Abstract
BRAT1 biallelic variants are associated with rigidity and multifocal seizure syndrome, lethal neonatal (RMFSL), and neurodevelopmental disorder associating cerebellar atrophy with or without seizures syndrome (NEDCAS). To date, forty individuals have been reported in the literature. We collected clinical and molecular data from 57 additional cases allowing us to study a large cohort of 97 individuals and draw phenotype-genotype correlations. Fifty-nine individuals presented with BRAT1-related RMFSL phenotype. Most of them had no psychomotor acquisition (100%), epilepsy (100%), microcephaly (91%), limb rigidity (93%), and died prematurely (93%). Thirty-eight individuals presented a non-lethal phenotype of BRAT1-related NEDCAS phenotype. Seventy-six percent of the patients in this group were able to walk and 68% were able to say at least a few words. Most of them had cerebellar ataxia (82%), axial hypotonia (79%) and cerebellar atrophy (100%). Genotype-phenotype correlations in our cohort revealed that biallelic nonsense, frameshift or inframe deletion/insertion variants result in the severe BRAT1-related RMFSL phenotype (46/46; 100%). In contrast, genotypes with at least one missense were more likely associated with NEDCAS (28/34; 82%). The phenotype of patients carrying splice variants was variable: 41% presented with RMFSL (7/17) and 59% with NEDCAS (10/17).
Subject terms: Paediatric neurological disorders, Disease genetics, Genetic testing
Introduction
BRCA1-associated protein required for ATM activation-1 (BRAT1) encodes a nuclear protein that interacts with the tumor suppressor protein BRCA1 (breast cancer 1) and ATM (ataxia telangiectasia mutated). It plays a role in DNA repair, particularly through ATM, a protein necessary for the early response to double-stranded DNA degrading agents, such as ionizing radiation. Ionizing radiations induce immediate phosphorylation of the Ser1981 residue of ATM, resulting in its activation [1]. BRAT1, which binds to ATM at double-strand breaks, is required for ATM activation [1]. Further functional studies showed that BRAT1 also interacts with the catalytic subunit of the DNA-dependent protein kinase DNA-PK (DNA-PKcs), the major player in DNA double-strand break repair [2].
BRAT1 is also involved in the cell cycle and growth, through its interaction with the protein SMC1 and its contribution to the stability and regulation of mTOR [2, 3]. In addition, BRAT1 has a role in apoptosis by the production of reactive oxygen species [4].
Biallelic BRAT1 variants were first described by Puffenberger et al. in 2012, after sequencing the exome of two individuals from an endogamous population of Amish origin in Pennsylvania. These individuals had a combination of severe drug-resistant epilepsy, limb rigidity, brain injury, and early death [5]. In 2015, Hanes et al. reported a patient with compound heterozygous BRAT1 variants and a less severe clinical phenotype characterized by global developmental delay and cerebellar atrophy [6]. Biallelic BRAT1 variants have therefore been associated with two distinct clinical pictures: BRAT1-related rigidity and multifocal seizure syndrome (RMFSL; MIM 614498) and BRAT1-related neurodevelopmental disorder associating cerebellar atrophy with or without seizures syndrome (NEDCAS; MIM 618056). A correlation between the severity of the disorder and the type of BRAT1 mutations has been suggested, with biallelic truncating variants appearing to be associated with the more severe phenotype [7, 8].
So far, 40 individuals from 29 families have been described with BRAT1 biallelic variants [5, 6, 8–29]. We report here a cohort of 57 additional individuals from 45 families, describe the clinical features of BRAT1-related disorders and discuss genotype-phenotype correlations.
Subjects and methods
Patients
Individuals with biallelic deleterious BRAT1 variants were collected from Australia, Belgium, Canada, France, Germany, Italy, Iran, Israel, the United Kingdom, Kazakhstan, Saudi Arabia, and the USA. Clinicians and biologists from the different centers were contacted through GeneMatcher [30] and at European congresses. Clinical and genetic data were transmitted by the referring clinicians in a detailed table (Supplementary data S1). Written informed consent was obtained for genetic testing and the use of photographs (if applicable).
In addition, we reviewed published clinical data [5, 6, 8–24, 26, 27, 31] to compare the phenotype to the one described in our patients (Supplementary data S2). The literature review was performed on Pubmed with the keywords “BRAT1” and “BAAT1” for articles published before March 31, 2022.
Individuals 38, 39, and 40 were briefly reported by Valence et al. [7] and individuals 19 and 20 by Cornet et al. [32]. Since we obtained an updated and detailed clinical description of these 5 cases from the referring clinicians, they were included in the current cohort.
Individuals were divided into two subgroups according to their phenotype. Patients with at least two of the following three criteria: (i) no psychomotor development (no words and no sitting); (ii) early-onset (<6 months) and/or drug-resistant epilepsy and (iii) early death (<3 years) were considered to have BRAT1-related RMFSL phenotype. All other patients were considered to have a BRAT1-related NEDCAS phenotype.
Methods
BRAT1 variants were identified by gene panel or exome sequencing (ES) and confirmed by targeted Sanger sequencing. Targeted Sanger sequencing of coding exons 1–14 of the BRAT1 gene was first performed in one family (Family 1). In the majority of cases, parental testing confirm that the variants found in the probands were in trans. For patient P23, the c.566dup; p.(Asp190Ter) variant occurred de novo. For two patients from the literature (PL5 and PL15) who were siblings of molecularly confirmed affected patients (PL4 and PL16), sequencing was not performed. However, they were considered to carry the familial variants.
BRAT1 variants are given according to mRNA reference number NM_152743.4. Genomic position (hg19) was determined by Mutalyzer. All the bioinformatics scores were obtained from Mobidetails interface (CADD (v1.6), Polyphen-2, SIFT scores, and ClinVar (v20230410) database), SeattleSeq Annotation138 website (for GERP and Grantham scores), GnomAD database (v3.1.2), HGMD database, LOVD database and PROVEAN web server. The effect on splicing was analyzed using SPIP (v2.1), splice AI (v1.3) scores and the UCSC database (Supplementary data S3). The graphical representation of the protein variants was performed with the IBS 1.0.3 software.
In our study, frameshifts and nonsense variants were considered as predicted loss-of-function variants with amorphous effects. Splice variants were considered separately as their amorphous or hypomorphic character is more challenging to assess. The variants with supportive splicing predictions by Splice AI (>0.2) and Spip were all considered in that group as affecting splicing. This corresponds to the following variations: c.431-10_431- 7delCCCTinsTGGGTAGGG; p.(?), c.803 G > A; p.(Arg268His), c.803+1 G > C; p.(?); c.925_930del;p.(Pro309_Gln310del), c.1015+2 T > C; p.(?), c.1134+1 G > A; p.(?), c.1395 G > C; p.(Thr465 = ), c.1395 G > A; p.(Thr465 = ), c.1498+1 G > A; p.(?), c.1499-1 G > T, p.(Glu500Alafs*36).
As SPIP and SpliceAI were not consistent to predict splicing effect of c.358 C > T; p.(Arg120Cys), c.458 A > C; p.(Gln153Pro) and c.(1564 G > A); p.(Glu522Lys) variants, they were considered as missense variants.
Results
Clinical data
Ninety-seven individuals belonging to 74 unrelated families were identified with BRAT1 biallelic variants. Forty-three patients were males (44%) and 52 were females (54%). Sex was unknown for two previously published patients. The median age at last follow-up was 20 months (6 days - 28 years).
The families were of Latin America, North Africa, Sub-Saharan Africa, Middle East, East and Southeast Asia, and Caucasian descent. Among the 74 families, 29 were consanguineous (39%).
BRAT1-related RMFSL (Table 1)
Table 1.
Clinical data of the 97 patients with BRAT1 biallelic variants.
| RMFSL | NEDCAS | Total | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Literature | Total | Cohort | Literature | Total | Cohort | Literature | Total | |||||||||||
| Epidemiological data | Number of families | 25/45 | 56% | 20/29 | 69% | 45/74 | 61% | 10/45 | 44% | 9/29 | 31% | 29/74 | 39% | 45/74 | 61% | 29/74 | 39% | 74 families | |
| Number of patients | 31/57 | 54% | 28/40 | 70% | 59/97 | 61% | 26/57 | 46% | 12/40 | 30% | 38/97 | 39% | 57/97 | 61% | 40/97 | 39% | 97 patients | ||
| Prenatal features | Prenatal features | 15/30 | 50% | 7/14 | 50% | 22/44 | 50% | 1/21 | 5% | 0/9 | 0% | 1/30 | 3% | 16/51 | 31% | 7/23 | 30% | 23/74 | 31% |
| Hydramnios | 2/30 | 7% | 0/14 | 0% | 2/44 | 5% | 0/21 | 0% | 0/9 | 0% | 0/30 | 0% | 2/51 | 4% | 0/23 | 0% | 2/74 | 3% | |
| Oligohydramnios | 1/30 | 3% | 1/14 | 7% | 2/44 | 5% | 1/21 | 5% | 0/9 | 0% | 1/30 | 3% | 2/51 | 4% | 1/23 | 4% | 3/74 | 4% | |
| Abnormal movements | 8/30 | 27% | 6/14 | 43% | 14/44 | 32% | 0/21 | 0% | 0/9 | 0% | 0/30 | 0% | 8/51 | 16% | 6/23 | 26% | 14/74 | 19% | |
| IUGR | 9/30 | 30% | 0/14 | 0% | 9/44 | 20% | 0/21 | 0% | 0/9 | 0% | 0/30 | 0% | 9/51 | 18% | 0/23 | 0% | 9/74 | 12% | |
| Developmental stages | No developmental milestones | 31/31 | 100% | 28/28 | 100% | 59/59 | 100% | 0/26 | 4% | 0/12 | 0% | 0/38 | 0% | 32/57 | 56% | 27/40 | 68% | 60/97 | 62% |
| Walk acquisition | − | − | 0/59 | 0% | 21/26 | 81% | 8/12 | 67% | 29/38 | 76% | 21/56 | 38% | 8/39 | 21% | 29/97 | 30% | |||
| Only few steps | 8/21 | 38% | 0/8 | 0% | 8/29 | 28% | 8/21 | 38% | 0/8 | 0% | 8/29 | 28% | |||||||
| Only with support | 11/21 | 52% | 4/8 | 50% | 15/29 | 52% | 11/21 | 52% | 4/8 | 50% | 15/29 | 52% | |||||||
| Ataxic gait | 17/21 | 81% | 3/4 | 75% | 20/25 | 80% | 17/21 | 81% | 3/4 | 75% | 20/25 | 80% | |||||||
| Language | − | − | 0/59 | 0% | 17/26 | 65% | 9/12 | 75% | 26/38 | 68% | 17/55 | 31% | 9/39 | 23% | 26/97 | 27% | |||
| Use few words | 6/14 | 43% | 5/6 | 83% | 11/20 | 55% | 6/14 | 43% | 5/6 | 83% | 11/20 | 55% | |||||||
| Use short sentences | 6/14 | 43% | 1/6 | 17% | 7/20 | 35% | 6/14 | 43% | 1/6 | 17% | 7/20 | 35% | |||||||
| Dysarthria | 8/10 | 80% | 6/6 | 100% | 14/16 | 88% | 8/10 | 80% | 6/6 | 100% | 14/16 | 88% | |||||||
| Intellectual disability | 26/26 | 100% | 11/12 | 92% | 37/38 | 97% | 28/28 | 100% | 12/13 | 92% | 40/41 | 98% | |||||||
| Epilepsy | Seizures | 31/31 | 97% | 28/28 | 100% | 59/59 | 100% | 2/26 | 8% | 4/12 | 33% | 6/38 | 16% | 33/57 | 58% | 32/40 | 80% | 65/97 | 67% |
| Age of onset (median) | 3 days (D0-M15) | 1 day (D0-M8) | 1 day (D0-M15) | 4 years (Y3-Y5) | 2,5 years (M3-Y13) | 3 years 1 month (M3-Y13) | 10 days (D0-Y5) | 1 day (D0-Y13) | 3,5 days (D0-Y13) | ||||||||||
| Pharmacoresistance | 25/28 | 89% | 26/26 | 100% | 51/54 | 94% | 1/2 | 50% | 3/4 | 75% | 4/6 | 67% | 26/30 | 87% | 29/30 | 97% | 55/60 | 90% | |
| Physical examination | Age at last exam (median) | 80 days | 5 months | 90 days | 7 years 7 months | 6 years 1/2 | 7 years 3 months | 3 years 1/2 | 1 year 1month | 1 year 8 months | |||||||||
| Microcephaly | 20/23 | 87% | 22/23 | 96% | 42/46 | 91% | 6/22 | 27% | 5/11 | 45% | 11/33 | 33% | 26/45 | 58% | 27/34 | 79% | 53/79 | 67% | |
| Dysmorphism | 13/26 | 50% | 17/22 | 77% | 30/48 | 63% | 9/20 | 45% | 4/9 | 44% | 13/29 | 45% | 22/46 | 48% | 21/31 | 68% | 43/77 | 56% | |
| Hypotonia | 15/30 | 50% | 14/26 | 54% | 29/56 | 52% | 17/20 | 85% | 6/9 | 67% | 23/29 | 79% | 32/50 | 64% | 20/35 | 57% | 52/85 | 61% | |
| Spasticity/hypertonia | 27/30 | 90% | 26/27 | 96% | 53/57 | 93% | 6/26 | 23% | 5/10 | 50% | 11/36 | 31% | 33/56 | 59% | 31/37 | 84% | 64/93 | 69% | |
| Ophtalmological features | Optic atrophy | 3/13 | 23% | 3/4 | 75% | 6/17 | 35% | 3/22 | 14% | 3/7 | 43% | 6/29 | 21% | 6/35 | 17% | 6/11 | 55% | 12/46 | 26% |
| Retinopathy | 1/13 | 8% | 0/4 | 0% | 1/17 | 6% | 7/22 | 32% | 1/7 | 14% | 8/29 | 28% | 8/35 | 20% | 1/11 | 9% | 9/46 | 20% | |
| Nystagmus | 1/6 | 17% | 0/5 | 0% | 1/11 | 9% | 15/19 | 79% | 8/9 | 89% | 23/28 | 82% | 16/25 | 64% | 8/14 | 57% | 24/39 | 62% | |
| Brain MRI | Normal | 7/29 | 24% | 15/25 | 60% | 22/54 | 41% | 0/26 | 0% | 0/11 | 0% | 0/37 | 0% | 7/55 | 13% | 15/36 | 42% | 22/91 | 24% |
| Cerebellar atrophy | 8/29 | 28% | 6/25 | 24% | 14/54 | 26% | 26/26 | 100% | 11/11 | 100% | 37/37 | 100% | 34/55 | 62% | 17/36 | 47% | 51/91 | 56% | |
| Cerebral atrophy | 16/29 | 55% | 15/25 | 60% | 31/54 | 57% | 0/26 | 0% | 0/11 | 0% | 0/37 | 0% | 16/55 | 29% | 15/36 | 42% | 31/91 | 34% | |
| Delayed myelinisation | 6/29 | 21% | 6/25 | 24% | 12/54 | 22% | 0/26 | 0% | 3/11 | 27% | 3/37 | 8% | 6/55 | 11% | 9/36 | 25% | 15/91 | 16% | |
| CC anomalies | 7/29 | 24% | 6/25 | 24% | 13/54 | 24% | 5/26 | 19% | 1/11 | 9% | 6/37 | 16% | 12/55 | 22% | 7/36 | 19% | 19/91 | 21% | |
| Survival | Death | 28/31 | 87% | 27/28 | 93% | 55/59 | 93% | 0/26 | 0% | 0/12 | 0% | 0/38 | 0% | 28/57 | 47% | 27/40 | 65% | 55/97 | 57% |
| Age of death (median) | 105 days (D11-Y23) | 150 days (D6-Y5M9) | 113 days (D6-Y23) | — | — | — | 105 days (D11-Y23) | 150 days (D6-Y5M9) | 113 days (D6-Y23) | ||||||||||
IUGR intra uterine growth retardation, D day, M month, Y year, CC corpus callosum.
Fifty-nine individuals from 45 families were included in this group. Twenty-seven were males and 30 were females. Consanguinity was noted in 19 families (19/45; 42%). The median age at last examination was 3 months (6 days - 14 years).
Prenatal signs were found in 50% of patients (22/44) with abnormal movements reported by the mother in 14 individuals (14/44; 32%), intra-uterine growth retardation (IUGR) in 9 (9/44; 20%), hydramnios in two (2/44; 5%) and oligohydramnios in two others (2/44; 5%). Ten individuals were born prematurely (10/48; 21%), including one at 27 weeks of gestation +6 days. Congenital microcephaly was reported in 14 individuals (14/49; 29%), axial hypotonia in 8 (8/22; 36%), and peripheral hypertonia in 16 (16/22; 73%).
From a neurodevelopmental perspective, these children had not acquired any psychomotor milestones (59/59; 100%). Of note, two of them showed initial psychomotor development before regression (Patients 24 and L26). Early death occurred in 54 patients (54/59; 92%) with a mean age at death of 274 days (median: 112 days; 6 days – 5 years). One patient died at age 23 (Patient 14). Four individuals in this cohort were still alive at the time of the study, two of them being less than one month old.
All individuals had epilepsy (59/59; 100%). Seizures were mostly drug-resistant (51/54; 94%). The mean age of onset was 39 days (median: 1 day) ranging from in utero to 15 months. For patient 7, who was born prematurely, epilepsy was considered to start at day 1 corrected age.
Brain magnetic resonance imaging (MRI) showed signs of cerebral atrophy in 57% of the cases (31/54). Other brain anomalies were cerebellar atrophy (14/54; 26%), abnormalities of the corpus callosum (13/54; 24%), and delayed myelination (12/54; 22%) (Fig. 1). One patient had a pattern of brain injury which could be related to neonatal hypoxic-ischemic injury (Patient 12). Brain MRIs were normal in 22 patients (22/54; 41%). Six patients presented signs of optic nerve injury (6/17; 35%).
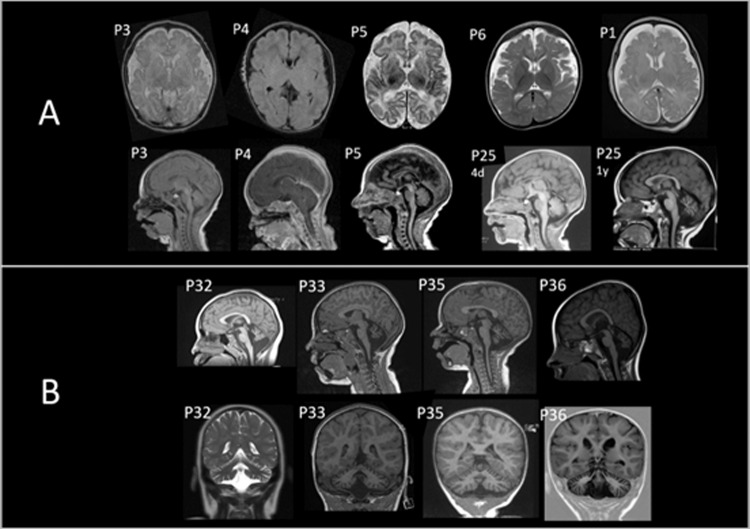
Fig. 1. Brain magnetic resonance image (MRI) of individuals with biallelic BRAT1 variants.
A Patients with RMFSL (P1, P3, P4, P5, P25). Note cerebral atrophy and enlarged subarachnoid spaces on axial and sagittal planes. B Patients with NEDCAS (P32, P33, P35, P36). Note cerebellar atrophy on sagittal and coronal planes.
On clinical examination, most individuals had microcephaly (42/46; 91%) and peripheral hypertonia or spasticity (53/57; 93%). Axial hypotonia was described in 52% of them (29/56). Two-thirds of the patients had dysmorphic features (30/48; 63%). The most common findings included micrognathia (7/30), round face (5/30), low-set ears (4/30), bulbous nose (4/30), and thick lips (4/30). Photographs are shown in Fig. 2.
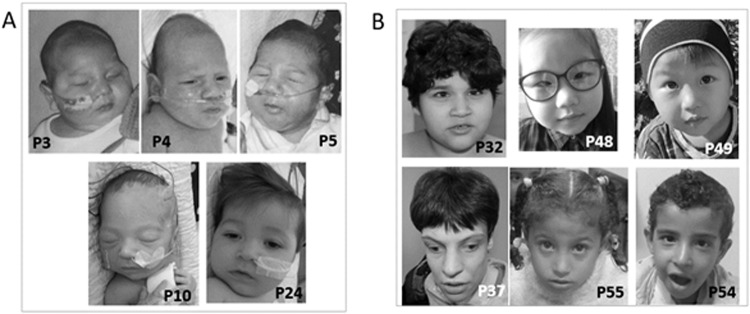
Fig. 2. Photographs of individuals with BRAT1-related disorders.
A Patients from RMFSL group. Coarse facies are noted without specific dysmorphic features. B Patients from NEDCAS group. A triangular face, a high nasal bridge and strabismus are noted in some patients.
Other associated findings were cardiac anomalies in seven individuals (7/59; 12%) and unilateral clubfoot in four patients (4/59; 7%).
BRAT1-related NEDCAS (Table 1)
Thirty-eight patients from 29 families were included in this group. Sixteen were males and 22 were females. Consanguinity was noted in one-third of the cases (10/29; 34%). The median age at last clinical examination was 7 years and 3 months (15 months - 28 years).
There were no prenatal signs, except in one individual who had oligohydramnios (1/30; 3%). Half of the newborns had neonatal hypotonia (14/27; 52%) and one had neonatal microcephaly (1/29; 3%).
All patients showed developmental delay and mild to severe intellectual disability, except one described as borderline. Almost all of them have acquired the sitting position (36/38), and the two remaining ones can sit with support. Seventy-six percent acquired walking (29/38). Among them, 28% were able to walk only a few steps (8/29), and 52% required support (15/29). Language skills were present in 26 patients (26/38; 68%), restricted to a few words or short sentences in 18 of them. Four individuals from two families acquired almost normal language late in life (Family 37 and Family L27). Fourteen individuals had dysarthria (14/16; 88%).
Epilepsy occured in 6 individuals (6/38; 16%). The mean age of onset was 4 years and 5 months (median: 3 years 1 month) ranging from 3 months to 13 years. Brain MRIs showed cerebellar atrophy in all patients (37/37; 100%) and abnormalities of the corpus callosum in six (6/37;16%) (Fig. 1).
Facial dysmorphism was reported in 13 individuals (13/29; 45%) (Fig. 2). The most common findings included long face (7/13), low-set ears (5/13), short philtrum (4/13), retrognathism (3/13), and prominent nose (2/13). Mean height was –0,39 SD (standard deviation) with 6 patients under −2 SD (6/27; 22%). Microcephaly (occipitofrontal circumference, OFC < −2 SD) was observed in 11 patients (11/33; 33%); hypotonia was noted in 23 patients (23/29; 79%) and hypertonia of the limbs in 11 patients (11/36; 31%). Ataxia was present in 31 individuals (31/35; 89%) and described as progressively resolutive in two.
Ophthalmological investigation revealed optic nerve injury in 6 patients (6/29; 21%) and retinopathy in 9 (9/29; 31%). Nystagmus was reported in 23 patients (23/28; 82%), oculomotor apraxia in 6 (6/17; 35%) and strabismus in 8 (8/16; 50%).
None of the patients in this group had died at the time of publication.
Molecular findings
(Fig. 3) BRAT1 variants were compound heterozygous in 46 families (46/97; 47%) and homozygous in 51 families (51/97; 53%).
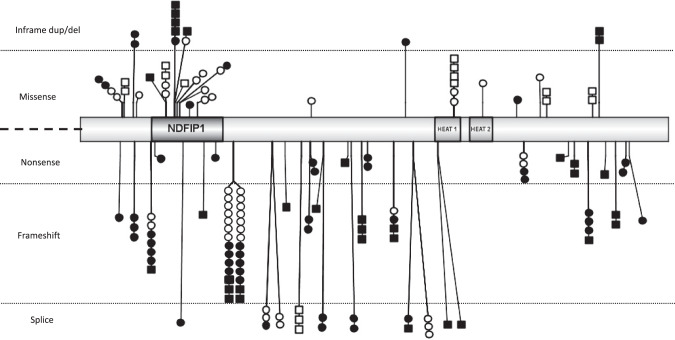
Fig. 3. Graphical representation of BRAT1 variants (IBS 1.0.3).
Missense and in-frame deletions are above; nonsense, frameshift and splice variants are below. Variants in black were identified in patients with RMFSL phenotype, those in white in patients with NEDCAS. Each square represents a patient with a homozygous variant, each circle a patient with a heterozygous one. Note that all the patients with a homozygous loss-of function variant are presenting a RMFSL phenotype and that the only one patient with a homozygous missense variant and a severe phenotype may have a second associated etiology. NDFIP1, NEDD4 family interacting protein 1; HEAT1/HEAT2, protein tandem repeat structural motif found in the four proteins: Huntingtin, Elongation factor 3 (EF3), protein phosphatase 2 A (PP2A) and TOR1.
In addition to the 28 variants and the deletion of 2 exons previously published, we identified 28 new variants in our cohort, including 12 missenses, 5 nonsense, 4 frameshift, 4 in-frame deletions, 1 in-frame duplication, and 2 intronic variants.
Nineteen BRAT1 variants were recurrent: c.638dup; p.(Val214Glyfs*189) was found in 20 families, c.294dup; p.(Leu99Thrfs*92) in seven families; c.359 G > A; p.(Arg120His), c.393_422dup; p.(Gln132_Ala141dup) and c.1564 G > A; p.(Glu522Lys) in four families; c.803 G > A; p.(Arg268His), c.925_930del; p.(Pro309_Gln310del), c.1313_1314del; p.(Gln438ArgfsTer51) and c.2125_2128del; p.(Phe709ThrfsTer17) in three families; p.(Leu59Pro), c.393_422del; p.(Gln132_Ala141del), c.419 T > C; p.(Leu140Pro), c.491 C > T; c.491 C > T; p.(Ala164Val), c.1203_1204del; p.(Cys401Ter), c.1395 G > C; p.(Thr465 = ), c.1395 G > A; p.(Thr465 = ), c.1825C>T; p.(Arg609Trp), c.1857G>A; p.(Trp619Ter) and c.2230_2237dup; p.(Ser747ThrfsTer36) in two families.
Bioinformatic tools results were non-consistent for the following variants: c.224_226del; p.(Phe75del) and c.406 G > A; p.(Ala136Thr) (each found in two siblings), c.491 C > T; p.(Ala164Val) (identified in two children from unrelated families), c.358 C > T; p.(Arg120Cys) and c.419 T > G; p.(Leu140Arg). Other missense variants identified in this study were classified as probably or possibly damaging by CADD, PolyPhen-2, and SIFT prediction tools (Supplementary data S3). None of these variants was present in the homozygous state in the GnomAD database (v.2.1.1).
Discussion
By combining previously published data and the results of our series, we were able to study the largest cohort described so far of 97 patients from 74 families with biallelic variants in BRAT1, which is a very large cohort size for such a rare condition.
Our study confirms that BRAT1 biallelic variants are associated with two distinct clinical pictures. On the first hand, 59 patients from 45 families presented with BRAT1-related RMFSL phenotype, which corresponds to a severe epileptic encephalopathy leading to early death. In our study, myoclonus was reported in several patients early in life, before the first seizures. However, it is difficult to assume that these neurologic manifestations are epileptic ones in all cases. Death, most frequently due to status epilepticus or dysautonomia, occurred in the first years of life for the majority of patients. An autopsy was performed for six individuals and showed multiple brain abnormalities including neuronal loss and gliosis. Nonspecific dysmorphic features were noted in some individuals but may be accentuated by intensive neonatal care.
On the second hand, 38 patients from 29 families had a milder phenotype of BRAT1-related NEDCAS phenotype (38/97; 39%), which corresponds to a clinical picture of intellectual disability associated with cerebellar ataxia. Facial dysmorphism was noted in some individuals, but none of the features seems specific to the disease or highly recognizable. We confirmed that all patients in this group presented with cerebellar anomalies and showed that the degree of intellectual disability was highly variable. Intrafamilial variability was previously suggested for BRAT-related disorders [18]. Even if the severity of the cognitive impairment could indeed be different, it appears in our large cohort that siblings always had an overlapping phenotype corresponding to either RMFSL or NEDCAS.
On a molecular basis, the variant c.925_930del; p.(Pro309_Gln310) was identified in three Tunisian families, which suggest a founder effect. The variants c.294dup; p.(Leu99ThrfsTer92) and c.638dup; p.(Val214GlyfsTer189) were respectively found in five Caucasian families and 20 families, mostly Caucasian (French, German, Italian, Spanish and Amish from Pennsylvania, descendants of Swiss immigrants) or Lebanese and Moroccan for four additional families. A founder effect seems possible for these variants but the high carrier frequency in the general population (respectively 42 and 66 individuals in the GnomAD database) makes it difficult to conclude.
Study of phenotype-genotype correlations shows that, among the 59 individuals with RMFSL, 35 harbored two frameshift or nonsense variants (35/59; 59%) and 6 had at least 1 missense (6/59; 10%). In the remaining 18 patients, at least one splice variant was found in 7 (7/59; 12%) and a deletion or in-phase insertion in 11 (11/59; 19%) (Fig. 4). Six individuals harbored the c.393_422del; p.(Gln132_Ala141del) (1/59; 2%) or c.393_422dup; p.(Gln132_Ala141dup) (5/59;8%) variants in a homozygous state or in trans of a loss-of-function variant (Fig. 4).
Fig. 4. Genotypes of patients with RMFSL and NEDCAS phenotypes.
Are considered in the groups “at least 1 splice variant” or “deletion/inframe insertion” the patients having respectively a splice variant or an inframe deletion/insertion in trans of any other variant (except a missense). Note that the majority of patients with NEDCAS presenting with at least one missense variant and that none of them are carrying two nonsense or frameshift variants. On the contrary, two third of the patients with RMFSL presenting with two frameshift, two nonsense or inframe deletion/duplication.
These data suggest that a genotype-phenotype correlation for BRAT1-related disorders may exist and depend on the mutation type, probably linked to the amount of residual protein. Although we could not collect data on gene expression and protein levels, we speculate that the most severe mutations are unable to produce BRAT1 protein and thus give rise to the RMFSL phenotype. Interestingly, we did not notice any phenotypic differences for patients with truncating variants that are predicted to escape NMD.
In-frame insertion and deletion variants are associated with the severe RMFSL phenotype. For the recurrent in-frame p.(Gln132_Ala141del)/p.(Gln132_Ala141dup) variants, located in the NDFIP1 domain, which is necessary for BRAT1 translocation to the nucleus [33], we could speculate that a modification of 10 amino acids in this domain leads to a conformational defect that makes the nuclear action of BRAT1 impossible. Thus, these variants probably act as null ones. Conformational studies could be useful to confirm this hypothesis and to study the changes caused by the other in-frame variants.
Among missense changes, we found some noteworthy cases as patient 12 who harbored the homozygous c.358 C > T; p.(Arg120Cys) variant. She presented with neonatal lactic acidosis and cerebral lesions suggestive of hypoxia on brain MRI and died prematurely. Bioinformatics tools predictions are non-consistent for this variant but an effect on splicing could be involved (Supplementary data S3). Her severe phenotype could be related to additional perinatal complications or even a dual genetic diagnosis that has not been proven so far. The exome identified another homozygous variant of undetermined significance in this patient in a gene not yet implicated in human disease (Supplementary data S4). More generally, some individuals may have a dual genetic diagnosis with a second gene involved, especially in consanguineous families, since it has been shown that dual diagnosis would concern 2% to 3% of patients [34, 35].
On the contrary, analysis of BRAT1 variants showed that 28 of the 38 patients with NEDCAS presented at least one missense variant (74%). The remaining ten presented with at least one splice variant (10/38; 26%) (Fig. 4). None of the patients presented with two nonsense or frameshift or inframe deletion or insertion, except for the recurrent variant c.925_930del; p.(Pro309_Gln310del) which seems to affect splicing and was therefore considered as such. We suggest that the NEDCAS phenotype is associated with hypomorphic variants that lead to residual protein activity. Variants predicted to affect splicing could thus have a milder effect and behave as hypomorphic and not as null variants.
Interestingly, the NDFIP1 domain seems to be a hotspot for missense variants, including variations with non-consistent predictions. This domain is not well conserved during evolution, but it has a major functional role. It is thus possible that localized variations in this domain decrease BRAT1 binding to NDFIP1, and lead to a decrease in BRAT1 nuclear translocation.
We also noticed that the presence of the c.1395 G > C; p.(Thr465 = ) synonymous variant is associated with the RMFSL phenotype while the c.1395 G > A; p.(Thr465 = ) variant is associated with the NEDCAS phenotype. Although functional studies done in HEK293T cells have shown that these two variants lead to a shorter isoform corresponding to the skipping of exon 10, fibroblasts were only studied in patients with the c.1395 G > A; p.(Thr465 = ) variant [31]. It would be interesting to know if the c.1395 G > C variant could result in a null variant, while c.1395 G > A would be hypomorphic.
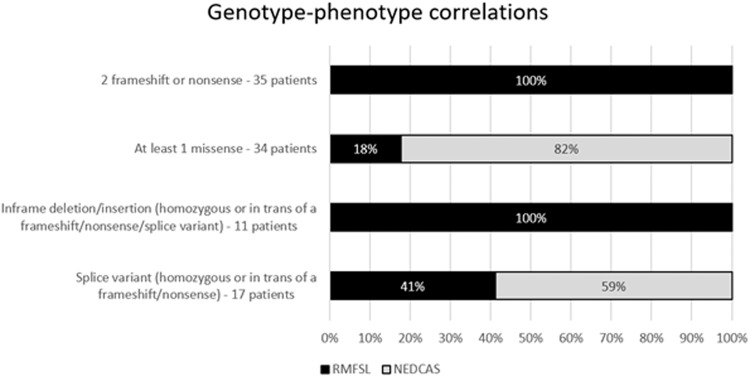
These observations allowed us to draw genotype-phenotype correlations for BRAT1-related disorders, which could be useful for genetic counselling (Fig. 5). Biallelic nonsense, frameshift or inframe deletion/insertion variants in a homozygous state or in trans with another nonsense or frameshift variant appeared to result in the severe BRAT1-related RMFSL phenotype (46/46;100%). In contrast, genotypes with at least one missense were more likely associated with NEDCAS (28/34; 82%). The phenotype of patients carrying splice variants was variable: 41% presented with RMFSL (7/17) and 59% with NEDCAS (10/17). Splice variants affecting invariant sites are generally considered severe changes and act as null variants, but alternative transcript or a residual normal amount of transcript can lead to a milder phenotype. Transcript analysis as well as functional studies would be valuable to investigate the null or hypomorphic effect of the identified BRAT1 splice site, missense, and in-frame variants on BRAT1 mRNA and protein function.
Fig. 5. Genotype-phenotype correlations for BRAT1 variants.
The percentage of patients with RMFSL are shown in black, and those with NEDCAS in grey. Note that all patients with two frameshift/nonsense or inframe insertions/deletions have an RMFSL phenotype, and that the majority of patients with at least one missense have a NEDCAS phenotype.
In conclusion, we studied a series of 97 patients with BRAT1 biallelic pathogenic variants, the largest cohort of patients described so far. Study of large cohort is very useful to define the clinical presentation of rare diseases, to better understand their natural history, to provide adequate genetic counselling to the extended family and to consider possible novel therapeutic perspectives.
Taken together, our results suggest that biallelic BRAT1 variants are associated with two distinct clinical presentations such as BRAT1-related RMFSL phenotype or BRAT1-related NEDCAS phenotype. The most severe clinical presentation is mainly seen in patients with two probably null variants and is associated with severe encephalopathy, drug-resistant epilepsy, cerebral atrophy, and early death. On the opposite, a phenotype of variable intellectual disability, cerebellar atrophy, ataxia, nystagmus, and higher life expectancy is mostly observed in patients with at least one missense variant. BRAT1-related neurodevelopmental disorders should therefore be considered at birth as a differential diagnosis of epileptic encephalopathy with rigidity due to ATAD1 [36], GRIA4 [37], NALCN [38], or MAGEL2 [39] variants, but also for conditions associating developmental delay and ataxia linked to WWOX [40, 41], PNKP [42], or SCA21 [43] variants.
Supplementary information
Acknowledgements
The authors would like to thank the families for their participation in this study. Funding: SS received funding from National Institute of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) (K23NS119666). FSA acknowledges the support of King Salman Center for Disability Research through Research Group no RG-2022-011.
Author contributions
CE, SV, GD, RM collected clinical and molecular data. CE, JP analyzed and interpreted data and wrote the manuscript. JP, LVM designed the study. SV, RM, AA, EA, FSA, VB, IB, MB-L, EB, IB, EB-B, A-LB, AB, DKB, CC, MRC, M-CC, CC, OD, VD, A-SD-P, MCDG, MD-F, HE, KC, MG, JGG, DH, JG, AG, FLH, HH, MI, RK, BK, EGK, DK, PK, KK, DL, LM, CM, DM-R, LN, SO, CO, JNP, LP, LP, CP, GP, CP, AP, MR, CR, VS, IS, AS, SS, RS, PS, EMV, PV, LV, AV, JW, MW, MSZ, FZ, GL, VRY, MM, FH-G, MB, FA, HG, CW, AN, MT, DS, YM, KK, CS, VC, MZ, LVM, LB characterized the clinical and molecular features of the disease. All the authors edited or commented the manuscript.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval
Individuals were referred by clinical geneticists for genetic testing as part of routine clinical care. All patients enrolled and/or their legal representative have signed informed consent for use of data and/or photographs. This study has been performed in accordance with the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-023-01410-z.
References
- 1.Aglipay JA, Martin SA, Tawara H, Lee SW, Ouchi T. ATM activation by ionizing radiation requires BRCA1-associated BAAT1. J Biol Chem. 2006;281:9710–8. doi: 10.1074/jbc.M510332200. [DOI] [PubMed] [Google Scholar]
- 2.Ouchi M, Ouchi T. Regulation of ATM/DNA-PKcs phosphorylation by BRCA1-associated BAAT1. Genes Cancer. 2010;1:1211–4. doi: 10.1177/1947601911404222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.So EY, Ouchi T. The potential role of BRCA1-associated ATM activator-1 (BRAT1) in regulation of mTOR. J Cancer Biol Res. 2013;1:1001. [PMC free article] [PubMed] [Google Scholar]
- 4.So EY, Ouchi T. BRAT1 deficiency causes increased glucose metabolism and mitochondrial malfunction. BMC Cancer. 2014;14:548. doi: 10.1186/1471-2407-14-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS ONE. 2012;7:e28936. doi: 10.1371/journal.pone.0028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanes I, Kozenko M, Callen DJA. Lethal neonatal rigidity and multifocal seizure syndrome—a misnamed disorder? Pediatr Neurol. 2015;53:535–40. doi: 10.1016/j.pediatrneurol.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Valence S, Cochet E, Rougeot C, Garel C, Chantot-Bastaraud S, Lainey E, et al. Exome sequencing in congenital ataxia identifies two new candidate genes and highlights a pathophysiological link between some congenital ataxias and early infantile epileptic encephalopathies. Genet Med. 2019;21:553–63. doi: 10.1038/s41436-018-0089-2. [DOI] [PubMed] [Google Scholar]
- 8.Colak FK, Guleray N, Azapagasi E, Yazıcı MU, Aksoy E, Ceylan N. An intronic variant in BRAT1 creates a cryptic splice site, causing epileptic encephalopathy without prominent rigidity. Acta Neurol Belg. 2020;120:1425–32. doi: 10.1007/s13760-020-01513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4:154ra135. doi: 10.1126/scitranslmed.3004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saitsu H, Yamashita S, Tanaka Y, Tsurusaki Y, Nakashima M, Miyake N, et al. Compound heterozygous BRAT1 mutations cause familial Ohtahara syndrome with hypertonia and microcephaly. J Hum Genet. 2014;59:687–90. doi: 10.1038/jhg.2014.91. [DOI] [PubMed] [Google Scholar]
- 11.Straussberg R, Ganelin-Cohen E, Goldberg-Stern H, Tzur S, Behar DM, Smirin-Yosef P, et al. Lethal neonatal rigidity and multifocal seizure syndrome – report of another family with a BRAT1 mutation. Eur J Paediatr Neurol. 2015;19:240–2. doi: 10.1016/j.ejpn.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 12.van de Pol LA, Wolf NI, van Weissenbruch MM, Stam CJ, Weiss JM, Waisfisz Q, et al. Early-onset severe encephalopathy with epilepsy: the BRAT1 gene should be added to the list of causes. Neuropediatrics. 2015;46:392–400. doi: 10.1055/s-0035-1564791. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Jáen A, Álvarez S, So EY, Ouchi T, de la Peña MJ, Duat A, et al. Mutations in BRAT1 cause autosomal recessive progressive encephalopathy: report of a Spanish patient. Eur J Paediatr Neurol. 2016;20:421–5. doi: 10.1016/j.ejpn.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn D, Weschke B, Knierim E, Fischer‐Zirnsak B, Stenzel W, Schuelke M, et al. BRAT1 mutations are associated with infantile epileptic encephalopathy, mitochondrial dysfunction, and survival into childhood. Am J Med Genet A. 2016;170:2274–81. doi: 10.1002/ajmg.a.37798. [DOI] [PubMed] [Google Scholar]
- 15.Mundy SA, Krock BL, Mao R, Shen JJ. BRAT1-related disease—identification of a patient without early lethality. Am J Med Genet A. 2016;170:699–702. doi: 10.1002/ajmg.a.37434. [DOI] [PubMed] [Google Scholar]
- 16.Smith NJ, Lipsett J, Dibbens LM, Heron SE. BRAT1-associated neurodegeneration: intra-familial phenotypic differences in siblings. Am J Med Genet A. 2016;170:3033–8. doi: 10.1002/ajmg.a.37853. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava S, Olson HE, Cohen JS, Gubbels CS, Lincoln S, Davis BT, et al. BRAT1 mutations present with a spectrum of clinical severity. Am J Med Genet A. 2016;170:2265–73. doi: 10.1002/ajmg.a.37783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava S, Naidu S. Epileptic encephalopathy due to BRAT1 pathogenic variants. Pediatr Neurol Briefs. 2016;30:45. doi: 10.15844/pedneurbriefs-30-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celik Y, Okuyaz C, Arslankoylu AE, Ceylaner S. Lethal neonatal rigidity and multifocal seizure syndrome with a new mutation in BRAT1. Epilepsy Behav Case Rep. 25 mai. 2017;8:2. doi: 10.1016/j.ebcr.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oatts JT, Duncan JL, Hoyt CS, Slavotinek AM, Moore AT. Inner retinal dystrophy in a patient with biallelic sequence variants in BRAT1. Ophthalmic Genet. 2017;38:559–61. doi: 10.1080/13816810.2017.1290118. [DOI] [PubMed] [Google Scholar]
- 21.Van Ommeren RH, Gao AF, Blaser SI, Chitayat DA, Hazrati LN. BRAT1 mutation: the first reported case of Chinese origin and review of the literature. J Neuropathol Exp Neurol. 2018;77:1071–8. doi: 10.1093/jnen/nly093. [DOI] [PubMed] [Google Scholar]
- 22.Szymańska K, Laure-Kamionowska M, Szczałuba K, Koppolu A, Furmanek M, Kuśmierska K, et al. Clinico-pathological correlation in case of BRAT1 mutation. Folia Neuropathol. 2018;56:362–71. doi: 10.5114/fn.2018.80870. [DOI] [PubMed] [Google Scholar]
- 23.Mahjoub A, Cihlarova Z, Tétreault M, MacNeil L, Sondheimer N, Caldecott KW, et al. Homozygous pathogenic variant in BRAT1 associated with nonprogressive cerebellar ataxia. Neurol Genet. 2019;5:e359. doi: 10.1212/NXG.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheffer IE, Boysen KE, Schneider AL, Myers CT, Mehaffey MG, Rochtus AM, et al. BRAT1 encephalopathy: a recessive cause of epilepsy of infancy with migrating focal seizures. Dev Med Child Neurol. 2020;62:1096–9. doi: 10.1111/dmcn.14428. [DOI] [PubMed] [Google Scholar]
- 25.Burgess R, Wang S, McTague A, Boysen KE, Yang X, Zeng Q, et al. The genetic landscape of epilepsy of infancy with migrating focal seizures. Ann Neurol. 2019;86:821–31. doi: 10.1002/ana.25619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pourahmadiyan A, Heidari M, Ardakani HS, Noorian S, Savad S. A novel pathogenic variant of BRAT1 gene causes rigidity and multifocal seizure syndrome, lethal neonatal. Int J Neurosci. 2021;131:875–8. doi: 10.1080/00207454.2020.1759589. [DOI] [PubMed] [Google Scholar]
- 27.Skafi O, Fawaz F, Merhi B, Jouni H, MMansour S, Harb R, et al. Rigidity with multifocal seizure syndrome, lethal neonatal in a lebanese neonate. A rare case report. J Pediatr Disord Neonatal Care. 2018;1:106. [Google Scholar]
- 28.Li W, Wu S, Xu H, Zhao X, Pan Y, Huang H, et al. Novel variant in BRAT1 with the lethal neonatal rigidity and multifocal seizure syndrome. Pediatr Res. 2021;1:7. doi: 10.1038/s41390-021-01468-9. [DOI] [PubMed] [Google Scholar]
- 29.Balasundaram P, Fijas M, Nafday S. A rare case of lethal neonatal rigidity and multi-focal seizure syndrome. Cureus. 2021;13:e13600. doi: 10.7759/cureus.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36:928–30. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuovo S, Baglioni V, De Mori R, Tardivo S, Caputi C, Ginevrino M, et al. Clinical variability at the mild end of BRAT1-related spectrum: evidence from two families with genotype-phenotype discordance. Hum Mutat. 2022;43:67–73. doi: 10.1002/humu.24293. [DOI] [PubMed] [Google Scholar]
- 32.Cornet MC, Morabito V, Lederer D, Glass HC, Santos SF, Numis AL, et al. Neonatal presentation of genetic epilepsies: early differentiation from acute provoked seizures. Epilepsia. 2021;62:1907–20. doi: 10.1111/epi.16957. [DOI] [PubMed] [Google Scholar]
- 33.Low LH, Chow YL, Li Y, Goh CP, Putz U, Silke J, et al. Nedd4 family interacting protein 1 (Ndfip1) is required for ubiquitination and nuclear trafficking of BRCA1-associated ATM activator 1 (BRAT1) during the DNA damage response. J Biol Chem. 2015;290:7141–50. doi: 10.1074/jbc.M114.613687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith ED, Blanco K, Sajan SA, Hunter JM, Shinde DN, Wayburn B, et al. A retrospective review of multiple findings in diagnostic exome sequencing: half are distinct and half are overlapping diagnoses. Genet Med. 2019;21:2199–207. doi: 10.1038/s41436-019-0477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AlAbdi L, Alrashseed S, Alsulaiman A, Helaby R, Imtiaz F, Alhamed M, et al. Residual risk for additional recessive diseases in consanguineous couples. Genet Med Off J Am Coll Med Genet. 2021;23:2448–54. doi: 10.1038/s41436-021-01289-5. [DOI] [PubMed] [Google Scholar]
- 36.Bunod R, Doummar D, Whalen S, Keren B, Chantot-Bastaraud S, Maincent K, et al. Congenital immobility and stiffness related to biallelic ATAD1 variants. Neurol Genet. 2020;6:e520. doi: 10.1212/NXG.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin S, Chamberlin A, Shinde DN, Hempel M, Strom TM, Schreiber A, et al. De novo variants in GRIA4 lead to intellectual disability with or without seizures and gait abnormalities. Am J Hum Genet. 2017;101:1013–20. doi: 10.1016/j.ajhg.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong JX, McMillin MJ, Shively KM, Beck AE, Marvin CT, Armenteros JR, et al. De novo mutations in NALCN cause a syndrome characterized by congenital contractures of the limbs and face, hypotonia, and developmental delay. Am J Hum Genet. 2015;96:462–73. doi: 10.1016/j.ajhg.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patak J, Gilfert J, Byler M, Neerukonda V, Thiffault I, Cross L, et al. MAGEL2-related disorders: a study and case series. Clin Genet. 2019;96:493–505. doi: 10.1111/cge.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aldaz CM, Hussain T. WWOX loss of function in neurodevelopmental and neurodegenerative disorders. Int J Mol Sci. 2020;21:8922. [DOI] [PMC free article] [PubMed]
- 41.Piard J, Hawkes L, Milh M, Villard L, Borgatti R, Romaniello R, et al. The phenotypic spectrum of WWOX-related disorders: 20 additional cases of WOREE syndrome and review of the literature. Genet Med. 2019;21:1308–18. doi: 10.1038/s41436-018-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gatti M, Magri S, Nanetti L, Sarto E, Bella DD, Salsano E, et al. From congenital microcephaly to adult onset cerebellar ataxia: distinct and overlapping phenotypes in patients with PNKP gene mutations. Am J Med Genet A. 2019;179:2277–83. doi: 10.1002/ajmg.a.61339. [DOI] [PubMed] [Google Scholar]
- 43.Riso V, Galatolo D, Barghigiani M, Galosi S, Tessa A, Ricca I, et al. A NGS-based analysis on a large cohort of ataxic patients refines the clinical spectrum associated with SCA21. Eur J Neurol. 2021;28:2784–8. doi: 10.1111/ene.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.