, PROX1 collaborates with the transcription factor, FOXC2. To proceed, VEGFR3 must be activated before being degraded by EPSIN (Gauvrit et al 2018). Lymphangiogenesis relies on the transcription factor ETV2 because of its capacity to directly control VEGFR3 expression. Moreover, it is well established that integrin-linked kinase regulates VEGFR3 signaling during lymphatic vascular development. Vascular endothelial phosphatase is the only known regulatory phosphatase for VEGFR3, and it may suppress downstream VEGFR3 signaling in LECs. Although VEGFR3 is often associated with vascular cells, it has been found in non-vascular cells such as osteoblasts, neural progenitor cells, and macrophages (Monaghan et al. 2021; Sarkar et al. 2022).
Fig. 1.
VEGFR3 signaling. A Ligand binding to VEGFR3 and activation of downstream signaling pathways. B Endothelial and endothelial-derived cell lines are subject to the regulation of several biological processes by heterodimerization of VEGFR3 and VEGFR2 and activation by VEGFA or VEGFC. VEGFR3 homodimers associate with neuropilin 2 (NRP2), and the activation of both VEGFR3 and NRP2 by their respective ligands, VEGFA and VEGF, respectively, regulates VEGFR3 activity. During the process of angiogenesis and haematopoiesis, VEGFR3 is able to serve as a receptor for VEGFC ligand binding when it is combined with VEGFR2 in the form of a heterodimer. In addition to this, it has been demonstrated that NRP2 is a coreceptor for both VEGFR2 and VEGFR3, and it is capable of acting in response to VEGFA/C
Signaling via VEGFR3 heterodimers
For VEGFC ligand binding, VEGFR3 may form a heterodimer with VEGFR2 during angiogenesis and hematopoiesis (Fig. 1B). Extremely reduced haematopoiesis and enhanced vascular bed formation were seen in VEGFR-3 mutant aortic mesoderm explants grown from murine embryos compared to wild type (Hamada et al. 2000; Suzuki et al. 2005). An increased amount of VEGFC signaling through VEGFR2 has been hypothesized to halt angiogenesis and blood cell formation during embryogenesis (Hamada et al. 2000; Suzuki et al. 2005). In response to VEGF, vascular progenitors derived from embryonic stem cells that express VEGFR2 may be encouraged to differentiate into endothelial cells. The ability of VEGFC to stimulate endothelial differentiation in LECs is dependent on the heterodimerization of VEGFR2 and VEGFR3 (Hamada et al. 2000; Suzuki et al. 2005).
In addition to being a coreceptor for VEGFR2 and VEGFR3, neuropilin 2 (NRP2) has been demonstrated to respond to VEGFA and VEGFC (Fig. 1B). Human microvascular endothelial cells that express NRP2 are more likely to migrate and survive in response to both ligands. At the plasma membrane, VEGFR3 and NRP2 interact in response to VEGFC, mediating vascular sprouting in the lymphatic system. Lymphatic vessel branching and sprouting occur normally in Vegfr2+/Nrp2+/double heterozygous mice but not in Vegfr3+/Nrp2+/animals throughout development (Xu et al. 2010). CLP24 is a hypoxia-regulated transmembrane protein that is essential for lymphatic channel sprouting because of its interaction with VEGFR-2/3 (Saharinen et al. 2010).
In the absence of a ligand, some VEGFR dimerization may take place, resulting in attenuated downstream signaling. VEGFA/VEGFC is demonstrated to stimulate heterodimerization of VEGFR2 and VEGFR3 in early lymphatic progenitor cells and in emerging blood vessels, respectively. Dimers also tend to cluster together spatially near the developing tip of angiogenic sprouts when the ligand is present (Tvorogov et al. 2010).
Endothelial cells undergo differentiation during angiogenesis, becoming either the tip or stalk cells of growing blood arteries. Postnatally, animals lacking VEGFR-3 in their endothelium had elevated angiogenic branching and sprouting and a decrease in Notch signaling. Even in the presence of VEGFR2 inhibitors, VEGFR3 controls angiogenic sprouting, indicating that this function may not need collaboration between VEGFR2 and VEGFR3. Although VEGFR2 is expressly necessary for this process in the retina, it has been demonstrated that VEGFR3 modulation through the Notch pathway may enhance angiogenesis without the need for VEGFR2 signaling (Benedito et al. 2012; Tammela et al. 2008).
During embryonic development and angiogenesis, VEGFR2/VEGFR3 is shown to function as part of a complex that facilitates the vascular endothelial cell response to fluid sheer stress. VE-cadherin functions as an adapter protein by attaching to the transmembrane region of both VEGFR3 and VEGFR2 (Baeyens et al. 2015; Zarkada et al. 2015). Flow response in the aortic endothelium is demonstrated to be affected by VEGFR-3. Interestingly, it is speculated that VEGFR-3 can regulate VEGFR-2 signaling, which would imply a ligand-independent role for VEGFR-3 in the mechanosensitive complex that is responsible for blood vessel formation (Baeyens et al. 2015; Zarkada et al. 2015). In addition to VEGFC and VEGFD, integrin/SRC may trigger VEGFR3 signaling; however, this results in a distinct phosphorylation pattern. This further increases the already intricate VEGFR3 signaling pathway by indicating ligand-independent activities for VEGFR3 (Baeyens et al. 2015; Zarkada et al. 2015).
Mechanism of VEGF-C/VEGFR-3 signaling in tumor cells
The majority of cells that express VEGFRs are endothelial cells. When bound and activated by VEGF, VEGFRs take on a pivotal function in pathological conditions. Many tumor cells and the vascular microenvironment surrounding tumors express VEGFRs and VEGFs, suggesting an autocrine/paracrine involvement for these molecules (Monaghan et al. 2021; Sarkar et al. 2022; Miller and Sewell-Loftin 2022). Despite the fact that there is substantial evidence relating the expression of VEGF-C and VEGFR-3 to a reduced risk of developing particular malignancies (Table 1), the role of the VEGF-C/VEGFR-3 signaling in tumors is still poorly understood. It is shown that VEGF-C and VEGFR-3 form an axis in tumors, with VEGFR-3 expression being linked to worse survival rates in colon cancer (Sha et al. 2018). Similarly, VEGFR-3 expression is strongly correlated with each step of cervical cancer (Van Trappen et al 2003). Although several studies failed to uncover evidence of VEGFR-3 expression by cancer cells; future more robust and sensitive approaches may reconcile these conflicting results (Su et al. 2007). Kaposi sarcoma cells express VEGFR-3 (Weninger et al. 1999). Kaposi sarcoma cells treated to VEGF-C recombinant protein or C156S mutant VEGF-C recombinant protein, selective ligands and activators of VEGFR-3, showed increased tyrosine phosphorylation of VEGFR-3 despite the absence of an Flt-1 activation signature (Marchio et al. 1999). In Kaposi sarcoma, stimulation of VEGF-C/VEGFR-3 signaling is responsible for regulating cellular functions including proliferation and migration (Marchio et al. 1999).
Table 1.
VEGFR3 signaling regulation in tumors
| Tumor types | Key findings | References |
|---|---|---|
| Breast cancer | Infiltration of macrophages bearing VEGFR3 at tumor site during chemotherapy causing lymphangiogenesis by releasing cathepsin, leading to vegf-c upregulation by heparanase | Alishekevitz et al. (2016) |
| Inhibition of notch signaling or silencing of Notchc4/Dll3 decreased the function of tumor-derived TP53+-CD31+ endothelial cells under chemotherapy treatment in breast cancer through VEGFR3 pathway. Claiming, VEGFR3 causes micro-vessel formation through notch and VEGF signaling | Zhang et al. (2016) | |
| Breast tumor-bearing mice treated with thalidomide develop a decreased expression of VEGFR3, because tumor-associated macrophage accumulation is inhibited | Alishekevitz et al. (2014) | |
| Chloropyramine hydrochloride blocks the binding site of focal adhesion kinase and VEGFR3 in breast cancer growth in vivo | Adam et al. (2008) | |
| There was no significant relation between VEGFR-3 expression in breast cancer tissue and tumor grade P1/4 0.063 | Longatto Filho et al. (2005) | |
| Higher level of VEGF was detected when VEGF-C/VEGFR-3 axis activated | Su et al. (2006) | |
| Doxorubicin's efficacy is decreased in triple-negative breast cancer when VEGFR3 expression is high | Torres-Ruiz et al. (2023) | |
| Ovarian cancer | Silencing of forkhead box F2 FOX2 activates the VEGFR3 transcription to promote the lymphatic metastases of aggressive basal-like breast cancer cells | Wang et al. (2018) |
| The ascites and serum of human ovarian cancer patients contain high levels of soluble VEGFC. After VEGFC expression, H0C8 cells infiltrate para-aortic lymphatic vessels. IGR0V1 metastatic growth was inhibited by Cediranib, an inhibitor of vegfr1-3 and c-kit, as well as autocrine tumor growth in vitro | Decio et al. (2014) | |
| CSCs with CD133 + are more likely to undergo cell cycle arrest when VEGFR3 is inhibited. In cell cycle arrest, p-ERK, E2F1, and both BRCA1 and BRCA2 expression are reduced. Additionally, both in vitro and in vivo, inhibition of VEGFR3 and its resultant decrease in expression of BRCA1 and BRCA2 increased chemosensitivity. As a result, VEGFR3 is a promising therapeutic target for ovarian cancer | Lim et al. (2014) | |
| Renal cancer | The upregulation of Ang-2 elevates the VEGFR3 in renal cell cancer patient | Lampinen et al. (2017) |
| High level of VEGFR3 associated with high MIB-1 and low level of bcl2 promotes cancer growth in renal cell cancer | Virman et al. (2016) | |
| Pancreatic cancer | The inhibitor of c-terminal FAK decreased the expression of VEGFR3 in pancreatic cell line isogenic MCF7 | Gogate et al. (2014) |
| Combination of silencing three VEGFRs vegf1, 2, 3 with artificial microRNA has improved the apoptosis, reduced proliferation, and inhibition of epithelial mesenchymal transition in pancreatic ductal adenocarcinoma. It shows synergistic effect with chemotherapy | Huang et al. (2017) | |
| The invasiveness of pancreatic neuroendocrine tumors is controlled by PTEN through the dephosphorylation of VEGFR3 by DUSP19 | Chang et al. (2022) | |
| Lymphangiogenesis in pancreatic cancer cells is stimulated by hypoxia through BANCR's upregulation of the HIF-1/VEGF-C/VEGFR-3 pathway | Hao et al. (2022) | |
| Colorectal cancer | VEGFC and VEGFR3 promote lymphangiogenesis and metastases, reducing expression of CDH5, increasing permeability, and promoting trans endothelial migration | Tacconi et al. (2015) |
| Monoclonal antibody LY3022856/IMC-3C5 inhibits the VEGFR3 expression in advanced colorectal cancer. Clinical trial: NCT01288989 | Saif et al. (2016) | |
| The expression of VEGFR-3 in 425% of the cancer cells was associated with significantly poorer overall survival, but not with lymph-node metastasis or depth of tumor invasion These results suggest that VEGFs promote cancer growth not only by stimulating angiogenesis, but also by acting on receptors present on the cancer cells themselves | Witte et al. (2002) | |
| Choriocarcinoma | Galectin-1 promotes VEGFR3 expression by phosphorylating tyrosine kinase residue in trophoblast tumor BeWo cells | Fischer et al. (2009) |
| Cervical cancer | Regulation of VEGFC/VEGFR3 in tumor cell increases lymph-node metastases under hypoxic tumor environment | Chaudary et al. (2011) |
| Cervical carcinoma cells expressed VEGF-C and VEGFR3 in significant correlation throughout the different stages of carcinogenesis. This suggests an autocrine growth stimulation pattern by VEGFR-3 | Van Trappen et al. (2003) | |
| Lymphangiogenesis | VEGFR3/Flt4 is directly activated by Etsrp/Etv2 binding to their promoter/enhancer region | Davis et al. (2018) |
| Through Ca2+ signaling and ERK1/2-Akt signaling, miR-128 promotes lymphatic endothelial cell proliferation by inhibiting the expression of VEGFC and VEGFR3 | Zhou et al. 2018 | |
| Endometrial carcinoma | The immunohistochemistry of endometrial carcinoma specimens expressed the vegfa correlated with vegfr2 and VEGFR3 with increase in micro-vessel density by 17.2-fold and 21.9-fold, respectively | Wang et al. (2014) |
| Patients at risk for poor outcomes may have increased levels of VEGF-D and VEGFR-3 in their endometrial carcinomas, which may predict metastasis to lymph nodes as well as myometrial invasion. Further, VEGF-D and VEGFR-3 might be promising targets for new treatments in endometrial cancer | Yokoyama et al. (2003) | |
| Corneal neovascular formation | Promotion of VEGFR3 expression by the down regulation of peroxisome proliferator—activated receptor alpha PPARα in keratocytes elevate corneal neovascularization | Wang et al. (2020) |
| Lung cancer | Non-small cell lung cancer NSCLC and small cell lung cancer SCLC patients do not experience any significant effects from overexpression of VEGFC and VEGFR3/flt-1. While other VEGFs have detrimental effects on stage I NSCLC, they can be used as prognostic tools at earlier stages of the disease | Zhan et al. (2009) |
| The status of VEGF-C and VEGFR-3 in patients with T1 lung cancer may indicate survival rates | Kojima et al. (2005) | |
| The expression of VEGF-C and VEGFR-3 in patients with NSCLC may be an indicator of survival | Arinaga et al. (2003) | |
| VEGF-C/VEGFR-3 is related to lymphangiogenesis, angiogenesis, and lung cancer development in NSCLC. NSCLC patients with high VEGF-C expression may have a poor prognosis | Li et al. (2003) | |
| Inflammation, mobility, and invasiveness of cancer cells are enhanced by the VEGF-C/VEGFR-3 axis, which promotes tumor metastasis. In tumor tissues from various types of cancer, VEGFR-3 and VEGF-C expression levels were found to correlate with clinical metastasis and patient survival | Su et al. (2006) | |
| Gastric cancer | Thymidine phosphorylase increases the VEGFC expression in association with increased vessel density and proliferation index at the lymph node and cause of hepatic metastasis in gastric cancer patients | Zhang et al. (2014) |
| p38 MAPK selective inhibitor prevents the peritoneal dissemination of gastric cancer with downregulation of flt4 VEGFR3, CXC chemokine receptor-4, non-receptor spleen tyrosine kinase SYK, and the collagen α2IV COL4A2 | Graziosi et al. (2012) | |
| Through activation of lymphatic endothelial cell VEGFR3, cancerous cells produce VEGFC that induces lymphangiogenesis | Yonemura et al. (2001) | |
| An independent prognostic parameter for carcinoma-specific survival was identified as VEGFR-3 expression via a Cox multivariate regression analysis. There was a poor survival rate associated with the VEGF-C/VEGFR-3 axis in gastric cancer patients with lymph nodes positive | Jüttner et al. (2006) | |
| Cholangiocarcinoma | VEGFR3 lies upstream of endothelial nitric oxide synthase eNOS, which increases the micro-vessel density and promotes metastasis in cholangiocarcinoma | Suksawat et al. (2017) |
| Stimulation of platelet-derived growth factor DPDGF-D express high level of VEGFC and vegfa in cancer associated fibroblast CAF to interact with their receptors VEGFR3 and vegfr2, respectively, in lymphatic endothelial cells, resulting in the expansion of lymphatic vasculature and tumor intravasation in cholangiocarcinoma | Cadamuro et al. (2019) | |
| Leukemia | Inhibiting HL-60 and NB4 promyelocytic leukemia cells' hyperacetylation with Belinostat and 3-Deazaneplanocin A, in conjunction with the granulocytic differentiation inducer retinoic acid, which downregulates the expression of VEGFR3 and VEGFA | Valiulienė et al. (2020) |
| Leukemia cells respond to VEGF-C/VEGFR-3 stimulation to proliferate, to survive, and to resist chemotherapy. The VEGF-C/VEGFR-3 pathway offers new therapeutic targets for the treatment of subsets of acute leukemia | Dias et al. (2002) | |
| Squamous cell carcinoma | mRNA expression of VEGF-C with VEGFR3 and vegfr1 affects the tumor differentiation largely in laryngeal squamous cell carcinoma | Pentheroudakis et al. (2012) |
| Neuropilin 2 Nrp2 binds to the VEGF-C/VEGFR3 to participate in the tumor metastases of oral squamous cell carcinoma | Ong et al. (2017) | |
| Head and neck squamous cell carcinoma contain a high expression of VEGF-C/VEGFR-3 which appears to promote tumor growth, angiogenesis, and cancer cell proliferation. Consequently, lymphangiogenesis inhibitors may be effective therapeutics | Neuchrist et al. (2003) | |
| Prostate cancer | Malignant epithelium/cancer cells expressed significantly higher levels of VEGF-A, VEGF-C, and VEGFR-3 compared with adjacent benign epithelium Po0.01. The expression of VEGF-C or VEGFR-3 in the tumor area of patients in stage D was significantly higher than that of patients in stages A, B, or C Po0.01 | Yang et al. (2006) |
| VEGF-C/VEGFR-3 expression was associated with prostate cancer progression and regional lymph-node metastasis | Jennbacken et al. (2005) | |
| Prostate cancer cells with high levels of VEGFR-3 have an increased risk of biochemical recurrence when treated with radical prostatectomy; VEGFR-3 expression has been associated with tumor progression and may facilitate lymphatic spread of prostate carcinomas | Li et al. (2004) | |
| Mesothelioma | Malignant mesothelioma cell growth was significantly inhibited by agents targeting VEGF/Flt-1 and VEGF-C/VEGFR-3 autocrine loops | Masood et al. (2003) |
| Kaposi sarcoma | Kaposi sarcoma cells migrate and proliferate in response to VEGF-C and VEGFR-3 | Marchio et al. (1999) |
| Melanoma | VEGF-C in activating its receptor-3 and chemokine receptor-4 in melanoma adhesion | Hlophe and Joubert (2022) |
Involvement of the VEGF-C/VEGFR-3 signaling in the pathogenesis of malignant pleural mesothelioma (MPM) is also shown (Masood et al 2003). Cell proliferation factors VEGF-A/VEGFR-2 and VEGF-C/VEGFR-3 are both expressed by many different MPM cell lines. The inhibition of VEGF-C to disrupt the VEGF-C/VEGFR-3 axis dramatically reduced cell viability in MPM cells (Su et al 2006; Hoshida et al. 2006; Skobe et al. 2001). Several studies show that tumor VEGF-C production does not promote tumor development in experimental settings but rather a tumor metastasis (Su et al 2006; Hoshida et al. 2006; Skobe et al. 2001). However, other research contradicts this, showing that producing VEGFR-3-Ig in lung cancer or a VEGF-C-siRNA in mammary tumor (Chen et al. 2005; He et al. 2002) did not influence tumor development.
The expression of VEGFR-3 and its interaction with VEGF-C are shown in leukemia (Dias et al. 2002). Increases in the growth and survival of cells (as measured by higher Bcl-2/Bax ratios) were seen in response to VEGF-C and a mutant version of the molecule lacking the KDR-binding motif. On top of that, chemotherapeutic drugs, including cytarabine, daunorubicin, and etoposide, could not induce apoptosis in leukemic cells because of the VEGF-C/VEGFR-3 axis' protective effects. Taken as a whole, these findings suggested that the VEGF-C/VEGFR-3 signaling might be a useful treatment option for various forms of blood cancer. VEGF-C/VEGFR-3 axis promotes tumor cell metastasis in a wide variety of cancers, including breast, lung, colorectal, cervical, and prostate cancers (Su et al. 2006). Cancer cell invasion and metastasis mediated by VEGF-C/VEGFR-3 were also demonstrated to need activation of the Src-p38 MAPK-C/EBP signaling. Studies of tumor tissues from a wide range of malignancies revealed high levels of VEGFR-3/VEG-C expression, which is significantly related to clinical metastasis and survival, lending credence to the role of the VEGF-C/VEGFR-3 signaling in invasion and metastasis. Neuropilin 2 (Nrp2) binds to the VEGF-C/VEGFR3 to participate in the tumor metastases of oral squamous cell carcinoma (Ong et al. 2017). Monoclonal antibody LY3022856/IMC-3C5 inhibits the homodimerization and heterodimerization of VEGFR3 in advanced colorectal cancer. However, the trial concluded a very low efficacy of this monoclonal antibody. Clinical trial no is NCT01288989 (Saif et al. 2016). In vivo and in vitro studies, as well as evaluations of patient outcomes, were used to infer the molecular mechanism and function of the VEGF-C/VEGFR-3 axis.
The VEGF-C/VEGFR-3 signaling controls cancer growth by controlling lymphangiogenesis and angiogenesis
Embryonic lymphatic vessel development relies on VEGF-C signaling via VEGFR-3 (Moe et al. 2017). By encouraging the development of new lymphatic vessels, VEGF-C plays a crucial role in tumor lymphangiogenesis, which may help in cancer metastasis. A poor prognosis, lymph-node metastasis, lymphatic invasion, and distant metastases are all associated with elevated VEGF-C expression in a tumor (Coso et al. 2012). Orthotopic implantation of human breast cancer cells overexpressing VEGF-C in the mammary ductal pads of severe combined immunodeficiency mice increased tumor dissemination through lymphatic channels (Ball et al. 2007). However, a soluble VEGFR-3 fusion protein may be able to stop the spread of tumors. Recent studies have shown that lymphangiogenesis suppression occurs in the tumor and surrounding tissues of mice's orthotopic xenografts of human lung cancer cells. High-resolution imaging demonstrates that VEGF-C increases lymphatic metastasis by promoting the dissemination of cancer cells to lymph nodes (Jha et al. 2018). Regulation of VEGFC/VEGFR3 in tumor cells increases lymph-node metastases under a hypoxic tumor environment (Chaudary et al. 2011). VEGFC and VEGFR3 promote lymphangiogenesis and metastases, reducing expression of CDH5, increasing permeability, and promoting transendothelial migration in colorectal cancer (Tacconi et al. 2015). Breast tumor-bearing mice treated with thalidomide develop a decreased expression of VEGFR3, because tumor-associated macrophage accumulation is inhibited (Alishekevitz et al. 2014). It is established that VEGF-A is responsible for angiogenesis and that VEGF-C promotes lymphangiogenesis. Increased lymphatic vasculature, but not vascular anatomy, was seen in transgenic mice overexpressing VEGF-C in the skin. Although VEGF-C has been primarily studied for its role in lymphangiogenesis (Achen et al. 1998), additional research shows that it may have a role in the control of healthy and pathological angiogenesis, as well. Recent research has shown that angiogenic sprouts may be induced by VEGF-C through VEGFR-2 and VEGFR-3 heterodimers. Further evidence links VEGF-C and its receptor VEGFR-3 to cancer-related angiogenesis (Jeltsch et al. 2006). To combat angiogenesis in cases when VEGF or VEGFR-2 inhibitors have failed, targeting VEGFR-3 may be a viable option. Pan et al. (2009) provide evidence that VEGF-C stimulates angiogenesis via an RhoA-mediated pathway. Important phases in tumor growth include lymphangiogenesis and ongoing angiogenesis. A tumor, much like angiogenesis, may create its own network of lymphatics that interact with the lymphatic vessels in the surrounding tissue. However, clinical data show that lymphatic transport of tumor cells is the most common initial spreading strategy for many carcinomas, traveling through afferent lymphatics via the body's natural drainage channels. Cancer researchers have just lately begun to consider tumor-induced lymphangiogenesis. Extra lymphatic channels might be provided by the overproduction of lymphangiogenic factors, which could aid in the metastasis of tumor cells. The VEGF family includes VEGF-C and VEGF-D, both of which are shown to promote lymphatic metastasis and serve as critical regulators of lymphangiogenesis in vivo (Skobe et al. 2001). Through Ca2+ signaling and ERK1/2-Akt signaling, miR-128 promotes LECs’ proliferation by inhibiting the expression of VEGFC and VEGFR3 (Zhou et al. 2018). The lymphangiogenic factor that has been shown to stimulate the growth of lymphatic vessels inside and surrounding tumors is VEGF-C. Research has also demonstrated that tumors that overexpress VEGF-C stimulate lymphatic vessel formation by stimulating the VEGF-C/VEGFR-3 signaling in LECs, hence facilitating the spread of cancer via the lymphatic system (Skobe et al. 2001). It is previously believed that the general invasive qualities of tumors were responsible for the recruitment of additional lymphatics, but the data show that VEGF-C or VEGF-D, which are produced by tumors, actively attract these vessels (Su et al. 2006). Animal models in which the VEGF-C/VEGFR-3 axis was activated showed increased lymphatic spread of tumors (Skobe et al. 2001). Multiple studies have linked VEGF-C expression in human tumors to metastatic cancer in nearby lymph nodes (Skobe et al. 2001; Su et al 2006; Hanrahan et al. 2003; Koyama et al. 2003). The study investigated the role of VEGF-C in the activation of VEGF receptor-3 and chemokine receptor-4 in the adhesion of melanoma cells. The researchers found that VEGF-C plays a crucial role in promoting the adhesion of melanoma cells through the activation of VEGF receptor-3 and chemokine receptor-4. The study provides valuable insights into the molecular mechanisms underlying melanoma adhesion and may contribute to the development of new therapeutic strategies for the treatment of this disease (Hlophe and Joubert 2022). The study investigated the role of TGF-β1 signaling in inducing VEGF-C expression in gastric cancer and its impact on tumor-induced lymphangiogenesis. The results showed that TGF-β1 signaling can upregulate the expression of VEGF-C in gastric cancer cells, leading to increased lymphatic vessel density and lymph-node metastasis. The study provides new insights into the molecular mechanisms underlying gastric cancer progression and suggests that targeting VEGF-C may be a potential therapeutic approach for treating this disease (Pak et al. 2019) Silencing of FOX2 activates the VEGFR3 transcription to promote the lymphatic metastases of aggressive basal-like breast cancer cells (Wang et al. 2018). The ascites and serum of human ovarian cancer patients contain high levels of soluble VEGFC. After VEGFC expression, ovarian carcinoma cells infiltrate para-aortic lymphatic vessels (Decio et al. 2014). Lymphangiogenesis is increased both inside and surrounding tumors that overexpress VEGF-C. Lymphatic vessels are documented to function around the periphery of tumors but not within them, indicating that these vessels may play a critical role in the lymphatic dissemination of cancer (Gombos et al. 2005). Lymphangiogenesis research relies heavily on determining whether lymphatic capillaries inside a tumor may transport cancer cells to lymph nodes.
Conclusion
The VEGF-C/VEGFR-3 axis is crucial in tumorigenesis, because it facilitates lymphangiogenesis and cancer cell movement (Su et al 2006). The VEGF-C/VEGFR-3 axis may have a role in the initiation and progression of cancer. Unlike the well-studied VEGF-A/VEGFR-2 axis, the VEGF-C/VEGFR-3 signaling may cause cancer growth through various unknown molecular pathways. Understanding how cancer instructs macrophages in tumor formation and how VEGF-C/VEGFR-3 signaling infiltrates/stimulates them are equally crucial. This is because invading macrophages negatively affect cancer patients' prognosis and survival (Schoppmann et al. 2006). Tumor-associated macrophages are critical for angiogenesis and lymphangiogenesis during periods of inflammation. Both transdifferentiation and the insertion of macrophages into the endothelium layer and stimulation of the division of previously extant local LECs have been demonstrated to increase lymphangiogenesis by macrophages (Maruyama et al. 2005). Unpublished data from our lab support the idea that radiosensitizing lung tumors requires VEGFR-3-driven macrophage invasion. VEGF-C has a role in this invasion.
There is a pressing need for further study on the issue of nuclear localization of VEGFR-3 and other VEGF receptors. The EGFR family, like with many other receptor tyrosine kinases, is nuclear-localized and functions as a transcription cofactor to activate gene promoters. Evidence shows that EGFR nuclear translocation is an important step in carcinogenesis (Brand et al. 2013). We found that VEGF-C induced nuclear translocation of VEGFR-3 in lung adenocarcinoma cells and primary LECs (Su et al. 2007). More study is required to reveal the chemical mechanisms and physical functions that lead to this nuclear localization.
VEGF-C/VEGFR-3 axis therapy may help certain cancers. To research VEGF-C and VEGFR-3 modulators, future cancer treatments must target them. In particular, novel anticancer medicines targeting the VEGF-C/VEGFR-3 signaling may provide benefits beyond preventing lymphangiogenesis. Tumor lymphangiogenesis factors and specific biomolecules stimulated the lymphatic endothelium to have potential as biomarkers for the diagnosis of highly aggressive metastatic malignancies and as therapeutic targets for avoiding tumor metastasis. By controlling lymphangiogenesis and angiogenesis, VEGF-C signaling via VEGFR-2 and VEGFR-3 plays a crucial role in the cancer development. A number of cancers are linked to enhanced invasion and metastasis when the VEGF-C/VEGFRs axis is highly activated. The creation of novel medicines that inhibit VEGF-C signaling is potentially improving the efficacy of current anti-tumor treatments. Potential new targets for antiangiogenic and anti-lymphangiogenic treatments are approved for clinical use as a result of clinical and preclinical research results. Research on the efficacy of these experimental treatments in humans is now underway (Monaghan et al. 2021). Here, we discuss the emerging possibility of translating our understanding of the VEGF-C/VEGFRs axis' function in regulating biological characteristics into innovative therapeutic options for the most lethal elements of cancer, and we evaluate the recent developments in this area of research. VEGFR3 is a fascinating target for future study due to its roles in angiogenesis and lymphangiogenesis, and the association of different genetic variations of the gene with clinical diseases. Researchers need to carefully weigh the methods they choose to reveal the physiologic activities and disease molecular functions linked to VEGFR3, since these processes are complex and difficult to disentangle experimentally.
Acknowledgements
This work was supported by the Department of Science and Technology, Science and Engineering Research Board (SERB) - Research Scientist Scheme, Government of India for the research Grant to S. Vimalraj (Grant no. SB/SRS/2022-23/109/LS). The authors acknowledge the Researchers Supporting Project Number (RSPD2023R674), King Saud University, Riyadh, Saudi Arabia for funding this research work.
Author contributions
SV: conceptualization, methodology, writing—original draft preparation, supervision, and validation. KNGH, MR, PG, KP, SS, and DG: original draft preparation.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci USA. 1998;95(2):548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alishekevitz D, Gingis-Velitski S, Kaidar-Person O, Gutter-Kapon L, Scherer SD, Raviv Z, Merquiol E, et al. Thalidomide suppresses breast cancer tumor growth by inhibiting tumor-associated macrophage accumulation in breast tumor-bearing mice. Clin Genitourin Cancer. 2014;15(4):105302. doi: 10.1016/j.clgc.2015.12.014. [DOI] [Google Scholar]
- Alishekevitz D, Gingis-Velitski S, Kaidar-Person O, Gutter-Kapon L, Scherer SD, Raviv Z, Merquiol E, Ben-Nun Y, Miller V, Rachman-Tzemah C, Timaner M, Mumblat Y, Ilan N, Loven D, Hershkovitz D, Satchi-Fainaro R, Blum G, Sleeman JP, Vlodavsky I, Shaked Y. Macrophage-induced lymphangiogenesis and metastasis following paclitaxel chemotherapy is regulated by VEGFR3. Cell Rep. 2016;17(5):1344–1356. doi: 10.1016/j.celrep.2016.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y. Clinical significance of vascular endothelial growth factor c and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer. 2003;97(2):457–464. doi: 10.1002/cncr.11073. [DOI] [PubMed] [Google Scholar]
- Baeyens N, Nicoli S, Coon BG, Ross TD, Van den Dries K, Han J, Lauridsen HM, Mejean CO, Eichmann A, Thomas JL, Humphrey JD, Schwartz MA. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. Elife. 2015;4:e04645. doi: 10.7554/eLife.04645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol. 2007;177(3):489–500. doi: 10.1083/jcb.200608093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signaling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ, Wheeler DL. Nuclear EGFR as a molecular target in cancer. Radiother Oncol. 2013;108(3):370–377. doi: 10.1016/j.radonc.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Cadamuro M, Brivio S, Mertens J, Vismara M, Moncsek A, Milani C, Fingas C, et al. Platelet-derived growth factor-D Enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol. 2019;70(4):700–709. doi: 10.1016/j.jhep.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TM, Chu PY, Lin HY, Huang KW, Hung WC, Shan YS, Chen LT, Tsai HJ. PTEN regulates invasiveness in pancreatic neuroendocrine tumors through DUSP19-mediated VEGFR3 dephosphorylation. J Biomed Sci. 2022;29(1):92. doi: 10.1186/s12929-022-00875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudary N, Milosevic M, Hill RP. Suppression of vascular endothelial growth factor receptor 3 (VEGFR3) and vascular endothelial growth factor C (VEGFC) inhibits hypoxia-induced lymph node metastases in cervix cancer. Gynecol Oncol. 2011;123(2):393–400. doi: 10.1016/j.ygyno.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Chen Z, Varney ML, Backora MW, Cowan K, Solheim JC, Talmadge JE, Singh RK. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65(19):9004–9011. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- Coso S, Zeng Y, Opeskin K, Williams ED. Vascular endothelial growth factor receptor-3 directly interacts with phosphatidylinositol 3-kinase to regulate lymphangiogenesis. PLoS ONE. 2012;7(6):e39558. doi: 10.1371/journal.pone.0039558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Koenig AL, Lubert A, Chestnut B, Liu F, Desai SP, Winkler T, Pociute K, Choi K, Sumanas S. ETS transcription factor Etsrp/Etv2 is required for lymphangiogenesis and directly regulates Vegfr3/Flt4 expression. Dev Biol. 2018;440(1):40–52. doi: 10.1016/j.ydbio.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decio A, Taraboletti G, Patton V, Alzani R, Perego P, Fruscio R, Jürgensmeier JM, Giavazzi R, Belotti D. Vascular endothelial growth factor C promotes ovarian carcinoma progression through paracrine and autocrine mechanisms. Am J Pathol. 2014;184(4):1050–1061. doi: 10.1016/j.ajpath.2013.12.030. [DOI] [PubMed] [Google Scholar]
- Dias S, Choy M, Alitalo K, Rafii S. Vascular endothelial growth factor (VEGF)-C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood. 2002;99(6):2179–2184. doi: 10.1182/blood.v99.6.2179. [DOI] [PubMed] [Google Scholar]
- Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, Claesson-Welsh L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells (LECs) regulates tyrosine phosphorylation sites. J Biol Chem. 2003;278:40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- Duffy AM, Bouchier-Hayes DJ, Harmey JH (2000–2013) Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signaling by VEGF. In: Madame Curie Bioscience Database [Internet]. Landes Bioscience, Austin. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6482/
- Filho L, Adhemar AM, Costa SMA, Schmitt FC. VEGFR-3 expression in breast cancer tissue is not restricted to lymphatic vessels. Pathol Res Pract. 2005;201(2):93–99. doi: 10.1016/j.prp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Fischer I, Schulze S, Kuhn C, Friese K, Walzel H, Markert UR, Jeschke U. Inhibiton of RET and JAK2 Signals and upregulation of VEGFR3 phosphorylation in vitro by galectin-1 in trophoblast tumor cells BeWo. Placenta. 2009;30(12):1078–1082. doi: 10.1016/j.placenta.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gauvrit S, Villasenor A, Strilic B, Kitchen P, Collins MM, Marin-Juez R, Guenther S, Maischein HM, Fukuda N, Canham MA, Brickman JM, Bogue CW, Jayaraman PS, Stainier DYR. HHEX is a transcriptional regulator of the VEGFC/FLT4/PROX1 signaling axis during vascular development. Nat Commun. 2018;9:2704. doi: 10.1038/s41467-018-05039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogate PN, Kurenova EV, Ethirajan M, Liao J, Yemma M, Sen A, Pandey RK, Cance WG. Targeting the C-terminal focal adhesion kinase scaffold in pancreatic cancer. Cancer Lett. 2014;353(2):281–289. doi: 10.1016/j.canlet.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos Z, Xu X, Chu CS, Zhang PJ, Acs G. Peritumoral lymphatic vessel density and vascular endothelial growth factor C expression in early-stage squamous cell carcinoma of the uterine cervix. Clin Cancer Res. 2005;11(23):8364–8371. doi: 10.1158/1078-0432.CCR-05-1238. [DOI] [PubMed] [Google Scholar]
- Graziosi L, Mencarelli A, Santorelli C, Renga B, Cipriani S, Cavazzoni E, Palladino G, et al. Mechanistic role of P38 MAPK in gastric cancer dissemination in a rodent model peritoneal metastasis. Eur J Pharmacol. 2012;674(2–3):143–152. doi: 10.1016/j.ejphar.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Hamada K, Oike Y, Takakura N, Ito Y, Jussila L, Dumont DJ, Alitalo K. Suda T (2000) VEGF-C signaling pathways through VEGFR-2 AND VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood. 2000;96:3793–3800. doi: 10.1182/blood.V96.12.3793. [DOI] [PubMed] [Google Scholar]
- Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, Fox SB. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200(2):183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- Hao S, Han W, Ji Y, Sun H, Shi H, Ma J, Yip J, Ding Y. BANCR positively regulates the HIF-1α/VEGF-C/VEGFR-3 pathway in a hypoxic microenvironment to promote lymphangiogenesis in pancreatic cancer cells. Oncol Lett. 2022;24(6):422. doi: 10.3892/ol.2022.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94(11):819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- Hlophe YN, Joubert AM. Vascular endothelial growth factor-C in activating vascular endothelial growth factor receptor-3 and chemokine receptor-4 in melanoma adhesion. J Cell Mol Med. 2022;26(23):5743–5754. doi: 10.1111/jcmm.17571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP, Jain RK. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66(16):8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- Huang J, Mei H, Tang Z, Li J, Zhang X, Yixiang Lu, Huang F, Jin Q, Wang Z. Triple-AmiRNA VEGFRs inhibition in pancreatic cancer improves the efficacy of chemotherapy through EMT regulation. J Control Release. 2017;245:1–14. doi: 10.1016/j.jconrel.2016.11.024. [DOI] [PubMed] [Google Scholar]
- Hughes DC. Alternative splicing of the human VEGFGR-3/FLT4 gene as a consequence of an integrated human endogenous retrovirus. J Mol Evol. 2001;53:77–79. doi: 10.1007/s002390010195. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Karpanen T, Strandin T, Aho K, Lankinen H, Alitalo K. Vascular endothelial growth factor (VEGF)/VEGF-C mosaic molecules reveal specificity determinants and feature novel receptor binding patterns. J Biol Chem. 2006;281(17):12187–12195. doi: 10.1074/jbc.M511593200. [DOI] [PubMed] [Google Scholar]
- Jennbacken K, Vallbo C, Wang W, Damber J-E. Expression of vascular endothelial growth factor C (VEGF-C) and VEGF receptor-3 in human prostate cancer is associated with regional lymph node metastasis. Prostate. 2005;65(2):110–116. doi: 10.1002/pros.20276. [DOI] [PubMed] [Google Scholar]
- Jha SK, Rauniyar K, Jeltsch M. Key molecules in lymphatic development, function, and identification. Ann Anat. 2018;219:25–34. doi: 10.1016/j.aanat.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Jüttner S, Wiβmann C, Jöns T, Vieth M, Hertel J, Gretschel S, Schlag PM, Kemmner W, Höcker M. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24(2):228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- Kojima H, Shijubo N, Yamada G, Ichimiya S, Abe S, Satoh M, Sato N. Clinical significance of vascular endothelial growth factor-C and vascular endothelial growth factor receptor 3 in patients with T1 lung adenocarcinoma. Cancer. 2005;104(8):1668–1677. doi: 10.1002/cncr.21366. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Kaneko K, Akazawa K, Kanbayashi C, Kanda T, Hatakeyama K. Vascular endothelial growth factor-C and vascular endothelial growth factor-D messenger RNA expression in breast cancer: association with lymph node metastasis. Clin Breast Cancer. 2003;4(5):354–360. doi: 10.3816/cbc.2003.n.041. [DOI] [PubMed] [Google Scholar]
- Lampinen AM, Virman JP, Bono P, Luukkaala TH, Sunela KL, Kujala PM, Saharinen P, Kellokumpu-Lehtinen PLI. Novel angiogenesis markers as long-term prognostic factors in patients with renal cell cancer. Clin Genitourin Cancer. 2017;15(1):e15–24. doi: 10.1016/j.clgc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Leppänen VM, Tvorogov D, Kisko K, Prota AE, Jeltsch M, Anisimov A, Markovic-Mueller S, Stuttfeld E, Goldie KN, Ballmer-Hofer K, Alitalo K. Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc Natl Acad Sci USA. 2013;110(32):12960–12965. doi: 10.1073/pnas.1301415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Dong X, Wei Gu, Qiu X, Wang E. Clinical significance of co-expression of VEGF-C and VEGFR-3 in non-small cell lung cancer. Chin Med J. 2003;116(05):727–730. [PubMed] [Google Scholar]
- Li R, Younes M, Wheeler TM, Scardino P, Ohori M, Frolov A, Ayala G. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in human prostate. Prostate. 2004;58(2):193–199. doi: 10.1002/pros.10321. [DOI] [PubMed] [Google Scholar]
- Lian L, Li XL, Xu MD, Li XM, Wu MY, Zhang Y, Tao M, Li W, Shen XM, Zhou C, Jiang M. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer. 2019;19(1):183. doi: 10.1186/s12885-019-5322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Yang K, Barbie Taylor-Harding W, Wiedemeyer R, Buckanovich RJ. VEGFR3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of BRCA1 and BRCA2. Neoplasia (united States) 2014;16(4):343–353.e2. doi: 10.1016/j.neo.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhai E, Liao B, Xu L, Zhang X, Peng S, He Y, Cai S, Zeng Z, Chen M. Autocrine VEGF signaling promotes cell proliferation through a PLC-dependent pathway and modulates Apatinib treatment efficacy in gastric cancer. Oncotarget. 2017;8(7):11990–12002. doi: 10.18632/oncotarget.14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchio S, Primo L, Pagano M, Palestro G, Albini A, Veikkola T, Cascone I, Alitalo K, Bussolino F. Vascular endothelial growth factor-C stimulates the migration and proliferation of Kaposi’s sarcoma cells. J Biol Chem. 1999;274(39):27617–27622. doi: 10.1074/jbc.274.39.27617. [DOI] [PubMed] [Google Scholar]
- Martins SF, Garcia EA, Luz MA, Pardal F, Rodrigues M, Filho AL. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genomics Proteomics. 2013;10(2):55–67. [PubMed] [Google Scholar]
- Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115(9):2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood R, Kundra A, Zhu ST, Xia G, Pierluigi Scalia D, Smith L, Gill PS. Malignant mesothelioma growth inhibition by agents that target the VEGF and VEGF-C autocrine loops. Int J Cancer. 2003;104(5):603–610. doi: 10.1002/ijc.10996. [DOI] [PubMed] [Google Scholar]
- Miller B, Sewell-Loftin MK. Mechanoregulation of vascular endothelial growth factor receptor 2 in angiogenesis. Front Cardiovasc Med. 2022;8:804934. doi: 10.3389/fcvm.2021.804934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe K, Heidecke H, Dechend R, Staff AC. Dysregulation of circulating autoantibodies against VEGF-A, VEGFR-1 and PlGF in preeclampsia—a role in placental and vascular health? Pregnancy Hypertens. 2017;10:83–89. doi: 10.1016/j.preghy.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Monaghan RM, Page DJ, Ostergaard P, Keavney BD. The physiological and pathological functions of VEGFR3 in cardiac and lymphatic development and related diseases. Cardiovasc Res. 2021;117(8):1877–1890. doi: 10.1093/cvr/cvaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser A, Range K, York DM, Manuscript A. The small molecule chloropyramine hydrochloride (C4) targets the binding site of focal adhesion kinase and vascular endothelial growth factor receptor 3 and suppresses breast cancer growth in vivo. Bone. 2008;23(1):1–7. doi: 10.1021/jm900159g.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuchrist C, Erovic BM, Handisurya A, Fischer MB, Steiner GE, Hollemann D, Gedlicka C, Saaristo A, Burian M. Vascular endothelial growth factor c and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck J Sci Spec Head Neck. 2003;25(6):464–474. doi: 10.1002/hed.10235. [DOI] [PubMed] [Google Scholar]
- Ong HS, Gokavarapu S, Xu Q, Tian Z, Li J, Ji T, Zhang CP. Cytoplasmic neuropilin 2 is associated with metastasis and a poor prognosis in early tongue cancer patients. Int J Oral Maxillofac Surg. 2017;46(10):1205–1219. doi: 10.1016/j.ijom.2017.03.035. [DOI] [PubMed] [Google Scholar]
- Onimaru M, Yonemitsu Y. Angiogenic and lymphangiogenic cascades in the tumor microenvironment. Front Biosci (schol Ed) 2011;3(1):216–225. doi: 10.2741/s146. [DOI] [PubMed] [Google Scholar]
- Pak KH, Park KC, Cheong JH. VEGF-C induced by TGF-β1 signaling in gastric cancer enhances tumor-induced lymphangiogenesis. BMC Cancer. 2019;19(1):799. doi: 10.1186/s12885-019-5972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan MR, Chang TM, Chang HC, Su JL, Wang HW, Hung WC. Sumoylation of Prox1 controls its ability to induce VEGFR3 expression and lymphatic phenotypes in endothelial cells. J Cell Sci. 2009;122(Pt 18):3358–3364. doi: 10.1242/jcs.050005. [DOI] [PubMed] [Google Scholar]
- Peach CJ, Mignone VW, Arruda MA, Alcobia DC, Hill SJ, Kilpatrick LE, Woolard J. Molecular pharmacology of VEGF-A isoforms: binding and signaling at VEGFR2. Int J Mol Sci. 2018;19(4):1264. doi: 10.3390/ijms19041264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentheroudakis G, Nicolaou I, Kotoula V, Fountzilas E, Markou K, Eleftheraki AG, Fragkoulidi A, et al. Prognostic utility of angiogenesis and hypoxia effectors in patients with operable squamous cell cancer of the larynx. Oral Oncol. 2012;48(8):709–716. doi: 10.1016/j.oraloncology.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Helotera H, Miettinen J, Norrmen C, D'Amico G, Jeltsch M, Langenberg T, Vandevelde W, Ny A, Dewerchin M, Carmeliet P, Alitalo K. Claudin-like protein 24 interacts with the VEGFR-2 and VEGFR-3 pathways and regulates lymphatic vessel development. Genes Dev. 2010;24:875–880. doi: 10.1101/gad.565010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif MW, Knost JA, Gabriela Chiorean E, Kambhampati SRP, Danni Yu, Pytowski B, Qin A, Kauh JS, O’Neil BH. Phase 1 study of the anti-vascular endothelial growth factor receptor 3 monoclonal antibody LY3022856/IMC-3C5 in patients with advanced and refractory solid tumors and advanced colorectal cancer. Cancer Chemother Pharmacol. 2016;78(4):815–824. doi: 10.1007/s00280-016-3134-3. [DOI] [PubMed] [Google Scholar]
- Sarkar J, Luo Y, Zhou Q, Ivakhnitskaia E, Lara D, Katz E, Sun MG, Guaiquil V, Rosenblatt M. VEGF receptor heterodimers and homodimers are differentially expressed in neuronal and endothelial cell types. PLoS ONE. 2022;17(7):e0269818. doi: 10.1371/journal.pone.0269818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139(6):839–846. doi: 10.1016/j.surg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Shah AA, Kamal MA, Akhtar S. Tumor angiogenesis and VEGFR-2: mechanism, pathways and current biological therapeutic interventions. Curr Drug Metab. 2021;22(1):50–59. doi: 10.2174/1389200221666201019143252. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7(2):192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Su J-L, Yang P-C, Shih J-Y, Yang C-Y, Wei L-H, Hsieh C-Y, Chou C-H, Jeng Y-M, Wang M-Y, Chang K-J. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9(3):209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Su JL, Yen CJ, Chen PS, Chuang SE, Hong CC, Kuo IH, Chen HY, Hung MC, Kuo ML. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007;96(4):541–545. doi: 10.1038/sj.bjc.6603487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksawat M, Techasen A, Namwat N, Yongvanit P, Khuntikeo N, Titapun A, Koonmee S, Loilome W. Upregulation of endothelial nitric oxide synthase (ENOS) and its upstream regulators in opisthorchis viverrini associated cholangiocarcinoma and its clinical significance. Parasitol Int. 2017;66(4):486–493. doi: 10.1016/j.parint.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Watabe T, Kato M, Miyazawa K, Miyazono K. Roles of vascular endothelial growth factor receptor 3 signaling in differentiation of mouse embryonic stem cell-derived vascular progenitor cells into endothelial cells. Blood. 2005;105:2372–2379. doi: 10.1182/blood-2004-07-2547. [DOI] [PubMed] [Google Scholar]
- Tacconi C, Correale C, Gandelli A, Spinelli A, Dejana E, D’Alessio S, Danese S. Vascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion. Gastroenterology. 2015;148(7):1438–1451.e8. doi: 10.1053/j.gastro.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz S, Tormo E, Garrido-Cano I, Lameirinhas A, Rojo F, Madoz-Gúrpide J, Burgués O, Hernando C, Bermejo B, Martínez MT, Lluch A, Cejalvo JM, Eroles P. High VEGFR3 expression reduces doxorubicin efficacy in triple-negative breast cancer. Int J Mol Sci. 2023;24(4):3601. doi: 10.3390/ijms24043601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvorogov D, Anisimov A, Zheng W, Leppanen VM, Tammela T, Laurinavicius S, Holnthoner W, Helotera H, Holopainen T, Jeltsch M, Kalkkinen N, Lankinen H, Ojala PM, Alitalo K. Effective suppression of vascular network formation by combination of antibodies blocking VEGFR ligand binding and receptor dimerization. Cancer Cell. 2010;18:630–640. doi: 10.1016/j.ccr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Uemura A, Fruttiger M, D'Amore PA, De Falco S, Joussen AM, Sennlaub F, Brunck LR, Johnson KT, Lambrou GN, Rittenhouse KD, Langmann T. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog Retin Eye Res. 2021;84:100954. doi: 10.1016/j.preteyeres.2021.100954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiulienė G, Vitkevičienė A, Navakauskienė R. The epigenetic treatment remodel genome-wide histone H4 hyper-acetylation patterns and affect signaling pathways in acute promyelocytic leukemia cells. Eur J Pharmacol. 2020 doi: 10.1016/j.ejphar.2020.173641. [DOI] [PubMed] [Google Scholar]
- Van Trappen PO, Steele D, Lowe DG, Baithun S, Beasley N, Thiele W, Weich H, Krishnan J, Shepherd JH, Pepper MS. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different stages of cervical carcinogenesis. J Pathol. 2003;201(4):544–554. doi: 10.1002/path.1467. [DOI] [PubMed] [Google Scholar]
- Virman JP, Bono P, Luukkaala TH, Sunela KL, Kujala PM, Kellokumpu-Lehtinen PLI. Combined angiogenesis and proliferation markers’ expressions as long-term prognostic factors in renal cell cancer. Clin Genitourin Cancer. 2016;14(4):e283–e289. doi: 10.1016/j.clgc.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Wang J, Taylor A, Showeil R, Trivedi P, Horimoto Y, Bagwan I, Ewington L, Lam EWF, El-Bahrawy MA. Expression profiling and significance of VEGF-A, VEGFR2, VEGFR3 and related proteins in endometrial carcinoma. Cytokine. 2014;68(2):94–100. doi: 10.1016/j.cyto.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Wang QS, He R, Yang F, Kang LJ, Li XQ, Li Fu, Sun B, Feng YM. FOXF2 deficiency permits basal-like breast cancer cells to form lymphangiogenic mimicry by enhancing the response of VEGF-C/VEGFR3 signaling pathway. Cancer Lett. 2018;420:116–126. doi: 10.1016/j.canlet.2018.01.069. [DOI] [PubMed] [Google Scholar]
- Wang X, Tang L, Zhang Z, Li W, Chen Y. Keratocytes promote corneal neovascularization through VEGFr3 induced by PPARα-inhibition. Exp Eye Res. 2020;193:107982. doi: 10.1016/j.exer.2020.107982. [DOI] [PubMed] [Google Scholar]
- Weninger W, Partanen TA, Breiteneder-Geleff S, Mayer C, Kowalski H, Mildner M, Pammer J, Stürzl M, Kerjaschki D, Alitalo K, Tschachler E. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi's sarcoma tumor cells. Lab Invest. 1999;79(2):243–251. [PubMed] [Google Scholar]
- Witte D, Thomas A, Ali N, Carlson N, Younes M. Expression of the vascular endothelial growth factor receptor-3 (VEGFR-3) and its ligand VEGF-C in human colorectal adenocarcinoma. Anticancer Res. 2002;22(3):1463–1466. [PubMed] [Google Scholar]
- Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivée B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas JL, Koch AW, Alitalo K, Eichmann A, Bagri A. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol. 2010;188(1):115–130. doi: 10.1083/jcb.200903137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GL, Li LY. Counterbalance: modulation of VEGF/VEGFR activities by TNFSF15. Signal Transduct Target Ther. 2018;10(3):21. doi: 10.1038/s41392-018-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hong-Fei Wu, Qian L-X, Zhang W, Hua L-X, Mei-Lin Yu, Wang Z, Zheng-Quan Xu, Sui Y-G, Wang X-R. Increased expressions of vascular endothelial growth factor (VEGF), VEGF-C and VEGF receptor-3 in prostate cancer tissue are associated with tumor progression. Asian J Androl. 2006;8(2):169–175. doi: 10.1111/j.1745-7262.2006.00120.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Stephen Charnock-Jones D, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Sakamoto A, Sakamoto T, Maruyama H. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res. 2003;9(4):1361–1369. [PubMed] [Google Scholar]
- Yonemura Y, Fushida S, Bando E, Kinoshita K, Miwa K, Endo Y, Sugiyama K, Partanen T, Yamamoto H, Sasaki T. Lymphangiogenesis and the vascular endothelial growth factor receptor (VEGFR)-3 in gastric cancer. Eur J Cancer. 2001;37(7):918–923. doi: 10.1016/S0959-8049(01)00015-6. [DOI] [PubMed] [Google Scholar]
- Zarkada G, Heinolainen K, Makinen T, Kubota Y, Alitalo K. VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc Natl Acad Sci USA. 2015;112:761–766. doi: 10.1073/pnas.1423278112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan P, Wang J, Lv XJ, Wang Q, Qiu LX, Lin XQ, Li Ke Yu, Song Y. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol. 2009;4(9):1094–1103. doi: 10.1097/JTO.0b013e3181a97e31. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zheng Z, Shin YK, Kim KY, Rha SY, Noh SH, Chung HC, Jeung HC. Angiogenic factor thymidine phosphorylase associates with angiogenesis and lymphangiogenesis in the intestinal-type gastric cancer. Pathology. 2014;46(4):316–324. doi: 10.1097/PAT.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Zhang P, He D, Chen Z, Pan Q, Fangfang Du, Zang X, Wang Y, et al. Chemotherapy enhances tumor vascularization via notch signaling-mediated formation of tumor-derived endothelium in breast cancer. Biochem Pharmacol. 2016;118:18–30. doi: 10.1016/j.bcp.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Zhou J, He Z, Guo Le, Zeng J, Liang P, Ren L, Zhang M, Zhang P, Huang X. MiR-128-3p directly targets VEGFC/VEGFR3 to modulate the proliferation of lymphatic endothelial cells through Ca2+ signaling. Int J Biochem Cell Biol. 2018;102:51–58. doi: 10.1016/j.biocel.2018.05.006. [DOI] [PubMed] [Google Scholar]