Abstract
Pyroptosis, apoptosis, necroptosis, and ferroptosis, which are the most well-studied regulated cell death (RCD) pathways, contribute to the clearance of infected or potentially neoplastic cells, highlighting their importance in homeostasis, host defense against pathogens, cancer, and a wide range of other pathologies. Although these four RCD pathways employ distinct molecular and cellular processes, emerging genetic and biochemical studies have suggested remarkable flexibility and crosstalk among them. The crosstalk among pyroptosis, apoptosis and necroptosis pathways is more evident in cellular responses to infection, which has led to the conceptualization of PANoptosis. In this review, we provide a brief overview of the molecular mechanisms of pyroptosis, apoptosis, necroptosis, and ferroptosis and their importance in maintaining homeostasis. We discuss the intricate crosstalk among these RCD pathways and the current evidence supporting PANoptosis, focusing on infectious diseases and cancer. Understanding the fundamental processes of various cell death pathways is crucial to inform the development of new therapeutics against many diseases, including infection, sterile inflammation, and cancer.
Subject terms: Cell death and immune response, Stress signalling
Cell death: Interactions between different pathways
The different pathways that cause cell death, which are activated under different conditions, have complex relationships and sometimes act together to fight infection or cancer. All the pathways eliminate cells damaged by disease or mutation and are important for development and disease resistance, but can affect immune responses to infection or cause damage if dysregulated. Rajendra Karki at Seoul National University in South Korea and co-workers review recent evidence revealing links, including common molecules, between the pathways. Because cancer cells can become resistant to anti-cancer drugs or to the usual closely regulated cell death pathway, the ability to trigger a different type of cell death or to activate all pathways holds great promise for cancer treatment. Improving our understanding of cell death could help in treating multiple diseases, from infection to cancer.
Introduction
Cell death is a conserved fundamental process that plays a central role in all aspects of life. It is involved in embryonic development, maintaining organismal homeostasis, and eliminating damaged cells. Cell death can be induced in response to physical damage and infection1,2. Based on their signal dependency, cell death can be classified into regulated or nonregulated cell death. While regulated cell death (RCD) is tightly regulated by intracellular signal transduction pathways, non-RCD is accidental and results from unexpected cell injury. Considering the morphological characteristics and molecular mechanisms, RCD can be further classified as nonlytic and lytic cell death3. Apoptosis is a nonlytic form of cell death in which the cell retains membrane integrity and exhibits cytoplasmic shrinkage, chromatin condensation, nuclear fragmentation, and plasma membrane blebbing (Table 1). In contrast, pyroptosis, necroptosis, and ferroptosis are classical lytic cell death processes, which result in the removal of dead cells and the release of potent inflammatory mediators (Table 1). Therefore, apoptosis is typically ‘immunologically silent’, whereas pyroptosis, necroptosis, and ferroptosis are referred to as relatively ‘violent’ types of cell death3. There are other RCD, including parthanatos, lysosome-dependent cell death, autophagy-dependent cell death, alkaliptosis, oxeiptosis, and cuproptosis4,5. As the field continues to progress, several molecules that regulate cell death have been identified that establish cell death as a regulated process. Although work over the past three decades has identified several distinct types of RCD, the molecular mechanisms responsible for the initiation, transduction, and execution of pyroptosis, apoptosis, necroptosis, and ferroptosis are the most well established. Molecularly, apoptosis is executed by activation of the executioner caspases caspase-3 (CASP3) and CASP7 downstream of the initiator caspases CASP8, CASP9, and CASP106–8 (Fig. 1). Plasma membrane pore formation by activated gasdermin family members such as GSDMD or GSDME leads to pyroptosis. Inflammatory caspases, CASP1 and CASP11 (mice) or CASP4/5 (humans) activate GSDMD9,10 (Fig. 2). Necroptosis is driven by the formation of mixed lineage kinase domain-like pseudokinase (MLKL) pores following MLKL phosphorylation downstream of the receptor-interacting protein kinase 1 (RIPK1) and RIPK3 signaling axis11,12 (Fig. 3). Although ferroptosis is usually accompanied by a large amount of iron accumulation and lipid peroxidation13, the molecules involved in the execution of ferroptosis are not known (Fig. 4). Plasma membrane rupture mediated by ninjurin 1 (NINJ1) is required for the release of larger DAMPs such as LDH and HMGB1 during lytic cell death14.
Table 1.
Morphological features of different types of regulated cell death.
| Regulated cell death (RCD) | Morphological Features | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lysis | Membrane rupture | Pore formation | Cell swelling | Organelle swelling | Cell shrinkage | Membrane blebbing | Chromatin condensation | DNA damage | Intact nucleus | |
| Pyroptosis | √ | √ | √ | √ | Χ | Χ | √ | √ | √ | √ |
| Apoptosis | Χ | Χ | Χ | Χ | Χ | √ | √ | √ | √ | Χ |
| Necroptosis | √ | √ | √ | √ | √ | Χ | Χ | Χ | √ | √ |
| PANoptosis | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
The table shows the morphological features of pyroptosis, apoptosis, necroptosis, and PANoptosis.
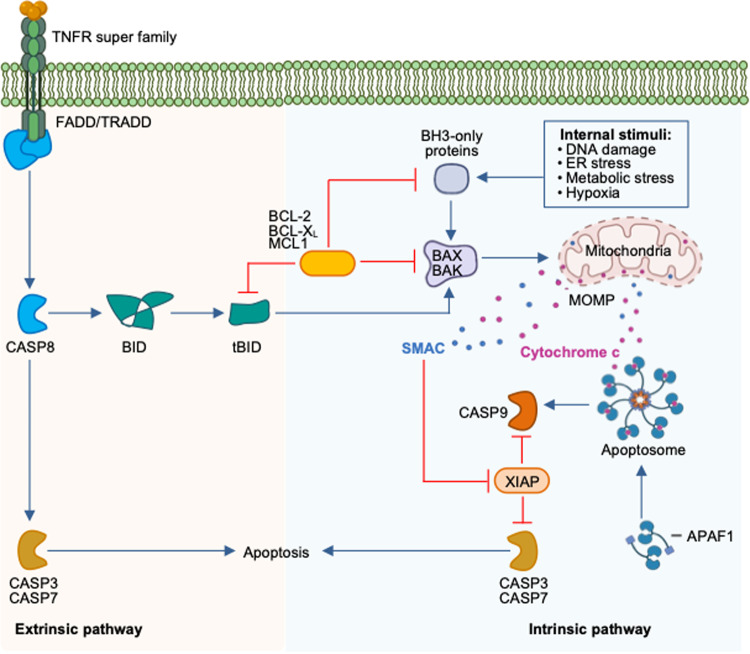
Fig. 1. Molecular mechanisms of extrinsic and intrinsic apoptosis.
Extrinsic apoptosis: Binding of a ligand such as FASL, TNF, TRAIL, and TWEAK to one of several death receptors (TNF receptor superfamily) initiates extrinsic apoptosis by triggering receptor oligomerization and the recruitment of adaptor proteins containing death domains such as TRADD and FADD. The resulting complexes activate caspase-8, which activates executioners caspase-3 and caspase-7. Intrinsic apoptosis: Diverse cytotoxic stimuli, such as DNA damaging agents and stress, activate BH3-only family members, thereby activating pro-apoptotic effectors BAX and BAK, which then disrupt the mitochondrial outer membrane. The cytochrome c released from the mitochondria interacts with APAF1 to form apoptosomes, which in turn activate the initiator caspase-9. Crosstalk between the extrinsic and intrinsic pathways can occur through BID cleavage by caspase-8, leading to activation of BAX and BAK. The two pathways converge at activation of the effector caspases (caspase-3 and caspase-7).
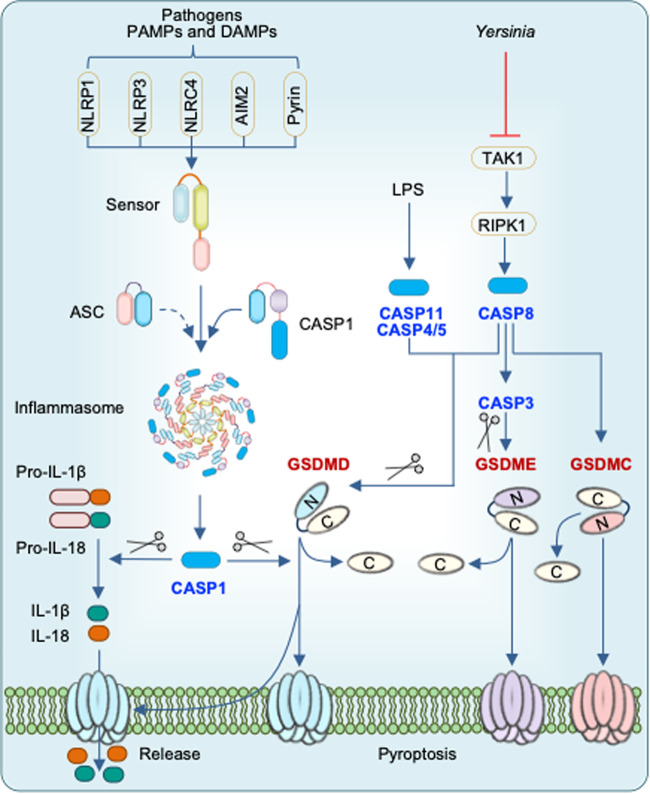
Fig. 2. Inflammasome activation and pyroptosis.
Certain pathogens, PAMPs, and DAMPs are sensed by specific sensors to assemble an inflammasome consisting of a sensor, ASC, and caspase-1. Active caspase-1 cleaves pro-IL-18 and pro-IL-1β into their mature forms. Active caspase-1, caspase-11, and caspase-8 cleave GSDMD to free the N-terminal region, which undergoes oligomerization to form pores in the plasma membrane. Active caspase-8 also cleaves GSDME and GSDMC. Pore formation in the plasma membrane by GSDMs causes cell lysis and release of intracellular contents and the inflammatory cytokines IL-18 and IL-1β following their maturation by caspase-1.
Fig. 3. Molecular mechanisms of necroptosis.
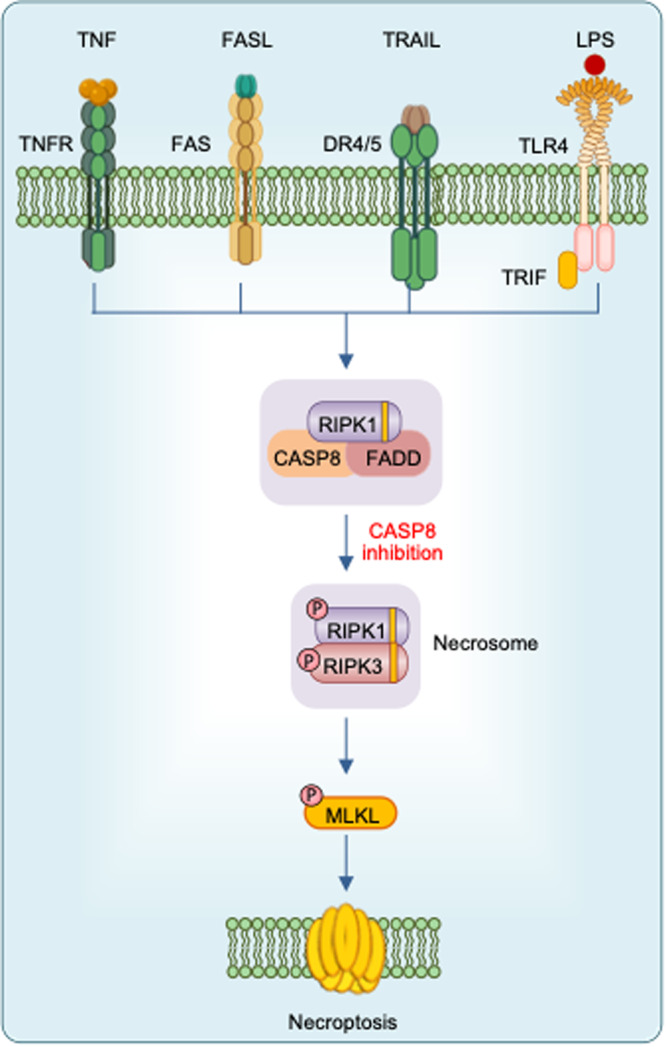
TNF ligation or LPS stimulation results in activation of NF-κB signaling. Inactivation of NF-κB signaling or engagement of death receptors triggers the assembly of an apoptosis-inducing complex consisting of FADD, caspase-8, and RIPK1. When caspase-8 is inhibited, RIPK1 and RIPK3 form necrosomes through homotypic interactions with RHIM, resulting in phosphorylation of MLKL. Phosphorylated MLKL undergoes oligomerization and induces membrane rupture.
Fig. 4. Molecular mechanisms of ferroptosis.
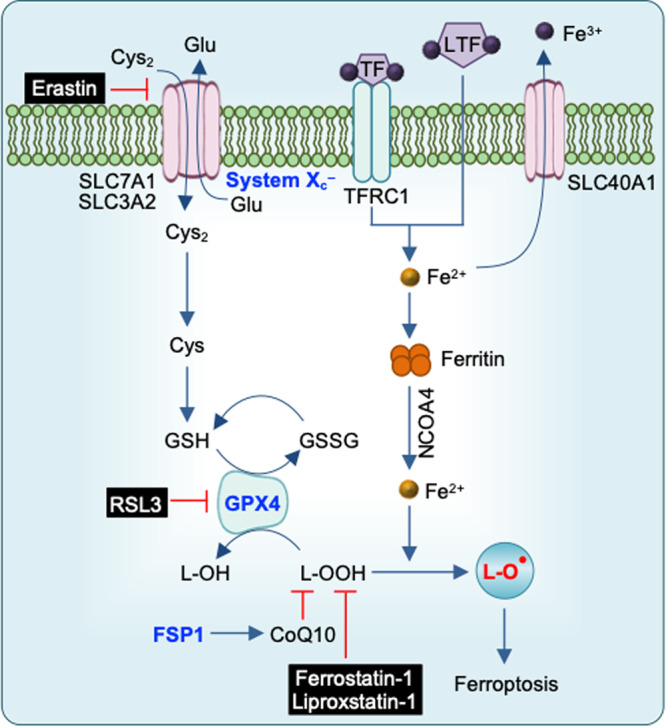
Ferroptosis is primarily driven by iron-dependent lipid peroxidation. Iron bound to transferrin is transported into cells by TFRC1. NCOA4-mediated ferritinophagy increases the free iron pool. Ferroptosis is inhibited by GSH, the synthesis of which involves the uptake of cystine via the cystine-glutamate antiporter (system Xc-). Using GSH as a cofactor, GPX4 reduces phospholipid hydroperoxides to their corresponding alcohols. The FSP1-CoQ10 system inhibits ferroptosis.
The identification of key regulators of pyroptosis, apoptosis, necroptosis, and ferroptosis has increased the understanding of cell death functions in multiple settings ranging from organismal homeostasis to infectious, inflammatory, and autoimmune diseases and cancer. While inflammatory cell death—pyroptosis, necroptosis, and ferroptosis—is involved in providing host defense against invading pathogens, apoptosis ensures normal development and cellular homeostasis1,2. For instance, mice with defective apoptosis, such as those with mutations in or lacking apoptotic regulators such as caspase-9, APAF1, BAK, BAX, and BOK, typically die during late stages of development or soon after birth15–17. Conversely, despite the impaired responses to certain pathogens or other external insults, mice deficient in pyroptosis, such as those lacking GSDMD and GSDME, or necroptosis, such as those lacking RIPK3 and MLKL, are born healthy9–12.
Although apoptosis, necroptosis, and pyroptosis have historically been considered independent, there is now mounting evidence that these RCD pathways are interconnected at multiple levels. Moreover, activation of biochemical markers from all three RCD pathways has been observed with several sterile triggers, such as the combination of interferon (IFN) and nuclear export inhibitors (NEI), the combination of TNF and IFN-γ and TAK1 inhibitors and nonsterile triggers, such as bacterial and viral infection1. The combined loss of these RCD pathways, but not individual RCD pathways, prevents the cell death induced by these triggers implying a united modality of death defined as PANoptosis (Fig. 5). The pathophysiological relevance of PANoptosis has been observed during infections as well as in autoinflammatory diseases, cytokine storms and cancer18–23.
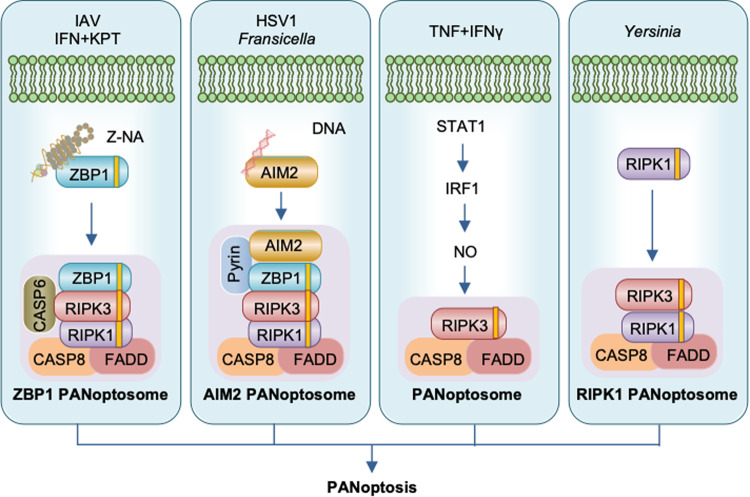
Fig. 5. Triggers and molecules involved in PANoptosome assembly.
Pathogens such as IAV, HSV1, Francisella, and Yersinia and other agents such as IFN + KPT and TNF + IFNγ have been identified to induce PANoptosis. ZBP1 senses IAV or endogenous Z-NA to assemble ZBP1 PANoptosomes consisting of ZBP1 and other cell death molecules. CASP6 potentiates the interaction between RIPK3 and ZBP1. AIM2 senses dsDNA during HSV1 or Francisella infection to assemble the AIM2 PANoptosome. TNF + IFNγ activates STAT1 to induce IRF1-dependent NO release, which activates CASP8 and RIPK3 to trigger PANoptosis. During Yersinia infection, RIPK1 assembles the RIPK1 PANoptosome.
In this review, we will provide a framework for understanding the different types of RCD pathways. We will discuss the integral components of pyroptosis, apoptosis, necroptosis, and ferroptosis and summarize the latest insights into molecular and functional connections among the different RCD pathways that have led to the conceptualization of PANoptosis. In addition, we will summarize the pathological contexts involving these RCD pathways and emerging therapeutic applications of modulating them. Finally, we will explain the concept of PANoptosis that will likely drive the next decade of cell death studies.
Regulated cell death
Apoptosis
Apoptosis was the first RCD to be described and is the most studied form of cell death24. It is primarily associated with development and homeostasis. Cell shrinkage and pyknosis are the characteristic features of apoptosis (Table 1). Extensive plasma membrane blebbing follows the formation of apoptotic bodies consisting of cytoplasm with tightly packed organelles. These bodies are subsequently phagocytosed by surrounding cells such as macrophages and parenchymal cells and degraded within phagolysosomes, thus likely preventing secondary necrosis. Based on molecular events, there are two main apoptotic pathways – the intrinsic or mitochondrial pathway and the extrinsic or death receptor pathway (Fig. 1). The proteolytic cascade leading to activation of caspases is one of the biochemical features of both extrinsic and intrinsic apoptosis2. Intrinsic apoptosis can be induced by various agents that can trigger a variety of microenvironmental perturbations, including DNA damage, ER and replication stress, microtubular alterations, or mitotic defects. Mitochondrial outer membrane permeabilization (MOMP), which is controlled by proapoptotic and antiapoptotic members of the BCL2 family, is a critical step in intrinsic apoptosis25. In response to apoptotic stimuli, BAX and BAK form pores across the outer mitochondrial membrane (OMM) and possibly other intracellular membranes in association with other proapoptotic BH3-only proteins. MOMP is antagonized by antiapoptotic members of the BCL2 family, which directly bind to proapoptotic members. The regulation of apoptosis by BCL-2 family members is critical and has been extensively reviewed elsewhere25. MOMP promotes the cytosolic release of apoptogenic factors, including cytochrome c and SMAC, that normally reside in the mitochondrial intermembrane space26. The cytosolic pool of cytochrome c binds to APAF1 and pro-caspase 9 (CASP9) to form the supramolecular complex called the apoptosome, which activates CASP927 (Fig. 1). Activated CASP9 is responsible for the activation of the downstream effector caspases CASP3 and CASP7. Extrinsic apoptosis is initiated through the engagement of two types of plasma membrane receptors: 1) death receptors, the activation of which depends on the binding of the cognate ligand, and 2) dependence receptors, which are activated upon dropping of their ligand level below a threshold28. Death receptors include Fas (CD95) and TNF receptor (TNFR). Upon ligand binding, a conformational change occurs that allows DD homotypic interactions between cytoplasmic adapter proteins such as FADD or TRADD and the receptors. The engagement of the Fas receptor results in the binding of FADD, whereas the binding of the TNF ligand to the TNF receptor engages TRADD with the recruitment of FADD and RIP. FADD then associates with pro-caspase-8 to form DISC, which leads to processing of caspase-8 to its active form29,30 (Fig. 1). The molecular mechanisms regulating CASP8 activity upon death receptor ligation have been extensively reviewed elsewhere28. Depending upon the cell type, the execution of extrinsic apoptosis follows two distinct pathways. The CASP8-dependent proteolytic activation of CASP3 and CASP7 is sufficient to execute cell death in type I cells such as thymocytes and mature lymphocytes31. However, in type II cells such as hepatocytes, pancreatic β cells and cancer cells, the activation of CASP3 and CASP7 is restrained by XIAP32. Therefore, type II cells require the proteolytic cleavage of BID by CASP8 to generate a truncated form of BID (tBID) that translocates to the OMM, leading to CASP9-driven cell death. Dependence receptors, which consist of 20 members, promote cell survival, proliferation, and differentiation when sufficient cognate ligands are available. However, these receptors activate the cell death cascade when ligand availability drops below a threshold value. For example, the dependence receptor DCC (deleted in colorectal cancer) promotes the activation of the CASP9-CASP3 cascade in the absence of its ligand33,34. Altogether, CASP9 and CASP8 are the initiator caspases of the intrinsic and extrinsic apoptosis pathways, respectively, and these pathways converge for activation of the same executioner enzymes: CASP3 and CASP7.
Pyroptosis
Pyroptosis is a form of RCD that occurs in response to perturbations associated with innate immunity. The term pyroptosis was first coined by Cookson and Brennan to define apoptosis as RCD, which is dependent on inflammatory CASP135. Depending on the initiating stimulus, pyroptosis is induced by inflammatory or apoptotic caspases, including CASP1, murine CASP11, human CASP4 and CASP5, and CASP336–39. The assembly of an inflammasome, which includes a multiprotein complex containing a sensor, the adaptor ASC, and CASP1, leads to the autoprocessing of CASP1 into its active form. The well-established canonical inflammasome sensors include NLRP1, NLRP3, NLRC4, AIM2, and Pyrin40 (Fig. 2). The inflammasome adaptor protein ASC bridges inflammasome sensors and CASP1, leading to CASP1 activation. Active CASP1 cleaves its downstream substrates, including the inflammatory cytokines pro-IL-1β and pro-IL-18 to produce their bioactive forms and GSDMD to facilitate plasma membrane pore formation40. In addition, LPS detection by murine CASP11 or human CASP4/5 induces GSDMD cleavage by CASP11 to form membrane pores, which facilitate NLRP3 inflammasome activation in a cell-intrinsic manner to induce IL-1β and IL-18 maturation36. Therefore, pyroptosis is often associated with inflammasome activation and the release of cytokines, including IL-1β and IL-18, conferring robust proinflammatory effects. In addition to GSDMD, the N-terminal domains of other members of the gasdermin family, including GSDMA, GSDMB, GSDMC, and GSDME, can also induce pyroptosis in a context-dependent manner39 (Fig. 2).
Necroptosis
Necroptosis is a form of the RCD pathway that is induced by specific death receptors, including Fas and TNFR1, or PRRs, such as TLR3 and TLR4, when caspase activation is inhibited (Fig. 3). The first genetic evidence of necroptosis was reported in T cells that underwent cell death in a FADD/RIPK1 manner without the release of cytochrome c41. Molecularly, necroptosis critically depends on the sequential activation of RIPK3 and MLKL12. In necroptosis initiated by TNFR1, the kinase activity of both RIPK1 and RIPK3 is essential for cell death, and RIPK1 and RIPK3 interact to form a necrosome through their RIP homotypic interaction motifs (RHIMs)42. Accordingly, chemical inhibitors of RIPK1, such as Nec-1, potently inhibit TNFR1-driven necroptosis43. Alternatively, RIPK3 can be activated following the RHIM-dependent interaction with TRIF upon either TLR3 or TLR4 activation44. Active RIPK3 phosphorylates MLKL, resulting in the formation of MLKL oligomers in the plasma membrane, which trigger plasma membrane permeabilization and cell death (Fig. 3). Considering that the inhibition of caspases, such as CASP8, is a prerequisite for necroptosis to occur, it is likely that necroptosis plays a “fail-safe” role in driving cell death in conditions that abrogate caspase activation45,46.
Ferroptosis
Distinct from pyroptosis, apoptosis, and necroptosis, ferroptosis is a form of cell death that depends on iron-dependent lipid peroxidation13. The identification of small molecules that induce a nonapoptotic form of cell death led to the discovery of ferroptosis, and the term ferroptosis was coined by Brent Stockwell in 201247. The major research areas that have provided the foundation for understanding ferroptosis are a) iron homeostasis, b) reactive oxygen species (ROS) biology, and c) amino acid and lipid metabolism, which are very much interconnected for inducing ferroptosis (Fig. 4). The oxidized form of cysteine, an amino acid that is required for the survival and growth of certain cells, causes glutathione (GSH) depletion and cell death48. GSH blocks the ability of oxidants such as hydrogen peroxide to cause oxidative stress. An antiporter Xc–, which imports cystine and is a building block for GSH, normally functions as a strong suppressor of ferroptosis47. A selenocystine protein, glutathione peroxidase 4 (GPX4), functions as a GSH-dependent peroxidase to prevent lipid oxidation in membranes49. PUFAs need to be incorporated into membrane lipids, such as phospholipids, which are key building blocks of membranes, to serve as essential substrates for ferroptosis. The enzymes involved in activating and incorporating polyunsaturated fatty acids (PUFAs) into membrane lipids promote ferroptosis. A mutagenesis screen in the KBM7 cell line, an analysis of ferroptosis-resistant cell lines, and a CRISPR suppression screen have revealed inactivation of acylcoenzyme A synthetase long-chain family member 4 and lysophosphatidylcholine acyltransferase 3 as a key mechanism for inhibiting ferroptosis in various contexts47. Consistent with the conclusion that oxidized PUFA tails must be associated with phospholipids to execute ferroptosis, the phospholipase A2 group VI suppresses ferroptosis by dissociating oxidized PUFA tails from phospholipids50. Therefore, free PUFAs or oxidized PUFAs are not intrinsically toxic to cells.
Identified in a high-throughput screen for HRASV12-selective lethal molecules, erastin (eradicator of RAS-transformed cells) and RSL3 (RAS-selective-lethal-3) induce ferroptosis by inhibiting cystine uptake through system Xc– and by inhibiting GPX4, respectively51,52. GPX4 degradation by ferroptosis-inducer-56 (FIN56), identified in a screen for caspase-independent lethal compounds, induces ferroptosis53. FIN56 also increases sensitivity to ferroptosis by depleting coenzyme Q10 (CoQ10). However, ferroptosis suppressor protein 1 inhibits ferroptosis independent of GPX4 by regenerating the reduced form of CoQ1013.
Crosstalk among RCD pathways
Although initially identified as independent pathways, pyroptosis, apoptosis, and necroptosis show extensive interactions among each other. The molecules that are involved in pyroptosis regulate apoptosis and vice versa. Activation of CASP7 observed in conditions known to induce pyroptosis, including Salmonella infection and LPS plus ATP, is abolished in macrophages deficient in CASP154,55, demonstrating a regulatory role for CASP1 in CASP7 activation. However, macrophages deficient in GSDMD, a downstream molecule of CASP1 and executioner of pyroptosis, undergo apoptosis accompanied by CASP3 activation in response to inflammasome stimuli56. CASP1 activation reroutes cell death responses to GSDME-mediated secondary necrosis or pyroptosis via the Bid-CASP9-CASP3 axis in the absence of GSDMD56. It is possible that inflammasome-driven apoptosis might be physiologically relevant to cells with low or no GSDMD expression, such as neurons and mast cells. In addition, the triggers of NLRP1b and NLRC4 promote CASP8 activation in the absence of inflammasome components. In WT macrophages infected with Salmonella, CASP1 and CASP8 colocalize with ASC specks, although CASP8 is dispensable for Salmonella-induced pyroptosis57. However, ASC colocalizes with FADD in Casp1–/– cells stimulated with the NLRC4 trigger FlaTox to promote Casp8-dependent apoptosis58. Furthermore, AIM2 inflammasome triggers, such as Francisella infection or DNA electroporation, lead to the recruitment and activation of CASP8 through ASC, resulting in CASP3 activation in Casp1–/– cells59,60. In addition to its classical role in extrinsic apoptosis, CASP8 has been shown to regulate inflammasome activation and pyroptosis in various conditions. Inflammasome activation and cell death are reduced in macrophages lacking RIPK3 and CASP8 or RIPK3 and FADD compared with cells deficient in RIPK3 in response to LPS + ATP stimulation and C. rodentium or Yersinia infection, indicating that FADD and CASP8 regulate inflammasome activation and pyroptosis during NLRP3 inflammasome stimuli61–63. CASP8 promotes the priming and posttranslational modification of NLRP3, which are essential for activation of the NLRP3 inflammasome. In addition to its role in the upregulation of Nlrp3 and Il1b, CASP8 is recruited into the inflammasome complex in response to NLRP3 triggers61. Furthermore, CASP8 can cleave GSDMD and activate the NLRP3 inflammasome upon TAK1 inhibition64. The crosstalk between pyroptosis and apoptosis has also been observed in conditions other than with inflammasome triggers. During chemotherapy, CASP3 cleaves GSDME to induce pyroptosis. CASP3 can also cleave GSDMD at its N-terminus to generate an inactive fragment that potentially limits GSDMD-mediated pyroptosis38,65. Bile acid-induced APAF1 apoptosomes containing CASP11 induce CASP3 cleavage to drive GSDME-dependent pyroptosis66. However, the regulatory mechanisms for switching between the APAF1-CASP11 pyroptosome and APAF1-CASP9 apoptosome remain elusive. Overall, these findings suggest that pyroptosis and apoptosis, which are functionally distinct cellular responses, mutually regulate each other.
There are few studies that suggest the interplay between pyroptosis and necroptosis. NLRP3 inflammasome activation occurs during necroptosis engaged by TLR3 signaling in combination with caspase inhibition. Cells deficient in RIPK3 or MLKL show impaired ASC oligomerization in response to Poly I:C and zVAD treatment, suggesting the involvement of RIPK367. Potassium efflux through MLKL pores acts as a signal for NLRP3 inflammasome activation during cell death induced by necroptotic triggers68. The interactions between necroptosis and apoptosis have been comparatively well documented compared with those between necroptosis and pyroptosis. The balance between necroptosis and apoptosis is crucial to maintain homeostasis. While its activation triggers apoptosis, deletion of CASP8 in mice leads to embryonic lethality, which can be rescued by loss of RIPK3 or MLKL69,70, suggesting a predominant role of apoptotic CASP8 in preventing necroptosis during development. Since RIPK1 is involved in the regulation of both necroptosis and apoptosis, embryonic lethality in Ripk1–/– mice can only be rescued upon deletion of both CASP8 and RIPK371. However, there are very limited studies on the intersection of ferroptosis with other RCD pathways. Nec-1, which blocks RIPK-1-dependent necroptosis, has been reported to inhibit erastin- or sulfasalazine-induced ferroptosis in Huh7 and SK-HEP-1 cells72. The inhibitory function of Nec-1 in ferroptosis could be an off-target effect on the ferroptosis pathway.
Overall, RCD processes such as pyroptosis, apoptosis, and necroptosis were originally thought of as distinct pathways. Emerging studies have suggested the existence of multiple interactions among these RCD pathways.
PANoptosis
As described above, the crosstalk among pyroptosis, apoptosis, and necroptosis indicates the existence of a dynamic molecular interaction network that has conceptualized PANoptosis as an inflammatory RCD activated by specific triggers and with the molecular characteristics of pyroptosis, apoptosis, and necroptosis73 (Fig. 5). A study that showed the activation of CASP1, CASP8, and CASP3 and phosphorylation of MLKL, the key molecular events of pyroptosis, apoptosis, and necroptosis, in macrophages infected with influenza A virus (IAV) was the first to provide evidence for PANoptosis74. Cells lacking individual components of the typical RCD pathways show a similar degree of cell death compared to that in WT cells during IAV infection. However, loss of the ZBP1 or Zα domain of ZBP1 provides protection against cell death, indicating that the sensing of IAV occurs through its Zα domain74–76. ZBP1 initiates the activation of the molecular machinery for PANoptosis execution. Another cytosolic sensor, AIM2, induces PANoptosis in macrophages upon infection with HSV1 or F. novicida. While loss of ZBP1 or pyrin reduces cell death, combined loss of ZBP1 and AIM2 abrogates cell death and activation of PANoptosis, indicating that ZBP1 and pyrin regulate AIM2 responses during HSV1 and F. novicida infection22. Moreover, the PAN apoptotic cell death in macrophages infected with murine hepatitis virus (MHV), a beta coronavirus, is potentiated by ZBP1 upon IFN treatment19. In addition to viral and bacterial infection, ZBP1 also mediates PANoptosis during fungal infection77. NAIP-NLRC4-engaged bacteria, Salmonella and Pseudomonas, and TAK1-inhibiting bacteria, Yersinia, have been shown to induce PANoptosis78,79. In addition to pathogens, sterile triggers induce PANoptosis in macrophages18,21,80. Accumulation of dsRNA in cells stimulated with IFN plus NEI, such as KPT or leptomycin, is sensed by the Zα domain of ZBP1 to drive RIPK3- and CASP8-dependent PANoptosis21,80. The combination of TNF and IFN-γ activates STAT1, induces IRF1-dependent iNOS and subsequently produces NO, which triggers CASP8-dependent PANoptosis in macrophages18,80. However, human cancer cells undergo PANoptosis in an IRF1-dependent but NO-independent manner23, suggesting that there may be cell type- or species-specific differences in regulation. PANoptosis has also been demonstrated in sterile inflammation. Mice carrying a mutation in Pstpip2 (Pstpip2cmo) develop osteomyelitis. Combined deletion of molecules involved in pyroptosis, apoptosis, and necroptosis can rescue these mice, implicating PANoptosis in this process81,82. Similarly, the expression of enzymatically inactive CASP8 (Casp8C362S/C362S) causes embryonic lethality in mice by inducing necroptosis and pyroptosis. However, Casp8C362S/C362SMlkl–/–Casp1–/– mice are viable83,84, suggesting that the enzymatic activity of caspase-8 controls PANoptosis. Overall, distinctive upstream triggers or sensors mostly converge on CASP8, which functions as a central node for the execution of PANoptosis.
Physiological relevance of cell death
Depending on the context, RCD pathways can be beneficial or detrimental. RCD pathways have mostly been implicated in combating infections by removing the replicative abilities of pathogens and preventing cancer by inducing cancer cell death. Conversely, aberrant cell death contributes to cytokine storm-associated and chronic degenerative diseases.
Development and homeostasis
Among the various RCD pathways, apoptosis is best characterized for its role in normal animal development and tissue homeostasis. Targeted disruption of caspases in mice has revealed differential requirements for individual caspases during mammalian development. Caspase-8 deficiency leads to embryonic lethality, which is associated with a regression of the extraembryonic yolk sac vasculature followed by abdominal hemorrhage due to cardiac puncture85. The conditional deletion of caspase-8 in endothelial cells results in embryonic lethal phenotypes similar to global caspase-8-deficient conditions86. Although mice lacking caspase-8 in T cells or myeloid cells are viable, these mice appear to have deficiencies in expansion and activation of T-cells and differentiation of myeloid progenitors into macrophages87. Deletion of the necroptotic effector molecules RIPK3 or MLKL rescues the embryonic lethality of Casp8–/– mice, suggesting that CASP8 prevents necroptosis during development88. Mice lacking RIPK1 die postnatally due to massive necroptosis in epidermal cells and apoptosis in the intestine89. Combined loss of CASP8 and RIPK3 in Ripk1–/– mice rescues lethality71. Mice carrying an uncleavable RIPK1 (D325A) also undergo embryonic lethality, which can be rescued by combined loss of both apoptosis and necroptosis. CASP8 cleaves RIPK1 to prevent excessive necroptosis and apoptosis90.
Caspase-9 deficiency also results in embryonic lethality due to severe brain malformation and hindbrain neural tube defects. The phenotypes observed in Casp9–/– mice are associated with reduced neuronal apoptosis, accumulation of necrotic tissues in the brain, and frequent intracerebral hemorrhages, highlighting the importance of CASP9 in brain development91. Caspase-3-deficient 129/Sv mice also show similar phenotypes as Casp9–/– mice92. These findings confirm that caspase-3 activation occurring downstream of caspase-9, which has been suggested from in vitro studies, is essential in mediating neural cell death that is required for brain development. Notably, Casp3–/– C57Bl/6 mice are normal, suggesting the possible presence of strain-specific genes that can actively suppress the phenotype caused by caspase-3 loss. Loss-of-function mutations in CASP9, APAF1, and CASP3 have been associated with neural tube defects93.
ADAR1- and ZBP1-regulated PANoptosis has been implicated in development and survival. Embryonic lethality in Adar1–/–, Adar1p150null/p150null, and Adar1E861A/E861A mice, which have a point mutation in the ADAR1 catalytic domain, is accompanied by hyperproduction of type I IFNs, upregulation of ISGs, and widespread cell death, particularly in liver hematopoietic cells80,94–96. The loss of MDA5 or the downstream adaptor protein MAVS rescues the embryonic lethality of Adar1–/– and Adar1p150null/p150null mice, although these mice still undergo lethality shortly after birth. With the concurrent loss of ZBP1, survival is significantly improved in Adar1–/–Mavs–/– mice or Adar1p150null/p150nullMavs–/–mice94–96, suggesting that developmental lethality is mediated by simultaneous activation of ZBP1, MDA5, and potentially other pathways. Beyond ADAR1-deficient conditions, ZBP1 also contributes to other developmental defects. The role of ZBP1 in driving lethality has further been shown in Adar1P195A/p150null mice, which carry a mutation within the Zα domain (P195A) on one allele in combination with deletion of ADAR1p150 in the second allele of Adar1, and in Adar1Zα/– mice95. Mice expressing RIPK1 with a mutated RHIM domain (mRHIM) undergo perinatal lethality, which can be rescued by deletion of ZBP197 or mutation of the ZBP1 Zα domain75.
Infectious diseases
All RCD pathways have been implicated in infectious diseases. Depending on the type of infection, these RCD pathways can be detrimental or beneficial. Lytic cell death pathways such as pyroptosis, necroptosis, ferroptosis, and PANoptosis are important for clearing invading pathogens. Pyroptosis releases intracellular bacteria residing within macrophages, such as Burkholderia thailandensis, Salmonella Typhimurium, and Legionella pneumophila, which are subsequently killed by neutrophils via a mechanism dependent on the production of ROS98. However, neutrophils in Nox2–/– mice infected with Pseudomonas aeruginosa undergo pyroptosis to compensate for deficiency of another major antimicrobial pathway99. The physical extrusion of infected enterocytes from the intestine depends on NLRC4 inflammasome activation during Salmonella infection100. Gsdmd–/– or Il18–/– mice are more susceptible to Salmonella infection, suggesting that pyroptosis-mediated cytokine release promotes host protection against Salmonella infection101. GSDMD can also damage and lyse bacteria directly by binding to cardiolipin and oligomerizing to form pores on the bacterial cell membrane102,103. HIV infection induces pyroptosis in quiescent lymphoid CD4 T cells, consequently leading to CD4 T-cell depletion and chronic inflammation104.
Many bacteria and viruses are known to induce necroptosis by activating RIPK3 and MLKL. Most studies have shown that necroptosis has a detrimental effect on bacterial infection. Necroptosis induced by Staphylococcus aureus leads to tissue damage and mortality105. Pore-forming toxins produced by various bacteria, such as Streptococcus marcescens, L. monocytes, S. aureus, and S. pneumonia, induce necroptosis to promote acute bacterial pneumonia106. Administration of RIPK1 or MLKL inhibitors has been shown to reduce morbidity and mortality during S. marcescens hemorrhagic pneumonia106. In contrast, necroptosis signaling is essential for host defense against S. pneumonia. Higher plasma concentrations of RIPK3 could serve as a potential marker of pneumococcal pneumonia107. EspL, which is an effector of the type III secretion system of enteropathogenic E. coli, degrades the RHIM-containing proteins RIPK1, RIPK3, TRIF, and ZBP1 to restrict pyroptosis, apoptosis and necroptosis during infection108. E. coli expressing NleB1 inhibits apoptosis and necroptosis by modifying arginine residues in death domain-containing proteins such as FADD and RIPK1109,110. NleB1-deficient E. coli fails to colonize the intestine of the host, suggesting the protective role of apoptosis and necroptosis during E. coli infection. The secreted effector of Toxoplasma gondii, TgNSM, prevents host cell necroptosis, thereby assuring the survival of intracellular cysts leading to chronic infection111. In addition, RIPK3 deficiency in combination with caspase-8 or FADD leads to increased susceptibility to Yersinia infection62.
Pathogens regulate ferroptosis by influencing host iron metabolism, iron transport, ROS production and antioxidant defenses13. Ferroptosis restricts hepatitis C viral replication in the host cell112. Patients with COVID-19 have high serum ferritin levels, indicating high iron exposure in tissues. Although the ferroptosis marker TfR1 is induced in Syrian golden hamsters following SARS-CoV-2 infection113, the significance of ferroptosis in SARS-CoV-2 infection and COVID-19 remains unclear. Mycobacterium and Pseudomonas infection induce ferroptosis, which upon inhibition increases the infection, indicating that ferroptosis promotes Mycobacterium and Pseudomonas infection114,115.
The role of PANoptosis has been well studied during IAV and SARS-CoV-2 infection19,74,116,117. IAV infection induces NLRP3 inflammasome activation and PANoptosis in macrophages, which is dependent on the Zα domain of ZBP174,75. The mortality caused by IAV infection in WT mice is prevented upon loss of the ZBP1 or Zα domain of ZBP119,118, suggesting that ZBP1-mediated PANoptosis provides host defense against IAV infection. In contrast to IAV infection, PANoptosis contributes to worse outcomes in COVID-19. SARS-CoV-2 infection-induced mortality is reduced in mice upon administration of neutralizing antibodies against the PANoptosis activating cytokine combination TNF and IFN-γ18. The synergism of TNF and IFN-γ induces NO via the STAT1/IRF1 axis to activate PANoptosis in a caspase-8-dependent manner. Indeed, deletion of Nos2 or Casp8 reduces SARS-CoV-2 infection-driven weight loss without impacting peak viral burdens in mice119, indicating the pathogenic role of the iNOS-caspase-8 axis in COVID-19. Moreover, treatment with IFNs potentiates ZBP1-dependent PANoptosis during β-coronavirus infection, including MHV and SARS-CoV-219. As a result of its antiviral properties, IFN therapy has been suggested to treat patients with viral infection. However, clinical studies have shown worse outcomes in patients with COVID-19 following IFN therapy116. Similarly, MHV-infected mice show increased mortality after administration of IFN-β. However, Zbp1–/– mice show reduced PANoptosis and mortality following IFN-β administration compared with WT mice19, suggesting that ZBP1-mediated PANoptosis impedes the therapeutic efficacy of IFN-β in COVID-19. Furthermore, murine BMDMs and human THP-1 macrophages undergo AIM2-dependent PANoptosis during infection with HSV1 or Francisella novicida. Confocal microscopy shows colocalization of ASC with CASP8 and RIPK3, and immunoprecipitation studies have found interactions of ASC with CASP1, CASP8, RIPK3, AIM2, and Pyrin in macrophages infected with HSV122.
Tumorigenesis
The role of pyroptosis, apoptosis, necroptosis, and ferroptosis in cancer remains controversial because they can be both tumor suppressive and tumor promoter depending on the context40,120. For instance, in an AOM-DSS model of colorectal tumorigenesis, Gsdme–/– mice show reduced tumor burden and HMGB1 release in the colon compared to their WT littermates121, implying that GSDME-mediated pyroptosis promotes colorectal tumorigenesis by releasing HMGB1. However, WT and GSDME-depleted cancer cells develop tumors of similar size in xenograft models of colorectal cancer, lung cancer, and melanoma122. Similarly, the role of necroptosis in tumorigenesis also seems to be controversial. The downregulation of RIPK3 or MLKL is associated with poor prognosis in patients with various types of cancer, such as colorectal cancer, acute myeloid leukemia, melanoma, breast cancer, ovarian cancer, and gastric cancer. On the other hand, the upregulation of RIPK3 or RIPK1 is associated with a promising prognosis in patients with lung cancer, glioma, and pancreatic cancer120. Blockade of the necrosome in vitro promotes proliferation of cancer cells and induces an aggressive oncogenic phenotype. Deletion of RIPK3 or blockade of RIPK1 has shown protection against pancreatic oncogenesis123, indicating that necroptosis promotes pancreatic tumorigenesis. Necroptosis can induce cancer cell death, leading to a promising prognosis, while it can also lead to inflammation and cancer.
Loss of apoptosis allows cancer cells to survive longer, resulting in the accumulation of mutations associated with different stages of tumorigenesis, such as proliferation, invasion, migration, and metastasis. There are several apoptosis-inducing anticancer drugs; however, cancer cells develop resistance to these agents124. Some agents, such as the combination of IFNs with nuclear export inhibitors or TNF and IFN-γ, have shown promising anticancer effects21,23. The combination of IFN with KPT induces ZBP1-dependent PANoptosis, which regresses melanoma growth in mice. PANoptosis induced by IFN plus KPT involves ADAR1, the only other human protein to contain a Zα domain. The interaction of ZBP1 with ADAR1 inhibits cell death, while its interaction with RIPK3 promotes cell death21. Similarly, ZBP1-dependent cell death can improve responsiveness to immune checkpoint blockade therapy in mouse models of melanoma125. Furthermore, PANoptosis-associated genes, including ZBP1, have been associated with a better prognosis in patients with skin cutaneous melanoma. TNF administration plus IFN-γ-induced PANoptosis has shown preclinical promise in promoting cell death in human cancer cells to reduce tumor size in murine ectopic transplant models23. In addition, IRF1-dependent PANoptosis inhibits colorectal tumorigenesis126. Overall, the role of pyroptosis and necroptosis in tumorigenesis is controversial. However, agents that induce PANoptosis are promising in treating cancer.
Autoimmune and inflammatory diseases
Deletion of GSDMD in MefvV726A/V726A, a mouse model of familial Mediterranean fever (FMF), an autoimmune disease driven by mutations in the gene Mefv, leads to normal growth and rescues inflammation127. In a mouse model of alcoholic hepatitis, loss of GSDMD mitigates the development of steatohepatitis128, indicating that pyroptosis contributes to sterile inflammation in liver disease. Gain-of-function mutations in NLRP3 have been associated with cryopyrin-associated periodic syndromes (CAPS)129. As GSDMD is activated downstream of NLRP3, it is possible that pyroptosis is involved in CAPS. Indeed, loss of GSDMD ameliorated inflammatory symptoms in a mouse model of NLRP3 gain-of-function mutations130.
Deficiency of RIPK3 or MLKL prevents airway inflammation in mice subjected to cigarette smoke-induced experimental chronic obstructive pulmonary disease (COPD)131, suggesting that necroptosis signaling contributes to inflammatory responses, airway remodeling and emphysema in COPD. The lungs of patients with COPD display active RIPK3 and MLKL. Administration of Nec-1 or loss of RIPK3 protects the liver from alcoholic and drug-induced liver injury132,133.
Ferroptosis has been associated with systemic lupus erythematosus (SLE). Patients with SLE and mice prone to lupus show low neutrophil counts and increased lipid ROS production. Neutrophil-specific Gpx4 haplosufficiency mirrors key clinical features of human SLE, including autoantibodies, neutropenia, skin lesions and proteinuria, suggesting that neutrophil ferroptosis leads to neutropenia and disease manifestations in SLE134. Indeed, administration of a ferroptosis inhibitor ameliorated disease severity in lupus-prone mice. Moreover, autoantibodies and IFN-α induce ferroptosis in neutrophils by suppressing Gpx4 expression134. Thus, ferroptosis in neutrophils promotes autoimmune disease, possibly by releasing autoantigens. Similarly, ferroptosis in human airway epithelial cells is associated with the release of mitochondrial DNA and consequent worse asthma patient outcomes135.
Several preclinical studies have indicated the role of PANoptosis in inflammatory diseases. Mutation of the proline-serine-threonine phosphatase-interacting protein 2 gene in mice (Pstpip2cmo) causes inflammatory lesions in the bones and various degrees of skin and paw inflammation, closely resembling the human disorder known as chronic recurrent multifocal osteomyelitis. While neutrophils and IL-1β are critical in the initiation of the disease, the combined deletion of RIPK3, caspase-1, and caspase-8 – all critical components of PANoptosis – prevents cytokine release and disease progression in Pstpip2cmo mice81. Induction of PANoptosis by proinflammatory cytokines, particularly the synergism of TNF and IFN-γ, contributes to lethal shock in a mouse model, which mirrors the major symptoms of cytokine storm associated diseases. The mortality driven by PANoptosis is prevented upon loss of STAT1 or codeletion of CASP8 and RIPK318. ZBP1-mediated PANoptosis contributes to the pathology of several diseases. In humans, ADAR1 loss-of-function or MDA5 gain-of-function mutations have been identified in rare autoimmune diseases such as AGS. Furthermore, mutations in the ADAR1 Zα domain cause AGS and BSN when combined with alleles that cause loss of ADAR1p150 expression. These conditions are mimicked in Adar1mZα/– or Adar1P195A/p150null mice, and these phenotypes are rescued by concomitant deletion of ZBP1 or the ZBP1 Zα domain94,95. Additionally, mice lacking SETDB1 in intestinal epithelial cells have severe bowel inflammation, which is prevented by deletion of ZBP1136. These findings have established a possible pathological role of ZBP1-dependent PANoptosis in common inflammatory diseases.
Summary
Innate immunity-mediated cell death plays critical roles across homeostasis, development, autoinflammatory diseases, host defense, and tumorigenesis. While nonlytic cell death–apoptosis is mostly implicated in homeostasis and development, lytic cell death pathways such as pyroptosis, necroptosis, ferroptosis, and PANoptosis are associated with infectious and autoinflammatory diseases. The RCD pathways discussed in this review are mostly distinguished by molecules, morphologies, and stimuli. Among the lytic cell death pathways, pyroptosis and PANoptosis are generally associated with the release of IL-1β and IL-18. Since cancer cells develop resistance to therapeutics, switching a mode of cell death to another or engaging more than one type of RCD could be beneficial. Importantly, agents that regulate PANoptosis show great potential for the treatment of cancer.
These RCD pathways share molecules that execute cell death. Depending on the complex assembled by the shared molecules, cells commit to one of these RCD pathways. For instance, inflammasomes execute pyroptosis, apoptosomes and complex II drive intrinsic and extrinsic apoptosis, necrosomes result in necroptosis, and PANoptosomes execute PANoptosis. The assembly of these complexes depends on the type of stimulus. However, the complex responsible for the execution of ferroptosis is not clear. While pyroptosis and necroptosis are accompanied by plasma membrane rupture mediated by NINJ1 for the release of larger DAMPs14, the role of NINJ1 in ferroptosis and PANoptosis has not been studied. While our understanding of the fundamental role of RCD pathways has improved, many questions remain. For instance, how does a dedicated molecule of an RCD pathway influence another RCD pathway? What is the identity of additional sensors and triggers? These RCD pathways also require further molecular characterization in different cell and tissue types. Since RCD pathways and their molecular components have been widely implicated across the disease spectrum, continued research on these RCD pathways is needed to discover therapeutic targets.
Acknowledgements
We apologize to our colleagues in the field whose work could not be cited owing to space limitations.
Funding
This work was supported by grant (RS-2023-00251395 to R.K.) from the National Research Foundation of Korea (NRF), by new faculty startup funds from Seoul National University (2023-0024 to R.K) and by a grant from the MD-PhD/Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (to E.L).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ein Lee, Chang-Hyun Song.
References
- 1.Wang Y, Kanneganti TD. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput Struct. Biotechnol. J. 2021;19:4641–4657. doi: 10.1016/j.csbj.2021.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green DR. The Coming Decade of Cell Death Research: Five Riddles. Cell. 2019;177:1094–1107. doi: 10.1016/j.cell.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020;21:678–695. doi: 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 4.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsvetkov P, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twiddy D, Cohen GM, Macfarlane M, Cain K. Caspase-7 is directly activated by the approximately 700-kDa apoptosome complex and is released as a stable XIAP-caspase-7 approximately 200-kDa complex. J. Biol. Chem. 2006;281:3876–3888. doi: 10.1074/jbc.M507393200. [DOI] [PubMed] [Google Scholar]
- 7.Stennicke HR, et al. Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson DW, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 10.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayagaki N, et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591:131–136. doi: 10.1038/s41586-021-03218-7. [DOI] [PubMed] [Google Scholar]
- 15.Ke FFS, et al. Embryogenesis and Adult Life in the Absence of Intrinsic Apoptosis Effectors BAX, BAK, and BOK. Cell. 2018;173:1217–1230.e1217. doi: 10.1016/j.cell.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Hakem R, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/S0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida H, et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/S0092-8674(00)81733-X. [DOI] [PubMed] [Google Scholar]
- 18.Karki R, et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184:149–168.e117. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karki R, et al. ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci. Immunol. 2022;7:eabo6294. doi: 10.1126/sciimmunol.abo6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mall R, et al. Pancancer transcriptomic profiling identifies key PANoptosis markers as therapeutic targets for oncology. NAR Cancer. 2022;4:zcac033. doi: 10.1093/narcan/zcac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karki R, et al. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 2021;37:109858. doi: 10.1016/j.celrep.2021.109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, et al. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 2021;597:415–419. doi: 10.1038/s41586-021-03875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malireddi RKS, et al. Inflammatory Cell Death, PANoptosis, Mediated by Cytokines in Diverse Cancer Lineages Inhibits Tumor Growth. Immunohorizons. 2021;5:568–580. doi: 10.4049/immunohorizons.2100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim HE, Du F, Fang M, Wang X. Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proc. Natl. Acad. Sci. USA. 2005;102:17545–17550. doi: 10.1073/pnas.0507900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 30.Scott FL, et al. The Fas-FADD death domain complex structure unravels signalling by receptor clustering. Nature. 2009;457:1019–1022. doi: 10.1038/nature07606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strasser A, Harris AW, Huang DC, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jost PJ, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehlen P, Tauszig-Delamasure S. Dependence receptors and colorectal cancer. Gut. 2014;63:1821–1829. doi: 10.1136/gutjnl-2013-306704. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, et al. Mediation of the DCC apoptotic signal by DIP13 alpha. J. Biol. Chem. 2002;277:26281–26285. doi: 10.1074/jbc.M204679200. [DOI] [PubMed] [Google Scholar]
- 35.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 36.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 37.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 39.de Vasconcelos, N. M. & Lamkanfi, M. Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis. Cold Spring Harb. Perspect. Biol.1210.1101/cshperspect.a036392 (2020). [DOI] [PMC free article] [PubMed]
- 40.Karki R, Kanneganti TD. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer. 2019;19:197–214. doi: 10.1038/s41568-019-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 42.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 43.Degterev A, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaiser WJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nailwal H, Chan FK. Necroptosis in anti-viral inflammation. Cell Death Differ. 2019;26:4–13. doi: 10.1038/s41418-018-0172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong Y, et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer. 2019;18:100. doi: 10.1186/s12943-019-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bannai S, Tsukeda H, Okumura H. Effect of antioxidants on cultured human diploid fibroblasts exposed to cystine-free medium. Biochem Biophys. Res. Commun. 1977;74:1582–1588. doi: 10.1016/0006-291X(77)90623-4. [DOI] [PubMed] [Google Scholar]
- 49.Seiler A, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Chen D, et al. iPLA2beta-mediated lipid detoxification controls p53-driven ferroptosis independent of GPX4. Nat. Commun. 2021;12:3644. doi: 10.1038/s41467-021-23902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/S1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 52.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimada K, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamkanfi M, et al. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol. Cell Proteom. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malireddi RK, Ippagunta S, Lamkanfi M, Kanneganti TD. Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J. Immunol. 2010;185:3127–3130. doi: 10.4049/jimmunol.1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuchiya K, et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat. Commun. 2019;10:2091. doi: 10.1038/s41467-019-09753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Man SM, et al. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1beta production. J. Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Opdenbosch N, et al. Caspase-1 Engagement and TLR-Induced c-FLIP Expression Suppress ASC/Caspase-8-Dependent Apoptosis by Inflammasome Sensors NLRP1b and NLRC4. Cell Rep. 2017;21:3427–3444. doi: 10.1016/j.celrep.2017.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pierini R, et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19:1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sagulenko V, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurung P, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Philip NH, et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc. Natl Acad. Sci. USA. 2014;111:7385–7390. doi: 10.1073/pnas.1403252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malireddi, R. K. S. et al. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J. Exp. Med.21710.1084/jem.20191644 (2020). [DOI] [PMC free article] [PubMed]
- 64.Orning P, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem. Biol. 2017;24:507–514.e504. doi: 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu W, et al. Apaf-1 Pyroptosome Senses Mitochondrial Permeability Transition. Cell Metab. 2021;33:424–436.e410. doi: 10.1016/j.cmet.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 67.Kang S, et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 2015;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conos SA, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl. Acad. Sci. USA. 2017;114:E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alvarez-Diaz S, et al. The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dillon CP, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuk, H., Abdullah, M., Kim, D. H., Lee, H. & Lee, S. J. Necrostatin-1 Prevents Ferroptosis in a RIPK1- and IDO-Independent Manner in Hepatocellular Carcinoma. Antioxidants (Basel)1010.3390/antiox10091347 (2021). [DOI] [PMC free article] [PubMed]
- 73.Christgen S, et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis) Front Cell Infect. Microbiol. 2020;10:237. doi: 10.3389/fcimb.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuriakose, T. et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol110.1126/sciimmunol.aag2045 (2016). [DOI] [PMC free article] [PubMed]
- 75.Kesavardhana S, et al. The Zalpha2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J. Biol. Chem. 2020;295:8325–8330. doi: 10.1074/jbc.RA120.013752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basavaraju S, Mishra S, Jindal R, Kesavardhana S. Emerging Role of ZBP1 in Z-RNA Sensing, Influenza Virus-Induced Cell Death, and Pulmonary Inflammation. mBio. 2022;13:e0040122. doi: 10.1128/mbio.00401-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banoth B, et al. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis) J. Biol. Chem. 2020;295:18276–18283. doi: 10.1074/jbc.RA120.015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doerflinger M, et al. Flexible Usage and Interconnectivity of Diverse Cell Death Pathways Protect against Intracellular Infection. Immunity. 2020;53:533–547.e537. doi: 10.1016/j.immuni.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sundaram B, Karki R, Kanneganti TD. NLRC4 Deficiency Leads to Enhanced Phosphorylation of MLKL and Necroptosis. Immunohorizons. 2022;6:243–252. doi: 10.4049/immunohorizons.2100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karki, R. & Kanneganti, T. D. ADAR1 and ZBP1 in innate immunity, cell death, and disease. Trends Immunol.10.1016/j.it.2023.01.001 (2023). [DOI] [PMC free article] [PubMed]
- 81.Gurung P, Burton A, Kanneganti TD. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1beta-mediated osteomyelitis. Proc. Natl Acad. Sci. USA. 2016;113:4452–4457. doi: 10.1073/pnas.1601636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lukens JR, et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014;516:246–249. doi: 10.1038/nature13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newton K, et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019;575:679–682. doi: 10.1038/s41586-019-1752-8. [DOI] [PubMed] [Google Scholar]
- 84.Fritsch M, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683–687. doi: 10.1038/s41586-019-1770-6. [DOI] [PubMed] [Google Scholar]
- 85.Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat. Rev. Mol. Cell Biol. 2011;12:757–763. doi: 10.1038/nrm3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang TB, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J. Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 87.Salmena L, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takahashi N, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 90.Newton K, et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019;574:428–431. doi: 10.1038/s41586-019-1548-x. [DOI] [PubMed] [Google Scholar]
- 91.Kuida K, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/S0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 92.Momoi T, Fujita E, Urase K. Strain-specific caspase-3-dependent programmed cell death in the early developing mouse forebrain. Neuroreport. 2003;14:111–115. doi: 10.1097/00001756-200301200-00021. [DOI] [PubMed] [Google Scholar]
- 93.Zhou X, et al. Rare mutations in apoptosis related genes APAF1, CASP9, and CASP3 contribute to human neural tube defects. Cell Death Dis. 2018;9:43. doi: 10.1038/s41419-017-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hubbard NW, et al. ADAR1 mutation causes ZBP1-dependent immunopathology. Nature. 2022;607:769–775. doi: 10.1038/s41586-022-04896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiao H, et al. ADAR1 averts fatal type I interferon induction by ZBP1. Nature. 2022;607:776–783. doi: 10.1038/s41586-022-04878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Reuver R, et al. ADAR1 prevents autoinflammation by suppressing spontaneous ZBP1 activation. Nature. 2022;607:784–789. doi: 10.1038/s41586-022-04974-w. [DOI] [PubMed] [Google Scholar]
- 97.Lin J, et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540:124–128. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryu JC, et al. Neutrophil pyroptosis mediates pathology of P. aeruginosa lung infection in the absence of the NADPH oxidase NOX2. Mucosal Immunol. 2017;10:757–774. doi: 10.1038/mi.2016.73. [DOI] [PubMed] [Google Scholar]
- 100.Sellin ME, et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe. 2014;16:237–248. doi: 10.1016/j.chom.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Karki R, et al. IRF8 Regulates Transcription of Naips for NLRC4 Inflammasome Activation. Cell. 2018;173:920–933.e913. doi: 10.1016/j.cell.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 103.Liu X, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Doitsh G, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou Y, et al. Inhibiting PSMalpha-induced neutrophil necroptosis protects mice with MRSA pneumonia by blocking the agr system. Cell Death Dis. 2018;9:362. doi: 10.1038/s41419-018-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonzalez-Juarbe N, et al. Pore-Forming Toxins Induce Macrophage Necroptosis during Acute Bacterial Pneumonia. PLoS Pathog. 2015;11:e1005337. doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang HR, et al. RIPK3 Activates MLKL-mediated Necroptosis and Inflammasome Signaling during Streptococcus Infection. Am. J. Respir. Cell Mol. Biol. 2021;64:579–591. doi: 10.1165/rcmb.2020-0312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pearson JS, et al. EspL is a bacterial cysteine protease effector that cleaves RHIM proteins to block necroptosis and inflammation. Nat. Microbiol. 2017;2:16258. doi: 10.1038/nmicrobiol.2016.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li S, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature. 2013;501:242–246. doi: 10.1038/nature12436. [DOI] [PubMed] [Google Scholar]
- 110.Pearson JS, et al. A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature. 2013;501:247–251. doi: 10.1038/nature12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosenberg A, Sibley LD. Toxoplasma gondii secreted effectors co-opt host repressor complexes to inhibit necroptosis. Cell Host Microbe. 2021;29:1186–1198.e1188. doi: 10.1016/j.chom.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamane D, et al. FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem. Biol. 2022;29:799–810.e794. doi: 10.1016/j.chembiol.2021.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bednash JS, et al. Syrian hamsters as a model of lung injury with SARS-CoV-2 infection: Pathologic, physiologic, and detailed molecular profiling. Transl. Res. 2022;240:1–16. doi: 10.1016/j.trsl.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Amaral EP, et al. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 2019;216:556–570. doi: 10.1084/jem.20181776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dar HH, et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Invest. 2018;128:4639–4653. doi: 10.1172/JCI99490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karki R, Kanneganti TD. Innate immunity, cytokine storm, and inflammatory cell death in COVID-19. J. Transl. Med. 2022;20:542. doi: 10.1186/s12967-022-03767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karki R, Kanneganti TD. The ‘cytokine storm’: molecular mechanisms and therapeutic prospects. Trends Immunol. 2021;42:681–705. doi: 10.1016/j.it.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thapa RJ, et al. DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell Host Microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simpson DS, et al. Interferon-gamma primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway. Immunity. 2022;55:423–441.e429. doi: 10.1016/j.immuni.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu X, et al. The role of necroptosis in disease and treatment. MedComm (2020) 2021;2:730–755. doi: 10.1002/mco2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan G, Huang C, Chen J, Zhi F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J. Hematol. Oncol. 2020;13:149. doi: 10.1186/s13045-020-00985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Schutter E, et al. GSDME and its role in cancer: From behind the scenes to the front of the stage. Int J. Cancer. 2021;148:2872–2883. doi: 10.1002/ijc.33390. [DOI] [PubMed] [Google Scholar]
- 123.Seifert L, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532:245–249. doi: 10.1038/nature17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 125.Muendlein HI, et al. ZBP1 promotes inflammatory responses downstream of TLR3/TLR4 via timely delivery of RIPK1 to TRIF. Proc. Natl Acad. Sci. USA. 2022;119:e2113872119. doi: 10.1073/pnas.2113872119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karki, R. et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight510.1172/jci.insight.136720 (2020). [DOI] [PMC free article] [PubMed]
- 127.Kanneganti A, et al. GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean fever. J. Exp. Med. 2018;215:1519–1529. doi: 10.1084/jem.20172060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu B, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 2018;68:773–782. doi: 10.1016/j.jhep.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 129.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xiao J, et al. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 2018;16:e3000047. doi: 10.1371/journal.pbio.3000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu Z, et al. Necroptosis Signaling Promotes Inflammation, Airway Remodeling, and Emphysema in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021;204:667–681. doi: 10.1164/rccm.202009-3442OC. [DOI] [PubMed] [Google Scholar]
- 132.Takemoto K, et al. Necrostatin-1 protects against reactive oxygen species (ROS)-induced hepatotoxicity in acetaminophen-induced acute liver failure. FEBS Open Bio. 2014;4:777–787. doi: 10.1016/j.fob.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramachandran A, et al. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li P, et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 2021;22:1107–1117. doi: 10.1038/s41590-021-00993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nagasaki, T. et al. 15LO1 dictates glutathione redox changes in asthmatic airway epithelium to worsen type 2 inflammation. J. Clin. Invest.13210.1172/JCI151685 (2022). [DOI] [PMC free article] [PubMed]
- 136.Wang R, et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature. 2020;580:386–390. doi: 10.1038/s41586-020-2127-x. [DOI] [PubMed] [Google Scholar]